Abstract
Although Th1-type cell-mediated immunity (CMI) is the predominant host defense mechanism against mucosal Candida albicans infection, CMI against a vaginal C. albicans infection in mice is limited at the vaginal mucosa despite a strong Candida-specific Th1-type response in the draining lymph nodes. In contrast, Th1-type CMI is highly effective against an experimental Chlamydia trachomatis genital tract infection. This study demonstrated through two independent designs that a concurrent Candida and Chlamydia infection could not accelerate or modulate the anti-Candida CMI response. Together, these results suggest that host responses to these genital tract infections are independent and not influenced by the presence of the other.
Recurrent vulvovaginal candidiasis caused by Candida albicans is common in women of child-bearing age (15, 16). Cell-mediated immunity (CMI) by Th1-type CD4+ T cells is considered the predominant host defense mechanism against C. albicans infection at mucosal sites and is thought to also play a role in maintaining the organism in its commensal state at those sites (13, 14). However, clinical studies of women with recurrent vulvovaginal candidiasis and studies from an experimental estrogen-dependent murine model of vaginal candidiasis have shown a lack of effects of systemic CMI locally at the vaginal mucosa despite the presence of Candida-specific Th1-type CD4+ T cells in the peripheral circulation (6–8). Likewise, the modest partial protection against a secondary vaginal infection in mice has also failed to provide any evidence for systemic CMI involvement (5). At the local level as well, studies to evaluate local tissues of mice showed no changes in a phenotypically distinct population of vaginal T cells during a vaginal infection and no evidence for systemic T-cell infiltration into the vaginal mucosa (4). Taken together, these results suggest either a complete absence for any role of CMI against vaginal C. albicans infections or that an immune regulatory mechanism prevents the contribution of anti-Candida CMI at the vaginal mucosa. In support of the latter, a profound local CMI response has been observed in rats given an experimental vaginal C. albicans infection (2).
In contrast to a limited response to Candida at the murine vaginal mucosa, mice given an experimental Chlamydia trachomatis genital tract infection exhibit a CD4+ T-cell infiltrate into the genital tract (11). This Th1-type response is critical for the resolution of infection and indicates that T cells can reach the genital mucosa and provide substantial protection (1). Accordingly, recruitment of T cells into the genital tract is dependent on the expression of adhesion molecules on the local endothelium and matched homing receptors on infiltrating T cells (9). Since C. trachomatis infection may modulate immunity to other sexually transmitted infections (10), this study addressed whether or not a dual infection with C. albicans and C. trachomatis could enhance the local recruitment of a CMI host response against C. albicans and facilitate clearance of the infection.
To assess whether a genital tract infection with C. trachomatis could affect an experimental infection with C. albicans at the vaginal mucosa by facilitating the recruitment of Candida-specific T cells normally present in the draining lymph nodes 7 to 14 days postinfection, we monitored the course of vaginal fungal burden in mice inoculated with both the mouse pneumonitis biovar of C. trachomatis (MoPn) and C. albicans. For this, we first established a MoPn infection in mice to induce an inflammatory response in the genital mucosa. The inflammatory response induces the appropriate signals for the up-regulation of adhesion molecules, which in turn results in recruitment of antichlamydial T cells (12). Accordingly, female BALB/c mice (6 to 8 weeks old; purchased from Harlan Sprague-Dawley [Indianapolis, Ind.] and housed in accordance with the American Association of Accreditation of Laboratory Animal Care guidelines) were first injected subcutaneously with 2.5 mg of Depo-Provera (Upjohn, Kalamazoo, Mich.) in 100 μl of sterile phosphate-buffered saline (PBS). Seven days later, while under sodium pentobarbital anesthesia, all mice were inoculated intravaginally with 107 inclusion-forming units of MoPn grown in McCoy cells. Infection was monitored every 3 days after inoculation by obtaining cervicovaginal swabs (Dacroswab type 1; Spectrum Laboratories, Houston, Tex.) as previously described (12). Seven days later, the mice were injected subcutaneously with 0.02 mg of β-estradiol 17-valerate (estrogen; Sigma, St. Louis, Mo.) in 0.1 ml of sesame oil. Estrogen treatments were continued at weekly intervals. Three days after the first estrogen treatment (10 days after MoPn inoculation), mice were inoculated with 5 × 104 stationary-phase C. albicans blastoconidia in a volume of 20 μl of PBS (5), using a laboratory-cultivated clinical isolate (3153A). Vaginal lavage samples were collected on days 4, 14, and 28 after Candida inoculation, and vaginal fungal burden was quantified as previously described (6). Lavage samples and swabs were not collected on the same day. Control groups were inoculated with C. albicans or MoPn alone under the same progesterone-estrogen treatment regimen or under an only estrogen (Candida) or progesterone (MoPn) regimen.
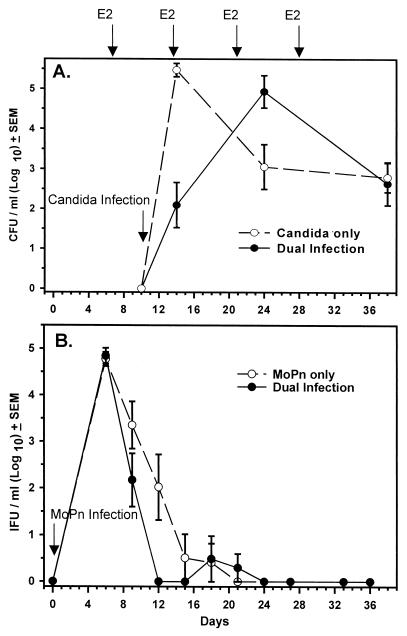
In mice infected with C. albicans and MoPn, clearance of the fungal infection was not significantly different from that in mice inoculated with C. albicans alone (Fig. 1A). C. albicans infection also had no effect on clearance of the MoPn infection (Fig. 1B). Positive Candida-specific delayed-type hypersensitivity (measured by footpad swelling 24 h after Candida antigen footpad challenge [6]) was equally present in both C. albicans-infected and dually infected mice, indicating that all mice produced a CMI response against C. albicans (data not shown). Therefore, MoPn infection did not appear to affect systemic or local anti-Candida immunity. The presence of progesterone would not be expected to have influenced these results since progesterone was recently shown to have no effect on a vaginal Candida infection in estrogen-treated mice (3). Likewise, estrogen injections did not alter the course of MoPn infection when given after MoPn infection was established (data not shown).
FIG. 1.
Natural history of a concomitant MoPn genital tract infection and Candida vaginal infection. Mice were treated with progesterone (day -7) and with MoPn (day 0), followed by estrogen (E2) treatment and Candida inoculation (day 10). Controls included Candida or MoPn inoculated alone under the same hormone treatment regimens. (A) Vaginal Candida burden (CFU). (B) Genital tract Chlamydia burden (IFU, inclusion forming units). Each data point represents the mean ± standard error of the mean of 4 to 12 mice per group. No significant differences were found among groups by two-way analysis of variance.
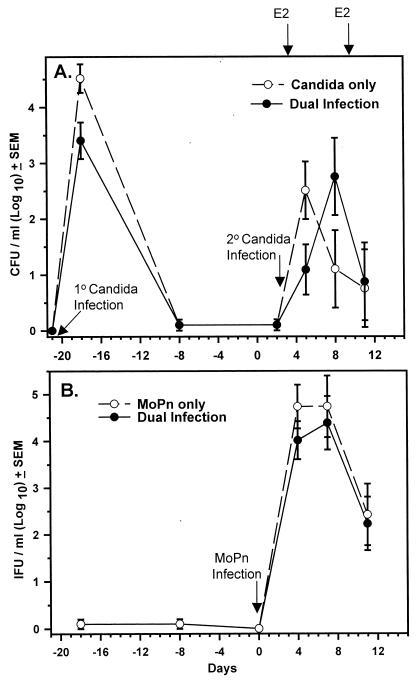
As a chlamydial infection had no influence on a primary vaginal C. albicans infection where Candida-specific CMI was generated after the chlamydial CMI induced by MoPn, we next sought to determine if a chlamydial infection could enhance the partial protection against a secondary C. albicans vaginal infection (5) where the Candida-specific T cells would be induced prior to the chlamydial infection. For this, mice were first vaginally inoculated with C. albicans in the absence of estrogen. Under these conditions, mice spontaneously resolve the vaginal infection by 14 to 21 days (Fig. 2A) (5). On day 14 after Candida inoculation, the recovered mice were given progesterone and 7 days later infected with MoPn. Two days after chlamydial inoculation, mice were treated with estrogen and reinoculated with Candida. Controls included mice inoculated with Candida or MoPn alone following the identical hormone treatments and primary Candida infection. As shown in Fig. 2A, the vaginal fungal burden was not different between C. albicans-infected and C. albicans-plus-MoPn-infected mice. Thus, the partial protection against the secondary Candida infection was not enhanced by the MoPn infection. Likewise, no differences were observed in the number of viable chlamydiae recovered from either group (Fig. 2B).
FIG. 2.
Ability of a MoPn genital infection to influence the fungal burden of a secondary Candida vaginal infection. Mice were inoculated with Candida (primary [1°] infection; day -21) followed by progesterone treatment (day -7) and inoculated with MoPn on day 0. On day 2, mice were treated with estrogen (E2) and inoculated with Candida a second time (secondary [2°] infection). Controls included mice inoculated with MoPn or Candida alone (day 2) following identical hormone treatments and primary Candida infection. (A) Vaginal Candida burden (CFU). (B) Genital tract Chlamydia burden (IFU, inclusion forming units). Each data point represents the mean ± standard error of the mean of eight mice per group. No significant differences were found among groups by two-way analysis of variance.
To determine if leukocyte recruitment was altered in mice with this second dual-infection design, we measured the number of CD4+ cells in the genital tracts of mice infected with Candida, Chlamydia, or both. The genital tracts were harvested, separated into the upper tract (oviducts and ovaries) and lower tract (cervicovaginal region), and treated with collagenase as previously described (12). Single-cell suspensions (2 × 105 to 4 × 105 cells) were stained with rat anti-mouse CD4 (L3T4; clone GK1.5; PharMingen, San Diego, Calif.) or an irrelevant control antibody (rat immunoglobulin G) as described previously (12). After being washed, the cells were resuspended in goat anti-rat immunoglobulin G conjugated to fluorescein isothiocyanate (BioSource International, Camarillo, Calif.) and fixed in PBS containing 1% paraformaldehyde until analyzed. Flow cytometry was performed on a FACScan (Becton Dickinson, Mountain View, Calif.) analyzer equipped with a 488-nm argon laser and Lysys II software. Dead cells were excluded on the basis of forward angle and 90° light scatter, and 5,000 cells were collected in the leukocyte region for each sample. For analysis, the percentage of positive cells was determined by subtracting the value obtained with the isotype control antibody.
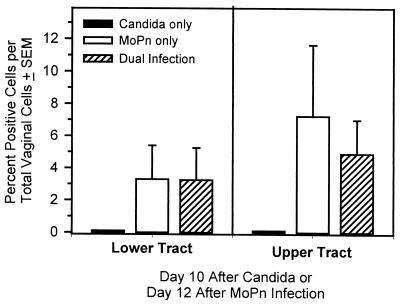
As shown in Fig. 3, we found elevated numbers of CD4+ cells in both the lower and upper tracts of mice 12 days after MoPn infection or after MoPn infection followed by secondary Candida infection. The percentages in the lower (3.1%) and upper (6.2%) tracts during a chlamydial genital tract infection were similar to those previously reported (12). However, no increases in CD4+ cells were noted in mice that received a challenge Candida infection alone. Previously, Wormley et al. (18) found in the vaginas of naive mice a phenotypically distinct population of CD4 cells that was not detected with the commonly used anti-CD4 monoclonal antibody against the GK1.5 epitope. Thus, using GK1.5 anti-CD4 antibodies, CD4+ cells will be detected in the vagina only if they have infiltrated from the systemic compartment. The lack of detectable CD4+ cells in Candida-infected mice in this study is consistent with those previous observations (18). In fact, based on the lack of a more enhanced protection against Candida in dually infected mice, one would predict that the infiltrating T cells were Chlamydia specific and not Candida specific, although formal confirmation will require in vitro antigen-specific blastogenesis of genital tract T cells.
FIG. 3.
Leukocyte recruitment to the genital tract during the single and dual MoPn and Candida infections in the secondary-challenge model. Leukocytes recovered from collagenase-treated upper and lower genital tracts were evaluated for CD4+ T cells by flow cytometry. Each data point represents the mean ± standard error of the mean of two to three mice per group harvested 12 days after MoPn vaginal inoculation and 10 days after Candida infection.
The lack of lymphocyte recruitment from the central circulation to the vaginal mucosa may be attributable not only to the pathogen itself (Chlamydia versus Candida) but also to differences in the ability of anatomically distinct regions of the genital tract to recruit lymphocytes. For instance, Kelly et al. (12) have recently found that CD4+ cells are preferentially recruited to the oviducts and uterine horns rather than the cervicovaginal region during chlamydial infection. This finding correlated with expression of adhesion molecules on the genital tract endothelium. Interestingly, adhesion molecules on the endothelium of the lower genital tract subsided within 7 to 10 days of an ongoing infection, while expression was maintained in the upper tract until resolution of infection. Therefore, lack of sustained adhesion molecule expression on the vaginal endothelium of dually infected mice may explain, in part, the inability of a concurrent chlamydial infection to enhance local anti-Candida immunity.
Alternatively, the stimulation of T cells by Candida antigens may, through some form of immunoregulation, down-regulate expression of homing receptors on Candida-specific CD4+ T cells such that they are unable to infiltrate from the draining lymph nodes into the vaginal mucosa despite increases of the reciprocal ligands on the vaginal endothelium. Support for this possibility comes from the observed increase in homing receptors on draining lymph node CD3+ cells during a MoPn genital tract infection versus a constant or diminution of the same molecules on CD3+ T cells during a Candida infection (unpublished data). If immunoregulatory mechanisms are functioning in the lower genital tract, one would predict them to be antigen specific based on the lack of any observed effects on the antichlamydial Th1-type CMI. Although it is unclear what the source of this putative immune regulation might be, high concentrations of transforming growth factor β, a potent immunoregulatory cytokine, have been observed in the vaginal mucosa of naive and Candida-infected mice and in the draining lymph nodes of infected mice (17). Regardless of the means, however, a concomitant Chlamydia infection induced before or after a Candida infection did not influence or modulate anti-Candida immunity against a vaginal C. albicans infection.
In summary, although a particular sexually transmitted infection may influence the susceptibility or immunity to another infectious agent (10), responses to concurrent experimental Chlamydia and Candida genital tract infections are independent. Such immunological independence may be manifested by antigen-specific immunoregulatory mechanisms and/or anatomical differences between the upper and lower genital tracts.
Acknowledgments
This work was supported by National Institutes of Health grants AI-26328 (Kelly) and AI-32556 (Fidel).
REFERENCES
- 1.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bernardis F, Santoni G, Boccanera M, Spreghini E, Adriani D, Morelli L, Cassone A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–3304. doi: 10.1128/iai.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidel P L, Jr, Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–657. doi: 10.1128/iai.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidel P L, Jr, Luo W, Steele C, Chabain J, Baker M, Wormley F., Jr Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67:3135–3140. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidel P L, Jr, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel P L, Jr, Lynch M E, Sobel J D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403–2408. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel P L, Jr, Lynch M E, Sobel J D. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun. 1994;62:1032–1038. doi: 10.1128/iai.62.3.1032-1038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins R A, Rank R G, Kelly K A. Expression of mucosal homing receptor α4β7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun. 2000;68:5587–5594. doi: 10.1128/iai.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho J L, He S, Hu A, Geng J, Basile F G, Almeida M G B, Saito A Y, Laurence J, Johnson W D., Jr Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-infected patients' mononuclear cells and cell lines: an in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med. 1995;181:1493–1505. [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly K A, Walker J C, Jameel S H, Gray H L, Rank R G. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamyida infection. Infect Immun. 2000;68:1519–1528. doi: 10.1128/iai.68.3.1519-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odds F C. Candida and candidosis. Baltimore, Md: University Park Press; 1998. pp. 104–110. [Google Scholar]
- 14.Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. In: Romagnani S, editor. Th1 and Th2 cells in health and disease. Farmington, Conn: Karger; 1996. pp. 114–137. [PubMed] [Google Scholar]
- 15.Sobel J D. Vaginal infections in adult women. Med Clin North Am. 1990;74:1573–1602. doi: 10.1016/s0025-7125(16)30496-5. [DOI] [PubMed] [Google Scholar]
- 16.Sobel J D, Faro S, Force R W, Foxman B, Ledger W J, Nyirjesy P R, Reed B D, Summers P R. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor B N, Saavedra M, Fidel P L., Jr Local Th1/Th2 cytokine production during experimental vaginal candidiasis: potential importance of transforming growth factor-β. Med Mycol. 2000;38:419–431. doi: 10.1080/mmy.38.6.419.431. [DOI] [PubMed] [Google Scholar]
- 18.Wormley F L, Jr, Scott M, Luo W, Baker M, Chaiban J, Fidel P L., Jr Evidence for a unique expression of CD4 on murine vaginal CD4+ cells. Immunology. 2000;100:300–308. doi: 10.1046/j.1365-2567.2000.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]