Abstract
Scaffold-based approach is a developed strategy in biomanufacturing, which is based on the use of temporary scaffold that performs as a house of implanted cells for their attachment, proliferation, and differentiation. This strategy strongly depends on both materials and manufacturing processes. However, it is very difficult to meet all the requirements, such as biocompatibility, biodegradability, mechanical strength, and promotion of cell-adhesion, using only single material. At present, no single bioprinting technique can meet the requirements for tissue regeneration of all scales. Thus, multi-material and mixing-material scaffolds have been widely investigated. Challenges in terms of resolution, uniform cell distribution, and tissue formation are still the obstacles in the development of bioprinting technique. Hybrid bioprinting techniques have been developed to print scaffolds with improved properties in both mechanical and biological aspects for broad biomedical engineering applications. In this review, we introduce the basic multi-head bioprinters, semi-hybrid and fully-hybrid biomanufacturing systems, highlighting the modifications, the improved properties and the effect on the complex tissue regeneration applications.
Keywords: Additive biomanufacturing, Hybrid bioprinter, Tissue regeneration, Scaffolds
1. Introduction
Tissue engineering is the most promising approach that could alleviate the shortage of organ donors as it aims for the development of biological substitutes that restore, maintain, or improve tissue function through an interdisciplinary effort between biologists, engineers, material scientists, and clinicians[1]. Scaffold-based approach is the main strategy in tissue engineering, which is based on the development of a temporal support structure (namely, the scaffold) to provide a suitable environment for tissue formation and regeneration. In this approach, scaffolds play a key role in enabling and modulating the cell behavior and enabling diffusion of nutrients and oxygen. Fundamental criteria in scaffold design to be considered include biocompatibility, biodegradability or bioresorbability, mechanical properties, scaffold architecture, and biomaterial selection[2,3].
Additive biomanufacturing (AB) provides the capability to rapidly produce complex three-dimensional (3D) structures with precision and reproducibility that exhibit functional gradients, multiple materials, and exquisite control over pore size distribution within the scaffold[4]. The advantages of additive manufacturing (AM) within tissue engineering have facilitated the fabrication of biomimetic and complex structures that more accurately reflect the in vivo environment. AM enables the precise spatial deposition of biological materials, viable cells, and biochemicals to fabricate 3D biological structures[5]. AB technologies have found extensive applications in the biomedical area, including skin (for example, full-thickness skin substitutes or wound dressings)[6], orthopedic[7], dental[8], osteochondral[9], cardiovascular[10], and other soft-tissue engineering applications (e.g., pancreas and liver)[10]. However, the complexity of tissues and organs prohibits the use of a single technique due to limitations inherent to each AM technology (e.g., relatively low printing resolution and limited material applicability of specific technologies). For these reasons, hybrid biomanufacturing techniques, combining different techniques and post-processes, have been developed and subsequently applied in various fields during the last 5 years. In this review, we discuss the basic types of AB, advanced hybrid biosystems, and current and future applications.
2. Additive biomanufacturing techniques
The process for material fabrication in AB includes the following steps: raw material preparation, computer-aided scaffold structure design and lay down pattern definition, program and G code generation by the control software, material deposition and if necessary, post-processing followed by extensive morphological, mechanical, and biological characterizations for validation. According to ASTM standards, AB techniques can be classified in three main categories: material jetting, material extrusion, and vat polymerization (Figure 1).
Figure 1.

Three main categories in additive biomanufacturing, including material jetting, (namely (A) inkjet bioprinting and (B) laser-assisted bioprinting), (C) material extrusion, and (D) vat photo polymerization.
(i) Material jetting refers to the technologies in which droplets of build and support materials are selectively jetted onto the build platform and cured by either ultraviolet (UV) light or heat to form a 3D object. Material jetting processes for biomanufacturing include inkjet bioprinting[11] and laser-assisted bioprinting[12].
(ii) Material extrusion refers to AM technique that uses a continuous filament of thermoplastic or composite material to construct 3D parts. Three main extrusion types have been widely used for biomanufacturing, including pressure-assisted material extrusion, piston-assisted material extrusion, and screw-assisted material extrusion[13].
(iii) Vat photopolymerization refers to the UV curing of materials using a light source during the printing process[14], including three main categories, namely, stereolithography (SLA)[15], digital light processing (DLP),[16] and two-photon polymerization (2PP)[17].
These processes allow a wide range of biomaterials to be deposited on the build platform by applying compressed air, light photopolymerization, thermal or mechanical effects. Table 1 summarizes the advantages and disadvantages of each technique.
Table 1.
Additive biomanufacturing techniques and their main characteristics.
| AB techniques | Material jetting | Material extrusion | Vat photo polymerization | |
|---|---|---|---|---|
|
| ||||
| Inkjet bioprinting | Laser-assisted bioprinting | |||
| Advantages | • High deposition resolution; • High printing accuracy • Low cost |
• Higher printing resolution (resolution: micron level) • High-throughput printing (up to 5000 droplets deposited per second) • Able to achieve in situ printing. |
• Large variety of material types and viscosities | • Superior resolution in the nanoscale region (~100 nm) • Highly complex surface topology • Hierarchical structures and high-resolution cell patterning |
|
| ||||
| Disadvantages | • Nozzle clogging • Low bioink viscosity • High possibility of cell sedimentation |
• Limited productivity and printing efficiency • High cost • Small number of biomaterials that can be transferred in each laser pulse |
• Nozzle clogging • Low printing resolution • Low cell viability due to pressure drop |
• Cell sedimentation effect resulting in poor cell homogeneity • High cost |
2.1. Material jetting
2.1.1. Inkjet bioprinting
Inkjet bioprinting, also known as drop-on-demand (DOD) printing, uses a cartridge of a biomaterial solution and cells (bioink) to precisely deposit small droplets (20 – 50 μm) to build up a multi-layer structure in a predesigned printing process. The size of the printed droplets is controlled by the pressure pulses generated by a thermal or piezoelectric actuator[11].
The thermal inkjet-based systems use a rapid temperature increase controlled by a heating element (ink temperature increase of up to ~300°C in a few μs[18] to generate vapor bubbles as the driving force to eject ink droplets onto the substrate[19]. The main concern associated with thermal inkjet is the negative effect of the high temperatures during printing on the survival of the biomolecules and cells to be printed[20]. However, it has been evaluated and confirmed that the short heating pulse (~2 μs) only increase a few degrees in temperature and not have a significant effect on cell viability[21]. Piezoelectric inkjet systems, based on the electromechanical system (MEMS), use the pressure increase generated by applying a piezoelectric change[22]. Both methods present poor ability to process bioinks with high density of cells, as cells tend to settle at the bottom of cartridge during the printing process, resulting in the clogging of the print head and inhomogeneous distribution of cells[23]. This may require cell agitators to be installed in the cartridge to prevent cell sedimentation[24]. Moreover, the thermal and shear stress during the biomanufacturing process also negatively affect the cell viability. Due to this attribute, this technique is only limited to materials with low viscosity (<10 mPa·s) and low cell concentration (<106 cells mL-1)[25], and as a result, the structures produced using this technique has relatively low mechanical strength.
The main advantages of inkjet printing include high deposition resolution with a controlled droplet size that can be adjusted to about the size of one cell (~10 μm), and high printing accuracy that can be tailored to <100 μm[13], allowing the printing of complex scaffolds[26]. Furthermore, the cost is lower and several print heads can be concurrently used to print multiple cell types in one construct[27].
2.1.2. Laser-assisted bioprinting
The first laser-based printing system, which was based on the laser-induced forward transfer (LIFT) effect and used a near-infrared laser to conduct cell patterning, was developed by Odde and Renn (1999)[28], and this technology was named laser-guided direct writing (LGDW). LGDW uses a laser source (from ultrafast pulsed lasers to continuous wave lasers) to enable the addition, removal, and modification of target materials in a layer-by-layer mode for tissue construct fabrication by transferring a bioink or cell suspension from a donor substrate onto a build platform[29]. In LGDW applied for tissue engineering, the bioink, such as cells in solution or hydrogels, is coated to the underside of the laser absorption substrate[30]. The laser excites the absorption layer and causes vaporization and microbubble formation within the cell suspension or hydrogel. This is used to eject a small droplet onto the parallel substrate in a predefined path[31]. The LGDW technique was subsequently developed into two types, namely, matrix-assisted pulsed laser evaporation direct writing (MAPLE-DW) and biological laser printing (BioLP)[32].
MAPLE-DW uses low powered pulse laser (usually near-UV wavelengths) to directly interact with a donor slide of matrix material, consisting of sacrificial water-based polymer, to achieve high light absorption, and energy transfer. However, MAPLE-DW limits in resolution and reproducibility[33]. To overcome the limitations, BioLP was developed by Barron et al. utilizing a three-layer approach by including a laser absorption interlayer (thickness: 75 – 100 nm) to prevent the laser direct interaction with the bioink[34], which improves the reproducibility of the droplet ejection and makes BioLP more efficient than other laser-assisted biomanufacturing techniques.
Laser-assisted biomanufacturing techniques have been widely applied in tissue regeneration due to its advantages in high printing resolution, high reproducibility, and feasibility for multi-biomaterial and biomaterial/cell mixtures processing[35]. Bourget et al. used LGDW to print human umbilical vein endothelial cells (HUVECs) and human bone mesenchymal stem cells (HBMSC) on to a collagen hydrogel substrate[36]. This study showed that LIFT allows easy patterning of cells on a collagen matrix, and endothelial cells tend to migrate randomly without HBMSCs but would stay within the printing lines when in co-culture. Moreover, Keriqual et al. (2017) conducted in situ LIFT to print mesenchymal stromal cells for bone tissue regeneration[37]. The in vivo results revealed that regeneration is homogeneous throughout the defect and long-term cell viability and proliferation was maintained up to 42 days post implantation.
The higher cell viability achieved by laser-based biomanufacturing, as compared with that in inkjet printing and material extrusion, is due to non-contact between the dispensing head and bioink. Furthermore, laser-based biomanufacturing is capable of high-viscosity bioink deposition and high printing resolution, allowing complex patterning and fabrication of structures that mimic the complexity of tissues and organs. However, despite all the above advantages, laser-based biomanufacturing shows great capabilities for cell patterning but has difficulty in building large 3D structures, especially in the vertical direction[38]. The equipment used in laser-based biomanufacturing is also more expensive due to the complexity of installing lasers and optics. Moreover, the heat generated from laser or light energy will negatively influence the cell survival, proliferation and degradation in the tissue constructs[39]. The introduction of new biomaterials to create crosslinked composite materials is beneficial for cell survival, which may help the development of laser-based biomanufacturing[40].
2.2. Material extrusion
Material extrusion is a versatile technique that operates with materials in the form of filaments, pellets, pastes, and solutions. Different methods, such as pneumatic (pressure), mechanical (piston), and screw-assisted systems, are currently being used in material extrusion[13]. These printing methods allow for the processing of a wide range of biomaterials and compositions, including polymer-ceramic composites, polymer-carbon nanomaterial composites, hydrogels, and bioinks containing cells and growth factors[41-43].
Pneumatic deposition process is simple and effective but can be slow due to the limited force applied to highly viscous material, which results in long printing time, presenting limitations in precisely controlling the volume of material deposited, especially during the lag time when the pressure is switched on and off. Also, the long printing time for high viscous material can reduce cell viability in the bioink. The limitation factor of the mechanical assisted extruders is related to the shear stress-induced deformation during the deposition process, which has a negative effect on cell viability[44]. High temperatures are typically required to process high-viscosity polymers using standard extrusion methods, and subsequently, screw-assisted extrusion, which can be termed precision extruding deposition (PED). PED was initially developed by Bellini et al. at Drexel University[45]. The system consists of a rotational screw, driven by stepper motors, to mix and force the material out of a nozzle. Almeida et al. (2010) improved this design by including an additional temperature-controlled material chamber in which the material can be liquefied and fed into the screw chamber using air pressure[46]. High-viscosity materials are more suitable to be processed with this technique using lower temperatures, which are less likely to induce material degradation in the polymer[47]. For example, a polycaprolactone (PCL)-graphene composite scaffold has been printed using screw-assisted extrusion, which has proven that it is difficult to extrude using pneumatic or piston-assisted extrusion with high speed and high precision[48].
The availability of a large variety of material types and viscosities, such as high-viscosity biomaterials and bioinks with high cell densities, that can be used in extrusion-based biomanufacturing is an apparent advantage of using the technique[49]. However, a big drop in pressure, especially for the screw-based bioextrusion method, can be harmful for the cell viability due to the disruption of the cell membranes[50]. Moreover, other drawbacks also include the potential risk of nozzle clogging and relatively low printing resolution (200 – 1000 μm)[51].
2.3. Vat photopolymerization (VP)
VP uses photopolymerization to cure the liquid ink hosted in a vat into a volumetric construct in a layer-by-layer manner[14]. It comprises mainly three categories:
(i) SLA[15]. As the primary technique of VP printing, SLA uses a laser beam that sweeps around to polymerize single lines of the ink to complete each layer in a raster scan mode. In the process of SLA printing, a series of UV light patterns are projected onto a vat containing the liquid ink to achieve photocrosslinking across the build volume.
(ii) DLP[16]. In DLP printing, a projector based on the digital micromirror device (DMD) or liquid crystal display (LCD) serves as the source to project an entire two-dimensional (2D) pattern for each layer, making the printing much quicker comparing to SLA.
(iii) 2PP[17]. 2PP utilizes the two-photon absorption of near-infrared (NIR) light to excite the same energy transition as UV photons. The polymerization process occurs when a molecule is excited from the ground state to the excited state by absorbing two photons for an extremely short time interval.
VP-based bioprinting process has been widely applied for tissue engineering due to its superior resolution and accuracy, which is up to 20 m in SLA and DLP and up to sub-100 nm region in 2PP. This enables fabrication of biomimetic microenvironment that closely emulates the complex extracellular matrices in native tissues, especially in creating surface topology[52], hierarchical structures[53], and high-resolution cell patterning[54].
Lee et al.[55] used an SLA system to fabricate neural scaffolds for nerve defects repair. The scaffolds contain encapsulated poly (lactic-co-glycolic acid) (PLGA) nanoparticles with nerve growth factors, and the results showed an enhancement in the neurite regeneration and improvement in the cell adhesion. DLP bioprinting has been used to fabricate nerve guidance conduits with micro-channels and the optimal elastic modulus is competitive to that of the native nerves, which is in the range of 0.5 – 13 MPa[56]. In addition to the applications in nerve repair, VP-based printing approaches have been used to fabricate synthetic bone scaffolds with similar mechanical properties as the native bones (0.22 – 10.44 MPa)[57], presenting highly complex nanoscale structures. Furthermore, VP-based bioprinting techniques have the potential to overcome the limitations in skin generation by fabricating high-resolution surface topography, for example, the physiological-relevant geometrical patterns to mimic the mesenchymal and epithelial compartmentalization for the regeneration of hair and other skin appendages[58]. Although the VP-based printing techniques have been well-established, due to the limited availability of biocompatible materials, the application of this technique in tissue engineering is still limited. With the development of bio-resin and improvement in the design complexity, it is promising for wider biomedical applications.
3. Classifications of current biomanufacturing systems
Tissue engineering aims for the repair and regeneration of tissues and organs through the facilitation of guided cellular growth and regeneration. A key aim in biomanufacturing is to fabricate a structure that can mimic the complexity and multi-material architecture of the native tissue. A single biomanufacturing technique or a single material-based construct cannot realize the requirements for fabricating an ideal tissue construct, thus requiring the improvements and combination of printing technologies as well as the development of advanced materials. An ideal bioprinter should satisfy the following requirements: high resolution, high-viscosity material processing, fabrication of complex printed structures, high cell viability, multiple biomaterial processing techniques, affordability, and ease of operation. Several biomanufacturing systems have been developed for producing advanced hybrid tissue constructs with improved properties. These sophisticated biomanufacturing systems can be divided into different categories due to the specific mechanisms incorporated:
(i) Basic multi-head biomanufacturing systems (BMBSs) (Figure 2A) use multiple printing techniques of the same type, such as pneumatic extrusion or inkjet. BMBSs have multi-head dispensing unit to selectively deposit different biomaterials and moving in Z-direction, with the build platform moving in the X-Y plane.
(ii) Semi-hybrid multi-head biomanufacturing systems (SMBSs) (Figure 2B) are similar to BMBSs, but combine different types of material extrusion techniques, such as pneumatic extrusion, piston extrusion, and screw-assisted extrusion. Hence, many types of biomaterials, including biomaterials of low viscosity and high viscosity, can be processed by the SMBSs.
(iii) Fully-hybrid biomanufacturing systems (FBSs) (Figure 2C) combine different AM techniques, electrospinning, or post-processing techniques within the same system. This enables the fabrication of functionalized, complex tissue constructs of not only multi-materials, but also in multi-scales.
Figure 2.
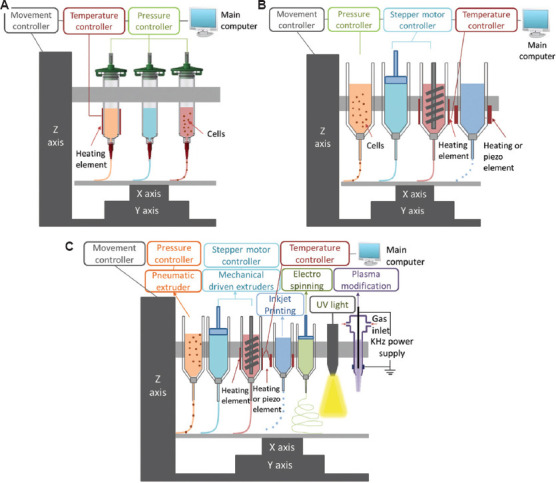
Categories of hybrid biomanufacturing systems, including (A) basic multi-head biomanufacturing systems (BMBSs), (B) semi-hybrid multi-head biomanufacturing systems (SMBSs), and (C) fully-hybrid biomanufacturing systems (FBSs).
Although there is no specific consensus on the definition of the term “hybrid processes,” researchers have explored a number of approaches to combine different AM processes and post-processing processes with the similar objectives of improving the complexity, functionality, and performance of printed tissue constructs. According to the above-mentioned definition, BMBSs are not hybrid biomanufacturing systems since they are using the same biomanufacturing techniques, while SMBSs and FBSs are hybrid biomanufacturing systems due to the incorporation of different AB techniques and post-processing techniques.
3.1. BMBSs
Various multi-head biomanufacturing systems have been under development to dispense multiple biomaterials with different viscosities, and thus multiple biomaterials, cell types or bioactive factors can be deposited within the same structure, which opens the route for multi-material and multi-functional scaffold fabrication. Key systems are reviewed here.
NovoGen MMX 3D Bioprinter™ (Organovo, San Diego, USA) (Figure 3A) is the first commercial extrusion-based 3D printing system applied in the bioengineering field. This machine was first created by Norotte et al.[59]. The system comprises two dispensing pumps with nozzles (i.e., micropipe) and contains temperature-controlled heating and cooling modes. This system ensures reproducibility of bioprinted tissues and cell-loaded scaffolds through high-precision controlling of the composition of tissue and the geometry; for example, small-diameter vascular reconstruction (outside diameter: 0.9 – 2.5 mm) has been performed using this system[59]. About 5% Gelatin methacryloyl (GelMA) hydrogel cylinders have also been printed by this system to induce ontogenesis for vascularized bone tissue regeneration[60]. Langer et al. (2019) used the NovoGen MMX 3D bioprinter to print scaffold-free tumor tissues with cancerous cells and several stromal cell types to aid in the in vitro modeling of cancer in three dimensions[61]. With this system, they produced multi-cell-type tissues and demonstrated that these bioprinted tissues can mimic aspects of in vivo neoplastic tissues.
Figure 3.
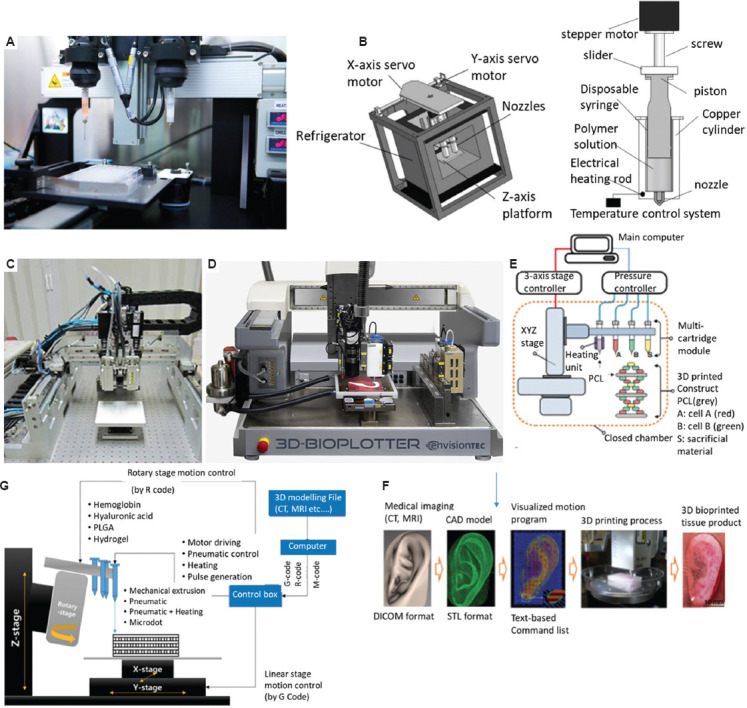
(A) The NovoGen MMX 3D Bioprinter (Organovo, San Diego, America). (B) A multi-nozzle low-temperature deposition and manufacturing (M-LDM) system with screw-driven piston actuated extruders[62], with permission from JOHN WILEY & SONS (publisher). (C) Multi-head deposition system (MHDS)[64], with permission from ELSEVIER BV (publisher). (D) 3D Bioplotter (EnvisonTEC). (E) Integrated tissue-organ printer (ITOP)[59], with permission from Nature Springer (publisher). (F) CAD/CAM process for automated printing of 3D shape imitating target tissue or organ using ITOP[59], with permission from Springer Nature (publisher). (G) A desktop multi-material biomanufacturing system[74], with permission from Springer Nature (publisher).
By replacing the pressure-assisted head with screw-driven piston actuated extruders, a multi-nozzle low-temperature deposition and manufacturing (M-LDM) system (Figure 3B) was developed by Liu et al. (2008) to fabricate scaffolds with heterogeneous materials and gradient hierarchical porous structures to coordinate biological properties[62], such as adhesion, proliferation, and differentiation of various cells in both time and space dimensions. M-LDM system can process various materials, such as PLGA, PLGA-TCP, collagen, chitosan, and gelatin[63].
Kim et al. (2009) increased the number of dispensing heads and developed a similar multi-head deposition system (MHDS) (Figure 3C) based on pressure-assisted extrusion[64], and able to process a wide range of biomaterials, such as PCL, PLGA, and various types of hydrogels (hydroxyapatite [HA], gelatin, and atelocollagen) for printing multi-material constructs, with resolution < 100 μm. PCL/PLGA scaffolds with controlled pore size and line width were fabricated, presenting good biocompatibility. Shim et al. (2011) used this system to fabricate scaffolds consisting of synthetic biopolymers and a natural hydrogel[65]. Moreover, bioactive ceramic particles, tricalcium phosphate (TCP), and hydroxyapatite (HA) were blended with PCL to fabricate scaffolds for bone tissue engineering[66].
3D Bioplotter is another commercial system based on multi-pneumatic extrusion heads and is one of the most popular systems. The machine was developed at the Freiburg Material Research Centre and commercialized by EnvisonTEC[67] (Figure 3D). The 3D bioplotter combines multiple temperature-controlled pneumatic extrusion heads, including low temperature head (2 – 70°C) and high temperature head (30 – 250°C), together with a temperature-controlled build platform (−10 – 80°C). The system has been used to print a wide range of materials, such as hydrogels, polymers, polymer-ceramics, and graphene composites[68,69]. For example, a composite bioink consisting of silk fibroin, gelatin, hyaluronic acid, and tricalcium phosphate (TCP) is used for producing hybrid scaffolds to promote osteogenic differentiation of stem cells for bone tissue engineering[70].
Kang et al. (2016) developed an integrated tissue-organ printer (ITOP) (Figure 3E) to deposit cell-laden hydrogels together with synthetic biopolymers to create tissue constructs with shape and scale comparable to native tissue (Figure 3F)[71]. The results showed that these patterns allow for sufficient nutrient exchange with maintenance of basic respiratory functions. The system using multiple pressure-assisted extrusion heads has the capability of fabricating human-scale mandible bone, ear cartilage and skeletal muscle tissue constructs of high structural integrity using multiple types of cells and biomaterials[72]. All the tissue constructs showed promising structural and functional characteristics in vitro and in vivo. In another study, Kim et al. (2018) used ITOP system to produce an implantable skeletal muscle tissue construct (10 × 7 × 3 mm3) composed of human primary muscle progenitor cells (hMPCs) and PCL[73]. The results showed 82% functional recovery in a rodent model of tibialis anterior muscle defect and the tissue construct was well integrated with host vascular and neural networks. All above studies showed that this system overcomes the biomanufacturing limitations by printing micro-channels for transportation of necessary nutrients, oxygen, and growth factors.
Due to the high cost of 3D biomanufacturing systems, which becomes a barrier to biomedical research, a low-cost biomanufacturing system (Figure 3G) with pneumatic print heads was developed by Lee et al. (2017) using open-source hardware and software[74]. The x-y-z axis moving system combined with a rotary mechanism has been applied for multi-material printing. The low cost of the whole system is attributed to the inexpensive linear stages, rotary table, and open-source software. Hybrid composite scaffolds with synthetic polymers and cell-laden hydrogels were fabricated to validate the performance of the system.
3.2. Hybrid biomanufacturing systems
3.2.1. SMBSs
The development of SMBSs is an important step in the realization of multi-material 3D-printed structures, which can more adequately reflect the complexity of native tissue and the requirement of multiple printing technologies to achieve this aim.
Shim et al. (2012) developed a multi-head tissue/organ building system (MtoBS) of six dispensing heads with an accuracy of ±2.4 μm and repeatability of ±1.0 μm (Figure 4A)[75]. The system has the capability to process a wide range of biomaterials, including thermoplastic biodegradable materials and hydrogels. Two of the heads were used to process thermoplastic biomaterials (such as PCL and PLGA; up to 150°C) driven by pneumatic pressure (up to 650 kPa). The remaining four heads (−5 – 100°C) governed by a stepper motor are used to dispense hydrogels with encapsulated cells and growth factors. The in vivo tests revealed that the viability of printed cells (osteoblasts and chondrocytes) was maintained up to 7 days. To improve the mechanical support at defects in various organs and tissues, new tissue matrix was developed by generating a custom (CAD/CAM) system for automatically generated CNC printing paths[76].
Figure 4.
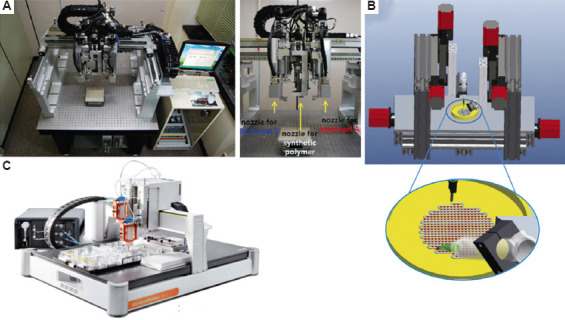
(A) Multi-head tissue/organ building system (MtoBS)[75], with permission from Institute of Physics Publishing (publisher). (B) Multi-arm bioprinter (MABP)[77], with permission from PERGAMON (publisher). (C) Bioscaffolder.
Ozbolat et al. (2014) developed a multi-arm bioprinter (MABP) (The University of Lowa, USA) to realize concurrent printing of both filament structures and cell spheroids using pressure-assisted and piston-driven print heads[77]. The MABP consists of two identical three-axis linear motion systems mounted on the table with a cell spheroid deposition nozzle and co-axial filament deposition nozzle that are separately fixed (Figure 4B). These two deposition systems can be independently set up in terms of motion path and dispensing parameters, such as filament distance, deposition speed and lay down pattern for the use in scaffold design and printing. Large-scale vascularized tissue spheroids in tandem with vessel-like microfluidic channels were fabricated using the MABP[78]. Bioink composite PCL/PLGA/HA was printed using MABP for large bone defect regeneration, presenting greater mechanical strength, better cell attachment and proliferation, faster degradation, and a higher level of bone repair and newly formed mineralized tissue with considerable vascularization compared with PCL ink[79].
The BioScaffolder (SYS+END, Salzgitter-Bad, Germany) (Figure 4C) is one of the most popular commercial BMBSs, which serves as a multi-head dispensing platform, and it can process a large variety of natural and synthetic materials with up to four independent print heads[80]. These print heads can be equipped with a variety of printing technologies, including pneumatic, piston, and screw-assisted extrusion, filament-based extrusion, and melt electrowriting (MEW) apparatus, that enable the fabrication of fibers at the microscale and hollow coaxial fibers. Thus, the BioScaffolder can create porous structures for tissue engineering with single and multi-materials, and even core-shell structure filaments. Schuurman et al. (2011) used the BioScaffolder to fabricate hybrid constructs by alternate deposition of thermoplastic fibers and hydrogels[80]. With this system, mechanical stiffness can be tailored by changing fiber spacing, orientation and/or thickness. The achieved Young’s moduli of printed constructs are within the same range as those of native tissues (e.g., cartilage: 4.1 MPa; trachea: 3.33 MPa). Moreover, complex anatomically shaped constructs were fabricated by co-depositing sacrificial components as a temporary support for overhanging geometries and removed after fabrication by immersing the constructs in aqueous solutions. The BioScaffolder was utilized since it can build 3D objects by the coordinated motion of several dispensing heads, which deposit on a stationary platform[81].
3.2.2. FBSs
FBSs are more advanced and complex systems that combine different techniques, such as electrospinning (or alternative electrowriting methods) and post-processing methods, to enable the fabrication of specifically functionalized, complex, multi-material, multi-scale, and hierarchical tissue constructs.
A popular hybrid biomanufacturing system is the 3D Discovery (RegenHU, Switzerland) (Figure 5A), which combines screw-assisted extruder, pneumatic-assisted extrusion head, inkjet printing, melt electrospinning writing, and UV light curing. Extrusion-based biomanufacturing with 3D Discovery has produced 30 wt.% GelAGE1MM (allyl: SH = 1:3, 0.05 wt.% I2 959 or 1/10×10−3 m Ru/SPS in PBS), which was irradiated with UV light and visible light during and after printing, respectively[82]. The UV light equipped with RegenHU enabled rapid gelation and gave rise to long-term mechanical stiffness. This produced cell-laden hydrogel constructs with more open and porous network. Using the 3D Discovery’s layer-by-layer UV curing system, Zhuang et al. (2019) built thick cell-laden constructs with high shape fidelity and mechanical properties suitable for soft tissue engineering applications[83] (Figure 5B). In addition, hybrid scaffold constructs were printed using the pressure-assisted extruder and screw-assisted extruder in 3D Discovery. Visscher et al. (2016) developed a contraction-free biocompatible PCL/collagen I/III hybrid scaffold (Figure 5C) for ear cartilage tissue engineering[84]. A PCL cage was printed with the screw-assisted extruder, and collagen was inserted into the cage using the pressure-assisted extruder before the cage printing was completed. The biomechanical results showed that the extracellular matrix deposition increased Young’s modulus in the hybrid structures.
Figure 5.
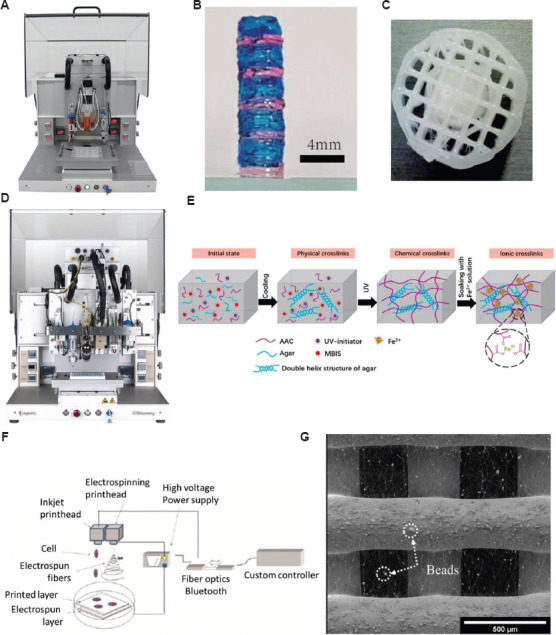
(A) 3D Discovery (RegenHU, Switzerland). (B) Multi-material cell-laden hydrogel constructs deposited with the layer-by-layer UV curing strategy[83], with permission from PLOS ONE (publisher). (C) Multi-material cage constructs (top view)[84], with permission from MARY ANN LIEBERT INC (publisher) (D) BioFactory (RegenHU, Switzerland). (E) Hybrid printing system shows the inkjet print head and the electrospinning print head[87], with permission from AMERICAN CHEMICAL SOCIETY (publisher). (F) Hybrid printing system combining the inkjet print head and the electrospinning print head[88], with permission from IOP Publishing (publisher). (G) SEM image of hybrid scaffolds printed with extrusion-based 3D printing and electrospinning technologies[89], with permission from Elsevier (publisher).
BioFactory (RegenHU, Switzerland) (Figure 5D) is a further iteration of the platform designed to create 3D organomimetic models for tissue engineering. The system is a versatile and cell-friendly biomanufacturing system that conbines biomanufacturing, electrospinning and bio stimulation functions in a single unit, allowing for embedded of cells, biomolecules, a range of soft and rigid biomaterials in 3D composite constructs to simulate natural environment[85,86]. The control technology at the micro-nano scale is dramatically promoted with various electrohydrodynamic technologies. A wide range of printing technologies, such as inkjet, pneumatic, thermoplastic extrusion, and piston-driven disposition heads for the fabrication of 3D porous structures, as well as UV light, photopolymerization, plasma pens, and nebulizers for surface modification are all available on this biofabrication platform. Self-healing and ultrastrechable double-network hydrogel have been fabricated through BioFactory by combining cross-linked k-carrageenan network and polyacrylamide (PAAm) network (Figure 5E)[87]. The printed 3D structure also presents remarkable sensitivity with a gauge factor of 0.63 at the strain of 1000%, which can be applied as a wearable strain sensor to effectively monitor and distinguish multifarious motions of a human body.
Xu et al. (2012) developed a system combining inkjet printing and electrospinning (Figure 5F) to fabricate nanofibrous viable tissues with enhanced mechanical and biological properties for cartilage tissue engineering[88]. The electrospinning print head is used to generate a polymeric fiber-based scaffold, and the inkjet print head is designed to deposit cells onto the scaffold with a x-y-z platform. The results revealed that both biological and mechanical properties were dramatically improved. First, chondrocytes survived with more than 80% viability 1 week after printing, and cells proliferated and maintained the original biological properties within the constructs. Moreover, the Young’s modulus of the hybrid constructs (1.76 MPa) is four times higher than alginate only constructs (0.41 MPa). Bo et al. proposed a simple and versatile hybrid printing process using a screw-assisted AM technique combined with rotational electrospinning to fabricate dual-scale anisotropic scaffolds. 3D microscale porous PCL structures with highly aligned nanoscale fibers were successfully produced in a layer-by-layer manner. The results showed improved cell distribution and tissue formation due to the controllable distributed electrospun fibers[89].
Liu et al. developed a plasma-assisted bioextrusion system (PABS)[47], which is able to produce smart scaffolds using a multi-head extrusion system and perform surface modification in a layer-by-layer fashion using low-temperature plasma jetting. It comprises two pressure-assisted extruder heads, one screw-assisted extruder head, and a plasma jet. Since many biopolymers are hydrophobic, plasma treatment is necessary to manipulate the surface chemistry of the polymer and tune properties, such as wettability and cell adhesion. The plasma treatment enables the design and fabrication of scaffolds matching the mechanical properties and surface characteristics of the surrounding tissue. PCL scaffolds with different zonal plasma treatments were fabricated. Wettability results confirmed that the hydrophilic character of the PCL samples increased due to the nitrogen groups introduced by the plasma jetting on the scaffold filaments. Furthermore, the plasma treatment positively influenced cell attachment and proliferation[90].
5. Conclusions and future perspectives
AB is a rapidly developing technology and is being widely applied in tissue engineering applications. Hybrid biomanufacturing techniques utilizing multiple biomaterials by combining multiple print heads or different printing techniques show great potential in fabricating multi-material gradient scaffolds which better reflect the complexity of native tissues and organs (summarized in Table 2). The available hybrid AM techniques can produce multi-material scaffolds using different types of material filaments printed with separate material chambers/cartridges, multi-scale structures, and surface modification to introduce specific chemistries to the scaffolds. The printed structures using these hybrid systems show improved behavior both in vitro and in vivo. Hybrid biomanufacturing systems are rapidly expanding due to the demanding requirements of tissue engineering, which requires complex material handling and processing to fabricate structures that resemble and mimic native tissues and organs.
Table 2.
Summary of hybrid biomanufacturing techniques comprising multi-dispensing jets or different printing techniques in fabricating multi-material gradient scaffolds.
| Systems | Bioprinters | Mechanisms | University/company | Materials | Applications |
|---|---|---|---|---|---|
| Multi-head biomanufacturing systems | NovoGen MXX 3D Biprinter | Dispense pumps with nozzles (micropipes) | Organovo, USA | • Hydrogel, bioinks | Bone, liver, breast cancer, vascularization |
|
| |||||
| 3D Bioplotter | Pneumatic extrusion | EnvisionTEC, Germany | • PCL, polymer/ceramics • Hydrogel, polymer/graphene • Food gradient |
Bone, cell-free scaffold, food, chocolate | |
|
| |||||
| A desktop multi-material biomanufacturing system | Piston extruder, pneumatic extruder | Seoul National University of Science and Technology | • Hydrogel • PLGA • Hyaluronic acid • Hemoglobin |
Bone blood vessel | |
|
| |||||
| Multi-head deposition system (MHDS) | Pneumatic extrusion | Pohang University of Science & Technology, Republic of Korea | • PCL, PLA, PLGA • Hydrogel |
Bone/cartilage | |
|
| |||||
| Integrated tissue–organ printer (ITOP) | Pneumatic extrusion | Wake Forest Institute for Regenerative Medicine, USA | • PCL, hydrogel | Cartilage, human-scale mandible bone, ear and skeletal muscle | |
|
| |||||
| Semi-hybrid systems | Multi-arm bioprinter (MABP) | Pneumatic extrusion, mechanical extrusion | University of Lowa, USA | • Sodium alginate, collagen chitosan | Vascularized tissue, bone and cartilage |
|
| |||||
| Multi-head tissue/organ building system (MtoBS) | Pneumatic extrusion, screw-driven extrusion | The Catholic University of Korea, Korea | • PCL, PLGA, Hydrogel | Liver, heart and adipose tissue, bone cartilage | |
|
| |||||
| BioScaffolder | Piston driven extrusion, screw-driven melt extrusion, pneumatic extrusion | Germany SYS+ENG | • Hydrogel • Synthetic polymers • GelMA-gellan |
Cartilage, vascular tree, human ear | |
|
| |||||
| Fully-hybrid systems | 3D Discovery | Screw-driven melt extrusion, pneumatic extrusion, UV light | RegenHU | • Biopolymers, collagen, polymer/ceramics, hydrogel, polymer/graphene | Bone, cartilage, vascularization |
|
| |||||
| Hybrid inkjet printing/electrospinning combined system | Micropipe, electrospinning | Wake Forest Institute for Regenerative Medicine, USA | • PCL, fibrin–collagen hydrogel, alginate | Muscle, cartilage | |
|
| |||||
| Plasma-assisted bioextrusion system (PABS) | Screw-driven melt extrusion, pneumatic extrusion, low-temperature plasma surface modification | University of Manchester, UK | • PCL • PCL/CNTs • PCL/HA • PCL/TCP • PCL/Hydrogel |
Bone and cartilage | |
|
| |||||
| Mixing extrusion head | Coaxial nozzle | Pneumatic extrusion | Massachusetts Institute of Technology (MIT), and Harvard University, USA | • CaCl2, GelMA • Alginate solution |
Heart-on-a-chip |
|
| |||||
| Variable Property Rapid Prototyping (VPRP) | Screw-assisted extrusion | Massachusetts Institute of Technology (MIT), USA | • Biopolymers, colored resins | Bioengineering | |
| Extrusion based gradient AM systems | Motor-driven Linear actuators | University of Wollongong, Australia University of Manchester, UK Jilin University, China | • Hydrogel • Polyurethane |
Simplified anatomical human muscle tendon, gradient scaffold | |
To mimic human tissues and organs, novel processing methods and new routes to produce functional scaffolds with adequate physical, chemical, and biological factors are still required to be developed and improved. The development of hybrid biomanufacturing systems and advanced print heads will be the main trend for the next decade. At present, since the hybrid printing systems are limited to extrusion-based techniques combined with electrospinning methods and post-processing techniques, the incorporation of more AM techniques, such as SLA, will be considered for further exploration and inclusion in a hybrid system. Another limitation is the poor capability of AM techniques to print vascularization within the constructs, which warrants more investigations in the future. Moreover, as the scale of the constructs are reducing to sub-micron or even nanoscale, imaging capabilities incorporated within the system can be considered for accurate control of the fabrication process. In addition, precise control over material properties during the printing is important so that a temperature gradient control nozzle with precise control of the crystallization process in semi-crystalline polymers to fabricate anisotropic structures can be considered. More advanced hybrid systems incorporating cell-culture systems can be developed for hospitals so that the patient-specific tissue constructs are able to be produced directly in the incubator and transplanted directly into the patients. The definition of biomanufacturing will be extended by incorporating material processing, printing/electrospinning, post-processing, and finally incubation and culturing bioreactor. To achieve this, it is imperative to design biomanufacturing systems that are able to produce a specific organ, for example, heart, with specific modules, and tools for the application.
Acknowledgments
None.
Funding
None.
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
Conceptualization: Fengyuan Liu
Data curation: Rixiang Quan, Enes Aslan, Fengyuan Liu
Supervision: Fengyuan Liu
Visualization: Rixiang Quan, Fengyuan Liu
Writing – original draft: Rixiang Quan, Cian Vyas, Enes Aslan, Fengyuan Liu
Writing – review & editing: Fengyuan Liu, Cian Vyas
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Bártolo P, Chua C, Almeida H, et al. Biomanufacturing for tissue engineering:present and future trends. Virtual Phys Prototyp. 2009;4:203–216. [Google Scholar]
- 2.Xu Y, Chen C, Hellwarth PB, et al. Biomaterials for stem cell engineering and biomanufacturing. Bioact Mater. 2019;4:366–379. doi: 10.1016/j.bioactmat.2019.11.002. https://doi.org/10.1016/j.bioactmat.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyas C, Pereira R, Huang B, et al. Engineering the vasculature with additive manufacturing. Curr Opin Biomed Eng. 2017;2:1–13. [Google Scholar]
- 4.Mota C, Puppi D, Chiellini F, et al. Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med. 2015;9:174–190. doi: 10.1002/term.1635. https://doi.org/10.1002/term.1635. [DOI] [PubMed] [Google Scholar]
- 5.Vyas C, Poologasundarampillai G, Hoyland J, et al. In:Biomedical Composites. 2nd ed. Netherlands: Elsevier; 2017. 3D printing of biocomposites for osteochondral tissue engineering; pp. 261–302. [Google Scholar]
- 6.Tavakoli S, Klar AS. Bioengineered skin substitutes:Advances and future trends. Appl Sci. 2021;11:1493. [Google Scholar]
- 7.Xue J, Singh S, Zhou Y, et al. A biodegradable 3D woven magnesium-based scaffold for orthopedic implants. Biofabrication. 2022;14:034107. doi: 10.1088/1758-5090/ac73b8. https://doi.org/10.1088/1758-5090/ac73b8. [DOI] [PubMed] [Google Scholar]
- 8.Pillai S, Upadhyay A, Khayambashi P, et al. Dental 3D-printing:Transferring art from the laboratories to the clinics. Polymers (Basel) 2021;13:157. doi: 10.3390/polym13010157. https://doi.org/10.3390/polym13010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafuente-Merchan M, Ruiz-Alonso S, García-Villén F, et al. Progress in 3D bioprinting technology for osteochondral regeneration. Pharmaceutics. 2022;14:1578. doi: 10.3390/pharmaceutics14081578. https://doi.org/10.3390/pharmaceutics14081578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonzo M, El Khoury R, Nagiah N, et al. 3D Biofabrication of a cardiac tissue construct for sustained longevity and function. ACS Appl Mater Interfaces. 2022;14:21800–21813. doi: 10.1021/acsami.1c23883. https://doi.org/10.1021/acsami.1c23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Liu B, Pei B, et al. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120:10793–10833. doi: 10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 12.Yusupov V, Churbanov S, Churbanova E, et al. Laser-induced forward transfer hydrogel printing:A defined route for highly controlled process. Int J Bioprint. 2020;6:271. doi: 10.18063/ijb.v6i3.271. https://doi.org/10.18063/ijb.v6i3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang T, Munguia-Lopez JG, Flores-Torres S, et al. Extrusion bioprinting of soft materials:An emerging technique for biological model fabrication. Appl Phys Rev. 6:011310. [Google Scholar]
- 14.Ng WL, Lee JM, Zhou M, et al. Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication. 12:022001. doi: 10.1088/1758-5090/ab6034. https://doi.org/10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 15.Gauvin R, Chen YC, Lee JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. https://doi.org/10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y, Tang H, Huang X, et al. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr Polym. 2020;235:115970. doi: 10.1016/j.carbpol.2020.115970. https://doi.org/10.1016/j.carbpol.2020.115970. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Michas C, Chen CS, et al. From simple to architecturally complex hydrogel scaffolds for cell and tissue engineering applications:Opportunities presented by two?photon polymerization. Adv Healthc Mater. 2020;9:1901217. doi: 10.1002/adhm.201901217. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P, Ebbens S, Zhao X. Inkjet printing of mammalian cells-theory and applications. Bioprinting. 2021;23:e00157. [Google Scholar]
- 19.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based biomanufacturing:past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Chen M, Fan X, et al. Recent advances in biomanufacturing techniques:Approaches, applications and future prospects. J Transl Med. 2016;14:271. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hölzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D biomanufacturing. Biofabrication. 2016;8:032002. doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Liu JK, Chen WS, et al. Controllable printing droplets on demand by piezoelectric inkjet:Applications and methods. Microsyst Technol. 2018;24:879–889. [Google Scholar]
- 23.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering:Biomanufacturing. Int Mater Rev. 2014;59:430–448. [Google Scholar]
- 24.Okubo N, Qureshi A, Dalgarno K, et al. Cost-effective microvalve-assisted bioprinter for tissue engineering. Biomanufacturing. 2019;13:e00043. [Google Scholar]
- 25.Yan X, Zhang L, Sett S, et al. Droplet jumping:Effects of droplet size, surface structure, pinning, and liquid properties. ACS Nano. 2019;13:1309–1323. doi: 10.1021/acsnano.8b06677. [DOI] [PubMed] [Google Scholar]
- 26.Graham AD, Olof SN, Burke MJ, et al. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci Rep. 2017;7:7004. doi: 10.1038/s41598-017-06358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negro A, Cherbuin T, Lutolf MP. 3D inkjet printing of complex, cell-laden hydrogel structures. Sci Rep. 2018;8:17099. doi: 10.1038/s41598-018-35504-2. https://doi.org/10.1038/s41598-018-35504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odde DJ, Renn MJ. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17:385–389. doi: 10.1016/s0167-7799(99)01355-4. https://doi.org/10.1016/s0167-7799(99)01355-4. [DOI] [PubMed] [Google Scholar]
- 29.Sears NA, Seshadri DR, Dhavalikar PS, et al. A review of three-dimensional printing in tissue engineering. Tissue Eng Part B:Rev. 2016;22:298–310. doi: 10.1089/ten.TEB.2015.0464. [DOI] [PubMed] [Google Scholar]
- 30.Kérourédan O, Rémy M, Oliveira H, et al. In:Laser Printing of Functional Materials:3D Microfabrication, Electronics and Biomedicine. United States: Wiley; 2018. Laser?assisted biomanufacturing of cells for tissue engineering; pp. 349–373. [Google Scholar]
- 31.Martinez-Rivas A, González-Quijano GK, Proa-Coronado S, et al. Methods of micropatterning and manipulation of cells for biomedical applications. Micromachines (Basel) 2017;8:347. doi: 10.3390/mi8120347. https://doi.org/10.3390/mi8120347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillemot F, Souquet A, Catros S, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta biomater. 2010;6:2494–500. doi: 10.1016/j.actbio.2009.09.029. https://doi.org/10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Koch L, Deiwick A, Chichkov B. Laser-based cell printing. Ovsianikov A, Yoo J, Mironov V, editors. 3D Printing and Biofabrication. Springer, Champaign. 2018:303–329. https://doi.org/10.1007/978-3-319-45444-3_11. [Google Scholar]
- 34.Vinson BT, Sklare SC, Chrisey DB. Laser-based cell printing techniques for additive biomanufacturing. Curr Opin Biomed Eng. 2017;2:14–21. [Google Scholar]
- 35.Pereira RF, Bártolo PJ. 3D biomanufacturing of photocrosslinkable hydrogel constructs. J Appl Polym Sci. 2015;132:42458. [Google Scholar]
- 36.Bourget JM, Kérourédan O, Medina M, et al. Patterning of endothelial cells and mesenchymal stem cells by laser-assisted biomanufacturing to study cell migration. Biomed Res Int. 2016;2016:3569843. doi: 10.1155/2016/3569843. https://doi.org/10.1155/2016/3569843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keriquel V, Oliveira H, Rémy M, et al. In situ printing of mesenchymal stromal cells, by laser-assisted biomanufacturing, for in vivo bone regeneration applications. Sci Rep. 2017;7:1778. doi: 10.1038/s41598-017-01914-x. https://doi.org/10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Luo Y, Ma L, et al. 3D biomanufacturing:An emerging technology full of opportunities and challenges. Bio Des Manuf. 2018;1:2–13. [Google Scholar]
- 39.Datta P, Ayan B, Ozbolat IT. Biomanufacturing for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. https://doi.org/10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 40.Mobaraki M, Ghaffari M, Yazdanpanah A, et al. Bioinks and bioprinting:A focused review. Bioprinting. 2020;18:e00080. [Google Scholar]
- 41.Qi X, Pei P, Zhu M, et al. Three-dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci Rep. 2017;7:42556. doi: 10.1038/srep42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakus AE, Shah RN. Multi and mixed 3D?printing of graphene?hydroxyapatite hybrid materials for complex tissue engineering. J Biomed Mater Res A. 2017;105:274–283. doi: 10.1002/jbm.a.35684. https://doi.org/10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]
- 43.Kim MH, Lee YW, Jung WK, et al. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based biomanufacturing. J Mech Behav Biomed Mater. 2019;98:187–194. doi: 10.1016/j.jmbbm.2019.06.014. https://doi.org/10.1016/j.jmbbm.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Blaeser A, Million N, Campos DF, et al. Laser-based in situ embedding of metal nanoparticles into bioextruded alginate hydrogel tubes enhances human endothelial cell adhesion. Nano Res. 2016;9:3407–3427. [Google Scholar]
- 45.Bellini A. Fused Deposition of Ceramics:A Comprehensive Experimental, Analytical and Computational Study of Material Behavior, Fabrication Process and Equipment Design. Drexel University, United States 2002 [Google Scholar]
- 46.Almeida H, Bartolo P, Mota C, et al. Processo e Equipamento de Fabrico Rápido por Bioextrusao. Portuguese Patent Application. 2010:104247. [Google Scholar]
- 47.Liu F, Wang W, Mirihanage W, et al. A plasma-assisted bioextrusion system for tissue engineering. CIRP Ann. 2018;67:229–232. [Google Scholar]
- 48.Wang W, Caetano G, Ambler WS, et al. Enhancing the hydrophilicity and cell attachment of 3D printed pcl/graphene scaffolds for bone tissue engineering. Materials. 2016;9:992. doi: 10.3390/ma9120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boularaoui S, Al Hussein G, Khan KA, et al. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting. 20:e00093. [Google Scholar]
- 50.Zhang T, Zhao W, Xiahou Z, et al. Bioink design for extrusion-based bioprinting. Appl Mater Today. 25:101227. [Google Scholar]
- 51.Chen DX. Extrusion biomanufacturing of scaffolds. In:Extrusion Biomanufacturing of Scaffolds for Tissue Engineering Applications. Berlin: Springer; 2019. pp. 117–145. [Google Scholar]
- 52.Zhang AP, Qu X, Soman P, et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater. 2012;24:4266–4270. doi: 10.1002/adma.201202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barraza B, Olate-Moya F, Montecinos G, et al. Superhydrophobic SLA 3D printed materials modified with nanoparticles biomimicking the hierarchical structure of a rice leaf. Sci Technol Adv Mater. 23:300–321. doi: 10.1080/14686996.2022.2063035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raman R, Bhaduri B, Mir M, et al. High?resolution projection microstereolithography for patterning of neovasculature. Adv Healthc Mater. 5:610–619. doi: 10.1002/adhm.201500721. https://doi.org/10.1002/adhm.201500721. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Zhu W, Heyburn L, et al. Development of novel 3-D printed scaffolds with core-shell nanoparticles for nerve regeneration. IEEE Trans Biomed Eng. 2016;64:408–418. doi: 10.1109/TBME.2016.2558493. https://doi.org/10.1109/TBME.2016.2558493. [DOI] [PubMed] [Google Scholar]
- 56.Luo Y, Le Fer G, Dean D, et al. 3D printing of poly (propylene fumarate) oligomers:Evaluation of resin viscosity, printing characteristics and mechanical properties. Biomacromolecules. 2019;20:1699–1708. doi: 10.1021/acs.biomac.9b00076. [DOI] [PubMed] [Google Scholar]
- 57.Dong D, Su H, Li X, et al. Microstructures and mechanical properties of biphasic calcium phosphate bioceramics fabricated by SLA 3D printing. J Manuf Processes. 2022;81:433–443. [Google Scholar]
- 58.Admane P, Gupta AC, Jois P, et al. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting. 2019;15:e00051. [Google Scholar]
- 59.Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using biomanufacturing. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. https://doi.org/10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Byambaa B, Annabi N, Yue K, et al. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6:1700015. doi: 10.1002/adhm.201700015. https://doi.org/10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langer EM, Allen-Petersen BL, King SM, et al. Modeling tumor phenotypes in vitro with three-dimensional biomanufacturing. Cell Rep. 2019;26:608–623. doi: 10.1016/j.celrep.2018.12.090. https://doi.org/10.1016/j.celrep.2018.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Xia Z, Han Z, et al. Novel 3D collagen scaffolds fabricated by indirect printing technique for tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;85:519–528. doi: 10.1002/jbm.b.30975. https://doi.org/10.1002/jbm.b.30975. [DOI] [PubMed] [Google Scholar]
- 63.Liu L, Yan Y, Xiong Z. A novel poly (lactic-co-glycolic acid)-collagen hybrid scaffold fabricated via multi-nozzle low-temperature deposition. Virtual Rapid Manuf. 20072019:57–61. [Google Scholar]
- 64.Kim JY, Cho DW. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron Eng. 2009;86:1447–1450. [Google Scholar]
- 65.Shim JH, Kim JY, Park M, et al. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication. 2011;3:034102. doi: 10.1088/1758-5082/3/3/034102. https://doi.org/10.1088/1758-5082/3/3/034102. [DOI] [PubMed] [Google Scholar]
- 66.Kim JY, Lee TJ, Cho DW, et al. Solid free-form fabrication-based PCL/HA scaffolds fabricated with a multi-head deposition system for bone tissue engineering. J Biomater sci Polym Ed. 2010;21:951–962. doi: 10.1163/156856209X458380. https://doi.org/10.1163/156856209X458380. [DOI] [PubMed] [Google Scholar]
- 67.Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer?assisted design combined with computer?guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282:17–21. [Google Scholar]
- 68.You F, Wu X, Zhu N, et al. 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2:1200–1210. doi: 10.1021/acsbiomaterials.6b00258. [DOI] [PubMed] [Google Scholar]
- 69.Jain S, Fuoco T, Yassin MA, et al. Printability and critical insight into polymer properties during direct-extrusion based 3D printing of medical grade polylactide and copolyesters. Biomacromolecules. 2019;21:388–396. doi: 10.1021/acs.biomac.9b01112. [DOI] [PubMed] [Google Scholar]
- 70.Wei L, Wu S, Kuss M, et al. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact Mater. 2019;4:256–260. doi: 10.1016/j.bioactmat.2019.09.001. https://doi.org/10.1016/j.bioactmat.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang HW, Lee SJ, Ko IK, et al. A 3D biomanufacturing system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 72.Merceron TK, Burt M, Seol YJ, et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication. 2015;7:035003. doi: 10.1088/1758-5090/7/3/035003. https://doi.org/10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 73.Kim JH, Seol YJ, Ko IK, et al. 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-29968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J, Kim KE, Bang S, et al. A desktop multi-material 3D bio-printing system with open-source hardware and software. Int J Precision Eng Manuf. 2017;18:605–612. [Google Scholar]
- 75.Shim JH, Lee JS, Kim JY, et al. Biomanufacturing of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng. 2012;22:085014. [Google Scholar]
- 76.Jung JW, Lee JS, Cho DW. Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Sci Rep. 2016;6:21685. doi: 10.1038/srep21685. https://doi.org/10.1038/srep21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozbolat IT, Chen H, Yu Y. Development of 'Multi-arm Bioprinter'for hybrid biofabrication of tissue engineering constructs. Robot Comput Integrated Manuf. 2014;30:295–304. [Google Scholar]
- 78.Yu Y, Moncal KK, Li J, et al. Three-dimensional biomanufacturing using self-assembling scalable scaffold-free “tissue strands”as a new bioink. Sci Rep. 2016;6:28714. doi: 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moncal KK, Heo DN, Godzik KP, et al. 3D printing of poly (ε-caprolactone)/poly (D, L-lactide-co-glycolide)/hydroxyapatite composite constructs for bone tissue engineering. J Mater Res. 2018;33:1972–1986. [Google Scholar]
- 80.Schuurman W, Khristov V, Pot MW, et al. Biomanufacturing of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3:021001. doi: 10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- 81.Visser J, Peters B, Burger TJ, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5(3):035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 82.Raphael B, Khalil T, Workman VL, et al. 3D cell biomanufacturing of self-assembling peptide-based hydrogels. Mater Lett. 2017;190:103–106. [Google Scholar]
- 83.Zhuang P, Ng WL, An J, et al. Layer-by-layer ultraviolet assisted extrusion-based (UAE) biomanufacturing of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS One. 2019;14:e0216776. doi: 10.1371/journal.pone.0216776. https://doi.org/10.1371/journal.pone.0216776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Visscher DO, Bos EJ, Peeters M, et al. Cartilage tissue engineering:preventing tissue scaffold contraction using a 3D-printed polymeric cage. Tissue Eng Part C Methods. 2016;22:573–584. doi: 10.1089/ten.TEC.2016.0073. https://doi.org/10.1089/ten.TEC.2016.0073. [DOI] [PubMed] [Google Scholar]
- 85.Müller M, Öztürk E, Arlov Ø, et al. Alginate sulfate-nanocellulose bioinks for cartilage biomanufacturing applications. Ann Biomed Eng. 2017;45:210–223. doi: 10.1007/s10439-016-1704-5. https://doi.org/10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- 86.Sultan S, Siqueira G, Zimmermann T, et al. 3D printing of nano-cellulosic biomaterials for medical applications. Curr Opin Biomed Eng. 2017;2:29–34. [Google Scholar]
- 87.Liu S, Li L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl Mater Interfaces. 2017;9:26429–26437. doi: 10.1021/acsami.7b07445. [DOI] [PubMed] [Google Scholar]
- 88.Xu T, Binder KW, Albanna MZ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 89.Huang B, Aslan E, Jiang Z, Daskalakis E, et al. Engineered dual-scale poly (ε-caprolactone) scaffolds using 3D printing and rotational electrospinning for bone tissue regeneration. Addit Manufac. 2020;36:101452. [Google Scholar]
- 90.Liu F, Wang W, Hinduja S, et al. Hybrid additive manufacturing system for zonal plasma treated scaffolds. 3D Print Addit Manuf. 2018;5:205–213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


