Abstract
This experiment evaluated the effects of roughage levels and supplemental fat on intake, growth performance, health, and physiological responses of newly received finishing cattle during 58-d receiving period. A total of 72 crossbred steers (initial body weight [BW] = 200 ± 13 kg) were used in a randomized complete block design with a 2 × 2 factorial arrangement of treatments, consisting of two roughage levels (wheat hay at 30% [R30] or 60% [R60]; dry matter [DM] basis) and two levels of supplemental fat (yellow grease at 0% [−FAT; no additional fat] or 3.5% [+FAT]; DM basis). Upon arrival, calves were individually weighed, blocked by off-truck shrunk BW, and assigned to 24 soil-surfaced pens (three calves per pen). Shrunk BW was also collected on day 58 for the calculation of average daily gain (ADG). Throughout the study, calves were assessed for bovine respiratory disease (BRD). Effects of roughage level × supplemental fat interaction were only observed for diet particle size distribution and estimated physically effective neutral detergent fiber (peNDF) of diets (P ≤ 0.10). Adding fat to R60 diets tended to increase the percentage of particles retained in the 8-mm screen (P = 0.06) and the estimated peNDF (P = 0.10), but did not affect R30 diets. Dietary roughage level did not affect DM intake (DMI; P = 0.85). Calves-fed R30 tended to have greater ADG and final BW than calves-fed R60 (P ≤ 0.08). Gain efficiency (gain:feed ratio; G:F) was greater for calves-fed R30 than calves-fed R60 (P = 0.01). Dietary roughage level did not affect morbidity and mortality (P ≥ 0.11). Supplemental fat did not affect DMI (P = 0.6) but tended (P = 0.09) to increase ADG compared to –FAT diets. The G:F was greater for calves-fed +FAT than –FAT (P = 0.03). The +FAT diet tended (P = 0.10) to increase the number of retreatments against BRD compared to −FAT, although the total number of antimicrobial treatments required to treat sick calves (P = 0.78) and the mortality rate (P = 0.99) were not affected by supplemental fat. Feeding +FAT diet tended (P ≤ 0.09) to increase plasma concentration of cortisol and immunoglobulin-G compared to –FAT. In summary, feeding 30% roughage diets or adding 3.5% yellow grease (DM basis) as supplemental fat increased G:F during the feedlot receiving period.
Keywords: beef cattle, fatty acids, feedlot receiving, fiber, morbidity
Supplementing 3.5% of yellow grease to receiving feedlot calves increased gain:feed.
Introduction
According to Blakebrough-Hall et al. (2020), bovine respiratory disease (BRD) is the single largest cause of clinical disease and death in the feedlot industry. The incidence of BRD is greater within the first days after feedlot arrival (often referred to as the receiving period; Snowder et al., 2006), usually the first 4 to 8 wk in which lightweight beef calves adjust to the feedlot environment, especially to new nutritional management (Richeson et al., 2019). During these first weeks, low dry matter intake (DMI; Hutcheson and Cole, 1986), associated with the stress of weaning, marketing, and road transportation, results in inadequate nutrient intake, especially energy, which increases the negative effects of stress on inflammation and immune function (Duff and Galyean, 2007; Richeson et al., 2019).
Feeding high-concentrate diets to newly received calves is an alternative to increase energy intake and average daily gain; however, the impacts on morbidity and mortality from BRD are still variable and deserve more investigation (Lofgreen et al., 1975, 1981; Rivera et al., 2005). Decreasing the amount of concentrate and increasing dietary roughage level could be an alternative to reduce BRD morbidity in lightweight, high-risk receiving calves (Lofgreen et al., 1981; Rivera et al., 2005). However, according to Rivera et al. (2005), the benefits of including higher amounts of roughage in the receiving diets on morbidity and mortality would not offset the loss in profit resulting from less average daily gain (ADG).
Adding fat to finishing diets is an alternative to increase energy intake (Zinn and Jorquera, 2007). Since energy is the first limiting component for newly arrived finishing cattle (Duff and Galyean, 2007; Richeson et al. 2019) and activation of the immune system is an energy-dependent process (Batistel et al., 2018), increasing fat concentration in receiving diets could be an alternative to overcome the negative effects of low DMI after feedlot arrival and improve animal performance and health. Information about sources and levels of fat in receiving diets, especially in low-starch feedlot diets, are scarce and variable (Cole and Hutcheson, 1987; Fluharty and Loerch, 1997; Zinn and Jorquera, 2007) and deserves further investigation.
Zinn and Jorquera (2007) recommended that receiving diets should not contain more than 2% supplemental fat due to some negative effects related to diet acceptability. According to Samuelson et al. (2016), only 18.2% of the nutritionists in the United States recommended their clients use either yellow grease or fat blends in receiving diets, and the maximum suggested total fat concentration in receiving diet was 3.75% of diet DM.
We hypothesized that adding fat to receiving diets containing a greater amount of roughage would be an alternative to increase energy intake and growth performance and reduce the negative effects of stress on the immune function of newly received calves compared to diets containing greater amounts of concentrate. Thus, the objective of this study was to evaluate the effects of supplemental yellow grease in diets containing different roughage levels on intake, growth performance, and health of newly received finishing calves.
Materials and Methods
This study was conducted at the Clayton Livestock Research Center, Clayton, NM. All animals used in this experiment were cared for in accordance with guidelines required by the New Mexico State University, Institutional Animal Care and Use Committee (approved protocol # 2020-001).
Animals and treatments
Seventy-two crossbred steers sourced from commercial auctions (British and British × Continental; initial shrunk body weight [BW]= 200 ± 13 kg) were used in this trial. Calves were transported approximately 1,300 km in one commercial trailer from Delhi, LA, to the Clayton Livestock Research Center in Clayton, NM (16 h on truck) and processed immediately after feedlot arrival using a “Bud Box” System (Daniels Bud Box System; AH-10 Stationary Squeeze Chute; Ainsworth, NE) before access to feed or water (off-truck shrunk BW). All calves were individually weighed (LPBWB, Avery Weigh-Tronix, Fairmont, MN), given a unique ID and pen tag, dewormed, (febendazole 10%; 10 mL/head orally; Safe-Guard, Merck Animal Health, Summit, NJ), vaccinated against respiratory disease (2.0 mL sc. injection of modified live virus, Vista Once SQ, Merck Animal Health) and clostridiosis (5.0 mL sc. injection of Covexin 8, Merck Animal Health), and growth-promoting implanted with 36 mg of Zeranol (3 to 12 mg pellets sc., Ralgro, Merck Animal Health). Once processed, calves were blocked by shrunk BW and assigned to 24 soil-surfaced pens (5 × 10 m; 3 calves/pen and 6 pens/treatment) equipped with water fountains and 1.5 m of feed bunk space.
The experiment was a randomized complete block design with a 2 × 2 factorial arrangement of treatments, consisting of two roughage levels (wheat hay at 30% [R30] or 60% [R60]; dry matter [DM] basis) combined with two levels of supplemental fat (yellow grease at 0% [−FAT; no supplemental fat] or 3.5% [+FAT]; DM basis). Diets were formulated to meet the nutrient requirements of beef calves as specified by NASEM (2016) and to contain equivalent concentration of crude protein (Table 1).
Table 1.
Ingredient and chemical composition of the experimental diets (DM basis)
| Item | Roughage level, % of DM | |||
|---|---|---|---|---|
| 30 | 60 | |||
| −FAT | +FAT | −FAT | +FAT | |
| Ingredient, % of DM | ||||
| Wheat hay | 30.0 | 30.0 | 60.0 | 60.0 |
| Steam flaked corn | 46.3 | 42.8 | 17.3 | 13.8 |
| Wet corn gluten feed1 | 18.0 | 18.0 | 18.0 | 18.0 |
| Soybean meal | 3.00 | 3.00 | 2.00 | 2.00 |
| Urea | 0.20 | 0.20 | 0.20 | 0.20 |
| Yellow grease | - | 3.50 | - | 3.50 |
| Mineral and vitamin supplement2 | 2.50 | 2.50 | 2.50 | 2.50 |
| Analyzed composition, % | ||||
| Dry matter | 80.5 | 80.9 | 82.0 | 82.5 |
| Crude protein | 13.7 | 13.2 | 13.4 | 13.3 |
| Ether extract | 2.68 | 5.40 | 2.31 | 5.39 |
| Neutral detergent fiber | 29.6 | 32.0 | 46.1 | 44.5 |
| In vitro NDFD3 | 65.0 | 52.8 | 58.0 | 52.6 |
| Total digestible nutrients4 | 80.0 | 82.9 | 69.8 | 72.2 |
| ME, Mcal/kg4 | 2.96 | 3.07 | 2.57 | 2.67 |
| NEm, Mcal/kg4 | 1.99 | 2.08 | 1.66 | 1.75 |
| NEg, Mcal/kg4 | 1.34 | 1.41 | 1.05 | 1.12 |
1 Sweet Bran (Cargill Sweet Bran, Dalhart, TX).
2 Formulated to meet mineral and vitamin requirements for receiving calves according to NASEM (2016). Containing (DM basis): 7 mg/kg Co, 440 mg/kg Cu, 24 mg/kg I, 1060 mg/kg Mn, 6.6 mg/kg Se, 200 mg/kg/Zn, 97,000 IU/kg Vit. A, 12,320 IU/kg Vit. D, 1,672 IU/kg Vit. E.
3 In vitro neutral detergent fiber digestibility (in vitro NDFD) was determined after 48 h incubation using the two-stage method described by Tilley and Terry (1963).
4 Total digestible nutrients (TDN), metabolizable energy (ME), net energy for maintenance, and gain (NEm and NEg, respectively) were calculated based on tabular values from NASEM (2016).
Management and sampling
Calves were fed once daily at 0700 h. Feed ingredients were individually weighed using a digital platform scale with 0.05 kg resolution (Angel SS-400, Angel POS, Burnaby, Canada), mixed using a stationary mixer (18 cubic feet) with a Davis ribbon and paddle agitator (Davis HD-3 Heavy Duty Mixer; H.C. Davis Sons Manufacturing Co., Inc., Bonner Springs, KS) for 3 min, weighed into 165-kg-capacity plastic containers using the same digital platform scale (Angel SS-400, Angel POS), and delivered manually to each pen. To prevent cross-contamination, −FAT diets were mixed prior +FAT diets, and the mixer was completely emptied between loads.
Feed bunks were monitored at 1300, 1800, and 0630 h daily to make assessments for the next feed calls. The amount of feed offered to each pen was adjusted based on the DMI of the previous day, and bunks were managed for a maximum of 5% orts. Orts were removed daily, weighed, sampled for DM determination and DMI calculation, and particle size analysis, and discarded. Samples of the diets were collected every other week and composed for analysis of DM and nutrient composition by a commercial laboratory (Cumberland Valley Analytical Services, Waynesboro, PA; DM at 105 °C [method 930.15; AOAC, 2000], total nitrogen [N; Leco FP-528 Nitrogen Combustion Analyzer; Leco Corp., St. Joseph, MI], neutral detergent fiber (NDF) using sodium sulfite and heat stable amylase [aNDF; Van Soest et al., 1991], acid detergent fiber and lignin [LIG, method 973.18; AOAC, 2000], ash [method 942.05; AOAC, 2000], and crude fat [method 2003.05; AOAC, 2000]). The fatty acid profile of yellow grease was analyzed using gas chromatography (Sukhija and Palmquist, 1988; Table 2). The crude protein concentration was calculated by multiplying nitrogen concentration by 6.25. The NDF digestibility of diets (IVNDFD) was determined using the two-stage method described by Tilley and Terry (1963). Hay, diets offered, and refusals were also collected on days 9, 20, 29, and 43 for particle size distribution analysis and particle sorting calculation. Particle size distribution was determined using the Penn State Particle Separator method described by Kononoff et al. (2003). Particle sorting was calculated as the actual intake of each fraction according to Leonardi and Armentano (2003), as follows: n intake/n predicted intake, in which n = particle fraction retained in each screen (19 mm, 8 mm, 4 mm, 1.18 mm, or bottom [<1.18 mm]). The predicted intake was calculated as the product of as-fed intake × as-fed intake of each fraction (screen). Selective consumption values equal to 1 indicate no sorting, <1 indicate selective refusals (sorting against), and >1 indicate preferential consumption (sorting for).
Table 2.
Fatty acid composition of yellow grease
| Item | |
|---|---|
| Dry matter, % as-fed | 98.7 |
| Total fatty acids, % of DM1 | 85.7 |
| Free fatty acids, % of DM2 | 14.8 |
| Fatty acid profile, g/100 g of fatty acid methyl esters1 | |
| Lauric (C12:0) | 0.09 |
| Myristic (C14:0) | 0.67 |
| Pentadecanoic (C15:0) | 0.10 |
| Palmitic (C16:0) | 12.7 |
| Palmitoleic (C16:1) | 0.84 |
| Heptadecanoic(C17:0) | 0.29 |
| Stearic (C18:0) | 6.19 |
| Oleic (C18:1 cis-9) | 27.0 |
| Vaccenic (C18:1 cis-11) | 1.77 |
| Linoleic (C18:2 n-6) | 30.5 |
| Linolenic (C18:3 n-3) | 4.05 |
| Arachidic (C20:0) | 0.29 |
| Eicosenoic (C20:1 cis-11) | 0.33 |
| Eicosadienoic (C20:2 n-6) | 0.07 |
| Behenoic (C22:0) | 0.21 |
| Lignoceric (C24:0) | 0.09 |
| Other (C12:0 to C24:0) | 0.63 |
| Total SFA | 20.63 |
| Total MUFA | 29.94 |
| Total PUFA | 34.62 |
1 Analyzed composition according to Sukhija and Palmquist (1988).
2 Maximum level according to the manufacturer (Texas Feed Fat Co., Inc., Hereford, TX).
The study was 58 d in length. Individual shrunk BW was collected on day 0 and again on day 58 after 16 h of feed and water withdrawal for ADG and gain efficiency (gain:feed ratio; G:F) calculation. Dietary net energy (NE) of maintenance (NEm) and gain (NEg) were estimated based on observed growth performance according to Zinn and Shen (1998). These calculated NE concentrations were compared with those predicted based on tabular values from NASEM (2016; Table 1). Blood samples were collected before feeding on days 0, 7, 14, 28, and 58 of the study from the jugular vein using 20-gauge BD Vacutainer needles into collection tubes containing 158 US pharmacopeia units of spray-coated lithium heparin as an anticoagulant for plasma or clot activator for serum collection (Vacutainer, 10 mL; Becton, Dickinson and Company, Franklin Lakes, NJ). After blood collection, samples were placed on ice until centrifugation (~30 min). Plasma and serum were obtained by centrifugation at 2500 × g for 30 min at 4 °C and aliquots were frozen (−80 °C) until further analysis.
Plasma samples were analyzed for concentration of cortisol (radioimmunoassay kit #07221106, MP Biomedicals, Santa Ana, CA; Colombo et al., 2020), haptoglobin (peroxidase activity; Cooke and Arthington, 2012), and glucose (colorimetry; Carysta High Volume Chemistry Analyzer; Zoetis), interleukin-6 (IL-6; colorimetric kit #MBS2609347; MyBioSource, San Diego, CA), tumor necrosis factor-α (TNF-α; colorimetric kit #MBS2609886; MyBioSource, San Diego, CA), immunoglobulin-G (IgG; colorimetric kit #MBS282946; MyBioSource, San Diego, CA), and urea nitrogen (PUN; colorimetric kit #EIABUN; Thermo Fisher Scientific; Waltham, MA). The aliquots used to determine IL-6 and TNF-α were concentrated using a vacuum concentrator before analysis. The LOD and LOQ for IL-6 were 1.3 and 4.0, respectively. The LOD and LOQ for TNF-α were 6.1 and 18.5, respectively. Samples for IgG were diluted 1/60 before analysis.
Serum samples were analyzed for antibodies against bovine viral diarrhea viruses type I using commercially available enzyme-linked immunosorbent assay (ELISA; BVDV Total Ab Test, #99-44000; IDEXX Switzerland AG, Liebefeld-Bern, Switzerland) as described by Gonda et al. (2012). Only samples from steers not diagnosed with BRD signs were analyzed for antibodies against BRD pathogens to ensure that this response was associated with vaccine efficacy rather than pathogenic infection (Callan, 2001). The intra- and interassay CV were, respectively, 2.59% and 4.19% for cortisol, 8.15% and 8.38% for haptoglobin, 4.33% and 6.61% for glucose, 3.76% and 5.27% for PUN, 5.60% and 9.45% for TNF-α, 4.37% and 7.84% for IL-6, and 4.76% and 8.14% for IgG.
Throughout the study, one trained evaluator conducted animal health evaluations implementing a four-point scale method based on depression, anorexia, respiratory, and temperature (“DART”) as described by Step et al. (2008) and Wilson et al. (2015). Briefly, calves were pulled from their pens for further assessment when showing depression, respiratory distress, or abnormal appetites. Any steer identified with a severity score of 1 or 2 and with a rectal temperature of 40 °C or greater received an antimicrobial according to label instructions. If a steer was identified with a severity score of 1 or 2 and with a rectal temperature of less than 40 °C, no antimicrobial treatment was administered. Any steer with severe clinical signs (severity score = 3 or 4) received an antimicrobial according to label instructions regardless of rectal temperature. Initial medical treatment for a sick animal was an injection of florfenicol antibiotic with flunixin meglumine (Resflor Gold, Merck Animal Health, Madison, NJ). If a second medical treatment was warranted, the steer received an injection of ceftiofur crystalline free acid (Excede, Zoetis, Florham Park, NJ), and a steer’s third medical treatment (if warranted) was an injection of oxytetracycline (Bio-Mycin 200, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO). After each medical treatment, the steer received an ear tag to demonstrate that it had been treated, and was assigned to a 5-d moratorium before receiving a subsequent medical treatment.
Statistical analysis
Quantitative data were analyzed using the MIXED procedure of SAS (version 9.4, SAS Inst. Inc., Cary, NC), whereas binary data were analyzed using the GLIMMIX procedure of SAS with a binomial distribution and logit link function. All data were analyzed as a randomized complete block design with a 2 × 2 factorial arrangement of treatments. Pen was the experimental unit, and the Satterthwaite approximation method was used to determine the correct denominator degrees of freedom for the test of fixed effects. The statistical model for DMI, ADG, G:F included the fixed effects of roughage level, fat, and roughage level × fat interaction. Pen (roughage level × fat), steer (pen), and BW block were considered random variables for BW and ADG analyses. The random variables for G:F included pen (roughage level × fat) and BW block. Serum and plasma variables were analyzed as repeated measurements. Sampling day was the specified term for the repeated measurement and pen (roughage level × fat) was the subject. The statistical model included the fixed effects of roughage level, fat, day, and roughage level × fat × day interaction. The covariance structure used was compound symmetry, based on the smallest Akaike information criteria. Data from day 0 were used as a covariate if differences between the treatments at day 0 were observed for any variable. When no significant interactions were detected, the main effects of roughage level and supplemental fat were evaluated. When significant interactions were observed, multiple comparisons were performed using the Tukey–Kramer test. Results are reported as least square means. Significance was set at P ≤ 0.05 and tendencies at P > 0.05 and ≤0.10.
Results
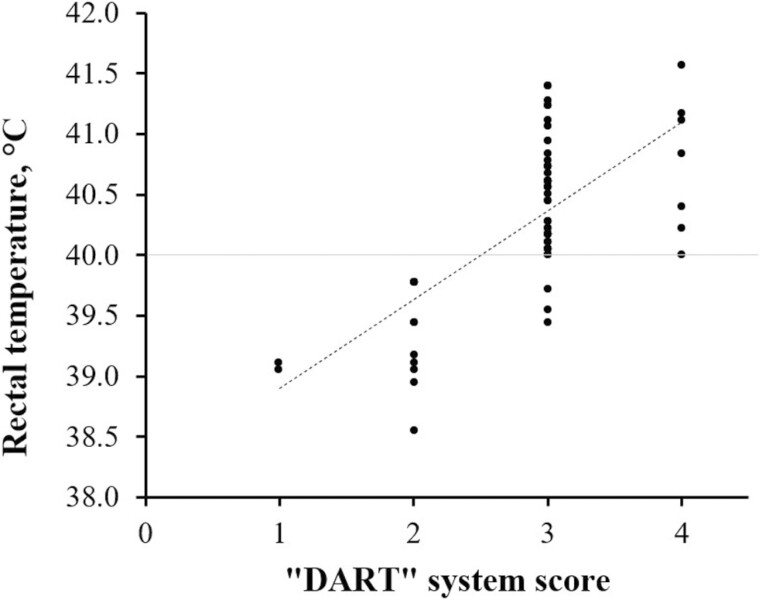
The descriptive data of rectal temperature and “DART” score of all calves pulled from their pens for examination of signs consistent with BRD are presented in Figure 1. None of the calves scored 1 or 2 in the current trial had rectal temperature greater than 40 °C, and rectal temperature increased with increasing severity score.
Figure 1.
Descriptive data of rectal temperature and “DART” score of all calves pulled from their pens for examination of signs consistent with bovine respiratory disease and treatment according to the procedures described by Step et al. (2008) and Wilson et al. (2015). Calves were monitored by one trained evaluator daily throughout the study (58 d).
Effects of roughage level × supplemental fat
Effects of roughage level × supplemental fat interaction were observed only for diet particle size distribution (P < 0.01) and a tendency was observed for estimated peNDF of diets (P = 0.10; Table 3). Adding fat to R60 diets tended to increase the percentage of particles retained on the 8-mm screen (P = 0.06; 26.5 vs. 35.3% for R60-FAT and R60+FAT, respectively) and the peNDF (P = 0.10; 23.9 vs. 25.6% for R60-FAT and R60+FAT, respectively), but did not affect R30 diets (P ≥ 0.05; average = 23.5% and 15.5% for particles retained on the 8-mm screen and peNDF, respectively; Table 3). As a result, the percentage of particles retained on the 4-mm screen was reduced when fat was added to R60 diets (P <0.01; 24 vs. 20.5% for R60-FAT and R60+FAT, respectively), but not in R30 diets (P ≥ 0.05; average = 24.8%; Table 3).
Table 3.
Particle size separation, estimated physically effective neutral detergent fiber (peNDF), and particle sorting of diets containing different roughage levels (wheat hay; 30% [R30] or 60% [R60]; DM basis) and supplemental fat (yellow grease; 0 (−FAT) or 3.5% [+FAT]; DM basis)
| Item | Wheat hay1 | R30 | R60 | SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| −Fat | +Fat | −Fat | +Fat | Roughage | Fat | Roughage × Fat | |||
| Pens (steers) | - | 6 (18) | 6 (18) | 6 (18) | 6 (18) | - | - | - | - |
| NDF, % DM | 58.8 | 27.4 | 27.0 | 41.8 | 41.4 | - | - | - | - |
| Diet offered | Retained/sieve (%) | ||||||||
| Particle size, % DM | |||||||||
| 19 mm | 41.1 | 7.66 | 11.1 | 6.60 | 6.02 | 3.00 | 0.33 | 0.65 | 0.52 |
| 8 mm | 9.33 | 25.6b | 21.4b | 26.5b | 35.3a | 3.12 | 0.04 | 0.48 | 0.06 |
| 4 mm | 21.4 | 23.2ab | 26.4a | 24.0a | 20.5b | 1.04 | 0.03 | 0.90 | <0.01 |
| 1.18 mm | 16.8 | 24.1b | 26.2a | 24.1b | 22.3c | 0.654 | 0.01 | 0.86 | 0.01 |
| <1.18 mm | 11.4 | 19.4 | 15.0 | 18.9 | 16.0 | 0.935 | 0.84 | 0.02 | 0.45 |
| peNDF3, % DM | 42.2 | 15.5c | 15.9c | 23.9b | 25.6a | 0.37 | <0.001 | 0.01 | 0.10 |
| Refusal, % of diet offered | - | 5.82 | 5.32 | 5.17 | 4.54 | 0.562 | 0.16 | 0.26 | 0.89 |
| Sorting | |||||||||
| Sieve size, mm | |||||||||
| 19 mm | - | 0.96 | 0.95 | 0.98 | 0.96 | 0.058 | 0.80 | 0.86 | 0.90 |
| 8 mm | - | 1.01 | 0.98 | 1.10 | 0.93 | 0.047 | 0.96 | 0.08 | 0.27 |
| 4 mm | - | 1.00 | 0.94 | 1.06 | 1.12 | 0.056 | 0.03 | 0.96 | 0.29 |
| 1.18 mm | - | 0.90 | 0.98 | 0.95 | 1.07 | 0.053 | 0.17 | 0.07 | 0.67 |
| <1.18 mm | - | 0.85 | 0.96 | 0.82 | 1.00 | 0.054 | 0.81 | <0.01 | 0.47 |
1 Descriptive data (n = 4).
2 Standard error of the mean.
3 peNDF was calculated by multiplying the percentage of particles ≤4 mm (top three sieves) by the percentage of Neutral Detergent Fiber (NDF) of the ingredient/sample prior to separation.
a,b,c Means without a common superscript differ (Tukey test; P < 0.05).
Adding fat to R30 diets increased the percentage of particles retained on the 1.18-mm screen (P = 0.01; 24.1 vs. 26.2% for R30-FAT and R30+FAT, respectively), and tended to decrease the percentage of particles retained on the 1.18-mm screen in R60 diets (P = 0.07; 24.1 vs. 22.3% for R60-FAT and R60+FAT, respectively; Table 3).
No other effects of roughage level × supplemental fat were observed in this experiment (P ≥ 0.27); therefore, the main effect of roughage level and supplemental fat was presented separately.
Effects of roughage level
Feeding R60 increased the extent of sorting for particles retained on the 4-mm screen compared to R30 (P = 0.03; Table 3). No other effects of roughage level were observed for particle sorting (P ≥ 0.17) or the percentage of feed refusals (P = 0.16).
Dietary roughage level did not affect DMI (P = 0.85; Table 4), but calves-fed R30 tended to have greater ADG and final BW than calves-fed R60 (P ≤ 0.08), and G:F was greater for calves-fed R30 than calves-fed diets R60 (P = 0.01). Observed NEm and NEg calculated from performance data were greater for R30 than R60 (P = 0.01), although no differences in observed:expected NE ratio were detected between R30 and R60 (P = 0.22).
Table 4.
Growth performance, observed net energy concentration, morbidity, and mortality of newly received feedlot calves-fed diets containing different roughage levels (wheat hay; 30% or 60%; DM basis) and supplemental fat (yellow grease; 0 [−FAT] or 3.5% [+FAT]; DM basis)
| Item | Roughage level | Supplemental fat | SEM1 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| 30% | 60% | −FAT | +FAT | Roughage | Fat | Roughage × Fat | ||
| Pens (steers) | 12 (36) | 12 (36) | 12 (36) | 12 (36) | - | - | - | - |
| Days on feed | 58 | 58 | 58 | 58 | - | - | - | - |
| Growth performance | ||||||||
| Initial body weight, kg | 200 | 199 | 200 | 200 | 6.00 | - | - | - |
| Final body weight, kg | 285 | 269 | 270 | 284 | 11.2 | 0.08 | 0.13 | 0.94 |
| Dry matter intake, kg/d | 6.00 | 5.92 | 5.87 | 6.04 | 0.297 | 0.85 | 0.64 | 0.94 |
| Average daily gain, kg | 1.47 | 1.20 | 1.21 | 1.46 | 0.144 | 0.07 | 0.09 | 0.91 |
| Gain efficiency2 | 0.249 | 0.204 | 0.207 | 0.246 | 0.016 | 0.01 | 0.03 | 0.80 |
| Observed NE, Mcal/kg3 | ||||||||
| Maintenance | 2.09 | 1.84 | 1.86 | 2.07 | 0.08 | 0.01 | 0.02 | 0.88 |
| Gain | 1.42 | 1.21 | 1.23 | 1.40 | 0.07 | 0.01 | 0.02 | 0.87 |
| Observed:expected NE ratio4 | ||||||||
| Maintenance | 1.02 | 1.09 | 1.02 | 1.08 | 0.05 | 0.22 | 0.18 | 0.80 |
| Gain | 1.03 | 1.11 | 1.03 | 1.11 | 0.06 | 0.22 | 0.16 | 0.76 |
| Health | ||||||||
| Cattle treated for respiratory disease5, % | ||||||||
| First Treatment | 50.0 | 47.2 | 44.4 | 52.8 | 9.61 | 0.82 | 0.49 | 0.82 |
| Second Treatment | 19.4 | 11.1 | 8.33 | 22.2 | 6.50 | 0.32 | 0.10 | 0.32 |
| Third treatment | 11.1 | 2.78 | 5.56 | 8.33 | 6.10 | 0.14 | 0.62 | 0.62 |
| Number of antimicrobial treatments required | 1.75 | 1.34 | 1.51 | 1.58 | 0.272 | 0.11 | 0.78 | 0.30 |
| Mortality, %, (n) | 8.33 (3) | 2.78 (1) | 5.56 (2) | 5.56 (2) | 3.87 | 0.31 | 1.00 | 0.31 |
1 Standard error of the mean.
2 Gain-to-feed ratio (G:F; kg/kg).
3 Calculated according to Zinn and Shen (1998).
4 Expected values were calculated using the NASEM (2016)—Beef Cattle Nutrient Requirements Model, empirical solution model, based on the diet composition presented in Table 1.
5 Calves were observed daily for bovine respiratory disease (BRD) signs according to the 4-point scale method based on depression, anorexia, respiratory, and temperature (“DART” system) as described by Step et al. (2008) and Wilson et al. (2015).
Dietary roughage level did not affect morbidity and mortality (P ≥ 0.11; Table 4), despite the numerical increase in the number of calves-fed R30 that required a second (19.1% vs. 11.1% for R30 and R60, respectively) and a third treatment for BRD (11.1% vs. 2.38% for R30 and R60, respectively).
Plasma concentration of haptoglobin tended (P = 0.09; Table 5) to be greater for R30 compared to R60. No other effects of roughage level were observed for blood analytes evaluated in the present study (P ≥ 0.13).
Table 5.
Blood analytes of newly received feedlot calves-fed diets containing different roughage levels (wheat hay; 30% or 60%; DM basis) and supplemental fat (yellow grease; 0 [−FAT] or 3.5% [+FAT]; DM basis)
| Item1 | Roughage level | Supplemental fat | SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| 30% | 60% | −FAT | +FAT | Roughage | Fat | Roughage × Fat | ||
| Pens (steers) | 12 (36) | 12 (36) | 12 (36) | 12 (36) | - | - | - | - |
| Days on feed | 58 | 58 | 58 | 58 | - | - | - | - |
| Blood analytes | ||||||||
| Plasma cortisol, ng/mL | 17.1 | 18.5 | 16.2 | 19.5 | 1.58 | 0.42 | 0.07 | 0.23 |
| Plasma haptoglobin, ng/ mL | 0.44 | 0.33 | 0.33 | 0.43 | 0.050 | 0.09 | 0.13 | 0.92 |
| Plasma immunoglobulin-G, g/L | 28.0 | 29.2 | 27.1 | 30.0 | 1.20 | 0.49 | 0.09 | 0.70 |
| Plasma tumor necrosis-α, pg/ mL | 67.2 | 70.5 | 68.0 | 70.0 | 1.75 | 0.13 | 0.44 | 0.38 |
| Plasma interleukin-6, pg/mL | 7.30 | 7.23 | 7.50 | 7.06 | 0.31 | 0.86 | 0.37 | 0.84 |
| Plasma glucose, mg/dL | 81.3 | 79.5 | 81.1 | 79.7 | 2.82 | 0.57 | 0.65 | 0.97 |
| Plasma urea nitrogen, mg/dL | 11.0 | 11.1 | 11.1 | 10.9 | 0.20 | 0.73 | 0.57 | 0.30 |
| Serum antibody titers | ||||||||
| Bovine viral diarrhea virus-1 | 0.875 | 0.845 | 0.870 | 0.851 | 0.113 | 0.84 | 0.90 | 0.30 |
1 Plasma Immunoglobulin-G, plasma tumor necrosis-α, and plasma interleukin-6 were analyzed on days 0 and 7. Plasma cortisol, haptoglobin, glucose, and urea nitrogen, and serum antibody titers were analyzed on days 0, 7, 14, 28, and 58. Serum concentrations of antibodies against bovine viral diarrhea virus (BVDV) were reported as sample:positive control ratio (Gonda et al., 2012).
2 Standard error of the mean.
Effects of supplemental fat
Adding 3.5% yellow grease in the receiving diet (+FAT) decreased the percentage of particles <1.18 mm (P = 0.02), tended to decrease the extent of sorting for particles retained on the 4-mm screen (P = 0.08) and tended to increase the sorting for particles retained on the 1.18-mm screen (P = 0.07; Table 3). Also, +FAT decreased the extent of sorting for particles <1.18 mm (P < 0.01). The percentage of feed refusal was similar between −FAT and +FAT diets (P = 0.26).
Feeding +FAT did not affect DMI (P = 0.64) but tended (P = 0.09) to increase ADG and increased G:F compared to −FAT (P = 0.03; Table 4). Observed NEm and NEg calculated from performance data were greater for steers-fed +FAT than −FAT (P = 0.02), but no effect of yellow grease supplementation was detected on the observed:expected NE ratio (P = 0.16).
Feeding +FAT diet tended (P = 0.10) to increase the number of retreatments (calves that received a second treatment) against BRD compared to −FAT (Table 4), although the total number of antimicrobial treatments required to treat sick calves (P = 0.78), and the mortality rate (P = 1.0) was not affected by supplemental fat.
Feeding +FAT diet tended (P ≤ 0.09) to increase plasma concentration of cortisol and immunoglobulin-G compared to –FAT (Table 5). No other effects of adding 3.5% yellow grease to the receiving diet were observed for blood analytes (P ≥ 0.13).
Effects of sampling day
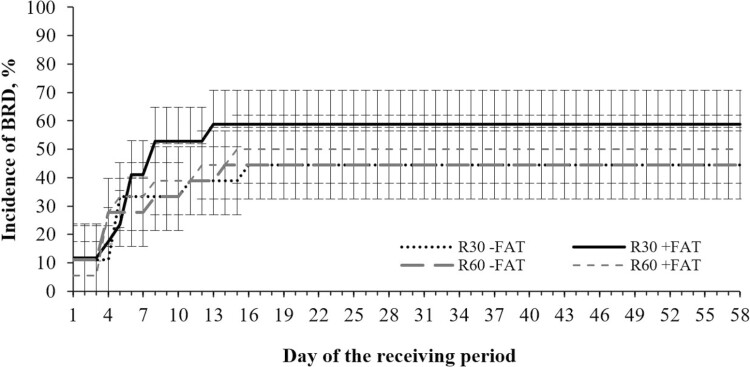
No effects of roughage level × supplemental fat × day were detected for morbidity (Figure 2) or any of the blood analytes (P ≥ 0.05) that were analyzed as repeated measures.
Figure 2.
Cumulative incidence of BRD signs in newly received feedlot calves-fed diets containing different roughage levels (wheat hay; 30% or 60%; DM basis) and supplemental fat (yellow grease; 0 [−FAT] or 3.5% [+FAT]; DM basis). Calves were observed daily for BRD signs according to the DART system (Step et al., 2008; Wilson et al., 2015). The graph represents the incidence of cattle treated with antimicrobials at least once during the experiment. No roughage level (P = 0.70), supplemental fat (P = 0.41), or roughage × fat × day interaction were detected (P = 0.90).
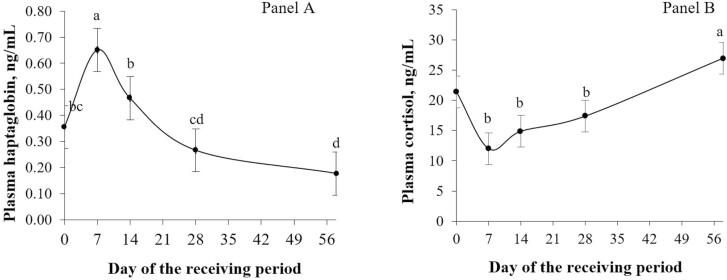
Effect of sampling day (P < 0.001; Figure 3) was observed for plasma concentration of haptoglobin (Panel A) and cortisol (Panel B). Plasma concentration of haptoglobin was greater on day 7 compared to all the other sampling days. The lowest concentration of plasma haptoglobin was observed on day 56 and was similar to day 28 (P > 0.05). Plasma concentration of cortisol was greater on day 56 compared to days 7, 14, and 28 (P ≤ 0.05).
Figure 3.
Effect of day of sampling (P < 0.001) on plasma haptoglobin (SEM = 0.067; panel A) and cortisol (SEM = 1.63; panel B) concentration (ng/mL) of newly received feedlot calves. Samples were collected on days 0 (feedlot arrival), 7, 14, 28, and 58. Means with different superscripts differ across sampling days (P ≤ 0.05). For plasma concentration of cortisol (panel B), day 0 values were used as a covariate, and it is included in this graph just as a reference for the initial concentration.
Discussion
The challenge of feeding lightweight, newly received feedlot calves has been intriguing veterinarians and animal scientists over the last decades. Although a lot of progress has been made to integrate animal nutrition and health (Duff and Galyean, 2007; Cook, 2017; Galyean et al., 2022) in such a way that improves growth performance while reducing morbidity from BRD during the receiving period, according to Richeson et al. (2019), the “receiving paradox” still does not allow scientists to thoroughly understand the mechanisms by which low dietary concentration of roughage (or high-concentrate diets) may negatively affect animal health. Corn is the primary source of energy in receiving and finishing diets (Samuelson et al., 2016) and the object of study on energy levels for receiving cattle. Although fat is also known to increase energy intake (Zinn and Jorquera, 2007), little and contradictory information on supplemental fat for newly received feedlot calves is currently available (Cole and Hutcheson, 1987; Fluharty and Loerch, 1997; Plascencia et al., 2022). In this study, it was hypothesized that adding fat to a high roughage diet (R60) would be an alternative to increase energy intake while decreasing the negative effects on morbidity from BRD that feeding diets with greater amounts of concentrate (R30) could cause.
According to Toaff-Rosenstein et al. (2016), the sickness response is greater as BRD severity increases and the fever is closely related to clinical score, although according to Theurer et al. (2015b), the rectal temperature had limited value as a prognostic indicator of whether calves would finish the production cycle. Although hyperthermia may provide some evidence of BRD in feedlot cattle, changes in body temperature are not only caused by BRD (Wolfger et al., 2015). Based on the data from this experiment, if the rectal temperature is the only objective criteria for treating BRD, calves that were visually scored DART 1 or 2 would not need to be pulled from their home pens to be further evaluated, since they all had rectal temperature lower than 40 °C. Two points need to be considered: 1) the choice of a critical rectal temperature—in the current study, 40 °C was the objective criterion for treating BRD. Although according to Theurer et al. (2015a), the rectal temperature of feedlot calves at the first treatment for BRD had limited value as a prognostic indicator of whether the calves would finish the production cycle, the target rectal temperature at the first BRD treatment (e.g., 40 vs. 39.5 °C) may affect the number of calves that will require a second treatement; 2) time between the first treatment and reevaluation of rectal temperature—it is still not clear what would be the best waiting period for reevaluation of rectal temperature or make a decision about the second treatment for BRD. According to Blakebrough-Hall et al. (2020), visual signs are the most important indicator to identify animals impacted by BRD. Contrary, White and Renter (2009) reported that current visual methods for identifying cattle with BRD have shown both low sensitivity (62%) and low specificity (63%). Further research is needed to identify the best objective criteria to determine if microbial therapy is needed to improve disease detection and treatment effectiveness.
Contrary to our hypothesis, no roughage level × supplemental fat interactions were observed in the current experiment. However, in agreement with published data (Lofgreen et al., 1975, 1981; Rivera et al., 2005; Crawford et al., 2022), increasing the level of concentrate in receiving diets increased growth performance due to increased energy intake. In the current study, steers-fed R30 had 22% greater G:F compared to R60. This is in agreement with the 19% increase in the dietary NEm and the 26% increase in the NEg presented in Table 1, based on the tabular values from NASEM (2016). Interestingly, observed:expected NE ratios in the current study reveal that the observed performance of steers-fed R30 is in agreement with the NASEM (2016) estimates, but R60 had 11% greater NEg than NASEM (2016) estimates. Based upon observed:expected dietary NE, although no differences between R30 and R60 were observed, steers-fed R60 had an 8% numerical improvement in the apparent utilization of the energy per unit of DM. Therefore, although steers-fed R60 had decreased G:F, calculated diet NE values based on growth performance were increased as compared to steers-fed R30.
The equations from Rivera et al. (2005) were used to estimate DMI and ADG for the dietary roughage levels used in the current experiment (30 and 60% of diet DM) and G:F calculated. According to these equations, steers-fed diets containing 30% roughage should have 40% greater G:F than steers-fed diets containing 60% of roughage. The discrepancy between our results and the estimates using the equations from Rivera et al. (2005) are probably due to the greater DMI and ADG observed in the current experiment compared to the dataset used by Rivera et al. (2005), maybe as a result of the short length (28 d) of the trials and the diets (ranged from all-hay to 75% concentrate) used in their studies.
Decreasing the roughage level in the current experiment tended to increase the number of animals that required a second treatment for BRD, in agreement with Lofgreen et al. (1975) and Rivera et al. (2005). According to Reuter et al. (2008), increasing dietary roughage levels increased the production of proinflammatory cytokines (PIC; e.g., interferon-γ, tumor necrosis factor-α, and IL-6) in response to an Escherichia coli lipopolysaccharide challenge in beef steers, which could be the mode of action for the slight decrease in morbidity that often occurs when newly received calves are fed roughage-based receiving diets (Lofgreen et al., 1975). However, in the current experiment, dietary roughage level did not affect PIC, but tended to increase haptoglobin in steers-fed R30 compared to R60. Haptoglobin is a key component of the acute-phase response (Carroll and Forsberg, 2007). Decreased concentration of plasma haptoglobin in the current experiment was not sufficient to impact the morbidity rate or the level of antibody titers of newly received calves. According to Whitney et al. (2006), early-weaned calves-fed bermudagrass hay during the backgrounding phase had greater IgG concentration than calves-fed 70% concentrate diet after the BHV-1 challenge, but the reason for that increase was unclear. However, in the current experiment, IgG concentration did not differ between R30 and R60. Immunoglobulin-G plays a major role in antibody-mediated defenses, and because it is the smallest of the immunoglobulin molecules, IgG can escape from the blood vessels more easily, which is especially important when inflammation is occurring (Tizard, 2016). Although our results differ from Whitney et al. (2006), the lack of effect of dietary roughage level on IgG concentration in the current experiment is in agreement with the lack of effect of roughage level on serum antibody titers and the minimal impact on morbidity.
According to Plascencia et al. (2022), yellow grease is the most common form of recycled supplemental fat in the United States and Mexico. It is composed of any combination of waste greases collected from restaurants (Zinn and Jorquera, 2007). The fatty acid profile (% of total fatty acids) of the yellow grease used in the current experiment was similar to the ones reported by Plascencia et al. (1999) and Zinn and Jorquera (2007).
Zinn and Jorquera (2007) reported that adding fat to feedlot diets can reduce particle size separation and help to improve the uniformity of feed mixes. This partially agrees with the results obtained in the current study, especially in R60 diets. Overall, feeding +FAT decreased the percentage of particles <1.18 mm. More specifically, feeding R60+FAT increased the estimated peNDF, mainly due to an increase in the percentage of particles retained on the 8-mm screen. As a result, supplementing fat decreased the sorting of particles <1.18 mm. According to Dykier et al. (2020), although steers sort to consume a different diet composition, diet sorting did not impact energy intake when steers are fed diets containing 12% roughage. However, when animals sort against longer forage particles, the intake of highly fermentable carbohydrates is associated with reduced rumen pH (Miller-Cushon and DeVries, 2017). Therefore, even if sorting does not affect energy intake, negative effects on performance can be observed due to changes in ruminal fermentation characteristics, and the benefits of improving the uniformity of TMRs using conditioning ingredients should be considered by nutritionists.
Feeding +FAT in the current experiment increased G:F by 18.8%, mainly due to increased ADG. Observed NEg of +FAT diets calculated based on performance was 14% greater than observed NEg of −FAT diets. Using the equations from Daley et al. (2020) and the nutrient composition of diets presented in Table 1, the total fatty acid (FA) concentration of each diet was estimated at 1.74 and 4.41% (DM basis). Using the equations from Zinn and Jorquera (2007) and the observed DMI of the current experiment, the FA digestion (%) was calculated (83.8 and 78.1%, respectively, for −FAT and +FAT diets). The energy value of fat was estimated assuming that 1 g of digestible fat has a metabolizable energy (ME) value of 9 Kcal and the partial efficiency of use of ME from dietary fat for BW gain of 67% (Zinn and Jorquera, 2007). Accordingly, NEg concentration of fat based upon FA intake is 5.05 and 4.71 Mcal/kg, respectively, for −FAT and +FAT diets. Corresponding NEm values are 6.23 and 5.84 Mcal/kg, respectively, for −FAT and +FAT diets. Thus, the predicted NE value for yellow grease based on FA intake is in agreement with the NASEM (2016).
Cole and Hutcheson (1987) evaluated the effects of including 4% fat blend (70% cottonseed oil + 30% tallow) on health and growth performance of newly received calves. Adding 4% fat to the diet improved G:F by 9%, without affecting DMI of newly received beef calves. Therefore, our results partially agree with the performance data described by Cole and Hutcheson (1987). Similarly, Fluharty and Loerch (1997) included 4% or 0% fat (animal–vegetable blend) in receiving diets and observed a 10% increase in G:F, with no differences in DMI in the diets containing 4% fat. These authors also observed a numerical decrease in morbidity for calves-fed diets containing 4% fat (animal–vegetable blend), which was contrary to our results, where feeding +FAT tended to increase the number of steers that required a second treatment for BRD. Although the number of animals used in the current experiment may have limited the ability to detect differences in categorical outcomes (e.g., morbidity and mortality), the blood analytes evaluated herein tended (IgG and cortisol) to increase in animals-fed +FAT, in agreement with the morbidity data. According to Roth (1985), increased plasma cortisol concentrations in cattle can cause neutrophilia and decrease antibody response. However, the magnitude of cortisol change in the current study didn’t result in changes in serum antibody titers.
Zinn and Jorquera (2007) recommended that receiving diets should not contain more than 2% supplemental fat, primarily due to some adverse effects related to diet palatability. However, similarly to the results from the current experiment, Plascencia et al. (2022) didn’t observe differences in DMI when Holstein steers were fed diets containing 3.5% supplemental yellow grease. Overall, according to the results from Cole and Hutcheson (1987), Plascencia et al. (2022), and the current experiment, feeding 3.5% supplemental fat to lightweight calves didn’t affect DMI and increased G:F. Since no interactions or additive effects between roughage and fat were detected in the current experiment, and G:F responses to increasing fat or decreasing roughage were generally not different, the economic and logistical aspects of feeding one or the other must be considered.
These results indicate that feeding newly received feedlot calves with diets containing 30% roughage (DM basis) increases ADG and G:F compared to diets containing 60% roughage, with no impact on animal health. Also, adding 3.5% of yellow grease (DM basis) as supplemental fat to receiving diets does not affect DMI, increases G:F, and has minimal impact on morbidity rate.
Acknowledgments
This project was funded by the New Mexico State Agricultural Experiment Station. Acknowledgment is made to Jim Bob Messer (Texas Feed Fat Co., Inc., Hereford, TX) and to Jeremy Page (Cargill Sweet Bran, Dalhart, TX) for providing discounted prices on the feed ingredients used in this project.
Glossary
Abbreviations
- +FAT
supplemental yellow grease at 3.5% of diet dry matter
- ADF
acid detergent fiber
- ADG
average daily gain
- BRD
bovine respiratory disease
- BW
body weight
- CP
crude protein
- DART
depression, anorexia, respiratory, and temperature
- DMI
dry matter intake
- -FAT
no yellow grease
- G:F
gain efficiency
- IgG
immunoglobulin-G
- IL-6
interleukin-6
- IVNDFD
in vitro neutral detergent fiber digestibility
- LIG
lignin
- NDF
neutral detergent fiber
- NEg
net energy of gain
- NEm
net energy of maintenance
- PSPS
Penn State Particle Separator
- PUFA
polyunsaturated fatty acids
- PUN
plasma urea nitrogen
- R30
wheat hay at 30% of diet dry matter
- R60
wheat hay at 60% of diet dry matter
- TNF-α
tumor necrosis factor alpha
Contributor Information
Vinícius N Gouvêa, Texas A&M AgriLife Research and Extension Center, Amarillo, TX 79106, USA; Department of Animal Science, Texas A&M University, College Station, TX 77845, USA.
Mario O Oliveira, Department of Animal Science, “Luiz de Queiroz” College of Agriculture, University of São Paulo, Piracicaba, SP 13418900, Brazil.
Hiam Jardel M Giacomelli, Department of Animal Science, Santa Catarina State University, Chapecó, SC 89815630, Brazil.
Eduardo A Colombo, Department of Animal Science, Texas A&M University, College Station, TX 77845, USA.
Fernanda Batistel, Department of Animal Science, University of Florida, Gainesville, FL 32611, USA.
Flávio A P Santos, Department of Animal Science, “Luiz de Queiroz” College of Agriculture, University of São Paulo, Piracicaba, SP 13418900, Brazil.
Glenn C Duff, Clayton Livestock Research Center, New Mexico State University, Clayton, NM 88415, USA.
Rodrigo S Marques, Department of Animal and Range Sciences, Montana State University, Bozeman, MT 59717, USA.
Reinaldo F Cooke, Department of Animal Science, Texas A&M University, College Station, TX 77845, USA.
Conflict of Interest Statement
The authors declare that they have no conflict of interest that could influence the subject matter of this manuscript.
References
- AOAC. 2000. Official methods of analysis. 16th ed. Gaithersburg (MD): AOAC International,. [Google Scholar]
- Batistel, F., Arroyo J. M., Garces C. I. M., Trevisi E., Parys C., Ballou M. A., Cardoso F. C., and Loor J. J.. . 2018. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 101:480–490. doi: 10.3168/jds.2017-13185. [DOI] [PubMed] [Google Scholar]
- Blakebrough-Hall, C., Dona A., D’occhio M. J., McMeniman J., and González L. A.. . 2020. Diagnosis of bovine respiratory disease in feedlot cattle using blood 1H NMR metabolomics. Sci. Rep. 10:1–12. doi: 10.1038/s41598-019-56809-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan, R. J. 2001. Fundamental considerations in developing vaccination protocols. Proceed. Thirty-Fourth Annual Conf. Am Assoc Bovine Practitioners. September 13-15, 2001. Vancouver, British Columbia. 34:14–22. doi: 10.21423/aabppro20015171. [DOI] [Google Scholar]
- Carroll, J. A., and Forsberg N. E.. . 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. N. Am. - Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Cole, N. A., and Hutcheson D. P.. . 1987. Influence of receiving diet fat on the health and performance of feeder calves. Nutr Rep Int. 965–970. [Google Scholar]
- Colombo, E. A., Cooke R. F., Brandão A. P., Wiegand J. B., Schubach K. M., Duff G. C., Gouvêa V. N., and Cappellozza B. I.. . 2020. Administering an appeasing substance to optimize performance and health responses in feedlot receiving cattle. J. Anim. Sci. 98:1–8. doi: 10.1093/JAS/SKAA339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, R. F., and Arthington J. D.. . 2012. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. (Berl) 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Cooke, R. F. 2017. Invited paper: Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573. [DOI] [Google Scholar]
- Crawford, D. M., Richeson J. T., Perkins T. L., and Samuelson K. L.. . 2022. Feeding a high energy finishing diet upon arrival to high-risk feedlot calves: effects on health, performance, ruminal pH, rumination, serum metabolites, and carcass traits. J Anim Sci. 100:1–12. doi: 10.1093/jas/skac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley, V. L., Armentano L. E., Kononoff P. J., and Hanigan M. D.. . 2020. Modeling fatty acids for dairy cattle: models to predict total fatty acid concentration and fatty acid digestion of feedstuffs. J. Dairy Sci. 103:6982–6999. doi: 10.3168/jds.2019-17407. [DOI] [PubMed] [Google Scholar]
- Duff, G. C., and Galyean M. L.. . 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykier, K. C., Oltjen J. W., Robinson P. H., and Sainz R. D.. . 2020. Effects of finishing diet sorting and digestibility on performance and feed efficiency in beef steers. Animal 14:59–65. doi: 10.1017/S1751731119001988. [DOI] [PubMed] [Google Scholar]
- Fluharty, F. L., and Loerch S. C.. . 1997. Effects of concentration and source of supplemental fat and protein on performance of newly arrived feedlot steers. J. Anim. Sci. 75:2308–2316. doi: 10.2527/1997.7592308x. [DOI] [PubMed] [Google Scholar]
- Galyean, M. L., Duff G. C., and Rivera J. D.. . 2022. Galyean Appreciation Club Review: revisiting nutrition and health of newly received cattle-what have we learned in the last 15 years? J. Anim. Sci. 100:1–7. doi: 10.1093/jas/skac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda, M. G., Fang X., Perry G. A., and Maltecca C.. . 2012. Measuring bovine viral diarrhea virus vaccine response: using a commercially available ELISA as a surrogate for serum neutralization assays. Vaccine 30:6559–6563. doi: 10.1016/j.vaccine.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Hutcheson, D. P., and Cole N. A.. . 1986. Management of transit-stress syndrome in cattle: nutritional and environmental effects. J. Anim. Sci. 62:555–560. doi: 10.2527/jas1986.622555x [DOI] [Google Scholar]
- Kononoff, P. J., Heinrichs A. J., and Buckmaster D. R.. . 2003. Modification of the Penn State Forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 86:1858–1863. doi: 10.3168/jds.S0022-0302(03)73773-4. [DOI] [PubMed] [Google Scholar]
- Leonardi, C., and Armentano L. E.. . 2003. Effect of quantity, quality, and length of alfalfa hay on selective consumption by dairy cows. J. Dairy Sci. 86:557–564. doi: 10.3168/jds.S0022-0302(03)73634-0. [DOI] [PubMed] [Google Scholar]
- Lofgreen, G. P., Dunbar J. R., Addis D. G., and Clark J. G.. . 1975. Energy level in starting rations for calves subjected to marketing and shipping stress. J. Anim. Sci. 41:1256–1265. doi: 10.2527/jas1975.4151256x. [DOI] [Google Scholar]
- Lofgreen, G. P., El Tayeb A. E., and Kiesling H. E.. . 1981. Millet and alfalfa hays alone and in combination with high-energy diets for receiving stressed calves. J. Anim. Sci. 52:959–968. doi: 10.2527/jas1981.525959x. [DOI] [PubMed] [Google Scholar]
- Miller-Cushon, E. K., and DeVries T. J.. . 2017. Feed sorting in dairy cattle: causes, consequences, and management. J. Dairy Sci. 100:4172–4183. doi: 10.3168/jds.2016-11983. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington (DC): National Academies of Sciences, Engineering, and Medicine. [Google Scholar]
- Plascencia, A., Estrada M., and Zinn R. A.. . 1999. Influence of free fatty acid content on the feeding value of yellow grease in finishing diets for feedlot cattle. J Anim Sci. 77:2603–2609. doi: 10.2527/1999.77102603x. [DOI] [PubMed] [Google Scholar]
- Plascencia, A., Latack B. C., v Carvalho P. H., and Zinn R. A.. . 2022. Feeding value of supplemental fat as a partial replacement for steam-flaked corn in diets for Holstein calves during the early growing phase. Transl. Anim. Sci. 6:1–5. doi: 10.1093/tas/txac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, R. R., Carroll J. A., Dailey J. W., Cook B. J., and M. L. Galyean. . 2008. Effects of dietary energy source and level and injection of tilmicosin phosphate on immune function in lipopolysaccharide-challenged beef steers. J. Anim. Sci. 86:1963–1976. doi: 10.2527/jas.2007-0838. [DOI] [PubMed] [Google Scholar]
- Richeson, J. T., Samuelson K. L., and Tomczak D. J.. . 2019. Energy and roughage levels in cattle receiving diets and impacts on health, performance, and immune responses. J. Anim. Sci. 97:3596–3604. doi: 10.1093/jas/skz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera, J. D., Galyean M. L., and Nichols W. T.. . 2005. Dietary roughage concentration and health of newly received cattle. Prof. Anim. Sci. 21:345–351. doi: 10.15232/S1080-7446(15)31231-6. [DOI] [Google Scholar]
- Roth, J. A. 1985. Cortisol as mediator of stress-associated immunosuppression in cattle. In: Gary P. M. (eds.) Animal stress. New York: Springer;. p. 225–243. doi: 10.1007/978-1-4614-7544-6_13. [DOI] [Google Scholar]
- Samuelson, K. L., Hubbert M. E., Galyean M. L., and Löest C. A.. . 2016. Nutritional recommendations of feedlot consulting nutritionists: the 2015 New Mexico State and Texas Tech University Survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas.2016-0282. [DOI] [PubMed] [Google Scholar]
- Snowder, G. D., van Vleck L. D., v. Cundiff L., and Bennett G. L.. . 2006. Bovine respiratory disease in feedlot cattle: environmental, genetic, and economic factors. J. Anim. Sci. 84:1999–2008. doi: 10.2527/jas.2006-046. [DOI] [PubMed] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Step, D. L., Krehbiel C. R., DePra H. A., Cranston J. J., Fulton R. W., Kirkpatrick J. G., Gill D. R., Payton M. E., Montelongo M. A., and Confer A. W.. . 2008. Effects of commingling beef calves from different sources and weaning protocols during a forty-two-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 86:3146–3158. doi: 10.2527/jas.2008-0883. [DOI] [PubMed] [Google Scholar]
- Sukhija, P. S., and Palmquist D. L.. . 1988. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 36:1202–1206. doi: 10.1021/jf00084a019. [DOI] [Google Scholar]
- Theurer, M. E., Renter D. G., and White B. J.. . 2015a. Using feedlot operational data to make valid conclusions for improving health management. Vet. Clin. North Am. Food Anim. Pract. 31:495–508. doi: 10.1016/j.cvfa.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Theurer, M. E., White B. J., and Renter D. G.. . 2015b. Optimizing feedlot diagnostic testing strategies using test characteristics, disease prevalence, and relative costs of misdiagnosis. Vet. Clin. North Am. Food Anim. Pract. 31:483–493. doi: 10.1016/j.cvfa.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Tilley, J. M. A., and Terry R. A.. . 1963. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 18:104–111. doi: 10.1111/j.1365-2494.1963.tb00335.x. [DOI] [Google Scholar]
- Tizard, I, editor.. 2016. Veterinary immunology. 10th ed. St. Louis (MO): Elsevier. [Google Scholar]
- Toaff-Rosenstein, R. L., Gershwin L. J., and Tucker C. B.. . 2016. Fever, feeding, and grooming behavior around peak clinical signs in bovine respiratory disease. J. Anim. Sci. 94:3918–3932. doi: 10.2527/jas.2016-0346. [DOI] [PubMed] [Google Scholar]
- White, B. J., and Renter D. G.. . 2009. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post-weaned beef calves. J. Vet. Diagn. Invest. 21:446–453. doi: 10.1177/104063870902100405. [DOI] [PubMed] [Google Scholar]
- Whitney, T. R., Duff G. C., Collins J. K., Schafer D. W., and Hallford D. M.. . 2006. Effects of diet for early-weaned crossbred beef steers on metabolic profiles and febrile response to an infectious bovine herpesvirus-1 challenge. Livest. Sci. 101:1–9. doi: 10.1016/j.livprodsci.2005.04.011. [DOI] [Google Scholar]
- Wilson, B. K., Step D. L., Maxwell C. L., Wagner J. J., Richards C. J., and Krehbiel C. R.. . 2015. Evaluation of multiple ancillary therapies used in combination with an antimicrobial in newly received high-risk calves treated for bovine respiratory disease. J. Anim. Sci. 93:3661–3674. doi: 10.2527/jas.2015-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfger, B., Timsit E., White B. J., and Orsel K.. . 2015. A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Vet. Clin. Food Anim. 31:351–365. doi: 10.1016/j.cvfa.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Zinn, R. A., and Jorquera A. P.. . 2007. Feed value of supplemental fats used in feedlot cattle diets. Vet. Clin. Food Anim. 23:247–268. doi: 10.1016/j.cvfa.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Zinn, R. A., and Shen Y.. . 1998. An evaluation of ruminally degradable intake protein and metabolizable amino acid requirements of feedlot calves. J. Anim. Sci. 76:1280–1289. doi: 10.2527/1998.7651280x. [DOI] [PubMed] [Google Scholar]