Abstract
This study investigated the effects of injectable vitamin C (VC) at weaning and prior to transit on growth performance and immune function in early-weaned beef steers. On day 0, 91 Angus × Simmental steers (92 ± 4 kg) were weaned (65 ± 11 d of age), given vaccination boosters, blocked by age, and randomly assigned to weaning (WEAN) treatments: intramuscular injections (20 mL per steer) of VC (250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL). From days 0 to 48, steers were housed at the Dixon Springs Agricultural Center (Simpson, IL) in pens (six pens; N = 14 to 16 steers per pen) equipped with two to three Vytelle bunks to measure individual daily feed disappearance. On day 49, half of the steers in each WEAN treatment were randomly assigned to an additional injection treatment (20 mL per steer) of VC or SAL prior to transport (TRANS). After administering pretransit injections, all steers were loaded onto a commercial livestock trailer with equal representation of treatments across compartments. Steers were transported for 6 h (approximately 480 km) to the Illinois Beef and Sheep Field Laboratory (Urbana, IL). Upon arrival, steers were sorted into pens (six pens; N = 13 to 17 steers per pen) with 2 Vytelle bunks per pen. Steers were weighed on days 0, 1, 14, 48, 49, 64, 78, 106, and 107. Blood was collected (WEAN = 24 steers per treatment; TRANS = 12 steers per treatment) on days 0, 1, 2, 14, 49 (pre- and posttransit), 50, and 51. Data were analyzed using the MIXED procedure of SAS 9.4 with fixed effects of age block, WEAN, TRANS, and WEAN × TRANS. Plasma ascorbate concentrations were greater (WEAN × time P < 0.01) on days 1 and 2 for steers that received VC at weaning. Similarly, for steers that received VC on day 49 pretransit, ascorbate concentrations were greater (TRANS × time P = 0.04) on days 49 posttransit, 50, and 51. Treatments did not affect (P ≥ 0.13) body weight, average daily gain, or gain to feed throughout the trial. Serum Bovine Viral Diarrhea Virus type 1 and 2 antibody titers on days 14 and 51 were not affected (P ≥ 0.32) by treatment. Injectable VC administered to early-weaned beef steers at the time of weaning or pretransit increased plasma ascorbate concentrations but did not improve growth performance or antibody response to vaccination booster.
Keywords: plasma ascorbate, transportation, weaning stress
Beef calves weaned at a younger age may encounter greater physiological or oxidative stress during weaning and transit. This research examined the ability of an injectable vitamin C to affect growth performance and immune function in early-weaned beef calves.
Introduction
Beef calves in the United States often encounter two significant stressors within the first 6 mo of life: weaning and transportation. At weaning, calves are separated from their dam, exposed to an unfamiliar environment, and transitioned to a new diet. During transport, calves are deprived of feed and water and may experience physical exhaustion. Weaning and transit elicit increases in inflammation markers, suppress the immune system, and decrease antioxidant status (Hughes, 1999; Deters and Hansen, 2020; Mattioli et al., 2020). These physiological responses may decrease growth and increase morbidity in beef calves (Marques et al., 2012; Cooke, 2017). The U.S. beef industry has adopted handling and welfare methods to reduce the stress of weaning and transportation, yet there is an opportunity for improvement. A proactive approach to these stress events is through nutritional modulation. Equipping the calf with adequate nutrients and antioxidants prior to a stress event may mitigate typical decreases in growth and immunity.
Vitamin C (VC) is the primary water-soluble antioxidant in plasma and tissues (Combs, 2012). The antioxidant capabilities of VC can prevent the oxidation of protein and DNA and aid in recycling vitamin E (Packer et al., 1979). There is no established dietary requirement for VC in cattle because of hepatic synthesis of VC from glucose. In newborn calves, plasma VC concentrations decrease from days 1 to 3 of life and then increase around 21 d of age when hepatic VC synthesis increases (Cummins and Brunner, 1991). Circulating VC concentrations may decrease in response to physiological and environmental stress (Cummins and Brunner, 1991; Padilla et al., 2006). Therefore, very young cattle experiencing stress from weaning or long-distance transit may not produce enough VC to meet their nutritional needs. Exogenous vitamin C delivered via intramuscular injection is easy to administer, affordable, and has been shown to increase plasma VC concentrations (Deters and Hansen, 2020). This study sought to determine the effectiveness of injectable VC at the time of weaning and before a 6-h transit event on growth performance and immune status of early-weaned beef steers. It was hypothesized that an injection of VC at the time of weaning and pretransit would sustain plasma ascorbate concentrations and improve subsequent growth performance of beef calves.
Materials and Methods
Animal and experimental design
All experimental procedures were approved by the University of Illinois Institutional Animal Care and Use Committee (#19242).
Ninety-one Angus × Simmental steer calves (92 ± 4 kg) from the University of Illinois Dixon Springs Agricultural Center (Simpson, IL) were early-weaned (65 ± 11 d of age) in December of 2019. Steers were used in a randomized complete block design with a 2 × 2 factorial treatment arrangement examining the effects of injectable VC at time of weaning (day 0) through the postweaning period (WEAN; days 0 to 48) and immediately prior to transport on day 49 through the posttransit period (TRANS; days 49 to 107).
Steers were blocked by age (two blocks; old ≥ 62 d, young = < 62 d) and stratified by sire, age, and weight per day of age. On day 0, steers were weaned and randomly assigned to an injection of VC (Vet One, Boise, ID; 250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL). The dosage was based on previous transit research in older weaned steers (Deters and Hansen, 2020). Saline and VC injections were delivered intramuscularly at a dose rate of 20 mL per steer (10 mL per injection site on opposite sides of the neck). On day 0, steers were given booster vaccinations with the corresponding vaccine they received prior to weaning (days −18 to −27). Steers were vaccinated against infectious bovine rhinotracheitis, bovine viral diarrhea types 1 and 2 viruses, bovine respiratory syncytial virus, parainfluenza-3 virus (2 mL subcutaneous; Bovishield Gold FP 5 VL5; Zoetis Inc., Parsippany, NJ), Mannheimia haemolytica (2 mL subcutaneous; Pulmo-guard PHM-1; Huvepharma, Peachtree City, GA), Mycoplasma bovis (2 mL subcutaneous; MpB Guard [Huvepharma], diseases caused by Clostridium chauboei, C. septicum, C. novyi type B, C. haemolyticum, C. tetani, and C. perfringens types C and D (2 mL subcutaneous; Covexin 8; Merck Animal Health, Rahway, NJ) and received an anthelmintic (2 mL oral of oxfendazole 22.5% solution; Synanthic, Boehringer Ingelheim Animal Health, Duluth, GA). From days 0 to 48, steers were housed at the Dixon Springs Agricultural Center in Simpson, IL and fed a complete total mixed ration (TMR; Table 1) once daily at 0800 hours in partially covered pens (six pens; 4.9 × 18.3 m; N = 14 to 16 steers per pen) equipped with 2 or 3 Vytelle bunks (Vytelle LLC, Calgary, AB, Canada) to measure individual daily feed disappearance.
Table 1.
Ingredient composition of common fed diet from days 0 to 48
| DM% | 87 |
| Ingredient, % DM basis | |
| Whole shelled corn | 30.5 |
| Dried distillers grains with solubles | 34.9 |
| Steakmaker Co-product Balancer1 | 4.4 |
| Ground grass hay | 30.2 |
| Analyzed composition | |
| CP | 16.2 |
| NDF | 38.0 |
| Ether extract | 6.3 |
| Net energy for gain, Mcal/kg2 | 1.28 |
1Pelleted protein and mineral supplement (Co-product balancer R700 T210; Land O’Lakes; Arden Hills, MN) containing not less than 25% crude protein, 1.5% crude fat, and 14% Ca.
2Calculated based on values in NASEM (2016).
On day 49, half of the steers in each WEAN, treatment were randomly assigned to one of two additional injection treatments (20 mL per steer; administered ~0500 hours) of VC or SAL prior to transport. After administration of pretransit injections, all steers were loaded at 0800 hours onto a commercial livestock trailer (Cattle Drive trailer; 15.8 m × 2.6 m; Henderson, CO). Cattle were transported for 6 h (approximately 480 km) and steers did not have access to feed or water during transport. Steers arrived at the Illinois Beef and Sheep Field Laboratory in Urbana, IL at approximately 1400 hours on day 49.
Upon arrival, steers were sorted into pens (six pens; 4.9 × 18.3 m; N = 13 to 17 steers per pen) outfitted with two Vytelle bunks per pen. Steers remained in the same pen grouping as prior to transit. Pens contained slatted concrete floors covered by rubber matting in a completely covered gable-roofed building. Steer were given ad libitum access to a common grass hay-based TMR and water. From days 49 to 56, steers were gradually transitioned from the hay-based TMR (35% grass hay inclusion, DM basis) to a corn silage-based TMR that was fed from days 56 to 107 (Table 2). Body weights (BW) were collected before feeding on days 0, 1, 14, 48, 49 (pretransit and posttransit), 64, 78, and 106, and 107. Two day average weights were used for initial and final BW. Steer average daily gain (ADG), gain:feed (G:F), and dry matter intake (DMI) were calculated from days 0 to 14, 14 to 48, 0 to 48, 49 to 107, and 0 to 107. Steers were monitored by farm personnel throughout the study for signs of illness including labored breathing, lethargy, nasal discharge, or reduced feed intakes. Steers displaying any specified signs were treated (Micotil, Elanco Animal Health, Indianapolis, IN) by farm personnel following veterinarian recommendations. Due to bloat or physical injury, four steers were removed from the study (2 SAL-SAL, 1 VC-SAL, and 1 VC-VC). Data from these steers were included in analysis until the time of removal from the study.
Table 2.
Ingredient composition of diet fed from days 56 to 107
| DM% | 47 |
| Ingredient, % DM basis | |
| High moisture corn | 30 |
| Modified distillers grains with solubles | 20 |
| Corn silage | 40 |
| Ground corn | 7.435 |
| Limestone | 1.708 |
| Urea | 0.650 |
| Rumensin 901 | 0.017 |
| Tylan 402 | 0.011 |
| Choice white grease | 0.081 |
| Trace mineral and vitamin premix3 | 0.098 |
| Analyzed composition | |
| CP | 13.6 |
| NDF | 28.6 |
| Ether extract | 5.8 |
| Net energy for gain, Mcal/kg4 | 1.32 |
1Provided monensin (Elanco Animal Health, Greenfield, IN).
2Provided tylosin (Elanco Animal Health, Greenfield, IN).
3Trace minerals provided as recommended by NASEM (2016).
4Calculated based on values in NASEM (2016).
Sample collection and analytical procedures
TMR samples were collected every 2 wk during the WEAN (days 0 to 48) and TRANS (days 49 to 107) periods. Samples were composited on an equal wet basis, freeze dried, and ground through a 1-mm screen using a Wiley mill (Arthur, H. Thomas, Philadelphia, PA). Ground samples were analyzed for dry matter (DM; 24 h at 105 °C), crude protein (Leco TruMac, LECO Corporation, St. Joseph, MI), crude fat by Ankom XT10 Fat Extractor (method 2; Ankom Technology, Macedon, NY), and neutral detergent fiber by using Ankom 200 Fiber Analyzer (method 5 including sodium sulfite and α-amylase; Ankom Technology). The dietary DM value was multiplied by daily individual as-fed intakes for each steer to determine DMI.
Blood samples were collected on 48 randomly selected steers, the same steers were sampled for the duration of the study (N = 24 steers per treatment during WEAN, N = 12 steers per treatment during TRANS), for various blood metabolite analyses. Blood samples were collected prior to feeding via jugular venipuncture into nonadditive and potassium EDTA vacuum tubes (Becton Dickinson, Franklin Lakes, NJ) on day 0 (prior to treatment injection and vaccination), 1, 14, 49 (prior to pretransit treatment injection), 49 (posttransit), 50, and 51 and transported to the laboratory on ice. Serum samples were centrifuged at 1,000 × g for 20 min at 4 °C and aliquoted into microcentrifuge tubes and stored at −80 °C for future analysis. Serum collected on days 0, 14, and 51 were sent to the Iowa State University Veterinary Diagnostic Laboratory for Bovine Viral Diarrhea Virus type 1 (BVDV1) and 2 (BVDV2) antibody titer analysis via virus neutralization (method 9.14; Kalkwarf, 2014). Serum samples were serially diluted (10−1 to 10−4) and incubated at 25 °C in a 96-well culture plate with virus for 2 h. The highest dilution with 100% neutralization of the challenge virus was considered the endpoint. Potassium EDTA tubes were centrifuged at 1,000 × g for 20 min at 4 °C; aliquoted into microcentrifuge tubes and stored at −80 °C for future analysis of ascorbate and cortisol. Plasma for ascorbate analysis was stabilized with diethylenetriaminepentaacetic acid prior to freezing to prevent ascorbate degradation, and all samples were analyzed within 45 d of collection. Commercial kits were used for analysis of plasma ascorbate (#700420, Cayman Chemical, Ann Arbor, MI; intra-assay CV = 2.95%, inter-assay CV = 7.87%) and cortisol (#K003-H5, Arbor Assays, Ann Arbor, MI; intra-assay CV = 7.28%, inter-assay CV = 8.46%) concentrations.
Statistical analysis
Performance and DMI data for the WEAN period (days 0 to 48) were analyzed as a randomized complete block design using the Mixed procedure of SAS 9.4 (SAS Institute Inc., Cary, NC.) Steer was the experimental unit (N = 45 to 46 per weaning treatment) with fixed effects of WEAN and age block. Plasma cortisol from day 1 was analyzed with a main effect of treatment and age block with plasma cortisol from day 0 considered as a covariate. Data collected following day 48 were analyzed as a 2 × 2 factorial utilizing the Mixed procedure of SAS with fixed effects of WEAN, TRANS, block, and interaction of WEAN and TRANS (N = 22 to 23 per treatment combination). Steer was the experimental unit for all blood analyses (N = 12 steers per treatment combination). Plasma ascorbate data were analyzed as repeated measures with time as the repeated effect and the heterogenous autoregressive variance structure was utilized to achieve the lowest Akaike information criterion value. Initial BW was included as covariate in performance and plasma ascorbate analysis. Data were tested for normality using the Shapiro–Wilks test; antibody titers were natural log-transformed to meet the assumption of normality and log-transformed means and SEM are presented. Initial values for serum antibody titers were used as covariates in analysis of subsequent sampling dates to reflect individual animal response to initial vaccine dose. Outliers were determined using Cook’s D statistic and removed if Cook’s D ≥ 0.5. The PDIFF statement in SAS was used to determine pairwise differences and data are reported as least square means ± SEM. When a significant interaction was observed, pairwise comparisons were considered across multiple time points for means separations given the few sampling times. Significance was declared at P ≤ 0.05 and tendencies from 0.05 < P ≤ 0.10.
Results
Weaning performance and blood metabolites
No differences (P ≥ 0.29) were detected for BW, ADG, or G:F when comparing VC vs. SAL during the WEAN period (days 0 to 48; Table 3). However, VC steers tended to have decreased (P = 0.06) DMI from days 0 to 14 compared with SAL, but DMI was not affected (P ≥ 0.29) by treatment from days 14 to 48 or 0 to 48.
Table 3.
Effect of injectable vitamin C (VC) treatment on postweaning performance of early-weaned (65 ± 11 d of age) beef steers
| WEAN1 | SEM2 | P-value | ||
|---|---|---|---|---|
| VC | SAL | |||
| Steers (N) | 45 | 46 | ||
| Body weight, kg | ||||
| Day 0 | 88 | 93 | 2.05 | 0.13 |
| Day 143 | 97 | 97 | 0.94 | 0.72 |
| Day 483 | 136 | 136 | 1.71 | 0.83 |
| ADG3, kg/d | ||||
| Day 0–14 | 0.52 | 0.56 | 0.072 | 0.71 |
| Day 14–48 | 1.65 | 1.58 | 0.059 | 0.44 |
| Day 0–48 | 0.98 | 0.97 | 0.036 | 0.83 |
| DMI3, kg/d | ||||
| Day 0–14 | 1.84 | 2.01 | 0.065 | 0.06 |
| Day 14–48 | 3.83 | 3.90 | 0.085 | 0.53 |
| Day 0–48 | 3.23 | 3.34 | 0.074 | 0.29 |
| G:F3 | ||||
| Day 0–14 | 0.263 | 0.278 | 0.0372 | 0.79 |
| Day 14–48 | 0.424 | 0.404 | 0.0128 | 0.29 |
| Day 0–48 | 0.301 | 0.289 | 0.0094 | 0.37 |
1WEAN: Treatments applied at weaning on day 0 including intramuscular injections (20 mL per steer) of VC (250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL).
2Greatest SEM of any treatment reported.
3Day 0 BW utilized as a covariate in performance analysis.
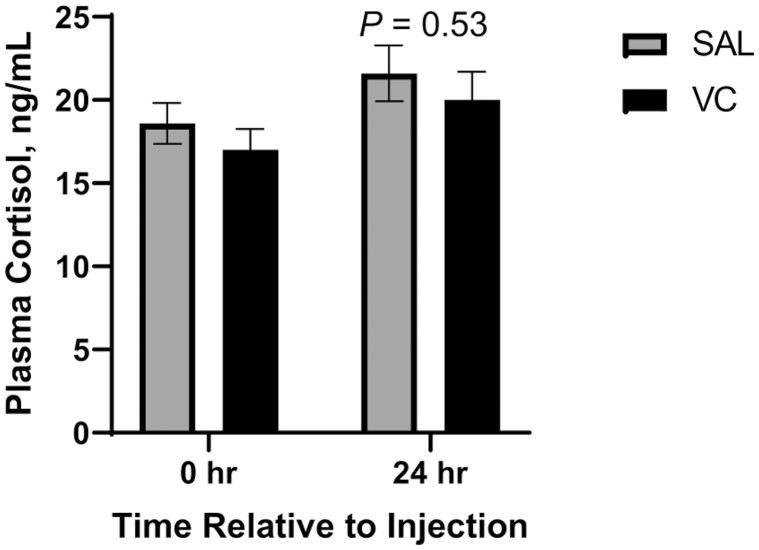
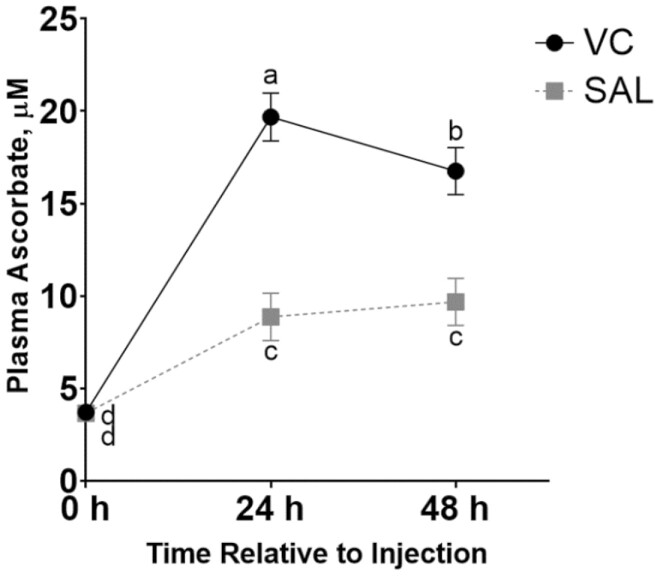
Plasma cortisol was not different between treatments on days 0 and 1 (P ≥ 0.40; Figure 1). Repeated measures analysis of blood samples collected on days 0 (pretreatment injection), 1, and 2, indicated a treatment × time effect (P < 0.01) for plasma ascorbate (Figure 2). Pretreatment injection (day 0) plasma ascorbate concentrations were not different between treatments. Both treatments had increased ascorbate concentrations on days 1 and 2, but steers that received injectable VC at time of weaning had the greatest plasma ascorbate concentrations on day 1 (24 h postweaning). Bovine Viral Diarrhea Virus Titers 1 and 2 for day 14, and 51 were not affected (P ≥ 0.32) by injectable VC at weaning (Table 4). However, titers for Bovine Viral Diarrheal Virus type 2 were different (P = 0.01) between treatments on day 0 despite the fact that treatments had not been administered prior to this blood collection.
Figure 1.
Effect of injectable vitamin C (VC) treatment on plasma cortisol concentration of early-weaned (65 ± 11 d of age) beef steers. Treatments were applied at weaning on day 0 including intramuscular injections (20 mL per steer) of VC (250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL; 20 mL per steer). Day 0 plasma cortisol concentration was used as a covariate in day 1 analysis but was not different between treatments (P = 0.40). N = 24 steers per treatment.
Figure 2.
Effect of injectable vitamin C (VC) treatment and time of sampling relative to weaning on day 0 on plasma ascorbate concentrations in early-weaned (65 ± 11 d of age) beef steers. Treatments were applied at weaning (WEAN) on day 0 including intramuscular injections (20 mL per steer) of VC (250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL; 20 mL per steer). Samples were collected on day 0 at 0 h (time of weaning and prior to treatment injection), 24 h (day 1), 48 h (day 2) postinjection. Day 0 plasma ascorbate concentration was used as a covariate. Means were separated across all time points and values with unlike superscripts differ (P ≤ 0.05). Effects of WEAN × time (P < 0.01), WEAN (P < 0.01), and time (P < 0.01) were observed. N = 24 steers per treatment.
Table 4.
Bovine viral diarrhea virus (BVDV) type 1 and 2 antibody titer response to injectable vitamin C (VC) on early-weaned (65 ± 11 d of age) beef steers
| WEAN1 | SEM2 | P-value | ||
|---|---|---|---|---|
| VC | SAL | |||
| Steers (N) | 24 | 24 | ||
| BVDV Type 13 | ||||
| Day 04 | 1.66 | 2.22 | 0.227 | 0.09 |
| Day 145 | 1.58 | 1.56 | 0.155 | 0.95 |
| Day 515 | 1.18 | 0.99 | 0.205 | 0.54 |
| BVDV Type 23 | ||||
| Day 04 | 2.38 | 3.95 | 0.292 | 0.01 |
| Day 145 | 2.62 | 2.59 | 0.218 | 0.93 |
| Day 515 | 2.80 | 2.36 | 0.297 | 0.32 |
1WEAN: Treatments applied at weaning on day 0 including intramuscular injections (20 mL per steer) of VC (250 mg sodium ascorbate per mL; 5 g per steer) or saline (SAL).
2Greatest SEM of any treatment reported.
3Natural log-transformed; transformed means and SEM presented.
4Steers were weaned on day 0. Blood samples collected prior to administration of booster vaccines.
5Values from day 0 utilized as covariate in analysis.
Posttransit feedlot performance and blood metabolites
BW on day 64 was greatest for SAL–SAL, intermediate for SAL–VC and VC–VC, and lowest for VC–SAL (WEAN × TRANS P = 0.03; Table 5). There were no other observed differences (P ≥ 0.27) for BW, ADG, and G:F during the posttransit period. Steers that received injectable VC at weaning had decreased (P = 0.03) DMI from days 49 to 107, resulting in lesser (P = 0.02) DMI overall (days 0 to 107). Final BW (day 107) and overall ADG and G:F were not affected by injectable VC at WEAN, TRANS, or WEAN × TRANS (P ≥ 0.24).
Table 5.
Effect of injectable vitamin C (VC) at weaning and pretransit on feedlot performance by beef steers
| WEAN1 | VC | SAL | SEM3 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| TRANS2 | VC | SAL | VC | SAL | WEAN | TRANS | WEAN × TRANS | |
| Steers (N) | 23 | 23 | 23 | 22 | ||||
| Body weight4, kg | ||||||||
| Day 495 | 132 | 132 | 131 | 133 | 2.23 | 0.96 | 0.68 | 0.52 |
| Day 64 | 151a,b | 145b | 148a,b | 158a | 4.02 | 0.21 | 0.69 | 0.03 |
| Day 78 | 165 | 163 | 163 | 168 | 3.35 | 0.63 | 0.79 | 0.28 |
| Final BW | 201 | 201 | 201 | 206 | 3.96 | 0.50 | 0.46 | 0.44 |
| ADG4, kg/d | ||||||||
| Day 49–64 | 1.06 | 0.79 | 1.03 | 1.38 | 0.293 | 0.32 | 0.88 | 0.27 |
| Day 49–107 | 1.15 | 1.19 | 1.20 | 1.22 | 0.050 | 0.42 | 0.53 | 0.84 |
| Day 0–107 | 1.04 | 1.04 | 1.04 | 1.09 | 0.037 | 0.48 | 0.46 | 0.45 |
| Posttransit (Day 49–107) | ||||||||
| DMI4, kg/d | 5.03 | 4.74 | 5.28 | 5.34 | 0.201 | 0.03 | 0.58 | 0.36 |
| G:F4 | 0.233 | 0.257 | 0.234 | 0.235 | 0.0131 | 0.43 | 0.32 | 0.37 |
| Overall (Day 0–107) | ||||||||
| DMI4, kg/d | 4.12 | 3.98 | 4.29 | 4.37 | 0.123 | 0.02 | 0.81 | 0.37 |
| G:F4 | 0.253 | 0.261 | 0.244 | 0.251 | 0.0084 | 0.24 | 0.32 | 0.95 |
1WEAN: Treatments applied at weaning on day 0 including intramuscular injections (20 mL per steer) of VC (250 mg sodium ascorbate per mL; 5 g per steer) or saline(SAL).
2TRANS: treatments applied immediately prior to 6-h transit on day 49 including intramuscular injections (20 mL per steer) of VC (250 mg sodium ascorbate per mL; 5 g per steer) or SAL.
3Greatest SEM of any treatment reported.
4Day 0 BW utilized as a covariate in performance analysis.
5Off-truck body weight.
a,bValues with unlike superscripts differ (P ≤ 0.05).
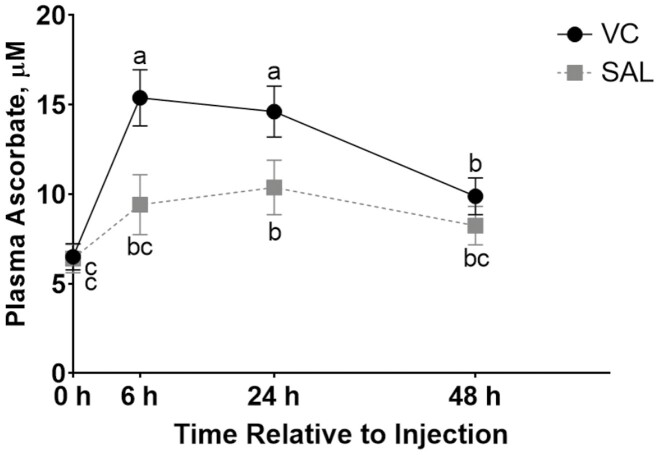
A TRANS × time effect (P = 0.04) was observed for plasma ascorbate samples collected on days 49 (pretransit and posttransit), 50 (24-h posttransit), and 51 (48-h posttransit; Figure 3). Pretransit plasma ascorbate concentrations were similar among treatments. Steers receiving injectable VC pretransit had greater plasma ascorbate concentrations posttransit (day 49), which persisted into day 50 compared with SAL steers. By day 51, concentrations did not differ between VC treatments and were beginning to decrease but had not reached baseline values. Plasma ascorbate was not affected (P ≥ 0.15) by WEAN, WEAN × TRANS, WEAN × time, or WEAN × TRANS × time effects.
Figure 3.
Effect of injectable vitamin C (VC) treatment and time of sampling relative to a 6-h transit event on day 49 on plasma ascorbate concentrations in early-weaned (65 ± 11 d of age) beef steers. Treatments were applied immediately prior to transit (TRANS) on day 49 including intramuscular injections (20 mL per steer) of VC (250-mg sodium ascorbate per mL; 5 g per steer) or saline (SAL; 20 mL per steer). Samples were collected on day 49 at hours 0 (pretransit), 6 (off-truck; day 49), and 24 and 48 postinjection (days 50 and 51). Means were separated across all time points and values with unlike superscripts differ (P ≤ 0.05). Effects of TRANS × time (P = 0.04), TRANS (P = 0.01), and time (P < 0.01) were observed. N = 24 steers per treatment.
Discussion
In the first few months of life, beef calves in the United States are exposed to multiple stressors. These stressors include weaning and transit which decrease antioxidant status, increase morbidity, and reduce overall performance (Chirase et al., 2004; Cooke et al., 2013; Deters and Hansen, 2019). Therefore, the present study compared injectable VC, a major water-soluble antioxidant, to saline at the time of weaning and before transport in early-weaned beef steers. For this study, injectable VC was given to determine if a dose of the antioxidant could modulate the negative stress response observed in beef calves.
A 5-g dose of sodium ascorbate was given intramuscularly to Angus × Simmental steer calves (92 kg; 65 d of age) on the day of weaning (day 0) and immediately prior to a 6-h transit event (day 49). Previous research in heavier beef steers (356 kg) reported pretransit plasma ascorbate concentrations of 18.3 ± 3.7 µM/L (Deters and Hansen, 2020) which falls within the reference range Matsui (2012) proposed for beef cattle (17.1 to 28.2 µM/L). However, steers in the present study had initial (day 0) plasma ascorbate concentrations that were markedly less (3.7 ± 0.27 µM/L). This may be due to age, body stores, or health status. Most animals produce endogenous VC except humans, guinea pigs, and primates due to a mutation in the L-gulonolactone oxidase gene (Drouin et al., 2011). Cattle produce VC from glucose in the liver (Chatterjee, 1973), and this is likely the reason cattle do not have an established VC requirement (NASEM, 2016). The endogenous synthesis of VC in young calves appears to begin around 3 wk of age (Lundquist and Phillips, 1943; Cummins and Brunner, 1991). Calves are born with greater hepatic VC (Watts, 1950) and greater plasma VC (Bouda et al., 1980; Toutain et al., 1997) than that of their dams. Hepatic VC concentrations decline within 24 to 48 h of birth, while plasma VC concentrations decline within 2 to 3 d of age (Lundquist and Phillips, 1943; Bouda et al., 1980). Interestingly, Bouda et al. (1980) reported calves reach plasma ascorbate concentrations similar to their dams around 14 to 21 d of age, but then plasma ascorbate concentrations decline from 6 wk to 3 mo of age. The combination of age with onset of VC production and low body stores could explain the low plasma ascorbate concentrations observed in this study.
Steers receiving a VC injection at weaning had a greater than 400% increase in plasma ascorbate concentrations 24 h postinjection (VC; 19.6 ± 1.31 µM/L) relative to baseline concentration at weaning (day 0), and plasma VC concentrations remained increased through 48 h postinjection. Interestingly, plasma ascorbate concentrations of SAL more than doubled from day 0 to 1 (8.7 ± 1.31 µM/L). This could be a physiological stress response due to weaning as serum cortisol concentrations were greater on day 1 than on day 0. A prior study reported stressed rats had greater plasma VC concentrations due to the release of VC from the liver and adrenal gland (Lahiri and Lloyd, 1962a). The adrenal gland has the highest concentration of VC in the body (Levine and Morita, 1985) with concentrations 473 times greater than plasma in dairy calves (Toutain et al., 1997). Additionally, stimulation of the hypothalamic-pituitary-adrenal axis also has been shown to increase circulating concentrations of VC (Lahiri andLloyd; 1962b; Kipp and Rivers, 1987).
No differences in BW, ADG, or G:F were observed during the WEAN period. Both treatments had greater plasma ascorbate concentrations following day 0, and even the small increase in circulating plasma ascorbate in SAL may have provided sufficient antioxidant capacity to scavenge reactive oxygen species produced during the stress of weaning, and to aid in regeneration of antioxidants such as vitamin E. From days 0 to 14, SAL steers tended to have greater DMI which contrasts prior observations where steers given injectable VC had greater DMI after transit (Deters and Hansen, 2020). However, in that study, steers were transported for a much longer duration (18 h) and cattle receiving saline decreased plasma VC posttransit suggesting the oxidative stress depleted physiologically available VC.
At the time of the second injection (day 49), baseline plasma ascorbate concentrations had increased to 6.4 ± 0.54 µM/L, a 73% increase from plasma VC concentrations on day 0 and likely a result of calf age as the calves were approximately 114 d of age on the day of transit. For steers receiving an injection of VC prior to transit, plasma VC increased 140% when measured after 6 h of transit, compared with pretransit baseline concentrations (VC − posttransit; 15.4 ± 1.57 µM/L). Once again, steers receiving saline prior to transit increased plasma VC (by 47%), presumably due to the physiological stress of the transit event (SAL − posttransit; 9.4 ± 1.67 µM/L). A longer transit event would likely be required to observe a similar decrease in plasma VC in lightweight calves receiving saline compared with previous research evaluating effects of an 18-h transit (Deters and Hansen, 2020). Within 48 h of receiving the pretransit injections, both treatments in the present study had similar plasma VC concentrations (9.0 ± 1.07 µM/L) which is similar to previously observed trends for water-soluble injectable VC (Beenken et al., 2021).
There were no differences observed for off-truck BW for either TRANS injection treatment. After 14 d posttransit, the SAL–SAL steers had greater BW, with VC–VC and SAL–VC being intermediate, and VC–SAL being the lightest. This was unexpected, as it was hypothesized that steers receiving injectable VC would have greater BW and ADG posttransit compared with steers given saline. In the current study, there was no difference in ADG or G:F during the TRANS period; however, the steers given SAL at WEAN had greater DMI during the posttransit period compared with VC steers with no effect of pretransit injection treatment on DMI from days 49 to 107. Overall, no differences were observed in final BW, ADG, or G:F among treatments. The steers given SAL at time of WEAN had greater DMI for the duration of the entire trial and this was mostly due to increased DMI during the posttransit period. In contrast to the current study, Deters and Hansen (2020) observed steers given injectable VC prior to an 18-h transit event had greater ADG and a 7-kg advantage in final BW during the 57-d posttransit period compared with steers given saline. Given the low plasma ascorbate concentrations noted in the young calves used in the present study, it is possible the dose of injectable VC used in the present study was too large.
The calves were given 5 g (54 mg/kg BW) of VC at weaning and transit, the same total dose Deters and Hansen (2020) administered to much heavier steers (14.1 mg VC/kg BW). The VC body pool in dairy calves at 7 d of age was estimated to be 23 ± 6.8 mg/kg BW (Toutain et al., 1997) but no estimates for calves representative of those used in the present study have been made. Any additional exogenous VC was likely excreted through the kidneys and not reabsorbed due to saturation of active transport mechanisms (Toutain et al., 1997). The large dose administered to the young and lightweight calves may have decreased endogenous VC synthesis. Bock and Shwartz (1974) and Bissell and Guzelian (1979) reported that VC synthesis could be controlled in perfused rat liver by the concentrations of VC, indicating that exogenous supplementation of VC may inhibit VC synthesis. Pogge and Hansen (2013) observed a decrease in plasma VC concentration in Angus-cross steers (341 kg) receiving rumen-protected dietary VC (10 g per steerper d) compared with nonsupplemented steers, which could be due to changes in endogenous production of VC due to VC supplementation. Given the low plasma ascorbate concentrations noted in the present study, other antioxidant pools in the body may be more critical for young calves. Further work is needed to determine the effects of lower doses of injectable VC across a range of young calf ages.
On the day of weaning, steers received several booster vaccinations, and BVDV1 and BVDV2 antibody titers were analyzed to determine if there was an antibody titer response to VC. Bovine viral diarrhea virus titers type 1 did not increase from days 0 to 14 and 51, indicating the booster vaccination was ineffective. The presence of maternal antibodies for BVDV1 and BVDV2 may have decreased the efficacy of the vaccine, thus limiting the titer response. Maternal antibodies for BVDV are effective for 3 to 6 mo after birth (Fulton et al., 2004). Calf age and antibody status are essential to consider when designing a vaccination protocol. The young age of the calves could explain the low plasma ascorbate concentrations, lack of performance differences due to VC treatment, and ineffectiveness of the booster vaccine. Early-weaned calves have different nutritional and immune needs than conventionally-weaned calves at approximately 7 mo of age. Future work should examine the influence of varying doses of VC given to calves experiencing varying degrees of weaning and transit stress.
The hypothesis that two injections of VC administered during stressful events in the early life of calves would influence growth and performance by improving antioxidant status was not supported by the results of this study. While steers had increased plasma VC concentrations when given an injection of VC, no performance differences were observed, potentially because neither the weaning nor transit event resulted in decreased plasma VC for steers receiving SAL. To conclude, 5 g of sodium ascorbate prior to or at the time of the stress event (weaning or transportation), had no positive influence on performance of early-weaned steers. More research is needed in young calves to investigate VC synthesis, metabolism, and to develop a useful reference range for plasma VC concentration for different ages of cattle. Several studies have shown VC to improve performance, but further investigation is needed to discover the ideal timing and dosage of exogenous VC, as limited information is available for beef cattle and injectable vitamins.
Glossary
Abbreviations
- ADG
average daily gain
- BVDV1
bovine viral diarrhea virus type 1
- BVDV2
bovine viral diarrhea virus type 2
- BW
body weight
- DM
dry matter
- DMI
dry matter intake
- G:F
gain to feed ratio
- SAL
saline
- TMR
total mixed ration
- TRANS
effect of treatments applied at transit
- VC
vitamin C
- WEAN
effect of treatments applied at weaning
Contributor Information
Aubree M Beenken-Bobb, Department of Animal Science, Iowa State University, Ames, IA 50011, USA.
Colten W Dornbach, Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Erin L Deters, Department of Animal Science, Iowa State University, Ames, IA 50011, USA.
Daniel W Shike, Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Stephanie L Hansen, Department of Animal Science, Iowa State University, Ames, IA 50011, USA.
Joshua C McCann, Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Conflict of Interest
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Beenken, A. M., Deters E. L., and Hansen S. L.. . 2021. The effect of injectable vitamin C and road transit duration on inflammation, muscle fatigue, and performance in pre-conditioned beef steer calves. J. Anim. Sci. 99:1–9. doi: 10.1093/jas/skab312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell, D. M., and Guzelian P. S.. . 1979. Ascorbic acid deficiency and cytochrome P-450 in adult rat hepatocytes in primary monolayer culture. Arch. Biochem. Biophys. 192:569–576. doi: 10.1016/0003-9861(79)90127-9 [DOI] [PubMed] [Google Scholar]
- Bock, K. W., and Shwartz L. R.. . 1974. Formation of L-ascorbic acid in perfused rat liver. Naunyn-Schmiedeberg’s Arch. Pharmacol. 284:307–310. doi: 10.1007/BF00500349 [DOI] [PubMed] [Google Scholar]
- Bouda, J., Jagos P., Dvorak R., and Ondrova J.. . 1980. Vitamin E and C in the blood plasma of cows and their calves fed from buckets. Acta Vet. Brno 49:53–58. doi: 10.2754/avb198049010053 [DOI] [Google Scholar]
- Chatterjee, I. B. 1973. Evolution and the biosynthesis of ascorbic acid. Science 182:1271–1272. doi: 10.1126/science.182.4118.1271 [DOI] [PubMed] [Google Scholar]
- Chirase, N. K., Greene L. W., Purdy C. W., Loan R. W., Auvermann B. W., Parker D. B., Walborg E. F., Stevenson D. E., Xu Y., and Klaunig J. E.. . 2004. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 65:860–864. doi: 10.2460/ajvr.2004.65.860 [DOI] [PubMed] [Google Scholar]
- Combs, G. F. 2012. Vitamin C. In: The vitamins: fundamental aspects in nutrition and health. 3rd ed. Burlington (MA):Elsevier Academic Press; p. 235–263. [Google Scholar]
- Cooke, R. F. 2017. Invited Paper: nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573 [DOI] [Google Scholar]
- Cooke, R. F., Guarnieri Filho T. A., Cappellozza B. I., and Bohnert D. W.. . 2013. Rest stops during road transport: impacts on performance and acute-phase protein responses of feeder cattle. J. Anim. Sci. 91:5448–5454. doi: 10.2527/jas.2013-6357 [DOI] [PubMed] [Google Scholar]
- Cummins, K. A., and Brunner C. J.. . 1991. Effect of calf housing on plasma ascorbate and endocrine and immune function. J. Dairy Sci. 74:1582–1588. doi: 10.3168/jds.S0022-0302(91)78320-3 [DOI] [PubMed] [Google Scholar]
- Deters, E. L., and Hansen S. L.. . 2019. Effect of supplementing a Saccharomyces cerevisiae fermentation product during a preconditioning period prior to transit on receiving period performance, nutrient digestibility, and antioxidant defense by beef steers. Trans. Anim. Sci. 3:1227–1237. doi: 10.1093/tas/txz140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deters, E. L., and Hansen S. L.. . 2020. Pre-transit vitamin C injection improves post-transit performance of beef steers. Animal 14:2083–2090. doi: 10.1017/S1751731120000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin, G., Godin J. R., and Page B.. . 2011. The genetics of vitamin C loss in vertebrates. Curr. Genomics 12:378. doi: 10.2174/138920211796429736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, R. W., Briggs R. E., Payton M. E., Confer A. W., Saliki J. T., Ridpath J. F., Burge L. J., and Duff G. C.. . 2004. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine 22:643–649. doi: 10.1016/j.vaccine.2003.08.033 [DOI] [PubMed] [Google Scholar]
- Hughes, D. A. 1999. Effects of dietary antioxidants on the immune function of middle-aged adults. Proc. Nutr. Soc. 58:79–84. doi: 10.1079/pns19990012 [DOI] [PubMed] [Google Scholar]
- Kalkwarf, E. 2014. Procedure for virus neutralization (VN) test. Ames (IA):Iowa State University Diagnostic Laboratory (ISUVDL). [Google Scholar]
- Kipp, D. E., and Rivers J. M.. . 1987. Uptake and release of adrenal ascorbic acid in the guinea pigafter injection of ACTH. J. Nutr. 117:1570–1575. doi: 10.1093/jn/117.9.1570 [DOI] [PubMed] [Google Scholar]
- Lahiri, S., and Lloyd B. B.. . 1962a. The form of vitamin C released by the rat adrenal. Biochem. J. 84:474–477. doi: 10.1042/bj0840474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri, S., and Lloyd B. B.. . 1962b. The effect of stress and corticotrophin on the concentrations of vitamin C in blood and tissue of the rat. Biochem. J. 84:483. doi: 10.1042/bj0840478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M., and Morita K.. . 1985. Ascorbic acid in endocrine systems. Vitam. Horm. 42:1–64. doi: 10.1016/S0083-6729(08)60060-6 [DOI] [PubMed] [Google Scholar]
- Lundquist, N. S., and Phillips R. H.. . 1943. Certain dietary factors essential for the growing calf. J. Dairy Sci. 26:1030. doi: 10.3168/jds.S0022-0302(43)92802-4 [DOI] [Google Scholar]
- Marques, R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. . 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90:5040–5046. doi: 10.2527/jas.2012-5425 [DOI] [PubMed] [Google Scholar]
- Matsui, T. 2012. Vitamin C nutrition in cattle. Asian-Australasian J. Anim. Sci. 25:597–605. doi: 10.5713/ajas.2012.r.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli, G. A., Rosa D. E., Turic E., Picco S. J., Raggio S. J., Minervino A. H. H., and Fazzio L. E.. . 2020. Effects of parenteral supplementation with minerals and vitamins on oxidative stress and humoral immune response of weaning calves. Animals 10:1–9. doi: 10.3390/ani10081298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle. 8th rev ed. Washington (DC):National Academies Press. [Google Scholar]
- Packer, J. E., Slater T. F., and Willson R. L.. . 1979. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 278:737–738. doi: 10.1038/278737a0 [DOI] [PubMed] [Google Scholar]
- Padilla, L., Matsui T., Kamiya Y., Kamiya M., Tanaka M., and Yano H.. . 2006. Heat stress decreases plasma vitamin C concentration in lactating cows. Livest. Sci. 101:300–304. doi: 10.1016/j.livprodsci.2005.12.002 [DOI] [Google Scholar]
- Pogge, D. J., and Hansen S. L.. . 2013. Supplemental vitamin C improves marbling in feedlot cattle consuming high sulfur diets. J. Anim. Sci. 91:4303–4313. doi: 10.2527/jas.2012-5638 [DOI] [PubMed] [Google Scholar]
- Toutain, P. L., Béchu D., and Hidiroglou M.. . 1997. Ascorbic acid disposition kinetics in the plasma and tissues of calves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273:R1585–R1597. doi: 10.1152/ajpregu.1997.273.5.r1585 [DOI] [PubMed] [Google Scholar]
- Watts, P. S. 1950. Studies on vitamins A and C in bovines: vitamin C in the liver, kidney, and plasma of cows, calves, and foetuses. J. Comp. Pathol. Ther. 60:283–293. doi: 10.1016/s0368-1742(50)80026-7 [DOI] [PubMed] [Google Scholar]