Abstract
Advanced visual computing solutions and three-dimensional (3D) printing are moving from engineering to clinical pipelines for training, planning, and guidance of complex interventions. 3D imaging and rendering, virtual reality (VR), and in-silico simulations, as well as 3D printing technologies provide complementary information to better understand the structure and function of the organs, thereby improving and personalizing clinical decisions. In this study, we evaluated several advanced visual computing solutions, such as web-based 3D imaging visualization, VR, and computational fluid simulations, together with 3D printing, for the planning of the left atrial appendage occluder (LAAO) device implantations. Six cardiologists tested different technologies in pre-operative data of five patients to identify the usability, limitations, and requirements for the clinical translation of each technology through a qualitative questionnaire. The obtained results demonstrate the potential impact of advanced visual computing solutions and 3D printing to improve the planning of LAAO interventions as well as the need for their integration into a single workflow to be used in a clinical environment.
Keywords: 3D printing, In silico simulations, Left atrial appendage occlusion, Pre-interventional planning, Virtual reality
1. Introduction
Computing solutions, such as three-dimensional (3D) imaging and visualization, virtual/augmented reality (VR/AR), in silico simulations, and visual analytics, among others, have seen an outstanding progress in recent years. Advances and improved access to big and high-resolution data, hardware infrastructure (e.g., high-performance computing clusters and graphical processing units), affordable devices (e.g., VR/AR headsets), and open-source codes thanks to the commitment to open and reproducible science by researchers, have made it possible. Biomedical applications are not an exception, with an unprecedented availability to medical datasets at different multi-scale and resolution levels. Therefore, some visual computing technologies are already disrupting traditional concepts of medical image exploration. Complex medical interventions are usually planned by exploring pre-operative medical images, where advanced visual computing solutions, in combination with technologies such as 3D printing, could contribute to reduce potential complications.
Until recently, the visualization and analysis of medical imaging data were always performed with visualization tools, usually as two-dimensional (2D) images and standard multiplanar reconstruction (MPR) visualization viewed in 2D monitors. Commonly, these are the only tools available during the intervention since they are fully integrated with the acquisition systems in the operating room, with clinicians mentally integrating the 3D structure and functional information provided by multiple sources. Offline image analysis can be performed with numerous tools, including open-source software such as 3D Slicer[1] or commercial solutions, which are usually used as the stand-alone software tools tailored to specific imaging modalities and type of pathologies.
In cardiology, patient-specific detailed information about the structure and function of the heart is a key for medical training, and for optimizing and personalizing clinical decisions involving diagnosis, treatment planning, and post-therapeutic monitoring of patients, specifically in structural heart disease, where transcatheter interventions are becoming a less invasive alternative to open surgery. However, the field of view in transcatheter interventions is limited, with the absence of a gold standard open cavity surgical field depriving physicians of the opportunity for tactile feedback and visual confirmation of cardiac anatomy[2]. At this juncture, there is a significant gap in understanding the 3D (plus time) anatomical and physiological relationships in the heart[3] that visual computing solutions can help to bridge. Recent studies have reviewed the added value of advanced visualization of cardiac data, including applications in conditions such as congenital heart disease[4,5], structural heart disease[2], or transcatheter mitral valve replacement[6].
The left atrial appendage occlusion (LAAO), along with a device inserted into the heart in a transcatheter intervention, has recently been proposed as an efficient alternative for patients in atrial fibrillation (AF) with contraindications to drug therapy. The interventional cardiologists need to decide the optimal type and size of the device, as well as the position where to implant it in each heart. During a LAAO procedure, if an implanted device seems inappropriate, the operator can recapture it and try a different one[7], at the expense of increasing costs, patient’s risk, and procedural time. Due to the large variability of LA and LAA anatomy in humans, and the wide range of device configurations, LAAO interventions can clearly benefit from pre-operative planning to reduce the need of device implantation attempts, thus maximizing intervention efficiency and minimizing costs.
During LAAO interventions, multimodal 2D images such as echocardiography and X-ray are mainly used. Nevertheless, the complexity and interpatient variability of the LAA 3D anatomy are unnoticeable under 2D imaging modalities. 3D echocardiography and, more recently, high-resolution computerized tomography (CT) images are increasingly becoming available, providing better spatial information of the LAA and reproducibility than 2D-based techniques[8] to optimize the implantation strategy and device selection before the intervention. However, there is still a debate on which is the most adequate imaging technique since there are discrepancies on the measurements from different modalities[9].
At present, commercial solutions are available with standard MPR visualization of the 3D imaging modalities (e.g., CT images) such as the 3mensio Structural Heart software (Pie Medical Imaging, Bilthoven, the Netherlands)[10], which also includes advanced 3D photorealistic rendering of the heart for an advanced exploration of the LAA, or Mimics (Materialise NV, Leuven, Belgium). Karagodin et al.[11] recently demonstrated an improved delineation of cardiac structures, including the LAA, with a new tissue transparency transillumination tool when visualizing 3D echocardiographic images, compared to standard 3D rendering in a system developed by Philips (Andover, MA). Most of these imaging tools to explore 3D LAA anatomies are based on stand-alone software but web-based frameworks with cloud-based engines are becoming modern and more flexible alternatives for medical image visualization and analysis. The Virtual Implantation and Device selection in the left Atrial Appendages (VIDAA) platform was recently developed, providing a clinician-friendly web-based tool to support the pre-operative planning of LAAO interventions[12]. It allows clinicians to interactively explore the LA geometry as a 3D mesh with different computer-aided design (CAD) LAAO models, together with some morphological indices and the original CT images in MPR format. However, these software tools are still limited in visualizing and analyzing imaging data with the standard MPR, volume rendering, and surface mesh views in 2D monitors; therefore, these tools have limited degrees of freedom interaction and could prevent a correct perception of the 3D nature of the studied anatomy (e.g., depth and scaling).
3D printing is a tool routinely used in certain cardiology fields, especially when dealing with abnormal heart anatomies[13], such as in congenital heart disease and pediatric applications[14], providing the clinician a better understanding of 3D cardiac anatomy. The clinical translation of 3D printing has been made possible due to the reduction of printer costs with simple rigid plastic materials, although more sophisticated printers and flexible materials mimicking tissue properties are available at a higher cost. Adaptation of 3D printing in clinical care and procedural planning has already demonstrated a reduction in early operator learning curve or in centers without previous experience on transcatheter interventions[2,15-17]. Several studies have evaluated the added value of 3D-printed models for training and planning of LAAO interventions[18]. However, Conti et al. recently compared 3D printing recommendations and implanted devices, with an agreement of only 35%[19]. Moreover, computational costs and time required for models to be printed with realistic materials are not negligible in the clinical workflow.
Although in an earlier phase of development and application in the biomedical field, there already exist proof-of-concept studies of using VR technologies for cardiac devices such as the pre-operative planning of transcatheter closure of cardiac deficiencies, such as ventricular septal[20] or sinus venous defects[21,22]. Nam et al.[23] used new functionalities of the 3D-Slicer open-source software (i.e., link with VR headsets) to develop a tool for the virtual testing, selection, and placement of transcatheter device closures of atrial and ventricular septal defects. Narang et al.[24] recently demonstrated a reduction in measurement variability and time required when exploring 3D echocardiography and CT images in different cardiac pathologies, with users reporting easy manipulation of VR models, diagnostic quality visualization of the anatomy, and high confidence in the measurements. As for LAAO devices, the EchoPixel True 3D VR Solution (EchoPixel, Inc., Mountain View, California, United States) allows to visualize CT scans and perform a “device-in-anatomy” simulation for LAAO pre-procedural planning[25]. Zbronski et al.[26] also visualized CT-derived LA anatomies before and during the occlusion procedure with the AR HoloLens headset, which is an enhancement according to clinicians. Finally, Medina et al.[27] developed a VR-based platform (VRIDAA) for the visualization/analysis of LAA anatomies and the most appropriate occlusion devices to be implanted; the platform is regarded by clinical users as a source of motivation for trainees who can better understand the required approach before the intervention.
In silico simulations from virtual physiological models of the heart, also known as digital twins, are emerging as a valuable technology in cardiology to support clinical decisions, such as interventional planning, diagnosis, and device optimization[28,29]. For instance, the commercial software HEARTguideTM (FEops NV, Gent, Belgium) provides patient-specific structural simulations of LAAO deployments with different device configurations[30], but without user interaction and lacking functional information. Computational fluid dynamics (CFD) solvers compute flow and pressure throughout a well-defined geometrical domain and can provide useful functional information about blood flow patterns with high spatial resolution, currently unattainable with in vivo imaging techniques. Different therapeutic scenarios can be in silico tested with CFD before the intervention, thus reducing operation costs with enhanced efficiency[31] and accelerating research and development understanding of fluid mechanics within device testing[2]. Regarding LAA applications, several researchers have run CFD simulations to study blood flow in the left atria[32-35], including after the implantation of LAAO to find the optimal configuration of device[12,36,37]. However, comprehensive pre-operative simulations may take between hours and days depending on the complexity of the anatomy and potential interactions between cardiac tissue and the blood flow to be modeled[2], thus limiting its application in a clinical environment.
In this paper, we present the evaluation of several advanced visual computing solutions for the planning of LAAO interventions (i.e., web-based platform with 3D imaging visualization, VR, and in silico fluid simulations), together with 3D printing with standard and affordable materials. 3D imaging data from CT scans of five patients, who were the candidates for LAAO implantation, were visualized with different visual computing and 3D printing technologies by six domain knowledge experts (three interventional and three imaging cardiologists). During the practical session, they were asked to decide the LAAO device settings after using each technology for each patient-specific case, and to fill in a usability questionnaire and some open questions to assess the adding value, limitations, and requirements for clinical translation of each one of the evaluated technologies.
2. Materials and methods
Figure 1 shows a general overview of the evaluation pipeline followed in our study. The original 3D CT scans of five patients, acquired before the LAAO intervention, were segmented to derive a binary mask of the left atria, including the left atrial appendage. Subsequently, a 3D model in the form of a surface mesh was generated and introduced, together with the gray-level 3D scan if required, to the specific processing workflow of each one of the evaluated computing technologies (e.g., web-based 3D imaging, 3D printing, VR, and in silico simulations). The LAAO devices selected for this study were the Amplatzer Amulet (St. Jude Medical-Abbott, St. Paul, Minnesota, United States) and the Watchman FLX (Boston Scientific, Marlborough, Massachusetts, United States), with different sizes available commercially. Therefore, the participants of the study tested the technologies with their available features (Section 2.3). After each technology, participants chose a given device configuration and were asked to give a final decision on device type, size, and position to implant. Subsequently, a System Usability Scale (SUS) questionnaire[38] as well as some open questions (Section 2.4.6) were filled in by each physician, focusing on the implantation of the tested technologies at their hospitals.
Figure 1.
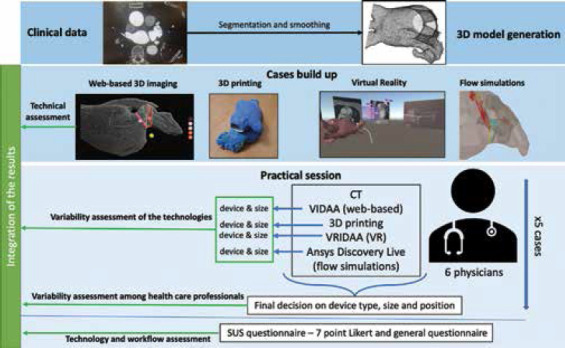
Overall pipeline for the evaluation of advanced computing technologies for the planning of the left atrial appendage occlusion (LAAO) interventions. The first step involved generating the three-dimensional (3D) surface model from the patient-specific medical images (i.e., computerized tomography, CT) of five cases. The resulting 3D model was the base for the setup of all models used in different technologies, which were tested by domain experts (i.e., physicians) in an experimental session where they needed to decide the device type, size, and position. Subsequently, the participants answered a system usability scale questionnaire and a general questionnaire with open questions.
2.1. Clinical data
The clinical data used in this work were provided by Hospital Haut-Lévêque (Bordeaux, France), including AF patients that underwent a LAAO intervention and with available pre-procedural high-quality CT scans. Five of them were randomly selected. The study was approved by the Institutional Ethics Committee; patients gave the informed consent for having their data used for research purposes, including tasks such as the ones presented in this study.
Cardiac CT studies were performed on a 64-slice dual source CT system (Siemens Definition, Siemens Medical Systems, Forchheim, Germany). Tube current was set to 120 kV in patients with body mass index (BMI) > 27 and 100 kV in those with BMI < 27. Acquisition was set on end systole using prospective ECG triggering, the delay being set in percentage of the RR interval in patients in sinus rhythm, and in ms in those with arrhythmia. Images were acquired using a biphasic injection protocol: 1 mL/kg of Iomeprol 350 mg/mL (Bracco, Milan, Italy) at the rate of 5 mL/s followed by a 1 mL/kg flush of saline at the same rate. A bolus tracking method was applied to acquire arterial phase images, and the region of interest was positioned within the LA.
2.2. 3D model generation
For each selected patient, the anatomy of the left atria, including its appendage, was extracted from the CT images using semi-automatic region growing and thresholding tools available in 3D slicer. The resulting binary mask of the LA was then introduced to the Marching Cubes algorithm to generate a 3D surface mesh model. Mesh smoothing was applied to correct irregularities from the segmentation, based on a Taubin filter smoothing operator (λ = 0.5, μ = −0.53), followed by the removal of self-intersecting faces and non-manifold edges wherever necessary using MeshLab 2016.12 (http://www.meshlab.net/). The same surface mesh of the heart was used across all the different approaches. The overall process of generating the 3D model took around 45 min per patient. A description of the set-up required for each evaluated computing technology is provided in the following. Furthermore, Table 1 illustrates the estimated preparation times and associated costs for each technology.
Table 1.
Set-up preparation (for each patient in brackets) and practical session times as well as the associated costs for each technology
| Technology | Set-up preparation times (per case) | Average practical session duration (per case) | Cost |
|---|---|---|---|
| 3D printing | 43 h (26.1 h) | 3 min | 6000+17.5 € |
|
| |||
| VIDAA | 7 min (1.24 min) | 8.13 min | RP |
|
| |||
| VRIDAA | 15 min (3 min) | 10 min | RP+1238€+2500 PC |
|
| |||
| In silico simulations | 21.6 h (4.3 h) | 12 min | Free license+2000 PC |
The time required to build the 3D models, including medical image segmentation (3.75 h), are not included. RP: Research platform (i.e., no cost)
2.3. Computing technologies
2.3.1. VIDAA: Web-based exploration of 3D imaging
Both CT scans and the LA meshes were introduced to the VIDAA platform for visualization (as MPR and 3D, respectively) and morphological analysis (Figure 2). The 3D surface mesh of the LA can be visualized with different levels of transparency, both in solid and wireframe formats. Some landmarks relevant to LAA interventions such as the circumflex artery can be manually selected by the user. The centerline of the LAA was computed using the Python’s VMTK library (https://www.vmtk.com) from the center of the LA mesh to a point on the LAA tip, the latter interactively selected by the user. Perpendicular 2D contours along the resulting centerline were then obtained to estimate morphological measurements on the LAA (i.e., maximum and minimum LAA diameters). These measurements were also visualized in 2D maps and a graph along the centerline depth to better identify local size variations in the LAA. The user can also define the ostium (i.e., intersection between the LA and LAA) and landing zone (i.e., where the device will be implanted) landmarks with small spheres along the centerline. Afterward, based on the estimated measurements, the VIDAA platform proposes a set of appropriate LAAO devices for the studied LAA geometry. The user can interactively manipulate the chosen LAAO device, changing its position in the LAA and its size. Once the CT and the mesh were uploaded into the VIDAA platform, the case was ready to be analyzed, with the centerline and morphological measurement calculation taking less than a minute. At present, the VIDAA platform is a research prototype, and it is not available commercially.
Figure 2.
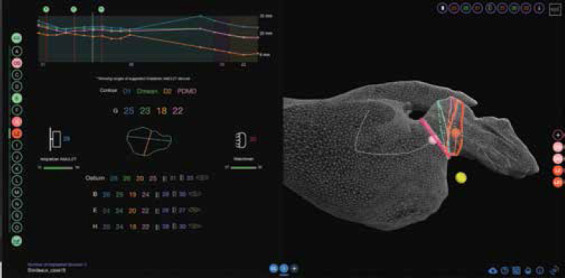
Web-based 3D imaging exploration (VIDAA platform). Left: Morphological parameters (e.g., diameters) of 2D contours along the centerline characterizing the left atrial appendage (LAA) anatomy and range of most appropriate devices to implant. Right: 3D visualization of the LAA anatomy in a 3D wire-frame mesh format, together with the LAA centerline (white), several 2D contours and some anatomical landmarks (pink, orange, and yellow small spheres corresponding to the ostium, the landing zone, and the circumflex artery, respectively) relevant for the device implantation.
2.3.2. 3D printing
The 3D model generated from the CT scan of each patient was printed at Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) with a Fused Deposition Modeling (FDM) 3D printer, the Ultimaker S5 (Ultimaker BV, Geldermalsen, Netherlands), which costs 6000 euros approximately. The 3D LA model was prepared for printing with the Ultimaker Cura 3 software from the printer provider. Typical rigid polylactic acid (PLA) was the material used for the LA models (Figure 3), with an associated cost of 1.5 euros for each model. Twenty hours were needed to print all LA cases (i.e., 4 h/case). Moreover, CAD models of Amplatzer and Watchman FLX LAAO devices corresponding to the different commercially available sizes were printed with thermoplastic polyurethane (TPU) to add more flexibility. Therefore, users could try to position the printed LAAO device into the 3D-printed model of the LA to have more insight into their interaction. The cost of all printed LAAO devices was of 10 euros, taking 22 h to print. An extra hour was added for time estimations due to pre- and post-processing tasks, such as adding thickness to the 3D models and removing the scaffolds of all the models.
Figure 3.
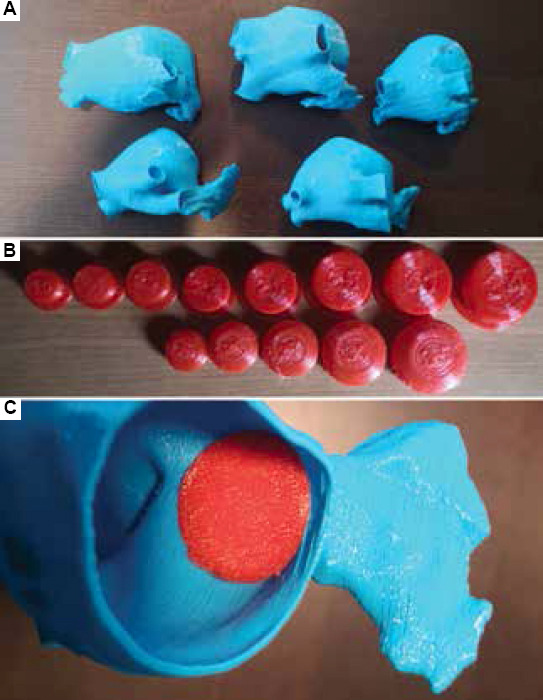
. (A) 3D-printed models of left atria (LA) analyzed in this study. (B) 3D-printed models of the left atrial appendage occluder (LAAO) devices. (C) Example of interaction between LA and LAAO 3D-printed models.
2.3.3. VRIDAA: VR tool
The VRIDAA platform developed by Medina et al.[27] was used to evaluate the use of VR technologies for the planning of LAAO interventions. VRIDAA allows the user to interact with the LA geometry, jointly visualize it with patient-specific medical images in standard MPR format and relevant morphological indices (Figure 4). Standard surface manipulation, including mesh clipping and transparency changes, as well as browsing along the CT scan slices are possible. Morphological measurements and landmarks imported from the web-based VIDAA platform, such as the centerline and a graph with the associated LAA contour diameters, can be also displayed in the VR environment. It is also possible to virtually place the LAAO device of choice (i.e., different designs and sizes) in any position. In addition, the user can also plan the optimal location for introducing the delivery catheter into the LAA, freely manipulating a catheter model, together with an endoscopic view to facilitate the visualization of the LA interior.
Figure 4.

Virtual reality VRIDAA platform to explore left atrial (LA) anatomies and occluder devices. A 3D LA geometry with axial, sagittal, and coronal slices of medical images visualized behind, together with the delivery catheter model (in white) and the 2D endoscopic view of the catheter’s tip camera for LAA interior visualization.
The computer used in this study was equipped with an Intel Core i5-8400 CPU @2.80 GHz processor, an NVIDIA GeForce RTX 2080 Ti graphic card and 16 GB of RAM, costing around 2000 euros. For the implementation of the VRIDAA platform, the Unity Engine version 2018.1.8f1 (64-bit) (Unity Technologies, San Francisco, California, United States) was used, with SteamVR as the runtime and OpenVR as the API to get full compatibility with all major VR display platforms. The employed VR headset was the HTC Vive Pro, with a resolution of 1440 × 1600 pixels for each eye, a 90 Hz refresh rate, and 110 degrees field of view. Its cost was of 1239 euros. Image processing outside the platform was performed using Python libraries. Once uploaded into VRIDAA, the user can interact with the 3D meshes using the HTC Vive Pro controller to freely move it (6 degrees of freedom, i.e., rotations and translations) or zoom it to navigate inside the patient’s LA. In its current implementation, the VRIDAA platform is intended to be used with the possibility of moving within the virtual environment. Thus, a clear space of around 2 m × 1.5 m is required.
All meshes and images were uploaded to VRIDAA before the practical session with participants. In VRIDAA, the centerline was not selected by the user; each case had a centerline previously generated in the web-based VIDAA platform, as well as the measurements and the proposed devices, which were transferred into the VR platform. Thus, if the system is already calibrated, the only required step for using the VR platform was the transfer and upload of the files to the VRIDAA system, making the workflow quite fast and straightforward.
2.3.4. In silico flow simulations
The participants were presented with the visualization of in silico fluid simulations in the studied LA anatomies and any possible LAAO device to be implanted, using the Ansys Discovery Live (ANSYS Inc., USA) software, under the Academic License (i.e., free of charge). It includes a GPU-based Lattice Boltzmann method that allows almost real-time simulations, once each case was set-up. Therefore, the participants could manually choose a given device (i.e., type and size) and place it in each position to analyze resulting blood flow patterns, which can be iterated as many times as desired.
The volumetric meshes required for the fluid simulations were generated from the 3D surface models using the Gmsh 4.5.4 software (https://gmsh.info/). The final meshes were around 800 thousand elements for all cases. The PC used to run the simulations was the one used for VR. The blood flow was treated as a Newtonian fluid, with a density of 1060 kg/m3 and a viscosity of 0.0035 Pa/s. The boundary conditions in the inlets and outlets of the 3D LA model of the LA were chosen as in our most recent LAA fluid simulation study[39], but without dynamic movement of the mitral ring plane since it was not allowed in the employed software. Basically, they were the same for all patients, which were extracted from Doppler echo, and pressure measurements of an AF patient are different from the ones processed in this study: A velocity profile in the outlet (i.e., the mitral valve) and a pressure wave at the pulmonary veins. The set-up for each simulation case included 30 min for meshing and building, accounting for 21.6 hours in total (with 13 devices per five patients).
2.4. Practical session
Before the practical session, the participants received a tutorial on the different technologies, approximately lasting 1 h, demonstrating their features. During the practical session, data from five typical LAAO patients (i.e., not particularly complicated LAA geometries) were presented to the physicians. For each case and technology, the participants chose the optimal device settings to identify consistent technology-related biases. At the end of the case analysis, they were asked to make a final decision with all knowledge gathered from all technologies. Finally, they answered the SUS questionnaire and open questions for a global assessment of each employed technology.
The order to present the technologies in the practical session is as follows (going from the most available to the most sophisticated technologies): CT medical images (optional), VIDAA, 3D printing, VRIDAA, and simulations. Initially, we offered the possibility of visualizing the raw CT medical images of each patient. Afterward, the web-based VIDAA platform followed, since it is similar to some of the most advanced software solutions commercially available in the market (e.g., 3mensio). Next, 3D printing and VRIDAA were presented to incorporate data visualization with enhanced depth perception, starting with 3D printing since most physicians are more familiar with it. Finally, the in silico simulations were shown in the Ansys Discovery Live software to include functional information complementary to the structural knowledge provided by the other technologies.
2.4.1. CT imaging
The visualization of CT medical images was optional since some of the physicians do not work on their daily basis with CT measurements. Although it was not assessed in SUS questionnaire, CT visualization was offered since it is the gold standard technique to explore LA anatomy, thus useful to plan LAAO interventions. Moreover, there were questions related to CT use in the general questionnaire. Physicians could explore the CT medical images with the Open-Source ITK-Snap software (http://www.itksnap.org/) to: (1) browse through the CT slices for inspecting the LAA shape and other anatomical landmarks such as the circumflex or the ostium and (2) take measurements of these anatomical landmarks.
2.4.2. Web-based VIDAA platform
Initially, after opening the 3D LA geometry in the VIDAA platform, participants were asked to select a point to create the optimal LAA centerline. Then, the contour diameters perpendicular to the LAA centerline were automatically computed (less than 30 s), as well as the selection of recommended LAAO devices. Next, participants interactively explored the LAA geometry, observing the contour diameters and looking for the best position and device for each case. In addition, physicians could select among the different (recommended or not) LAAO devices and move them freely to decide.
2.4.3. 3D printing
The 3D-printed model of the LA of each studied case was offered to the physician to manipulate with their hands, together with the full range of 3D-printed replicas of the Amplatzer Amulet and Watchman FLX LAAO devices available in the market. Physicians could then interact with both types of 3D-printed models to decide which device would fit each LA anatomy better.
2.4.4. Virtual reality VRIDAA platform
The tasks performed by the participants on the VRIDAA platform were very similar to VIDAA. Nevertheless, the LAA centerline was already provided by default. Once the participant wore the VR glasses, the LA appeared, together with its centerline, and the physician could move it, grab it, or go inside to better explore the interior of the anatomical structure. Then, the user could select the type and size of a given device, which would be placed at the beginning of the centerline, which was able to move along. In addition, the LAAO device could also be grabbed and moved freely.
2.4.5. In silico simulations
Initially, the participant was asked to select a device type and size, after which it could be freely placed in any location of the LAA using the interface of the Ansys Discovery Live software. The LA anatomy could also be moved. Once a device position was chosen, the simulation was launched, requiring several minutes to visualize the resulting blood flow patterns (Figure 5). Therefore, blood flow velocities near the device could be estimated, as well as flow recirculations and leaks due to the chosen LAAO positioning, potentially leading to DRT.
Figure 5.
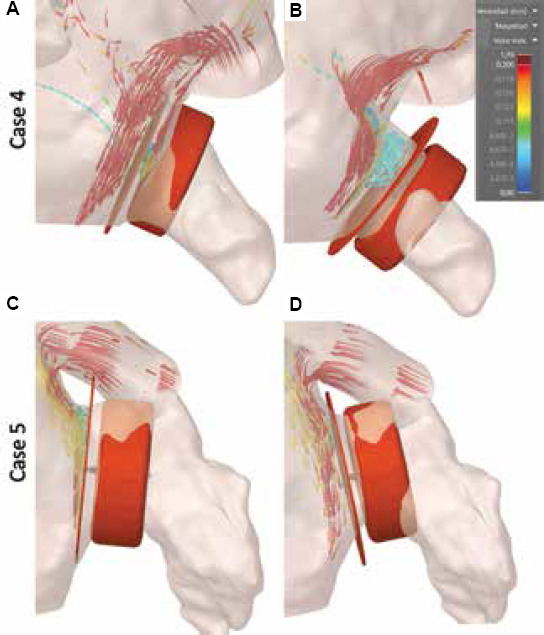
(A-D) Examples of in silico fluid simulations using Ansys Discovery Live with optimal device settings according to a given participant. 3D streamline-based visualization simulations were used to illustrate blood flow patterns of in the left atrium and left atrial appendage. Blue and red represent low velocity (< 10 m/s) and high velocities (> 20 m/s), respectively.
2.4.6. Evaluation questionnaires
Once the participants had gone through all the patients, they answered two questionnaires: A SUS questionnaire and a more general questionnaire with open questions. The SUS questionnaire, developed by Brooke[38], was used to give a more quantitative assessment on the usability of the technologies. It is a 10-item questionnaire using, in our case, a 7-point Likert scale. Consequently, the physicians answered the SUS questionnaire for each technology at the end of the session. Details on the SUS questions and the answers for each technology are included in the Supplementary File.
The aim of the questionnaire with open questions was to profile the participants and to know more about how these technologies could be implemented in their current workflow, according to their point of view. The questions were the following:
Years of experience in LAAO interventions.
Current position at the hospital.
Did you know about the application of these technologies to LAAO planning? If yes, which one?
Have you tested any of these technologies before? If yes, which one?
Have you participated in the development of these technologies? If yes, which one?
Which technologies would you add in your ideal workflow for LAAO (disregarding economical and equipment restrictions)?
Which technology did influence your final decision on device election the most?
If your hospital is mainly using ultrasound (US) imaging to plan LAAO interventions instead of CT, would you consider acquiring CT data only to be able to use these technologies?
3. Results
3.1. Participant profile
Out of the six participants, three were interventional cardiologists (P2, P3, and P4), that is, physicians who are implanting the device, while the remaining three are imaging cardiologists, who were responsible for the medical image acquisition and analysis before and during LAAO procedures. On average, they had 5.08 years of experience in LAAO interventions (with 10 years and < 1 year for the most and less experienced, respectively). The participants work in three different hospitals, with two of them using CT images for LAAO planning while US imaging is the choice in the remaining clinical center.
None of the participants have taken part in the development of VIDAA, VRIDAA, or Ansys Discovery Live. However, one participant (P6) helped on the 3D printing process but being blind to critical data in the study (i.e., which device was implanted, clinical output). Most participants (5/6) were familiar with 3D printing, as they have tested it before. Moreover, only one participant did not know about the use of any of these technologies for LAAO planning, while only one knew about all of them. Not a single participant had tested the VIDAA and VRIDAA platforms before the practical session and only two physicians had some experience with fluid simulations beforehand, although not with the Ansys Discovery Live software.
3.2. SUS questionnaire
The results of the SUS questionnaire are summarized in Table 2 and Figure 6. Overall, all the evaluated computing technologies passed the acceptability and user-friendliness threshold (as defined in Bangor et al.[40]).
Table 2.
SUS score for each technology and participant
| Participant | VIDAA | VRIDAA | 3D printing | Simulations |
|---|---|---|---|---|
| P1 (I) | 88.0* | 70.4 | 72.0 | 51.2 |
|
| ||||
| P2 (IC) | 72.0* | 62.4 | 64.0 | 70.4 |
|
| ||||
| P3 (IC) | 67.2* | 59.2 | 64.0 | 28.8 |
|
| ||||
| P4 (IC) | 72.0 | 80.0* | 78.4 | 73.6 |
|
| ||||
| P5 (I) | 80.0* | 62.4 | 68.8 | 48.0 |
|
| ||||
| P6 (I) | 89.6* | 75.2 | 92.8* | 77.6 |
|
| ||||
| Mean (STD) | 78.13 (9.24) | 62.2 (8.26) | 73.33 (10.96) | 58.27 (18.87) |
I: Imaging cardiologists; IC: Interventional cardiologists.
The best computing technology according to each participant
Figure 6.
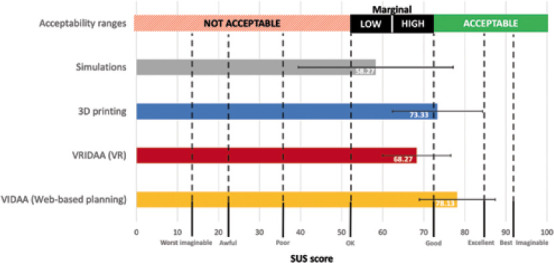
Overall results of the SUS questionnaire. Acceptable ranges were extracted from Bangor et al.[40]. Error bars show the standard deviation.
The web-based VIDAA platform was the best technology, according to the participants, with an average score of 78.13 and two physicians considering it excellent (scores above 85). Interestingly, based on Table 2, the imaging cardiologists valued VIDAA more (average of 85.87) than the interventional cardiologists (average of 70.4) did, although the latter still labeled the technology within the marginal high range of acceptability and user-friendliness. The strongest points of the VIDAA platform, based on the SUS questions, were that it was easy to use and fast to learn without any support, with all participants agreeing on their willingness to use VIDAA frequently. On the other hand, the participants found that there were too many features and steps in the platform, which could be simplified, to perform the final LAAO planning.
3D printing was the second most valued technology (score of 73.3, i.e., within the acceptable range of usability), with good marks on easiness of usability and complexity. However, it failed on the confidence of use, consistency of the system, and a proper integration of all features.
The VR VRIDAA platform was the technology with a wider range of answers from the participants regarding its use daily, with one strongly agreeing to use it and the remaining ones with no clear opinion. Moreover, there was no agreement in the participants on any major flaw of the technology. Five participants consider it easy to use, although three of them reported that they might need support at some point. Participants also mentioned that the devices recommended by the VRIDAA system were slightly bigger than expected. Finally, according to the participants, most of them were confident with the device positioning due to the possibility of freely moving.
The visualization of in silico fluid simulations was the technology with the lowest score (58.27), barely passing the usability test, in the low marginal area of acceptability ranges. However, it was the technology presenting the largest variance between participants (from 77.6 to 28.8), some evaluating it at the level of the remaining technologies and others in the not acceptable usability area. Furthermore, five participants would like to use the technology frequently, with the remaining one providing an inconclusive answer. Moreover, they found most of the simulation-based features useful, especially in identifying possible leaks after LAAO implantation, which is well integrated and without inconsistencies, and they felt quite confident on its use at the end of the practical session. The main reason for the overall low score was the poor easiness of use of the Ansys Discovery Live interface; almost all participants required support and claimed that it is difficult to learn the interface, which is also cumbersome to use.
3.3. Open questions on each technology
Figure 7 summarizes the answers from participants to the open questions on each evaluated technology, focusing on their incorporation into the clinical workflow. Three physicians (all imaging cardiologists, not interventionists) would add the VIDAA platform into the clinical workflow, one of them only for planning complex anatomies and the two remaining would include also the platform for their regular cases. The VR platform, VRIDAA, was included in the workflow by only 2/6 clinicians; both used it for regular and complex planning, while half of the participants would use 3D printing. However, 5/6 participants found the 3D-printed models very useful for exploring the anatomy and were willing to use this technology in a frequent basis, provided the printer and flexible materials would be cheaper. Despite the low values in the SUS questionnaire, 4/6 clinicians (including the three imaging ones) would use in silico fluid simulations for planning complex anatomies, mainly to avoid leaks and device-related thrombus (DRT) after LAAO device implantation. It is worthy to point out that in silico fluid simulations are the only technology positively rated for follow-up purposes (2/6 participants), especially if the relationship between low blood flow velocities and DRT is confirmed in more extensive clinical studies.
Figure 7.
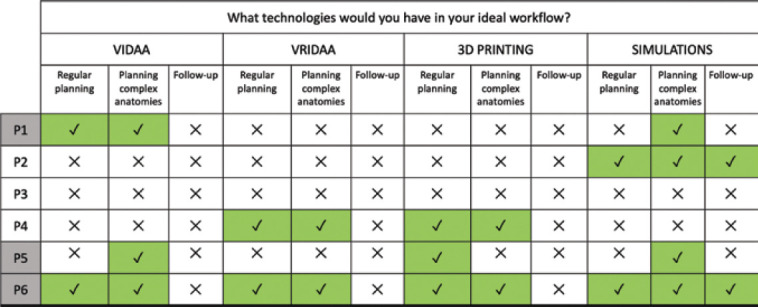
Ideal workflow according to the participants. On the left, gray shading represents imaging cardiologists, white shading represents interventional cardiologists.
One participant (P1) was from a hospital where CT is not routinely used for LAAO planning; US imaging is preferred since most patients are elderly people with other comorbidities (e.g., renal dysfunction), often having contraindications to CT acquisition. However, the same physician would be interested in acquiring CT scans to have access to the evaluated computing technologies when younger patients are eligible for LAAO implantation.
Figure 7 also illustrates the extremes in physician approaches toward the use of these technologies, with one of them (P3) not willing to add any of them into the clinical workflow, and the counterpart one (P6) opting for incorporating all of them. We would like to point out that P6 was the physician with more previous experience and interest on computational tools. An important remark from P6 was that a single software integrating the access to all different technologies is necessary.
3.4. Device selection comparison
Table 3 illustrates the interparticipant variation in LAAO device selection after testing each computing technology, where different patterns can be observed. For instance, participants P1 and P5 (both imaging specialists) tended to select smaller devices with 3D printing, which they attributed to the rigidity of LA walls in the printed model. Therefore, they mainly used other technologies for their final LAAO device decision. Participants P2 and P4 (both interventional cardiologists) also followed the same pattern, without much LAAO size variation between different technologies. However, they were inclined to select a larger device in the VIDAA platform since it is not obvious to check for potential leaks in it, thus overestimating the size. On the other hand, features in the VRIDAA platform (e.g., being within the LA cavity) and in silico simulations (e.g., functional flow information) made these two technologies better suited for a more optimal device position to avoid leaks. Finally, participant P6 rarely changed the selected device after testing each technology.
Table 3.
Devices selected by the participants (P1 – P6) after using each computing technology
| Case no. | P1 (I) Web/VR/3Dpr/Sim | P2 (IC) Web/VR/3Dpr/Sim | P3 (IC) Web/VR/3Dpr/Sim | P4 (IC) Web/VR/3Dpr/Sim | P5 (I) Web/VR/3Dpr/Sim | P6 (I) Web/VR/3Dpr/Sim |
|---|---|---|---|---|---|---|
| C1 | A25/A22/A16/A22 | W24/W24/A25/A22 | A28/A22/A20/A22 | A25/A22/A22/A22 | A25/A28/A20/A28 | A22/A22/A22/A22 |
|
| ||||||
| C2 | A20/A18/A16/A16 | A22/A18/W20/A18 | A22/A22/A20/A22 | A20/A16/A16/A16 | A20/A22/A16/A22 | A18/A18/A18/A18 |
|
| ||||||
| C3 | A22/A20/A16/A18 | W24/A22/W20/A22 | A22/A22/A20/A22 | A18/A18/A18/A18 | A18/A18/A18/A18 | A20/A18/A18/A18 |
|
| ||||||
| C4 | A20/A20/A18/A18 | W27/W27/W20/W24 | A22/A22/A20/A20 | A20/A18/A18/A18 | A20/A22/A18/A22 | W20/A18/A18/A18 |
|
| ||||||
| C5 | A25/A25/A18/A22 | A28/A25/A22/A25 | A28/A28/A25/A25 | A25/A25/A25/A25 | A25/A25/A20/A25 | W24/W20/W20/W20 |
Web: Web-based VIDAA platform; VR: Virtual reality VRIDAA platform; 3Dpr: 3D printing; Sim: Fluid simulations; W: Watchman flex; A: Amplatzer Amulet. Numbers refer to the device size (in mm). In bold, when the device settings coincide with the final decision made by the clinician
In three of the studied cases (C2, C3, and C4), there were substantial intraparticipant variations (e.g., more than 2 device sizes), while in the remaining cases, final decisions were quite similar. The main reason for these variations was the different LAA morphologies of the two groups of cases; the first group had a so-called chicken wing-type morphology that allows a different interventional technique (e.g., sandwich) with larger LAAO device sizes, which is preferred by some physicians. Unfortunately, the sandwich technique was not considered in any of the studied technologies. On the other hand, the agreement in the non-CW LAA cases (i.e., C1 and C5) was higher, as can be seen in Table 3. Shockingly, none of the participants proposed the LAAO device, which was finally implanted in case C4.
Table 4 shows the final LAAO devices selected for each participant in all studied cases, compared to the device effectively implanted in the patient. Most LAAO devices selected by participants were the Amplatzer Amulet since it was the device mainly used in their training period and they had more experience with it. Therefore, they felt more comfortable using it. It can be observed in Table 4 that in most cases, participants chose up to three different LAAO device sizes, generally (63% of the times) not matching the implanted one and with some extreme choices (C4 in Table 4, with A18 and A25 being selected).
Table 4.
Devices finally selected by the participants
| Case | P1 (I) | P2 (IC) | P3 (IC) | P4 (IC) | P5 (I) | P6 (I) | Implanted |
|---|---|---|---|---|---|---|---|
| C1 | A22* | A25 | A22* | A22* | A28 | A22* | A22 |
|
| |||||||
| C2 | A18 | A18 | A22 | A16* | A22 | A18 | A16 |
|
| |||||||
| C3 | A20 | A22 | A22 | A18* | A22 | A18* | A18 |
|
| |||||||
| C4 | A20 | W24 | A20 | A18 | A22 | A18 | A25 |
|
| |||||||
| C5 | A22 | A25* | A25* | A25* | A25* | W20 | A25 |
W: Watchman flex; A: Amplatzer Amulet. Numbers refer to the device size (in mm).
The cases that matched the size of the device implanted to the patient
4. Discussion
The fields of visual computing and 3D printing have seen a considerable progress over the last few years, slowly providing solutions for advanced visualization in some biomedical applications. According to a recent review by Wang[2], a well-known cardiology leader in the field of LAAO interventions, 3D printing, computational modeling, and artificial intelligence (AI) plays a role in bridging the dichotomy of real-world in the trenches imaging and futuristic capabilities of computer science and biomedical engineering. Pre-planning complex cardiology procedures such as LAAO with visual computing and 3D printing technologies can be beneficial in maximizing interventional efficiency and minimizing costs, which are estimated by procedural time and device expenditure. However, the clinical translation of advanced visualization technologies is not straightforward, needing to fulfill the demanding requirements to be embedded in the existing workflows in hospitals. For instance, clinicians will not invest more than a few minutes on planning cases that can take 30 min for the intervention. Moreover, pre-planning should not add excessive complexity and cost to the overall clinical workflow; therefore, sufficient cost-effectiveness or patient safety impact must be proven to justify their routine use.
Despite recent generic reviews of visual computing solutions in cardiology applications[2,4-6], there is a lack of complete studies testing the different visualization methodologies on the same patient-specific data for benchmarking purposes. To the best of our knowledge, our study is the first attempt on this direction focusing on LAAO interventions, aiming at evaluating the added value, limitations, and requirements for the clinical translation of these technologies. The results obtained in the practical session demonstrated that the tested visual computing and 3D printing solutions are complementary, all providing added value in different steps of the current LAAO clinical workflow. All the evaluated technologies passed the threshold of acceptability range on usability; the web-based VIDAA platform and 3D printing were better rated, and the former getting excellent marks from some participants. The VR-based platform (VRIDAA) and in silico simulations were placed in the high and low marginal ranges, respectively, but huge discrepancies between participants existed in the latter. However, we need to consider that previous experience on the technologies could influence the usability score (e.g., P4 and P6 being the only users with some knowledge on simulations gave them the best scores). Nonetheless, the main overall conclusions among the participants were the complementarity of the technologies and the need for an integrative unique platform of the visual computing technologies (i.e., VIDAA, VRIDAA, and fluid simulations) to be incorporated into the clinical workflow and used on a daily basis. In addition, a more realistic elastic behavior of the 3D-printed LAAO devices would increase the precision on the selected settings.
One of the most valued features in the web-based VIDAA platform was the detailed characterization of the LAA anatomy, with the diameters along the centerline, since it can be used to identify the optimal implantation or to better plan special strategies such as the sandwich technique. However, the manual selection of seed points for the centerline was an important factor to properly characterize the LAA anatomy and select the appropriate device and its position. In addition, based on the SUS questionnaire, VIDAA was fast and intuitive, which covered several manual steps but with a fast learning curve. Participants stressed the added value of the web-based platform in complex cases, proposing to incorporate functional information from fluid simulations for a more complete solution. The current commercial solutions comparable to VIDAA are the stand-alone 3mensio Structural Heart software (Pie Medical Imaging, Bilthoven, the Netherlands) or Mimics (Materialise NV, Leuven, Belgium), which include MPR visualization, 3D rendering, and 3D surface visualization, and HEARTguideTM (FEOPS NV, Gent, Belgium), which offers simulations of device deployment. The VIDAA platform provides a more comprehensive and interactive morphological characterization of the LAA, as well as interaction with 3D models of the LAA devices in a web-based environment that does not require any software installation and easily allows multicentric studies and collaborative decisions. Moreover, none of these solutions offer in silico fluid simulations. The price of these commercial software tools can also be an obstacle for including them in the clinical workflow of some hospitals.
Participants in our study acknowledged the better exploration of the 3D LAA anatomy with the VR system, due to an enhanced depth perception, 6 degrees of freedom interaction with 3D objects (both the LA geometry and the device) and views from the interior of the cavity (not easy to see even in 3D-printed models), all points important for the device implantation[27]. For example, it was challenging for participants to truly grasp the depth and scaling of human organs and device sizes (as well as their relation) only from 2D monitors, especially to detect possible leaks. Although the learning times for using the VRIDAA platform were short (i.e., a few minutes), the participants preferred the combination of web-based 3D imaging software in conjunction with 3D printing since it would be easier to fit in the current clinical workflow. The evaluated VR setup that requires a certain allocated physical space is not adequate for use in most hospitals. However, affordable VR headsets with reasonable performance and resolution, including wireless solutions without requiring much room space (e.g., the Oculus brand or even AR glasses), would be a more appropriate alternative.
3D printing emerged as a useful technology for rapid prototyping, testing, and pre-operative planning. However, the use of cheap materials in our study was a limiting factor since it did not realistically mimic the left atrial wall elastic properties, which are important to determine the interaction with the device once implanted. Specifically, it made physicians pick sizes smaller than the one implanted or selected with the other technologies. The use of more realistic materials, such as the transparent and flexible HeartFlex from Materialise NV (Leuven, Belgium) or resin, was also noted by the participants, but would dramatically increase the costs of the technology (approximately 200 euros per piece vs. 1.5 euros with PLA). In addition, the use of real LAA occluders rather than 3D replicas could improve the realism of the planning, but it will require access to all LAAO designs and sizes, which is often not possible. Moreover, it was difficult to manipulate the LAAO device inside the 3D-printed model since the only open holes were the pulmonary veins and the mitral valve; the geometries should be opened in half to facilitate free movement of the device in the printed model. Despite its limitations, most physicians thought 3D printing was the best technology to recognize the shape and do a mental quick strategy of the intervention for regular planning.
In silico fluid simulations including LAAO devices were a unique source of valuable functional information, not available from current imaging modalities or other computing solutions. Imaging cardiologists particularly valued this option for evaluating regions with potential leaks and complex flow after the LAAO implantation. However, the Ansys Discovery Live interface, which is difficult to interact with or move the CAD model of the device, was not as user-friendly as the remaining technologies, as quantified in the SUS questionnaire. Participants would not include this technology in its current form, but they would recommend incorporating it in other tools such as VIDAA or VRIDAA. However, to integrate in silico fluid simulations in device-related decision-making, enough credibility still needs to be built following verification, validation, and uncertainty quantification standards such as the V&V40 guidelines[41], including sensitivity analysis to identify the boundary conditions to provide more the realistic fluid simulations[39]. Moreover, the employed fluid solver allowed user-interaction for estimating changes in blood flow patterns with different device positions, but at the expense of simplifications (e.g., absence of wall motion) that could be relevant to better mimicking of the interaction between the device and the anatomy[42].
The comparison between the devices selected by the participants after each technology demonstrated the relevance for interventional cardiologists to explore the data and anatomy fully in three dimensions with systems such as VR and including functional information from flow simulations. In addition, there were consistent differences in device sizes selected with 2D-based tools compared to 3D alternatives. Beyond computational tools, there are other reasons related to the current LAAO clinical workflow and training that could explain the large variation on device sizing by different clinicians. For instance, the existing manufacturer’s sizing recommendations overlap, so a situation where a given LAA measurement could be covered by more than 1 device size might occur. The consequence is that some clinicians would favor larger or smaller sizes in a subjective way (e.g., device manufacturer’s recommendation of 22 mm, some opting for 20 mm, and others for 25 mm), increasing the importance of their experience on the procedure for a successful LAAO implantation. Even with industry-supported training, experienced clinicians selected a wide range of LAAO device sizes. The integration of pre-operative planning techniques into the industrial training programs would help further standardize LAAO implantation procedures. Effectively, physicians highlighted the potential role of 3D printing and the VR VRIDAA platform for training clinical fellows, especially on challenging cases. A complementary and very important use of these technologies according to domain experts involves patient education, which could contribute to reduce the levels of stress and anxiety before the intervention thanks to a better understanding of the procedure. A final comment was related to the application of the assessed computing solutions to other structural heart disease procedures involving medical devices such as transaortic valvular interventions since the technologies would only need slight modifications from the current LAAO-based use case.
There are some limitations in our study. First, only a small cohort consisting of six participants was able to evaluate the computing technologies. Moreover, all participants were trained with a single LAAO device (Amplatzer Amulet), thus undoubtedly creating some bias favoring such a device. Furthermore, the disagreements found between the final selected and the implanted devices may be misleading since follow-up information was not available for the analyzed cases; therefore, the implanted device could not be ideal, eventually leading to abnormal events at some point.
Another possible factor that could impact the evaluation of the technologies is that the same five cases were analyzed by the users, following the same order, which could lead to a model learning effect: Participants remembered their choices with previously tested technologies, making their analysis not fully independent. The rationale of the chosen order was going from the most clinically available to the most sophisticated technologies, but a more randomized study that includes more cases should be performed in the future to alleviate the learning effect. Using computational tools that all had been developed by the authors provided an unbiased analysis toward the preferred technology or which one should be considered the best. On the other hand, the maturity of the individual instance of each technology certainly had an influence on the evaluation results, not necessarily reflecting the technology potential. For instance, in silico fluid simulations could obtain better scores with a more mature user interface while 3D printing would be better assessed with more realistic, soft, and elastic materials[43]. However, the present study successfully identified the most relevant aspects to consider in each technology for clinical translation, beyond individual instances.
In addition, we evaluated all computing technologies on 3D models built from CT medical images, which are not always available for LAAO planning. Echocardiography images could also be used in the presented visual computing solutions, mainly with advanced 3D rendering or in VR setups[11,24], but anatomical details of the structures under study will be lost, which could negatively affect the medical decisions on the devices to implant. Otherwise, the manual steps and time required for creating the 3D models from the original medical images could be a limitation for the clinical translation of the evaluated tools. Nevertheless, the use of automatic deep learning techniques for LA segmentation and mesh creation is being developed and should be available soon. Furthermore, it would have been useful to include new photorealism and advanced cinematic rendering visualization[11] in our study, which will be planned in the future. In addition, other anatomical structures, relevant for the LAAO procedure, such as the fossa ovalis for guiding catheters into the LA, should be incorporated in different computing technologies for a more complete planning of the intervention. Finally, an evaluation study where the advanced visual computing solutions are implemented and tested in the hospital would complete the current work, where the technologies were tested by domain experts in a research laboratory.
5. Conclusions
In this work, we evaluated several computing technologies to assess their added value, limitations, and requirements before they are translated to a clinical environment, particularly for the planning of left atrial appendage occlude (LAAO) interventions. All the evaluated technologies could be beneficial in different steps of the LAAO clinical workflow, even if most need some adaptation to fit in the hospital routine. Specifically, the web-based 3D imaging VIDAA platform provided a complete morphological characterization and excellent user interaction to manipulate and test multiple device configurations. Economical 3D-printed models, although lacking completely realistic device-LAA interaction, were useful to have a better perception of the 3D LAA anatomy and can be easily integrated in the current clinical workflows. VR technologies, which are especially suited for educational or pre-operative planning purposes, were also very helpful for 3D perception, but only simple VR headsets would be suitable for daily clinical routine. In silico fluid simulations with LAAO devices have potential to reduce the risk of leaks and device-related thrombus after the implantation but required more user-friendly interfaces. In consequence, all the evaluated computing technologies could contribute to better personalization of LAA intervention and post-interventional treatment to each patient, which helps ensure better outcomes. Advanced versions of the studied computing solutions will be properly embedded in clinical workflows soon, especially if they can be all integrated into a single space or software platform, as most participants of this study demanded.
Acknowledgments
The authors of this manuscript want to thank the physicians, Dr. L.S., Dr. X.M., Dr. L.A., Dr. P.L., and Dr. V.A., who accepted our invitation to take part in this study as participants.
Funding
This work was supported by the Spanish Ministry of Science, Innovation and Universities under the Retos I+D Programme (RTI2018-101193-B-I00), the Maria de Maeztu Units of Excellence Programme (MDM 2015-0502), and the Spanish Ministry of Economy and Competitiveness under the Programme for the Formation of Doctors (PRE2018-084062). In addition, this work was supported by the H2020 EU SimCardioTest project (Digital transformation in Health and Care SC1-DTH-06-2020; grant agreement No. 101016496).
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
Conceptualization: Jordi Mill, Helena Montoliu, Xavier Freixa, Dabit Arzamendi, and Oscar Camara.
Data Curation: Jordi Mill, Helena Montoliu, Abdel H. Moustafa, Carlos Albors, and Andy L. Olivares.
Investigation and formal analysis: Jordi Mill, Helena Montoliu, Carlos Albors, and Andy L. Olivares.
Methodology: Jordi Mill and Helena Montoliu.
Resources: Abdel H. Moustafa.
Software: Elodie Medina, Ainhoa Aguado, and Mario Ceresa.
Supervision and validation: Dabit Arzamendi, Xavier Freixa, and Oscar Camara.
Writing – Original draft: Jordi Mill
Writing – review and editing: Andy L. Olivares and Oscar Camara
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee; patients gave the informed consent for having their data used for research purposes, including tasks such as the ones presented in this study.
Consent for publication
The authors have the consent of the participants to publish their data obtained from this study.
Availability of data
Data is available under request.
References
- 1.Kikinis R, Pieper SD, Vosburgh KG. 3D slicer:A platform for subject-specific image analysis, visualization, and clinical support. In: jolesz fa., editor. Intraoperative imaging and image-guided therapy. New York: springer; 2014. pp. 277–289. [Google Scholar]
- 2.Wang DD, Qian Z, Vukicevic M, et al. 3D printing, computational modeling, and artificial intelligence for structural heart disease. JACC Cardiovasc Imaging. 2021;14:41–60. doi: 10.1016/j.jcmg.2019.12.022. https://doi.org/10.1016/j.jcmg.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Wang DD, Geske J, Choi AD, et al. Navigating a career in structural heart disease interventional imaging. JACC Cardiovasc Imaging. 2018;11:1928–1930. doi: 10.1016/j.jcmg.2018.07.010. https://doi.org/10.1016/j.jcmg.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Salavitabar A, Figueroa CA, Lu JC, et al. Emerging 3D technologies and applications within congenital heart disease:teach, predict, plan and guide. Future Cardiol. 2020;16:695–709. doi: 10.2217/fca-2020-0004. https://doi.org/10.2217/fca-2020-0004. [DOI] [PubMed] [Google Scholar]
- 5.Goo HW, Park SJ, Yoo SJ. Advanced medical use of three-dimensional imaging in congenital heart disease:Augmented reality, mixed reality, virtual reality, and three-dimensional printing. Korean J Radiol. 2020;21:133–145. doi: 10.3348/kjr.2019.0625. https://doi.org/10.3348/kjr.2019.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohli K, Wei ZA, Sadri V, et al. Framework for planning TMVR using 3-D imaging, in silico modeling, and virtual reality. Struct Hear. 2020;4:336–341. https://doi.org/10.1080/2474∂.2020.1762268. [Google Scholar]
- 7.Devgun J, De Potter T, Fabbricatore D, et al. Pre-cath laboratory planning for left atrial appendage occlusion optional or essential? Interv Cardiol Clin. 2022;11:143–152. doi: 10.1016/j.iccl.2021.11.003. https://doi.org/10.1016/j.iccl.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Vivoli G, Gasparotti E, Rezzaghi M, et al. Simultaneous functional and morphological assessment of left atrial appendage by 3D virtual models. J Healthc Eng. 2019;2019:7095845. doi: 10.1155/2019/7095845. https://doi.org/10.1155/2019/7095845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saw J, Fahmy P, Spencer R, et al. Comparing measurements of ct angiography, tee, and fluoroscopy of the left atrial appendage for percutaneous closure. J Cardiovasc Electrophysiol. 2016;27:414–422. doi: 10.1111/jce.12909. https://doi.org/10.1111/jce.12909. [DOI] [PubMed] [Google Scholar]
- 10.Hascoet S, Smolka G, Bagate F, et al. Multimodality imaging guidance for percutaneous paravalvular leak closure:Insights from the multi-centre ffpp register. Arch Cardiovasc Dis. 2018;111:421–431. doi: 10.1016/j.acvd.2018.05.001. https://doi.org/10.1016/j.acvd.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Karagodin I, Addetia K, Singh A, et al. Improved delineation of cardiac pathology using a novel three-dimensional echocardiographic tissue transparency tool. J Am Soc Echocardiogr. 2020;33:1316–1323. doi: 10.1016/j.echo.2020.08.005. https://doi.org/10.1016/j.echo.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguado AM, Olivares AL, Yagüe C, et al. In silico optimization of left atrial appendage occluder implantation using interactive and modeling Tools. Front Physiol. 2019;10:237. doi: 10.3389/fphys.2019.00237. https://doi.org/10.3389/fphys.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vukicevic M, Mosadegh B, Min JK, et al. Cardiac 3D printing and its future directions. JACC Cardiovasc Imaging. 2017;10:171–184. doi: 10.1016/j.jcmg.2016.12.001. https://doi.org/10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte MNV, Hussain T, Roest A, et al. Living the heart in three dimensions:applications of 3D printing in CHD. Cardiol Young. 2019;29:733–743. doi: 10.1017/S1047951119000398. https://doi.org/10.1017/S1047951119000398. [DOI] [PubMed] [Google Scholar]
- 15.Fan Y, Yang F, Cheung GSH, et al. Device sizing guided by echocardiography-based three-dimensional printing is associated with superior outcome after percutaneous left atrial appendage occlusion. J Am Soc Echocardiogr. 2019;32:708–719.e1. doi: 10.1016/j.echo.2019.02.003. https://doi.org/10.1016/j.echo.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang DD, Gheewala N, Shah R, et al. Three-dimensional printing for planning of structural heart interventions. Interv Cardiol Clin. 2018;7:415–423. doi: 10.1016/j.iccl.2018.04.004. https://doi.org/10.1016/j.iccl.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Eng MH, Wang DD. Computed tomography for left atrial appendage occlusion case planning. Interv Cardiol Clin. 2018;7:367–378. doi: 10.1016/j.iccl.2018.03.003. https://doi.org/10.1016/j.iccl.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim WD, Cho I, Kim YD, et al. Improving left atrial appendage occlusion device size determination by three-dimensional printing-based preprocedural simulation. Front Cardiovasc Med. 2022;9:830062. doi: 10.3389/fcvm.2022.830062. https://doi.org/10.3389/fcvm.2022.830062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti M, Marconi S, Muscogiuri G, et al. Left atrial appendage closure guided by 3d computed tomography printing technology:a case control study. J Cardiovasc Comput Tomogr. 2019;13:336–339. doi: 10.1016/j.jcct.2018.10.024. https://doi.org/10.1016/j.jcct.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Mendez A, Hussain T, Hosseinpour AR, et al. Virtual reality for preoperative planning in large ventricular septal defects. Eur Heart J. 2019;40:1092. doi: 10.1093/eurheartj/ehy685. https://doi.org/10.1093/eurheartj/ehy685. [DOI] [PubMed] [Google Scholar]
- 21.Tandon A, Burkhardt BEU, Batsis M, et al. Sinus venosus defects. JACC Cardiovasc Imaging. 2019;12:921–924. doi: 10.1016/j.jcmg.2018.10.013. https://doi.org/10.1016/j.jcmg.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Southworth MK, Silva JR, Silva JN. Use of extended realities in cardiology. Trends Cardiovasc Med. 2020;30:143–148. doi: 10.1016/j.tcm.2019.04.005. https://doi.org/10.1016/j.tcm.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam HH, Herz C, Lasso A, et al. Simulation of transcatheter atrial and ventricular septal defect device closure within three-dimensional echocardiography-derived heart models on screen and in virtual reality. J Am Soc Echocardiogr. 2020;33:641–644.e2. doi: 10.1016/j.echo.2020.01.011. https://doi.org/10.1016/j.echo.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narang A, Hitschrich N, Mor-Avi V, et al. Virtual reality analysis of three-dimensional echocardiographic and cardiac computed tomographic data sets. J Am Soc Echocardiogr. 2020;33:1306–1315. doi: 10.1016/j.echo.2020.06.018. https://doi.org/10.1016/j.echo.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Sanon S, Lim DS. Update on left atrial appendage occlusion an overview of innovative preprocedural and intraprocedural imaging techniques, trial data, and current laao devices and devices in development. Struct Heart Dis. 2019;13:35–41. [Google Scholar]
- 26.Lindner S, Behnes M, Wenke A, et al. Assessment of peri-device leaks after interventional left atrial appendage closure using standardized imaging by cardiac computed tomography angiography. Int J Cardiovasc Imaging. 2019;35:725–731. doi: 10.1007/s10554-018-1493-z. https://doi.org/10.1007/s10554-018-1493-z. [DOI] [PubMed] [Google Scholar]
- 27.Medina E, Aguado AM, Mill J, et al. VRIDAA:Virtual reality platform for training and planning implantations of occluder devices in left atrial appendages. Eurographics Work Vis Comput Biol Med. 2020:31–35. https://doi.org/10.2312/vcbm.20201168. [Google Scholar]
- 28.Ribeiro JM, Astudillo P, de Backer O, et al. Artificial intelligence and transcatheter interventions for structural heart disease:A glance at the (near) future. Trends Cardiovasc Med. 2022;32:153–159. doi: 10.1016/j.tcm.2021.02.002. https://doi.org/10.1016/j.tcm.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Corral-Acero J, Margara F, Marciniak M, et al. The “Digital Twin”to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556–4564. doi: 10.1093/eurheartj/ehaa159. https://doi.org/10.1093/eurheartj/ehaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garot P, Iriart X, Aminian A, et al. Value of feops heartguide patient-specific computational simulations in the planning of left atrial appendage closure with the amplatzer amulet closure device:Rationale and design of the predict-laa study. Open Hear. 2020;7:e001326. doi: 10.1136/openhrt-2020-001326. https://doi.org/10.1136/openhrt-2020-001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naci H, Salcher-Konrad M, Mcguire A, et al. Impact of predictive medicine on therapeutic decision making:A randomized controlled trial in congenital heart disease. NPJ Digit Med. 2019;2:17. doi: 10.1038/s41746-019-0085-1. https://doi.org/10.1038/s41746-019-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otani T, Al-Issa A, Pourmorteza A, et al. A computational framework for personalized blood flow analysis in the human left atrium. Ann Biomed Eng. 2016;44:3284–3294. doi: 10.1007/s10439-016-1590-x. https://doi.org/10.1007/s10439-016-1590-x. [DOI] [PubMed] [Google Scholar]
- 33.Fanni BM, Capellini K, Di Leonardo M, et al. Correlation between laa morphological features and computational fluid dynamics analysis for non-valvular atrial fibrillation patients. Appl Sci. 2020;10:1448. https://doi.org/10.3390/app10041448. [Google Scholar]
- 34.Pons MI, Mill J, Fernandez-Quilez A, et al. Joint analysis of morphological parameters and in silico haemodynamics of the left atrial appendage for thrombogenic risk assessment. J Interv Cardiol. 2022;2022:9125224. doi: 10.1155/2022/9125224. https://doi.org/10.1155/2022/9125224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng L, Gao H, Griffith B, et al. Analysis of a coupled fluid-structure interaction model of the left atrium and mitral valve. Int J Numer Method Biomed Eng. 2019;35:e3254. doi: 10.1002/cnm.3254. https://doi.org/10.1002/cnm.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mill J, Olivares AL, Arzamendi D, et al. Impact of flow-dynamics on device related thrombosis after left atrial appendage occlusion. Can J Cardiol. 2020;36:968.e13–968.e14. doi: 10.1016/j.cjca.2019.12.036. https://doi.org/10.1016/j.cjca.2019.12.036. [DOI] [PubMed] [Google Scholar]
- 37.Mill J, Agudelo V, Li CH, et al. Patient-specific flow simulation analysis to predict device-related thrombosis in left atrial appendage occluders. REC Interv Cardiol. 2021;3:278–285. https://doi.org/10.24875/RECICE.M21000224. [Google Scholar]
- 38.Brooke J. In:Usability Evaluation in Industry. Vol. 189. Boca Raton, Florida: CRC Press; 1996. SUS-A quick and dirty usability scale; pp. 4–7. [Google Scholar]
- 39.Mill J, Agudelo V, Olivares AL, et al. Sensitivity analysis of in silico fluid simulations to predict thrombus formation after left atrial appendage occlusion. Mathematics. 2021;9:2304. https://doi.org/10.3390/math9182304. [Google Scholar]
- 40.Bangor A, Kortum P, Miller J. Determining what individual sus scores mean;Adding an adjective rating. J Usability Stud. 2009;4:114–123. [Google Scholar]
- 41.Viceconti M, Pappalardo F, Rodriguez B, et al. In silico trials:Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods. 2021;185:120–127. doi: 10.1016/j.ymeth.2020.01.011. https://doi.org/10.1016/j.ymeth.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaccaria A, Danielli F, Gasparotti E, et al. Left atrial appendage occlusion device:development and validation of a finite element model. Med Eng Phys. 2020;82:104–108. doi: 10.1016/j.medengphy.2020.05.019. https://doi.org/10.1016/j.medengphy.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Celi S, Gasparotti E, Capellini K, et al. 3D printing in modern cardiology. Curr Pharm Des. 2021;27:1918–1930. doi: 10.2174/1381612826666200622132440. https://doi.org/10.2174/1381612826666200622132440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available under request.


