Abstract
Understanding the human hypothalamic-pituitary-gonadal (HPG) axis presents a major challenge for medical science. Dysregulation of the HPG axis is linked to infertility and a thorough understanding of its dynamic behaviour is necessary to both aid diagnosis and to identify the most appropriate hormonal interventions. Here, we review how quantitative models are being used in the context of clinical reproductive endocrinology to: 1. analyse the secretory patterns of reproductive hormones; 2. evaluate the effect of drugs in fertility treatment; 3. aid in the personalization of assisted reproductive technology (ART). In this review, we demonstrate that quantitative models are indispensable tools enabling us to describe the complex dynamic behaviour of the reproductive axis, refine the treatment of fertility disorders, and predict clinical intervention outcomes.
Keywords: Assisted reproductive technology, Machine learning, Mathematical modelling, Pulsatility analysis, Clinical decision making, In vitro fertilization, Artificial intelligence, Reproductive endocrinology, Quantitative modelling
Abbreviations: HPG, hypothalamic-pituitary gonadal; ART, assisted reproductive technology; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PCOS, polycystic ovary syndrome; HA, hypothalamic amenorrhea; BSA, Bayesian Spectrum Analysis; AI, artificial intelligence; ML, machine learning; IVF, in vitro fertilization; AMH, anti-Müllerian hormone; OHSS, ovarian hyperstimulation syndrome; E2, estradiol; P4, progesterone
Introduction
The reproductive system is a complex endocrine system, involving non-linear feedback and feed-forward interactions conveyed by dynamic hormone signals [1], as well as multifaceted crosstalk with other endocrine axes and the central nervous system [2]. Such complexity makes it challenging to decipher how the system behaves in normal physiological conditions, under acute perturbations, or during chronic disease. To this end, quantitative modelling is an indispensable tool for solidifying our understanding of the system, analysing its dynamic behaviour, and designing medical interventions.
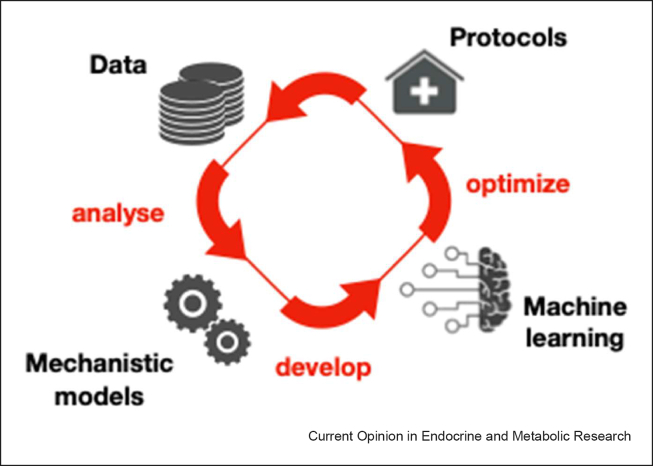
This review aims to provide an update on how quantitative models are being used in the context of clinical reproductive endocrinology (Figure 1). We focus on computational methods that assist in profiling the dynamics of reproductive hormones, mechanistic models that assist the quantitative assessment of drugs in reproductive medicine, as well as machine learning approaches that are currently used in assisted reproductive technology (ART).
Figure 1.
Utility of quantitative models in reproductive medicine. This flowchart provides an overview of the workflow of quantitative modelling in reproductive medicine. The first step involves the collection of data, such as hormonal and imaging data. Mathematical models aid the analysis of the data, facilitating extraction of meaningful information. Furthermore, processed data can be used to develop machine learning models with the aim of optimizing current procedures and protocols. The workflow is iterative, enabling continuous model evaluation and improvement.
Computational model for the analysis of hormone pulsatile dynamics
The hypothalamic-pituitary-gonadal (HPG) axis is a complex endocrine system controlling sexual development (throughout fetal, neonatal, and pubertal stages) and reproduction [3]. The system relies on dynamic hormone signals to serve its role. Most notably, gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile manner from the hypothalamus into the anterior pituitary gland, and stimulates the release of gonadotropins (luteinizing hormone, LH; and follicle-stimulating hormone, FSH), which in turn trigger gonadal processes involved in gametogenesis and sex-steroid production [4]. Hence, pulsatile GnRH dynamics are crucial for the onset of puberty and subsequent healthy reproductive function in adults. Disruption in the frequency of GnRH/LH pulses is observed in common reproductive disorders, such as polycystic ovary syndrome (PCOS), in which the frequency and amplitude of GnRH pulses are increased [5], and hypothalamic amenorrhea (HA), in which GnRH pulses are reduced [6]. Therefore, accurate assessment of hormone pulsatility could facilitate the diagnosis and treatment of patients presenting with reproductive endocrine disorders [7].
In clinical research, LH is measured as the gold standard surrogate for GnRH (as it is not possible to measure GnRH in the peripheral circulation at high enough levels). Measuring serum levels of LH at regular intervals (e.g., every 10 minutes) enables quantification and assessment of pulsatile dynamics. However, analysing hormone pulsatility is challenging as pulse-to-pulse variability combined with measurement error often obscure the underlying hormone dynamics [8]. Several computational methods have been proposed in the literature to facilitate the analysis of LH pulsatility [8∗, 9, 10, 11, 12, 13∗∗] (see Table 1). Among these, the deconvolution analysis method is considered the gold standard in clinical research [8]. The method uses a mathematical model describing the time-varying secretion and clearance dynamics of LH, which seeks to fit data and deconvolve the two processes. Data-fitting is achieved via maximum likelihood estimation, providing estimates of the times at which pulses of LH have occurred alongside estimates of the secretion and clearance rates. Bayesian Spectrum Analysis (BSA) presents a different approach to pulsatility analysis, allowing one to quantify the frequency of LH pulses while ignoring mechanistic parameters (e.g., secretion and clearance rates), as well as the actual timing of pulses [14,15]. BSA relies on an abstract model describing generic periodic signals and estimates the frequency from LH data using Bayesian inference [11]. A key strength of the BSA method is that frequency estimates come in the form of Bayesian posterior distributions, facilitating the estimation of uncertainty and hypothesis testing. Finally, Bayesian extensions to the deconvolution method [13,16, 17, 18] as well as a recently proposed Bayesian framework for inference of LH dynamics based on mechanistic models of pulse generation [19] enable uncertainty estimation of parameters as well as estimation of latent hypothalamic dynamics.
Table 1.
Summary of methods used in LH pulsatility analysis.
| Method/Tool | Model | Outputs | Open-source Implementation | Reference |
|---|---|---|---|---|
| Deconvolution analysis | Mechanistic model | Position of pulses and pulse parameters (point estimates) | Unavailable | [8] |
| Cluster analysis | Statistical pattern matching | Position of pulses (point estimates) | Unavailable | [9] |
| DynPeak | Mechanistic model | Position of pulses (point estimates) | Python | [10] |
| BaSAR | Harmonic functions | Pulse frequency (posterior distribution) | R package | [11] |
| Bayesian Deconvolution Analysis | Mechanistic model | Position of pulses; pulse parameters (posterior distribution) | Unavailable | [13] |
| HormoneBayes | Mechanistic model | Model parameters; position of pulses (posterior distribution) | C++ | [19] |
The potential of artificial intelligence in assisted reproductive technology
The broad field of artificial intelligence (AI) encompasses machine learning (ML), which specifically refers to statistical models that are leveraged to automatically detect patterns from large and complex datasets in order to make predictions regarding an outcome of interest [20]. AI and ML methods have a wide scope for improving ART [21, 22∗, 23, 24], which includes in vitro fertilization (IVF) treatment; a procedure that, for example, inherently requires the classification and selection of both male and female gametes, as well as several complex decisions that are made during the cycle with respect to the dosage, and timing, of hormonal interventions.
Key for the successful application of ML is high-quality substantial datasets that contain strong predictors, capture the variance in the population, and are accurately annotated [25,26]. For this reason, early ML models of predicting live birth after IVF treatment using neural networks achieved a modest accuracy (59%) [27], as they relied on small datasets lacking key predictors. More recently, the accuracy of predictive models trained on richer datasets has increased to 84.4% [28]. Even where ML techniques provide an ability to predict outcomes, some methodologies can remain unexplainable (‘black-box’) [26], such that mechanistic insights into the decision processes carried out by such models may not be evident. Others harness more interpretable methods e.g., random forests [29,30] or linear regression [31], where the most important predictors can be identified. For example, top predictors of live birth after IVF treatment included female partner age, anti-Müllerian hormone (AMH) [32], number of high-quality embryos, and serum estradiol level (reflective of cumulative follicle size and, in turn, the number of eggs that will be retrieved) on the day of administration of the trigger for oocyte maturation [33].
With the recent influx of literature surrounding the use of AI and ML in ART, there is a clear interest in the academic community on how such models can be used to improve treatment strategies in clinical workflows [34].
AI to support decision-making in in vitro fertilization
In vitro fertilization (IVF) is a complex procedure involving hormonal interventions to act upon specific processes during the treatment cycle. These include: 1. Ovarian stimulation [35], 2. Prevention of premature ovulation [36], 3. Induction of oocyte maturation [29,30], 4. Fertilization in vitro and embryo selection for transfer [21,23,24], to hopefully result in live birth [37]. The timings of these interventions can vary depending on the specific IVF protocol carried out by the clinician [38]. In the initial stages of IVF, preparations containing FSH are used to induce the growth of multiple ovarian follicles, whilst a GnRH antagonist, or continuous non-pulsatile administration of a GnRH agonist (which desensitizes the GnRH receptor), is used to prevent a premature LH surge and, in turn, untimely ovulation [38]. Once the follicles grow to the required size, a hormonal trigger, namely either human chorionic gonadotropin (hCG) or a GnRH agonist, is administered to provide LH-like exposure and induce oocyte maturation (i.e., oocytes attain the capacity for fertilization by losing half of their genetic material as the polar body) [38].
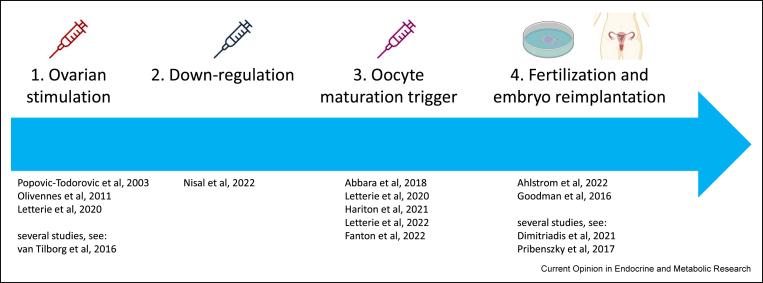
The vast amount of complex data generated before and during an IVF treatment cycle has the potential to be analysed more precisely and objectively using ML techniques. Consequently, there are several processes in the IVF cycle wherein decision-making can potentially benefit from AI pipelines (Figure 2), and have been explored in recent literature [39,40].
Figure 2.
Potential AI-based interventions during IVFtreatment. This pipeline outlines the processes carried out during IVF treatment cycles, where interventions using AI and ML techniques could be used to support clinical decision-making. The references provided at each stage indicate literature exploring efforts in quantitative modelling of these stages. The four stages in the figure above correspond to the numbered sections under ‘AI to support decision-making in in vitro fertilization’. Of the four stages presented, the first three pertain endocrinological interventions, where optimizations with respect to dose and timing are of value.
Selection of gonadotropin doses for ovarian stimulation
Quantitative modelling can aid in the selection of the appropriate dose of gonadotropins for ovarian stimulation as the ovarian response to the same dose can vary by baseline characteristics such as age and ovarian reserve (represented by AMH level [32] or antral follicle count [41]). There are several algorithms derived to estimate the optimal initial dose of FSH for ovarian stimulation taking into account baseline factors [42,43]. Studies using such algorithms, and other markers reflective of ovarian reserve [44, 45, 46, 47, 48], have been explored in a systematic review by van Tilborg et al. [49]. Excessive dosing can increase the risk of ovarian hyperstimulation syndrome (OHSS), whereas insufficient dosing can increase the risk of a suboptimal ovarian response [50]. Furthermore, a physician's reaction to an insufficient initial response with a subsequent increase in dose can increase variability in follicle sizes and hamper response to triggering oocyte maturation [35]. Therefore, using AI to optimize the initial dose, and subsequent dose adjustment [40], is likely to improve the success of treatment, although the extent of its impact on later outcomes (e.g., live birth rate) remains undetermined [50].
Prevention of premature ovulation
Accurate measurement of LH, FSH, estradiol (E2), and progesterone (P4) levels across the normal cycle facilitated the development of a mechanistic mathematical model of the human menstrual cycle [51], incorporating key interactions in the HPG axis. This model described how timing and dosing of GnRH analogues affect hormonal responses: reproducing clinical findings of Nafarelin (GnRH agonist) delaying ovulation when administered in the early follicular phase, while immediately triggering ovulation if administered in the late follicular phase [52]; and predicting that the length of the delay in ovulation after Cetrorelix (GnRH antagonist) administration in the follicular phase depends on the dose used [53].
Nagaraja et al. modelled the inhibitory effect of Cetrorelix (GnRH antagonist) on LH secretion, as well as the induced delay of the LH surge based on the pharmacokinetics of the drug [36,54,55]. Later mathematical models also incorporated mechanistic features of the HPG axis (such as feedback control from the gonads), hence providing a more complete description of the endocrine system and predicting the response to both GnRH agonists and antagonists [56].
Further, in the context of specifically using a GnRH antagonist for pituitary downregulation during IVF treatment cycles, Nisal et al. were able to present the potential application of a quantitative FSH dosing algorithm in a local pilot study [57]. There is scope for the dose and timing of GnRH antagonist to be personalized according to patient characteristics, using more sophisticated AI and ML techniques. Optimized approaches to dose and timing of downregulatory protocols have the potential to reduce costs whilst maintaining, or even improving, pregnancy outcomes as both over and under-suppression of endogenous LH levels can be deleterious.
Induction of oocyte maturation
The trigger to induce oocyte maturation is administered once follicles grow to the required size to be able to respond appropriately and yield oocytes. Typically, simple rules are used to guide the timing of this step, such as the presence of at least two to three follicles more than 17 or 18 mm in diameter. However, this approach assumes uniform growth of the follicles behind these lead follicles, rather than a more diverse set of follicle sizes [35]. By harnessing ML techniques such as bagged decision trees [58], random forests [30], and linear regression [31], found in the literature, the size of follicles on the day of trigger most likely to yield oocytes has been estimated, and indicates the potential to support the optimization of the timing of trigger administration during clinical workflows [39]. Identification of this follicle size range enables the quantification of oocyte maturation [29], and can provide a target for response to gonadotropins when evaluating response to ovarian stimulation. In essence, ML techniques have the potential to increase the precision, objectivity, and reproducibility of decision-making during IVF protocols.
Embryo selection for transfer
An example of complex data generated during IVF treatment is image analysis of embryos growing over several days assessed via time-lapse technology, which has the potential to aid in the selection of embryos that are most likely to implant. This represents a large amount of data which can be challenging and impractical for an embryologist to capture manually [21,22]. Additionally, prediction of outcomes based on oocyte quality has been attempted based on their morphology [59,60], texture [61, 62, 63], and morpho-kinetic [64] information. Furthermore, researchers have shown that the mechanical properties of human zygotes are predictive of embryo survival during the blastocyst stage, allowing one to predict within hours after fertilization whether the zygote will arrest with 90% precision [65]. However, the benefit of using AI technology in the embryo selection process has yet to be proven as superior to current means in double-blind randomized controlled trials [66,67], whereby no significant improvement was shown in clinical pregnancy rates when selecting day five blastocysts for transfer with a time-lapse algorithm. These studies highlight the necessity for the accuracy of predictions made via ML techniques to be prospectively tested and validated prior to adoption into clinical practice, with appropriate mitigations of study biases [68].
Conclusions
Quantitative models enable data-driven support in clinical decision-making. In the context of reproductive endocrinology, mechanistic mathematical models enable the analysis of hormone data and the effect of endocrine interventions, while ML models can facilitate outcome predictions in ART protocols.
Importantly, quantitative models enable us to move away from one-size-fits-all approaches and design patient-optimized protocols. Ultimately, this can reduce operational costs by improving the efficiency and efficacy of treatment to further enhance treatment outcomes, and reduce psychological morbidity associated with unsuccessful treatment. The use of AI in this context remains nascent, however, is expected to continue to burgeon with the inclusion of large diverse multi-center datasets to ensure model generalizability, undergo appropriate validation studies, as well as presenting viable integration into well-established clinical workflows [26].
Editorial disclosure
Given their role as Guest Editor, Krasimira Tsaneva-Atanasova and Margaritis Voliotis had no involvement in the peer review of the article and has no access to information regarding its peer-review. Full responsibility for the editorial process of this article was delegated to Karen Chapman.
Conflict of interest statement
Nothing declared.
Acknowledgements
MV and KTA acknowledge the financial support of the EPSRC via grants EP/T017856/1 and EP/N014391/1, and BBSRC via grants BB/S000550/1 and BB/S001255/1. SH is supported by the UKRI CDT in AI for Healthcare http://ai4health.io (Grant No. EP/S023283/1). AA is supported by an NIHR Clinician Scientist Award (CS-2018-18-ST2-002). WSD is supported by an NIHR Senior Investigator Award and the NIHR Imperial Biomedical Research Centre.
This review comes from a themed issue on Mathematical Modelling of Endocrine Systems
Edited by Craig McArdle, Krasimira Tsaneva-Atanasova and Margaritis Voliotis
Data availability
No data was used for the research described in the article.
References
- 1.Zavala E., Wedgwood K.C.A., Voliotis M., Tabak J., Spiga F., Lightman S.L., Tsaneva-Atanasova K. Mathematical modelling of endocrine systems. Trends Endocrinol Metabol. 2019;30:244–257. doi: 10.1016/j.tem.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zavala E., Voliotis M., Zerenner T., Tabak J., Walker J.J., Li X.F., Terry J.R., Lightman S.L., O'Byrne K., Tsaneva-Atanasova K. Dynamic hormone control of stress and fertility. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.598845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.T.M. Plant, and Anthony J. Zeleznik, Knobil and Neill's physiology of reproduction, Academic Press, 2014.
- 4.Stamatiades G.A., Kaiser U.B. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. doi: 10.1016/j.mce.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbara A., Dhillo W.S. Targeting elevated GnRH pulsatility to treat polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;106:e4275–e4277. doi: 10.1210/clinem/dgab422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayasena C.N., Abbara A., Veldhuis J.D., Comninos A.N., Ratnasabapathy R., De Silva A., Nijher G.M., Ganiyu-Dada Z., Mehta A., Todd C., Ghatei M.A., Bloom S.R., Dhillo W.S. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of Kisspeptin-54. J Clin Endocrinol Metab. 2014;99:E953–E961. doi: 10.1210/jc.2013-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phylactou M., Clarke S.A., Patel B., Baggaley C., Jayasena C.N., Kelsey T.W., Comninos A.N., Dhillo W.S., Abbara A. Clin Endocrinol (Oxf); 2020. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan D.M., Veldhuis J.D. Pulsatility of hypothalamo-pituitary hormones: a challenge in quantification. Physiology. 2016;31:34–50. doi: 10.1152/physiol.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gives an overview of the challenges involved in quantifying hormonal dynamics.
- 9.Veldhuis J.D., Johnson M.L. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 10.Vidal A., Zhang Q., Medigue C., Fabre S., Clement F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granqvist E., Hartley M., Morris R.J. BaSAR-A tool in R for frequency detection. Biosystems. 2012;110:60–63. doi: 10.1016/j.biosystems.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan D.M., Veldhuis J.D., Yang R. Joint recovery of pulsatile and basal hormone secretion by stochastic nonlinear random-effects analysis. Am J Physiol. 1998;275:R1939–R1949. doi: 10.1152/ajpregu.1998.275.6.R1939. [DOI] [PubMed] [Google Scholar]
- Johnson T.D. Bayesian deconvolution analysis of pulsatile hormone concentration profiles. Biometrics. 2003;59:650–660. doi: 10.1111/1541-0420.00075. [DOI] [PubMed] [Google Scholar]; Illustrates how novel measurment technologies and quantification algorithms can facillitate continuous profiling of hormones improving the the diagnosis and treatment of patients with endocrine disorders.
- 14.Liang S., Kinghorn A.B., Voliotis M., Prague J.K., Veldhuis J.D., Tsaneva-Atanasova K., McArdle C.A., Li R.H.W., Cass A.E.G., Dhillo W.S., Tanner J.A. Measuring luteinising hormone pulsatility with a robotic aptamer-enabled electrochemical reader. Nat Commun. 2019;10:852. doi: 10.1038/s41467-019-08799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prague J.K., Voliotis M., Clarke S., Comninos A.N., Abbara A., Jayasena C.N., Roberts R.E., Yang L., Veldhuis J.D., Tsaneva-Atanasova K., McArdle C.A., Dhillo W.S. Determining the relationship between hot flushes and LH pulses in menopausal women using mathematical modeling. J Clin Endocrinol Metab. 2019;104:3628–3636. doi: 10.1210/jc.2018-02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson T.D. Analysis of pulsatile hormone concentration profiles with nonconstant Basal concentration: a bayesian approach. Biometrics. 2007;63:1207–1217. doi: 10.1111/j.1541-0420.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 17.Carlson N.E., Grunwald G.K., Johnson T.D. Using Cox cluster processes to model latent pulse location patterns in hormone concentration data. Biostatistics. 2016;17:320–333. doi: 10.1093/biostatistics/kxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson N.E., Johnson T.D., Brown M.B. A Bayesian approach to modeling associations between pulsatile hormones. Biometrics. 2009;65:650–659. doi: 10.1111/j.1541-0420.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voliotis M., Abbara A., Prague J.K., Veldhuis J.D., Dhillo W.S., Tsaneva-Atanasova K. HormoneBayes: a novel Bayesian framework for the analysis of pulsatile hormone dynamics. medRxiv. 2022 doi: 10.1101/2022.03.14.22272000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkomar A., Dean J., Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347–1358. doi: 10.1056/NEJMra1814259. [DOI] [PubMed] [Google Scholar]
- 21.Dimitriadis I., Zaninovic N., Badiola A.C., Bormann C.L. Artificial intelligence in the embryology laboratory: a review. Reprod Biomed Online. 2022;44:435–448. doi: 10.1016/j.rbmo.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Wang R., Pan W., Jin L., Li Y., Geng Y., Gao C., Chen G., Wang H., Ma D., Liao S. Artificial intelligence in reproductive medicine. Reproduction. 2019;158:R139–R154. doi: 10.1530/REP-18-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough review of machine learning approaches to assisted reproductive technology.
- 23.Fernandez E.I., Ferreira A.S., Cecilio M.H.M., Cheles D.S., de Souza R.C.M., Nogueira M.F.G., Rocha J.C. Artificial intelligence in the IVF laboratory: overview through the application of different types of algorithms for the classification of reproductive data. J Assist Reprod Genet. 2020;37:2359–2376. doi: 10.1007/s10815-020-01881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riegler M.A., Stensen M.H., Witczak O., Andersen J.M., Hicks S.A., Hammer H.L., Delbarre E., Halvorsen P., Yazidi A., Holst N., Haugen T.B. Artificial intelligence in the fertility clinic: status, pitfalls and possibilities. Hum Reprod. 2021;36:2429–2442. doi: 10.1093/humrep/deab168. [DOI] [PubMed] [Google Scholar]
- 25.Prior F., Almeida J., Kathiravelu P., Kurc T., Smith K., Fitzgerald T.J., Saltz J. Open access image repositories: high-quality data to enable machine learning research. Clin Radiol. 2020;75:7–12. doi: 10.1016/j.crad.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiens J., Saria S., Sendak M., Ghassemi M., Liu V.X., Doshi-Velez F., Jung K., Heller K., Kale D., Saeed M., Ossorio P.N., Thadaney-Israni S., Goldenberg A. Do no harm: a roadmap for responsible machine learning for health care. Nat Med. 2019;25:1337–1340. doi: 10.1038/s41591-019-0548-6. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann S.J., Eastaugh J.L., Snowden S., Smye S.W., Sharma V. The application of neural networks in predicting the outcome of in-vitro fertilization. Hum Reprod. 1997;12:1454–1457. doi: 10.1093/humrep/12.7.1454. [DOI] [PubMed] [Google Scholar]
- 28.Guvenir H.A., Misirli G., Dilbaz S., Ozdegirmenci O., Demir B., Dilbaz B. Estimating the chance of success in IVF treatment using a ranking algorithm. Med Biol Eng Comput. 2015;53:911–920. doi: 10.1007/s11517-015-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbara A., Hunjan T., Ho V.N.A., Clarke S.A., Comninos A.N., Izzi-Engbeaya C., Ho T.M., Trew G.H., Hramyka A., Kelsey T., Salim R., Humaidan P., Vuong L.N., Dhillo W.S. Endocrine requirements for oocyte maturation following hCG, GnRH agonist, and kisspeptin during IVF treatment. Front Endocrinol (Lausanne) 2020;11 doi: 10.3389/fendo.2020.537205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbara A., Vuong L.N., Ho V.N.A., Clarke S.A., Jeffers L., Comninos A.N., Salim R., Ho T.M., Kelsey T.W., Trew G.H., Humaidan P., Dhillo W.S. Follicle size on day of trigger most likely to yield a mature oocyte. Front Endocrinol (Lausanne) 2018;9:193. doi: 10.3389/fendo.2018.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanton M., Nutting V., Solano F., Maeder-York P., Hariton E., Barash O., Weckstein L., Sakkas D., Copperman A.B., Loewke K. An interpretable machine learning model for predicting the optimal day of trigger during ovarian stimulation. Fertil Steril. 2022;118:101–108. doi: 10.1016/j.fertnstert.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Nelson S.M., Yates R.W., Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles--implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 33.Hafiz P., Nematollahi M., Boostani R., Namavar Jahromi B. Predicting implantation outcome of in vitro fertilization and intracytoplasmic sperm injection using data mining techniques. Int J Fertil Steril. 2017;11:184–190. doi: 10.22074/ijfs.2017.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curchoe C.L., Bormann C.L. Artificial intelligence and machine learning for human reproduction and embryology presented at ASRM and ESHRE 2018. J Assist Reprod Genet. 2019;36:591–600. doi: 10.1007/s10815-019-01408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbara A., Patel A., Hunjan T., Clarke S.A., Chia G., Eng P.C., Phylactou M., Comninos A.N., Lavery S., Trew G.H., Salim R., Rai R.S., Kelsey T.W., Dhillo W.S. FSH requirements for follicle growth during controlled ovarian stimulation. Front Endocrinol (Lausanne) 2019;10:579. doi: 10.3389/fendo.2019.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pechstein B., Nagaraja N.V., Hermann R., Romeis P., Locher M., Derendorf H. Pharmacokinetic-pharmacodynamic modeling of testosterone and luteinizing hormone suppression by cetrorelix in healthy volunteers. J Clin Pharmacol. 2000;40:266–274. doi: 10.1177/00912700022008937. [DOI] [PubMed] [Google Scholar]
- 37.McLernon D.J., Steyerberg E.W., Te Velde E.R., Lee A.J., Bhattacharya S. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ. 2016;355:i5735. doi: 10.1136/bmj.i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbara A., Clarke S.A., Dhillo W.S. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39:593–628. doi: 10.1210/er.2017-00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterie G., MacDonald A., Shi Z. An artificial intelligence platform to optimize workflow during ovarian stimulation and IVF: process improvement and outcome-based predictions. Reprod Biomed Online. 2022;44:254–260. doi: 10.1016/j.rbmo.2021.10.006. [DOI] [PubMed] [Google Scholar]; Presents a machine learning algorithm designed to improve workflow during IVF, including optimizing visits to the clinic for minimal monitoring requirements.
- 40.Letterie G., Mac Donald A. Artificial intelligence in in vitro fertilization: a computer decision support system for day-to-day management of ovarian stimulation during in vitro fertilization. Fertil Steril. 2020;114:1026–1031. doi: 10.1016/j.fertnstert.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Broekmans F.J., Verweij P.J., Eijkemans M.J., Mannaerts B.M., Witjes H. Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2014;29:1688–1697. doi: 10.1093/humrep/deu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivennes F., Howies C.M., Borini A., Germond M., Trew G., Wikland M., Zegers-Hochschild F., Saunders H., Alam V. Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod Biomed Online. 2011;22(Suppl 1):S73–S82. doi: 10.1016/S1472-6483(11)60012-6. [DOI] [PubMed] [Google Scholar]
- 43.Popovic-Todorovic B., Loft A., Bredkjaeer H.E., Bangsboll S., Nielsen I.K., Andersen A.N. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a 'standard' dose of 150 IU/day in 'standard' patients undergoing IVF/ICSI treatment. Hum Reprod. 2003;18:2275–2282. doi: 10.1093/humrep/deg472. [DOI] [PubMed] [Google Scholar]
- 44.Lefebvre J., Antaki R., Kadoch I.J., Dean N.L., Sylvestre C., Bissonnette F., Benoit J., Menard S., Lapensee L. 450 IU versus 600 IU gonadotropin for controlled ovarian stimulation in poor responders: a randomized controlled trial. Fertil Steril. 2015;104:1419–1425. doi: 10.1016/j.fertnstert.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Klinkert E.R., Broekmans F.J., Looman C.W., Habbema J.D., te Velde E.R. Expected poor responders on the basis of an antral follicle count do not benefit from a higher starting dose of gonadotrophins in IVF treatment: a randomized controlled trial. Hum Reprod. 2005;20:611–615. doi: 10.1093/humrep/deh663. [DOI] [PubMed] [Google Scholar]
- 46.Jayaprakasan K., Hopkisson J., Campbell B., Johnson I., Thornton J., Raine-Fenning N. A randomised controlled trial of 300 versus 225 IU recombinant FSH for ovarian stimulation in predicted normal responders by antral follicle count. BJOG. 2010;117:853–862. doi: 10.1111/j.1471-0528.2010.02545.x. [DOI] [PubMed] [Google Scholar]
- 47.Harrison R.F., Jacob S., Spillane H., Mallon E., Hennelly B. A prospective randomized clinical trial of differing starter doses of recombinant follicle-stimulating hormone (follitropin-beta) for first time in vitro fertilization and intracytoplasmic sperm injection treatment cycles. Fertil Steril. 2001;75:23–31. doi: 10.1016/s0015-0282(00)01643-5. [DOI] [PubMed] [Google Scholar]
- 48.Berkkanoglu M., Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders? Fertil Steril. 2010;94:662–665. doi: 10.1016/j.fertnstert.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 49.van Tilborg T.C., Broekmans F.J., Dolleman M., Eijkemans M.J., Mol B.W., Laven J.S., Torrance H.L. Individualized follicle-stimulating hormone dosing and in vitro fertilization outcome in agonist downregulated cycles: a systematic review. Acta Obstet Gynecol Scand. 2016;95:1333–1344. doi: 10.1111/aogs.13032. [DOI] [PubMed] [Google Scholar]
- 50.Broekmans F.J. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne) 2019;10:181. doi: 10.3389/fendo.2019.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblitz S., Stotzel C., Deuflhard P., Jones H.M., Azulay D.O., van der Graaf P.H., Martin S.W. A mathematical model of the human menstrual cycle for the administration of GnRH analogues. J Theor Biol. 2013;321:8–27. doi: 10.1016/j.jtbi.2012.11.020. [DOI] [PubMed] [Google Scholar]; Presents a comprhensive model of the human menstrual cycle that predicts hormonal changes following administration of GnRH analogues.
- 52.Monroe S.E., Henzl M.R., Martin M.C., Schriock E., Lewis V., Nerenberg C., Jaffe R.B. Ablation of folliculogenesis in women by a single dose of gonadotropin-releasing hormone agonist: significance of time in cycle. Fertil Steril. 1985;43:361–368. doi: 10.1016/s0015-0282(16)48432-3. [DOI] [PubMed] [Google Scholar]
- 53.Duijkers I.J., Klipping C., Willemsen W.N., Krone D., Schneider E., Niebch G., Hermann R. Single and multiple dose pharmacokinetics and pharmacodynamics of the gonadotrophin-releasing hormone antagonist Cetrorelix in healthy female volunteers. Hum Reprod. 1998;13:2392–2398. doi: 10.1093/humrep/13.9.2392. [DOI] [PubMed] [Google Scholar]
- 54.Neal-Perry G., Nejat E., Dicken C. The neuroendocrine physiology of female reproductive aging: an update. Maturitas. 2010;67:34–38. doi: 10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagaraja N.V., Pechstein B., Erb K., Klipping C., Hermann R., Niebch G., Derendorf H. Pharmacokinetic and pharmacodynamic modeling of cetrorelix, an LH-RH antagonist, after subcutaneous administration in healthy premenopausal women. Clin Pharmacol Ther. 2000;68:617–625. doi: 10.1067/mcp.2000.111481. [DOI] [PubMed] [Google Scholar]
- 56.Tornoe C.W., Agerso H., Senderovitz T., Nielsen H.A., Madsen H., Karlsson M.O., Jonsson E.N. Population pharmacokinetic/pharmacodynamic (PK/PD) modelling of the hypothalamic-pituitary-gonadal axis following treatment with GnRH analogues. Br J Clin Pharmacol. 2007;63:648–664. doi: 10.1111/j.1365-2125.2006.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nisal A., Diwekar U., Hobeika E. Personalized medicine for GnRH antagonist protocol in in vitro fertilization procedure using modeling and optimal control. Comput Chem Eng. 2022;156 doi: 10.1016/j.jtbi.2019.110105. [DOI] [PubMed] [Google Scholar]
- 58.Hariton E., Chi E.A., Chi G., Morris J.R., Braatz J., Rajpurkar P., Rosen M. A machine learning algorithm can optimize the day of trigger to improve in vitro fertilization outcomes. Fertil Steril. 2021;116:1227–1235. doi: 10.1016/j.fertnstert.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Faramarzi A., Khalili M.A., Omidi M. Morphometric analysis of human oocytes using time lapse: does it predict embryo developmental outcomes? Hum Fertil (Camb) 2019;22:171–176. doi: 10.1080/14647273.2017.1406670. [DOI] [PubMed] [Google Scholar]
- 60.Omidi M., Faramarzi A., Agharahimi A., Khalili M.A. Noninvasive imaging systems for gametes and embryo selection in IVF programs: a review. J Microsc. 2017;267:253–264. doi: 10.1111/jmi.12573. [DOI] [PubMed] [Google Scholar]
- 61.Saeedi P., Yee D., Au J., Havelock J. Automatic identification of human blastocyst components via texture. IEEE Trans Biomed Eng. 2017;64:2968–2978. doi: 10.1109/TBME.2017.2759665. [DOI] [PubMed] [Google Scholar]
- 62.Santos Filho E., Noble J.A., Poli M., Griffiths T., Emerson G., Wells D. A method for semi-automatic grading of human blastocyst microscope images. Hum Reprod. 2012;27:2641–2648. doi: 10.1093/humrep/des219. [DOI] [PubMed] [Google Scholar]
- 63.Singh A., Au J., Saeedi P., Havelock J. Automatic segmentation of trophectoderm in microscopic images of human blastocysts. IEEE Trans Biomed Eng. 2015;62:382–393. doi: 10.1109/TBME.2014.2356415. [DOI] [PubMed] [Google Scholar]
- 64.Storr A., Venetis C., Cooke S., Kilani S., Ledger W. Time-lapse algorithms and morphological selection of day-5 embryos for transfer: a preclinical validation study. Fertil Steril. 2018;109:276–283 e3. doi: 10.1016/j.fertnstert.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 65.Yanez L.Z., Han J., Behr B.B., Pera R.A.R., Camarillo D.B. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun. 2016;7 doi: 10.1038/ncomms10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahlström A., Lundin K., Lind A.K., Gunnarsson K., Westlander G., Park H., Thurin-Kjellberg A., Thorsteinsdottir S.A., Einarsson S., Astrom M., Lofdahl K., Menezes J., Callender S., Nyberg C., Winerdal J., Stenfelt C., Jonassen B.R., Oldereid N., Nolte L., Sundler M., Hardarson T. Vol. 37. Hum Reprod; 2022. A double-blind randomized controlled trial investigating a time-lapse algorithm for selecting Day 5 blastocysts for transfer; pp. 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodman L.R., Goldberg J., Falcone T., Austin C., Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275–285 e10. doi: 10.1016/j.fertnstert.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Pribenszky C., Nilselid A.M., Montag M. Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta-analysis. Reprod Biomed Online. 2017;35:511–520. doi: 10.1016/j.rbmo.2017.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.