Abstract
Three-dimensional (3D) printing, which is a valuable technique for the fabrication of tissue-engineered constructs and biomedical devices with complex architectures, has brought about considerable progress in regenerative medicine, drug delivery, and diagnosis of diseases. However, because of the static and inanimate properties of conventional 3D-printed structures, it is difficult to use them in therapies for active and precise medicine, such as improved tissue regeneration, targeted or controlled drug delivery, and advanced pathophysiological monitoring. The integration of stimuli-responsive biomaterials into 3D printing provides a potential strategy for designing and building smart constructs that exhibit programmed functions and controllable changes in properties in response to exogenous and autogenous stimuli. These features make 3D-printed smart constructs the next generation of tissue-engineered products. In this review, we introduce the prevalent 3D printing techniques (with an emphasis on the differences between 3D printing and bioprinting, and biomaterials and bioink), the working principle of each technique, and the advantages of using 3D printing for the fabrication of smart constructs. Stimuli-responsive biomaterials that are widely used for 3D printing of smart constructs are categorized, followed by a summary of their applications in tissue regeneration, drug delivery, and biosensors. Finally, the challenges and future perspectives of 3D-printed smart constructs are discussed.
Keywords: Three-dimensional printing, Stimuli-responsive biomaterials, Smart constructs, Tissue engineering, Biomedical application
1. Introduction
Three-dimensional (3D) bioprinting is a revolutionary manufacturing technology that enables the flexible and precise assembly of living cells, biomaterials, and biological factors to build tissue-engineered structures for use in therapeutics, pharmaceuticals, and diagnostics[1]. The constructs are designed and biofabricated with biomimetic structures into specific shapes, sizes, and compositions to perform tasks, such as tissue repair, drug release, or signal acquisition, when implanted in patients. These constructs can serve as supportive bio-scaffolds that escort cells and biomolecules toward the target sites[2], or as stiff sensors and transducers placed in the vicinity of biological substances (e.g., sweat, tears, and blood) to detect pathophysiological changes of these substances in the body[3]. However, conventional 3D-printed constructs are restricted to rigid, static, and passive processes because they cannot promote regeneration, achieve targeted drug delivery, and monitor physiological changes, making it difficult for them to meet the demands of biomedical and clinical applications. To fill this gap, the focus has been on smart constructs that can detect environmental conditions and stimuli (e.g., mechanical, chemical, electrical, or magnetic signals) and react to them by performing specific functions.
To achieve smart performance, 3D-bioprinted constructs are fabricated with specific microstructures, topology, geometry, and composition; therefore, under a given stimuli, a designed transformation, specific property, or programmed functionality of the responsive biomaterials is triggered. Recently developed intelligent materials include stimuli-responsive hydrogels[4], shape-memory polymers[5], liquid crystal polymers[6], and responsive additives (e.g., graphene oxide[7], magnetic medium[8], and electroconductive compounds[9]). The emergence of intelligent biomaterials has opened new avenues for engineering various smart structures (such as self-control mechanics, soft robots, adaptive optics, and actuators) that have been utilized in the fields of defense, aerospace, and industry. However, to build smart bioproducts, the selected biomaterials must fulfill several critical requirements so that they can be adapted to 3D printing/bioprinting techniques.
The fundamental requirement for biomedical applications is that the biomaterial used for building smart constructs should be non-toxic, biocompatible, and biodegradable. The flexibility of the 3D printing techniques can facilitate the fabrication of biological structures with intricate designs (e.g., microstructure, topology, geometry, and composition). Although many printing techniques are available, it is not possible to bioprint all types of biomaterials, and the materials used require several suitable properties, including viscosity, rheological features, and polymerization[10]. Furthermore, to impart 3D-bioprinted products with intelligence, biomaterials must show prompt and tunable responses to specific stimuli that can be endured by patients. These stimuli can be exogenous or autogenous signals. Due to the advancements in modern medical instrumentation, diverse signals, including magnetism, electricity, irradiation, heat, and acoustics, can be readily used as external stimuli for biological devices and medicines to realize precise, customized health care[11]. However, the human body is a sophisticated system that naturally and continuously experiences complex stress and changes in temperature, moisture, pH, enzymes, ion concentrations, and electrical activity, which can be used as internal impulses[12]. These physical, chemical, and biological cues can be utilized to control various aspects of the smart constructs by triggering shape-morphing[13], navigating targeted delivery[14], programming release kinetics[15], and controlling the degradation rate[16]. Due to their flexibility, 3D-bioprinted smart constructs have great potential as next-generation therapeutic tools and biomedical devices (Figure 1).
Figure 1.
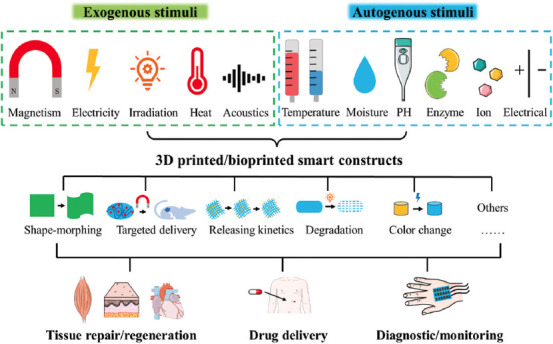
Illustration of 3D-printed or -bioprinted smart constructs with various intelligent functions in response to external or internal stimuli and their biomedical applications for tissue regeneration, drug delivery, and diagnosis or monitoring.
This review focuses on the progress of 3D-bioprinted smart constructs using stimuli-responsive biomaterials for biomedical and clinical applications. It explains the definition and classification of 3D-printable biomaterials and bioinks, outlines prevalent 3D printing and bioprinting techniques, and elaborates on the advantages of 3D printing and bioprinting in creating smart constructs. Subsequently, various advanced responsive biomaterials that have been explored for fabricating smart constructs are discussed, and typical applications of these 3D-bioprinted smart constructs, such as in regenerative medicine, drug delivery, and biosensors, are systematically summarized.
2. Biomaterials and 3D bioprinting techniques
2.1. Biomaterials and bioinks
For a long time, confusion regarding the definitions of “biomaterials” and “bioinks” has led to incorrect use of these two terms for quite some time. Biomaterials have been extensively studied over the past 50 years. Biomaterials are defined as substances engineered to interact with the biological systems for biomedical applications, mainly for therapeutic (treatment, augmentation, repair, or replacement) or diagnostic purposes[17]. Biomaterials is, therefore, a broad term that includes biocompatible metals, ceramics, glass, polymers, biomolecules, and biological products, such as enzymes, growth factors, DNA, and exosomes.
Groll et al.[18] defined bioinks as “a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active components and biomaterials. “ This definition distinguishes bioinks from other types of biomaterials. Although biomaterials must support cellular behaviors and functions, they are not designated to encapsulate cells for the fabrication of constructs. Bioinks must contain living cells as a fundamental element, irrespective of the other materials selected as the basal matrix. Even microcarriers of cells (e.g., micro-spheroids and -cylinders) and cell aggregates (composed of only cells) qualify as bioinks. However, to support cell viability and performance, bioinks should provide a friendly environment that optimally mimics the native microenvironment of the human body. Therefore, bioactive hydrogels that resemble the structure and composition of the natural extracellular matrix (ECM) are commonly used in bioinks (Figure 2). Based on their source, hydrogel bioinks can be divided into two categories, natural (e.g., collagen, gelatin, alginate, chitosan, cellulose, fibrin, and their derivatives) and synthetic bioinks (e.g., polyethylene glycol (PEG) and Pluronic F127), which have been comprehensively reviewed[19].
Figure 2.
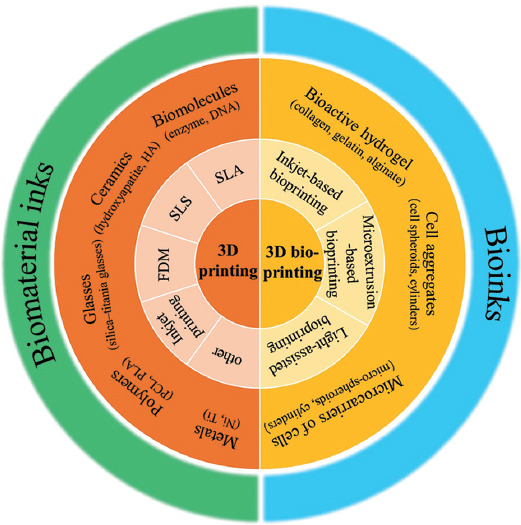
A schematic for categorizing prevalent 3D printing and bioprinting techniques and distinguishing bioinks from biomaterial inks.
In addition, the fabrication techniques designed for each biomaterial and bioink should be classified accordingly. Since cells are vulnerable to harsh manufacturing conditions, such as heat, moisture, pH, osmotic pressure, and irradiation, 3D printing techniques used for building structures using biomaterials might not be applicable for bioinks. 3D bioprinting, which is based on several conventional 3D printing techniques, has been developed to utilize bioinks to fabricate living constructs.
2.2. General overview of conventional 3D printing techniques
3D printing, also known as additive manufacturing (AM) or rapid prototyping, is a versatile fabrication technique that can assemble a wide range of materials using various principles (e.g., deposition, binding, sintering, or polymerization) in a layer-by-layer manner to rapidly construct 3D objects by following complex designs that are difficult to produce using traditional manufacturing approaches, such as milling, cutting, drilling, and lathing[20]. Since its invention in the 1980s, 3D printing techniques have been applied to a broad range of domains, such as engineering, medicine, military, food industry, and education. Its distinctive advantages and vast demands in relevant fields have spawned various 3D printing techniques with unique working mechanisms. As defined in the ASTM F2792 standard, AM techniques are divided into seven categories: Binder jetting, directed energy deposition, material extrusion, material jetting, powder bed fusion, sheet lamination, and vat photopolymerization[21]. This section focuses on relevant techniques frequently applied to precision medicine and discusses their principles, benefits, and limitations, as well as the materials used in each technique.
2.2.1. Stereolithography
Stereolithography (SLA) was the first 3D printing technique developed and was patented by Charles Hulk in 1986[22]. As SLA is a typical technique based on vat photopolymerization, the exposed photocurable resins used in this technique are selectively polymerized through several types of resin chemical reactions (e.g., free radical, methacrylate, and cationic reactions) under light irradiation. By sketching the profile of each layer using ultraviolet (UV), infrared, or visible lasers, a solid slice of a 3D object can be generated. The vertical movement of the working platform at a certain distance induces the flow of liquid resins to form another “blank paper” for printing the next layer[23] (Figure 3A(i)). Due to the precision of computer-controlled laser beams, complex geometries and submicron printing resolutions can be obtained[24]. The emergence of two photon polymerization (TPP) technique further refines the printing resolution down to nanometer scale. However, the point-by-point scanning of laser beams substantially limits the printing efficiency of early SLA.
Figure 3.

Schematic of the 3D printing technique. (A) (i) Stereolithography, (ii) digital light processing (DLP)[25] (reproduced with permission from Mu et al.[25]; copyright 2021, John Wiley and Sons), (iii) computed axial lithography volumetric fabrication[26] (reproduced with permission from Kelly et al.[26]; copyright 2019, AAAS). (B) Selective laser sintering. (C) 3D inkjet printing. (D) Fused deposition modeling.
To accelerate the fabrication speed, digital light processing (DLP) introduced a digital projector consisting of micro-mirror arrays to flash an image of a layer across the entire platform, curing all the targeted resin simultaneously (Figure 3A(ii))[25]. This improvement converts the scanning manner from point-by-point to layer-by-layer, facilitating the efficient printing of 3D constructs. Kelly et al.[26] reported a volumetric additive manufacturing approach (VAM) by rotating a photopolymer in a dynamically evolving light field, allowing for the printing of an entire complex structure through a complete revolution, skipping the need for layering (Figure 3A(iii)). Using this novel technique, several centimeter-scale objects can be printed in seconds.
With this working principle, the materials applicable to photopolymerization-based 3D printing techniques are generally compatible to photocurable materials, enabling a wide range of materials to be adoptable for engineering smart structures, including hydrogels, shape-memory polymers, and liquid crystal elastomers[27]. Without impeding key parameters in a printing procedure (e.g., irradiation intensity, light penetration, and polymerization), functional additives, such as magnetic particles, conductive compounds, biochemical reagents, and chromogenic payloads, can be flexibly incorporated into constructs, which further expand environment-responsive intelligence.
Because of the advantage for shaping materials with high resolution and complex architectures, these techniques are useful for constructing ultrafine and delicate smart biomedical devices, such as microneedles and micro-/nano-biorobots. In combination with programmed dynamic changes, these devices are equipped with advanced performances. For instance, relying on intensity decays as light penetrates the resin precursor solution during a DLP process, a microneedle arrays with back-facing barbs can be created through the desolvation-induced deformation of multiple horizontal struts (100 μm thickness and 450 μm length) on microneedles as a post-printing procedure, which enhances the tissue adhesion effect by 18 times compared with those of barb-free products[28]. As another representative example, micro-/nano-biorobots with exquisite designs can be easily constructed through polymerization-based 3D printing techniques[29]. When propelled in response to physical or chemical stimuli, these biorobots may complete various medical tasks (e.g., cancer therapy, targeted drug delivery, track imaging, and microsurgery).
2.2.2. Selective laser sintering (SLS)
As shown in Figure 3B, SLS is a powder bed fusion technique in which a laser beam is used on the surface of a thermoplastic powder to produce a designed image. The powder is then recovered from the surface, and the procedure is repeated. The laser produces a high temperature and selectively melts the powder such that the scaffold structure has low porosity. Modulating the laser power and scanning speed can result in different phenomena. Decreasing the laser scanning speed could result in a dense structure because the powder is exposed to the laser beam for longer duration; however, the fabricated structure would be more imprecise. Increasing the laser scanning speed could result in a porous structure because the powder absorbs less laser power[30].
Many types of materials can be used in SLS. Thermoplastic polymers, including natural and synthesized polymers, such as cellulose[31] and polycaprolactone (PCL)[32], have been used to manufacture scaffolds. Bioactive glass, ceramics, and metals can be used in SLS. Because polymers possess elasticity and low stiffness and ceramics have a greater stiffness than polymers, mixing polymers and ceramics (e.g., PCL and hydroxyapatite [HA]) can improve the mechanical properties of the structure[33]. The SLS technique applied in biomedical engineering can be used for manufacturing patient-specific anatomical models and implantable devices, including bone implantation, tissue engineering, and the development of bioceramic and bioactive scaffolds[34].
Another merit of SLS is the implementation of a metallurgical effect during printing. The enhanced interatomic/intermolecular forces drastically improve mechanical strength and stability. Combining with shape-memory polymers, metals, and alloys, smart constructs that can both exhibit programmed deformation (e.g., self-expansion and self-compression) and endure bear-loading environment have been developed for biomedical uses, such as artificial bone, cardiovascular stent, and orthopedic apparatus[35].
2.2.3. 3D inkjet printing
Inkjet printing is a binder jetting technique used to print computer data or information on paper[36]. In recent years, it has been used to fabricate 3D objects. During the inkjet printing process, a liquid material is printed from an inkjet nozzle onto a powder bed or a receiving substrate according to a computer-generated design. When an electrical pulse is applied to the nozzle, a vapor bubble is generated, and the droplet ejects from the nozzle as the pressure increases. The droplets either combine with the particles in the powder bed or are deposited on the substrate to create a layer. Repetition of such a drop-on-demand procedure leads to the building of a 3D structure (Figure 3C). One limitation of inkjet printing is the relatively narrow range of materials, because using highly viscous inks may increase the risk of nozzle blockage. However, because the fabrication unit includes single liquid droplets, the printing resolution of inkjet can be as high as micrometers when appropriate materials and fine nozzles are used[37,38].
Due to the high-resolution and pinpointing deposition of droplets with a small volume, inkjet 3D printing is widely used to produce smart constructs. For example, micro-electrodes and micro-actuators can be fabricated through accurate jetting of conductive polymer-loaded inks; consequently, a glucose sensor can be successfully constructed on the glucose sensor can be successfully constructed on a paper to produce an integrated wearable biomedical device[39]. Moreover, by controlling the printing parameters, droplets with uniform dimensions, shapes, and amount payloads can be produced, which are conducive to engineering responsive drug carriers with controlled dosage formulation and release kinetics. In a representative study, thermoresponsive core-shell polymer microcapsules for controlled drug release were fabricated through inkjet printing[40]. As the sensing temperature decreased to around 37°C, the thermoresponsive capsule dynamically changed from a swollen structure to a collapsed structure, resulting in the appearance of nanopores, which served as a retractable gate to control drug release and retention.
2.2.4. Fused deposition modeling
Fused deposition modeling (FDM) is a commonly used material extrusion technology for rapid prototyping. The principle of the FDM process is shown in Figure 3D. A thermoplastic polymer (e.g., polycaprolactone [PCL], polylactic acid [PLA][41], and polyurethane [PU]) is melted into a liquid state at a temperature higher than glass transition point and then extruded through a head nozzle to form a filament. These filaments can be directly deposited following computer-generated design in a layer-by-layer manner to generate a 3D structure. The thickness of the layers, the diameter of nozzles, and printing speed (speed of nozzle movement) are important factors in modulating the resolution of printing. Reducing the diameter or increasing the moving velocity of nozzles produces thinner filaments, thereby improving the printing resolution. However, the determination of these parameters depends on the properties of applied materials (e.g., glass transition point and viscosity of molten polymers). Taking acrylonitrile butadiene styrene (ABS) as an example, empirical studies have explored optimal printing parameters, the layer height is 0.1 – 0.3 mm, and the melting extrusion temperature is above 180 – 200°C[42].
Unlike other 3D printing techniques, FDM is a relatively simple fabrication process that does not require any solvent or sophisticated laser system, but it needs polymeric fiber coils that continuously supply materials and mechanical platforms to control motions[43]. This convenient approach permits FDM to be a convenient 3D printing technique for fabricating smart constructs, if environment-responsive materials or shape-memory polymers technically possess thermoplastic characteristics. Conversely, if the primary input material does not possess any stimuli-responsive ability, other functional additives should be used before extrusion (e.g., nano carbon tubes/fibers, magnetic particles, and conductive materials) to induce smart behaviors in the printed 3D constructs. Moreover, through the direct writing feature of FMD, multiple materials can be collaboratively deposited using different printing heads in a single process to produce a construct with complex architecture and heterogeneous compositions[42,44]. The coprinting manner can unify the mechanical property of structural materials and the responsive capacity of smart inks. Thus, various smart devices, such as strain sensors, smart tires, and cable-driven soft fingers, can be produced.
2.3. 3D bioprinting techniques
The fields of tissue engineering and regenerative medicine aim to facilitate the repair and regrowth of damaged tissues and organs, which usually requires key participants, such as engineered cells and biomolecules, to improve the interactions between the engineered constructs and the body of the host. The aforementioned 3D printing techniques can be used to build complex 3D constructs using various biomaterials, including bioactive polymers, metals, ceramics, and glass, which help in producing implants and surgical instruments for personalized medicine. However, their hash printing environments render them unsuitable for use with bioinks. For instance, the application of UV irradiation in SLA can trigger cell apoptosis due to DNA damage; the inkjet printing binders are usually cytotoxic, and the high temperatures used in FDM and SLS inevitably cause cell death and denaturation of proteins. Although cells and biological factors can be introduced in 3D-printed structures through post-processing methods, such as seeding, binding, and coating, these approaches are limited because precision is needed to produce biomimetic living constructs. To overcome these challenges, the 3D bioprinting techniques were designed to directly use living cells, biomaterials, and biomolecules as fundamental building blocks to fabricate 3D constructs[45]. The prevalent 3D bioprinting techniques, such as light-assisted, microextrusion-based, and inkjet-based approaches, are summarized in this section.
2.3.1. Light-assisted 3D bioprinting
Despite the high resolution of laser-based 3D printing techniques, the detrimental effects of commonly used UV irradiation on cell viability and molecular stability limit their application in engineering biological constructs (Figure 4A). UV light is electromagnetic radiation with wavelengths ranging from 10 to 400 nm, which can be further categorized into UVA (320 – 400 nm), UVB (275 – 320 nm), and UVC (<275 nm). UVA is the least harmful to the human body compared to the other shorter wavelengths. Treatments involving UVA irradiation for a short period of time, such as ophthalmological therapy for corneal disorders, have been studied in clinical trials[46]. Given the relatively high safety of UVA irradiation, SLA-based 3D printing methods that use light at a wavelength of 365 nm have been explored to process bioinks containing living cells[47]. However, UVA may induce significant cell injury during printing. To achieve high cell viability, the exposure time and irradiation intensity are critical parameters.
Figure 4.
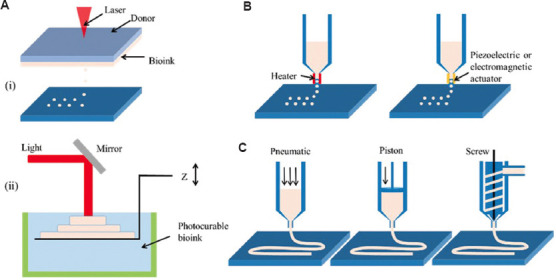
Schematic of 3D bioprinting techniques. (A) (i) Laser-assisted jetting and (ii) stereolithography. (B) Inkjet-based 3D bioprinting. (C) Extrusion-based 3D bioprinting.
Visible light (e.g., blue[48], green[49], and white lights[50]) is more biocompatible than UV irradiation. Most photocurable bioinks require photoinitiators. Since many types of photoinitiators have identical absorbance peaks but different levels of toxicities to cells, selecting a photoinitiator is the main consideration for visible light-based 3D polymerization. Based on the catalytically active species in polymerization, photoinitiators can be classified as free radicals and cations. Cationic photoinitiators are unlikely to be utilized in 3D bioprinting because they can produce protonic acid, which harms the cells[51]. Therefore, 3D bioprinting of biological constructs using visible light mostly relies on free radical photoinitiators, such as lithium phenyl-2,4,6-trimethylbenzoyl phosphinate (LAP)[52], hexahydrate/sodium persulfate (RU/SPS)[53], and eosin Y (EY)[54]. The use of visible light irradiation considerably expands the applicability of SLA for 3D bioprinting of living constructs. For instance, Wang et al.[54] reported an SLA-based 3D bioprinting approach that polymerizes polyethylene glycol diacrylate and gelatin methacrylate (GelMA) hybrid hydrogels with the assistance of an EY photoinitiator under visible light irradiation. The printed structure reached a resolution as fine as 50 μm and preserved high cell viability (>85%) for over 5 days. In addition, Bernal et al.[55] applied visible light (405 nm) to print complex centimeter-scale structures in short periods (seconds) using the volumetric photopolymerization bioprinting method and achieved high cell viability (>85%).
As an alternative to switching the light source, changing the interaction between light and the bioinks used can circumvent the damage caused by cell irradiation. The laser-assisted bioprinting approach utilizes UV irradiation as an activator for ejecting bioink droplets, instead of inducing polymerization. This bioprinting system is designed with a laser beam, a ribbon consisting of a laser energy absorbing stratum, a layer of bioink, and a receiving substrate to collect cell-laden materials[45]. While the laser beam is focused locally on the ribbon, vapor pockets are generated in the absorbing layer, inducing the formation of bioink droplets and propelling them to the receiving substrate. Patterned structures can eventually be fabricated by combining them with a moving platform. Light-assisted bioprinting is nozzle free and, therefore, avoids nozzle clogging, which is a distinct advantage in printing bioinks with high cell concentrations (up to 108 cells mL−1)[56].
2.3.2. Inkjet-based 3D bioprinting
3D inkjet bioprinters can be categorized into two types: Thermal printers, which rely on heat to produce air pressure pulses, and piezoelectric printers, which rely on acoustic pulses. Both bioprinters result in droplets being forced out of the printer heads (Figure 4B). Compared to 3D inkjet printing, the mild printing process of the inkjet-based 3D bioprinting technique allows for the application of bioinks containing cells and biomolecules. However, the materials selected for this technique should undergo stringent tests to determine their rheological and gelation properties. The inkjet-based printing method has the advantage of producing ultra-tiny droplets that can achieve single-cell deposition. However, a fine resolution is achieved using a small diameter nozzle (<100 μm), which can easily be blocked. Therefore, to facilitate the ejection of droplets from the nozzles, bioinks must have low viscosity (~0.1 Pa·s). Moreover, the successful fabrication of shaped structures using droplets of fluidic materials relies on the rapid sol-gel transition of the printed inks (e.g., through ionic gelation and photopolymerization[57,58]). Because of these prerequisites, inkjet-based 3D bioprinting is limited to depositing materials that are viscous or undergo a long-term crosslinking process. However, its advantages of being low cost, having high resolution, controllable droplet size, uniform cell density, and a rapid deposition rate allow for the fabrication of layers with patterned cellularized structures.
2.3.3. Microextrusion-based 3D bioprinting
Microextrusion-based 3D bioprinting inherits the working principle of FDM, which squeezes bioinks from a sharp nozzle in the form of filaments (Figure 4C). In contrast to roller-driven methods for the continuous material delivery of FDM, microextrusion-based 3D bioprinting applies either pneumatic pressure[59,60] or mechanical pressure, which uses pistons and screws, to force the bioink out of the needles[61,62]. A strong extrusion force (up to MPa) permits this technique to print a wide range of biomaterials, including hydrogels, polymers, and cell aggregates. Therefore, in terms of material adaptation, microextrusion-based 3D bioprinting is the most versatile of the available 3D bioprinting techniques.
The printing resolution of this technique is governed by several printing parameters: Moving speed, extrusion rate, and nozzle gauge. Changing the moving speed and extrusion rate can change the dimensions of the filaments by defining the volume of bioink deposited in a certain printing period. However, rapid motion or slow extrusion can lead to intermittent or non-uniform filaments and affect the printing quality. Although using a small nozzle helps refine the resolution, it may adversely affect the cellular activity due to the higher shear stress generated during the extrusion process. Therefore, during the process of cell-laden constructs, the bioink should exhibit shear-thinning properties to alleviate the shear stress-induced damage to cells. However, strong shear stress can be applied to orient fibrous materials residing in the bioink along the printing direction[63]. The generated anisotropic alignment of fibers can trigger the programmed dynamic changes in hydrogels (swollen and shrunk) when printed structures are exposed to stimuli (e.g., temperature, humidity, pH, and irradiation); consequently, smart functions, such as shape-morphing and locomotion, can be achieved[13]. Gong et al. reported that post-printing stimuli could reduce the scaffold dimensions and generate higher-resolution constructs[64]. The previous studies demonstrated that fiber orientation in hydrogels can guide the corresponding alignment of cells through biomechanical signaling[65]. Therefore, as cells form anisotropic functional tissues (e.g., neurons and muscles), constructs can be moved by supplying bioelectric and biochemical stimuli.
In general, 3D bioprinting has a key merit that differentiates it from 3D printing, that is, living cells can be used as building bricks. Printing flow innovations and parameters are made to protect cells. However, because of similar working principles, 3D bioprinting techniques inherit the advantages of their progenitor 3D printing methods in building smart constructs. Undoubtedly, the recruitment of vulnerable cells shrinks the pool of adaptable smart biomaterials and requires a carefully designed fabrication process. However, the involved cells and generated tissues impart lives to the 3D-bioprinted structures, enabling accurate sensing and reacting toward stimuli comparable with the response of the human body. Thus, it provides a basis for engineering smart constructs.
Table 1 summarizes and compares the main advantages and limitations of each 3D bioprinting technique in terms of the bioink properties, printing capacity, and cell-friendly performance.
Table 1.
Comparison of performances of commonly used 3D bioprinting techniques.
| Technique | Advantages | Drawbacks | References |
|---|---|---|---|
| Inkjet based | • Low cost • High cell viability (75% – 90%) • Fine printing resolution (~30 μm) • Rapid printing speed (up to 10,000 droplets per second) |
• Low cell density (<106 cells mL-1) • Low-viscosity bioinks (~3 – 12 mPa·s) • Risk of nozzle clogging |
[45,154-157] |
|
| |||
| Microextrusion | • High cell density (~108 cells mL-1, e.g., cell spheroids) • High viscosity bioinks (30 mPa·s-6×107 mPa·s×107 mPa·s) • Medium cell viability (40% – 95%) |
• Low resolution (~100 μm) • Slow printing speed (~10 – 50 μm s-1) • High shear stress • Nozzle clogging |
[158-162] |
|
| |||
| Light assisted | • Ultrafine printing resolution • High cell viability (>95%) • High cell density (108 cells mL-1) • Low-viscosity cell suspensions (1 – 300 mPa·s), • Free from nozzle clogging |
• High cost • Post-printing cell damage • Difficulty in assembling multiple types of bioinks |
[159,163,164] |
3. Stimuli-responsive biomaterials toward precision medicine
3D bioprinting enables the precise deposition of various cells and biomaterials for engineering living artificial tissues and organs with structural complexities. However, as the interest in personalized medicine increases, merely locating cells and biomaterials are not sufficient in constructing an ideal tissue or organ. Therefore, many endeavors have been implemented to develop novel biomaterials for tissue engineering[66]. Several biomaterials capable of responding to certain stimuli (e.g., temperature, pH, light, ultrasound, and magnetic fields) have been briefly introduced before their application to 3D bioprinting (Figure 5 and Table 2).
Figure 5.
Stimulation-responsive biomaterials in tissue engineering.
Table 2.
Responsive biomaterials and bioinks for 3D printing of smart constructs.
| Stimuli | Materials | Responses | Fabrication methods | Applications |
|---|---|---|---|---|
| Temperature | PLA/PCL/SOEA | Shape-morphing | FDM | Muscle tissue engineering[165] |
|
| ||||
| Polyurethane | Shape-morphing | Microextrusion | Scaffolds for tissue engineering[166] | |
|
| ||||
| PLA/hydroxyapatite | Shape-morphing | Microextrusion | Bone tissue repair[167] | |
|
| ||||
| SOEA | Shape-morphing | SLA | Muscle tissue engineering[168] | |
|
| ||||
| SOEA | Shape-morphing | SLA | Cardiac tissue repair[169] | |
|
| ||||
| pH | mPEG-silane | Programming release kinetics | Microextrusion | Protein delivery for bone repair[170] |
|
| ||||
| Polyvinylpyrrolidone | Programming release kinetics | FDM | Producing delayed release tablets[171] | |
|
| ||||
| Hydrogel-based dressing | Programming release kinetics | Microextrusion | Biosensor for monitoring pH of the wound[172] | |
|
| ||||
| Single-walled carbon nanotubes | Changing resistance values | Inkjet printing | Biosensor to measure the change in pH and fluid content in a wound[144] | |
|
| ||||
| Electricity | Pluronic F127 and aniline tetramer-grafted-polyethyleneimine | Changing the conductivity | Microextrusion | Muscle, cardiac, and nerve tissue repair[147] |
|
| ||||
| Graphene/Poly (trimethylene Carbonate) | Changing the conductivity | Microextrusion | Developing scaffolds for tissue engineering[173] | |
|
| ||||
| Light | Polyurethane | Shape-morphing | FDM | Soft robotics[174] |
|
| ||||
| Poly (lactic-co-glycolic) acid/plasmonic gold nanorods | Programming release kinetics | Microextrusion | Programmable Release Capsules[175] | |
|
| ||||
| Alginate/polydopamine | Shape-morphing | Microextrusion | Artificial tissues and organs[13] | |
|
| ||||
| Magnetic field | PLA/Fe3O4 | Shape-morphing | Microextrusion | Intravascular stent[176] |
|
| ||||
| Fe3O4/bioactive glass/PCL | Programming release kinetics | Microextrusion | Local anticancer drug delivery[177] | |
|
| ||||
| PCL/iron-doped hydroxyapatite | Changing cells behaviors | Microextrusion | Bone regeneration[178] | |
|
| ||||
| Collagen/agarose | Alignment of collagen fibers | Inkjet printing | Cartilage tissue engineering[179] | |
|
| ||||
| Ultrasound | Alginate | Programming release kinetics | Microextrusion | Drug delivery[106] |
|
| ||||
| Arginine-glycine-aspartic acid-serine peptide/nanocrystalline hydroxyapatite | Changing cells behaviors | SLA | Bone regeneration[180] | |
SOEA: Soybean oil epoxidized acrylate
3.1. Temperature-responsive biomaterials
Thermoresponsive materials respond to exogenous temperature changes and undergo shape transformations[67]. The transformation principle of thermoresponsive biomaterials is based on how their solubility and wettability change with temperature. Temperature responsiveness enables a user-defined functionality. For example, double-network hydrogels were fabricated by combining two compositions with substantially different thermal properties through crystallization[68-70]. The novel hydrogel was formulated with polyvinyl alcohol (PVA), which is capable of interpenetrating with chemically cross-linked PEG during crystallization. After three cycles of freezing and thawing, the hydrogel composed of 70% PVA and 30% PEG exhibited a stable helical morphology. The results of these studies showed that the degree of thermoresponsiveness was affected by the crystallinity of the polymers. Xu et al.[71] merged dynamic ionic and covalent bonds to formulate a novel thermally responsive polybutadiene (PB) rubber. More specifically, the ionic bonds were formed through the combination between PB-COOH and PB-NH2. Afterward, they were cross-linked with different concentrations of the trithiol cross-linker. The SPB-6% hydrogel exhibited a permanent spiral-like structure when the SPB film was photocrosslinked after rolling on a rod. As the spiral biomaterial ink was heated to 60°C, the structure was changed into a temporary flat shape, which reverted to spiral shape on cooling. This transformation may have been caused by the thermoreversibility of the ionic bonds. Different types of thermoresponsive materials are widely utilized in various tissue engineering applications. Therefore, they have great potential in smart 3D bioprinting, in which the shape of biomaterial ink can be transformed by altering the temperature within ideal ranges.
3.2. pH-responsive biomaterials
The development of pH-sensitive biomaterial inks to improve the efficiency of drug release for tumor therapy is underway[72,73]. Polymers that are used to study pH responsiveness include polyacrylate, poly(N-isopropylacrylamide), n-vinylcaprolactam, sodium alginate, and carrageenan[74]. Bivalent copper can be used in combination with alginate to form biomaterial ink. Notably, it has proven in this study that the encapsulation system is able to retain its structural integrity at pH = 1.2. The contents were then released slowly only at a pH > 5, suggesting slow release of the contents in the intestinal tract[75,76]. Furthermore, novel biomaterial inks have been developed using a gelation mechanism that crosslinks polyvinylpyrrolidone polymer and crotonic acid under gamma (γ) radiation[76]. The drug release rate from the resulting gel was much lower in an acidic medium than in a neutral medium. Some biomaterial inks must also include the stability of their pores to avoid swelling. Silica nanocomposites on top of poly(N-isopropylacrylamide) have been employed for this purpose[77]. The pH-responsive biomaterial inks depend on the ionic side chains, that is, they depend on the protonation of electrostatic repulsion with the surrounding environment[78]. pH-responsive cellulose biomaterial inks have also been used in wound dressings for better skin tissue engineering. These are biomaterial inks that can be degraded on acidic skin and have the ability to self-heal. For example, Akhlaghi et al.[79] revealed that the functionalized cellulose nanocrystals (CNCs) with amine (CNC-NH2) moieties could lead to pH-responsive characteristics. The biomaterial ink was degraded at a low pH. This ink can be added into a poly(vinyl acetate) to obtain pH-responsive composite nanofilms. In addition, pH-responsive characteristics can be used to improve micropores within a biomaterial ink[80,81]. For example, Bao et al.[80] manufactured micropore-forming viscoelastic hydrogels stimulated by pH-triggered phase separation. The fabricated structure can be tuned independently of porosity and stiffness while superior mechanical robustness can be included despite high porosity. With these features, cell spreading, migration, and proliferation can be improved.
3.3. Electrically responsive biomaterials
Many tissues or organs in our body generate endogenous electrical signals critical for various mechanisms, including mitosis, cell signaling, migration, wound healing, and angiogenesis[82]. Therefore, the ability to respond to electrical cues is essential for enhancing the functionality and homeostasis of the native tissues and organs. In addition, applying exogenous electrical signals are shown to affect stem cell differentiation and the maturity of engineered tissues/organs[82]. Recently, the use of conductive biomaterials has been a focus when studying neural interfacing, drug or molecule release from engineered structures, and mechanical actuation/stimulation acting on the cells[83]. Conductive polymers are among the most commonly used biomaterials to interface with cells[83]. This type of polymer is used as a biomaterial and in tissue engineering applications and has several advantages over conventional conductive materials (e.g., metals).
Conductive polymers are used for mechanical stimulation on cells in “organ-on-a-chip” setups. They can provoke electrical activity of internal calcium concentration of stimulated cells[84]. The actuation properties of conductive polymers in 3D structures have also been investigated. For example, PPy-coated poly(lactic-co-glycolic acid) (PLGA) fibers experienced cyclical shrinking and swelling when an electrical signals were applied to the tissue structure. It induced directional contraction and flowed through the pores of the structure to the seeded cells. The electrical stimulation significantly downregulated Oct4 and upregulated the cardiomyocyte-specific genes NKX2.5 and GATA4 with or without electrical stimulation. These results could influence stem cell differentiation and functionalities.
Electrically responsive biomaterials have recently been shown to be capable of drug release with electrical signals triggering rapid, localized, responsive, and controllable drug release[85]. This facilitates localized burst release, which is appropriate for long-term implants. Several growth factors and drugs are accommodated into conductive polymers for controlled release, including dopamine[86], naproxen[87], heparin[88], nerve growth factor (NGF)[89], and dexamethasone[90]. Specifically, drug release can be controlled stepwise, and payload volume can be controlled through electrical stimulation by modifying the electric potential used. Although controlled drug release for cell manipulation within 3D structures has not been widely studied, electrical stimulation and drug release have been shown to have remarkable effects on neuronal cell development and growth. An electroactive structure capable of controlled release of BMP4 has also been shown in a rabbit model with electrodes inserted into the bone defects. Enhanced bone healing in the tested animals was demonstrated with the synergistic benefits of electrical stimulation and drug release.
3.4. Light-responsive biomaterials
Light is a versatile stimuli for the control of engineered structures due to the easiness of use[82]. Optical stimuli were used to a localized region with a high spatial resolution. The light intensity can affect the behavior of biomaterial inks mainly by manipulating the polymer chains. Light-responsive biomaterials are an emerging class of materials for biomedical engineering applications, including photothermal[91] and photodynamic therapy[92], drug delivery[93], and regenerative medicine[94].
Light-responsive biomaterials not only enable spatiotemporal tunability of biomaterial ink but also provide biological cues that significantly affect cellular function and behavior. For instance, the mechanical property of the biomaterial ink has a part in tumor cell migration[95] since ECM stiffness is related with cellular invasion and cell phenotype. Since these processes take a long time to complete, a dynamic change of the stiffness by an external source is required. Lee et al.[96] presented a novel method to manipulate cell adhesion through light-triggered activation of RGD peptide. Growth factors that are delivered by light-responsive nanoparticles through a biomaterial ink could also promote cell function and activity. Therefore, recent achievements in the study of novel applications of light-responsive biomaterial inks in 3D cell culture hold great promise for future clinical applications. Carbon nanotube (CNT), gold nanoparticles, graphene oxide (GO), and graphite carbon nitride are widely used for designing light-responsive structures that regulate the extracellular environment[97]. The use of light-responsive graphene-based materials has also been reported[98]. A polydopamine-functionalized GO nanosheet (GO-PDA) was used to fabricate the micro pattern. The GO-PDA structure transformed to form a box after 2.6 seconds of near-infrared (NIR) light exposure. A microbot and an artificial hand were also assembled. The artificial hand could fold and grasp an object when stimulated by NIR light, whereas the microbot moved forward upon the NIR stimulation and showed precise remote control.
In addition to mechanical changes, light-responsive biomaterial inks can control the patterning of biochemical signals and define signaling areas that affect cell behavior or cell fate[99]. Biomaterial inks can be considered to respond to two-photon excitation by employing NIR light[100]. Using a PEG hydrogel cross-linked with allyl sulfide groups, Gandavarapuet et al.[101] reported a ligand attachment and subsequent patterning within a 3D structure through two photons. This technique formed an RGD peptide pattern, and the results demonstrated the selective attachment of human mesenchymal stem cells (hMSCs) to the patterned region. Similarly, Wylie et al.[102] showed the patterning of multiple growth factors within 3D biomaterial inks through the two photons. A coumarin-photocaged thiols-included agarose was activated through the reaction of photon with the two distinct maleimide-conjugated linkers. It eventually allowed the independently controlled immobilization of two stem cell differentiation factors. Light-responsive biomaterials can also be used to time the presentation of bioactive cues[103]. Kloxin et al. designed a platform with a time-controlled presentation of adhesive peptides and photo-controlled degradation. An RGD adhesive peptide linked to a PEG-based structure was incorporated using a nitrobenzyl ether-derived photolabile tether. Photocleavage of RGDs from the structure on day 10 after the culture increased the production of glycosaminoglycan and induced the differentiation of hMSCs through a chondrogenic pathway with their viability retained during the culture.
3.5. Ultrasound stimulation
Ultrasound pressure waves at frequencies above 20 kHz are used as controllable remote stimuli for 3D structures[82]. The pressure caused by oscillation and the resultant mechanical effects have been widely used in various tissue engineering applications, including controlling the release from acoustically responsive carriers, enhancing the transdermal permeability of agents through sonophoresis, and manipulating cells in vitro into defined geometric assemblies. Ultrasound waves are readily penetrated through engineered tissues and tissue-like constructs[82], allowing for the in situ manipulation of materials and cells. Ultrasound can be employed with high biocompatibility by managing the duration and intensity of the stimulating ultrasound[104]. Therefore, the design of tissue structures capable of cellular control using ultrasound stimuli is a promising area in the field of tissue engineering approaches.
One strategy to create ultrasound-responsive biomaterial inks involves incorporating acoustic-responsive delivery particles into the biomaterial ink. The sustained delivery of certain growth factors might benefit effective tissue regeneration[105]. Kennedy et al.[82] demonstrated that ultrasound-burstable capsules with high retention could be integrated into bulk biomaterial inks for controlled release. Alginate-based capsules with a 4 mm diameter were formulated for loading the nanoparticle-based payload solutions. The formulated alginate capsules showed near-complete retention of the gold nanoparticle (AuNP) payload for 7 days. The ability to rupture weak capsules with lower-intensity ultrasound was evaluated while ensuring sustained release from stronger walled capsules. AuNPs were further modified with BMP2 to better induce osteogenic differentiation.
Ultrasound stimulation can be employed to control crosslinks within a biomaterial ink, which can influence the release profile[106,107]. As an example, Huebsch et al.[106] addressed this issue by formulating an ionically cross-linked alginate biomaterial ink to which ultrasound exposure could increase the drug release rate, facilitating repeated release. Ultrasound stimulation enables the degradation of the guluronic acid chains of alginate polymers, increasing the payload release. When the ultrasound was completed, the crosslinks were reformed through the calcium binding. It eventually reduces the release rate. Furthermore, they exhibited the pulse release of ECM-binding cytokine and stromal cell-derived factor-1α. Biomaterial inks that allow ultrasound-induced growth factor release provide a method for personalized remote control of bioactive cues from engineered constructs, which can lead to optimal tissue regeneration depending on the bodily conditions of patients.
In addition to photothermal-activated biomaterial inks, ultrasound-activated reactions are unique candidates of biomaterial ink sources for precision medicine. Specifically, ultrasound can be used as an energy source to control a fabricated construct remotely. Habibi et al.[108] demonstrated the 3D printing of structures using acoustic cavitation through the concentrated ultrasound and showed its potential by printing ear and nose constructs. This finding based on ultrasound-activated reactions highlights the variability in the selection of biomaterial inks for precision medicine.
3.6. Magnetic stimulation
Magnetically responsive biomaterials have been introduced into biomedicine to improve the biological activity of cells, tissues, and organs[109]. This is mainly due to their responsiveness to external magnetic fields. This stimulation remotely regulates the biochemical and physical-mechanical properties toward native tissues and organs[109]. Several outcomes have demonstrated that magnetic biomaterial inks can function as superior drug release and targeted systems. Gao et al.[11] developed a magnetic biomaterial ink based on ferromagnetic vertex domain iron oxide. The result demonstrated that the developed magnetic ink could remarkably suppress the local recurrence of breast tumors. In addition, Manjua et al.[110] developed a magnetically responsive PVA hydrogel that could be activated by an on/off magnetic field to regulate motility and sorption non-invasively. This magnetism-based biomaterial can be used as a promising drug delivery system or biosensor. Chen et al.[111] prepared a novel magnetic biomaterial ink by combining self-healing chitosan/alginate biomaterial inks with magnetic gelatin microspheres.
Most studies on this topic have evaluated magnetic nanoparticle (MNP) incorporated hydrogels for bone regeneration[112]. Since hydroxyapatite (HAP) is the well-qualified inorganic component of native bone tissue, a magnetic HAP composite biomaterial ink was fabricated for enhanced bone tissue regeneration. Specifically, nano-HAP-coated γ-Fe2O3 nanoparticles (m-nHAPs) were formulated and then added into a PVA solution. As a result, PVA exhibits excellent biocompatibility, slow biodegradation, and excellent mechanical properties, which are essential for personalized application. The pore sizes of the hydrogels gradually increased, followed by an increase in m-nHAP content. The proliferation and function of human osteoblasts were significantly enhanced when the concentration of m-nHAP increased. Zhang et al.[16] also studied magnetic biomaterial ink for enhanced cartilage tissue engineering. In this study, PVA-conjugated Fe3O4 MNPs were prepared using the grafting-on method. Subsequently, it was mixed with a hybrid biomaterial ink (MagGel) composed of PEG, HA, and type II collagen using a mechanical method. The in vitro results showed that the MagGel lost its structural integrity after incubation at 37°C for 21 days. The findings demonstrated that the magnetic nanocomposite biomaterial ink had a microstructure and chemical components similar to those of natural hyaline cartilage and supported bone mesenchymal stem cell behavior in vitro.
4. 3D-bioprinted smart constructs using stimuli-responsive biomaterials for precision medicine
Studies have attempted to develop personalized treatments by controlling the regenerative capabilities of implants in vivo. Recently, 3D bioprinting-based biofabrication and stimuli-responsive biomaterials have been used to engineer 3D smart constructs that can be controlled after the fabrication process. This section introduces several advancements in 3D bioprinting-based approaches that use functionalized and smart bioinks.
4.1. Tissue regeneration and repair
3D bioprinting is a promising biofabrication tool for generating a 3D engineered tissue structure for use in biomedical tissues and organs. Biomaterial inks are regarded as excellent materials for tissue engineering because of their soft, porous, and water-resistant extracellular matrices. Biomaterial inks employed in tissues are composed of collagen, alginate, gelatin, chitosan, PEG, and polyethylene glycol diacrylate. Due to their ability to support complex microenvironments for better functionality, these inks require dynamic and time following performances in vivo as observed in the original tissues.
In combination with 3D bioprinting, stimuli-responsive biomaterials inks, termed bioinks, are developed to emulate the structural shape and dynamic behavior of native tissues and organs. For example, Kirillova et al.[94] suggested a 3D-bioprinted form changing bioink by mixing methacrylated alginate (AA-HA) and bone marrow stromal cells to open new pathways for developing personalized cell-encapsulated form changing structure and tailoring targeted tissues/organs. Specifically, harnessing the printing and post-printing parameters through water, calcium chloride, and EDTA solutions allows the attainment of internal tubes with average diameters as low as 20 μm, which is appropriate for capillaries (Figure 6A). This process did not affect cell viability and supported cell survival for 7 days (Figure 6B). Ko et al.[113] revealed that oxidized hyaluronate (OHA) and glycol chitosan (GC) could be used to create a self-curing ferrogel without the use of further chemical cross-linkers. The GC/OHA ferrogel bioink was magnetic field responsive (Figure 6C). This ferrogel may benefit the design and fabrication of controllable tissue-engineered constructs. Gua et al.[114] created a nanoclay-incorporated double-network (NIDN) bioink using 3D bioprinting. Nanoclays interact with methacrylated hyaluronic acid (HAMA) and alginate as physical cross-linkers (Alg). The nanoclay played a key role as a physical cross-linker between HAMA and Alg. This endows the bioink with the ability to form complex structures (Figure 6D). Their findings also showed that the NIDN bioink could be easily transformed into a new type of magnetic-reactive biomaterial ink supporting the growth of bone-derived stem cells (Figure 6E). The ability to repair calvarial defects was also observed (Figure 6F).
Figure 6.
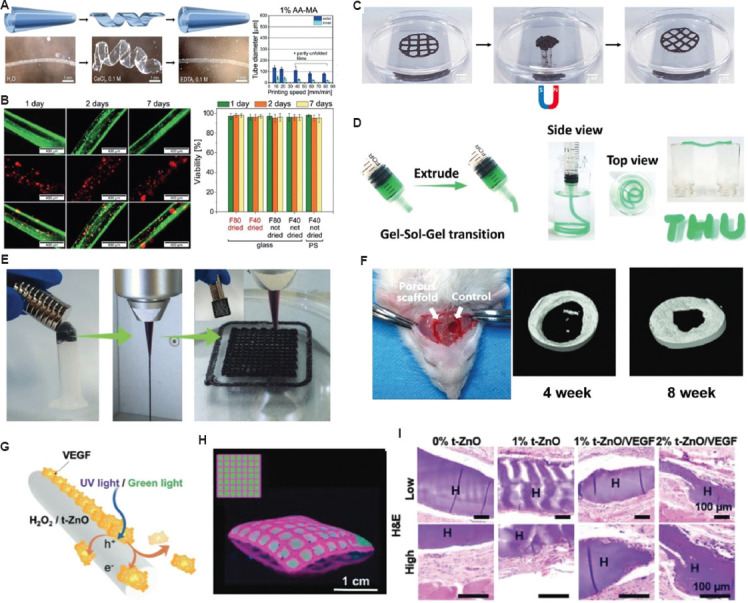
Potential applications of stimuli-responsive bioinks for tissue regeneration and repair. (A) AA-MA tube responsiveness in immersed solutions and tube diameters according to printing speed. (B) Representative fluorescent images of cell-laden AA-MA tubes for 7 days: fluorescence images in green (upper row) representing live cells, fluorescence images in red (middle row) showing dead cells within self-folded tubes, and overlays of green and red fluorescence images (lower row) with live cells (orange) and dead cells (red)[152]. (Figure A and B reproduced with permission from Kirillova et al.[152]; copyright 2017, John Wiley and Sons). (C) Alteration in the fabricated 3D structure by applying a magnetic field; the originally printed shape was returned when the magnetic field was removed (reproduced with permission from Ko et al.[113]; copyright 2020, Elsevier). (D) Images showing the injected bioink through a syringe to create self-supporting structure. (E) The images of magnetic ferrofluids, extrusion filament, and 3D-fabricated structure with magnetic-responsive behavior. (F) Micro-CT images after implantation on 4 weeks and 8 weeks. (Figure D, E, and F reproduced with permission from Guo et al.[114]; copyright 2021, John Wiley and Sons). (G) Working principle showing drug-release through UV/green light stimulations. (H) Micropatterned patch by 3D bioprinting process. (I) Hematoxylin and eosin staining of skin tissue collected after 28 days to show the wound healing process. (Figures G, H, and I reproduced with permission from Siebert et al.[116]; copyright 2021, John Wiley and Sons).
Electrical stimulation can manipulate cell maturation and responses through electroactive bioinks. Dister et al.[115] studied the 3D printability of bioinks in building cytocompatible and conductive hydrogels by the formulation of pyrrole and oxidized alginate-gelatin (ADA-GEL) bioink. The mechanical and electrical features, 3D bioprintability, and biocompatibility of the developed bioink were evaluated. In contrast to a 2D structure, 3D bioprinting allows for the creation of open porous structures with electrically conductive properties and provides higher cell proliferation efficacy. More recently, Siebert et al.[116] suggested a GelMA-based light controllable bioink for wirelessly triggering the release of vascular endothelial growth factor (VEGF). To induce light-triggered activation, a 3D-bioprinted patch was fabricated. In the patch, VEGF was coated with photoactive tetrapodal zinc oxide (t-ZnO) microparticles (Figure 6G). This light-controlled wound patch was activated at different concentrations by exposure to UV or visible light. The elastic modulus and degradation of the patch can be adjusted by changing the t-ZnO concentration. Its potential as a bioink source was demonstrated by printing the desired micropattern (Figure 6H). In vivo tests showed that the printed wound patch is a promising tool for enhancing wound healing (Figure 6I). This approach demonstrates a smart wound dressing platform that can be controlled after application. Banche-Niclot et al.[117] proposed large-pore mesoporous silicas (LPMSs) that deliver large biomolecules, which are released on pH stimulation, for bone regeneration. The presented pH-triggered bioink was intended to imitate the release of growth factors, along with a decrease in pH during bone remodeling. To achieve this, LPMSs were formulated using 1,3,5-trimethyl benzene as the swelling agent. The synthesis solution was hydrothermally treated to determine how the process temperature and duration affected the resultant meso-structure. Subsequently, the LPMSs were coated with pH-responsive PEG to enable the transfer of the incorporated biomolecules in response to pH reduction. These findings indicate that in an acidic environment, PEG-coated carriers could rapidly release horseradish peroxidase because of the protonation of PEG at low pH, suggesting that LPMSs could be used as functional carriers. Although this bioink has not been used in 3D printing, this delivery method can be adapted for 3D bioprinting of pH-responsive tissue constructs such as skin and bone.
4.2. Drug delivery for therapy of disease
Conventional delivery systems require frequent and high-dose administration for success[118]. However, the adverse effects could badly influence the body and decrease the drug efficacy. In line with precision medicine, an optimal drug delivery system should release pre-planned drugs under harsh in vivo environmental conditions. Stimuli-responsive biomaterials have the potential to change their form in response to external stimuli and changes in variables, such as pH and biomolecule concentration. Therefore, 3D-bioprinted constructs with stimuli-responsive biomaterials are under development as smart drug delivery systems. Wang et al.[119] proposed a hybrid bioink with shape memory for drug delivery. Sodium alginate and Pluronic F127 diacrylate macromers (F127DA) were mixed to formulate shape-memory hydrogels (SMHs). 3D printing was utilized as an effective tool to control the internal structure of the shape memory hydrogels. This bioink included two network structures: A permanent network created by F127DA photo-crosslinking and a reversible lattice shape created by alginate (Figure 7A). The printed construct with the temporal state had the higher release profile of drug when compared with the one with original state, indicating the curved shape could increase the drug release. Shape-memory hydrogels (SMHs) can be loaded with methotrexate (MTX), which is a representative anticancer agent, indicating that SMHs can be used for drug delivery. They evaluated the release profile under in vitro conditions and found that the printed SMHs can release more drugs (Figure 7B), probably because of the 3D-bioprinted interior patterns, which increase the surface area of the drug. Larush et al.[120] utilized the DLP bioprinting method to develop drug delivery systems with shape-dependent swelling- and pH-triggered drug release. This study revealed that 3D printing was utilized to ameliorate the efficacy of conventional shapes, which were used in drug administration by controlling the geometric criteria (Figure 7C). Due to the responsiveness of the printed bioink, drug release in oral delivery systems can be manipulated according to variations in the pH of the system. Melocchi et al.[121] fabricated a device for intravenous drug delivery. Engineered SMHs were used to maintain a temporal form for bladder administration. As the device contacted water in the bladder, it reverted to its original shape (Figure 7D). Increased treatment time was able to improve the efficiency of treatment by facilitating localized and extended drug delivery. Next, Ceylan et al.[122] investigated the degradation properties of gelatin methacryloyl bioink to manufacture a biodegradable microrobot for the detection of the matrix metalloproteinase 2 (MMP-2) enzyme. Their findings showed that MMP-2 could entirely degrade the microswimmer after 118 h to solubilize non-toxic materials (Figure 7E). After the injection of microswimmers into the tumor, an external magnetic field allowed the remote control to reach a targeted location (Figure 7F). During the process of tissue remodeling, MMP-2 destroyed collagen matrix and formed the structure. Taken together, these findings show that stimuli-responsive bioinks can be utilized to create 3D engineered structures with localization and control over drug release.
Figure 7.
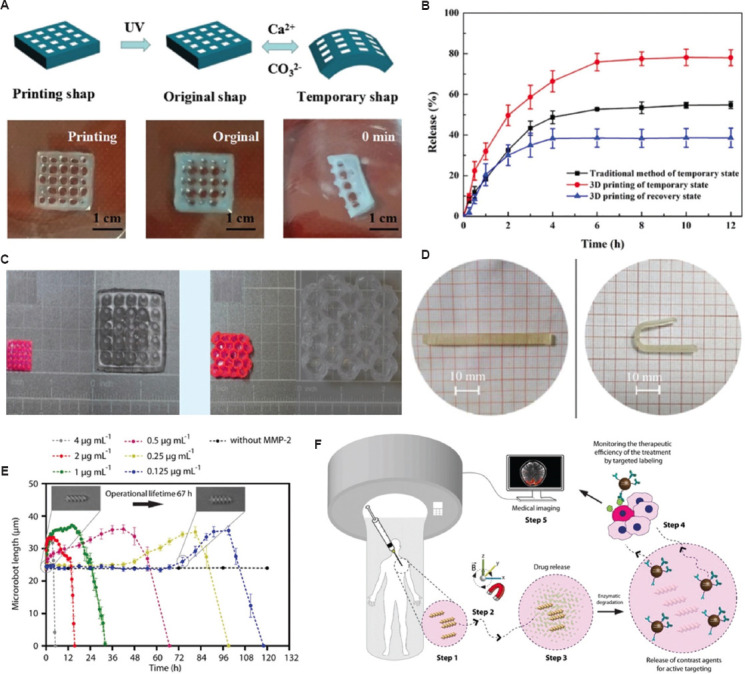
Potential applications for drug delivery. (A) The images displaying the shape-memory behavior of F127DA-alginate hydrogels: (left) Printing shape, (middle) UV 365 nm fixed the original shape of bioink, (right) the temporary shape of the biomaterial ink. (B) In vitro drug release profiles from 3D-printed structure. (C) Images of drug-loaded 3D-printed tablets with different shapes before and after 24 h swelling in phosphate buffer (pH 7.4) (reproduced with permission from Wang et al.[119]; copyright 2021, John Wiley and Sons). (D) Photographs obtained during shape recovery (room temperature) on PVA05GLY samples with their original I-shape[153]. (Figures A, B, and D reproduced with permission from Wang et al.[153], copyright 2021, John Wiley and Sons). (E) Enzymatic degradation of fabricated microswimmers; MMP-2 is degraded from microswimmers within 118 h at the physiological level. (F) Application scenario of the 3D-printed and biodegradable microrobotic swimmers.[122]. (Figures E and F reproduced with permission from Ceylan et al.[122], copyright 2021, John Wiley and Sons).
4.3. Biosensors
Due to the increasing interest in precision medicine and personalized therapy, studies on 3D bioprinting of living cells have focused on the real-time monitoring of cell growth in bioprinted structures after implantation. Trampe et al.[123] formulated a functionalized smart hydrogel with luminescent optical sensor nanoparticles for use in combination with 3D bioprinting (Figure 8A). They developed a novel functionalized bioink incorporated sensor that enabled spatiotemporal mapping of oxygen dynamics in 3D-bioprinted constructs with living cells. Oxygen is a key element for the survival of encapsulated cells and a crucial indicator to determine metabolic activity, which is the most important for tissue functionalities. After printing was completed, the oxygen level in the printed construct was measured using a ratiometric RGB camera system. Specifically, sensor particles were excited using a 445 nm customized LEP chip equipped with a bandpass filter. The combination of sensing nanoparticles with green microalgae did not impair the viability of stem cells (Figure 8B). Moreover, the rheological properties of the bioink enabled layer-by-layer 3D bioprinting of the developed sensing bioink with a smart response to oxygen concentration. Using the printed bioink with microalgae and oxygen sensor nanoparticles, the ability to use luminescence imaging to map the spatiotemporal chemical heterogeneity was explored (Figure 8C). Oxygen dynamics resulting from respiration and photosynthesis could be captured, and the metabolic activity of different cell types could be distinguished within the engineered 3D structures (Figure 8D). Based on these results, the authors stated that the suggested bioinks with sensor nanoparticles enabled non-invasive monitoring of cell metabolism and spatiotemporal dynamics in 3D-bioprinted structures. This major advantage facilitates swift evaluations of cell activities in bioprinted constructs without post-processing, including a function of structural complexity, metabolic interactions, and response to external incubation conditions. This is the first attempt to combine a 3D bioprinting technique with a luminescent sensor nanoparticle. In another example, Iversen et al.[124] suggested a wearable, flexible smart patch through 3D printing acting as a pH and hydration sensor for wound healing. Wound healing is a complex biological regeneration process with the physical-chemical microenvironments[125]. Therefore, real-time monitoring would offer strong benefits, particularly for bedridden patients. Their study reported low-cost, flexible, and printed sensors that can be attached to the skin for the measurement of pH change and fluid contents in a wound. The components of the printed patch are shown in Figure 8E; specifically, components 1, 2, 3, 4, and 6 were printed, and components 5, 7, and 8 were then used to conduct the measurements without interfering with the printed parts. Eventually, a sensor consisting of different electrodes was printed on a polydimethylsiloxane substrate to sense pH and moisture (Figure 8F). For the pH sensor, patches were submerged in a buffer solution, and changes in resistance were detected with a digital Keithley model 2110. Afterward, the sensor output was recorded using Kickstart (version 2.4). The printed patch was maintained in buffer solution until the detected resistance was in a steady state. In addition, the repeatability of the printed sensor was investigated with time. The maximum difference between the first maximum and minimum resistance values was 0.9. With the fabricated hydration sensors, the hydration (or fluid) level could be measured with respect to the measured resistance of the sensor ranging from 0% to 100%. The developed sensor patch had included a sensitivity in wound pH levels of 7.1 ohm/pH. In addition, the results of the fabricated hydration sensor demonstrated that the moisture levels could be evaluated on a semi-porous surface based on the change in resistance.
Figure 8.
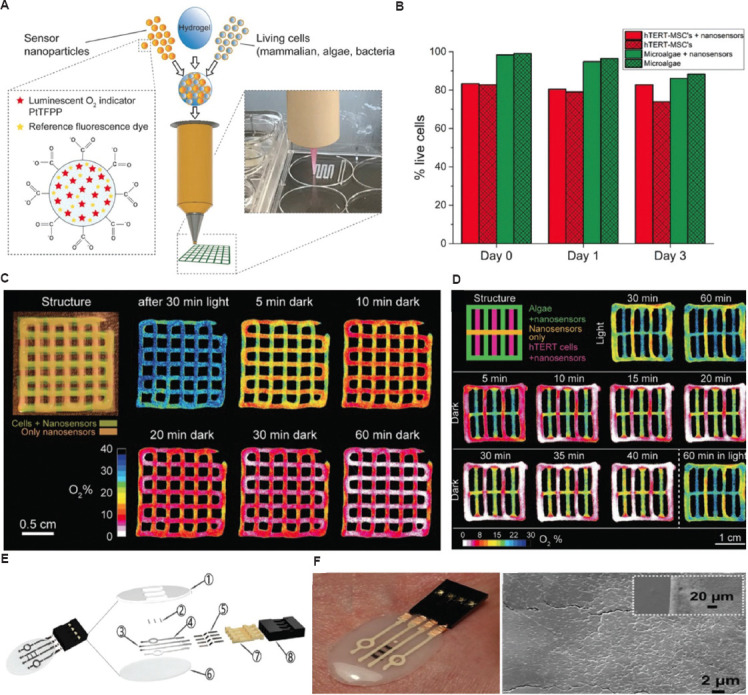
Potential applications for biosensors. (A) A new bioprinting method with functionalized bioink with oxygen-sensing nanoparticles. (B) Viability of the microalga Chlorella sorokiniana and cell line during incubation in 3D-bioprinted structure containing oxygen sensor nanoparticles. (C) Structural image of 3D-bioprinted constructs with the two different layers and images of oxygen concentrations according to time-dependent illumination. (D) Spatiotemporal dynamics of oxygen concentrations in a multilayered 3D construct with sensor nanoparticles only, nanoparticles + microalga, as well as cell line. (Figures A, B, C, and D reproduced with permission from Trampe et al.[123]; copyright 2018, John Wiley and Sons). (E) Exploded view of the patch showing all the components necessary for fabrication processes. (F) The gross images and scanning electron microscope image of fabricated patch with polydimethylsiloxane (PDMS) containing 20 wt% of SiO2 NPs and Ag and single-walled carbon nanotubes electrodes.
5. Advantages and considerations of 3D-printed and 3D-bioprinted smart constructs
5.1. Advantages over other manufacturing techniques
Smart constructs are considered next-generation tools in precision medicine because they can responsively perform predesigned functions (e.g., shape-morphing, navigating, sensing, and providing feedback). Although various manufacturing techniques, such as molding, stamping, etching, filament winding, and cutting, have been used, processing responsive biomaterials and living units to obtain smart constructs with acceptable quality (e.g., high resolution, heterogeneous composition, complex architecture, and good viability) remain challenging. Therefore, the unique advantages of 3D printing and bioprinting techniques, such as the high compatibility of materials, the flexibility of building complex constructs, and the capacity for engineering living systems, turn out to be compelling.
Numerous intelligent materials, including shape memory, photovoltaic, photochemical, electroactive, magnetostrictive, thermo-electric, dielectric, halochromic, and chromogenic materials, have been intensively explored for engineering smart constructs[126]. Several categories of 3D printing and bioprinting techniques are available for full exploitation of their functions (section 2). Each of these methods shows a unique ability to process different types and forms of biomaterials. For instance, photocurable polymers, shape-memory alloys, low viscosity liquids, and thermoplastic polymers are specifically adaptable to SLA, SLS, 3D inkjet, and FDM techniques, respectively. Furthermore, commonly used 3D bioprinting techniques can embed living cells within the printed structures. Therefore, 3D printing and bioprinting technique cover a wide range of smart biomaterials. Liquid metals[127], liquid crystal polymers[128], hydrogels[129], shape-memory polymers[130], and smart composites[131] have been used as printable materials in pioneering studies, demonstrating the unparalleled material compatibility of 3D printing and bioprinting techniques.
Complex structural and compositional designs can further enhance the intelligence of some stimuli-responsive materials; consequently, advanced smart structures can be developed. For example, smart construct structures can be designed in bilayers or multiple layers, anisotropic alignments, and programmed patterns to achieve the desired shape transformation responsive morphing behaviors, such as rolling[132], compression[133], torsion[134], stretching[135], folding[136], and complex actions (e.g., swimming, walking, and crawling[137]). In addition, the distribution or gradient of multiple components has been applied to smart composites to establish stimuli-triggered transformation[138], degradation[139], and payload release[140]. Despite these advanced functions, developing sophisticated designs using other manufacturing approaches are difficult. 3D printing and bioprinting have become pivotal techniques for producing smart constructs because they provide the flexibility to precisely position multiple biomaterials and bioinks to construct a 3D assembly with the desired structures and compositions.
Cell therapy, cell-based drug delivery system, and functional in vitro tissue models, organ-on-a-chip, and organoids have fully demonstrated that living systems are desirable for biomedical and clinical applications because they can comply with physiological processes and respond to natural signals from the human body (e.g., pH, ion, bioelectricity, body motion strain, and infectious signals). Equipped with smart properties, these living constructs may achieve unique performances. 3D bioprinting technique is a tailor-made technology that can combine smart biomaterials, cells, and biomolecules to achieve this goal. For example, Schmidt et al. developed a biohybrid sperm micromotor (Spermbot) as a novel targeted drug delivery system for the treatment of diseases within the female reproductive tract. A sperm can be unleashed to deliver drugs to cancer cells by magnetically navigating sperm-embedded microrobots fabricated using a TPP 3D printing technique toward a HeLa tumor spheroid. Other studies have also innovatively investigated the shape-morphing of cell-laden 3D constructs bioprinted using stimuli-responsive bioinks[141]. For instance, Luo et al.[13] 3D bioprinted a bilayered scaffold with an orthogonal structure using alginate/polydopamine and alginate/GelMA-containing human embryonic kidney cells (HEK 293T). On near-infrared irradiation-induced dehydration, the biphasic scaffold can undergo tailored structural transformation within several minutes while retaining high cell viability after 14 days of culturing. With the application of 3D bioprinting in engineering live smart constructs, 4D bioprinting and biofabrication of advanced artificial tissues may be achieved.
5.2. Considerations of 3D-printed smart constructs in precision medicine applications
Despite the great potential for precision medicine application, 3D-printed smart constructs should be further developed and enhanced, awaiting future efforts for continuous innovations. Fundamental material formulation and desired smart functions should be matched to develop advanced smart constructs for tissue regeneration, drug delivery, and health monitoring.
In general, artificial tissue, drug carrier, and biosensors are usually implanted into the patients’ body or placed in close contact with human tissues. Therefore, biosafety becomes the principal requirement for smart constructs. Regardless of smart properties, the applied biomaterials must possess several critical performances, including non-immunogenicity, non-toxicity, and biocompatibility. Conversely, good printability is an indispensable performance of smart biomaterials in 3D printing techniques. In addition, strong magnetoelectric signal, extreme pH, intense irradiation, and high ion concentration may trigger cell death and tissue damage[28]. Considering the patients’ health, the stimuli applied to activate smart constructs must be mild.
There are also additional specific requirements for the design of materials in each type of application. For tissue repair and regeneration, biomaterials and bioinks must be cell-friendly to promote cell activities and generate functional tissues. Accordingly, mechanical, compositional, and structural features of materials matrices should mimic those of the tissue ECM so that a cell-favorable microenvironment can be established[10]. Moreover, hyperplasia and mechanical-failure can be initiated by the unmatched mechanical properties between implants and soft or hard tissues[142]. Hence, key material properties (e.g., strength, elasticity, hardness, and fatigue) should be carefully modulated.
One of the most important considerations for applying 3D-printed smart construct as a carrier for targeted drug delivery is the maintenance of drug nature and stability. Although various responsive biomaterials show intriguing potential for engineering smart drug delivery systems, the type of stimuli should not compromise the therapeutic efficacy of loaded drugs. Numerous biomolecule-based drugs (e.g., growth factors, enzymes, hormones, antibodies) have been used for disease treatment. However, the structure and function of these proteins are susceptible to environmental factors, such as temperature, light, and pH[143]. Therefore, appropriate stimuli-responsive biomaterials for carrying these molecules must be selected. Constituent biomaterials should be completely excluded from the patients’ body after the necessary tasks are completed. Therefore, controllable biodegradation and safe metabolism of byproduct or at least retrievable materials from the human body are important requirements for such applications.
Implantable or wearable biosensors have stringent requirement in terms of sensitivity and stability of applied smart biomaterials. In many cases, biosensors collect a small volume (e.g., microliters by microneedles) of biological samples from body fluids (sweater and blood) or tissue (cells) and acquire imperceptible physical or chemical signals (motion, heat, electricity, ion, and pH) to monitor and analyze the health of patients[144]. To achieve real-time and precise diagnosis, smart materials must show high sensitivity and rapid response toward the targeted stimuli. In addition, biomaterials or bioinks used for fabricating biosensors should possess acceptable stability to avoid the interference of signal acquisition and analysis. For instance, stimuli-responsive hydrogels are widely applied in biosensing fields. However, hydrogel-based biosensors are mainly applied in aqueous environments to avoid dehydration. Solution-induced hydrogel swelling may drastically affect the evaluation of analyte level and the precision of detection.
5.3. Design of smart functions
At present, most of the achievements that apply 3D-printed smart constructs for tissue generation rely on the shape-morphing ability of stimuli-responsive materials to generate complex structures or adapt the architecture of native tissues. However, with the continuous innovations of fabrication strategies, 3D printing technique alone may be applied to reproduce the structural features of tissues and organs. Using FRESH 3D printing strategies, researchers have engineered an artificial heart[145]. Therefore, further efforts should be devoted to exploring the advantages of smart constructs to support organ functions and improve tissue repair. For example, the shape-morphing property can be applied to assist the function of motile organs, such as the heart, stomach, and bladder. In a previous study, a 3D-printed implantable device has been developed using a shape-memory alloy actuator to void an underactive bladder[146]. With this achievement, various implantable constructs, such as heart and stomach patches as pacemakers or support for gastrointestinal peristalsis, can be developed. Furthermore, the deformation-electricity transition function of piezoelectric and triboelectric materials can involve electromechanical stimuli that trigger cell and tissue activities[147]; consequently, neural and muscular tissues may regenerate. These applications, combined with 3D-bioprinted cell-laden constructs, are promising therapeutic tools for tissue regeneration.
In comparison with conventional drug administration, targeted drug delivery is advantageous because of the precise navigation of drugs to disordered sites and the controlled dosage release. An elaborate design of smart constructs potentially helps improve the performances of drug delivery systems. Thus far, various smart drug carriers responsive to pH, redox reaction, heat, magnetism, ultrasound, hypoxia, and infection have been developed[148]. For example, thermoresponsive liposomes and magnetic-responsive iron oxide nanoparticles[149,150] have been approved for clinical applications. A remarkable advantage of these products, based on the previous U.S. Food and Drug Administration (FDA) evaluations, is their simple designs and formulations. Conversely, many developed drug delivery systems are equipped with complex structures and compositions to gain smart functions. However, such sophisticated designs inevitably limit scale-up manufacturing in the pharmaceutical industry. Therefore, design simplicity is a critical consideration for translating smart drug carriers to clinical applications.
With the assistance of 3D printing technique, a wide range of wearable and implantable smart biosensors, including glucose, neurotransmitter, sweat, strain, lactate, and oxygen sensors, have been successfully engineered[151]. Considering the continuous emergence of printable smart materials with excellent properties, such as superior elasticity, flexibility, recyclability, self-healing, self-fueling, and conductivity, more advanced 3D printing technologies may be explored to integrate these properties so as to improve the performances of existing biosensors.
6. Conclusion and future perspectives
The convergence of 3D printing techniques and intelligent biomaterials has enabled the creation of various smart constructs that exhibit tunable alterations in their properties, such as shape, architecture, position, degradation, and color, in response to the given signals. In this review, we provide an overview of the classifications and divisions of 3D printing or bioprinting techniques, biomaterials or bioinks, and commonly used stimuli-responsive biomaterials, followed by a summary of recent biomedical applications of 3D-printed or -bioprinted smart constructs in regenerative medicine, drug delivery, and pathophysiology monitoring. Although these achievements have resulted in the dawn of smart constructs, several critical challenges in using this innovative technology remain.
First, the limited number of available intelligent biomaterials is a major challenge to produce smart constructs. Despite the abundant emergence of stimuli-responsive materials, most of them are not eligible candidates for biomedical applications because they do not fulfill two important criteria, biocompatibility and 3D printability. A few pioneering studies have demonstrated that properties such as adhesion, migration, and contraction can be utilized to actuate 3D-bioprinted structures, showing that it is possible to create living smart constructs. When producing constructs using cell-laden bioinks, 3D bioprinting techniques have additional requirements for the properties of biomaterials, including cell-friendliness, rheology, and crosslinking performance. In addition, when executing specific functions, such as shape transformation, biomaterials should exhibit reasonable mechanical strength. Therefore, an increased focus should be placed on developing novel, applicable intelligent biomaterials to advance smart constructs.
Second, the smart constructs summarized in this review are categorized into three groups: Tissue regeneration, drug delivery, and monitoring. However, to alleviate the suffering of patients and clinical burdens, the constructs are expected to be multifunctional and should be able to serve both therapeutic and diagnostic purposes when stimuli are supplied on demand. For instance, conventional vascular stents are usually designed to be delivered using a catheter to mechanically expand the stenotic blood vessels. Several studies have developed 3D-printed vascular stents using heat- and magnetically-sensitive biopolymers. Designs utilizing shape-memory transformations or magnetic field-triggered navigation have significantly eased and improved the precision of the deployment process. However, the ability to provide radial force to support and regain the diameter of the narrowed vessels is just one of the many properties required for vascular stents. An ideal smart stent should encompass other critical features, such as the programmable release of drugs for recovering atherosclerotic plaques, controllable degradation to prevent the generation of hyperplastic tissues, and trackable signals to reflect the condition of the lesions. Despite the absence of multifunctional stimuli-responsive biomaterials, the integration of available candidates and corresponding types of stimuli may be an optional approach to engineering versatile smart constructs.
Third, numerous problems await when translating 3D printing and bioprinting from laboratory to industrial settings. Although the automated fabrication method is highly reproducible, large-scale production remains challenging. Considering medical and clinical applications, a standardized production process, including material preparation, fabrication, and packaging, should be established in accordance with good manufacturing practices to avoid unexpected contaminations. Moreover, for products containing cells and fragile biological or chemical components, their viability and stability during storage and transportation should be considered.
Finally, although the existing literature has demonstrated the potential of smart bioconstructs and biomedical devices, most of them are proof-of-concept studies that are still in their infancy. The most of the reviewed reports describe works that are still in the fundamental research phase, and no prototypes have been developed for clinical trials. Hence, FDA-approved smart constructs are currently not available. Advancing the smart constructs into clinical scenarios requires in vitro and in vivo experiments to validate their safety and efficacy.
In conclusion, 3D printing of smart constructs is an emerging research subject that has proven its extraordinary potential for the next generation of bioproducts and biomedical devices and as a future direction of tissue engineering. Overcoming the current challenges requires multidisciplinary collaborations to enrich the pool of smart biomaterials, clarify clinical demands, and complete the pre-clinical evaluations.
Acknowledgments
None.
Funding
This study was funded by the Beijing Institute of Technology Research Fund Program for Young Scholars (XSQD-202123003) and the Fundamental Research Funds for the Central Universities (No. LY2022-22). This research was also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2022R1C1C1004803 and 2022R1A5A2027161).
Conflict of interest
The authors declare they have no competing interests.
Author contributions
Writing – original draft: Qiqi Gao and Jae-Seong Lee
Writing – review & editing: Byoung Soo Kim and Ge Gao
All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Somma M, Schaafsma W, Grillo E, et al. Natural histogel-based bio-scaffolds for sustaining angiogenesis in beige adipose tissue. Cells. 2019;8:1457. doi: 10.3390/cells8111457. https://doi.org/10.3390/cells8111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han T, Kundu S, Nag A, et al. 3D printed sensors for biomedical applications:A review. Sensors. 2019;19:1706. doi: 10.3390/s19071706. https://doi.org/10.3390/s19071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sood N, Bhardwaj A, Mehta S, et al. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23:748–770. doi: 10.3109/10717544.2014.940091. https://doi.org/10.3109/10717544.2014.940091. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, He Y, Zhang F, et al. A review of shape memory polymers and composites:Mechanisms, materials, and applications. Adv Mater. 2021;33:e2000713. doi: 10.1002/adma.202000713. https://doi.org/10.1002/adma.202000713. [DOI] [PubMed] [Google Scholar]
- 6.Pang X, Lv JA, Zhu C, et al. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv Mater. 2019;31:e1904224. doi: 10.1002/adma.201904224. https://doi.org/10.1002/adma.201904224. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Cheng H, Zhang M, et al. Graphene-based smart materials. Nat Rev Mater. 2017;2:17046. https://doi.org/10.1038/natrevmats.2017.46. [Google Scholar]
- 8.Liu Z, Liu J, Cui X, et al. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front Chem. 2020;8:124. doi: 10.3389/fchem.2020.00124. https://doi.org/10.3389/fchem.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghovvati M, Kharaziha M, Ardehali R, et al. Recent advances in designing electroconductive biomaterials for cardiac tissue engineering. Adv Healthc Mater. 2022;11:2200055. doi: 10.1002/adhm.202200055. https://doi.org/10.1002/adhm.202200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D bioprinting:An overview. Biomater Sci. 2018;6:915–946. doi: 10.1039/c7bm00765e. https://doi.org/10.1039/C7BM00765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao B, Yang Q, Zhao X, et al. 4D Bioprinting for biomedical applications. Trends Biotechnol. 2016;34:746–756. doi: 10.1016/j.tibtech.2016.03.004. https://doi.org/10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Aimetti AA, Langer R, et al. Bioresponsive materials. Nat Rev Mater. 2016;2:16075. https://doi.org/10.1038/natrevmats.2016.75. [Google Scholar]
- 13.Luo Y, Lin X, Chen B, et al. Cell-laden four-dimensional bioprinting using near-infrared-triggered shape-morphing alginate/polydopamine bioinks. Biofabrication. 2019;11:045019. doi: 10.1088/1758-5090/ab39c5. https://doi.org/10.1088/1758-5090/ab39c5. [DOI] [PubMed] [Google Scholar]
- 14.Raza A, Hayat U, Rasheed T, et al. “Smart”materials-based near-infrared light-responsive drug delivery systems for cancer treatment:A review. J Mater Res Technol. 2019;8:1497–1509. https://doi.org/10.1016/j.jmrt.2018.03.007. [Google Scholar]
- 15.Izadifar M, Haddadi A, Chen X, et al. Rate-programming of nano-particulate delivery systems for smart bioactive scaffolds in tissue engineering. Nanotechnology. 2015;26:012001. doi: 10.1088/0957-4484/26/1/012001. https://doi.org/10.1088/0957-4484/26/1/012001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Wang S, Zhou C, et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9. https://doi.org/.10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. https://doi.org/10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Groll J, Burdick JA, Cho DW, et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication. 2018;11:013001. doi: 10.1088/1758-5090/aaec52. https://doi.org/10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 19.Khoeini R, Nosrati H, Akbarzadeh A, et al. Natural and synthetic bioinks for 3D bioprinting. Adv NanoBiomed Res. 2021;1(8):2000097. https://doi.org/10.1002/anbr.202000097. [Google Scholar]
- 20.Hull CW. Apparatus for Production of Three-dimensional Objects by Stereolithography. California: Uvp I; 1986. [Google Scholar]
- 21.ASTM International. Standard Terminology for Additive Manufacturing Technologies:Designation F2792-12a. West Conshohocken PA: ASTM International; 2012. [Google Scholar]
- 22.Cho W, Job AV, Chen J, et al. A review of current clinical applications of three-dimensional printing in spine surgery. Asian Spine J. 2018;12:171–177. doi: 10.4184/asj.2018.12.1.171. https://doi.org/10.4184/asj.2018.12.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. https://doi.org/10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 24.Maruo S, Ikuta I. Submicron stereolithography for the production of freely movable mechanisms by using single-photon polymerization. Sens Actuators A. 2002;100:70–76. [Google Scholar]
- 25.Mu Q, Wang L, Dunn CK, et al. Digital light processing 3D printing of conductive complex structures. Addit Manuf. 2017;18:74–83. https://doi.org/10.1016/j.addma.2017.08.011. [Google Scholar]
- 26.Kelly BE, Bhattacharya I, Heidari H, et al. Volumetric additive manufacturing via tomographic reconstruction. Science. 2019;363:1075–1079. doi: 10.1126/science.aau7114. https://doi.org/10.1126/science.aau7114. [DOI] [PubMed] [Google Scholar]
- 27.Layani M, Wang X, Magdassi S. Novel materials for 3D printing by photopolymerization. Adv Mater. 2018;30:1706344. doi: 10.1002/adma.201706344. https://doi.org/10.1002/adma.201706344. [DOI] [PubMed] [Google Scholar]
- 28.Karimi M, Ghasemi A, Zangabad PS, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–1501. doi: 10.1039/c5cs00798d. https://doi.org/10.1039/C5CS00798D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YW, Ceylan H, Yasa IC, et al. 3D-printed multi-stimuli-responsive mobile micromachines. ACS Appl Mater Interfaces. 2021;13:12759–12766. doi: 10.1021/acsami.0c18221. https://doi.org/10.1021/acsami.0c18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amorim FL, Lohrengel A, Neubert V, et al. Selective laser sintering of Mo-CuNi composite to be used as EDM electrode. Rapid Prototyp J. 2014;20:59–68. https://doi.org/10.1108/rpj-04-2012-0035. [Google Scholar]
- 31.Salmoria GV, Klauss P, Paggi RA, et al. Structure and mechanical properties of cellulose based scaffolds fabricated by selective laser sintering. Polym Test. 2009;28:648–652. https://doi.org/10.1016/j.polymertesting.2009.05.008. [Google Scholar]
- 32.Williams JM, Adewunmi A, Schek RM, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. https://doi.org/10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 33.Wiria FE, Leong KF, Chua CK, et al. Poly-epsilon-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007;3:1–12. doi: 10.1016/j.actbio.2006.07.008. https://doi.org/10.1016/j.actbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoli A. Selective laser sintering in biomedical engineering. Med Biol Eng Comput. 2013;51:245–256. doi: 10.1007/s11517-012-1001-x. https://doi.org/10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 35.Subash A, Kandasubramanian B. 4D printing of shape memory polymers. Euro Polym J. 2020;134:109771. https://doi.org/10.1016/j.eurpolymj.2020.109771. [Google Scholar]
- 36.Calvert P. Inkjet printing for materials and devices. Chem. Mater. 2001;13:3299–3305. [Google Scholar]
- 37.Park JY, Gao G, Jang J, et al. 3D printed structures for delivery of biomolecules and cells:tissue repair and regeneration. J Mater Chem B. 2016;4:7521–7539. doi: 10.1039/c6tb01662f. https://doi.org/10.1039/c6tb01662f. [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Haverinen HM, Dhagat P, et al. Inkjet printing-process and its applications. Adv Mater. 2010;22:673–685. doi: 10.1002/adma.200901141. https://doi.org/10.1002/adma.200901141. [DOI] [PubMed] [Google Scholar]
- 39.Bihar E, Wustoni S, Pappa AM, et al. A fully inkjet-printed disposable glucose sensor on paper. Npj Flex Electron. 2018;2:30. https://doi.org/10.1038/s41528-018-0044-y. [Google Scholar]
- 40.Yang J, Katagiri D, Mao S, et al. Inkjet printing based assembly of thermoresponsive core-shell polymer microcapsules for controlled drug release. J Mater Chem B. 2016;4:4156–4163. doi: 10.1039/c6tb00424e. https://doi.org/10.1039/C6TB00424E. [DOI] [PubMed] [Google Scholar]
- 41.Belaid H, Nagarajan S, Teyssier C, et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater Sci Eng C Mater Biol Appl. 2020;110:110595. doi: 10.1016/j.msec.2019.110595. https://doi.org/10.1016/j.msec.2019.110595. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed OA, Masood SH, Bhowmik JL. Optimization of fused deposition modeling process parameters:A review of current research and future prospects. Adv Manuf. 2015;3:42–53. https://doi.org/10.1007/s40436-014-0097-7. [Google Scholar]
- 43.Zeina I, Hutmacher DW, Tan KC, et al. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23:1169–1185. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 44.Ahn SH, Montero M, Odell D, et al. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp J. 2002;8:248–257. https://doi.org/10.1108/13552540210441166. [Google Scholar]
- 45.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 46.O'Brart DP. Corneal collagen cross-linking:A review. J Optom. 2014;7:113–124. doi: 10.1016/j.optom.2013.12.001. https://doi.org/10.1016/j.optom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar H, Kim K. Stereolithography 3D bioprinting. In: Crook JM, editor. 3D Bioprinting:Principles and Protocols. New York: Springer US; 2020. pp. 93–108. [Google Scholar]
- 48.Feng M, Hu S, Qin W, et al. Bioprinting of a blue light-cross-linked biodegradable hydrogel encapsulating amniotic mesenchymal stem cells for intrauterine adhesion prevention. ACS Omega. 2021;6:23067–23075. doi: 10.1021/acsomega.1c02117. https://doi.org/10.1021/acsomega.1c02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petta D, Armiento AR, Grijpma D, et al. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication. 2018;10:044104. doi: 10.1088/1758-5090/aadf58. https://doi.org/10.1088/1758-5090/aadf58. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Kang B, Cui X, et al. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv Funct Mater. 2021;31:2011252. https://doi.org/10.1002/adfm.202011252. [Google Scholar]
- 51.Wang Y, Zhang S, Wang J. Photo-crosslinkable hydrogel and its biological applications. Chinese Chem Lett. 2021;32:1603–1614. https://doi.org/10.1016/j.cclet.2020.11.073. [Google Scholar]
- 52.Pahoff S, Meinert C, Bas O, et al. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J Mater Chem B. 2019;7:1761–1772. doi: 10.1039/c8tb02607f. https://doi.org/10.1039/c8tb02607f. [DOI] [PubMed] [Google Scholar]
- 53.Lim KS, Schon BS, Mekhileri NV, et al. New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks. ACS Biomater Sci Eng. 2016;2:1752–1762. doi: 10.1021/acsbiomaterials.6b00149. https://doi.org/10.1021/acsbiomaterials.6b00149. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Kumar H, Tian Z, et al. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl Mater Interfaces. 2018;10:26859–26869. doi: 10.1021/acsami.8b06607. https://doi.org/10.1021/acsami.8b06607. [DOI] [PubMed] [Google Scholar]
- 55.Bernal P N, Delrot P, Loterie D, et al. volumetric bioprinting of complex living-tissue constructs within seconds. Adv Mater. 2019;31:1904209. doi: 10.1002/adma.201904209. https://doi.org/10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 56.Guillotin B, Souquet A, Catros S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31:7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. https://doi.org/10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 57.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013;101:272–284. doi: 10.1002/jbm.a.34326. https://doi.org/10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 58.Khalil S, Wei SJ. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater Sci Eng C. 2007;27:469–478. [Google Scholar]
- 59.Chang CC, Boland ED, Williams SK, et al. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011;98:160–170. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil S, Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater Sci Eng C. 2007;27:469–478. https://doi.org/10.1016/j.msec.2006.05.023. [Google Scholar]
- 61.Kolesky DB, Truby RL, Gladman S, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:2966–2966. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 62.Visser J, Peters B, J Burger T, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5:035007. doi: 10.1088/1758-5082/5/3/035007. https://doi.org/10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 63.Nerger BA, Brun PT, Nelson CM. Microextrusion printing cell-laden networks of type I collagen with patterned fiber alignment and geometry. Soft Matter. 2019;15:5728–5738. doi: 10.1039/c8sm02605j. https://doi.org/10.1039/C8SM02605J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong J, Schuurmans CC, Genderen AM, et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat Commun. 2020;11:1267. doi: 10.1038/s41467-020-14997-4. https://doi.org/10.1038/s41467-020-14997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwab A, Hélary C, Richards RG, et al. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater Today Bio. 2020;7:100058. doi: 10.1016/j.mtbio.2020.100058. https://doi.org/10.1016/j.mtbio.2020.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakiyama-Elbert S, Hubbell J. Functional biomaterials:Design of novel biomaterials. Ann Rev Mater Res. 2001;31:183–201. [Google Scholar]
- 67.He Y, Guo S, Liu Z, et al. Pattern transformation of thermo-responsive shape memory polymer periodic cellular structures. Int J Solids Struct. 2015;71:194–205. [Google Scholar]
- 68.Chen Q, Chen H, Zhu L, et al. Fundamentals of double network hydrogels. J Mater Chem B. 2015;3:3654–3676. doi: 10.1039/c5tb00123d. [DOI] [PubMed] [Google Scholar]
- 69.Haque MA, Kurokawa T, Gong JP. Super tough double network hydrogels and their application as biomaterials. Polymer. 2012;53:1805–1822. [Google Scholar]
- 70.Li G, Zhang H, Fortin D, et al. Poly (vinyl alcohol)–poly (ethylene glycol) double-network hydrogel:A general approach to shape memory and self-healing functionalities. Langmuir. 2015;31:11709–11716. doi: 10.1021/acs.langmuir.5b03474. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Guo J, Zhang H, et al. Intelligent rubber with tailored properties for self-healing and shape memory. J Mater Chem A. 2015;3:12864–12872. https://doi.org/10.1039/c5ta01915j. [Google Scholar]
- 72.Liu J, Huang Y, Kumar A, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Zhuo S, Zhang F, Yu J, et al. pH-sensitive biomaterials for drug delivery. Molecules. 2020;25:5649. doi: 10.3390/molecules25235649. https://doi.org/10.3390/molecules25235649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shim JK, Lee YB, Lee YM. pH?dependent permeation through polysulfone ultrafiltration membranes prepared by ultraviolet polymerization technique. J Appl Polym Sci. 1999;74:75–82. [Google Scholar]
- 75.Mukhopadhyay P, Sarkar K, Bhattacharya S, et al. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr Polym. 2014;112:627–637. doi: 10.1016/j.carbpol.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 76.Xu Q, Huang W, Jiang L, et al. KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohydr Polym. 2013;97:565–570. doi: 10.1016/j.carbpol.2013.05.007. https://doi.org/10.1016/j.carbpol.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Gupta P, Vermani K, Garg S. Hydrogels:from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7:569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 78.Ullah F, Othman MB, Javed F, et al. Classification, processing and application of hydrogels:A review. Mater Sci Eng C. 2015;57:414–433. doi: 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 79.Akhlaghi SP, Zaman M, Mohammed N, et al. Synthesis of amine functionalized cellulose nanocrystals:Optimization and characterization. Carbohydr Res. 2015;409:48–55. doi: 10.1016/j.carres.2015.03.009. https://doi.org/10.1016/j.carres.2015.03.009 . [DOI] [PubMed] [Google Scholar]
- 80.Bao G, Jiang T, Ravanbakhsh H, et al. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater Horizons. 2020;7:2336–2347. doi: 10.1039/d0mh00813c. https://doi.org/10.1039/D0MH00813C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yi S, Liu Q, Luo Z, et al. Micropore-forming gelatin methacryloyl (GelMA) bioink toolbox 2.0:Designable tunability and adaptability for 3D bioprinting applications. Small. 2022;18:2106357. doi: 10.1002/smll.202106357. https://doi.org/10.1002/smll.202106357. [DOI] [PubMed] [Google Scholar]
- 82.Gelmi A, Schutt CE. Stimuli?responsive biomaterials:Scaffolds for stem cell control. Adv Healthc Mater. 2021;10:2001125. doi: 10.1002/adhm.202001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higgins MJ, Wallace GG, Molino PJ, et al. Conducting Polymers:Their Route to Nanobionics Applications via Atomic Force Microscopy. New York: Nova Science Publishers, Inc; 2015. [Google Scholar]
- 84.Du L, Li T, Jin F, et al. Design of high conductive and piezoelectric poly (3, 4-ethylenedioxythiophene)/chitosan nanofibers for enhancing cellular electrical stimulation. J Coll Interface Sci. 2020;559:65–75. doi: 10.1016/j.jcis.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Yi YT, Sun JY, Lu YW, et al. Programmable and on-demand drug release using electrical stimulation. Biomicrofluidics. 2015;9:022401. doi: 10.1063/1.4915607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng T, Ji W, Tang Q, et al. Low-fouling nanoporous conductive polymer-coated microelectrode for in vivo monitoring of dopamine in the rat brain. Anal Chem. 2019;91:10786–10791. doi: 10.1021/acs.analchem.9b02386. [DOI] [PubMed] [Google Scholar]
- 87.Kaewchingduang R, Paradee N, Sirivat A, et al. Effects of conductive polyazulene and plasticizer embedded in deproteinized natural rubber transdermal patch on electrically controlled naproxen release-permeation. Int J Pharm. 2019;561:296–304. doi: 10.1016/j.ijpharm.2019.02.046. https://doi.org/10.1016/j.ijpharm.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 88.Meng S, Rouabhia M, Zhang Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin?bioactivated conductive scaffolds. Bioelectromagnetics. 2013;34:189–199. doi: 10.1002/bem.21766. [DOI] [PubMed] [Google Scholar]
- 89.Kim DH, Richardson?Burns SM, Hendricks JL, et al. Effect of immobilized nerve growth factor on conductive polymers:Electrical properties and cellular response. Adv Func Mater. 2007;17:79–86. [Google Scholar]
- 90.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Rel. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 91.Bao Z, Liu X, Liu Y, et al. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J Pharm Sci. 2016;11:349–364. [Google Scholar]
- 92.Algorri J F, Ochoa M, Roldán-Varona P, et al. Light technology for efficient and effective photodynamic therapy:A critical review. Cancers (Basel) 2021;13:3484. doi: 10.3390/cancers13143484. https://doi.org/10.3390/cancers13143484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alvarez?Lorenzo C, Bromberg L, Concheiro A. Light?sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 94.Mantha S, Pillai S, Khayambashi P, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials (Basel) 2019;12:3323. doi: 10.3390/ma12203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rapp TL, DeForest CA. Visible light?responsive dynamic biomaterials:Going deeper and triggering more. Adv Healthc Mater. 2020;9:1901553. doi: 10.1002/adhm.201901553. https://doi.org/10.1002/adhm.201901553. [DOI] [PubMed] [Google Scholar]
- 96.Lee TT, García JR, Paez JI, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–360. doi: 10.1038/nmat4157. https://doi.org/10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aleksandrzak M, Kukulka W, Mijowska E. Graphitic carbon nitride/graphene oxide/reduced graphene oxide nanocomposites for photoluminescence and photocatalysis. Appl Surface Sci. 2017;398:56–62. [Google Scholar]
- 98.Du W, Jin Y, Lai S, et al. Multifunctional light-responsive graphene-based polyurethane composites with shape memory, self-healing, and flame retardancy properties. Composites Part A Appl Sci Manuf. 2020;128:105686. [Google Scholar]
- 99.Qazi TH, Blatchley MR, Davidson MD, et al. Programming hydrogels to probe spatiotemporal cell biology. Cell Stem Cell. 2022;29:678–691. doi: 10.1016/j.stem.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng Y, Chen Z, Jiang Q, et al. Near-infrared-light regulated angiogenesis in a 4D hydrogel. Nanoscale. 2020;12:13654–13661. doi: 10.1039/d0nr02552f. [DOI] [PubMed] [Google Scholar]
- 101.Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo?click living strategy for controlled, reversible exchange of biochemical ligands. Adv Mater. 2014;26:2521–2526. doi: 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wylie RG, Ahsan S, Aizawa Y, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. https://doi.org/10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 103.Hammer JA, West JL. Dynamic ligand presentation in biomaterials. Bioconjug Chem. 2018;29:2140–2149. doi: 10.1021/acs.bioconjchem.8b00288. https://doi.org/10.1021/acs.bioconjchem.8b00288. [DOI] [PubMed] [Google Scholar]
- 104.Chiriaco F, Conversano F, Soloperto G, et al. Epithelial cell biocompatibility of silica nanospheres for contrast-enhanced ultrasound molecular imaging. J Nanoparticle Res. 2013;15:1–13. [Google Scholar]
- 105.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. https://doi.org/10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huebsch N, Kearney CJ, Zhao X, et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Natl Acad Sci. 2014;111:9762–9767. doi: 10.1073/pnas.1405469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koetting MC, Peters JT, Steichen SD, et al. Stimulus-responsive hydrogels:Theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1–49. doi: 10.1016/j.mser.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Habibi M, Foroughi S, Karamzadeh V, et al. Direct sound printing. Nat Commun. 2022;13:1800. doi: 10.1038/s41467-022-29395-1. https://doi.org/10.1038/s41467-022-29395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Z, Liu J, Cui X, et al. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front Chem. 2020;8:124. doi: 10.3389/fchem.2020.00124. https://doi.org/10.3389/fchem.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manjua AC, Alves VD, Crespo JO, et al. Magnetic responsive PVA hydrogels for remote modulation of protein sorption. ACS Appl Mater Interfaces. 2019;11:21239–21249. doi: 10.1021/acsami.9b03146. https://doi.org/10.1021/acsami.9b03146. [DOI] [PubMed] [Google Scholar]
- 111.Chen X, Fan M, Tan H, et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater Sci Eng C Mater Biol Appl. 2019;101:619–629. doi: 10.1016/j.msec.2019.04.012. https://doi.org/10.1016/j.msec.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Farzaneh S, Hosseinzadeh S, Samanipour R, et al. MNP. J Drug Deliv Sci Technol. 2021;64:102525. [Google Scholar]
- 113.Ko ES, Kim C, Choi Y, et al. 3D printing of self-healing ferrogel prepared from glycol chitosan, oxidized hyaluronate, and iron oxide nanoparticles. Carbohydr Polym. 2020;245:116496. doi: 10.1016/j.carbpol.2020.116496. https://doi.org/10.1016/j.carbpol.2020.116496. [DOI] [PubMed] [Google Scholar]
- 114.Guo Z, Dong L, Xia J, et al. 3D printing unique nanoclay?incorporated double?network hydrogels for construction of complex tissue engineering scaffolds. Adv Healthc Mater. 2021;10:e2100036. doi: 10.1002/adhm.202100036. https://doi.org/10.1002/adhm.202100036. [DOI] [PubMed] [Google Scholar]
- 115.Distler T, Polley C, Shi F, et al. Electrically conductive and 3D?printable oxidized alginate?gelatin polypyrrole:PSS hydrogels for tissue engineering. Adv Healthc Mater. 2021;10:2001876. doi: 10.1002/adhm.202001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siebert L, Luna?Cerón E, García?Rivera LE, et al. Light?controlled growth factors release on tetrapodal ZnO?incorporated 3D?printed hydrogels for developing smart wound scaffold. Adv Funct Mater. 2021;31:2007555. doi: 10.1002/adfm.202007555. https://doi.org/10.1002/adfm.202007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maan Z, Masri NZ, Willerth SM. Smart bioinks for the printing of human tissue models. Biomolecules. 2022;12:141. doi: 10.3390/biom12010141. https://doi.org/10.3390/biom12010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tiwari G, Tiwari R, Sriwastawa B, et al. Drug delivery systems:An updated review. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. https://doi.org/10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang M, Li W, Tang G, et al. Engineering (bio) materials through shrinkage and expansion. Adv Healthc Mater. 2021;10:2100380. doi: 10.1002/adhm.202100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pawar AA, Saada G, Cooperstein I, et al. High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Sci Adv. 2016;2:e1501381. doi: 10.1126/sciadv.1501381. https://doi.org/10.1126/sciadv.1501381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melocchi A, Uboldi M, Maroni A, et al. 3D printing by fused deposition modeling of single-and multi-compartment hollow systems for oral delivery-a review. Int J Pharm. 2020;579:119155. doi: 10.1016/j.ijpharm.2020.119155. https://doi.org/10.1016/j.ijpharm.2020.119155. [DOI] [PubMed] [Google Scholar]
- 122.Ceylan H, Yasa IC, Yasa O, et al. 3D-printed biodegradable microswimmer for theranostic cargo delivery and release. ACS Nano. 2019;13:3353–3362. doi: 10.1021/acsnano.8b09233. https://doi.org/10.1021/acsnano.8b09233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trampe E, Koren K, Akkineni AR, et al. Functionalized bioink with optical sensor nanoparticles for O2 imaging in 3D?bioprinted constructs. Adv Funct Mater. 2018;28:1804411. [Google Scholar]
- 124.Iversen M, Monisha M, Agarwala S. Flexible, wearable and fully-printed smart patch for pH and hydration sensing in wounds. Int J Bioprint. 2022;8:447. doi: 10.18063/ijb.v8i1.447. https://doi.org/10.18063/ijb.v8i1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dargaville TR, Farrugia BL, Broadbent JA, et al. Sensors and imaging for wound healing:A review. Biosens Bioelectron. 2013;41:30–42. doi: 10.1016/j.bios.2012.09.029. https://doi.org/10.1016/j.bios.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 126.Bahl S, Nagar H, Singh I, et al. Smart materials types, properties and applications:A review. Mater Today Proc. 2020;28:1302–1306. https://doi.org/10.1016/j.matpr.2020.04.505. [Google Scholar]
- 127.Yu Y, Zhang J, Liu JJ. Biomedical implementation of liquid metal ink as drawable ECG electrode and skin circuit. PLoS One. 2013;8:e58771. doi: 10.1371/journal.pone.0058771. https://doi.org/10.1371/journal.pone.005≃. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gantenbein S, Masania K, Woigk W, et al. Three-dimensional printing of hierarchical liquid-crystal-polymer structures. Nature. 2018;561:226–230. doi: 10.1038/s41586-018-0474-7. https://doi.org/10.1038/s41586-018-0474-7. [DOI] [PubMed] [Google Scholar]
- 129.Mantha S, Pillai S, Khayambashi P, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials (Basel) 2019;12:3323. doi: 10.3390/ma12203323. https://doi.org/10.3390/ma12203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zarek M, Layani M, Cooperstein I, et al. 3D printing of shape memory polymers for flexible electronic devices. Adv Mater. 2016;28:4449–4454. doi: 10.1002/adma.201503132. https://doi.org/10.1002/adma.201503132. [DOI] [PubMed] [Google Scholar]
- 131.Khoo ZX, Teoh JE, Liu Y, et al. 3D printing of smart materials:A review on recent progresses in 4D printing. Virtual Phys Prototyp. 2015;10:103–122. https://doi.org/10.1080/17452759.2015.1097054. [Google Scholar]
- 132.Janbaz S, Hedayati R, Zadpoor AA. Programming the shape-shifting of flat soft matter:from self-rolling/self-twisting materials to self-folding origami. Mater Horizons. 2016;3:536–547. https://doi.org/10.1039/c6mh00195e. [Google Scholar]
- 133.Zhao W, Huang Z, Liu L, et al. Porous bone tissue scaffold concept based on shape memory PLA/Fe3O4. Composites Sci Technol. 2021;203:108563. https://doi.org/10.1016/j.compscitech.2020.108563. [Google Scholar]
- 134.Wang Y, Cui H, Wang Y, et al. 4D printed cardiac construct with aligned myofibers and adjustable curvature for myocardial regeneration. ACS Appl Mater Interfaces. 2021;13:12746–12758. doi: 10.1021/acsami.0c17610. https://doi.org/10.1021/acsami.0c17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ge Q, Sakhaei AH, Lee H, et al. Multimaterial 4D printing with tailorable shape memory polymers. Sci Rep. 2016;6:31110. doi: 10.1038/srep31110. https://doi.org/10.1038/srep31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao Z, Wu J, Mu X, et al. Origami by frontal photopolymerization. Sci Adv. 2017;3:e1602326. doi: 10.1126/sciadv.1602326. https://doi.org/10.1126/sciadv.1602326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li C, Lau GC, Yuan H, et al. Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Sci Robot. 2020;5:eabb9822. doi: 10.1126/scirobotics.abb9822. https://doi.org/10.1126/scirobotics.abb9822. [DOI] [PubMed] [Google Scholar]
- 138.Yang G, Wang J, Yan Y, et al. Multi-stimuli-triggered shape transformation of polymeric filaments derived from dynamic covalent block copolymers. Biomacromolecules. 2020;21:4159–4168. doi: 10.1021/acs.biomac.0c00956. https://doi.org/10.1021/acs.biomac.0c00956. [DOI] [PubMed] [Google Scholar]
- 139.Won JY, Kim J, Gao G, et al. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone:A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020;116:174–185. doi: 10.1016/j.actbio.2020.09.015. https://doi.org/10.1016/j.actbio.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 140.Ruskowitz ER, Comerford MP, Badeau BA, et al. Logical stimuli-triggered delivery of small molecules from hydrogel biomaterials. Biomater Sci. 2019;7:542–546. doi: 10.1039/c8bm01304g. https://doi.org/10.1039/c8bm01304g. [DOI] [PubMed] [Google Scholar]
- 141.Yang GH, Yeo M, Koo YW, et al. 4D bioprinting:Technological advances in biofabrication. Macromol Biosci. 2019;19:e1800441. doi: 10.1002/mabi.201800441. https://doi.org/10.1002/mabi.201800441. [DOI] [PubMed] [Google Scholar]
- 142.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. https://doi.org/10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Narupai B, Smith PT, Nelson A. 4D printing of multi-stimuli responsive protein-based hydrogels for autonomous shape transformations. Adv Funct Mater. 2021;31:2011012. https://doi.org/10.1002/adfm.202011012. [Google Scholar]
- 144.Iversen M, Monisha M, Agarwala S. Flexible, wearable and fully-printed smart patch for pH and hydration sensing in wounds. Int J Bioprint. 2022;8:447. doi: 10.18063/ijb.v8i1.447. https://doi.org/10.18063/ijb.v8i1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. https://doi.org/10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 146.Hassani FA, Peh WY, Gammad GG, et al. A 3D printed implantable device for voiding the bladder using shape memory alloy (SMA) actuators. Adv Sci (Weinh) 2017;4:1700143. doi: 10.1002/advs.201700143. https://doi.org/10.1002/advs.201700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dong SL, Han L, Du CX, et al. 3D printing of aniline tetramer-grafted-polyethylenimine and pluronic F127 composites for electroactive scaffolds. Macromol Rapid Commun. 2017;38:1600551. doi: 10.1002/marc.201600551. https://doi.org/10.1002/marc.201600551. [DOI] [PubMed] [Google Scholar]
- 148.Kaur MD, Mir B, Noor A, et al. 4D printing:the dawn of “smart”drug delivery systems and biomedical applications. J Drug Deliv Ther. 2021;11:131–137. https://doi.org/10.22270/jddt.v11i5-S.5068. [Google Scholar]
- 149.Xu L, Zhang W, Park HB, et al. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J Immunother Cancer. 2019;7:220. doi: 10.1186/s40425-019-0702-1. https://doi.org/10.1186/s40425-019-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu L, Zhou Z, Mao H, et al. Magnetic nanoparticles for precision oncology:Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine (Lond) 2017;12:73–87. doi: 10.2217/nnm-2016-0316. https://doi.org/10.2217/nnm-2016-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu Y, Wu X, Guo X, et al. The boom in 3D-printed sensor technology. Sensors (Basel) 2017;17:1166. doi: 10.3390/s17051166. https://doi.org/10.3390/s17051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kirillova A, Maxson R, Stoychev G, et al. 4D biofabrication using shape-morphing hydrogels. Adv Mater. 2017;29:1703443. doi: 10.1002/adma.201703443. https://doi.org/10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Miao Y, Zhang J, et al. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;84:44–51. doi: 10.1016/j.msec.2017.11.025. https://doi.org/10.1016/j.msec.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 154.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting:Past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. https://doi.org/10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 155.Christensen K, Xu C, Chai W, et al. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015;112:1047–1055. doi: 10.1002/bit.25501. https://doi.org/10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- 156.Cui X, Breitenkamp K, Finn MG, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18:1304–1312. doi: 10.1089/ten.tea.2011.0543. https://doi.org/10.1089/ten.TEA.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heinrich MA, Liu W, Jimenez A, et al. 3D Bioprinting:From benches to translational applications. Small. 2019;15:e1805510. doi: 10.1002/smll.201805510. https://doi.org/10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pedde RD, Mirani B, Navaei A, et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater. 2017;29:1606061. doi: 10.1002/adma.201606061. https://doi.org/10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- 160.Malda J, Visser J, Melchels FP, et al. 25th anniversary article:Engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. https://doi.org/10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 161.Duan B, Hockaday LA, Kang KH, et al. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2012;101:1255–1264. doi: 10.1002/jbm.a.34420. https://doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A. 2008;14:41–48. doi: 10.1089/ten.a.2007.0004. https://doi.org/10.1089/ten.a.2007.0004. [DOI] [PubMed] [Google Scholar]
- 163.Hopp B, Smausz T, Kresz N, et al. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005;11:1817–1823. doi: 10.1089/ten.2005.11.1817. https://doi.org/10.1089/ten.2005.11.1817. [DOI] [PubMed] [Google Scholar]
- 164.Schiele NR, Corr DT, Huang Y, et al. Laser-based direct-write techniques for cell printing. Biofabrication. 2010;2:032001. doi: 10.1088/1758-5082/2/3/032001. https://doi.org/10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miao S, Nowicki M, Cui H, et al. 4D anisotropic skeletal muscle tissue constructs fabricated by staircase effect strategy. Biofabrication. 2019;11:035030. doi: 10.1088/1758-5090/ab1d07. https://doi.org/10.1088/1758-5090/ab1d07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hendrikson WJ, Rouwkema J, Clementi F, et al. Towards 4D printed scaffolds for tissue engineering:Exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication. 2017;9:031001. doi: 10.1088/1758-5090/aa8114. https://doi.org/10.1088/1758-5090/aa8114. [DOI] [PubMed] [Google Scholar]
- 167.Senatov FS, Niaza KV, Zadorozhnyy MY, et al. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J Mech Behav Biomed Mater. 2016;57:139–148. doi: 10.1016/j.jmbbm.2015.11.036. https://doi.org/10.1016/j.jmbbm.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 168.Miao S, Zhu W, Castro NJ, et al. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:27226. doi: 10.1038/srep27226. https://doi.org/10.1038/srep27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miao S, Cui H, Nowicki M, et al. Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation. Biofabrication. 2018;10:035007. doi: 10.1088/1758-5090/aabe0b. https://doi.org/10.1088/1758-5090/aabe0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Banche-Niclot F, Montalbano G, Fiorilli S, et al. PEG-coated large mesoporous silicas as smart platform for protein delivery and their use in a collagen-based formulation for 3D printing. Int J Mol Sci. 2021;22:1718. doi: 10.3390/ijms22041718. https://doi.org/10.3390/ijms22041718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okwuosa TC, Pereira BC, Arafat B, et al. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm Res. 2017;34:427–437. doi: 10.1007/s11095-016-2073-3. https://doi.org/10.1007/s11095-016-2073-3. [DOI] [PubMed] [Google Scholar]
- 172.Mirani B, Pagan E, Currie B, et al. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv Healthc Mater. 2017;6:1700718. doi: 10.1002/adhm.201700718. https://doi.org/10.1002/adhm.201700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sayyar S, Bjorninen M, Haimi S, et al. UV cross-linkable graphene/poly(trimethylene Carbonate) composites for 3D printing of electrically conductive scaffolds. ACS Appl Mater Interfaces. 2016;8:31916–31925. doi: 10.1021/acsami.6b09962. https://doi.org10.1021/acsami.6b09962. [DOI] [PubMed] [Google Scholar]
- 174.Yang H, Leow WR, Wang T, et al. 3D printed photoresponsive devices based on shape memory composites. Adv Mater. 2017;29:1701627. doi: 10.1002/adma.201701627. https://doi.org/10.1002/adma.201701627. [DOI] [PubMed] [Google Scholar]
- 175.Gupta MK, Meng F, Johnson BN, et al. 3D printed programmable release capsules. Nano Lett. 2015;15:5321–5329. doi: 10.1021/acs.nanolett.5b01688. https://doi.org/10.1021/acs.nanolett.5b01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei H, Zhang Q, Yao Y, et al. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl Mater Interfaces. 2017;9:876–883. doi: 10.1021/acsami.6b12824. https://doi.org10.1021/acsami.6b12824. [DOI] [PubMed] [Google Scholar]
- 177.Zhang J, Zhao S, Zhu M, et al. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J Mater Chem B. 2014;2:7583–7595. doi: 10.1039/c4tb01063a. https://doi.org/10.1039/C4TB01063A. [DOI] [PubMed] [Google Scholar]
- 178.D'Amora U, Russo T, Gloria A, et al. 3D additive-manufactured nanocomposite magnetic scaffolds:Effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioactive Mater. 2017;2:138–145. doi: 10.1016/j.bioactmat.2017.04.003. https://doi.org/10.1016/j.bioactmat.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Betsch M, Cristian C, Lin YY, et al. Incorporating 4D into bioprinting:Real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Advanced Healthcare Materials. 2018;7(21):1800894. doi: 10.1002/adhm.201800894. https://doi.org/10.1002/adhm.201800894. [DOI] [PubMed] [Google Scholar]
- 180.Zhou X, Castro NJ, Zhu W, et al. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci Rep. 2016;6:32876. doi: 10.1038/srep32876. https://doi.org/10.1038/srep32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


