Abstract
Escherichia coli have served as important model organisms for over a century—used to elucidate key aspects of genetics, evolution, molecular biology, and pathogenesis. However, defining which strains actually belong to this species is erratic and unstable due to shifts in the characters and criteria used to distinguish bacterial species. Additionally, many isolates designated as E. coli are genetically more closely related to strains of Shigella than to other E. coli, creating a situation in which the entire genus of Shigella and its four species are encompassed within the single species E. coli. We evaluated all complete genomes assigned to E. coli and its closest relatives according to the biological species concept (BSC), using evidence of reproductive isolation and gene flow (i.e., homologous recombination in the case of asexual bacteria) to ascertain species boundaries. The BSC establishes a uniform, consistent, and objective principle that allows species-level classification across all domains of life and does not rely on either phenotypic or genotypic similarity to a defined type-specimen for species membership. Analyzing a total of 1,887 sequenced genomes and comparing our results to other genome-based classification methods, we found few barriers to gene flow among the strains, clades, phylogroups, or species within E. coli and Shigella. Due to the utility in recognizing which strains constitute a true biological species, we designate genomes that form a genetic cohesive group as members of E. coliBIO.
Keywords: Escherichia coli, Shigella, enteric bacteria, recombination, speciation
Introduction
When initially isolated, Escherichia coli was designated Bacillus coli communis, a latinization describing its prominent characteristic as a “common colon bacterium” that could be readily cultured in a variety of substrates. The original specimen, as first described in 1885, was distinguished by its colony and cellular morphology, and its ability to ferment glucose, produce acid, and sour milk (Escherich 1885). Upon its rechristening in 1,919 to acknowledge its discoverer, and in the decades that ensued, features used for assignment to this species were expanded to include a suite of characters that distinguish E. coli from other enteric species (Koser 1923; Kauffmann 1944). Most notably, E. coli are lactose, catalase, and indole positive, and oxidase, urease, and citrate negative, although there is a low level of polymorphism for many of these properties.
Genetic and genomic features entered into the classification of E. coli in the 1960s with the application of DNA–DNA hybridization (DDH) procedures (Marmur et al. 1963). By this method, strains were considered as members of E. coli if they displayed ≥70% DNA similarity to the reference strains (Brenner et al. 1972)—noting that although DDH percentages do not match the actual amount of DNA identity between strains (Rosselló-Mora 2006), this method pioneered a threshold-based approach for defining bacterial species. Subsequently, other nucleic-acid-based cutoffs were applied to the delineation of bacterial species, such as ≥97% (Tindall et al. 2010; Yarza et al. 2014) and more recently ≥99% (Edgar 2018) 16S RNA sequence identity, or ≥95% average nucleotide identity (ANI) (Konstantinidis and Tiedje 2005) for the core set of genes shared among strains (Jain et al. 2018). Naturally, there is a certain circularity to this approach since sequence-identity thresholds were ascertained from strains that were already assigned to E. coli based on metabolic, morphological, or biochemical features, thereby constraining the genetic cutoffs to species boundaries that were already established. And unfortunately, hybridization and sequence-identity thresholds are convenient rather than universal, their biological basis remains unclear.
Phylogenetic analysis of E. coli strains that were considered to span the diversity in the species at large defined six main clades (A, B1, B2, D, E, and F) and several rarer clades (Herzer et al. 1990; Chaudhuri and Henderson 2012). However, expanding the set to include strains from additional animal and environmental sources yielded five “cryptic” clades (termed CI to CV) that were all more closely related to E. coli than to its sister species Escherichia fergusonii (Walk et al. 2009; Luo et al. 2011). The taxonomic status of these five unclassified clades remains uncertain: they cannot be differentiated from E. coli based on phenotypic characters, but they are genetically divergent, which led to a proposal that a least some of these clades (e.g., Clades III + IV and Clade V) might represent distinct species (Walk 2015).
As additional full genomes were integrated into the analyses, the phylogenetic structure and evolutionary relationships of E. coli became more refined, with recognition of increased numbers of subspecific groups (Lu et al. 2016; Abram et al. 2021) and suggestions that some might represent actual or incipient species (Didelot et al. 2012; Kang et al. 2021). To accommodate the burgeoning numbers of sequenced strains in all taxa, the Genome Taxonomy Database (GTDB; gtdb.ecogenomic.org/) recommended the application of a genome-wide identity threshold (analogous to ANI) to define bacterial species (Parks et al. 2018). Imposing their metrics, strains currently classified as E. coli would be split into six species—E. coli, E. coli_E, Escherichia ruysiae, Escherichia marmotae, Escherichia sp001660175, and Escherichia sp005843885—with the majority consigned to E. coli (Parks et al. 2021).
Classification of E. coli has also been confounded by the intransigence of Shigella as a separate genus. Every strain assigned to Shigella appears to fall within the variation spanned by E. coli (Brenner et al. 1973; Ochman et al. 1983), and the four Shigella species originated independently, and multiple times, from within E. coli (Rolland et al. 1998; Pupo et al. 2000; Lan and Reeves 2002). Clearly, the taxonomy of E. coli is idiosyncratic and often supports conflicting results. To resolve these incongruencies, and to apply consistent and objective criteria to identifying species boundaries, we analyze and classify a comprehensive set of Escherichia and Shigella genomes according to the biological species concept (BSC) (Mayr 1942), a universally accepted procedure that circumscribes species based on homologous gene exchange. Although asexuality is often assumed to render bacteria immune to classification by the BSC (Donoghue 1985; Rosselló-Mora and Amann 2001; Costechareyre et al. 2009), the patterns of recombination in E. coli and related enteric bacteria provide a consistent and robust signal for species assignment (Brenner and Falkow 1971; Shen and Huang 1986; Dykhuizen and Green 1991; Lawrence and Retchless 2009; Didelot et al. 2012) and allow the application of a single biological feature to define species across all branches of the Tree of Life.
Results
To establish the species boundaries of E. coli and determine which sequenced genomes should be assigned to this species, we implemented three genome-based methods, including two (ConSpeciFix and PopCOGenT) that adhere to the precepts of the BSC. We considered 1,635 complete genomes designated as E. coli in the National Center for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov/) as of August 2020. To ensure that the breadth of variation in the species at large is represented, we included the genomes of strains classified to the five Escherichia phylogroups (Clades I–V) described by Walk et al. (2009), to the E. coli phylogroups resolved by Abram et al. (2021), and to the newly designated Escherichia species proposed by the GTDB (E. albertii, E. coli, E. coli_E, E. fergusonii, E. marmotae, E. ruysiae, Escherichia sp001660175, Escherichia sp002965065, Escherichia sp004211955, and Escherichia sp005843885) (gtdb.ecogenomic.org). Additionally, we analyzed all other fully sequenced genomes assigned to the genus Escherichia as well as representatives of the four designated species of Shigella, which are known to have originated from within E. coli but have mostly maintained their status as a separate genus for historical reasons.
ConSpeciFix
Using gene flow as a condition for species membership, this method calculates recombination based on homoplasies in genes common to the strains under consideration (Bobay et al. 2018).
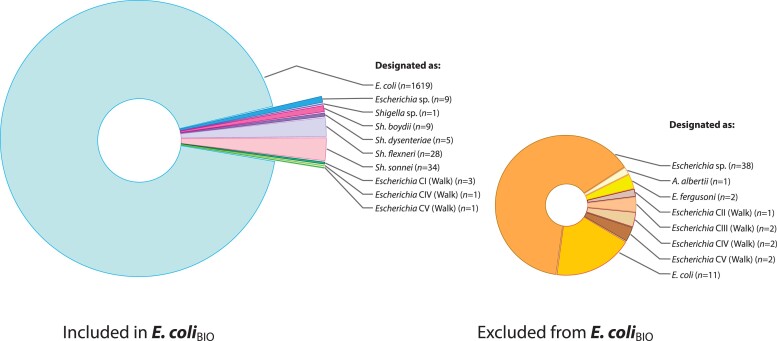
(i) Applying ConSpeciFix to assess species status of strains designated as E. coli in NCBI. Genomes classified as E. coli in the NCBI database form more than one species, with 12 strains that are sexually isolated from the rest of the E. coli genomes. All remaining genomes designated as E. coli in the NCBI database constitute a true biological species (hereafter referred to as E. coliBIO to identify membership as a biological species) and serve as a reference to evaluate genomes classified by other means. The numbers and distribution of strains that are members of this biological species, and those that are reproductively isolated and excluded from E. coliBIO, are shown in figures 1 and 2.
(ii) Applying ConSpeciFix to assess species status of phylogroups defined by Walk et al. (2009) and Abram et al. (2021). All the studied strains classified to the E. coli phylogroups of Abram et al., and to the E. coli taxonomic group and Clade I specified by Walk et al., which together include strains classified as E. coli and Escherichia sp. in NCBI, are members of E. coliBIO based on ConSpeciFix. Genomes in their remaining clades (Clades II–V) constitute different species by ConSpeciFix, with the exception of one genome in Clade IV (E. coli B49-2 serovar O157:H7) and one in Clade V (E. coli strain E620 serovar ON5), both of which are members of E. coliBIO (supplementary table S1, Supplementary Material online).
(iii) Applying ConSpecFix to assess the status of E. coli species defined by GTDB. The GTDB recognizes E. coli and nine additional species (E. ruysiae, E. marmotae, E. coli_E. E. fergusonii, Escherichia albertii, Escherichia sp001660175, Escherichia sp002965065, Escherichia sp004211955, and Escherichia sp005843885). Of these nine additional species, only Escherichia coli_E, one strain of Escherichia sp001660175 (based on ANI), three strains of Escherichia sp005843885 (one of them based on ANI), and three strains of E. ruysiae (two of them based on ANI) were classified as E. coli in the NCBI database (supplementary table S1, Supplementary Material online). Although listed as differently by the GTDB, one strain of E. albertii and one strain of E. marmotae are classified as E. coli in the NCBI. Of the 12 genomes assigned to E. coli in the NCBI but excluded from E. coliBIO, nine were also excluded from E. coli by the GTDB (supplementary table S1, Supplementary Material online). ConSpeciFix assigned the GTDB species Escherichia sp001660175 (n = 1), sp004211955 (n = 2), and sp005843885 (n = 38) to a separate biological species. No members of these three GTDB species belong to E. coliBIO when used as test lineages, but they form a biological species distinct from E. coliBIO when Escherichia sp005843885 is used as a reference lineage. None of the other GTDB species is a member of either E. coliBIO or this new species.
(iv) Other enteric species. All tested genomes of the four Shigella species are members of E. coliBIO. In contrast, none of the genomes currently classified to any of the other Escherichia species [E. albertii (n = 1), E. fergusonii (n = 2), E. marmotae (n = 1)] or to any of the other enteric genera considered [Proteus (n = 2), Citrobacter (n = 2), Cronobacter (n = 2), Salmonella (n = 111), Enterobacter (n = 6), and Klebsiella (n = 4)] is a member of E. coliBIO. Genomes from genera other than Escherichia were included as controls.
Fig. 1.
Assignment of sequenced genomes to the biological species E. coliBIO. Wedges are labeled according to their taxon designation in the NCBI database or their assignment to an Escherichia phylogroup by Walk et al., with the number of genomes in each taxon indicated. Note that genomes that are both assigned to an Escherichia phylogroup (CI–CV) and taxonomically defined in the NCBI are excluded from counts of NCBI genomes. For example, one of the genomes assigned to E. coli by NCBI but excluded from E. coliBIO belongs to Walk Clade IV and was therefore excluded from the count of E. coli.
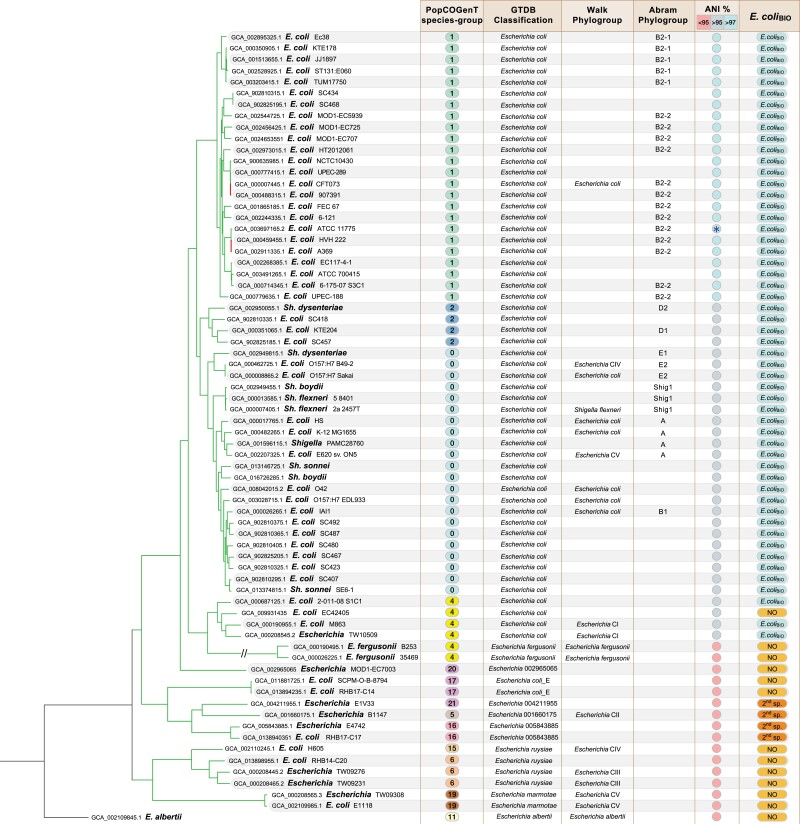
Fig. 2.
Maximum-likelihood phylogenetic tree of selected Escherichia genomes. Genomes were selected to represent the extent of diversity present in the genus and have <99.8% ANI to their nearest relative. For each genome, strain accession number and designation in the NCBI database is followed, from left to right, by PopCOGenT species-group, GTDB v207 classification, Walk et al. phylogroup (Clades I–V) and Abram et al. phylogroups wherever possible, ANI to E. coli ATCC 11775, and membership status in E. coliBIO. (Note that GCA_002109985_1, E. marmotae E1118, is labeled as E. coli in the PATRIC database). PopCOGenT species-groups are distinguished by number, and ANI % denotes extent of sequence identity to the reference genome (marked with asterisk): the first 24 genomes in the tree present an ANI > 97%; the following 30 an ANI between 95 and 97%, and the last 16 an ANI <95%. All branches have bootstrap support values >90% except for the strains GCA_000007445.1, GCA_000488315.1, GCA_000459455.1 and GCA_002911335.1, which are <60%. Seven of the 12 NCBI-classified E. coli strains that were excluded from E. coliBIO are NCBI pathogen detection assemblies (i.e., surveillance genomes) and were not classified by the GTDB classification: those genomes lacking taxonomic assignation in GTDB were classified to the same species as their closest relative having an ANI >95%.
PopCOGenT
PopCOGenT is an alternate method for grouping genomes based on gene flow (Arevalo et al. 2019). For the representative set of genomes evaluated by this method (n = 128), there were a total of 21 species-groups, of which 10 contained strains designated as E. coli in the NCBI database (supplementary table S1, Supplementary Material online). The phylogenetic relationships of a dereplicated subset of these genomes, along with their nomenclature, strain and species designations in different databases, and species-groupings based on several metrics, are presented in figure 2.
(i) Applying PopCOGenT to assess species status of clades defined by Walk et al. (2009) and Abram et al. (2021). Genomes from the E. coli taxonomic group specified by Walk are assigned to PopCOGenT species-groups 0 and 1 (fig. 2; supplementary table S1, Supplementary Material online), and Clades I, II, and III of Walk are each classified as different PopCOGenT species-groups (4, 5, and 6, respectively). Genomes from Walk Clade IV assort into two species-groups: one of which contains only Clade IV genomes, and another that contains genomes from both Clade V and the canonical E. coli and Shigella flexneri taxonomic groups specified by Walk. Similarly, genomes from Clade V of Walk segregate into two species-groups—the aforementioned one that contains genomes from Clade IV and the canonical E. coli and S. flexneri taxonomic groups, and a unique species-group (19) that contains only Clade V genomes. Several of the E. coli phylogroups defined by Abram et al. were distinguished as different species-groups by PopCOGenT.
(ii) Applying PopCOGenT to assess the status of E. coli species defined by GTDB. The five GTDB-recognized species within E. coli (E. coli, E. coli_E, E. ruysiae, Escherichia sp001660175, and Escherichia sp005843885) and the five other Escherichia species (E. albertii, E. fergusonii, E. marmotae, E. sp002965065, and E. sp004211955) were classified to multiple species-groups by PopCOGenT (supplementary table S1, Supplementary Material online). Each of the Escherichia species recognized by the GTDB forms a unique PopCOGent species-group, except 1) E. ruysiae, whose members were distributed into two PopCOGent species-groups (6, 15), 2) E. coli, whose members were distributed into four PopCOGent species-groups (0, 1, 2, 4), and 3) E. coli_E, whose members were distributed into two PopCOGent species-groups (8, 17) (fig. 2; supplementary table S1, Supplementary Material online).
(iii) Other enteric species. Whereas PopCOGenT separated the NCBI-designated strains of E. coli into 10 species-groups, all Shigella genomes considered by PopCOGenT, except Sh. dysenteriae (accession GCA_002950055.1), were classified as members of species-group 0. Most strains that were assigned to Escherichia species other than E. coli (or whose species status went unassigned) were deemed separate species by PopCOGenT, although many partitioned in species-groups that also contained members of E. coli. Though not included in figure 2, PopCOGenT distinguished Salmonella enterica, Salmonella bongori, Enterobacter cloacae, Enterobacter carcerogenus, and Proteus mirabilis as distinct species.
FastANI
This metric is based on sequence-identity thresholds (typically 95%) to delineate strains that constitute a species (Jain et al. 2018).
(i) Applying ANI to assess species status of clades defined by Walk et al. (2009) and Abram et al. (2021). Genomes from S. flexneri and the E. coli taxonomic group specified as Clade I by Walk, and one genome each from Clades IV and V, are all classified as members of the same species based on 95% ANI to the reference genome, E. coli ATCC 11775. Applying this 95% ANI threshold, the studied phylogroups distinguished by Abram et al. are also included in this species (supplementary table S1, Supplementary Material online). All genomes in this ANI species were originally designated as E. coli or S. flexneri in NCBI, except in the case of one genome classified as Escherichia sp. All remaining members of Clades IV and V, and all other members of the other clades defined by Walk et al., are sufficiently distant from E. coli ATCC 11775 and are not considered members of the species at this ANI threshold.
(ii) Applying ANI to assess the status of E. coli species defined by GTDB. Because the GTDB circumscribes species based on sequence-identity thresholds, the majority of the genomes assigned to E. coli have an ANI > 95% to the E. coli ATCC 11775 reference genome; however, there are a few exceptions due to the normalization applied by this database (supplementary table S1, Supplementary Material online).
(iii) Other enteric species. applying a sequence-identity threshold of 95% to E. coli ATCC 11775, all tested genomes of the four Shigella spp. are members of E. coli. None of the genomes classified to other Escherichia species (E. albertii and E. fergusonii) or to any of the other enteric genera (Proteus, Citrobacter, Cronobacter, Salmonella, Enterobacter, and Klebsiella) is a member of E. coli at this sequence-identity threshold.
Applying the many-to-many option in ANI returned results that were virtually identical to those recovered with the one-to-many comparisons to the single reference genome. For example, all other enteric species yielded ANI values <95% to members of both E. coli and Shigella. With regard to the biological species (E. coliBIO) defined ConSpeciFix, most genomes displayed ANI values <95% using the many-to-many option; however, the minimum ANI of 93.90% occurred between two strains having 97% and 98% ANI with the reference genome.
Maximum-Likelihood (ML) Phylogeny
To examine the evolutionary relationships among strains, we constructed a phylogeny on the dereplicated set of 70 genomes having <99.8% ANI to one another.
(i) Applying an ML phylogeny to assess species status of clades defined by Walk et al. (2009) and Abram et al. (2021). Our results broadly confirm the phylogroups distinguished by Walk et al. (2009), which is not surprising given that their phylogroups represent phylogenetically resolved clades. All E. coli and Shigella genomes that they defined were monophyletic, and Clades I, II, III, IV, and V each formed monophyletic groups, with the exception of one strain from each of Clade IV and Clade V, which grouped with E. coli and Shigella. In our tree, the clade containing E. coli and Shigella is most closely related to Walk Clade I, which is a sister group to E. fergusonii, and Walk Clades III, IV, and V together form a separate clade. In addition, each of the phylogroups resolved Abram et al. (2021) is monophyletic (fig. 2).
(ii) Applying an ML phylogeny to assess the status of E. coli species defined by GTDB. The clades defined in the ML phylogeny are consistent with the species distinguished by GTDB, and each is monophyletic. The only exceptions are the two strains classified as E. fergusonii, which reside on a very long branch, have low ANI (<95%) to the E. coli reference strain, and are not members of E. coliBIO based on ConSpeciFix (fig. 2; supplementary table S1, Supplementary Material online). The high bootstrap support of this branch suggests an ancient separation followed by limited recombination with divergent members of E. coli, as exemplified by the inclusion of E. fergusonii genomes in PopCoGenet species-group 4.
(iii) Other enteric species. Based on the ML phylogeny, the only members of other Escherichia species that occur in the monophyletic group that contains E. coli and Shigella are the two aforementioned strains of E. fergusonii.
Discussion
Bacterial strains were originally typed as E. coli based on their growth characteristics and possession of specific metabolic properties, and, more recently, based on their sequence similarity to one another or to a canonical strain. In addition, there are sufficiently high levels of recombination among strains, despite their asexual mode of reproduction, to warrant the classification of strains to this species based on the BSC. Using homologous exchange as the sole criterion for species assignment, we found that the vast majority of strains currently designated as E. coli, or as any of the species of Shigella, are all members of a single biological species, which we term E. coliBIO. Species-level definitions for the genus Escherichia have already been described by Walk (2015) and by Denamur et al. (2021), who have recently proposed a dichotomy between E. coli sensu stricto and E. coli sensu lato. However, such a classification scheme is inadequate because it does not have a biological basis and it can be universally applied (and, moreover, the new species, E. coli sensu lato, does not include Shigella, which belongs to the same species based on all genetic-based methods).
The species boundaries of E. coliBIO, which are based solely on homologous recombination within the set of core genes shared by all strains, largely agree with the classifications proposed by other schemes. For example, all methodologies, except PopCOGenT, consider the E. coli phylogroups of Abram et al. (2021) as comprising a single species, whereas PopCOGenT separates them into multiple species. That PopCOGenT, which also uses gene flow to delineate species, distinguishes more species than ConSpeciFix is due to the fact that PopCOGenT considers entire genomes when assigning species membership and can include horizontally transferred regions that are confined to subsets (or even pairs) of strains. Given that events of horizontal gene transfer occur over broad phylogenetic distances (and even between organisms classified to different domains or kingdoms), we chose exclude regions that are sporadically distributed among genomes and to confine analyses to core genes present in all genomes considered.
Strains typed to Shigella have been viewed as distinct from E. coli because they exhibited certain defining characteristics, including the absence of motility (due to a deletion in the fliF operon or insertion in the flhD operon) (Al Mamun et al. 1997) and an inability to ferment lactose (due to the lack of one or more lac fermentation or permease genes) (Luria and Burrous 1957; Khot and Fisher 2013). Moreover, the four species of Shigella are conventionally distinguished from one another by their O serotypes (Wheeler and Stuart 1946; Lan and Reeves 2002) because many of the other diagnostic properties, such as the utilization of mannitol and decarboxylation of ornithine, can be shared among species. However, the traits used to discriminate species of Shigella, and Shigella from E. coli, are often observed in enteroinvasive E. coli, which blurs the distinction between these species and genera.
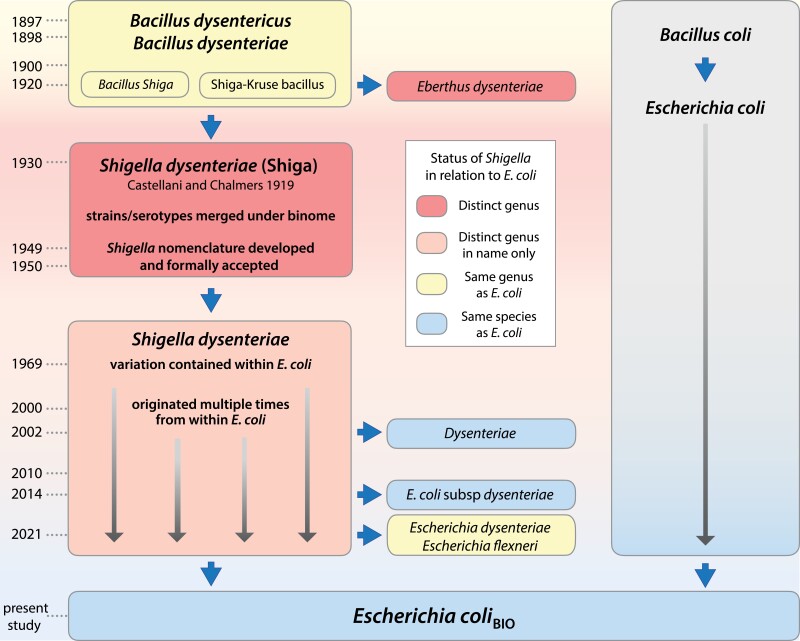
In actuality, E. coli and Shigella were initially assigned to the same genus due to their similarities but to different species to distinguish pathogenic and nonpathogenic forms (Bacillus dysenteriae and B. coli, respectively) (Shiga 1898). But as chronicled in figure 3, due to their medical significance, pathogenic strains were elevated to a separate genus in the following decades despite their resemblance to enteroinvasive E. coli (Ewing et al. 1952). The close genetic relationship between E. coli and Shigella was initially recognized in the 1950s based on their ability to reciprocally recombine (Luria and Burrous 1957), but because Shigella recombined with E. coli at lower frequencies than observed among strains of E. coli, each taxon maintained its status as a separate genus. However, subsequent analyses of genetic and genomic characters by DNA hybridization (Brenner et al. 1969, 1972), multilocus enzyme electrophoresis (Ochman et al. 1983), and chromosomal and plasmid gene phylogenies (Pupo et al. 2000; Lan and Reeves 2002) all indicated that strains typed as Shigella fall within the variation observed in E. coli.
Fig. 3.
Chronological changes in the S. dysenteriae and E. coli nomenclature. References used to produce this figure are listed in Supplementary material online.
The fact that Shigella remains classified as a distinct genus, despite its genetic and phenotypic overlap with E. coli, is further complicated by the fact that other named species within the genus Escherichia (e.g., E. albertii or E. fergusonii) do not recombine with E. coli, and can be differentiated based on such metabolic characters as 1) the lack of acid production from D-xylose, melibiose, L-rhamnose, and dulcitol for E. albertii (Hinenoya et al. 2019) and (2) an incapacity to ferment sorbitol and lactose, coupled with the ability to ferment adonitol, amygdalin, and cellobiose for E. fergusonii (Farmer et al. 1985). Taken together, this creates a situation in which the genus Escherichia contains multiple distinguishable species, whereas the four named species of Shigella should be subsumed within E. coli.
To mitigate confusion that might stem from abolishing the genus Shigella, Brenner et al. (1973) proposed the use of two separate nomenclatures—one for diagnostic purposes and one for genetic purposes—though it is difficult to see how this serves as an improvement. Lan and Reeves (2002) regarded the species of Shigella as serotypes within E. coli and removed the generic name, referring to them simply as Boydii, Sonnei, Flexneri, and Dystenteriae. Meier-Kolthoff et al. (2014) proposed including the four Shigella species as subspecies of E. coli, with nomenclature following guidelines of the Bacteriological Code (Lapage et al. 1992): In this system, for example, Shigella dysenteriae would be renamed as E. coli subsp. dysenteriae, and current members of E. coli as E. coli subsp. coli. Along similar lines, Parks et al. (2020) suggested including the four species of Shigella within the genus Escherichia, creating E. sonnei, E. boydii, E flexneri, and E. dysenteriae. However, based on DNA similarity threshold that they routinely use to define species, these newly named Escherichia species should remain within E. coli (Parks et al. 2021).
To circumvent issues surrounding the elimination or amendment of species names, we propose that conspecifics defined by the BSC be classified under the heading of a single biological species, as denoted by a subscripted suffix “BIO” adjoined to the latin biome. This procedure would place strains of E. coli and Shigella under the umbrella of a single biological species, in this case, E. coliBIO, but would retain their full names to maintain clinically and historically relevant information. As such, S. dysenteriae would be labeled EcoliBIOS. dysenteriae, and current members of E. coli as E. coliBIO followed by their strain designation. This resolution mimics the nomenclature developed for serovars of S. enterica and does not impose a taxonomic revision but is nevertheless useful in indicating which strains are members of the same biological species. The retention of strain appellations in the proposed scheme maintains consistency with the traditional nomenclature and avoids conflict with clinical identification and applications.
Despite the ability of E. coli and other bacteria to acquire genes from distant sources, recombination between shared homologs occurs primarily among sequences with high levels of similarity (Shen and Huang 1986; Rayssiguier et al. 1989; Roberts and Cohan 1993; Matic et al. 1995; Zawadzki et al. 1995; Majewski and Cohan 1999). This feature enables a natural classification of bacteria into species based on their propensity for homologous exchange, a biological criterion that can be applied to all lifeforms. To assure the universality of species definition, it is, therefore, necessary to confine analyses of recombination to the core set of genes shared among genomes. Those sequences with rare or sporadic distributions, as might originate from infrequent or independent events of horizontal gene transfer between taxa, occur in eukaryotes as well as bacteria (Akanni et al. 2015; Husnik and McCutcheon 2018; Wu et al. 2022), and can involve very distant taxa. Thus, such genes are best excluded from consideration when delineating species boundaries
Species, when defined by their capacity for gene flow, constitutes the only taxonomic rank based on a biological process rather than an arbitrary or subjective criterion (Bapteste and Boucher 2009; Lawrence and Retchless 2009). The recent availability of genome sequence data now allows the application of the same parameters for delineating species boundaries to asexual lifeforms (bacteria, archaea, viruses), all of which were previously considered as not amenable to classification based on the BSC (Donoghue 1985; Rosselló-Mora and Amann 2001; Costechareyre et al. 2009). This uniformity in defining species has implications beyond taxonomic classification in that the formation of equivalently defined species allows comparisons of evolutionary processes across all lifeforms (Staley 2009) and more accurate inferences about the rates and patterns of speciation in different groups of organisms.
The ANI divergence between strains in E. coliBIO can be as much as 6.1%. This relatively high level of divergence between members of the same species is evident at other taxonomic ranks: for example, between E. coliBIO and other species of Escherichia (E. albertii, E. fergusonii, and Clades II, III, IV, and V), sequence divergence ranges from 8% to 12%, and between Escherichia and its sister genus, Salmonella, the divergence among shared genes averages 15%. This degree of variation within and among species sharply contrasts the situation in, say, humans, in which the sequence divergence between homologs from two individuals is a mere 0.1% (Lek et al. 2016), and there is only a 0.5% difference to our sister species Homo neanderthalensis (Noonan et al. 2006) and 1.2% difference to our sister genus Pan (Carroll 2003).
The genetic approach to bacterial identification and classification, which began in the 1960s (Marmur et al. 1963), is more instructive than metabolic typing, which relies on a subjective set of diagnostic features (which themselves can originate by different means within and across species, and are often not discrete) (Priest et al. 1993). Moreover, a genetic delineation of biological species divulges the actual extent of phenotypic variation that is present in a species. For example, E. coli is traditionally distinguished from S. enterica as being Lac-positive and Citrate-negative; however, many members of E. coli, including most Shigella and many pathogenic strains, are lactose nonfermenters, and citrate-positive strains of E. coli have been reported (Ishiguro et al. 1978) and evolved (Blount et al. 2012). All of the classification methods that we evaluated indicate that the majority of E. coli and Shigella represent a single species; however, our analyses, based on the propensity for homologous exchange, provide the genetic basis for this conclusion.
Reports that strains within some phylogenetic clades of E. coli recombine at higher frequencies within one another than with members of other clades—as might be expected if homologous exchange relied wholly on the degree of sequence similarity—has been interpreted as evidence of incipient speciation (Didelot et al. 2012; Kang et al. 2021). However, applying the principles of the BSC, we established the genetic boundaries of E. coli, termed E. coliBIO, which was found to include all members of the genus Shigella, exclude only 12 genomes currently classified as E. coli in the NCBI database, and to be distinct from the other named species within the genus. Aside from its utility in classification and systematics, applying a universal species concept and identifying populations that readily engage in gene flow is valuable for studying novelty and diversity within species, and the mechanisms by which bacterial species form.
Materials and Methods
Genomes Analyzed
We downloaded a total of 1,635 genome sequences classified as E. coli by the NCBI database (www.ncbi.nlm.nih.gov/), which included representatives of the species within E. coli recognized by the GTDB v207 (April 8, 2022; gtdb.ecogenomic.org/) (Parks et al. 2018) and the E. coli phylogroups of Abram et al. (2021). To maximize core-genome size, we restricted our analyses to all complete, ungapped genomes available at the time of analysis. Additionally, we retrieved complete genome sequences for Escherichia species other than E. coli (E. albertii, n = 1; E. fergusonii, n = 2; and 53 Escherichia strains not assigned to species), the five Escherichia phylogroups (CI–CV) described by Walk et al. (2009) (n = 12), the four named species of Shigella (S. flexneri, n = 28; S. boydii, n = 9; S. dysenteriae, n = 5; S. sonnei, n = 34), one unassigned strain of Shigella, S. enterica (n = 106), S. bongori (n = 5), E. cloacae (n = 3), Klebsiella pneumoniae (n = 3), P. mirabilis (n = 2), and one strain each of Citrobacter koseri, Citrobacter rodentium, Cronobacter sakazakii, Cronobacter turicensis, Enterobacter cancerogenus, Enterobacter lignolyticus, Enterobacter sp., and Klebsiella variicola. Accession numbers, strain, and species assignments and nomenclature in the NCBI and GTDB databases (and Walk et al. and Abram et al. phylogroups, where applicable), and taxonomic classification based on the schemes implemented in this study, are presented in supplementary table S1, Supplementary Material online.
Initial assignment of genomes to a named species followed the nomenclature designated in the NCBI database. Currently, the NCBI database uses an ANI metric to assign genomes to species, with species-level assignments representing strains having >95% ANI for at least 90% of the shared portions of their genomes (Ciufo et al. 2018). Assignments to bacterial genera do not rely on fixed ANI cutoffs, and accommodations are made for certain genera, such as Shigella, which is known to be polyphyletic and contained within E. coli. The GTDB also defines species based on >95% ANI to a representative strain, except in cases in which representatives from different species, as obtained from cross-referencing the LPSN, BacDive, StrainInfo, and NCBI databases, are very closely related and a higher threshold must be applied.
Classification Methods and Detecting Gene Flow Among Strains
Complete genomes were partitioned into sets according to their nomenclature, phylogenetic groupings, or degree of DNA similarity. For each selected set, we evaluated the extent of recombination among genomes and the consistency among the taxonomic assignments based on different methods and criteria. We applied and compared the following methodologies for species-level classification:
(i) Average Nucleotide Identity (ANI). We calculated ANI, a whole-genome metric for evaluating the degree of DNA sequence identity, using FastANI (Jain et al. 2018). When assigning strains to E. coli by this approach, we applied the “one-to-many” option and used the type strain E. coli ATCC 11775 (https://lpsn.dsmz.de/species/escherichia-coli), which was fully sequenced in 2019 (Wadley et al. 2019), as the species representative to which all other genomes were compared. As such, all genomes with an ANI ≥ 95% to ATCC 11775 would be designated members of E. coli. We also applied the “many-to-many” option in FastANI employing the same DNA identity threshold.
(ii) ConSpeciFix. To identify species boundaries according to the precepts of the BSC, we used the ConSpeciFix v1.3.0 pipeline (Bobay et al. 2018), which recognizes genomes as belonging to the same species based on their capacity for gene flow. In ConSpeciFix, gene flow is estimated by assessing the extent of homologous recombination among genes in the core genome. The core genome is built with single-copy orthologs that occur in at least 85% of all strains considered, with single-copy orthologs aligned in MAFFT v7 (Katoh and Standley 2013) and merged into a single concatenate. Based on the core-genome phylogeny, ConSpeciFix calculates the number of homoplastic alleles (h, recombinant sites, i.e., those not related by vertical ancestry) relative to the number of nonhomoplastic alleles (m, vertically transmitted mutations), using a distance-based approach, with higher h/m ratios indicative of more recombination (Bobay et al. 2018).
To calculate h/m ratios, which estimates the limits of recombination among genomes, a representative of a different species or phylogenetic clade (the “test lineage”) is included in a set of genomes previously determined by ConSpeciFix to recombine with one another (the “reference lineages”). Disruptions or reductions in h/m values caused by the inclusion of the test lineage indicate that the test lineage does not recombine with the reference lineages and, thus, belongs to a different species based on the BSC. Analyses were extended to include different combinations of reference genomes and test lineages in order to define species boundaries.
To define the set of E. coli genomes that constitute the reference lineages, we initially examined the 1,635 complete genomes available in the NCBI database. Because it was computationally infeasible to run the entire set of genomes through the ConSpeciFix pipeline as a single group, we randomly subdivided those strains designated as E. coli into subgroups of 150 genomes and analyzed each subgroup separately. These analyses identified 12 genomes that were reproductively isolated from the rest of the E. coli genomes and, therefore, removed from the set of NCBI-designated E. coli strains that were randomly sampled to produce new sets of reference lineages for assessing recombination with test lineages. Within the ConSpeciFix pipeline, we also tested the extent of gene flow between E. coli and representative genomes of other species of Escherichia, the four species of the genus Shigella, and several non-Escherichia species of Enterobacteriaceae (supplementary table S1, Supplementary Material online).
(iii) PopCOGenT. Another approach for defining bacterial species based on gene exchange, PopCOGenT (Arevalo et al. 2019), uses the presence of anomalously similar regions to infer events of gene transfer between genomes. Unlike ConSpeciFix, which deduces the source and ancestry of each polymorphic site, PopCOGenT is based on the premise that SNPs occur more frequently in vertically inherited genes than in recently transferred regions, and that genomes engaging in gene exchange will have longer and more frequent stretches of identical regions. In both ConSpeciFix and PopCOGenT, genomes connected through gene exchange are considered members of the same species, but the methods differ in criteria for identifying recombination, and possibly, species boundaries.
We applied PopCOGenT to a total of 128 genomes, including many that were originally assigned to E. coli but whose species status has been questioned or changed. In addition to strains consistently classified as E. coli, this set included strains labeled as E. ruysiae and E. marmotae by the GTDB, the 12 E. coli genomes from the NCBI database recognized by ConSpeciFix as being reproductively isolated from the rest of the species, representatives of the CI–CV phylogroups (Walk et al. 2009) as well as representatives of other species (E. albertii, E. fergusonii, Shigella sp. PAMC 28760, Shigella sonnei, S. flexneri, S. dysenteriae, Shigella boydii, S. enterica, and S. bongori) and the phylogroups of Abram et al. (2021) (supplementary table S1, Supplementary Material online).
To compare the species assignments and nomenclature of Escherichia and Shigella strains across different classification schemes, we first dereplicated the dataset, retaining only a single representative strain for cases in which multiple genomes averaged >99.8% nucleotide identity. This dereplication yielded a reduced, but otherwise identical, phylogeny that was used for ConSpeciFix. The maximum-likelihood phylogeny of the 70 genomes remaining after dereplication was generated with RaxML (Stamatakis, 2014) using the analysis tool PhaME (Shakya et al. 2020). To generate this phylogeny, PhaME uses nucmer2 (Delcher et al., 2002) to first aligns each genome against itself in order to identify and eliminate the repeated regions within a genome, and then aligns the repeat-free genomes against the selected reference genome. The RaxML phylogeny of the aligned genomes with associated bootstrap branch-support values was built using the evolutionary model GTR and the rate heterogeneity model GAMMA with an estimation of invariable sites (GTRGAMMAI).
Supplementary Material
Acknowledgments
We thank Kim Hammond for assistance with figures. This work was supported by the National Science Foundation Dimensions (grant number 1831730) and the National Institutes of Health (R35GM118038) to H.O.
Contributor Information
Marta Cobo-Simón, Department of Molecular Biosciences, University of Texas at Austin, Austin, TX.
Rowan Hart, Department of Molecular Biosciences, University of Texas at Austin, Austin, TX.
Howard Ochman, Department of Molecular Biosciences, University of Texas at Austin, Austin, TX.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Author Contributions
H.O. conceived the study; H.O. and M.C.S. supervised the research activity planning and execution; M.C.S. and R.H. analyzed and interpreted the data; M.C.S., R.H., and H.O. wrote the paper, read, and approved the final manuscript.
Data Availability Statement
All complete genomes used in this analysis are available from the NCBI database (https://www.ncbi.nlm.nih.gov/) using accession numbers listed in supplementary table S1, Supplementary Material online.
References
- Abram K, Udaondo Z, Bleker C, Wanchai V, Wassenaar TM, Robeson MS, Ussery DW. 2021. Mash-based analyses of Escherichia coli genomes reveal 14 distinct phylogroups. Commun Biol. 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanni WA, Siu-Ting K, Creevey CJ, McInerney JO, Wilkinson M, Foster PG, Pisani D. 2015. Horizontal gene flow from Eubacteria to Archaebacteria and what it means for our understanding of eukaryogenesis. Philos Trans R Soc B Biol Sci. 370:20140337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mamun AA, Tominaga A, Enomoto M. 1997. Cloning and characterization of the region III flagellar operons of the four Shigella subgroups: genetic defects that cause loss of flagella of Shigella boydii and Shigella sonnei. J Bacteriol. 179:4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo P, VanInsberghe D, Elsherbini J, Gore J, Polz MF. 2019. A reverse ecology approach based on a biological definition of microbial populations. Cell. 178:820–834. [DOI] [PubMed] [Google Scholar]
- Bapteste E, Boucher Y. 2009. Epistemological impacts of horizontal gene transfer on classification in microbiology. Clifton, New Jersey: Humana Press. [DOI] [PubMed] [Google Scholar]
- Blount ZD, Barrick JE, Davidson CJ, Lenski RE. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 489:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobay LM, Ellis BSH, Ochman H. 2018. Conspecifix: classifying prokaryotic species based on gene flow. Bioinformatics. 34:3738–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, Falkow S. 1971. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 16:81–118. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Fanning GR, Johnson KE, Citarella RV, Falkow S. 1969. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 98:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, Fanning GR, Miklos GV, Steigerwalt AG. 1973. Polynucleotide sequence relatedness among Shigella species. Int J Syst Evol Microbiol. 23:1–7. [Google Scholar]
- Brenner DJ, Fanning GR, Skerman FJ, Falkow S. 1972. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 109:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. 2003. Genetics and the making of Homo sapiens. Nature. 422:849–857. [DOI] [PubMed] [Google Scholar]
- Chaudhuri RR, Henderson IR. 2012. The evolution of the Escherichia coli phylogeny. Infect Genet Evol. 12:214–226. [DOI] [PubMed] [Google Scholar]
- Ciufo S, Kannan S, Sharma S, Badretdin A, Clark K, Turner S, Brover S, Schoch CL, Kimchi A, DiCuccio M. 2018. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int J Syst Evol Microbiol. 68:2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costechareyre D, Bertolla F, Nesme X. 2009. Homologous recombination in Agrobacterium: potential implications for the genomic species concept in bacteria. Mol Biol Evol. 26:167–176. [DOI] [PubMed] [Google Scholar]
- Delcher AL, Phillippy A, Carlton J, Salzberg SL. 2002. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 30:2478–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denamur E, Clermont O, Bonacorsi S, Gordon D. 2021. The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol. 19:37–54. [DOI] [PubMed] [Google Scholar]
- Didelot X, Méric G, Falush D, Darling AE. 2012. Impact of homologous and non-homologous recombination in the genomic evolution of Escherichia coli. BMC Genomics. 13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ. 1985. A critique of the biological species concept and recommendations for a phylogenetic alternative. Bryologist. 88:172–181. [Google Scholar]
- Dykhuizen DE, Green L. 1991. Recombination in Escherichia coli and the definition of biological species. J Bacteriol. 173:7257–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2018. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics. 34:2371–2375. [DOI] [PubMed] [Google Scholar]
- Escherich T. 1885. Die Darmbakterien des Neugeborenen und Säuglings. Fortschr Med. 3:515–522. [Google Scholar]
- Ewing WH, Hucks MC, Taylor MW. 1952. Interrelationship of certain Shigella and Escherichia cultures. J Bacteriol. 63:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer JJ, Fanning GR, Davis BR, O’Hara CM, Riddle C, Hickman-Brenner FW, Asbury MA, Lowery VA, Brenner DJ. 1985. Escherichia fergusonii and Enterobacter taylorae, two new species of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 21:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzer PJ, Inouye S, Inouye M, Whittam TS. 1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol. 172:6175–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinenoya A, Ichimura H, Awasthi SP, Yasuda N, Yatsuyanagi J, Yamasaki S. 2019. Phenotypic and molecular characterization of Escherichia albertii: further surrogates to avoid potential laboratory misidentification. Int J Med Microbiol. 309:108–115. [DOI] [PubMed] [Google Scholar]
- Husnik F, McCutcheon JP. 2018. Functional horizontal gene transfer from bacteria to eukaryotes. Nat Rev Microbiol. 16:67–79. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Oka C, Sato G. 1978. Isolation of citrate-positive variants of Escherichia coli from domestic pigeons, pigs, cattle, and horses. Appl Environ Microbiol. 36:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90 K prokaryotic genomes reveals clear species boundaries. Nat Commun. 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Yuan L, Shi X, Chu Y, He Z, Jia X, Lin Q, Ma Q, Wang J, Xiao J, et al. 2021. A fine-scale map of genome-wide recombination in divergent Escherichia coli population. Brief Bioinform. 22:bbaa335. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann F. 1944. Zur Serologie Der Coli-Gruppe. Acta Pathol Microbiol Scand. 21:20–45. [PubMed] [Google Scholar]
- Khot PD, Fisher MA. 2013. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 51:3711–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis KT, Tiedje JM. 2005. Towards a genome-based taxonomy for prokaryotes. J Bacteriol. 187:6258–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koser SA. 1923. Utilization of the salts of organic acids by the colon-aerogenes group. J Bacteriol. 8:493–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Reeves PR. 2002. Escherichia coli in disguise: molecular origins of Shigella. Microbes Infect. 4:1125–1132. [DOI] [PubMed] [Google Scholar]
- Lapage SP, Sneath PHA, Lessel EF, Skerman VBD, Seeliger HPR, Clark WA. 1992. International code of nomenclature of bacteria bacteriological code, 1990 revision. Washington: (DC: ): ASM Press. [PubMed] [Google Scholar]
- Lawrence JG, Retchless AC. 2009. The interplay of homologous recombination and horizontal gene transfer in bacterial speciation. In: Gogarten MB, Gogarten JP, Olendzenski LC, editors. Horizontal gene transfer. Methods in molecular biology. Vol. 532. Pittsburgh: Humana Press. p. 29–53. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Jin D, Wu S, Yang J, Lan R, Bai X, Liu S, Meng Q, Yuan X, Zhou J, et al. 2016. Insights into the evolution of pathogenicity of Escherichia coli from genomic analysis of intestinal E. coli of Marmota himalayana in Qinghai-Tibet plateau of China. Emerg Microbes Infect. 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Walk ST, Gordon DM, Feldgarden M, Tiedje JM, Konstantinidis KT. 2011. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc Natl Acad Sci U S A. 108:7200–7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria SE, Burrous JW. 1957. Hybridization between Escherichia coli and Shigella. J Bacteriol. 74:461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J, Cohan FM. 1999. DNA sequence similarity requirements for interspecific recombination in Bacillus. Genetics. 153:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur J, Falkow S, Mandel M. 1963. New approaches to bacterial taxonomy. Annu Rev Microbiol. 17:329–372. [DOI] [PubMed] [Google Scholar]
- Matic I, Rayssiguier C, Radman M. 1995. Interspecies gene exchange in bacteria: the role of SOS and mismatch repair systems in evolution of species. Cell. 80:507–515. [DOI] [PubMed] [Google Scholar]
- Mayr E. 1942. Systematics and the origin of species. Cambridge: Harvard University Press. [Google Scholar]
- Meier-Kolthoff JP, Hahnke RL, Petersen J, Scheuner C, Michael V, Fiebig A, Rohde C, Rohde M, Fartmann B, Goodwin LA, et al. 2014. Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand Genomic Sci. 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan JP, Coop G, Kudaravalli S, Smith D, Krause J, Alessi J, Chen F, Platt D, Pääbo S, Pritchard JK, et al. 2006. Sequencing and analysis of Neanderthal genomic DNA. Science. 314:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Whittam TS, Caugant DA, Selander RK. 1983. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. Microbiology. 129:2715–2726. [DOI] [PubMed] [Google Scholar]
- Parks DH, Chuvochina M, Chaumeil PA, Rinke C, Mussig AJ, Hugenholtz P. 2020. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat Biotechnol. 38:1079–1086. [DOI] [PubMed] [Google Scholar]
- Parks DH, Chuvochina M, Reeves PR, Beatson SA, Hugenholtz P. 2021. Reclassification of Shigella species as later heterotypic synonyms of Escherichia coli in the Genome Taxonomy Database. bioRxiv. preprint bioRxiv:2021.09.22.461432. [Google Scholar]
- Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 36:996–1004. [DOI] [PubMed] [Google Scholar]
- Priest FG, Tsubota K, Austin B. 1993. Modern bacterial taxonomy. Berlin, Heildelberg: Springer Science & Business Media. [Google Scholar]
- Pupo GM, Lan R, Reeves PR. 2000. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A. 97:10567–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayssiguier C, Thaler DS, Radman M. 1989. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 342:396–401. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Cohan FM. 1993. The effect of DNA sequence divergence on sexual isolation in Bacillus. Genetics. 134:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland K, Lambert-Zechovsky N, Picard B, Denamur E. 1998. Shigella and enteroinvasive Escherichia coli strains are derived from distinct ancestral strains of E. coli. Microbiology. 144:2667–2672. [DOI] [PubMed] [Google Scholar]
- Rosselló-Mora R. 2006. DNA–DNA reassociation methods applied to microbial taxonomy and their critical evaluation. In: Stackebrandt E, editor. Molecular identification, systematics, and population structure of prokaryotes. Berlin, Heidelberg: Springer. p. 23–50. [Google Scholar]
- Rosselló-Mora R, Amann R. 2001. The species concept for prokaryotes. FEMS Microbiol Rev. 25:39–67. [DOI] [PubMed] [Google Scholar]
- Shakya M, Ahmed SA, Davenport KW, Flynn MC, Lo CC, Chain PSG. 2020. Standardized phylogenetic and molecular evolutionary analysis applied to species across the microbial tree of life. Sci Rep. 10:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Huang H V. 1986. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 112:441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga K. 1898. Ueber den Erreger der Dysenterie in Japan. Zentralbl Bakteriol Mikrobiol Hyg (Vorläufige Mitteilung). 23:599–600. [Google Scholar]
- Staley JT. 2009. Universal species concept: pipe dream or a step toward unifying biology? J Ind Microbiol Biotechnol. 36:1331–1336. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall BJ, Rosselló-Móra R, Busse HJ, Ludwig W, Kämpfer P. 2010. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 60:249–266. [DOI] [PubMed] [Google Scholar]
- Wadley TD, Jenjaroenpun P, Wongsurawat T, Ussery DW, Nookaew I. 2019. Complete genome and plasmid sequences of Escherichia coli type strain ATCC 11775. Microbiol Resour Announc. 8:e00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST. 2015. The “Cryptic” Escherichia. EcoSal Plus. 6. doi: 10.1128/ecosalplus.ESP-0002-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST, Alm EW, Gordon DM, Ram JL, Toranzos GA, Tiedje JM, Whittam TS. 2009. Cryptic lineages of the genus Escherichia. Appl Environ Microbiol. 75:6534–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler KM, Stuart CA. 1946. The mannitol-negative Shigella group. J Bacteriol. 51:317–325. [DOI] [PubMed] [Google Scholar]
- Wu F, Speth DR, Philosof A, Crémière A, Narayanan A, Barco RA, Connon SA, Amend JP, Antoshechkin IA, Orphan VJ. 2022. Unique mobile elements and scalable gene flow at the prokaryote–eukaryote boundary revealed by circularized Asgard archaea genomes. Nat Microbiol. 7:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer KH, Whitman WB, Euzéby J, Amann R, Rosselló-Móra R. 2014. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 12:635–645. [DOI] [PubMed] [Google Scholar]
- Zawadzki P, Roberts MS, Cohan FM. 1995. The log-linear relationship between sexual isolation and sequence divergence in Bacillus transformation is robust. Genetics. 140:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All complete genomes used in this analysis are available from the NCBI database (https://www.ncbi.nlm.nih.gov/) using accession numbers listed in supplementary table S1, Supplementary Material online.