Abstract
The outer membrane protein p66 of the Lyme disease agent, Borrelia burgdorferi, has been identified as a candidate ligand for β3-chain integrins. To identify portions of p66 required for integrin recognition, fusions of maltose-binding protein to fragments of p66 were tested for binding to integrin αIIbβ3, and synthetic peptides derived from the p66 amino acid sequence were tested for the ability to inhibit B. burgdorferi attachment to the same integrin. The data identify two noncontiguous segments of p66 that are important for αIIbβ3 recognition, suggesting that, as is true for other integrin ligands, the tertiary structure of p66 is important for receptor recognition.
Lyme disease is caused by the tick-borne spirochete Borrelia burgdorferi. Clinical manifestations of B. burgdorferi infection may affect the skin, joints, heart, and nervous system. These manifestations are complex and reflect both the spread of the organism from the site of the tick bite to the affected tissues and the host response to B. burgdorferi (12, 18, 29). In the absence of appropriate antibiotic therapy, B. burgdorferi can establish persistent infection in humans, in animal models of infection, and in the wild rodents that serve as reservoirs of the organism. Interactions with host tissue matrix components and cells are likely to play key roles in the dissemination of B. burgdorferi and the establishment of persistent infection.
In support of this hypothesis, a number of laboratories have demonstrated that B. burgdorferi binds to several types of cultured mammalian cells, including endothelial cells, glial cells, epithelial cells, and fibroblasts (10, 15, 30, 31). B. burgdorferi has also been shown to bind to platelets (6, 9) and to cultured tick cells (20). Recent studies have documented several adhesion pathways used by B. burgdorferi to bind to cell surfaces or to components of the extracellular matrix. Two related B. burgdorferi proteins that bind to the collagen-associated proteoglycan decorin have been characterized (13, 14); in addition, B. burgdorferi proteins that bind to heparan and dermatan sulfate proteoglycans (17, 23) and fibronectin (11, 19, 25) have been identified. Less is known of the bacterial molecules involved in binding to glycosphingolipids (1) or of the relevance of any of the aforementioned interactions to human disease. It is likely that, as for other pathogenic bacteria, the interaction between B. burgdorferi and the host is complex and mediated by multiple bacterial virulence factors.
B. burgdorferi also binds to integrins αIIbβ3 and αvβ3 (6, 7). Integrins are divalent cation-dependent, heterodimeric receptors that normally mediate a variety of cell-cell and cell-extracellular matrix interactions. αvβ3 (vitronectin receptor) expression is widespread, but αIIbβ3 (fibrinogen receptor) is expressed only by platelets and megakaryocytes, and requires activation prior to binding B. burgdorferi and its normal mammalian ligands (16, 28). The ligand specificities of the β3-chain integrins overlap but are not identical (16). Several integrins, including αIIbβ3 and αvβ3, recognize the amino acid sequence Arg-Gly-Asp (RGD) (16), and peptides containing this sequence can block receptor function. Binding of B. burgdorferi to each of the β3-chain integrins can be inhibited by EDTA, cyclic and linear RGD peptides, and appropriate blocking monoclonal antibodies (6, 7). Determination of the pathogenic role of β3-chain integrin binding by Lyme disease spirochetes awaits the definitive identification of the B. burgdorferi protein(s) involved and the generation of appropriate mutant strains derived from an infectious parent.
We previously identified a B. burgdorferi protein, p66, that is an excellent candidate ligand for the β3-chain integrins (5). p66 is recognized by a majority of Lyme disease patient sera and is therefore expressed by the spirochete during infection. p66 was previously shown to be localized on the surface of B. burgdorferi (4, 24), a critical prerequisite for any candidate adhesin, and is one of two putative porins identified in B. burgdorferi to date (27). Although p66 does not contain any previously identified integrin recognition motifs, we showed that in recombinant form, the protein binds specifically to β3-chain integrins and competes with B. burgdorferi for attachment to the same integrins (5). When expressed on the surface of Escherichia coli, p66 increases attachment of the E. coli cells to a transfected human cell line that expresses αvβ3 but not to the parental cell line which expresses no β3-chain integrins (5). The central portion of p66, comprising amino acids 142 to 384 and denoted p66M, contains all of the information required for binding to β3-chain integrins (5).
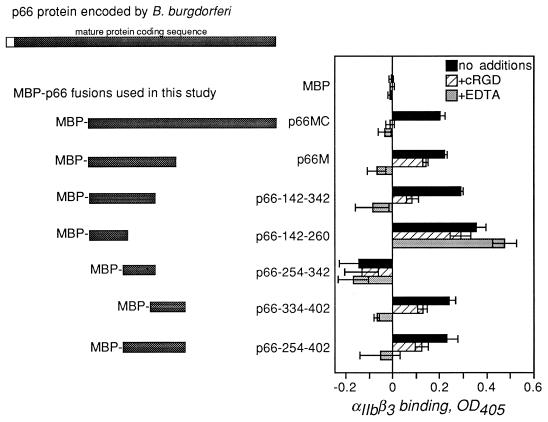
To further understand the basis of β3-chain integrin binding by p66, which contains none of the known integrin recognition motifs, smaller fragments of p66 were tested for binding to αIIbβ3. We constructed a series of recombinant proteins in which fragments of the integrin-binding domain of p66 were fused to the E. coli maltose-binding protein (MBP), which facilitates the production and purification of soluble protein (Fig. 1). Fragments of the p66 gene were generated by PCR using the primers listed in Table 1. After amplification, each fragment was digested with the appropriate restriction enzymes and cloned into pMalC2 (New England Biolabs, Beverly, Mass.) which had been digested with the same enzymes. After induction with isopropyl-β-d-thiogalactopyranoside, the recombinant fusion proteins were purified by amylose affinity chromatography as previously described (5). The MBP-p66 fusions tested were p66M (amino acids 142 to 384) and p66MC (amino acids 142 to 618), both of which were described previously (5), plus p66-142-260 (amino acids 142 to 260), p66-142-342, p66-254-402, p66-334-402, and p66-254-342 (Fig. 1).
FIG. 1.
The recombinant fusions of p66 to MBP used in this study, and the integrin αIIbβ3-binding activities of the fusion proteins. The full-length p66 as encoded by B. burgdorferi is shown at the top; the unfilled segment denotes the secretion signal, which is not present in the mature protein. MBP-p66M and MBP-p66MC were previously described (5). The other fusions are denoted by amino acid numbers corresponding to the B. burgdorferi strain B31 sequence (8). The oligonucleotides used for amplification of B. burgdorferi sequences are listed in Table 1. For quantification of binding activity, purified αIIbβ3 (3 μg/ml) was immobilized in microtiter wells as previously described (5). All wells were blocked with 25 mM HEPES (pH 7.8)–150 mM NaCl–1 mM MgCl2–1 mM MnCl2–0.25 mM CaCl2–1% bovine serum albumin–0.1% dextrose (5) and then incubated in the same buffer alone (no additions) or with the addition of cRGD peptide G4120 (2) (15 μM) or EDTA (10 mM) for 30 min at ambient temperature. MBP fusion proteins were then added to 3 μg/ml (final concentration), and incubation continued for 3 h. Unbound protein was removed by washing; bound protein was quantified by enzyme-linked immunosorbent assay using an anti-MBP rabbit antiserum (1:10,000; New England Biolabs) followed by anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (1:10,000; Promega). Binding to uncoated wells was subtracted to give the integrin-specific signals displayed. In each case, the integrin-specific signal was at least three times the signal obtained with no αIIbβ3. Shown are the means + standard deviations of the four replicates performed in each experiment; similar results were observed in multiple experiments. OD405, optical density at 405 nm.
TABLE 1.
Synthetic oligonucleotides used for generation of recombinant p66 fragments
| Oligonucleotide | Sequencea (5′→3′) |
|---|---|
| oC66Ib | ACGCGTCGACCTTCTTTTGCTATTAGCTTCCGCTGTA |
| oJLC42b | GGAGGATCCGCACCTATGACTGGATTTAAAAGCACTTAC |
| oJLC43b | GGACTGCAGTTAAAAACCTATGCTTGCTCCTGTTGAAAATG |
| oJLC53 | ACGCGTCGACTACTCGTTTCCATAGGCTCCTGACAAG |
| oJLC54 | ACGCGTCGACTAGAAATTCGCTGCTTTTGAGATGTGTC |
| oJLC55 | ACGTGTCGACTAATCAGATCCTTTAATCGCCCA |
| oJLC56 | GGAAGGATCCTCAGGAGCCTATGGAAACGAGAC |
| oJLC57 | GGAAGGATCCGGACACATCTCAAAAGCAGCGAA |
| oJLC58 | GATCCAATCAAGAGAATGACAAAGACACTCCATAGTAG |
| oJLC59 | TCGACTACTATGGAGTGTCTTTGTCATTCTCTTGATTG |
Restriction sites used for cloning PCR products are underlined; overhangs used for cloning annealed oligonucleotides are italicized.
As described in reference 5.
The MBP-p66 fusion proteins were each tested for the ability to bind to microtiter wells coated with purified αIIbβ3 (Fig. 1) as described previously (5). Binding was quantified by enzyme-linked immunosorbent assay using a polyclonal anti-MBP rabbit serum (New England Biolabs) followed by an anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Promega, Madison, Wis.). Attachment of each protein to uncoated wells was subtracted to give the integrin-specific signals shown. To determine whether the observed binding was inhibitable by known integrin antagonists, EDTA and a cyclic RGD (cRGD) peptide (G4120 [2, 5]) were added to parallel wells. Both reagents inhibit attachment of recombinant p66 and of B. burgdorferi to αIIbβ3 and αvβ3 (5, 7).
As demonstrated previously, p66M and p66MC both bound efficiently to αIIbβ3, and this attachment was significantly inhibited by both EDTA and cRGD (Fig. 1) (5). Similarly, the two largest fragments tested here, p66-142-342 and p66-254-402, which overlap by 88 amino acids, displayed αIIbβ3-binding activity that was inhibitable by the integrin antagonists (Fig. 1). These results initially suggested that the 88 amino acids common to these recombinant proteins might contain all of the information required for αIIbβ3 recognition. However, when this portion of p66 alone, i.e., p66-254-342, was tested for αIIbβ3 binding, no integrin-specific attachment was observed (Fig. 1). In contrast, p66-142-260 and p66-334-402, which do not overlap, both displayed αIIbβ3-binding activity, and attachment of p66-334-402 to αIIbβ3 was inhibited by EDTA and cRGD (Fig. 1). Attachment of p66-142-260 αIIbβ3 was not inhibited by the integrin antagonists at concentrations that inhibit B. burgdorferi attachment to the same receptor.
These results suggest that integrin recognition by p66 is complex and dependent on the conformation of the protein. This conclusion is based on the observation that two distinct regions of p66M appear to recognize αIIbβ3 in different ways. The region encompassed by amino acids 334 to 402 appears to contain information that allows binding to αIIbβ3 in a manner that resembles binding by mammalian integrin ligands, because attachment of all MBP-p66 fusion proteins containing these amino acids is inhibited by EDTA and cRGD (Fig. 1, fusion proteins p66M, p66MC, p66-254-402, and p66-334-402). However, portions of p66 amino terminal to amino acids 334 to 402 also appear to recognize αIIbβ3. p66-142-260 binds efficiently to αIIbβ3 but is not inhibited by EDTA or cRGD, while p66-142-342 not only binds to αIIbβ3 but also is inhibited by the integrin antagonists. This cannot be explained simply by the presence of residues that determine specific integrin recognition within amino acids 254 to 342 in the latter fusion protein, because this region of p66 did not appear to have any αIIbβ3-binding activity on its own (Fig. 1). It is also unlikely that the binding activity of p66-142-260 is simply due to nonspecific protein-protein interactions, since the buffer contains bovine serum albumin in excess. Rather, it is more likely that p66 residues 142 to 260 bind to a domain of αIIbβ3 that is not directly affected by EDTA or cRGD and that additional amino acids carboxy terminal to this portion of the protein contribute to recognition of domains of αIIbβ3 that are affected by the integrin antagonists. This hypothesis is supported by data presented below, which demonstrate that B. burgdorferi attachment to αIIbβ3 can be inhibited by synthetic peptides that correspond to specific portions of p66.
A second approach to the identification of p66 sequences that are important for integrin recognition was based on previous studies with RGD-containing peptides (reviewed in reference 16). If a peptide contains an integrin recognition sequence, we would expect that peptide to competitively inhibit the attachment of B. burgdorferi to the integrin. Therefore, a series of synthetic peptides corresponding to portions of the integrin-binding domain of p66 (p66M) derived from B. burgdorferi strain N40 was generated. Each p66-derived peptide contained a central aspartic acid (D) residue flanked by at least two additional amino acids on either side (Table 2). The approach of targeting D residues was taken because of the importance of this amino acid in the known integrin recognition motifs present in several ligands, including fibrinogen, fibronectin, and the invasin protein of Yersinia pseudotuberculosis, which binds several β1-chain integrins (16, 22). Peptide stocks diluted in the assay buffer were adjusted to bring the pH to that of the buffer with no added peptide, then diluted to the concentrations indicated, and incubated with αIIbβ3 immobilized in microtiter wells (5–7). Radiolabeled B. burgdorferi strain N40 was then added and allowed to bind as described previously (6). Peptides GRGDSP and GRGESP, which are not derived from p66, were included as positive and negative controls, respectively, in all experiments.
TABLE 2.
Synthetic peptides tested for inhibition of B. burgdorferi attachment to integrin αIIbβ3
| Peptidea | Sequenceb |
|---|---|
| 185-91 | KLDLTFA |
| 202-8 | QENDKDT |
| 203-9 | ENDKDTP |
| 203-9S | DNEKPDT |
| 203-9 (NH3) | ENDKDTP(NH3) |
| 229-36 | KNLLDQNE |
| 235-40 | NEDTKS |
| 270-5 | SLKDKS |
| 278-84 | GNDLLSP |
| 305-11 | KINDKNT |
| 317-22 | MGTDFG |
| 326-31 | FASDFS |
| 346-52 | TPSDPNK |
| 354-60 | AEIFDPN |
| D205E | ENEKDTP |
| D207E | ENDKETP |
| RGD | GRGDSP |
| RGE | GRGESP |
Denoted by positions of amino acids derived from the B. burgdorferi strain N40 p66 sequence (5). All except 203-9 (NH3) were synthesized with a carboxylate group at the C terminus. Peptides D205E and D207E contain single conservative amino acid substitutions, (aspartic acid at positions 205 and 207 to glutamic acid) of the p66 sequence. Peptides RGD and RGE are not related to any p66 sequence but were used as controls for integrin specificity (16).
In single-letter amino acid code.
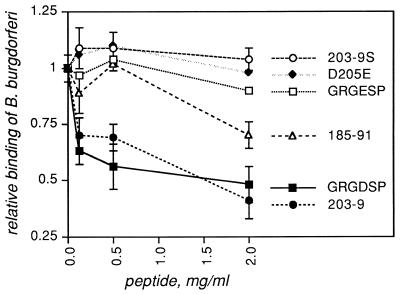
One of the p66-derived peptides, representing residues 203 to 209, inhibited B. burgdorferi attachment to αIIbβ3 with an activity comparable to that of a linear RGD peptide (Fig. 2). Similar inhibition profiles were observed with three independently synthesized batches of peptide 203-9, but a scrambled version of this peptide showed no inhibitory activity (Fig. 2, 203-9S). This result demonstrates that it is the sequence of amino acids, not the overall composition of peptide 203-9, that is important to the ability of this peptide to compete with B. burgdorferi for integrin attachment. We were unable to detect direct binding to αIIbβ3 by an MBP fusion to p66 residues 201 to 209 (the product of cloning and expressing annealed oligonucleotides oJLC58 and oJLC59 in pMalC2 [data not shown]), but it is possible that this small portion of p66 is not exposed to the solvent in the context of the 42-kDa MBP fusion partner or that the affinity of the peptide fused to MBP is too low to allow stable attachment.
FIG. 2.
Inhibition of B. burgdorferi attachment to αIIbβ3 by p66-derived synthetic peptides. Integrin αIIbβ3 was immobilized in microtiter wells at 1 μg/ml and then incubated with synthetic peptides at the concentrations shown for 30 min at ambient temperature. 35S-labeled B. burgdorferi strain N40 was then added to the wells, and the plates were centrifuged, incubated at ambient temperature, and washed as described previously (6, 7). Bound bacteria were quantified by liquid scintillation counting. Data points represent the means ± standard deviations of four replicates and represent at least two independent experiments. Relative binding efficiency is defined as the level of attachment in the presence of each reagent divided by the level seen in the absence of any peptide. Peptide sequences are given in Table 2; peptides listed in Table 2 but not shown in this figure did not affect B. burgdorferi attachment to αIIbβ3. For the sake of clarity, error bars are shown only for GRGDSP, N203-9, N203-9S, and N185-91, but GRGDSP and N203-9 were always significantly different from all other peptides (P < 0.05) by the two-tailed t test.
Several additional experiments suggest that structural features of peptide 203-9 may be important for integrin recognition. Conservative substitution of E for either one of the two D residues in peptide 203-9 (denoted D205E and D207E) resulted in no significant inhibition of B. burgdorferi binding to αIIbβ3 (Table 2, Fig. 2, and data not shown). These results suggest that it is the specific amino acid side chain, rather than the overall charge, that determines integrin recognition by this peptide. In addition, a peptide from which the proline at the C terminus had been deleted (203-8) had no inhibitory activity (data not shown). With the terminal proline intact, the C-terminal linkage (carboxylate versus amide) made no difference (Table 2 and data not shown). Given these results, it is interesting that for RGD peptide inhibition of mammalian cells to fibronectin, GRGDSP was more potent than RGDS, GDSP, and GRGESP (26), although peptide inhibition profiles vary with the particular ligand-integrin pair.
Peptide 185-91 also inhibited B. burgdorferi attachment to αIIbβ3, but to a significantly lesser extent than did either 203-9 or GRGDSP (Fig. 2). None of the other p66-derived peptides tested inhibited B. burgdorferi binding to αIIbβ3 (Table 2, Fig. 2, and data not shown), leaving 203-9 and 185-91 as the only peptides that competed with B. burgdorferi N40 for integrin attachment. These results are intriguing because both of these peptides are encompassed within the p66-142-260 fusion protein, which binds to integrin αIIbβ3 but is not affected by the integrin antagonist EDTA or cRGD. The results obtained with the p66-142-260 fusion protein and with peptides 185-91 and 203-9 suggest that this portion of the protein is important for αIIbβ3 recognition by p66. It is possible that p66-142-260 binds with high affinity to αIIbβ3 and therefore cannot be inhibited by either EDTA or cRGD. It is also possible that p66-142-260 binds to a site on the integrin that, while not affected by EDTA or cRGD, is nevertheless involved in the formation of a stable attachment complex by B. burgdorferi.
The results of our studies with p66-derived peptides and recombinant protein fragments suggest that two noncontiguous portions of p66 each contain information required for recognition of integrin αIIbβ3 and that the overall conformation of the protein is likely to be important for αIIbβ3 binding by B. burgdorferi. It is not surprising that by implication, the tertiary structure of p66 would be critical for integrin recognition. Despite the ability of small peptides to inhibit binding to integrins, maintenance of the overall structure of the mature protein ligand has been shown to be critical for stable integrin attachment by other ligands, e.g., fibronectin and the Y. pseudotuberculosis invasin protein (3, 21). The identification of p66 residues required for integrin interaction will facilitate the generation of targeted mutants in B. burgdorferi that will allow rigorous testing of the role of this interaction in infection, with minimal disruption to the biology of the organism.
Acknowledgments
We thank Mia Spugnardi, Wambui Chege, Hyacinthe Ntchobo, and Daniel Caffrey for their contributions to this work, Sarah Bodary for reagents and advice, and John Leong and Allen Steere for review of the manuscript.
This work was supported by a Biomedical Science Grant from the Arthritis Foundation and by PHS grant AI-40938 (to J.C.) and the Center for Gastroenterology Research on Absorptive and Secretory Processes at New England Medical Center, PHS grant 1 P30DK39428 awarded by NIDDK.
REFERENCES
- 1.Backenson P B, Coleman J L, Benach J L. Borrelia burgdorferi shows specificity of binding to glycosphingolipids. Infect Immun. 1995;63:2811–2817. doi: 10.1128/iai.63.8.2811-2817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker P, Bullens S, Bunting S, Burdick D, Chan K, Deisher T, Eigenbrot C, Gadek T, Gantzos R, Lipari M, Muir C, Napier M, Pitti R, Padus A, Quan C, Stanley M, Struble M, Tom J, Burnier J. Cyclic RGD peptide analogues as antiplatelet antithrombotics. J Med Chem. 1992;35:2040–2048. doi: 10.1021/jm00089a014. [DOI] [PubMed] [Google Scholar]
- 3.Bowditch R, Halloran C, Aota S, Obara M, Plow E, Yamada K, Ginsberg M. Integrin αIIbβ3 (platelet GPIIb-IIIa) recognizes multiple sites in fibronectin. J Biol Chem. 1991;266:23323–23328. [PubMed] [Google Scholar]
- 4.Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- 5.Coburn J, Chege W, Magoun L, Bodary S C, Leong J M. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 6.Coburn J, Leong J, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn J, Magoun L, Bodary S C, Leong J M. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 9.Galbe J L, Guy E, Zapatero J M, Peerschke E I, Benach J L. Vascular clearance of Borrelia burgdorferi in rats. Microb Pathog. 1993;14:187–201. doi: 10.1006/mpat.1993.1019. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Monco J C, Fernandez-Villar B, Benach J L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989;160:497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- 11.Grab D J, Givens C, Kennedy R. Fibronectin-binding activity in Borrelia burgdorferi. Biochim Biophys Acta. 1998;1407:135–145. doi: 10.1016/s0925-4439(98)00038-6. [DOI] [PubMed] [Google Scholar]
- 12.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 13.Guo B P, Brown E L, Dorward D W, Rosenberg L C, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hechemy K E, Samsonoff W A, Harris H L, McKee M. Adherence and entry of Borrelia burgdorferi in Vero cells. J Med Microbiol. 1992;36:229–238. doi: 10.1099/00222615-36-4-229. [DOI] [PubMed] [Google Scholar]
- 16.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Investig. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalish R A, Leong J M, Steere A C. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp P A, Schmitt M, Wellensiek H J, Blobel H. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect Immun. 1995;63:3804–3808. doi: 10.1128/iai.63.10.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtti T J, Munderloh U G, Ahlstrand G G, Johnson R C. Borrelia burgdoferi in tick cell culture: growth and cellular adherence. J Med Entomol. 1988;25:256–261. doi: 10.1093/jmedent/25.4.256. [DOI] [PubMed] [Google Scholar]
- 21.Leong J M, Morrissey P E, Isberg R R. A 76-amino acid disulfide loop in the Yersinia pseudotuberculosis invasin protein is required for integrin receptor recognition. J Biol Chem. 1993;268:20524–20532. [PubMed] [Google Scholar]
- 22.Leong J M, Morrissey P E, Marra A, Isberg R R. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parveen N, Leong J M. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- 24.Probert W S, Allsup K M, LeFebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Probert W S, Johnson B J. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 26.Pytela R, Pierschbacher M D, Argraves S, Suzuki S, Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- 27.Skare J T, Mirzabekov T A, Shang E S, Blanco D R, Erdjument-Bromage H, Bunikis J, Bergstrom S, Tempst P, Kagan B L, Miller J N, Lovett M A. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 29.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 30.Szczepanski A, Furie M B, Benach J L, Lane B P, Fleit H B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Investig. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas D D, Comstock L E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989;57:1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]