Abstract
In situ bioprinting has emerged as a promising technology for tissue and organ engineering based on the precise positioning of living cells, growth factors, and biomaterials. Rather than traditional in vitro reconstruction and recapitulation of tissue or organ models, the in situ technology can directly print on specific anatomical positions in living bodies. The requirements for biological activity, function, and mechanical property in an in vivo setting are more complex. By combining progressive innovations of biomaterials, tissue engineering, and digitalization, especially robotics, in situ bioprinting has gained significant interest from the academia and industry, demonstrating its prospect for clinical studies. This article reviews the progress of in situ bioprinting, with an emphasis on robotic-assisted studies. The main modalities for in situ three-dimensional bioprinting, which include extrusion-based printing, inkjet printing, laser-based printing, and their derivatives, are briefly introduced. These modalities have been integrated with various custom-tailored printers (i.e., end effectors) mounted on robotic arms for dexterous and precision biofabrication. The typical prototypes based on various robot configurations, including Cartesian, articulated, and parallel mechanisms, for in situ bioprinting are discussed and compared. The conventional and most recent applications of robotic-assisted methods for in situ fabrication of tissue and organ models, including cartilage, bone, and skin, are also elucidated, followed by a discussion on the existing challenges in this field with their corresponding suggestions.
Keywords: In situ bioprinting, Robot configurations, Robotic-assisted bioprinting
1. Introduction
Three-dimensional (3D) printing is a technique that deposits and accumulates materials through computer-aided design and manufacturing to construct physical entities[1]. In the early stages of its technological development, 3D printing was considered merely befitting for the fabrication of functional or aesthetic prototypes, and thus the term rapid prototyping was often adopted to represent this technique[2]. 3D printing is now used synonymously with additive manufacturing since its precision, efficiency, reproducibility, and robustness have been greatly enhanced to the industrial-production level[3]. 3D printing simplifies the processing procedure and minimizes the cost of personalized production. As an extension of additive manufacturing, bioprinting is a state-of-the-art technology that involves layer-by-layer deposition of a mixture of cells, matrix, and nutrients to produce living tissues and potentially whole organs, such as blood vessels, bones, heart, and skin[4]. By means of this, sophisticated 3D tissues and organs with recapitulated biological functions can be constructed for numerous applications, including drug screening[5], disease modeling[6], pathological and pharmacological analysis[7], as well as regenerative medicine[8]. The use of bioprinting in medical training and testing tasks has advanced in the past two decades. Manifold reports have demonstrated the successful fabrication of various tissues and organs[9] for streamlining early surgical planning models and permanent implants, as well as cell-seeded biocompatible scaffolds or in vitro biological models (Figure 1). To create an environment that supports fast and efficient cell growth, cells are often seeded around scaffolds made of biodegradable polymers or collagen, which eventually grow into functional tissue[10]. However, in vitro 3D scaffolds have many inherent limitations with regard to their actual clinical applications[11]. Since 2007, in situ bioprinting (i.e., in vivo bioprinting) has been proposed based on inkjet technology[12]. In situ bioprinting can be defined as the direct printing of living cells, growth factors, and biomaterials to create or repair living tissues or organs at a defect site[13]. This technology involves complex shapes, curved surfaces, or even more intricate geometries with heterogeneous compositions, whereas conventional 3D printing usually adds materials layer-by-layer to a flat substrate[14]. Robotic-assisted automated printers or handheld printers are the leading platforms for in situ bioprinting. Among these, computer-controlled robots, which can be programmed to aid in biomaterials positioning and manipulation, have shown effectiveness in simplifying and improving the in situ operation[15]. Robotic-assisted operation facilitates in situ bioprinting with higher accuracy, flexibility, and control. To date, robotic arms with Cartesian, articulated, and parallel configurations have been developed for biofabrication. Moreover, technologies of robotic-assisted minimally invasive surgery can be integrated with 3D bioprinting to improve printing accuracy and dexterity. Particularly, by combining progressive innovations of biomaterials, automation, digitalization, and tissue engineering, robotic-assisted in situ bioprinting is becoming more attractive and realistic[16,17], and a number of studies have verified its exceptional potential for use in clinical settings[18-20].
Figure 1.
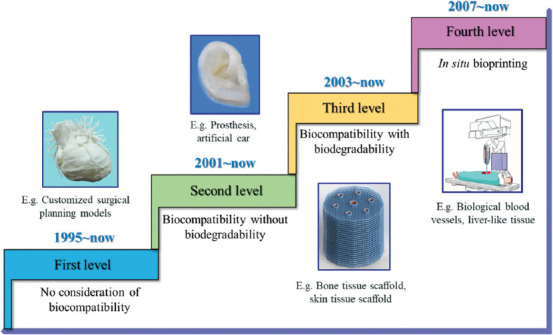
Development of bioprinting.
In this review, we discuss the progress of in situ bioprinting, with emphasis on robotic-assisted approaches and platforms. The mainstream modalities and advanced methodologies for in situ 3D bioprinting are introduced, and the prototypes and commercial products based on different configurations, including Cartesian coordinate, articulated, and parallel robots, for in situ fabrication are compared and discussed. The classic utilizations and potential application models for robotic-assisted fabrication of in situ tissues and organs, such as cartilage, bone, skin, and liver, are elucidated. In addition, we briefly discuss the existing challenges and provide suggestions for future improvements from the perspectives of individualized medicine, robotics, and information science.
2. In situ bioprinting modalities
2.1. Extrusion-based bioprinting
Extrusion-based bioprinting involves the continuous deposition of bio-ink through syringes or nozzles to construct 3D tissues or organs[21]. Applied pressure, piezoelectric effect, and solenoid dispensing have been employed by bioprinters of this type. Extrusion-based bioprinting generally offers gentle fabrication with high regard for cell viability. One of the most promising features of this technology lies in the fact that multiple cells and biocompatible materials can be simultaneously applied through different nozzles. Furthermore, it is regarded as the most mature solution for in vivo clinical applications, owing to its decadal recognition in arthroscopy repair. Commercial bioprinters that are based on this technology have been successfully developed.
2.2. Inkjet bioprinting
In inkjet bioprinting, bio-ink is sprayed onto the deposition substrate via droplet or continuous ejection to establish 3D living constructs[22]. Similar to traditional inkjet printing, this technology has certain merits, including a broad selection of commercial apparatus due to the low cost of machine modification. Ease of multiple printer heads installation facilitates heterogeneous architectures of tissue or organ and ensures a sound printing resolution. An ability to keep integrity is critical as newly printed cells are expected to have long-term survival in the in vivo environment. A prompt establishment of mechanical properties through supporting biomaterials is valuable. Since the printing conditions and size are limited, inkjet bioprinting is merely practical for in vivo repair or fabrication of exterior structures, such as skin.
2.3. Laser-assisted bioprinting
Laser-assisted bioprinting employs a laser to polymerize bio-ink into solid structures[23]. Laser direct-write techniques have been widely used in this approach. By laser pulses, living cells are selectively transferred from the supply container to defect locations. Stereolithography can also be used for in vivo bioprinting to allow precise fabrication of structures with micro or nanoscale resolution. The fact that the heat generated by the laser or exposure to ultraviolet lights may impair cell viability should be considered. Moreover, laser or stereolithography-based techniques may be unsuitable for in vivo scenarios due to the machine size. Although the advantage of optics-assisted bioprinting in ultrahigh resolution and precision to meet the requirements of clinical settings, there is still room for improvement in terms of photocrosslinkable biomaterials and photonics techniques.
The aforementioned methods are the most common modalities in bioprinting. Their derivatives, which include acoustic droplet ejection[24], direct-write assembly[25], fused deposition modeling[26], and powder printing[27], have also been developed recently. These printing modalities can be further applied to a variety of printers (or end effectors) mounted on robotic arms for dexterous and precision biofabrication.
3. Bioprinting robots
Robots and handheld devices are commonly employed to achieve in situ fabrication of 3D structures with complex shapes and curved surfaces[15]. Robotics can facilitate bioprinting tasks with high accuracy and automation level without exhaustion. Robots have been routinely used in minimally invasive surgical settings[28], thereby paving the way for in situ bioprinting[29]. Robot configurations determine the working space, deposition flexibility, and operational precision of bioprinting, of which Cartesian coordinate, articulated, and parallel robots are the main configurations[30]. The typical robotic-assisted bioprinting process is shown in Figure 2.
Figure 2.
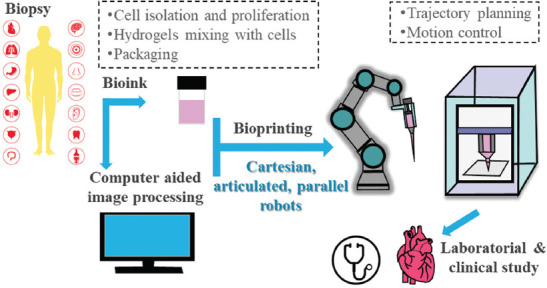
Typical process of robotic-assisted bioprinting.
3.1. Configurations
3.1.1. Cartesian coordinate robots
Conventional 3D printers deposit materials layer-by-layer along the vertical direction (Figure 3A) using the axis-aligned slicing method. A planar surface is often needed to support the printed structure. Adopting this mechanism allows for individualized modeling and rapid fabrication. The procedure involves 3D computer model design and slicing followed by layer deposition of biomaterials through force, sound, light, electricity, and heat. Extrusion-, inkjet-, and optics-based methods can be readily combined with Cartesian coordinate robots. The advantages of this technology for bioprinting include low cost, technology transferability from conventional 3D printing, and a high degree of stiffness of the printing platform, whereas the challenges are evident in anisotropic bioprinting[31]. Since body tissues are anisotropic, different anisotropic material properties along an axis are needed. Moreover, the stair-step effect is non-negligible[32]. During the fabrication of each layer, the motion of the nozzle is restricted to a two-dimensional plane along the direction of gravity. This inevitably results in the staircase effect, where surface distortion occurs between neighboring layers. To improve printing flexibility, Edward Shi et al. proposed a method combining Cartesian and curvilinear printing head motion for in vivo bioprinting. A biomimetic “tendon cable” soft robot arm was added to a conventional Cartesian three-axis 3D printer to facilitate motion along six independent degrees of freedom (DOF)[31]. O’Neill et al. demonstrated the feasibility of robotic deposition of biocompatible materials directly onto unconstrained, moving human anatomy (Figure 3B). The robotic platform employed the XYZ gantry system, in which the motions along the axis were actuated by stepper motors[33].
Figure 3.
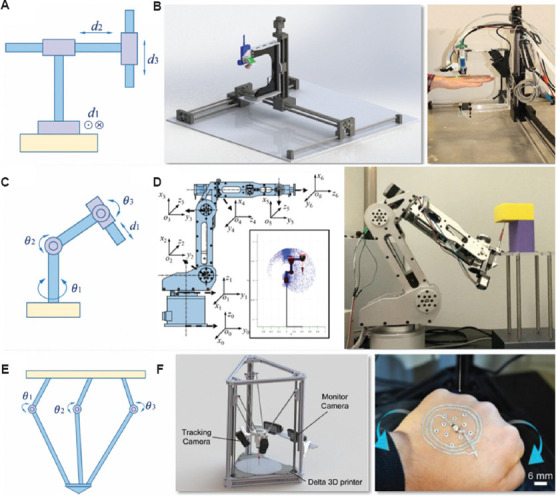
Typical robot configurations for in situ 3D bioprinting. (A) Cartesian. (B) Design and material deposition onto a moving hand by the robot[33] (from ref. [33] licensed under IEEE license). (C) Articulated. (D) Design and construction on inverted surface by the robotic bioprinter[41] (from ref. [41] licensed under Creative Commons Attribution 4.0 license). (E) Parallel. (F) Design and adaptive printing on a human hand, which can move freely in the workspace[46] (from ref. [46] licensed under John Wiley and Sons license).
3.1.2. Articulated robots
Articulated robots with 360° rotating joints (Figure 3C) have been developed to overcome the limitations of fixed axes. The number of rotary joints ranges from two to ten or more, and these rotary joints are often powered by servo motors. Most robotic arms have three to six axes, which allow biomaterials to be placed onto curved surfaces with sophisticated profiles from all directions[34]. Articulated robots are more versatile and flexible than other platforms as they have multiple axes and degrees of freedom. Other merits of this anthropomorphic technology include its deployable/foldable ability to reduce the footprint. Moreover, the advanced kinematics algorithms also help to improve the precision of movement[35]. Particularly, as demonstrated by the da Vinci surgical system, articulated robots enable surgeons to perform delicate operations through small incisions[36]. Articulated robots can also enhance in situ bioprinting for potential clinical applications. One of the main concerns in the development of the articulated robotic system is the low intraoperative correction ability if the controller fails[37]. In addition, a singularity (a robot end effector becomes blocked in certain directions) may exist[38]. Compared with Cartesian robots, the controlling and programming of articulated robots are more complicated. For instance, redundancy can be exploited to improve manipulability and achieve more dexterous motions, but it may complicate the inverse kinematics[39]. Li et al. demonstrated the feasibility of using the industrial 6-DOF robot for direct in situ 3D printing in living animal models for injury repair. The osteochondral defect in rabbits could be repaired in about 1 min[40]. Zhao et al. used a novel design and an adaptive in situ bioprinting robot for rapid biomaterial fabrication on an excisional wound in mice (Figure 3D). The 6-DOF robot successfully provided immediate, precise, and complete wound coverage through stereotactic bioprinting[41]. Zhang et al. equipped a printer with a 6-DOF robotic arm, which enabled cell printing on 3D complex-shaped vascular scaffolds from all directions, and proposed an oil bath-based cell printing method to preserve the natural functions of cell after printing[42].
3.1.3. Parallel robots
Parallel robots or delta robots have multiple arms (usually three) connected to a single base mounted above the workspace (Figure 3E). These robots employ articulated robots that use similar mechanisms for movement, and they tend to move delicately and precisely. Since each joint of the end effector is directly controlled by multiple arms, these robots have high efficiency with respect to their moving speed[43]. Other advantages of the parallel configuration include simple structure design and easy installation. The replacement of machine elements is also relatively straightforward. In contrast, issues such as massive linkages and singularity due to parallel linkages may exist in ordinary parallel robots[44]. Zhu et al. employed a delta robot printer to print cell-laden hydrogels on live mice to investigate the potential of bioprinting for wound healing[45]. The method also demonstrated feasibility in fabricating smart wearable devices directly on the human body (Figure 3F). Zhao et al. developed a micro bioprinting platform that can be installed on an endoscope to enter the human body and process bioprinting. A delta robot was leveraged as the configuration of the printing platform. The delta robot can fold itself down into smaller size when entering the patient’s body and unfold before bioprinting[46].
The comparison of robot configurations for in situ bioprinting is shown in Table 1.
Table 1.
Comparisons of typical robot configurations for in situ 3D bioprinting
| Configuration | Advantages | Disadvantages | Workspace |
|---|---|---|---|
| Cartesian | • Simpler mechanical engineering design | • Nonflexible operation restricted by axes | Restricted |
|
| |||
| • Transferability from conventional 3D printing | • Non-negligible staircase effect | ||
|
| |||
| • High stiffness compared with articulated design | • Unable to print on curvilinear or irregular surfaces | ||
|
| |||
| Articulated | • Excellent flexibility, owing to multiple DOFs | • Low intraoperative correction ability if the controller fails | Large |
| • Enhanced by cutting-edge control algorithms | • Singularity issue | ||
|
| |||
| • Compatible with minimally invasive surgery | • Relatively complex inverse kinematics | ||
|
| |||
| • High foldability and small footprint | |||
|
| |||
| Parallel | • Simple structure and easy installation | • Massive linkages | Medium |
|
| |||
| • Easy replacement of machine elements • High precision at fast speed |
• Singularity issue due to parallel linkages | ||
DOF: Degrees of freedom
4. Three-dimensional bioprinted tissues and organs
4.1. Cartilage
Cartilage is an important structural component of the human body. Cartilage injuries are very common, affecting millions of people, and they may result in joint dysfunction. Cartilage is firm but softer and much more flexible than bone. However, blood vessels and nerves are absent in the tissue. Hence, damaged articular cartilage has poor self-healing capacity, and it is difficult to detect early articular cartilage damage. Although autologous chondrocyte implantation, mosaicplasty, and periosteal grafts have been widely adopted as conventional treatments for repairing chondral defects, the reproduction of normal hyaline cartilage with long-term stability and reliable functionality must be improved. The direct repair of cartilage by developing large-scale biomimetic anisotropic constructs with structural integrity, mimicking the native tissue, is challenging. Cui et al. developed a 3D bioprinting system with photopolymerization that is capable of cartilage tissue engineering. For repairing defects in osteochondral plugs, poly(ethylene glycol) dimethacrylate with human chondrocytes was printed layer-by-layer, revealing the significance of direct cartilage repair through bioprinting[47]. Sun et al. demonstrated anisotropic cartilage regeneration through 3D bioprinting dual-factor releasing and gradient-structured constructs. The fabricated anisotropic cartilage structures showed fine integrity, superficial lubrication, and nutrient supply within deep layers[48]. The dual-factor releasing and gradient-structured cartilage scaffold demonstrated better repairing effect in the rabbit knee cartilage defect model in vivo (Figure 4A). Ma et al. developed a 6-DOF robot for in situ 3D bioprinting to regenerate cartilage and explored its potential application in clinical settings. The in vivo experiment was conducted on rabbits. The arrangement of chondrocytes in the hydrogel implantation and in situ bioprinting groups was closer to native cartilage[49].
Figure 4.
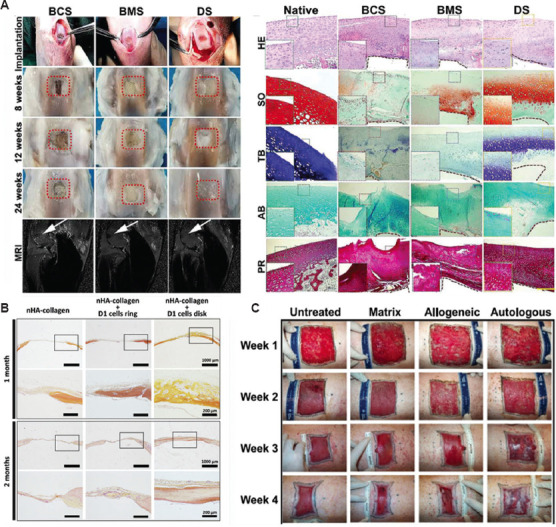
Tests of robotic-assisted bioprinted cartilage, bone, and skin. (A) Scaffold implantation process, and gross appearance of the repaired cartilage at different weeks (left); histological evaluation of the repaired cartilage (right)[49] (from ref. [49] licensed under Creative Commons Attribution 4.0 license). (B) Histology tests of bone repair in a calvaria defect in mice at 1 and 2 months post printing using hematoxylin-eosin-safran staining[40] (from ref. [40] licensed under Creative Commons Attribution 4.0 license). (C) In situ bioprinted autologous and allogeneic fibroblasts and keratinocytes compared to bioprinted fibrinogen/collagen (matrix only) and untreated control over weeks[54] (from ref. [54] licensed under Creative Commons Attribution 4.0 license).
4.2. Bone
Bone fracture healing and the realization of the function of bones to withstand and adapt to mechanical stresses are results of the synergic effect of bone cells, extracellular matrix, and bioactive molecules. Vascularized bone graft has been recognized as the gold standard in the field of bone healing for four decades. Approximately a couple of million bone grafts are performed yearly across the globe to treat bone lesions. These traditional technologies for repairing defects based on autogenous or allogeneic bone grafts have several limitations, including donor-site availability and morbidity, graft incorporation and remodeling, low biological properties, and high cost. 3D bioprinting provides novel solutions to these enormous clinical challenges. In particular, repairing bone damage by direct in situ 3D bioprinting has been viewed as a promising entrance for applying 3D bioprinting in clinical settings. Some reports have evaluated in situ 3D bioprinting for clinical use or injury repair, demonstrating the employability of this technology in healing damaged bones. According to Keriquel et al., automatic robotic bioprinting can be employed by surgeons to achieve precise cellular implantation at a micron or millimeter scale. Mesenchymal stromal cells with collagen and nano-hydroxyapatite were successfully printed for in vivo bone regeneration in a calvaria defect model in mice[50]. After hematoxylin-eosin-safran staining, the histologic evaluation of in vivo bone repair in a calvaria defect in mice at 1 and 2 months is shown in Figure 4B. Li et al. developed an in situ 3D bioprinting technology based on a robotic manipulator to repair long segmental bone defects in a living swine model. By robotic-assisted means, the operation time was significantly reduced, which may be beneficial to patients[40]. Lipskas et al. combined 3D bioprinting and robotic-assisted minimally invasive surgery techniques to improve regenerative medicine. They investigated the remote center of motion, which is critical to minimally invasive surgery, followed by biomaterial development. The repair of knee defects was used as an example of the application of in vivo 3D printing[51].
4.3. Skin
Skin, which consists of epidermis, dermis, and subcutaneous tissue, is the largest organ in the human body. It serves as a protective barrier against mechanical, thermal, and physical injuries as well as hazardous substances. The skin performs physiological functions, including physiological metabolism and nerve conduction. Its self-regeneration process is slow, in which wounds beyond 4 cm in diameter do not repair well without intervention. Conventional methods for repairing skin wounds include autologous skin transplantation and artificial skin substitutes. The former, which covers the excised total thickness wound with autologous skin graft, has been considered the gold standard treatment. However, the applicability of grafts is limited by the supply of available donor sites; thus, it is difficult to repair skin damage covering a large area. 3D bioprinting is able to deliver bio-inks to specific sites for the reconstruction of damaged skin with biomimetic functions and activities. Recently, there has been remarkable progress in the field of skin bioprinting, which shows great potential in revolutionizing the paradigm of treatment in injury and surgery. By vividly mimicking the layered architecture, consisting of epidermis and dermis, damaged skins have been repaired successfully through bioprinting. Lee et al. revealed the potential of 3D bioprinting for tissue engineering using human skin as a prototypical example. The fabricated constructs were cultured and exposed to the air-liquid interface to promote maturation and stratification. The fabricated skin can be viewed as morphologically and biologically representative of in vivo human skin tissue, as indicated by histology and immunofluorescence characterization results[52]. Cubo et al. performed 3D bioprinting of human bilayered skin using bio-inks containing human plasma, primary human fibroblasts, and keratinocytes. Long-term in vivo analysis of the structure and function of the printed skin using an immunodeficient mice model verified that the bioengineered skin obtained by the Cartesian printer was very similar to human skin[53]. Albanna et al. conducted validation testing of a mobile skin bioprinting system that offers rapid on-site management of extensive wounds. Through printing layered autologous dermal fibroblasts and epidermal keratinocytes in a hydrogel carrier, the excisional wounds showed rapid closure, reduced contraction, and accelerated re-epithelialization[54].
4.4. Other tissues or organs
Repair and regeneration of other tissues or organs, including muscle, vascular, neural structures, and liver, through 3D bioprinting have also been successfully developed, thus providing potential clinical applications. Chen et al. used a combination of 3D printing with digital near-infrared photopolymerization to perform proof-of-concept in vivo noninvasive bioprinting. The bio-ink was printed in situ into a customized ear-like construct, with chondrification and a muscle tissue, layer-by-layer without surgical implantation[55]. Lee et al. constructed vascular channels and created adjacent capillary networks through a natural maturation process based on 3D bioprinting. The connection of capillary networks to the large perfused vascular channels was realized by the presented means[56]. Owens et al. fabricated fully biological grafts, composed of cells and cell-secreted material, with reliable reproducibility through bioprinting. The motor and sensory functions of grafts have been tested using a rat sciatic nerve injury model. The practicability of bioprinting for nerve regeneration has been validated[57]. Zhou et al. developed a ferromagnetic soft catheter robot (FSCR) system capable of performing in situ computer-controlled bioprinting in a minimally invasive manner. The FSCR was guided by the magnetic field to complete printing with high precision. The in situ printing of curved surfaces on a porcine tissue phantom and the liver of a living rat demonstrated the advantages of the intelligent and minimally invasive approach[58].
5. Challenges and suggestions
Although robotic-assisted systems have high operating accuracy and automation and are compatible with minimally invasive surgeries, their applications in clinical settings remain a challenge. In situ bioprinting robots are now in the prototype testing phase. Three issues should be addressed before promoting their applications in clinical settings. First, defect scanning, digital model reconstruction, code programming, trajectory planning, and printer calibration are all time-consuming. Furthermore, professional skills are required for human-controlled robotic-assisted operations during intraoperative work; therefore, they may be impractical for resource-limited areas. In addition, in situ bioprinting approaches are still restricted to locations near the skin; otherwise, surgery is required for printing on internal organs.
Industry 4.0 technologies, including artificial intelligence (AI), 5G, big data, and cloud computing, have revolutionized many fields. Healthcare and medical sectors are also benefiting from these technologies. For example, the aforementioned time-consuming issue can be minimized by AI-based systems, and teleoperation combined with 5G can help to scale and accelerate the applications of robotic-assisted 3D bioprinting in resource-limited areas. Miniature robotics may be more useful for minimally invasive or noninvasive surgeries. Selectively biodegradable robots with bio-inks for target tissues and organs will be useful for internal repair tasks. Four-dimensional bioprinting technologies, which add time as the fourth dimension, can be integrated with miniature robots to modulate their shapes or functionalities with time. Interdisciplinary collaborations across various fields are essential for fostering more innovations and promoting clinical applications.
6. Conclusions
The potential of in situ regeneration of cartilage, skin, and bone in animal models through robotics has been widely recognized. This article reviews the advancements in the field of robotic-assisted automated in situ bioprinting. The primary modalities of 3D bioprinting, robot configurations, and the applications in cartilage, bone, and skin repair are discussed. With the accelerated growth of knowledge and advancements of technologies in computer science and manufacturing engineering, in situ bioprinting is believed to be feasible in the near future.
Acknowledgments
The authors gratefully thank Professor Hui Guo, the Director of Shanghai Neuromedical Centre, and Yi Huang, the Director of Experimental Research Centre in Clinical Medicine, Fujian Provincial Hospital for their helpful suggestions.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Numbers: 62173093, 61604042] and Fujian Provincial Nat. Sci. Foundation [Grant Numbers: 2020Y0014].
Conflict of interest
There are no conflicts to declare.
Author contributions
Conceptualization: Hui Dong, Hao Sun, Junyi Shang
Supervision: Hao Sun
Writing – original draft: Bo Hu, Weikang Zhang, Wantao Xie, Jin Mo
Writing – review & editing: Bo Hu, Weikang Zhang, Wantao Xie, Jin Mo, Hao Sun
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Gao W, Zhang Y, Ramanujan D, et al. The status, challenges, and future of additive manufacturing in engineering. Comput Aided Des. 2015;69:65–89. https://doi.org/10.1016/j.cad.2015.04.001. [Google Scholar]
- 2.Kruth JP, Leu MC, Nakagawa T. Progress in additive manufacturing and rapid prototyping. Cirp Ann. 1998;47:25–540. https://doi.org/10.1016/S0007-8506(07)63240-5. [Google Scholar]
- 3.Dilberoglu UM, Gharehpapagh B, Yaman U, et al. The role of additive manufacturing in the era of industry 4.0. Proc Manuf. 2017;11:545–554. https://doi.org/10.1016/j.promfg.2017.07.148. [Google Scholar]
- 4.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Liu J, Zhu W, et al. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev. 2018;132:235–251. doi: 10.1016/j.addr.2018.06.011. https://doi.org/10.1016/j.-addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pati F, Gantelius J, Svahn HA. 3D bioprinting of tissue/organ models. Angew Chem Int Ed Engl. 2016;55:4650–4665. doi: 10.1002/anie.201505062. https://doi.org/10.1002/anie.201505062. [DOI] [PubMed] [Google Scholar]
- 7.Peng W, Datta P, Ayan B, et al. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017;57:26–46. doi: 10.1016/j.actbio.2017.05.025. https://doi.org/10.1016/j.actbio.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzio N, Eglin D, Serra T, et al. Bio-fabrication:convergence of 3D bioprinting and nano-biomaterials in tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2020;8:326. doi: 10.3389/fbioe.2020.00326. https://doi.org/10.3389/fbioe.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4:370–380. doi: 10.1038/s41551-019-0471-7. https://doi.org/10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 10.Sun W, Starly B, Daly AC, et al. The bioprinting roadmap. Biofabrication. 2020;12:022002. doi: 10.1088/1758-5090/ab5158. https://doi.org/10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 11.Samandari M, Mostafavi A, Quint J, et al. In situ bioprinting:Intraoperative implementation of regenerative medicine. Trends Biotechnol. 2022;40:1229–1247. doi: 10.1016/j.tibtech.2022.03.009. https://doi.org/10.1016/j.tibtech.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther. 2007;7:1123–1127. doi: 10.1517/14712598.7.8.1123. https://doi.org/10.1517/14712598.7.8.1123. [DOI] [PubMed] [Google Scholar]
- 13.Hong N, Yang GH, Lee J H, et al. 3D bioprinting and its in vivo applications. J Biomed Mater Res B Appl Biomater. 2018;106:444–459. doi: 10.1002/jbm.b.33826. https://doi.org/10.1002/-jbm.b.33826. [DOI] [PubMed] [Google Scholar]
- 14.Prendergast ME, Burdick JA. Recent advances in enabling technologies in 3D printing for precision medicine. Adv Mater. 2020;32:1902516. doi: 10.1002/adma.201902516. https://doi.org/10.1002/adma.201902516. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Choudhury D, Yu F, et al. In situ bioprinting-bioprinting from benchside to bedside? Acta Biomater. 2020;101:14–25. doi: 10.1016/j.actbio.2019.08.045. https://doi.org/10.1016/j.actbio.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, He J, Liu Y, et al. The trend towards in vivo bioprinting. Int J Bioprint. 2015;1:15–26. https://doi.org/10.18063/IJB.2015.01.001. [Google Scholar]
- 17.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33:395–400. doi: 10.1016/j.tibtech.2015.04.005. https://doi.org/10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Ding H, Chang RC. Simulating image-guided in situ bioprinting of a skin graft onto a phantom burn wound bed. Addit Manuf. 2018;22:708–719. https://doi.org/10.1016/j.addma.2018.06.022. [Google Scholar]
- 19.Samandari M, Quint J, Rodríguez?delaRosa A, et al. Bioinks and bioprinting strategies for skeletal muscle tissue engineering. Adv Mater. 2022;34:2105883. doi: 10.1002/adma.202105883. https://doi.org/10.1002/adma.202105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Ravnic DJ, Ozbolat IT. Intraoperative bioprinting:Repairing tissues and organs in a surgical setting. Trends Biotechnol. 2020;38:594–605. doi: 10.1016/j.tibtech.2020.01.004. https://doi.org/10.1016/j.tibtech.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Jia Y, Dong H, et al. Combining additive manufacturing with microfluidics:An emerging method for developing novel organs-on-chips. Curr Opin Chem Eng. 2020;28:1–9. https://doi.org/10.1016/j.coche.2019.10.006. [Google Scholar]
- 23.Dou C, Perez V, Qu J, et al. A state?of?the?art review of laser?assisted bioprinting and its future research trends. Chem Bio Eng Rev. 2021;8:517–534. https://doi.org/10.1002/cben.202000037. [Google Scholar]
- 24.Jentsch S, Nasehi R, Kuckelkorn C, et al. Multiscale 3D bioprinting by nozzle?free acoustic droplet ejection. Small Methods. 2021;5:2000971. doi: 10.1002/smtd.202000971. https://doi.org/10.1002/smtd.202000971. [DOI] [PubMed] [Google Scholar]
- 25.Masaeli E, Marquette C. Direct-write bioprinting approach to construct multilayer cellular tissues. Front Bioeng Biotechnol. 2020;7:478. doi: 10.3389/fbioe.2019.00478. https://doi.org/10.3389/fbioe.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darwish LR, El-Wakad MT, Farag MM. Towards an ultra-affordable three-dimensional bioprinter:A heated inductive-enabled syringe pump extrusion multifunction module for open-source fused deposition modeling three-dimensional printers. J Manuf Sci Eng. 2021;143:125001. https://doi.org/10.1115/1.4050824. [Google Scholar]
- 27.Salehi MM, Ataeefard M. Micro powder poly lactic acid/carbon black composite as a bio printing ink. J Composite Mater. 2019;53:2407–2414. https://doi.org/10.1177/0021998319828154. [Google Scholar]
- 28.Kalan S, Chauhan S, Coelho RF, et al. History of robotic surgery. J Robot Surg. 2010;4:141–147. doi: 10.1007/s11701-010-0202-2. https://doi.org/10.1007/s11701-010-0202-2. [DOI] [PubMed] [Google Scholar]
- 29.Simeunović A, Wolf K, Tierling K, et al. A surgical robot for intracorporeal additive manufacturing of tissue engineering constructs. IEEE Robot Automation Lett. 2022;7:7495–7502. https://doi.org/10.1109/LRA.2022.3183752. [Google Scholar]
- 30.Tan B, Kuang S, Li X, et al. Stereotactic technology for 3D bioprinting:From the perspective of robot mechanism. Biofabrication. 2021;13:043001. doi: 10.1088/1758-5090/ac1846. https://doi.org/10.1088/1758-5090/ac1846. [DOI] [PubMed] [Google Scholar]
- 31.Shi E, Lou L, Warburton L, et al. 3D printing in combined Cartesian and curvilinear coordinates. J Med Devices. 2022;16:044502. https://doi.org/10.1115/1.4055064. [Google Scholar]
- 32.Urhal P, Weightman A, Diver C, et al. Robot assisted additive manufacturing:A review. Robot Comput Integr Manuf. 2019;59:335–345. https://doi.org/10.1016/j.rcim.2019.05.005. [Google Scholar]
- 33.O'Neill JJ, Johnson RA, Dockter RL, et al. In:2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) New Jersey: IEEE; 2017. 3D bioprinting directly onto moving human anatomy; pp. 934–940. https://doi.org/10.1109/IROS.2017.8202257. [Google Scholar]
- 34.Fortunato GM, Rossi G, Bonatti AF, et al. Robotic platform and path planning algorithm for in situ bioprinting. Bioprinting. 2021;22:e00139. https://doi.org/10.1016/j.bprint.2021.e00139. [Google Scholar]
- 35.Dong H, Du Z, Chirikjian GS. Workspace density and inverse kinematics for planar serial revolute manipulators. Mech Machine Theory. 2013;70:508–522. https://doi.org/10.1016/j.mechmachtheory.2013.08.008. [Google Scholar]
- 36.Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg. 2004;188:19–26. doi: 10.1016/j.amjsurg.2004.08.020. https://doi.org/10.1016/j.amjsurg.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 37.D'Souza M, Gendreau J, Feng A, et al. Robotic-assisted spine surgery:History, efficacy, cost, and future trends. Robot Surg Res Rev. 2019;6:9–23. doi: 10.2147/RSRR.S190720. https://doi.org/10.2147/RSRR.S190720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torabi A, Khadem M, Zareinia K, et al. Using a redundant user interface in teleoperated surgical systems for task performance enhancement. Robotica. 2020;38:1880–1894. https://doi.org/10.1017/-S0263574720000326. [Google Scholar]
- 39.Zhao Q, Guo J, Hong J, et al. An enhanced moment-based approach to time-dependent positional reliability analysis for robotic manipulators. Mech Machine Theory. 2021;156:104167. https://doi.org/10.1016/j.mechmachtheory.2020.104167. [Google Scholar]
- 40.Li L, Shi J, Ma K, et al. Robotic in situ 3D bio-printing technology for repairing large segmental bone defects. J Adv Res. 2021;30:75–84. doi: 10.1016/j.jare.2020.11.011. https://doi.org/10.1016/j.jare.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao W, Chen H, Zhang Y, et al. Adaptive multi?degree?of?freedom in situ bioprinting robot for hair?follicle?inclusive skin repair:A preliminary study conducted in mice. Bioeng Transl Med. 2022;2022:e10303. doi: 10.1002/btm2.10303. https://doi.org/10.1002/btm2.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Wu C, Dai C, et al. A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioactive Mater. 2022;18:138–150. doi: 10.1016/j.bioactmat.2022.02.009. https://doi.org/10.1016/j.bioactmat.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding J, Lyu S, Da T, et al. Error space estimation of three degrees of freedom planar parallel mechanisms. J Mech Robot. 2019;11:031013. https://doi.org/10.1115/1.4042633. [Google Scholar]
- 44.Yang Y, Peng Y, Pu H, et al. Deployable parallel lower-mobility manipulators with scissor-like elements. Mech Machine Theory. 2019;135:226–250. https://doi.org/10.1016/j.mechmachtheory.2019.01.013. [Google Scholar]
- 45.Zhu Z, Guo S Z, Hirdler T, et al. 3D printed functional and biological materials on moving freeform surfaces. Adv Mater. 2018;30:1707495. doi: 10.1002/adma.201707495. https://doi.org/10.1002/adma.201707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao W, Xu T. Preliminary engineering for in situ in vivo bioprinting:A novel micro bioprinting platform for in situ in vivo bioprinting at a gastric wound site. Biofabrication. 2020;12:045020. doi: 10.1088/1758-5090/aba4ff. https://doi.org/10.1088/1758-5090/aba4ff. [DOI] [PubMed] [Google Scholar]
- 47.Cui X, Breitenkamp K, Finn MG, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18:1304–1312. doi: 10.1089/ten.tea.2011.0543. https://doi.org/10.1089/ten.tea.-2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, You Y, Jiang W, et al. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci Adv. 2020;6:eaay1422. doi: 10.1126/sciadv.aay1422. https://doi.org/-10.1126/sciadv.aay1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma K, Zhao T, Yang L, et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel:An in vivo study. J Adv Res. 2020;23:123–132. doi: 10.1016/j.jare.2020.01.010. https://doi.org/10.1016/j.jare.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keriquel V, Oliveira H, Rémy M, et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-01914-x. https://doi.org/10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipskas J, Deep K, Yao W. Robotic-assisted 3D bio-printing for repairing bone and cartilage defects through a minimally invasive approach. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-38972-2. https://doi.org/10.1038/s41598-019-38972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee V, Singh G, Trasatti JP, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 2014;20:473–484. doi: 10.1089/ten.tec.2013.0335. https://doi.org/10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cubo N, Garcia M, Del Canizo JF, et al. 3D bioprinting of functional human skin:Production and in vivo analysis. Biofabrication. 2016;9:015006. doi: 10.1088/1758-5090/9/1/015006. https://doi.org/10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 54.Albanna M, Binder KW, Murphy SV, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep. 2019;9:1586. doi: 10.1038/s41598-018-38366-w. https://doi.org/10.1038/s41598-018-38366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Zhang J, Liu X, et al. Noninvasive in vivo 3D bioprinting. Sci Adv. 2020;6:eaba7406. doi: 10.1126/sciadv.aba7406. https://doi.org/10.1126/sciadv.aba7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee VK, Lanzi AM, Ngo H, et al. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng. 2014;7:460–472. doi: 10.1007/s12195-014-0340-0. https://doi.org/10.1007/s12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owens CM, Marga F, Forgacs G, et al. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5:045007. doi: 10.1088/1758-5082/5/4/045007. https://doi.org/10.1088/1758-5082/5/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou C, Yang Y, Wang J, et al. Ferromagnetic soft catheter robots for minimally invasive bioprinting. Nat Commun. 2021;12:5072. doi: 10.1038/s41467-021-25386-w. https://doi.org/10.1038/s41467-021-25386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


