Abstract
Winter and spring precipitation are predicted to increase in the Midwest region of the United States, causing muddy conditions. In a previous experiment, Angus cows (8 per treatment) were paired based on initial body weight (BW) and one cow from each pair was randomly allocated to either the mud or control treatment. Though cows consumed the same amount of dry matter, cows in the mud treatment weighed 37.4 kg less than cows in the control treatment by day 269 of gestation. The objective of this experiment was to evaluate developmental programming effects of steers born to cows in the mud treatment (MUD; n = 7) or the control treatment (CON; n = 6). Steers were weighed at birth and then weekly from approximately 56 d of age until weaning and were subjected to a glucose tolerance test (GTT) and adrenocorticotropic hormone (ACTH) challenge after weaning. Steers were then placed in the feedlot for an 84-d growing phase and were weighed weekly and 12th rib back fat (BF) and ribeye area (REA) were imaged every 28 d using ultrasonography. Data were analyzed as a randomized complete block design with repeated measurements when appropriate (SAS 9.4). Although there was a 37.4 kg decrease in BW of cows by the end of gestation, there was no evidence of a pen treatment effect on calf birth weight (P = 0.60) or weaning weight (P = 0.99). Additionally, there was no evidence of a pen treatment × day effect for steer BW from birth to weaning (P = 0.67) or growing phase BW (P = 0.60). There was evidence of a treatment × day of growing phase effect (P = 0.02) for BF, such that CON steers had greater BF on day 28 of the growing phase; however, there was no evidence of a treatment × day effect for REA (P = 0.20). Furthermore, there was no evidence of a pen treatment effect for the growing phase average daily gain (P = 0.74), dry matter intake (P = 0.65), gain:feed (P = 0.48), plasma glucose concentration (P = 0.67) or plasma insulin concentration (P = 0.61) in response to the GTT, or plasma cortisol concentration in response to the ACTH challenge (P = 0.51). These results indicate that while mud increased net energy requirements for cows in the MUD treatment, there were no subsequent effects observed for steer BW, gain:feed, or response to glucose and ACTH during the growing phase.
Keywords: beef cow, calf growth, developmental programming, late gestation, nutrient restriction
This research evaluated the effects of cows being housed in muddy environmental conditions during late gestation on fetal growth, postnatal growth, and postnatal response to glucose and adrenocorticotropic hormone. Based on the results, it seems that mature cows housed in a muddy environment during late gestation can mobilize body stores without negatively affecting fetal growth, postnatal growth, and gain:feed, or response to glucose and adrenocorticotropic hormone.
Introduction
Developmental programming of the offspring is believed to occur when maternal nutrition and/or endocrine status during gestation are altered. These alterations can cause long-term changes in the offspring’s structure, physiology, and metabolism that can lead to postnatal metabolic and endocrine diseases (Godfrey and Barker, 1995; Barker and Clark, 1997). Specifically, nutrient or energy restriction of the dam during the last trimester of gestation can cause intrauterine growth retardation in the bovine fetus, resulting in negative effects on growth and carcass quality of the offspring (Maresca et al., 2019). In addition to nutrient or energy restriction, exposure of dams to stress or disease during gestation can result in an adverse intrauterine environment that can negatively affect fetal and postnatal development, growth, and metabolism of the offspring (Barker and Clark, 1997). It has previously been demonstrated in humans that low birth weight is associated with insulin resistance in adult life which commonly results in impaired glucose tolerance, increased blood pressure, and disturbed lipoprotein metabolism (Barker et al., 1993). Therefore, it is possible that animals exposed to adverse intrauterine environments can have impaired growth and metabolism in utero, at birth, and later in life compared with animals exposed to normal intrauterine environments.
A dam’s dietary intake and nutrient stores combined with both the nutrient delivery to the placenta and the nutrient transfer through the placenta affects nutrient supply to the fetus (Owens et al., 1989). When nutrient supply to the fetus is reduced during critical periods of development that coincide with rapid cell division, the rate of cell division slows (Barker and Clark, 1997). Slowed cell division can then result in deleterious developmental programming of the offspring. Long et al. (2021) provided cows only 70% of the NRC (2000) recommendation for energy and 100% of the NRC (2000) protein recommendations starting on day 158 of gestation, and harvested cows and their fetuses at 265 d of gestation. The authors reported a reduction in fetal pancreatic mass as well as decreased fetal umbilical vein plasma insulin concentration in fetuses from the energy-restricted cows compared with fetuses from nonrestricted cows (Long et al., 2021). Similarly, Tipton et al. (2018) managed cows, limiting forage accessibility, to lose 1 to 1.5 body condition score (BCS) units during the last 100 d of gestation. The authors (Tipton et al., 2018) found that 15-mo-old heifer offspring had increased plasma glucose concentration in a 10-wk high energy feeding trial, and also had a greater area under the curve for plasma glucose after a glucose tolerance test (GTT). These previous studies indicate that undernourishment of the dam during late gestation may result in altered pancreatic development and glucose regulation later in life.
In humans, a relationship has been described such that men with lower birth weights are more likely to have impaired glucose tolerance or diabetes when they are 59 to 70 yr of age (Barker and Clark, 1997). As impaired glucose metabolism and diabetes often results in obesity, it is possible that if a fetus is exposed to an adverse intrauterine environment and/or is not provided adequate fetal nutrition, the offspring will be lighter at birth and have a predisposition to diabetes and obesity later in life. In ruminants, maternal nutrition during pregnancy affects pancreatic function (Trotta and Swanson, 2021). Radunz et al. (2012) showed that calves born from cows fed diets with various energy sources have different initial insulin responses during a GTT. However, we are not aware of any studies that have compared insulin metabolism of calves born from cows that lost body weight (BW) due to an environmental stressor during late gestation. Previous work has demonstrated that when sheep are selected for enhanced responsiveness to an adrenocorticotropic hormone (ACTH) challenge, they are more likely to become obese (Lee et al., 2014a) and have greater adiposity than low cortisol responders (Tilbrook et al., 2008; Lee et al., 2014b). Therefore, fetuses that are undernourished during critical periods of gestation may become obese later in life and could potentially have greater adiposity and greater responses to ACTH challenges and other stressors they may experience.
Nickles et al. (2022) previously investigated the effects of housing cows in mud from day 213 to 269 of gestation on BW, conceptus free live weight, BCS, 12th rib back fat (BF) thickness, and rump fat thickness of the cow. The authors estimated cows housed in mud have an increased requirement for net energy of 3.9 Mcal/d to maintain conceptus free live weight, although there was no effect on calf birth weight (Nickles et al., 2022). As there was no treatment effect on calf birth weight, Nickles et al. (2022) assumed that fetal growth did not differ between treatments when making calculations for conceptus free live weight. However, the cows in the mud treatment were undernourished and while calf birth weight was not different between the treatments, there may have been alterations in fetal development through adaptation to undernutrition while in utero (Barker and Clark, 1997).
We hypothesized that steers born to dams exposed to muddy environmental conditions and energy restricted by an average of 3.9 Mcal/d during the last trimester of gestation would have decreased plasma insulin responses to a glucose infusion, increased adiposity, increased plasma cortisol responsiveness to an ACTH challenge, and decreased growth and gain:feed (G:F) during an 84-d growing phase compared with steers born to dams housed in pens without mud during late gestation. The objective of this experiment was to determine the effects of energy restriction of the cow during the last 56 d of gestation because of muddy environmental conditions on the response to glucose and ACTH, growth, and G:F during an 84-d growing phase in steers born from those cows.
Materials and Methods
All procedures were approved by The Ohio State University Institutional Animal Care and Use Committee (Animal Use Protocol # 2019A00000142).
Animals, experimental design, treatments
Steers (Simmental × Angus crossbred) used in this experiment were born from dams that were used in a previous study by our research group to evaluate the effects of a muddy environment on energy requirements of cows during late gestation (Nickles et al., 2022). The dams were used in a randomized complete block design at the Eastern Agricultural Research Station (Caldwell, OH), where they were individually housed and fed. Sixteen cows were paired based on initial BW, and one cow from each pair was randomly allocated to either the mud (MUD) or control treatment (CON) from day 213 to 269 of gestation. Cows in the CON treatment were housed in pens bedded with wood chips and not exposed to mud, while cows in the MUD treatment were housed in pens containing mud (average depth of 23.6 ± 5.8 cm). After water was initially added to the pens at the start of the treatment period to create the muddy conditions, the external environment (temperature and precipitation) continued to maintain the mud in the pens. The 16 individual pens were located in the same outdoor lot and were uncovered and housed away from all buildings to avoid a windbreak effect, so that all cows were exposed to the same environmental conditions except for the allocated pen treatment. The outdoor lot was scraped and graded using a skid loader before the treatment period began to create a flat surface for the pens. The area occupied by the pens was previously used for holding pens outside of the chute area and had a geotextile fabric and stone base. Before the treatment period began and after the outdoor lot was scraped and graded, soil from the same area of the research station was added to the pens at a depth of approximately 30 cm as a target depth in all eight of the mud treatment pens. The target depth of 30 cm was based on the depth of mud that cows were typically subjected to at the research station in previous years. The soil that was used to create the mud in the mud treatment pens was previously analyzed by the National Resources Conservation Service as a part of the National Cooperative Soil Survey and is classified as Vandalia-Guernsey silty clay loam. The CON pens were bedded weekly with saw dust and wood chips as needed such that no mud formed in those pens and to the same target depth as the mud pens of 30 cm. Cows were pair fed during the treatment period, such that cows in each pair consumed the same amount of dry matter throughout the treatment period. Each week, dry matter allowances for each pair of cows was adjusted based on the control cow’s BW and week of gestation. Diets were formulated to meet or exceed NASEM (2016) recommendations for maintenance plus gestation. Of the 16 multiparous cows that were used, 13 bull calves were born and subsequently used in this study (CON, n = 6; MUD, n = 7).
Cows were maintained as one herd and treated similarly before initiation of the study. All cows entered an estrous synchronization protocol to allow for fixed-time artificial insemination with conventional semen in June 2019. Pregnancy status was diagnosed using transrectal ultrasonography approximately 31 d after artificial insemination at the initiation of the study. All cows were confirmed to be bred to the first artificial insemination date and all cows had an expected calving date of March 22nd, 2020. Only cows that had conceived to the first artificial insemination were used to ensure that all cows were at the same days of gestation throughout the treatment period. All cows were removed from their pens on day 269 of gestation to prevent any calves from being born in the mud pens. At parturition, calf weight was recorded within 24 h after birth. A total of six bulls were born from dams in the control treatment and seven bulls were born from dams in the mud treatment. Bull calves were castrated at birth. After all calves were born, cows and calves were maintained as one herd and were managed on pasture. Calf weight was recorded at birth and then weekly from approximately 56 d of age until weaning. The authors were not able to record calf weight after birth until the research farm was reopened to researchers on May 19th, 2020 due to university restrictions during the COVID-19 pandemic. Calves were weaned at approximately 196 ± 3 d of age. At weaning, all calves were vaccinated against bovine viral diarrhea virus, infectious bovine rhinotracheitis, parainfluenza 3, bovine respiratory syncytial virus, and leptospirosis (Bovi-Shield Gold FP 5 L5, Zoetis, Parsippany, NJ), clostridial diseases (Ultrachoice 7, Zoetis, Parsippany, NJ), and dewormed (Dectomax, Zoetis, Parsippany, NJ). The steers were then moved to a feedlot pen (7.3 m × 37.2 m) in which they were housed together at the research station and were transitioned to consuming a growing diet (Table 1). The transition to the growing phase diet lasted a total of 2 wk until the steers were consuming no hay and only the growing diet. The feedlot pen had two GrowSafe feed bunks that allowed for daily collection of individual dry matter intake (DMI) throughout the growing phase. The growing diet in the current experiment was formulated to allow a growth rate of 0.75 kg/d. Byproducts, instead of hay, were used as a fiber source to avoid sorting in the GrowSafe feed bunks.
Table 1.
Composition and nutritional profile of the diet offered for ad libitum consumption in the GrowSafe system to steers during the 84-d growing phase
| Item | Value |
|---|---|
| Composition, as-fed basis | |
| Soyhulls (pelleted), % | 63.00 |
| Dried distillers grains (pelleted), % | 11.00 |
| Whole shelled corn, % | 11.00 |
| Wheat middlings, % | 10.00 |
| Blended animal-vegetable fat, % | 1.00 |
| Mineral mix1, % | 4.00 |
| Nutritional profile2, dry matter basis | |
| Net energy for maintenance3, Mcal/kg | 1.53 |
| Net energy for gain3, Mcal/kg | 0.95 |
| Total digestible nutrients, % | 65.42 |
| Neutral detergent fiber, % | 42.89 |
| Acid detergent fiber, % | 30.86 |
| Ash, % | 7.05 |
| Ether extract, % | 4.04 |
| Starch, % | 15.62 |
| Crude protein, % | 14.43 |
1Containing 12.5% urea, 24.999% limestone, 21.812% dicalcium phosphate, 12.5% white salt, 0.185% vitamin A-30, 0.185% vitamin D-3, 0.555% vitamin E, 17.5% gypsum, 0.95% selenium, 0.425% Rumensin 90, 7.5% potassium chloride, 0.15% copper sulfate, 0.5% zinc sulfate, 0.237% manganese sulfate, 0.002% cobalt carbonate.
2Based on wet chemistry procedures by a commercial laboratory (Rock River Laboratory, Wooster, OH).
3Calculations for net energy for maintenance and gain of the diet using the feed composition estimates provided by NASEM (2016).
GTT and ACTH challenge
Immediately after the 2-wk adaptation period to the growing diet, a GTT and an ACTH challenge was performed on all steers. During the 2-wk dietary adjustment period before the GTT and ACTH challenge, steers were trained in the working facilities 5 d/wk to allow for the steers to become acclimated to the handling process that was to occur during the GTT and ACTH challenge. On the first day, steers were in the working facility for 10 min, and this time increased up to 1 h by the last day of acclimation.
During the GTT, jugular catheters were inserted and steers were allowed a 1-h rest period in their pen before the GTT began. Steers were fasted for 24 h before the GTT but were allowed ad libitum access to water. On the morning of the GTT, calves were weighed to determine bolus size (0.25 g of glucose/kg BW delivered in a 50% weight/volume dextrose solution). Blood samples were collected 5 and 2 min before administration of the glucose bolus to determine basal plasma glucose concentration. Subsequent blood samples were collected immediately after glucose bolus infusion, 5, 10, 15, 20, 30, 60, and 120 min after glucose bolus infusion. A 10-mL blood sample was then collected, and after collection, the catheter line was flushed again with 4 to 5 mL of heparinized saline. All blood samples were transferred to a tube containing K2 EDTA and then immediately placed on ice. The K2 EDTA tubes were transferred back to the laboratory and centrifuged for 25 min at 2,500 g and 4 °C. The plasma was then aliquoted into individual microcentrifuge tubes for later determination of plasma glucose and plasma insulin concentrations. Quantification of plasma glucose concentration was completed using a colorimetric assay (Stanbio Glucose LiquiColor Oxidase Procedure, Stanbio Laboratory, Boerne, TX). As all of the samples in this study were obtained during a GTT, the concentration for many of the time points was above the linear portion of the standard curve. Therefore, any sample that was outside of the linear portion of the standard curve was diluted in deionized water using a 1:2 dilution such that 5 µL of unknown sample and 5 µL of deionized water was added to the well per the assay protocol. All samples were run in duplicates and the intra- and inter-assay coefficient of variations were 2.9% and 3.0%, respectively. Plasma insulin was quantified using a commercial Porcine insulin radioimmunoassay kit previously validated for bovine by Miqueo et al. (2019) (90% bovine insulin specificity, MilliporeSigma, Burlington, MA). The intra- and inter-assay coefficient of variations were 8.3% and 7.3%, respectively.
One week after the GTT was completed, jugular catheters were placed in the opposite jugular vein as the GTT and the ACTH challenge was performed on all steers. Baseline blood samples were collected −30 and −15 min before ACTH administration to determine basal cortisol concentration. ACTH (Cosyntropin, 0.25 mg/vial, Sandoz, Princeton, NJ) was reconstituted with sterile saline solution (1 mL per vial) and administered intravenously (0.16 µg/kg BW; Schwinn et al., 2018), and blood was sampled immediately after ACTH administration, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, and 180 min after ACTH administration. Blood samples were transferred to tubes containing lithium heparin and were then immediately placed on ice. The lithium heparin tubes were transferred back to the laboratory and centrifuged for 25 min at 2,500 g and 4 °C. The plasma was then aliquoted into individual microcentrifuge tubes for later determination of plasma cortisol concentrations. Plasma cortisol concentration was quantified using a commercially available radioimmunoassay kit (MP Biomedicals, LLC., Solon, OH), previously validated for cattle (Wagner et al., 2020). The minimum level of detection was 1 µg/dL. All samples were run duplicate in a single assay, and the intra-assay coefficient of variation was 1.8%.
Growth and G:F measurements
After the GTTs and ACTH challenges were completed, all steers were placed in the same pen and allowed a 3-wk acclimation period to ensure that all steers were acclimated to eating out of the GrowSafe bunks. Once the acclimation period was completed, GrowSafe data were collected for 84 d while steers consumed the growing diet (Table 1). At the start of the 84-d growing phase, steers were 245 ± 3.1 d of age. Using the GrowSafe system, we obtained 84-d DMI for each steer. This allowed us to calculate average daily DMI, and G:F. Throughout the 84-d growing trial, steers were weighed weekly and B-mode ultrasonography was used to determine BF thickness and ribeye area (REA) every 4 wk. Ultrasound measurements of BF and REA were made between the 12th and 13th ribs over the longissimus muscle (Brethour, 1992) by the same trained technician.]
Statistical analysis
In this experiment, each individual calf was considered the experimental unit as the original treatment (control vs. mud) was applied to the dam. Dam BW pair was considered the blocking criteria, as cows were ranked by initial BW and put into BW pairs. Therefore, plasma glucose and insulin concentration during the GTT, plasma cortisol concentration during the ACTH challenge, calf BW up to weaning, growing phase BW, growing phase BF, and growing phase REA data were analyzed using the MIXED procedure of SAS with repeated measures (9.4, SAS Inst. Inc., Cary, NC). For calf BW up to weaning, growing phase BW, BF, and REA, the model included pen treatment, day, and the interaction as the fixed effects. For plasma glucose and cortisol concentrations, the model included treatment, time of the GTT and ACTH challenge, and the interaction as fixed effects. For all of these variables, the model included block and calf ID nested within block by pen treatment as the random effects. For the equally spaced time points (plasma cortisol concentration, growing phase BW, growing phase BF, and growing phase REA), the first-order autoregressive structure with heterogenous variances was used as the covariance structure to account for the error’s correlation due to the repeated measures over time, as it produced the lowest AIC for each model. For the unequally spaced time points (plasma glucose concentration and calf BW up to weaning), a spatial power covariance structure was used. For all of the models with repeated measures, the Kenward-Roger degrees of freedom was used to calculate the denominator degrees of freedom. The data that only had one time point (calf birth weight, weaning weight, and growing phase average daily gain [ADG], DMI, and G:F) were analyzed using the MIXED procedure of SAS. The model included pen treatment as the fixed effect, and block and calf ID nested within block by treatment as the random effects.
Based on a power analysis using the variability in the steers’ BWs and the differences due to treatment of the dam (Nickles et al., 2022), to observe a difference at a P-value = 0.05, and a power of 80%, an N of 4 animals per treatment was required. The assumptions of normality and homogeneity of variance were evaluated using the residuals plots in SAS for all variables. No variables violated these assumptions, therefore, there were no transformations. Differences were considered significant if P ≤ 0.05. The PDIFF option of SAS was used for mean separation, and data is presented as least-square means (LSM) ± SEM.
RESULTS
Steer birth weight, growth from birth to weaning, weaning weight, and DMI
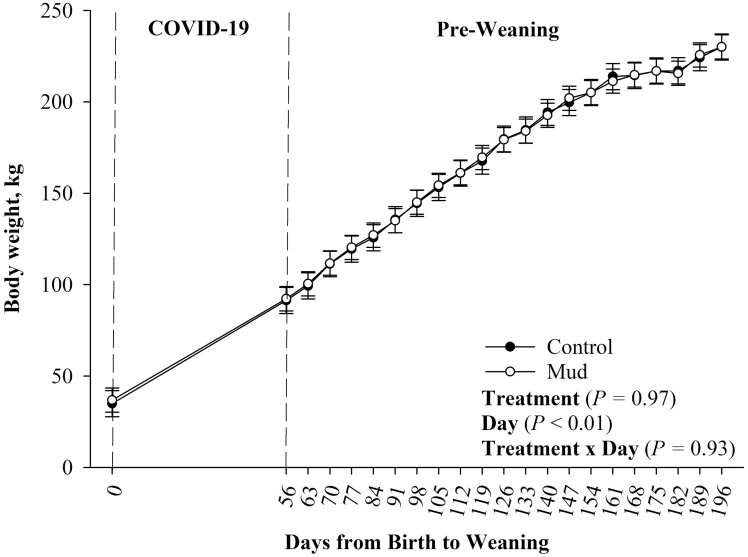
There was no evidence of a pen treatment effect on birth weight for the 13 bull calves (Table 2; P = 0.60), growth up to weaning (Figure 1; P = 0.97) nor an effect on weaning weight (Table 2; P = 0.99) of the steers used in this study. While there was no evidence of a treatment × day effect (Figure 1; P = 0.93), there was evidence of a day effect on calf growth up to weaning such that all calves increased their BW from birth to weaning (Figure 1; P < 0.01).
Table 2.
Least squares mean ± the standard error of the mean for birth weight, weaning weight, average daily gain (ADG), dry matter intake (DMI), and gain:feed during an 84-d growing phase for steers born to cows housed in 23.6 ± 5.8 centimeters of mud (MUD, n = 7) or wood chips (Control, n = 6)
| Control | MUD | SEM | P-value | |
|---|---|---|---|---|
| Birth weight, kg | 35.43 | 36.45 | 1.83 | 0.60 |
| Weaning weight, kg | 230.07 | 230.13 | 13.43 | 0.99 |
| ADG1, kg/d | 1.15 | 1.21 | 0.13 | 0.74 |
| DMI2, kg/d | 8.43 | 8.88 | 0.66 | 0.65 |
| Gain:feed | 0.31 | 0.29 | 0.02 | 0.48 |
1Average daily gain calculated from day 0 to 84 of the growing phase.
2Average dry matter intake calculated from day 0 to 84 of the growing phase.
Figure 1.
Mean body weight ± SEM measured weekly from birth (day 0) to weaning (day 196) of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
There was no evidence of a pen treatment effect (Table 2; P = 0.65) on average DMI during the growing phase, with steers born to dams in the CON treatment consuming 8.43 ± 0.66 kg/d and steers born to dams in the MUD treatment consuming 8.88 ± 0.66 kg/d. Similarly, there was no evidence of a pen treatment effect on ADG Table 2; P = 0.74), or G:F (Table 2; P = 0.48).
GTT and ACTH challenge
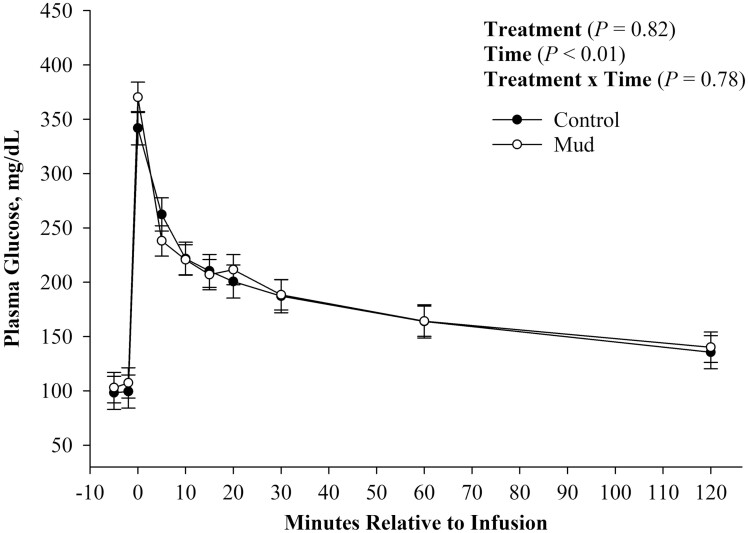
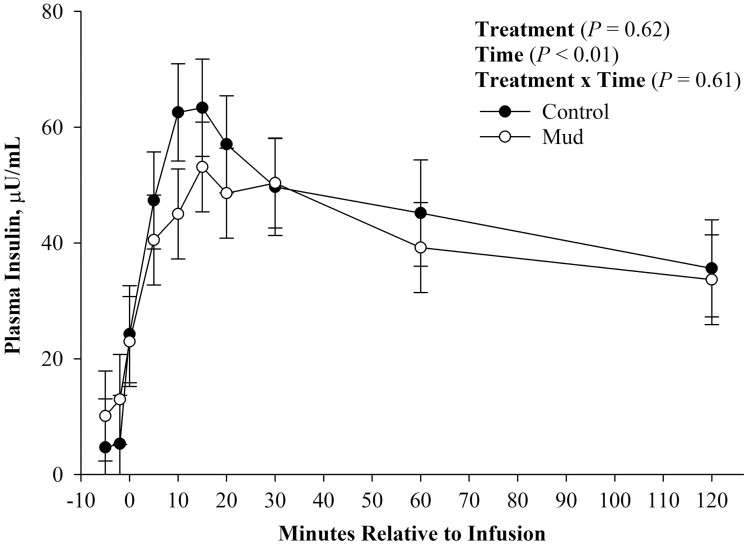
There was no evidence of a treatment (Figure 2; P = 0.82) or treatment × time effect (Figure 2; P = 0.78) observed for plasma glucose concentration. There was evidence of a time effect (Figure 2; P < 0.01) for plasma glucose concentration, such that both treatment groups started at similar fasting plasma glucose concentration, experienced peak plasma glucose concentration at time 0 immediately after infusion, and then returned to baseline concentrations by 120 min after infusion. Similarly, there was no evidence of a treatment (Figure 3; P = 0.62) or treatment × time effect (Figure 3; P = 0.61) observed for plasma insulin concentration. There was, however, evidence of a time effect (Figure 3; P < 0.01) observed for plasma insulin concentration. Fasting plasma insulin concentration was similar between treatments. Both treatments experienced peak plasma insulin concentration 15 min after glucose infusion, and then began to return to baseline concentration after peak concentration was reached.
Figure 2.
Mean plasma glucose concentration ± SEM measured during a glucose tolerance test of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
Figure 3.
Mean plasma insulin concentration ± SEM measured during a glucose tolerance test of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
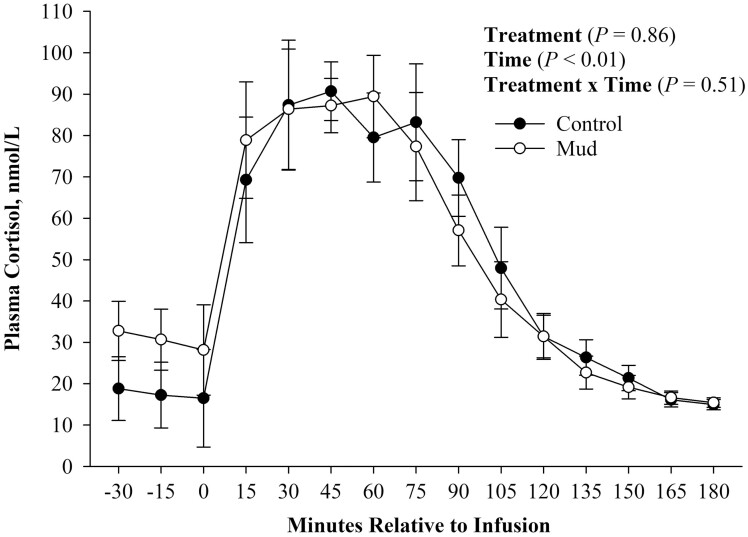
Similar to the GTT, there was no evidence of a treatment (Figure 4; P = 0.86) or treatment × time effect (Figure 4; P = 0.51) observed for plasma cortisol concentration. There was evidence of a time effect (Figure 4; P < 0.01), as both treatments started at similar baseline concentrations and after infusion of ACTH at 0 min continued to increase their plasma cortisol concentrations. After peak plasma cortisol concentration was reached, calves from both treatments decreased plasma cortisol concentration.
Figure 4.
Mean plasma cortisol concentration ± SEM measured during an adrenocorticotropin hormone challenge of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
Growing phase measurements
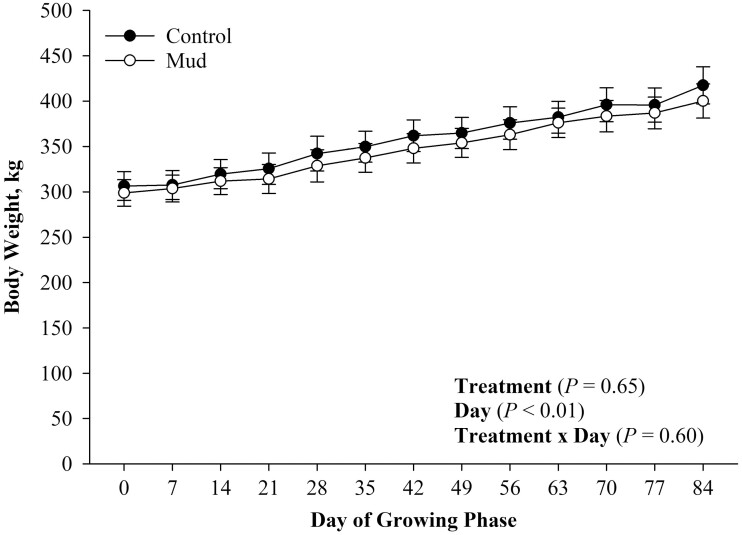
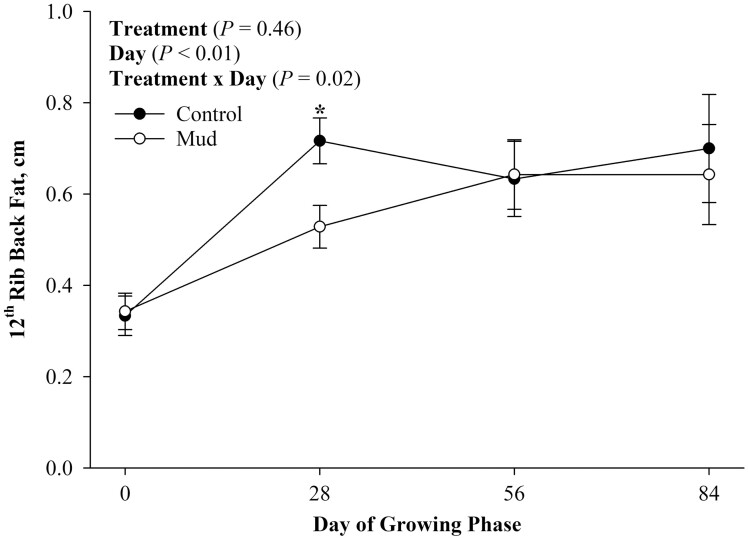
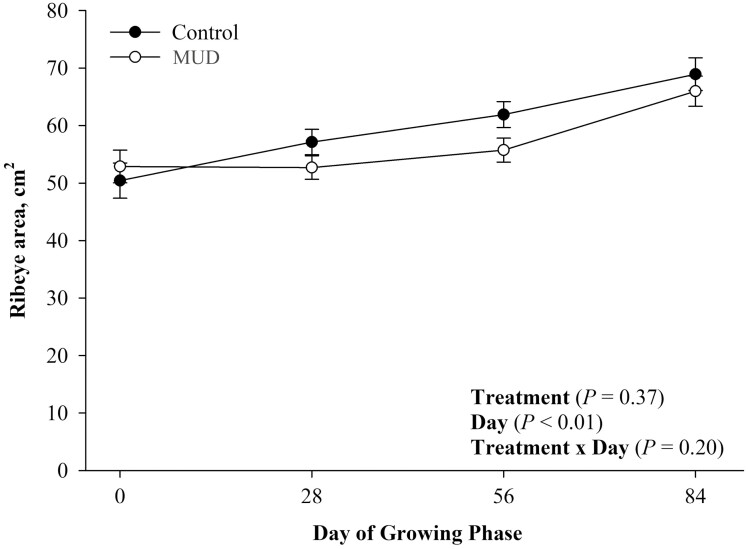
There was no evidence of a treatment effect (Figure 5; P = 0.65) nor a treatment × day effect for steer BW (Figure 5; P = 0.60). There was evidence of a day effect (Figure 5; P < 0.01) for steer BW during the 84-d growing phase such that steers born from both pen treatments increased their BWs as the growing phase progressed. There was evidence of a treatment × day effect (Figure 6; P = 0.02) for BF thickness. Steers born to dams from both treatments started the growing phase with similar BF thickness. However, steers born to dams from the CON treatment had greater BF thickness on day 28 of the growing phase (P = 0.02). Additionally, there was no evidence of a treatment (Figure 7; P = 0.37) or a treatment × day effect (Figure 7; P = 0.20) for REA. There was, however, evidence of a day effect (Figure 7; P < 0.01) for REA, such that steers increased their REA as the growing phase progressed.
Figure 5.
Mean body weight ± SEM measured weekly during the 84-d growing phase of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
Figure 6.
Mean 12th rib back fat thickness ± SEM measured every 28 d during the 84-d growing phase of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips (Control) during late gestation.
Figure 7.
Mean ribeye area ± SEM measured every 28 d during the 84-d growing phase of steers born to pair fed cows housed in 23.6 ± 5.8 centimeters of mud (MUD) or wood chips.
Discussion
Based on the present results, we reject our hypotheses that steers born to cows that were housed in mud and energy restricted during late gestation would have decreased birth weights and growth after birth compared with calves born to cows that were not housed in mud nor energy restricted. Though we previously completed a power test, we do wish to acknowledge the sample size consideration of the present study. This limitation is expected with fetal programming studies, and therefore the small number of steers used from each pen treatment should be considered as the implications of this study are extrapolated. The dams in the MUD treatment weighed approximately 37.4 kg less than the CON dams by the end of the late gestation treatment period (Nickles et al., 2022). Long et al. (2021) reported that cow weight loss was 30 kg, similar to the 37 kg decrease observed in the present study for the MUD treatment, in cows provided 70% compared with 100% of NRC (2000) net energy recommendation from day 158 until day 265 of gestation at harvest. At harvest, there were no observed differences in gravid uterus weight, empty uterus, number of placentomes, or fetal weight (Long et al., 2021). However, while the bull calves’ birth weight in this study agree with Long et al. (2021), the literature is equivocal when evaluating the effects of dam nutrient restriction on calf birth weight. Several studies have alternatively reported decreased birth weights, specifically in response to maternal energy restriction during late gestation (Perry et al., 1991; LeMaster et al., 2017; Tipton et al., 2018; Ramírez et al., 2020). Cows in these previous studies (Perry et al., 1991; Tipton et al., 2018; Ramírez et al., 2020) started at a BCS of approximately 5 on a scale of 1 to 9, which is greater than the cows in the present study which started the treatment period at a BCS of approximately 4. Cows in the previous studies that demonstrated decreased calf birth weights decreased their condition scores by approximately 1 to 2 BCS units, whereas cows in the MUD treatment in the current study (Nickles et al., 2022) decreased their BCS by approximately 0.5 of a BCS unit. Both the starting BCS and decrease in BCS over late gestation in these previous studies is greater than in the cows in Nickles et al. (2022). The inconsistencies in the literature when evaluating birth weight are likely because of the differences in length of undernourishment and the type of undernourishment of the cow (i.e., protein or energy). Starting BCS as well as how much body condition is decreased during late gestation may also influence results obtained when evaluating fetal development in response to undernourishment. In addition to the type of nutritional stress that the dams experienced in these previous studies, the type of environmental stress endured could have also affected fetal development and birth weight. Chronic exposure to elevated environmental temperatures during gestation can decrease uterine blood flow (McGuire et al., 1989) and may decrease birth weight (Holland and Odde, 1992). Alternatively, cold stress may increase DMIs and possibly increase uterine blood flow resulting in greater birth weight of calves (Holland and Odde, 1992). Furthermore, previous nutrition and nutrient stores of the dam can have an effect on fetal nutrient supply (Holland and Odde, 1992). However, when reviewing the literature, there is often a lack of information regarding the nutritional status of the dam either before or after the gestational treatment period. This does not allow us to discern if the females were on an ascending or descending plane of nutrition during various stages of gestation outside of the treatment period, which can greatly affect fetal growth patterns. A BW or BCS at the start of the treatment period does not provide indication of the dam’s previous nutrition earlier in gestation that could have effects on fetal nutrition.
Similar to birth weight, there was no evidence of a pen treatment effect on calf growth from birth to weaning, weaning weight, or growth after weaning during the growing phase in the current study. Ramírez et al. (2020) observed that as net energy allowance to cows in late gestation decreased, calf birth weight also linearly decreased. However, this difference was not evident at weaning and ADG from birth to weaning was not different among calves born form cows supplied different net energy during late gestation (Ramírez et al., 2020). Mulliniks et al. (2015) similarly reported no difference in weaning weight from calves that were born to cows that lost approximately 25 kg during late gestation compared with calves that were born to cows that lost 1 kg or gained 25 kg during late gestation. Although Ramírez et al. (2020) and Mulliniks et al. (2015) did not observe a difference in postnatal growth in response to maternal energy restriction or maternal BW loss during late gestation, several authors have reported decreased weaning weights. Tipton et al. (2018) reported a tendency for calves born to cows that lost 47 kg during late gestation to have decreased weaning weights. Similarly, Perry et al. (1991) observed a prepartum treatment effect for calf weight at 70 d of age such that calves born to cows that were provided only 70% of NRC (1984) recommendations for dietary energy during late gestation weighed less than calves born to cows that were provided 150% of the recommended dietary energy. While the present results contradict Tipton et al. (2018) and Perry et al. (1991), it is possible that before the pen treatment period began (Nickles et al., 2022), cows in the MUD treatment had great enough maternal energy reserves to withstand the nutrient restriction that was imposed on them because of the muddy conditions. The cows in the MUD treatment began the gestation treatment period at a BCS of approximately 4, and ended the treatment period at a BCS of approximately 3.5. This is below the recommendation of a BCS of 5 to 6 for cows at calving to avoid negative consequences on reproduction after calving (Richards et al., 1986; Soca et al., 2013). However, this data demonstrates that a BCS of 4 for a mature cow at the start of the last trimester may still be adequate to avoid negative consequences on fetal growth and development. We consider a BCS of 4 to be adequate in this experiment, as cows in the CON treatment also started the treatment period at a BCS of 4, but increased their BCS to approximately a 5 by the end of the treatment period; yet, there were no differences in calf birth or weaning weights. Additionally, body condition scoring is a subjective measurement, and this could contribute to the inconsistencies in the literature in gestational nutrient restriction. While it is common to assess BCS in beef cows, we believe it is more accurate to evaluate BW losses of the cows in this experiment. The cows in the MUD treatment decreased their conceptus free live weight by approximately 5.2 kg/wk, while the cows in the CON treatment only decreased their conceptus free live weight by 0.3 kg/wk. This difference in cow conceptus free live weight loss over the treatment period when cows were provided the same dry matter allowance daily further demonstrates that the cows in the MUD treatment were in adequate condition at the start of the treatment period to withstand the energy restriction placed on them. Based on the results observed with the steers in this study, it is likely that mature beef cows in adequate condition have a sufficient amount of body stores to mobilize during a period of restriction in late gestation because of muddy conditions without negatively affecting fetal growth and development.
As there was no difference in calf birth weight, growth from birth to weaning, weaning weight, or growth during the growing phase, it was not surprising that there were no differences in REA or growing phase ADG, DMI, or G:F. There was, however, a treatment × day effect for 12th rib BF. This could be a measurement error, as the only difference was found on day 28 of the growing phase such that control steers had greater BF compared with mud steers; however, this difference was not detected by day 56 and 84 of the growing phase. We reject our hypothesis that calves born to cows from the mud treatment would have lesser BWs throughout the growing phase and would be less efficient. The lack of evidence of a difference in efficiency (Tipton et al., 2018; Ramírez et al., 2020), ADG during either lactation (Ramírez et al., 2020), the growing phase (Ramírez et al., 2020), or the finishing phase (Mulliniks et al., 2015; Ramírez et al., 2020), and DMI is consistent with other studies (Mulliniks et al., 2015; Tipton et al., 2018; Ramírez et al., 2020). However, Tipton et al. (2018) did observe a tendency for calves born to cows that lost 47 kg of BW during late gestation to have decreased ADG from birth to weaning when compared with calves born to cows that were not undernourished. Similarly, Mulliniks et al. (2015) reported that calves born to cows that lost 25 kg of BW during late gestation had decreased ADG during the finishing phase compared with calves born to cows that only lost 1 kg or gained 25 kg during late gestation. Previous literature is equivocal when discussing maternal undernourishment during late gestation on calf growth and efficiency later in life. It seems that while moderate loss of maternal BW during late gestation can reduce offspring BW gain, severe maternal restriction can alternatively result in compensatory growth by offspring later in life (Ramírez et al., 2020). This catch-up growth exhibited by calves later in life could be why some authors as well as the present results indicate that although cows undergo a period of energy restriction during late gestation, there are no negative effects on postnatal calf growth. Likewise, part of this discrepancy in the literature regarding postnatal calf growth and efficiency measures may be caused by the inconsistencies in both the knowledge of and reporting of dam nutrient intakes during gestation.
We hypothesized that as cows in the MUD treatment would be energy restricted during late gestation, fetal organogenesis would be affected, specifically the fetal pancreas. We further hypothesized that the maternal energy restriction would alter glucose and insulin responses, directly impacting glucose uptake and steer G:F. We reject our hypothesis, however, as we did not observe any differences in the steers’ plasma glucose or plasma insulin concentration in response to the GTT. We also hypothesized that as steers born to cows in the MUD treatment would have impaired glucose uptake, this would predispose the steers to metabolic syndrome later in life (Barker andClark, 1997) and cause these steers to have more body fat and increased responsiveness to an ACTH challenge. We reject our hypothesis, however, as steers born to cows in the MUD treatment did not have a greater response to the ACTH challenge and did not have greater basal cortisol concentrations compared with steers born to cows in the CON treatment. However, it is possible that these types of metabolic changes could appear later in life and since we only followed these steers until the end of the growing phase, we have not yet observed these differences.
Based on our results, while cows in the mud treatment weighed 37.4 kilograms less than the cows in the control treatment by day 269 of gestation (Nickles et al., 2022), the demand for energy for the developing fetus was met before the cow’s demand to maintain herself. Since the cows in the Nickles et al. (2022) study started the treatment period at adequate BCSs, it is possible that they were able to sufficiently mobilize their own body stores to meet the demands of the growing fetus and avoid any negative developmental programming effects while the fetus was in utero. After the treatment period, the cows from Nickles et al. (2022) were placed on pasture and housed as one group and supplemented, therefore, making it likely that the cows had adequate nutrient intakes to increase their own BW and produce enough milk for the steers in the present study, as calf growth to weaning was not different between the two treatments. While it seems that mature cows are able to withstand a late gestation energy restriction resulting from a muddy environment without negatively impacting calf growth and G:F later in life.
Acknowledgments
We would like to thank the staff at Eastern Agricultural Research Station for their continuous help with this project.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- ADG
average daily gain
- BCS
body condition score
- BF
12th rib back fat
- BW
body weight
- CFLW
conceptus free live weight
- DMI
dry matter intake
- G:F
gain to feed ratio
- GTT
glucose tolerance test
- REA
ribeye area
Contributor Information
Kirsten R Nickles, Department of Animal Sciences, The Ohio State University, Wooster, OH 44691, USA.
Alejandro E Relling, Department of Animal Sciences, The Ohio State University, Wooster, OH 44691, USA.
Alvaro Garcia-Guerra, Department of Animal Sciences, The Ohio State University, Columbus, OH 43210, USA.
Francis L Fluharty, Department of Animal and Dairy Science, University of Georgia, Athens, GA 30602, USA.
Anthony J Parker, Department of Animal Sciences, The Ohio State University, Wooster, OH 44691, USA.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Literature Cited
- Barker, D. J., and Clark M. P... 1997. Fetal undernutrition and disease in later life. Rev. Reprod 2:105–112. doi: 10.1530/ror.0.0020105 [DOI] [PubMed] [Google Scholar]
- Barker, D. J. P., Hales C. N., Fall C. H. D., Osmond C., Phipps K., and Clark P. M. S... 1993. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67. doi: 10.1007/bf00399095 [DOI] [PubMed] [Google Scholar]
- Brethour, J. R. 1992. The repeatability and accuracy of ultrasound in measuring backfat of cattle. J. Anim. Sci. 70:1039–1044. doi: 10.2527/1992.7041039x [DOI] [PubMed] [Google Scholar]
- Godfrey, K. M., and Barker D. J... 1995. Maternal nutrition in relation to fetal and placental growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 61:15–22. doi: 10.1016/0028-2243(95)02148-l [DOI] [PubMed] [Google Scholar]
- Holland, M. D., and Odde K. G... 1992. Factors affecting calf birth weight: A review. Therio 38:769–798. doi: 10.1016/0093-691X(92)90155-K [DOI] [PubMed] [Google Scholar]
- Lee, T. K., Clarke I. J., St. John J., Ross Young I., Leury B. L., Rao A., Andrews Z. B., and Henry B. A... 2014b. High cortisol responses identify propensity for obesity that is linked to thermogenesis in skeletal muscle. Fed. Am. Soc. Exper. Biol. 28:35–44. doi: 10.1096/fj.13-238345 [DOI] [PubMed] [Google Scholar]
- Lee, T. K., Lee C., Bischof R., Lambert G. W., Clarke I. J., and Henry B. A... 2014a. Stress-induced behavioral and metabolic adaptations lead to an obesity-prone phenotype in ewes with elevated cortisol responses. Psychoneuroendocrin 47:166–177. doi: 10.1016/j.psyneuen.2014.05.015 [DOI] [PubMed] [Google Scholar]
- LeMaster, C. T., Taylor R. K., Ricks R. E., and Long N. M... 2017. The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves. Therio 87:64–71. doi: 10.1016/j.theriogenology.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Long, J. M., Trubenbach L. A., Pryor J. H., Long C. R., Wickersham T. A., Sawyer J. E., and Satterfield M. C... 2021. Maternal nutrient restriction alters endocrine pancreas development in fetal heifers. Dom. Anim. Endocrin. 74:106580. doi: 10.1016/j.domaniend.2020.106580 [DOI] [PubMed] [Google Scholar]
- Maresca, S., Lopez Valiente S., Rodriguez A. M., Testa L. M., Long N. M., Quintans G. I., and Pavan E... 2019. The influence of protein restriction during mid- to late gestation on beef offspring growth, carcass characteristic and meat quality. Meat Sci. 153:103–108. doi: 10.1016/j.meatsci.2019.03.014 [DOI] [PubMed] [Google Scholar]
- McGuire, M. A., Beede D. K., DeLorenzo M. A., Wilcox C. J., Huntington G. B., Reynolds C. K., and Collier R. J... 1989. Effects of thermal stress and level of feed intake on portal plasma flow and net fluxes of metabolites in lactating Holstein cows. J. Anim. Sci. 67:1050–1060. doi: 10.2527/jas1989.6741050x [DOI] [PubMed] [Google Scholar]
- Miqueo, E., Chiarle A., Giuliodori M. J., and Relling A. E... 2019. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Dom. Anim. Endocrinol. 69:62–67. doi: 10.1016/j.domaniend.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Mulliniks, J. T., Sawyer J. E., Harrelson F. W., Mathis C. P., Cox S. H., Löest C. A., and Petersen M. K... 2015. Effect of late gestation bodyweight change and condition score on progeny feedlot performance. Anim. Prod. Sci 56:1998–2003. doi: 10.1071/AN15025 [DOI] [Google Scholar]
- NASEM. 2016. Nutrient requirements of beef cattle, 9th rev. ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Nickles, K. R., Relling A. E., Garcia-Guerra A., Fluharty F. L., Kieffer J., and Parker A. J... 2022. Beef cows housed in mud during late gestation have greater net energy requirements compared with cows housed on wood chip bedding. Transl. Anim. Sci 6:txac045. doi: 10.1093/tas/txac045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 1984. Nutrient requirements of beef cattle (6th Ed.). Washington, DC: National Academy Res. [Google Scholar]
- NRC. 2000. Nutrient requirements of beef cattle. 7th rev. ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Owens, J. A., Owens P., and Robinson J... 1989. Experimental fetal growth retardation: metabolic and endocrine aspects. In: Gluckman G.D., Johnston B. M., and Nathanielsz P. W., editors. Advances in fetal physiology. Ithaca (NY): Perinatology Press; p. 263–286. [Google Scholar]
- Perry, R. C., Corah L. R., Cochran R. C., Beal W. E., Stevenson J. S., Minton J. E., Simms D. D., and Brethour J. R... 1991. Influence of dietary energy on follicular development, serum gonadotropins, and first postpartum ovulation in suckled beef cows. J. Anim. Sci. 69:3762–3773. doi: 10.2527/1991.6993762x [DOI] [PubMed] [Google Scholar]
- Radunz, A. E., Fluharty F. L., Relling A. E., Felix T. L., Shoup L. M., Zerby H. N., and Loerch S. C... 2012. Prepartum dietary energy source fed to beef cows: II. Effects on progeny postnatal growth, glucose tolerance, and carcass composition. J. Anim. Sci. 90:4962–4974. doi: 10.2527/jas.2012-5098 [DOI] [PubMed] [Google Scholar]
- Ramírez, M., Testa L. M., López Valiente S., Latorre M. E., Long N. M., Rodriguez A. M., Pavan E., and Maresca S... 2020. Maternal energy status during late gestation: effects on growth performance, carcass characteristics and meat quality of steers progeny. Meat Sci. 164:108095. doi: 10.1016/j.meatsci.2020.108095 [DOI] [PubMed] [Google Scholar]
- Richards, M. W., Spitzer J. C., and Warner M. B... 1986. Effect of varying levels of postpartum nutrition and body condition at calving on subsequent reproductive performance in beef cattle. J. Anim. Sci. 62:300–306. doi: 10.2527/jas1986.622300x [DOI] [Google Scholar]
- Schwinn, A. C., Sauer F. J., Gerber V., Bruckmaier R. M., and Gross J. J... 2018. Free and bound cortisol in plasma and saliva during ACTH challenge in dairy cows and horses. J. Anim. Sci. 96:76–84. doi: 10.1093/jas/skx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soca, P., Carriquiry M., Keisler D. H., Claramunt M., Carmo M. D., Olivera-Muzante J., Rodriguez M., and Meikle A... 2013. Reproductive and productive response to suckling restriction and dietary flushing in primiparous grazing beef cows. Anim. Prod. Sci 53:283–291. doi: 10.1071/AN12168 [DOI] [Google Scholar]
- Tilbrook, A. J., Rivalland E. A. T., Turner A. I., Lambert G. W., and Clarke I. J... 2008. Responses of the hypothalamopituitary adrenal axis and the sympathoadrenal system to isolation/restraint stress in sheep of different adiposity. Neuroendocrin. 87:193–205. doi: 10.1159/000117576 [DOI] [PubMed] [Google Scholar]
- Tipton, J. E., Ricks R. E., LeMaster C. T., and Long N. M... 2018. The effects of late gestation nutrient restriction of dams on beef heifer intake, metabolites and hormones during an ad libitum feeding trial. J. Anim. Physiol. and Anim. Nutr. 102:e877–e884. doi: 10.1111/jpn.12849 [DOI] [PubMed] [Google Scholar]
- Trotta, R. J., and Swanson K. C... 2021. Prenatal and postnatal nutrition influence pancreatic and intestinal carbohydrase activities of ruminants. Animals 11:171. doi: 10.3390/ani11010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, B. K., Relling A. E., Kieffer J. D., and Parker A. J... 2020. Intranasal oxytocin treatment does not attenuate the hypothalamo-pituitary-adrenal axis in beef heifers subjected to isolation stress or restraint and isolation stress. Domest Anim. Endocrinol. 70:106379. doi: 10.1016/j.domaniend.2019.07.007 [DOI] [PubMed] [Google Scholar]