Abstract
Introduction:
Hyalocytes have been recognized as resident tissue macrophages of the vitreous body since the mid-19th century. Despite this, knowledge about their origin, turnover, and dynamics is limited.
Areas covered:
Historically, initial studies on the origin of hyalocytes used light and electron microscopy. Modern investigations across species including rodents and humans will be described. Novel imaging is now available to study human hyalocytes in vivo. The shared ontogeny with retinal microglia and their eventual interdependence as well as differences will be discussed.
Expert opinion:
Owing to a common origin as myeloid cells, hyalocytes and retinal microglia have similarities, but hyalocytes appear to be distinct as resident macrophages of the vitreous body.
Keywords: Hyalocytes, Vitreous, Macrophages, Monocytes, Origin, Turnover, Imaging
1. Introduction
Vitreous (Fig 1) is an enigmatic structure [1] with important relevance to ocular health and disease [2]. Hyalocytes are mononuclear phagocytes (macrophages) of the vitreous body that derive from myeloid precursors (Fig 2). During the past decades the origin and turnover of macrophages in development and adulthood has been intensively investigated throughout the body, fueled by the availability of novel fate mapping approaches labeling myeloid precursor cells at narrow and defined time frames [3–11]. Studies in highly complex organs that contain distinct compartments (each having its own anatomical barriers and molecular characteristics) have found a differential origin and turnover of myeloid cells [7,10,12,13]. This is particularly notable in the brain and its associated tissues including the meninges, the perivascular Virchow-Robin-spaces, the choroid plexus [7,14,15], and the circumventricular organs which lack a blood-brain-barrier [16]. The eye has comparably complex compartmentalization that was recently found (similarly to the brain) to result in differential origin and turnover of myeloid cells. A mouse study comparing retinal microglia with macrophages residing in the ciliary body and cornea employed single-cell analysis combined with fate mapping and parabiosis to characterize heterogeneity of myeloid subsets and their dynamics in the eye [10].
Figure 1. Human vitreous body.
This sclera, choroid, and retina were dissected off this specimen from a 9-month-old child. The vitreous body remains attched to the anterior segment and maintains in gel turgenscence in spit of being situtated onto a surgical towel in room air. Image courtesy of New England Eye Bank, Boston, MA; reproduced with permission from [2], © 1989 Springer-Verlag New York Inc. and [121], © 2014 Springer Science+Business Media New York.
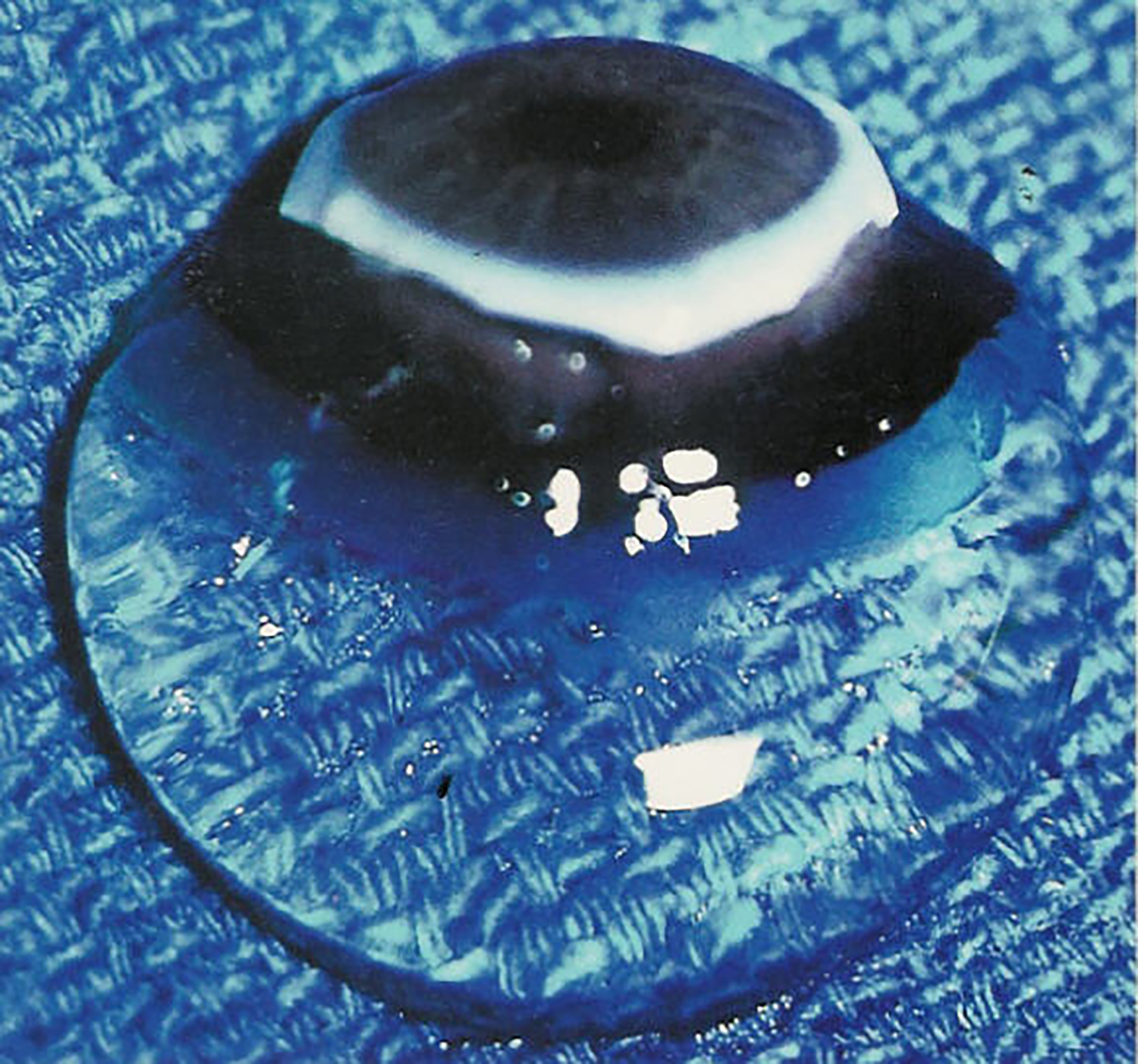
Figure 2. Human hyalocytes.
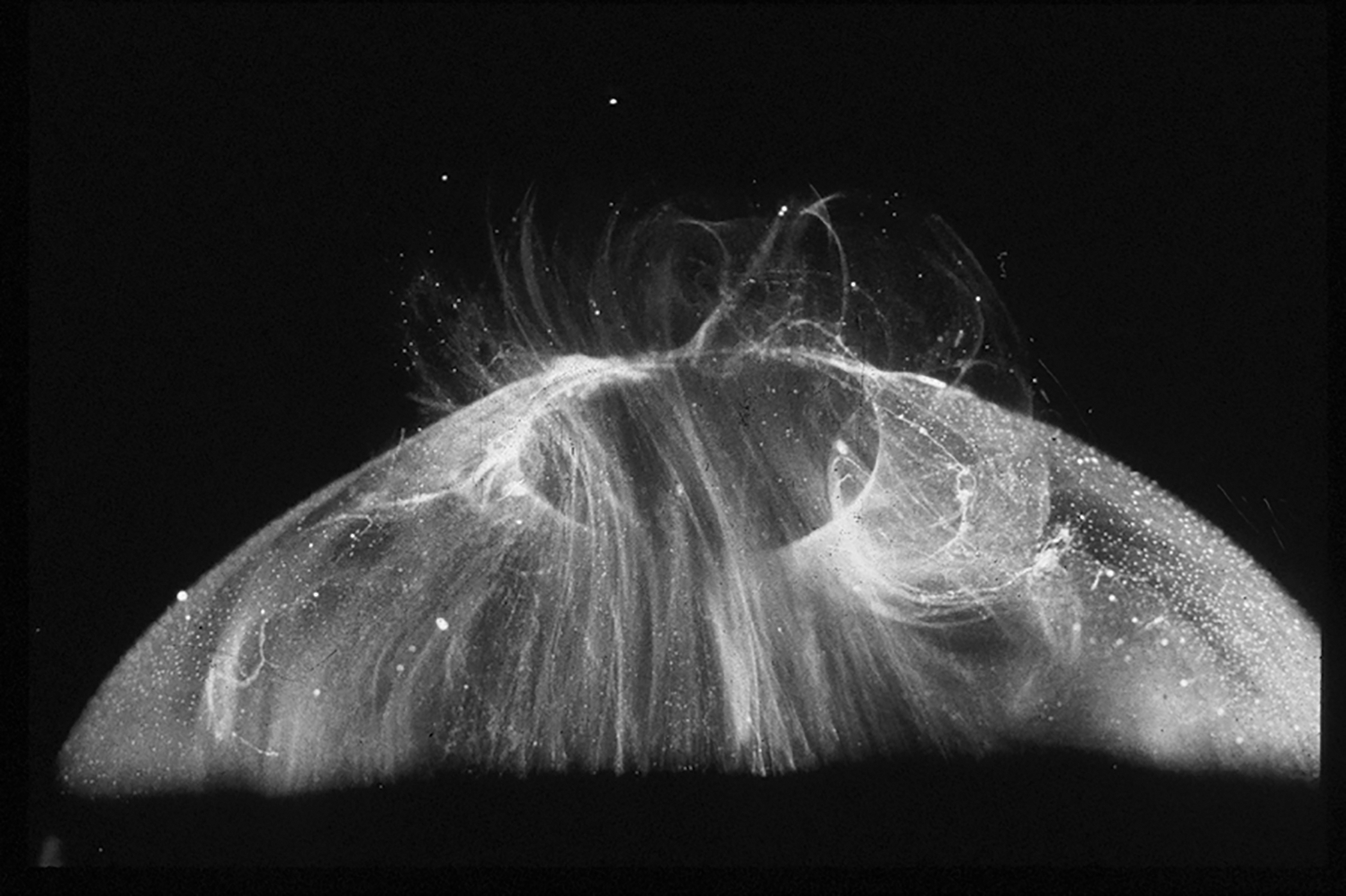
LEFT: Dark-field slit microscopy of whole vitreous body from a 59-year-old male reveals hyalocytes embedded within the posterior vitreous cortex, appearing as the white dots. RIGHT: Phase contrast microscopy of flat mount preparation of hyalocytes in the vitreous cortex from the eye of an 11-year-old girl, obtained at autopsy (courtesy of the New England Eye Bank, Boston, MA). No stains or dyes were used in this preparation. (A) Monoculear cells are distributed in a single layer within the vitreous cortex (x 115). (B) Higher magnification (x 290) demonstrates the mononuclear, round appearance of these cells. Pseudopodia are present in some cells. Reproduced with permission from [2], © 1989 Springer-Verlag New York Inc.
Vitreous macrophages, known as hyalocytes, are found in 3 distinct locations within the vitreous body: posteriorly, close to the inner limiting membrane (ILM) separating the vitreous from the retina [17]; anteriorly, close to the ciliary body epithelium where aqueous is produced [18]; and as free cells in the vitreous body, although in far fewer numbers compared to preretinal hyalocytes [19,20]. The origin, structure, and imaging of hyalocytes will be discussed in this first review article on hyalocytes. The second article will focus on the functional role during development and in the adult complemented by a broad picture of the immunological role of hyalocytes in health and vitreo-retinal immune diseases. The third article will close the special issue with a strong focus on proliferative vitreo-retinal diseases such as proliferative diabetic retinopathy, highlighting the importance of a deeper understanding of all aspects of the nature of hyalocytes as an attractive target in novel therapeutic approaches of the future.
1.1. History
There is a long history of scientific investigation seeking to identify the true nature and origin of hyalocytes, with contradictory results and interpretations. Hannover is often credited as being the first to identify hyalocytes in 1840 [21]. In 1852 Rudolf Virchow described these as nucleated cells with granular inclusions in the vitreous body [22]. He investigated the “mucoid tissue” of the eye during embryonic development that reminded him of the umbilical cord, and proposed that these cells produce the vitreous body [22]. Other microscopic studies of the adult vitreous body during the 19th century brought more attention to hyalocytes but their true cellular identity became the subject of debate [23]. Only in 1874 were hyalocytes recognized as macrophages on the basis of their morphological resemblance to leukocytes [24,25] and their increase in numbers after inoculation of foreign antigen [25]. The hypothesis of a close developmental relationship between hyalocytes and retinal microglia, also termed Hortega cells at that time, was first proposed in 1931 [23,26]. In fact, the interdependence of hyalocytes, microglia and later also monocytes [27], was experimentally addressed with a variety of methods and models but still remains elusive today as will be discussed beow.
The following will also discuss how the localization, structure, and expression profiles of hyalocytes have been investigated with dark-field slit, light, phase contrast, and electron microscopies, immunhistochemistry, immunofluorescent labeling, transgenic reporter lines with fluorescent microscopy, and confocal microscopy, finally peaking with the use of imaging mass cytometry [28–32]. As a result of these studies, hyalocytes can now be reliably regarded as members of the mononuclear phagocyte system, established in the 1970’s [33,34]. Since then, the investigation of the development and homeostasis of tissue-resident macrophages has experienced a rejuvenation fueled by the availability of transgenic mice suitable for fate mapping approaches and, more recently, RNA-sequencing of purified cell populations, including human hyalocytes [30], or at single-cell level [3,7,8,10]. While these techniques have provided valuable insights into hyalocytes, the advent of optical coherence tomography, scanning light ophthalmoscopy, and adaptive optics, ushers in a new era opening the possibility to follow individual hyalocytes over time in vivo in humans [35–37].
2. Origin of hyalocytes
2.1. Historical perspective
Original studies investigating the origin of hyalocytes employed cell labeling by ink or vital dyes due to the lack of superior methodologies and the ready availability of light microscopy for analysis. Confounding this approach is the fact that apoptotic cells can also release dyes that are then taken up by resident macrophages [38] leading to false interpretations. Several different dyes were used in the past, such as Pyrrol, that lead to the term “Pyrrolzellen” to refer to all cells that displayed positive staining with this particular vital dye [39]. As part of the reticulo-endothelial system that includes cells capable of ingesting extracellular components, the presence of the morphologically defined tissue-resident macrophages termed “histiocytes” with granular dye inclusions, was investigated in the different compartments of the eye. Besides Pyrrol, other dyes like Trypan Blue were used to gain more insight into the biology of the vitreous body [40,41]. Interestingly, after intravenous trypan blue injection in adult rabbits no labeling was observed in hyalocytes, while the iris and sclera turned blue rapidly [40]. In another study, the same observations were made leading to the conclusion that the vitreous, retina, and optic nerve were devoid of labeled histiocytes, even after several repeat trypan blue injections [41]. This lack of dye labeling by hyalocytes was interpreted as evidence against cell turnover and suggested the existence of a tightly sealed blood-ocular barrier that did not allow the dye to access the vitreous body. These findings were further corroborated by using the incorporation of 3H-Thymidine that only occurs during cell cycle progression.
Long-lived macrophages (such as brain and retinal microglia) undergo cell divisions at a comparably low frequency that only accelerates during pathologic conditons [10,42]. The rapid turnover of monocytes (circulating half-life of less than 24 hours) has to be considered when it comes to the use of radioactive 3H-Thymidine or thymidin analogues like 5′-bromo-2′-deoxyuridine (BrdU) or 5-ethynyl-2’-deoxyuridine) (EdU) [43,44], since there can be confounding effects resulting from labeling of progenitor cells during their development in the bone marrow leading eventually to labeled cells in various tissues. The labeling pattern of hyalocytes treated with 3H-Thymidine and the rare observance of mitotic figures favor a long-lived nature of these cells, rather than a continuous replenishment with peripheral blood monocytes as previously suggested [19,45,46]. In contrast, after intravitreal injection of radio-labeled peritoneal macrophages into rabbit eyes, a turnover rate of less than a week was suggested [47]. However, the injected macrophages clearly acted as sole surrogates for local vitreous body macrophages, so these results should be interpreted with care.
From a historical point of view, these techniques provided valuable insights in the past, but are no longer performed today. Only the azo dye Evan’s blue labeling of circulating Albumin is occasionally used to investigate barrier functions in different organs including the retina [48,49], but there are alternative methods to visualize leakage of serum albumin into tissues after barrier breakdown [50,51]. Nevertheless, the absence of vital dye and radiolabeling in hyalocytes suggests self-maintenance comparable to other long-lived macrophages, pointing to a pre- or perinatal origin, before the barrier functions of the eye are established.
2.2. Hematopoiesis
Hematopoiesis can be subdivided into primitive hematopiesis that is restricted to early embryonic development, and defintive hematopiesis that takes place at later stages of development in the aorto-gonad-mesonephros (AGM) and the fetal liver (FL) as well as in the bone marrow established at the perinatal stages that remains active throughout life [52,53]. Primitive hematopoiesis is established in the hemogenic endothelium/hemangioblast of the extra-embryonic yolk sac that provides the blood island where precursors of both nucleated embryonic erythrocytes and primitive macrophages are created via the erythro-myeloid precursors (EMP) [54]. During development, EMPs further differentiate and their descendants colonize organs such as the brain or eye, before the intrinsic blood-brain or blood-retina-barriers are formed, to become local tissue-resident macrophages including microglia [7,8,10,11,53,54]. Besides the developing organs that are reached via the embryonic circulation [3], they transiently colonize the fetal liver where progenitors of definitive hematopoiesis, derived from the AGM, can be found and establish the bone marrow later, prior to birth [53,55,56]. With regard to ciliary body macrophages, primitive hematopoiesis has been identified as the main source with only limited contribution from other hematopoietic organs including the fetal liver and the bone marrow [10]. With respect to hyalocytes, no embryonic fate mapping has been performed to date. However, human embryonic developmental stages have been investigated with respect to the local leukocyte populations by immunophenotyping [57]. The results suggest the presence of several markers that were attributed to hemangioblasts and suggested the existence of local blood islands remote from their well described localization in the yolk sac. Future experiments involving more contemporary methods including transcriptional profiling would help to decipher the former microscopic observations.
Despite the scarce body of literature involving modern mouse models and the existence of only a limited number of transcriptional studies of human hyalocytes, conclusive experiments have been performed to study the degree of longevity of hyalocytes in mice and humans, as will be discussed below. Resident tissue macrophages of embryonic origin tend to be long-lived and a self-maintained population, not necessarily excluding a replenishment after birth by bone marrow-derived cells. Microglia in the brain and retina, as well as CNS-associated macrophages have clearly been determined to be long-lived [7,8,10,11,14]. In certain other organs or specialized niches in such organs including the cornea, resident macrophages are continuously renewed over time and can be regarded as short-lived [10,58,59]. The first experiments addressing hyalocyte longevity used the persistence or accessability of dyes in hyalocytes or the vitreous body respectively. These are still relevant to the ongoing discussion about the origin and turnover of hyalocytes.
2.3. Retinal microglia and hyalocytes in the zebrafish
The zebrafish represents a model organism that is suitable for the investigation of macrophage development with the advantage of live imaging approaches using fluorescent reporter strains, specifically labeling macrophages or vessels [60–62]. Such experiments were conducted in the past to study the nature of brain microglia (precursors) during development [63–65]. Regarding the eye, previous studies investigated the development of the hyaloid vasculature [66] but also of retinal microglia in the context of gene expression patterns [67] or phagocytosis and apoptosis [68].
The first descriptions of hyalocytes in fish go back to the 1850’s [69]. More recently, the eyes of zebrafish with labeled macrophages and endothelial cells during development were found to have intimate contacts between both cell types, as expected [26,60]. This finding led to the assumption that increasing numbers of microglial precursors enter the eye via the bloodstream, in line with the need of a functional circulation observed in mice [3,62]. After entry of the microglial precursors, they moved posteriorly to colonize the developing retina, with no further connection to the hyaloid vasculature [62]. The proliferation rate was low in this study suggesting a migratory colonization rather than a local expansion of few cells, at least in the zebrafish [62]. This conclusion re-opens the question if microglia and hyalocytes actually share a common precursor entering the eye that distributes to its respective tissue environment directed by local cues, determining the final cell identity [26,70,71] [70,71]. In support of this postulate, the deletion of interferon-regulatory factor 8 (key to microglial development and homeostasis) in mice affected the distribution of retinal microglia already during development leading to reduced numbers of retinal microglia specifically in the outer plexiform layer but not in the inner plexiform layer [72]. The significant reduction in purinergic receptor expression has led to the hypothesis that at this stage the immature microglia can’t detect signals from deeper retinal layers during postnatal stratification, as was previously shown in the quail [73], suggesting a shared precursor following specific environmental cues to finally colonize distinct niches in the vitreous body and retina.
3. Turnover of hyalocytes
The turnover of macrophages can be investigated by two different experimental approaches: labeling of the resident macrophage population in the tissue of interest or labeling of the putative progenitor such as peripheral blood monocytes. Each strategy can be conducted by several different ways including immunolabeling of differential surface receptor expression, cell type-specific fluorescent protein labeling or fate mapping in transgenic mice, transfer of labeled bone marrow after lethal irradiation distinguishable from the recipients bone marrow or a shared circulation between a wildtype mouse, and fluorescently labeled partner mouse in the parabiosis model [74,75]. All of these experimental approaches were used in the past to decipher the nature of eye macrophages regarding their turnover characteristics and will be discussed below in the context of hyalocyte research.
3.1. Bone marrow chimeras
The use of mice expressing a fluorescent protein under a defined and well-known promotor were mainly used to distinguish myeloid cells from other immune cells. As an example, the Cx3cr1GFP/+ model was frequently used for that approach but the wide expression of Cx3cr1 across myeloid cells renders this model unsuitable to distinguish between central and peripheral myeloid cells in the context of the eye, except when additional markers are used [76]. Therefore, fluorescently labeled cells from transgenic mice were used in another fashion to use their full potential at a time when more advanced transgenic mice were rare and not commonly used. The transfer of full bone marrow from Cx3cr1GFP/+ mice labeling mostly myeloid cells or Acta1GFP/+ mice labeling all cells of the body was intended to label peripheral blood cells but spare resident tissue-macrophages [48,77]. An essential prerequisite is the depletion of the recipients’ bone marrow to create a free niche for the labeled and transfered bone marrow from the donor mice. Unfortunately, the lethal irradiation that was frequently used for the depletion created severe side effects affecting the interpretation of the experimental outcomes. The strong irradiation led to a breakdown of the intrinsic barrier function of exposed organs including the blood-brain and blood-retinal barriers by affecting the tight junctions. Only head-shielding prevented the observed effects in previous studies of the retina [78,79]. It is important to note that the intravenous application of full bone marrow introduces artifact due to the artificial presence of exogenous hematopoietic stem cells and myeloid progenitors that would not enter the circulation under homeostatic circumstances. This would result in infiltration of the (damaged) tissues and the erroneous interpretation that they originated directly from mature monocytes. Furthermore, the turnover itself is likely to be accelerated by the irradiation-induced local expression of the chemokine ligand 2 (CCL2) promoting the attraction and extravasation of peripheral blood monocytes expressing chemokine receptor 2 (CCR2) [51]. Interestingly, first experiments of irradiating newborns rabbits and cats showed a radiation-induced depletion of hyalocytes that were observed to be quickly replenished which suggests monocyte dependence [46].
In the context of the eye, lethally irradiated bone marrow reconstituted rodents were employed in several studies investigating ocular macrophages [78–92] but only few investigated hyalocytes [29,93]. In the first study taking advantage of labeling peripheral cells by bone marrow reconstitution with transgenic mice expressing GFP under the beta-Actin promotor [80], GFP+ hyalocytes were quantified across all stained nuclei in the vitreous body [93]. Within 7 months, a turnover of >90% was reported, evaluated by histological assessment [93]. Comparable to the observation in the brain [51], CCL2 plays in important role in the recruitment of peripherally-derived cells in the retina after low-grade inflammation induced by irradiation followed bone marrow reconstitution peaking into an observed turnover or resident CD11b+ cells within 6 months [89]. Under pathologic conditions, in streptotocozin-induced diabetic Cx3cr1GFP/+ mice, an accumulation of morphologically distinct macrophages could be observed including highly ramified microglia, hyalocytes with an amoeboid shape, and circular cells resembling monocytes [94]. The same observation was made after intraocular injection of CCL2 and was absent in diabetic Cx3cr1GFP/+Ccl2−/− mice, corroborating the role for CCL2 in the retina and but also in the vitreous body after preconditioning [94]. The authors concluded that the vascular damage prior to entering the retina was more dependent on the CCL2-dependent recruitment than on CCL2 alone acting on endothelial cells building the bood-retinal barrier. Also in humans, increased number of cells resembling macrophages were found in patients suffering from proliferative diabetic retinopathy suggesting a translational relevance of the findings made in mice to the human condition [95].
Infiltrating cells can aquire features that resemble the original resident tissue macrophage population including the morphology, longevity, and radio-resistance in the case of microglia [10,29,85,96,97]. Care should be taken when it comes to expression profiles and the chromatin landscape where resident cells could be clearly separated from infiltrating cells in the tissue [97]. In the context of hyalocytes, bone marrow from Cx3cr1GFP/+ mice was transplanted into BALB/c or C57BL/6J wild type and allowed the identification of GFP+ cells [29]. Moreover, GFP-expressing hyalocytes showed strong morphologic resemblance to native (resident) hyalocytes, some expressing MHCII (indicative of turnover), with the aforementioned technical limitations [29]. The expression of MHCII is an important finding due to the fact that MHCII+ hyalocytes are usually not found in healthy mice but were observed to be more numerous after lipopolysaccharide challenge at the junction between retina and ciliary body [17], speaking against a physiological environment after irradiation and bone marrow reconstitution.
3.2. Parabiosis
The model of parabiosis is conducted by establishing a shared circulation between two individual mice [77,98]. After the surgery, the parabiotic partners remain connected for up to several months and leukocytes can shuttle between the mice via the anastomotic connections [77]. Comparable to bone marrow chimerism, a wildtype and a transgenic reporter mouse were used in the past but also other modes exist, e.g. diseased and non-diseased [77,96] or heterochronic using young and old mice [98,99]. Analogously, the specificity is determined by the properties of the transgenic mice labeling mostly myeloid cells (Cx3cr1GFP/+) or all leukocytes (Acta1GFP/+) [48,77,96]. Two advantages should be considered in comparison to the use of bone marrow chimeras. First, parabiosis can be regarded as an experimental setup closer to the physiological condition without the need for lethal irradiation. Second, the only source of labeled cells found in the blood or tissue of the wildtype parabiotic partner is the peripheral blood of the transgenic reporter mouse. At no time do immature myeloid cells artificially enter the blood stream as it does during bone marrow reconstitution. In the context of the eye, parabiosis was only rarely used under homeostatic conditions [10], in depletion [48] or disease models including optic nerve crush [100] and experimental autoimmune uveitis [101]. Unfortunately, none of these studies considered hyalocytes and the contribution of peripheral cells to the local vitreous body macrophage pool. In contrast to the findings made with bone marrow chimeras showing a contribution of peripheral blood myeloid cells to retinal microglia, no replenishement could be observed by using parabiotic mice. Given analogous artificial turnover of both retinal microglia and hyalocytes in irradiated and bone marrow reconstituted mice, it can be tentatively suggested that the findings made for retinal microglia made in parabiotic mice could also apply to hyalocytes [10,29,86,89,91,93].
3.3. Conditional transgenic reporter mouse lines
In the recent years, several transgenic mouse models were created that allow inducible removal of a certain gene sequence that is flanked by loxP-sites using tamoxifen-mediated activity of a bacteriophage Cre recombinase fused to a mutated estrogen receptor (ER) with an increased affinity to tamoxifen over estrogen [74,75,102]. Besides the possibility to create cell type-specific CreER-mediated conditional knock-out mice [6], the induction of fluorescent reporters allowed the investigation of the turnover of resident macrophages in any organ, tissue, or compartment of interest [6–8,10,11,44,59,103]. The broadly expressed Cx3cr1CreER-T2 model has been widely used to investigate macrophages in general, but also specifically retinal and brain microglia [6–8,10]. Other models took advantage of genes more specific for microglia including spalt-like transcription factor 1 (Sall1), transmembrane protein 119 (Tmem119), and purinergic receptor P2Y12 (P2ry12) driving CreER expression [104–106]. With regard to hyalocytes, these novel models were not applied to further investigate the turnover under steady or diseased conditions until now. To date, it is even unknown if hyalocytes express markers considered microglia-specific like SALL1, TMEM119 and P2RY12. This would be helpful to delineate the yet elusive relationship between retinal microglia, hyalocytes, and other tissue macrophages. Regarding the close morphologic resemblance of hyalocytes to epiplexus cells in the choroid plexus of the brain, the exclusive YFP-labeling of epiplexus cells in Sall1CreER:R26-YFP mice, with no labeling of stromal macrophages in the choroid plexus, has rendered this specific CNS-associated macrophage population more similar to microglia [14]. Furthermore, the use of the Cx3cr1CreER-T2 model found no evidence of turnover in ciliary body macrophages analyzed by flow cytometry [10]. These samples could have contained a small fraction of anterior vitreous body macrophages (also considered hyalocytes), due to the experimental tissue manipulation employed. Indeed, based on past studies with the Cx3cr1GFP/+ model, CX3CR1 was shown to be expressed by hyalocytes before and therefore label hyalocytes together with the stromal macrophages in the ciliary body as well, despite being not further investigated in the study [10,29]. To what extent this model can be used to study diseases involving hyalocytes (like the aforementioned streptozocicin-induced model of diabetic retinopathy) has yet to be examined [94].
Under diseased conditions, distinctive morphology that distinguishes hyalocytes from highly ramified retinal microglia, primarily based upon activation and the presence of amoeboid microglia is lost, together with the loss of their typical expression signature and differentiating into (disease-associated) subpopulations [10]. This increase of complexity could only be addressed by taking advantage of a validated hyalocyte-specific gene not expressed by microglia or monocytes that has yet to be identified. Other strategies using a binary Cre approach with the need of two promotors active at the same time in the same cell could be an alternative to one single hyalocyte-specific gene which eventually does not exist [107,108]. Knowledge about such a distinctive gene expression pattern would convincingly allow the use of conditional transgenic reporter mice to further investigate this underinvestigated ocular macrophage population in the future.
3.4. Depletion
Macrophage depletion was intensively studied in the past [74,75,109], revealing new findings on a functional level but also shedding light on origin and turnover by creating a free niche in tissues usually densely populated by tissue-resident macrophages [109]. Limitations in interpreting these findings derive from experimental artifacts due to using receptor tyrosine kinase inhibtors primarily targeting macrophage-related receptors such as colony-stimulating factor 1 receptor (CSF1R) [110], or inhibitors primarily targeting CKIT and only secondarily CSF1R [74]. This eventually affects CSF1R-expressing cells in the bone marrow and peripheral blood as well, including monocytes [111,112]. However, inhibitors targeting CSF1R are undoubtedly capable of depleting retinal microglia very efficiently with only minor changes in peripheral mononuclear cell populations [48]. A study investigating the source of re-population after depletion in whole retinas showed the total absence of GFP+ positive cells after the intended depletion of retinal microglia in Cx3cr1GFP/+ mice which seems to have depleted hyalocytes as well [48]. In this study, the contribution of extra-retinal macrophages to the (depleted) retinal microglia pool was elegantly investigated by a differential ex vivo culturing of the retina with or without the optic nerve or ciliary body and iris attached, respectively [48]. Given the fact that there is a continuum between the hyalocytes in the anterior part of the vitreous body (in close proximity to the ciliary epithelium) and preretinal hyalocytes [17], it is reasonable to implicate the re-population of depleted microglia in the peripheral retina by macrophages from the ciliary body/iris complex [48]. The transition zone between the ciliary body and the retina looks surprisingly similar to the repopulated mice study and in an earlier study identifying an accumulation of macrophages after lipopolysaccharide challenge, both showing a high variety of morphologic states ranging from amoeboid to moderately ramified [17,48]. Most importantly, the depleted retina remained completely devoid of re-populated cells during cultivation without the ciliary body and iris, owing perhaps to the lack of a circulation due to the primary tissue culture setup [48]. The observations made in these two studies further corroborate a very close relationship between retinal microglia and hyalocytes in the vitreous body. Of note, the fetal hyaloid vessels are transiently formed during eye development supporting the lens development but undergo macrophage-dependent regression at later stages, always in close contact to hyalocytes [27].
4. Hyalocyte morphology
4.1. Electron microscopy
Visualization of hyalocytes by scanning electron microscopy showed an appearance similar to macrophages in other organs [113] displaying a stellate, sometimes bipolar shape with long protrusions [114]. Transmission electron microscopy of a human hyalocyte is shown in Figure 3. The strong resemblance to the Kolmer’s epiplexus cells of the choroid plexus [18] in the brain’s ventricles that shares some histological characteristics with the ciliary body and produces the liquid that fills the ventricles and subarachnoidal spaces was brought to attention in the 1970s [113]. The so-called “cytoblasts” in the vitreous body were described for the first time by Henle in 1841 and in close proximity to the zonules arising from the ciliary body [23,115]. Choroid plexus macrophages were intensively investigated in recent years, also in the context of origin and turnover [8,14,15]. It turned out that macrophages in the choroid plexus can be subdivided into distinct subpopulation consisting of the epiplexus cells located on the apical surface, comparable to the anterior hyalocytes apically located on the ciliary epithelium, and two further subpopulations situated in the stroma [14]. These similarities based on structural and anatomical findings could be further indicative of a prenatal origin, with the need to be validated with modern methodologies.
Figure 3. Transmission electron microspcipy of human hyalocyte.
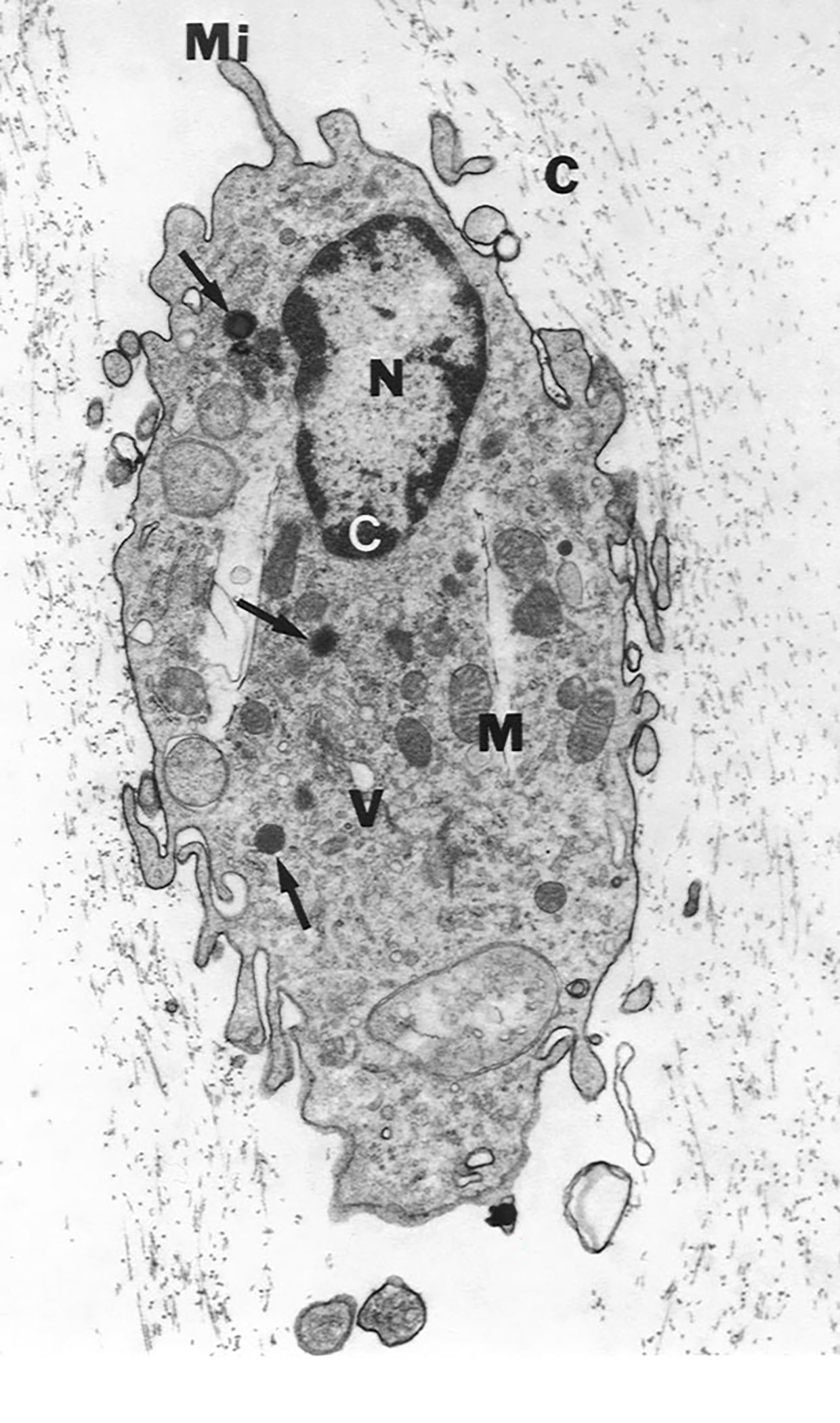
A mononuclear cell is seen embedded within the dense collagen fibril (black C) network of the vitreous cortex. There is a lobulated nucleus (N) with dense marginal chromatin (white C). In the cytoplasm there are mitochondria (M), dense granules (arrows), vacuoles (V), and microvilli (Mi) (X 11,670). Image courtesy of JL Craft, DM Albert and DG Cogan (Laboratory of Ophthalmic Pathology, Harvard Medical School, Boston, MA). Reproduced with permission from [2], © 1989 Springer-Verlag New York Inc.
4.2. Laboratory studies of human hyalocyte morphology
Initial experiments to study the nature of the vitreous body early in the 19th and 20th century took advantage of relatively unspecific vital dyes [116] or other simple staining techniques. However, the use of immunohistochemical stains revealed morphological subtypes of hyalocytes and where they are located inside the eye. Since then, labeling and imaging techniques have significantly improved and now allow the identification of, in this case, myeloid cells in tissues of different origins and their respective subpopulations. In particular, the use of multichannel antibody-mediated immunofluorescent labeling has some advantages over immunohistochemical dye precipitation, such as the use of horse radish peroxidase and labeling with diaminobenzidin (DAB). Of note, both fluorescent and histochemical labeling can also be performed with lectins acting as plant-derived proteins specifically labeling certain glycosylated surface or intracellular proteins of hyalocytes [117]. However, in the context of macrophage research it can become difficult to use labeling with antibodies or lectins as the only method to distinguish resident from infiltrating macrophages, but it does provide valuable insights in a complementary fashion. For example, TMEM119 is restricted to brain and retinal microglia in the myeloid compartment and not expressed by monocytes under homeostatic conditions. Yet, it can be reduced in expression under pathological conditions, like in the retina [10,118]. This finding would not have been made without the combination of antibody labeling and transgenic flourescent reporter lines [10] which currently remains restricted to research conducted in mice.
In humans, the use of antibodies, lectins or other proteins is the only way to study hyalocytes and other myeloid cells at the microscopic level in tissue sections. Hyalocytes have the advantage of being the only cell population inside the vitreous body and can be easily identified by nuclear counterstaining (Fig 4). The use of immunofluorescent labeling allows the study of protein expression profiles with a much higher sensitivity than by the use of immunohistochemical staining in combination with light microscopy [30]. By applying pan-macrophage markers such as IBA1, hyalocytes can be specifically identified, for example to validate findings made with RNA-seq, as it has been done to confirm the expression of HLA-DR involved in antigen presentation [30]. The transparency of the vitreous body allows visible light to easily pass through and reach the photoreceptors of the retina. To visualize biological phases that are transparent under visible light, the phase contrast or differentical interference contrast (DIC) between the specimen and the surrounding embedding medium or remaining constitutents of the vitreous body allow the detection of hyalocytes without any prior labeling (Fig 4).
Figure 4. Confocal microscopy of fluorescently labeled hyalocytes.
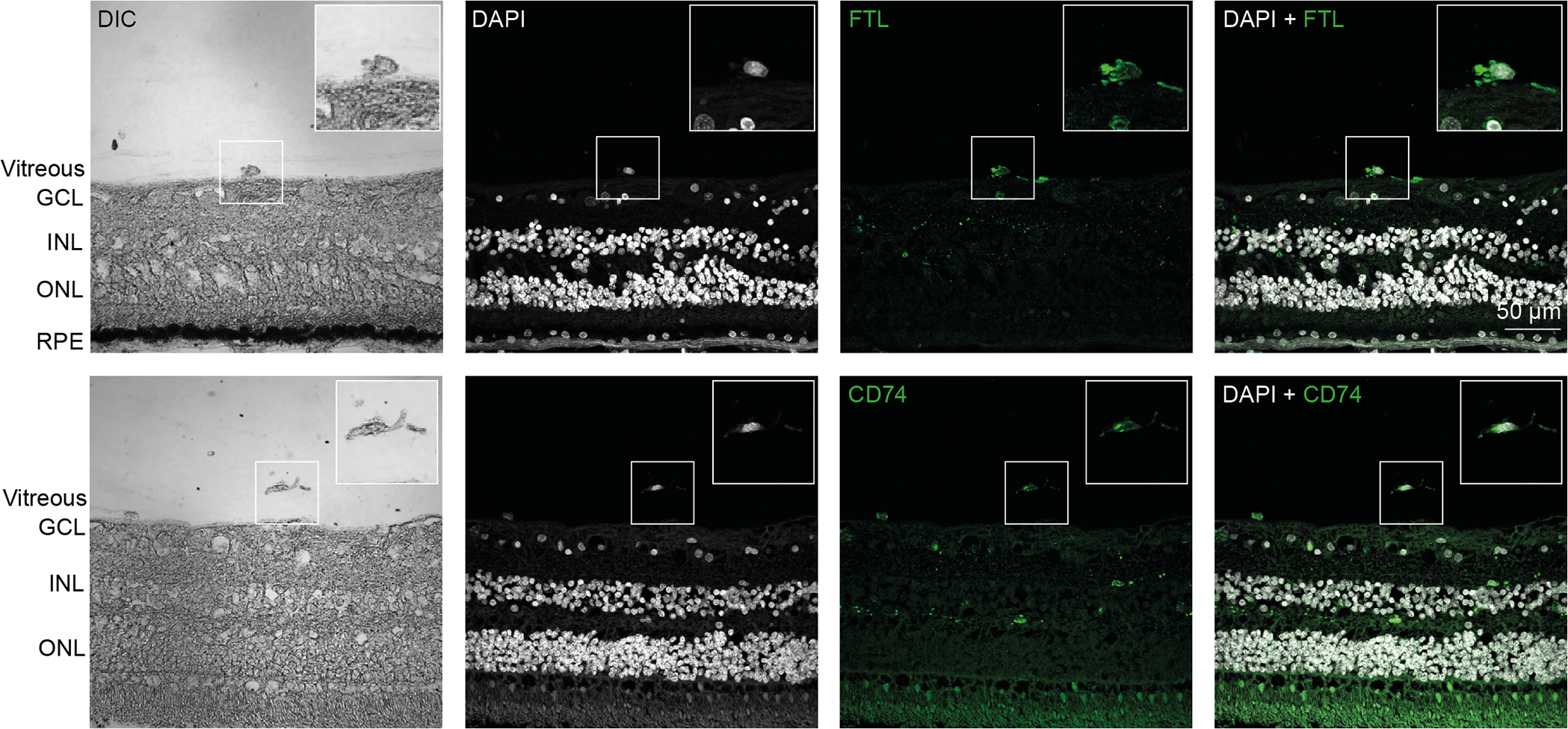
TOP: A preretinal hyalocyte close to the inner limiting membrane is shown. The hyalocyte was identified by its location via differential interference contrast (DIC) and a nuclear counterstain with DAPI to investigate the expression of the ferritin light chain (FTL). BOTTOM: A free hyalocyte with filopodia is shown. The hyalocyte was identified by its location via differential interference contrast and a nuclear counterstain with DAPI to investigate the expression of CD74. GCL = ganglion cell layer, INL = inner nuclear layer, ONL = outer nuclear layer, RPE = retinal pigment epithelium. Reproduced from [30], licensed under CC-BY 4.0 (http://creativecommons.org/licenses/by/4.0/)
In another experimental setup, hyalocytes were purified by flow cytometry after vitrectomy of a patient diagnosed with proliferative diabetic retinopathy and in vitro cultivated [32] (Fig 5). The combination of antibody labeling with other fluorescently labeled proteins, or in this case the toxic cyclic peptide Phalloidin from fungi which stains Actin, illustrates the structure of the cytoskeleton of hyalocytes at a subcellular level and different morphological states [32] (Fig 5).
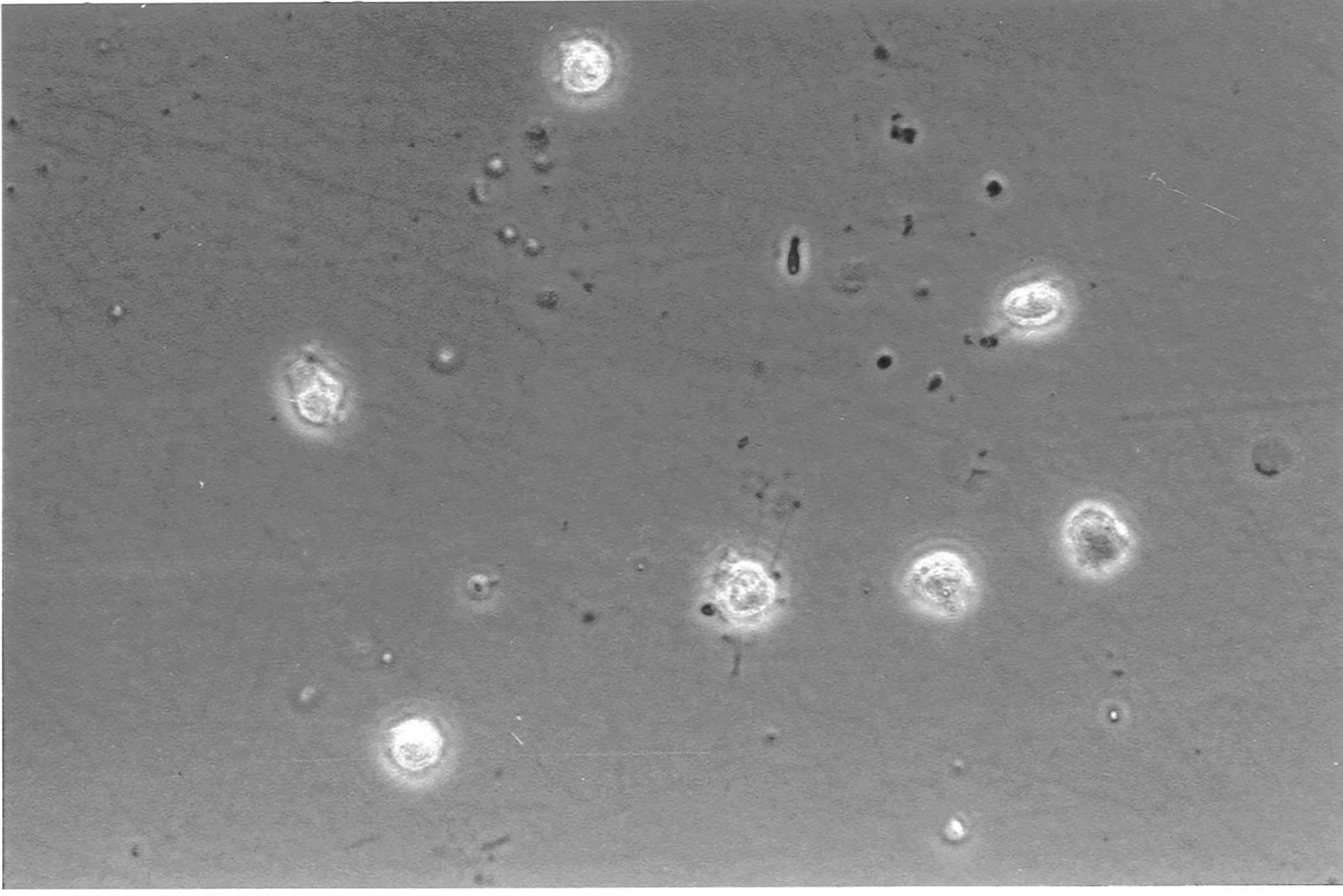
Figure 5. In vitro cultured human hyalocytes.
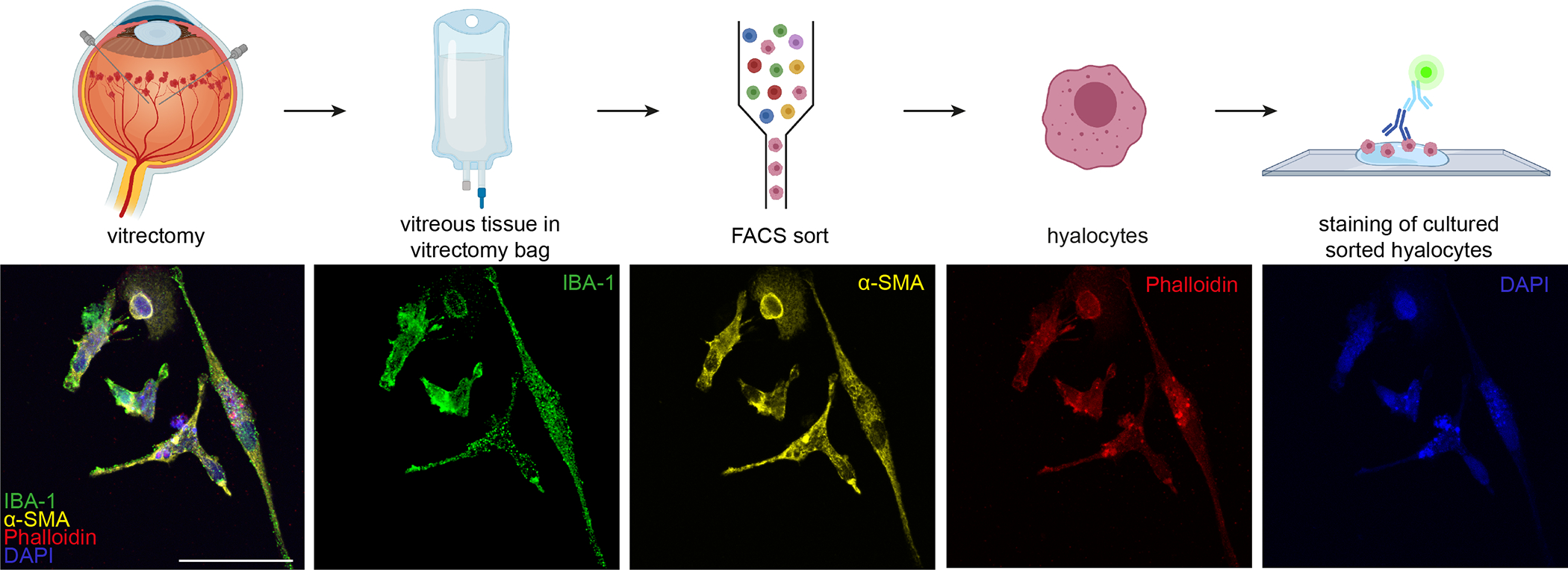
Primary hyalocytes were purified by flow cytometry after vitrectomy, cultured in vitro and stained via immunofluorescence. Hyalocytes are labeled with the macrophage marker IBA1, alpha smooth muscle actin and phalloidin labeling the cytoskeleton protein actin. Scale Bar = 50 μm. Reproduced from [32], licensed under CC-BY 4.0 (http://creativecommons.org/licenses/by/4.0/)
Recent advances in novel imaging approaches include the combination of labeling antibodies with different elements instead of fluorescent dyes that can be detected by time-of-flight (TOF) mass spectrometry (MS), leading to the term “CyTOF” or “imaging mass cytometry” (IMC). This overcomes the limitations of light imaging that only allows a certain number of dyes to be used at the same time without spectral interference. In principle, the only limiting factor is the number of elements in the universe. This method was recently applied for the first time to study hyalocytes contributing to retinal neovascularisation into the posterior vitreous cortex in patients with proliferative diabetic vitreo-retinopathy [32]. Here, the multiplexed approach enabled simultaneous imaging of 20 markers followed by segmentation of individual cells to identify up to 24 distinct cell clusters according to their protein expression profiles in areas of retinal neovascularization [32]. Thus, hyalocyte microscopy has evolved from the sole use of dyes beginning in the 1840’s into a complex methodology combining the analysis of multiple markers at the same time supported by computional-based identification of cell subtypes found in healthy as well as diseased tissues.
5. Hyalocyte imaging
In contrast to other organs such as the brain, the eye has the advantage of direct access by light for imaging of myeloid cells [35]. Nonetheless, vitreous has has long posed great challenges to imaging, perhaps because it’s invisible ‘by design’ [119–122]. Conventional imaging has provided some insights, primarily limited to the posterior vitreous and vitreo-retinal interface [123,124].
5.1. Optical coherence tomography (OCT)
5.1.1. Clinical OCT Imaging
Hyalocytes can be readily visualized with both spectral-domain and swept-source OCT. Since they appear as hyperreflective round dots, image enhancement is not usually necessary. However, to localize them within the cortical vitreous fiber meshwork, there is benefit from modifications to routine image acquisition and post-acquisition processing. Previous studies [125] developed an acquisition mode termed enhanced vitreous imaging (EVI) characterized by averaging a large number of scans (for example, 45 to 70 by automatic real time-function on the Heidelberg Spectralis), as well as pulling the focus back into the vitreous body and positioning the retinal images inferiorly in the scan window. This highlights cortical vitreous structures including hyalocytes embedded within the posterior vitreous cortex (Fig 6A). Since retinal nerve fiber analysis relies on high averaging, hyalocytes appear routinely in the peripapillary area (Fig 6B). A similar acquisition algorithm using 32 averaged images and vitreous focusing using Swept-Source OCT yields scans with greater depth into the vitreous body, particularly when using the Zeiss PLEX Elite device (Fig 7A). Coronal plane (en face) OCT imaging [20] in particular visualizes an abundance of hyalocytes in the cortical vitreous close to the retinal surface (Fig 7B), whereas they are less prevalent farther anteriorly into the vitreous body (Fig 7C).
Figure 6. Spectral domain OCT imaging of human hyalocytes in situ.
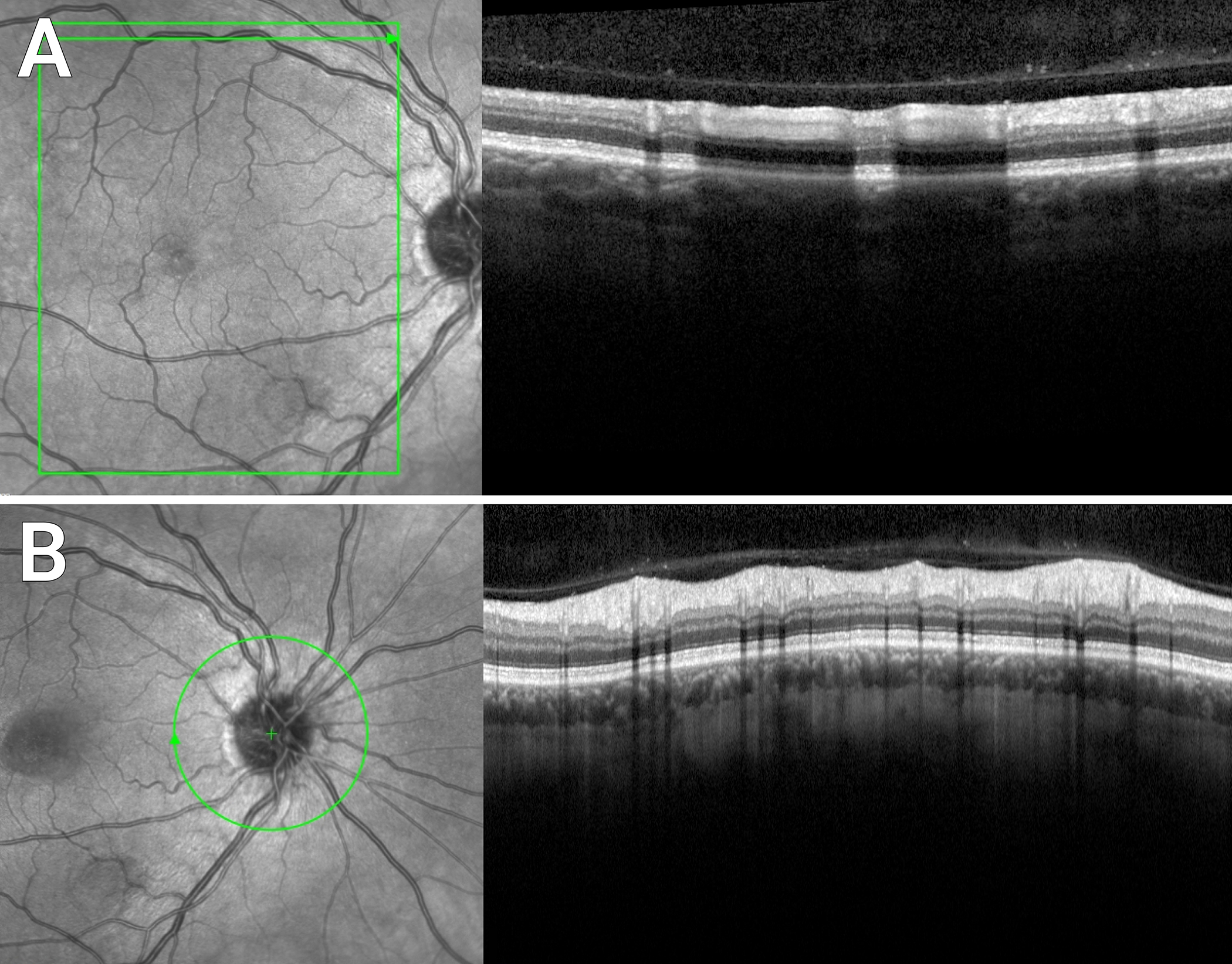
Enhanced vitreous imaging with the Heidelberg Spectralis demonstrates hyalocytes overlying the superior arcade (A) and the peripapillary retina (B) in a 52-year-old male with central serous chorioretinopathy. Images courtesy of author M Engelbert.
Figure 7. Swept source OCT imaging of human hyalocytes in situ.
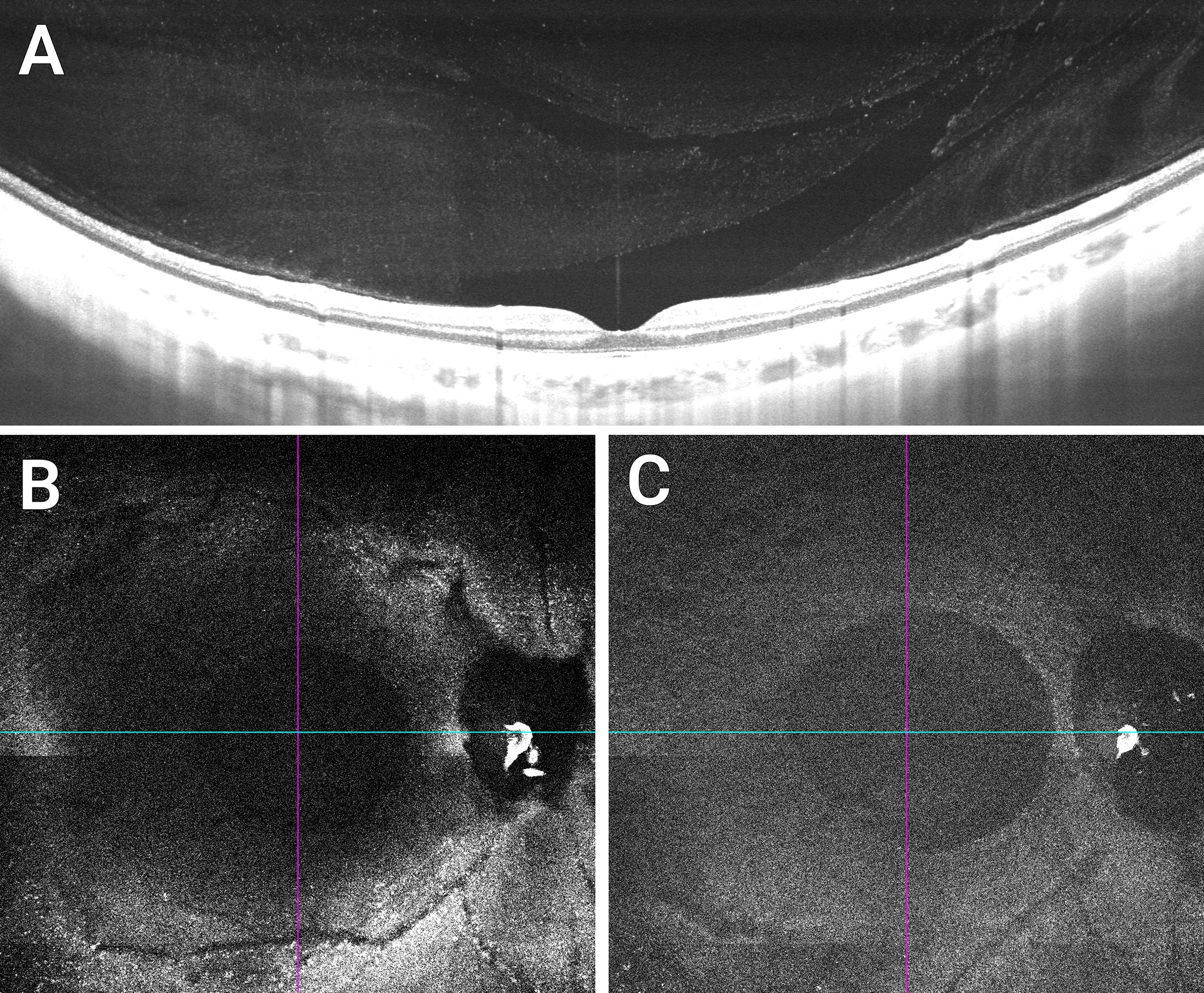
Swept-Source OCT visualizes not only the Bursa Premacularis of Worst, but other lacunae of liquefied vitreous in the vitreous body in proximity to the retina. Hyalocytes appear as hyperreflective dots (A). En face imaging demonstrates a greater abundance of hyalocytes in a 20 μm slab just over the retinal surface (B) than in an equivalent slab 100 μm away from the retina (C). Images courtesy of author M Engelbert.
5.1.2. Laboratory OCT Imaging (experimental models)
OCT imaging is also suitable for laboratory research (with rodents), allowing the quantification of the number of vitreous cells after toll-like receptor activation [126]. Although a limitation is that this method does not allow discrimination between local proliferation of resident hyalocytes and infiltrating monocytes by the use of specific myeloid markers, OCT elegantly takes advantage of the relatively cell-free environment of the vitreous body in a non-invasive and repeatable way [126]. Another advantage is the comparability with the human situation where this method has significantly improved in recent years by taking advantage of the physical parameters of distinct cell neuronal and non-neuronal cells for visualization of hyalocytes rich in details, and allowing for the first time in vivo imaging kinetics of human hyalocytes over time (Fig 8) [35,125].
Figure 8. Imaging human hyalocytes in vivo.
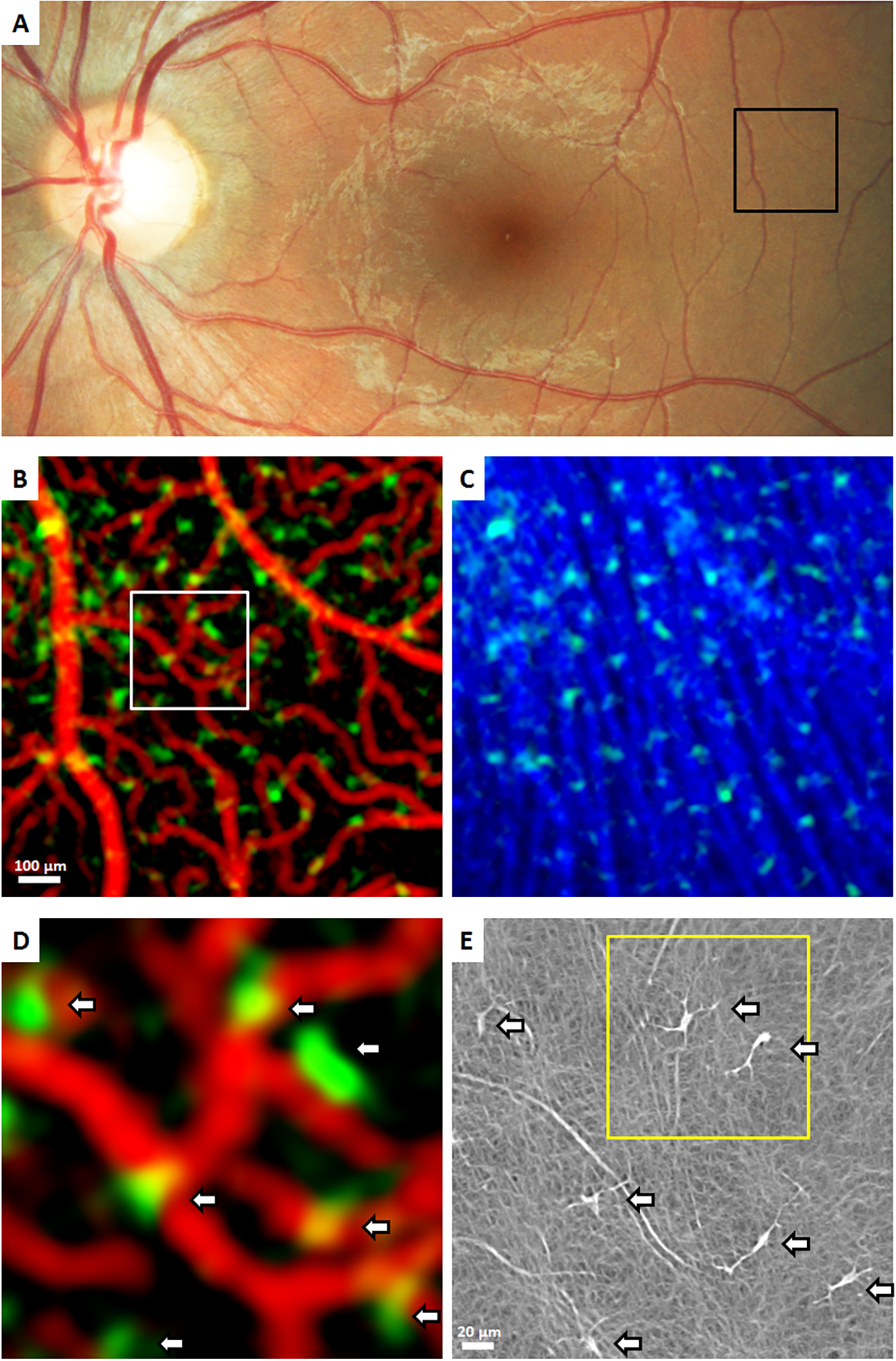
A 32-year-old male was imaged with clinical OCT & Adaptive Optics Scanning Light Ophthalmoscopy (AOSLO). A: Color fundus photo, black box indicates a region imaged using clinical OCT. B & C: OCT reflectance and OCT angiography (OCTA) color overlays of the black box in (A). Clinical OCT color overlays of (B) superficial retinal vascular network (red) and hyalocytes (green), and (C) hyalocytes (green) seen anterior to the retinal nerve fiber bundles (blue) show the spatial relationships among structures. D: Magnified color overlay of the superficial retinal vascular network (red) and hyalocytes (green) of the white box in (B). White arrows indicate seven hyalocytes imaged within this region of interest. E: Corresponding AOSLO image also revealed the same number of hyalocytes (white arrows) with better visibility of their cell somas and processes. Hyalocyte locations appear to match between imaging modalities, but the cell size and shape were different. Yellow box indicates a region imaged over 2 hours in Figure 9. Images courtesy of authors TYP Chui and RB Rosen.
A novel transgenic reporter mouse model opens the possibility to specifically label resident tissue macrophages in defined region by photoconversion [127]. In Cx3cr1Dendra2/+ mice, the photoconvertible Dendra2 that is similar to GFP can be converted from green into red by blue light and allows the observation of Cx3cr1-expressing cells, in this study retinal microglia, by in vivo fluorescence imaging [127]. Within 7 days, the converted red Dendra2 molecules are fully replaced within individual cells by the continuous expression of the green variant, but allow the observation of the converted cells as well as potentially infiltrating cells or recruited non-converted retinal microglia in the observed field of view during that time frame [127]. In theory, individually identified groups of hyalocytes could be converted as well and followed under native or diseased conditions, in combination with microscopic techniques of fixed and stained tissue.
5.2. Adaptive optics scanning light ophthalmoscopy
Recently, coronal plane imaging of human hyalocytes has been enhanced to study hyalocyte movement in vivo without exogenous labeling (Figs 8 and 9, Movie 1) [128]. A recent study [37] has demonstrated the use of non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy (AOSLO) [129] and coronal plane (en face) OCT [128] to observe the dynamic morphological changes and the variable motility of these cells over extended time intervals in the living human eye (Fig. 8). In this study, hyalocytes demonstrated variability in movement, fluidity of cell soma configuration, and degree of activation over time. (Fig 9, Movie 1) Cell processes appeared to reach out and retract upon achieving their intended purpose [37]. The detail of hyalocyte morphology and complexity of movements observable with this imaging technique brings single cell imaging in living subjects to a new level not previously demonstrated.
Figure 9. Coronal plane imaging of human hyalocytes in vivo.
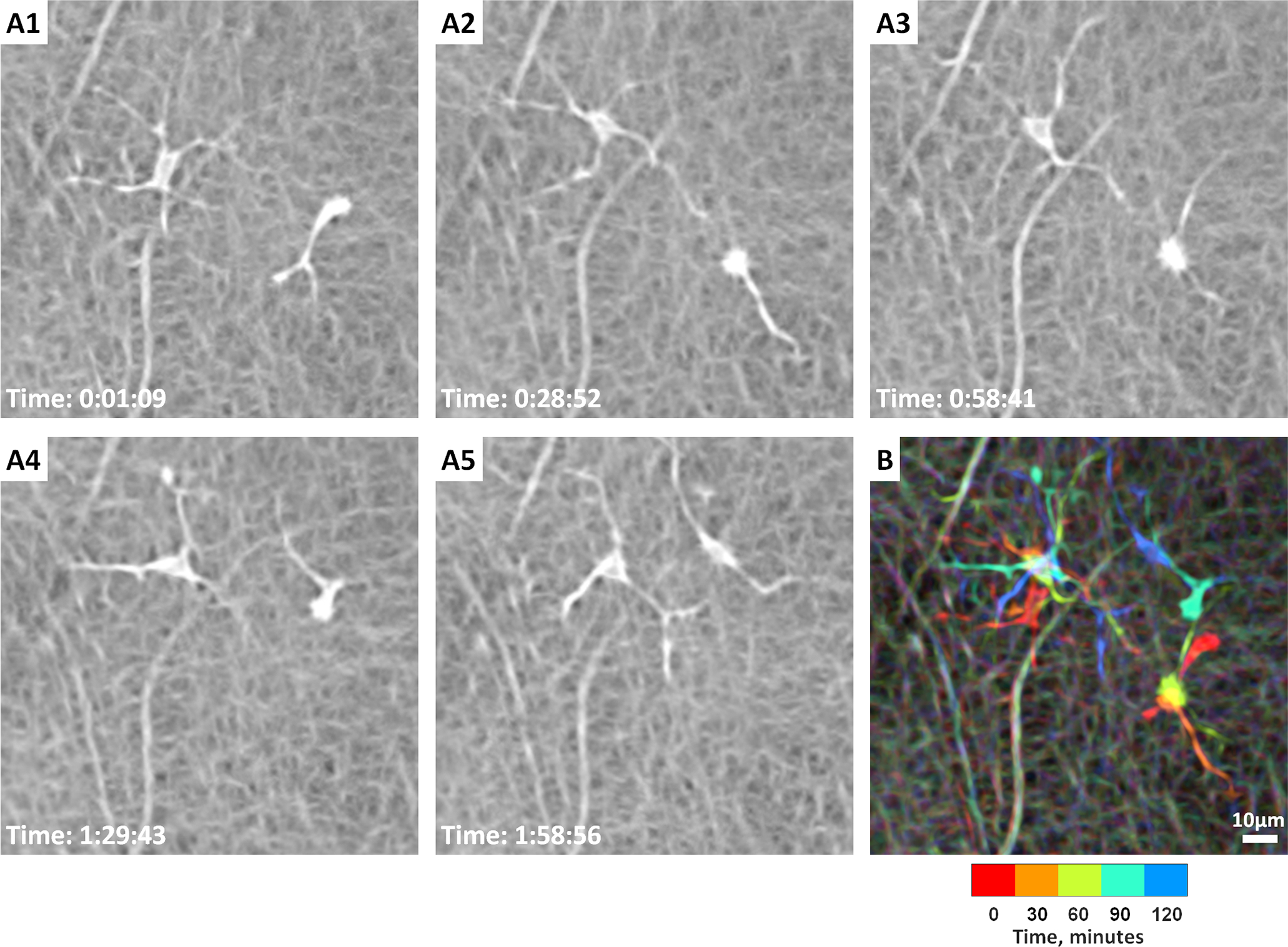
In vivo imaging of hyalocyte morphology and dynamics in a 32-year-old male using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy. A1-A5: Two ramified hyalocytes show noticeable differences in shape and movement over 2 hours. Time of acquisition in the lower-left corner of each image denotes the hrs:mins:secs. B: A chromo-temporal map composite of the 5 time points (each approximately 30 minutes apart) demonstrates the movements of cell somas and processes over 2 hours. While the more ramified cell (left) displays relatively stationary soma, the less ramified cell (right) shows relatively greater movement of the soma as evidenced by the long chromatic trail in the chromo-temporal map. The processes of both cells, however, appear to move significantly over 2 hours. See Movie 1 for the AOSLO time-lapse video of the two hyalocytes and their processes movement over 2 hours. Images courtesy of authors TYP Chui and RB Rosen.
6. Conclusions
Hyalocytes were first discovered in 1840 and recognized as macrophages shortly thereafter, long before microglia were described in the early 20th century [116,130]. The presence and active role of hyalocytes during development, adulthood, and under pathologic conditions speaks for a distinct and non-redundant role throughout the lifespan of different species including rodents, fish, and humans. Early labeling experiments in the 19th centrury already pointed to the long-lived nature of these macrophages in a unique environment, being the major cell type inhabiting the vitreous body. However, more recent experimental findings (including irradiation and bone marrow reconstitution to follow blood-derived cells into tissues) showed contradictory results compared to earlier concepts, in favor of a peripherally-derived (circulatory) monocytic origin [29,93]. With the advent of inducible transgenic reporter mouse labeling that is devoid of irradiation artefacts and enables study of resident macrophages in an exclusive way, the findings made in bone marrow chimera studies were revisited for all kinds of resident tissue-macrophage populations across many organs including the eye [7,8,10,59,131].
Beside traditional microscopic methods such as light and electron microscopy, multicolor confocal imaging and imaging mass cytometry have expanded our knowledge regarding the expression pattern of human hyalocytes on the protein level in high resolution [30–32]. The transparent nature of vitreous makes it directly accessible with light-based imaging such as OCT, both clinically in humans and experimentally in mice. Ongoing improvements of algorithms for the analysis of OCT-derived images allows previously never-seen spatial and temporal resolution of hyalocytes in patients in vivo [35,125], complemented by Adaptive Optics Scanning Light Ophthalmoscopy. Despite different morphologies, both hyalocytes and microglia demonstrate motile behavior rather than being static. This again begs the question if and to what extent these two cells can be regarded as close relatives or just neighbors on two sides of the fence, represented by the ILM separating the neural retina from the vitreous body.
On a transcriptional level, however, recent studies applied RNA sequencing of hyalocytes purified by flow cytometry to shed more light upon the nature of human hyalocytes under homeostatic and diseased conditions, allowing comparisons to retinal microglia [30,32]. More research is needed to gain more insights into the species-specific differences, as has been done recently for retinal microglia [132]. Current expression patterns show overlap between the expression profiles of hyalocytes and retinal microglia. In humans, these similarities seem to be a consequence of a common origin during early development, as was suggested by live imaging in zebrafish. For mammals, however, further investigation in mouse models and advanced coronal plane imaging in humans [35,37] are likely to be the most suitable approaches to gain further insight into this special cell that has fascinated researchers for almost two centuries, and appropriately continues to do so.
7. Expert opinion
Although the vitreous body is the largest structure within the human eye, knowledge about its roles in health and disease is perhaps the least of all ocular tissues. Consequently, little is known about various aspects of vitreous physiology and how it performs different functions to promote a stable microenvironment within the eye. Not the least of these functions is the maintenance of transparency within the center of the eye, which enables the unhindered transmission of light to the retina. Thus, the organization of vitreous macromolecules minimizes light scattering, although this changes with aging and in certain diseases. Transparency also requires a relatively cell-free environment, yet there are some cells within the vitreous body whose origin and nature have been the subject of controversy ever since their discovery nearly 200 years ago. This review has attempted to determine what evidence exists regarding the origin, turnover, and morphology of hyalocytes as a unique population of cells.
Based upon the evidence presented in this review, it is reasonable to conclude that hyalocytes represent a population of cells that reside within the vitreous body, which are distinct from retinal microglia, though the two possess many similarities. Indeed, a weakness in this expert opinion is that there are currently no definitive ways to uniquely identify either of these two cells, although the preponderance of evidence presented in this review suggests that hyalocytes are indeed a distinct if not unique cell type. While found throughout the vitreous body, the “most important” hyaloctes might be those embedded within the posterior vitreous cortex anterior to the inner limiting membrane of the retina. There, hyalocytes act as sentinel cells and as such would be the first to respond to any noxious circumstances threatening the health of the posterior segment as well as the entire eye. Hyalocytes in the anterior vitreous and at the vitreous base might serve a similar function with respect to the lens and ciliary body. The reactions of posterior hyalocytes to trauma, infection, aging, neurodegenerative, and systemic diseases probably play important roles in the pathophysiology of various diseases of the posterior segment of the eye. These aspects will be considered in the second [133] and third [134] articles of this series of expert reviews on hyalocytes.
As the eye has long been considered a window to the body, the interplay of the eye and central nervous system is particularly intriguing. Thus, inspecting and evaluating hyalocytes in the posterior vitreous and their interaction(s) with the neural retina could afford insights to the brain in neurodegenerative disorders. The advent of new imaging modalities offers scientists and clinicians the opportunity to study human hyalocytes in vivo, enabling better understanding of cell activity (e.g. movement) during normal physiology, as well as changes in various diseased states. With further information concerning hyalocyte physiology and their departure(s) from normality as part of the pathophysiology of various pathologic conditions, the role of hyalocytes in pathogenesis can be further elucidated. This could usher in new therapeutic strategies designed to mitigate the role of these critical cells in the early stages of disease, thus stopping progression to more advanced stages. In fact, advanced knowledge could one day help to develop strategies to prevent many vitreo-retinal diseases entirely.
Supplementary Material
Movie 1. Video showing the AOSLO time-lapse video of the two hyalocytes presented in Figure 9 and their processes movement over 2 hours. Images courtesy of authors TYP Chui and RB Rosen.
Article highlights.
This article reviews the origin and turnover of hyalocytes.
Hyalocytes are tissue macrophages residing in the vitreous body, investigated by phase, electron, and confocal microscopies.
Transcriptional profiling has identified overlapping expression signatures in both vitreous hyalocytes and retinal microglia, owing to common myeloid cell identity.
Preretinal vitreous hyalocytes are important as sentinel cells, ready to respond to noxious circumstances.
In vivo imaging of human hyalocytes promises to shed more light on the role of hyalocytes in health and disease.
Funding
This paper was funded by the VMR Research Foundation (J Sebag), Research to Prevent Blindness (TYP Chui and RB Rosen), the Marrus Family Foundation (TYP Chui and RB Rosen), the New York Eye and Ear Infirmary Foundation (TYP Chui and RB Rosen) and the National Institutes of Health (R01EY027301, R01HL159116; TYP Chui and RB Rosen).
Abbreviations
- BrdU
5′-bromo-2′-deoxyuridine
- EdU
5-ethynyl-2’-deoxyuridine
- AOSLO
adaptive optics scanning light ophthalmoscopy
- AGM
aorto-gonad-mesonephros
- CNS
central nervous system
- CCL2
chemokine ligand 2
- CCR2
chemokine receptor 2
- DAPI
4’,6-Diamidino-2-phenylindol
- DAB
diaminobenzidin
- DIC
differentical interference contrast
- EVI
enhanced vitreous imaging
- EMP
erythro-myeloid precursor
- ER
estrogen receptor
- FL
fetal liver
- FTL
Ferritin Light Chain
- GCL
Ganglion Cell Layer
- GFP
green fluorescent protein
- HLA
human leukocyte antigen
- ILM
inner limiting membrane
- IMC
imaging mass cytometry
- INL
Inner Nuclear Layer
- MHC II
major histocompatibility complex II
- MS
mass spectrometry
- OCT
Optical Coherence Tomography
- ONL
Outer Nuclear Layer
- P2RY12
purinergic receptor P2Y12
- RPE
retinal pigment epithelium
- SALL1
spalt-like transcription factor 1
- TOF
time-of-flight
- TMEM119
transmembrane protein 119
- YFP
yellow fluorescent protein
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- [1].Sebag J Vitreous: the resplendent enigma. British Journal of Ophthalmology. 2009;93:989–991. [DOI] [PubMed] [Google Scholar]
- [2]. Sebag J Structure of the Vitreous. In: Sebag J, editor. The Vitreous: Structure, Function, and Pathobiology [Internet]. New York, NY: Springer; 1989. [cited 2022 Feb 23]. p. 35–58. Available from: 10.1007/978-1-4613-8908-8_4. * This publication features phase contrast microscopy of unstained human hyalocytes in situ, as well as transmission electron microscopy of human hyalocytes.
- [3].Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. [DOI] [PubMed] [Google Scholar]
- [5].Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldmann T, Wieghofer P, Müller PF, et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. [DOI] [PubMed] [Google Scholar]
- [7].Hagemeyer N, Kierdorf K, Frenzel K, et al. Transcriptome-based profiling of yolk sac-derived macrophages reveals a role for Irf8 in macrophage maturation. EMBO J. 2016;35:1730–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goldmann T, Wieghofer P, Jordão MJC, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wieghofer P, Prinz M. Genetic manipulation of microglia during brain development and disease. Biochim Biophys Acta. 2015; [DOI] [PubMed] [Google Scholar]
- [10].Wieghofer P, Hagemeyer N, Sankowski R, et al. Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J. 2021;40:e105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Koren EG, Yu C, Klingeborn M, et al. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity. 2019;50:723–737.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dawson CA, Pal B, Vaillant F, et al. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat Cell Biol. 2020; [DOI] [PubMed] [Google Scholar]
- [13].Wang M, Yang Y, Cansever D, et al. Two populations of self-maintaining monocyte-independent macrophages exist in adult epididymis and testis. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–1035. [DOI] [PubMed] [Google Scholar]
- [15].Jordão MJC, Sankowski R, Brendecke SM, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363. [DOI] [PubMed] [Google Scholar]
- [16].Kaur C, Ling E-A. The circumventricular organs. Histol Histopathol. 2017;32:879–892. [DOI] [PubMed] [Google Scholar]
- [17].Vagaja NN, Chinnery HR, Binz N, et al. Changes in murine hyalocytes are valuable early indicators of ocular disease. Invest Ophthalmol Vis Sci. 2012;53:1445–1451. [DOI] [PubMed] [Google Scholar]
- [18].Ogawa K Scanning electron microscopic study of hyalocytes in the guinea pig eye. Arch Histol Cytol. 2002;65:263–268. [DOI] [PubMed] [Google Scholar]
- [19].Haddad A, André JC. Hyalocyte-like cells are more numerous in the posterior chamber than they are in the vitreous of the rabbit eye. Exp Eye Res. 1998;66:709–718. [DOI] [PubMed] [Google Scholar]
- [20].Leong BCS, Fragiotta S, Kaden TR, et al. OCT en face analysis of the posterior vitreous reveals topographic relationships among premacular bursa, prevascular fissures, and cisterns. Ophthalmol Retina. 2020;4:84–89. [DOI] [PubMed] [Google Scholar]
- [21]. Hannover A Ueber die netzhaut und ihre gehirnsubstanz bei wirbelthieren, mit ausnahme des menschen. Arch f Anat, Phys u wiss Med (Müllers Arch). 1840;320. * Hannover was the first to identify hyalocytes as an independent cell population.
- [22].Virchow R Archiv für pathologische Anatomie und Physiologie und für klinische Medizin. Springer; 1852. [PMC free article] [PubMed] [Google Scholar]
- [23].SZIRMAI JA, BALAZS EA. Studies on the structure of the vitreous body: III. Cells in the cortical layer. AMA Archives of Ophthalmology. 1958;59:34–48. [DOI] [PubMed] [Google Scholar]
- [24].Schwalbe GA. Mikroskopische anatomie des sehnerven, der netzhaut und des glaskörpers, in handbuch der gesamten augenheilkunde. 1874;1:321–479. [Google Scholar]
- [25].Potiechin A Ueber die Zellen des Glaskörpers. Archiv f pathol Anat. 1878;72:157–165. [Google Scholar]
- [26].Lopéz Enríquez M, Costero I Sobre los carateres de la microglia retinania emigrada al humor vítreo. Bol soc espan hist nat. 1931;425–431. [Google Scholar]
- [27].Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. [DOI] [PubMed] [Google Scholar]
- [28].Bloom GD, Balazs EA. An electron microscopic study of hyalocytes. Exp Eye Res. 1965;4:249–255. [DOI] [PubMed] [Google Scholar]
- [29].Kezic JM, McMenamin PG. The effects of CX3CR1 deficiency and irradiation on the homing of monocyte-derived cell populations in the mouse eye. PLoS ONE. 2013;8:e68570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Boneva SK, Wolf J, Rosmus D-D, et al. Transcriptional profiling uncovers human hyalocytes as a unique innate immune cell population. Front Immunol. 2020;11:567274. ** This study was the first to provide a transcriptional analysis by RNA-sequencing of human hyalocytes after vitrectomy.
- [31].Schlecht A, Boneva S, Salie H, et al. Imaging mass cytometry for high-dimensional tissue profiling in the eye. BMC Ophthalmol. 2021;21:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Boneva SK, Wolf J, Hajdú RI, et al. In-depth molecular characterization of neovascular membranes suggests a role for hyalocyte-to-myofibroblast transdifferentiation in proliferative diabetic retinopathy. Front Immunol. 2021;12:757607. ** In this study RNA-sequencing and single-cell based imaging mass cytometry was applied to epiretinal membranes and membranes of retinal neovascularization providing new molecular insights into diabetic vitreoretinal diseases.
- [33].Schwalbe GA. Lehrbuch der Anatomie des Auges. Verlag von Eduard Besold; 1887. [Google Scholar]
- [34].van Furth R Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970;7:125–141. [PubMed] [Google Scholar]
- [35].Hammer DX, Agrawal A, Villanueva R, et al. Label-free adaptive optics imaging of human retinal macrophage distribution and dynamics. Proc Natl Acad Sci USA. 2020;117:30661–30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kurokawa K, Crowell JA, Zhang F, et al. Suite of methods for assessing inner retinal temporal dynamics across spatial and temporal scales in the living human eye. Neurophotonics. 2020;7:015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Migacz JV, Otero-Marquez O, Zhou R, et al. Imaging of vitreous cortex hyalocyte dynamics using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy in human subjects. Biomed Opt Express, BOE. 2022;13:1755–1773. ** This study used non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy (AOSLO) to non-invasively visualize the movement and morphological changes of the hyalocyte cell bodies and processes over 1–2 hour periods in the living human eye.
- [38].Baranska A, Shawket A, Jouve M, et al. Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal. J Exp Med. 2018;215:1115–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goldmann EE. Die äussere und innere Sekretion des gesunden und kranken Organismus im Lichte der “vitalen Färbung”: T. 1. Tübingen: H. Laupp; 1909. [Google Scholar]
- [40].Schnaudigel O Die vitale Färbung mit Trypanblau am Auge. Graefes Arhiv für Ophthalmologie. 1913;86:93–105. [Google Scholar]
- [41].Towbin BG. Zur Lehre von der Vitalfärbung des Auges in Verbindung mit dem Reticulo-Endothelialsystem. Graefes Arhiv für Ophthalmologie. 1933;129:387–412. [Google Scholar]
- [42].Tay TL, Mai D, Dautzenberg J, et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017;20:793–803. [DOI] [PubMed] [Google Scholar]
- [43].Goto Y, Hogg JC, Suwa T, et al. A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5′-bromo-2′-deoxyuridine. American Journal of Physiology-Cell Physiology. 2003;285:C253–C259. [DOI] [PubMed] [Google Scholar]
- [44].Yona S, Kim K-W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gloor BP. Cellular proliferation on the vitreous surface after photocoagulation. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1969;178:99–113. [DOI] [PubMed] [Google Scholar]
- [46].Balazs EA, Toth LZ, Ozanics V. Cytological studies on the developing vitreous as related to the hyaloid vessel system. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213:71–85. [DOI] [PubMed] [Google Scholar]
- [47].van Meurs JC, Sorgente N, Gauderman WJ, et al. Clearance rate of macrophages from the vitreous in rabbits. Curr Eye Res. 1990;9:683–686. [DOI] [PubMed] [Google Scholar]
- [48].Huang Y, Xu Z, Xiong S, et al. Dual extra-retinal origins of microglia in the model of retinal microglia repopulation. Cell Discov. 2018;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rubbino F, Garlatti V, Garzarelli V, et al. GPR120 prevents colorectal adenocarcinoma progression by sustaining the mucosal barrier integrity. Sci Rep. 2022;12:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Krueger M, Härtig W, Frydrychowicz C, et al. Stroke-induced blood-brain barrier breakdown along the vascular tree - No preferential affection of arteries in different animal models and in humans. J Cereb Blood Flow Metab. 2017;37:2539–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kierdorf K, Katzmarski N, Haas CA, et al. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS ONE. 2013;8:e58544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kierdorf K, Prinz M, Geissmann F, et al. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wieghofer P, Prinz M. Genetic manipulation of microglia during brain development and disease. Biochim Biophys Acta. 2016;1862:299–309. [DOI] [PubMed] [Google Scholar]
- [54].Kierdorf K, Erny D, Goldmann T, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. [DOI] [PubMed] [Google Scholar]
- [55].Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. [DOI] [PubMed] [Google Scholar]
- [56].Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311. [DOI] [PubMed] [Google Scholar]
- [57].Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kolter J, Feuerstein R, Zeis P, et al. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity. 2019;50:1482–1497.e7. [DOI] [PubMed] [Google Scholar]
- [59].Ydens E, Amann L, Asselbergh B, et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat Neurosci. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yu T, Guo W, Tian Y, et al. Distinct regulatory networks control the development of macrophages of different origins in zebrafish. Blood. 2016; [DOI] [PubMed] [Google Scholar]
- [61].Hamilton L, Astell KR, Velikova G, et al. A Zebrafish live imaging model reveals differential responses of microglia toward glioblastoma cells in vivo. Zebrafish. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ranawat N, Masai I. Mechanisms underlying microglial colonization of developing neural retina in zebrafish. eLife. 10:e70550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. [DOI] [PubMed] [Google Scholar]
- [64].Casano AM, Peri F. Microglia: Multitasking specialists of the brain. Developmental Cell. 2015;32:469–477. [DOI] [PubMed] [Google Scholar]
- [65].Casano AM, Albert M, Peri F. Developmental apoptosis mediates entry and positioning of microglia in the zebrafish Brain. Cell Rep. 2016;16:897–906. [DOI] [PubMed] [Google Scholar]
- [66].Halabi R, Watterston C, Hehr CL, et al. Semaphorin 3fa controls ocular vascularization from the embryo through to the adult. Invest Ophthalmol Vis Sci. 2021;62:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mazzolini J, Le Clerc S, Morisse G, et al. Gene expression profiling reveals a conserved microglia signature in larval zebrafish. Glia. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Blume ZI, Lambert JM, Lovel AG, et al. Microglia in the developing retina couple phagocytosis with the progression of apoptosis via P2RY12 signaling. Dev Dyn. 2020;249:723–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Doncan A Dissertatio continens de corporis vitrei structura disquitiones anatomicas, entopicas et pathologicas. 1854;
- [70].Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Epigenomic Gosselin D. and transcriptional determinants of microglial cell identity. Glia. 2020; [DOI] [PubMed] [Google Scholar]
- [72].Zhang P, Schlecht A, Wolf J, et al. The role of interferon regulatory factor 8 for retinal tissue homeostasis and development of choroidal neovascularisation. J Neuroinflammation. 2021;18:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martín-Estebané M, Navascués J, Sierra-Martín A, et al. Onset of microglial entry into developing quail retina coincides with increased expression of active caspase-3 and is mediated by extracellular ATP and UDP. PLoS ONE. 2017;12:e0182450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wieghofer P, Knobeloch K-P, Prinz M. Genetic targeting of microglia. Glia. 2015;63:1–22. [DOI] [PubMed] [Google Scholar]
- [75].Dumas AA, Borst K, Prinz M. Current tools to interrogate microglial biology. Neuron. 2021;109:2805–2819. [DOI] [PubMed] [Google Scholar]
- [76].Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ajami B, Bennett JL, Krieger C, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. [DOI] [PubMed] [Google Scholar]
- [78].Müther PS, Semkova I, Schmidt K, et al. Conditions of retinal glial and inflammatory cell activation after irradiation in a GFP-chimeric mouse model. Invest Ophthalmol Vis Sci. 2010;51:4831–4839. [DOI] [PubMed] [Google Scholar]
- [79].London A, Itskovich E, Benhar I, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hisatomi T, Sakamoto T, Sonoda K-H, et al. Clearance of apoptotic photoreceptors: elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin alphavbeta3. Am J Pathol. 2003;162:1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Albini TA, Wang RC, Reiser B, et al. Microglial stability and repopulation in the retina. Br J Ophthalmol. 2005;89:901–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Caicedo A, Espinosa-Heidmann DG, Piña Y, et al. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. [DOI] [PubMed] [Google Scholar]
- [83].Xu H, Chen M, Mayer EJ, et al. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. [DOI] [PubMed] [Google Scholar]
- [84].Chinnery HR, Humphries T, Clare A, et al. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kaneko H, Nishiguchi KM, Nakamura M, et al. Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest Ophthalmol Vis Sci. 2008;49:4162–4168. [DOI] [PubMed] [Google Scholar]
- [86].Kezic J, McMenamin PG. Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. J Leukoc Biol. 2008;84:721–729. [DOI] [PubMed] [Google Scholar]
- [87].Joly S, Francke M, Ulbricht E, et al. Cooperative phagocytes: resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am J Pathol. 2009;174:2310–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kezic J, McMenamin PG. The monocyte chemokine receptor CX3CR1 does not play a significant role in the pathogenesis of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51:5121–5127. [DOI] [PubMed] [Google Scholar]
- [89].Chen M, Zhao J, Luo C, et al. Para-inflammation-mediated retinal recruitment of bone marrow-derived myeloid cells following whole-body irradiation is CCL2 dependent. Glia. 2012;60:833–842. [DOI] [PubMed] [Google Scholar]
- [90].Alt C, Runnels JM, Mortensen LJ, et al. In vivo imaging of microglia turnover in the mouse retina after ionizing radiation and dexamethasone treatment. Invest Ophthalmol Vis Sci. 2014;55:5314–5319. [DOI] [PubMed] [Google Scholar]
- [91].O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep. 2016;6:20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ebneter A, Kokona D, Schneider N, et al. Microglia activation and recruitment of circulating macrophages during ischemic experimental branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2017;58:944–953. [DOI] [PubMed] [Google Scholar]
- [93].Qiao H, Hisatomi T, Sonoda K-H, et al. The characterisation of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol. 2005;89:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rangasamy S, McGuire PG, Franco Nitta C, et al. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE. 2014;9:e108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ong JX, Nesper PL, Fawzi AA, et al. Macrophage-like cell density is increased in proliferative diabetic retinopathy characterized by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2021;62:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ajami B, Bennett JL, Krieger C, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. [DOI] [PubMed] [Google Scholar]
- [97].Shemer A, Grozovski J, Tay TL, et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun [Internet]. 2018. [cited 2019 Jan 15];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6284018/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- [100].Heuss ND, Pierson MJ, Roehrich H, et al. Optic nerve as a source of activated retinal microglia post-injury. Acta Neuropathol Commun. 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].McPherson SW, Heuss ND, Abedin M, et al. Parabiosis shows that interplay of donor effector and regulatory t cells influences the outcome of disease induction in the partner mouse. Investigative Ophthalmology & Visual Science. 2019;60:786. [Google Scholar]
- [102].Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. [DOI] [PubMed] [Google Scholar]
- [103].Masuda T, Amann L, Sankowski R, et al. Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol. 2020; [DOI] [PubMed] [Google Scholar]
- [104].Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016; [DOI] [PubMed] [Google Scholar]
- [105].Kaiser T, Feng G. Tmem119-EGFP and Tmem119-CreERT2 Transgenic mice for labeling and manipulating microglia. eNeuro. 2019;6:ENEURO.0448–18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McKinsey GL, Lizama CO, Keown-Lang AE, et al. A new genetic strategy for targeting microglia in development and disease. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hirrlinger J, Requardt RP, Winkler U, et al. Split-CreERT2: temporal control of DNA recombination mediated by split-Cre protein fragment complementation. PLoS ONE. 2009;4:e8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kim J-S, Kolesnikov M, Peled-Hajaj S, et al. A binary cre transgenic approach dissects microglia and cns border-associated macrophages. Immunity. 2021;54:176–190.e7. [DOI] [PubMed] [Google Scholar]
- [109].Green KN, Crapser JD, Hohsfield LA. To kill a microglia: A case for CSF1R inhibitors. Trends Immunol. 2020;41:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lei F, Cui N, Zhou C, et al. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. PNAS. 2020;117:23336–23338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Spiteri AG, Ni D, Ling ZL, et al. PLX5622 Reduces Disease Severity in lethal CNS infection by off-target inhibition of peripheral inflammatory monocyte production. Front Immunol. 2022;13:851556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Schwarze EW. The origin of (Kolmer’s) epiplexus cells. A combined histomorphological and histochemical study. Histochemistry. 1975;44:103–104. [DOI] [PubMed] [Google Scholar]
- [114].Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech. 1998;41:43–56. [DOI] [PubMed] [Google Scholar]
- [115].Henle J Allgemein Anatomie. Leipzig: Verlag L. Vos; 1841. [Google Scholar]
- [116].Hamburg A Some investigations on the cells of the vitreous body. OPH. 1959;138:81–107. [DOI] [PubMed] [Google Scholar]
- [117].Llombart C, Nacher V, Ramos D, et al. Morphological characterization of pecteneal hyalocytes in the developing quail retina. J Anat. 2009;215:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA. 2016;113:E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sebag J Classifying posterior vitreous detachment: a new way to look at the invisible. Br J Ophthalmol. 1997;81:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sebag J Seeing the invisible: the challenge of imaging vitreous. J Biomed Opt. 2004;9:38–46. [DOI] [PubMed] [Google Scholar]
- [121].Sebag J To see the invisible: the quest of imaging vitreous. Dev Ophthalmol. 2008;42:5–28. [DOI] [PubMed] [Google Scholar]
- [122].Sebag J, Silverman RH, Coleman DJ. To see the invisible - the quest of imaging vitreous. In: Vitreous - in Health & Disease (Sebag J, ed.); Springer, New York, 2014, pp 193–222 [Google Scholar]
- [123].Sebag J Vitreous biochemistry, morphology, and clinical examination. In: Clinical Ophthalmology (Tasman W, Jaeger EA, eds). JB Lippincott Co, Philadelphia, Vol 5, Chapter 38, 1992 [Google Scholar]
- [124].Wang MY, Sebag J. Combined SD-OCT/SLO imaging of vitreous & the vitreo-retinal interface. In: Essentials of Ophthalmology: Medical Retina - Focus on Retinal Imaging (Holz Spaide, eds). Springer, 2010 [Google Scholar]
- [125].Pang CE, Freund KB, Engelbert M. Enhanced vitreous imaging technique with spectral-domain optical coherence tomography for evaluation of posterior vitreous detachment. JAMA Ophthalmol. 2014;132:1148–1150. [DOI] [PubMed] [Google Scholar]
- [126].Chinnery HR, NaranjoGolborne C, Leong CM, et al. Retinal microglial activation following topical application of intracellular toll-like receptor ligands. Investigative Ophthalmology & Visual Science. 2015;56:7377–7386. [DOI] [PubMed] [Google Scholar]
- [127].Miller EB, Karlen SJ, Ronning KE, et al. Tracking distinct microglia subpopulations with photoconvertible Dendra2 in vivo. J Neuroinflammation. 2021;18:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Castanos MV, Zhou DB, Linderman RE, et al. Imaging of macrophage-like cells in living human retina using clinical OCT. Invest Ophthalmol Vis Sci. 2020;61:48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sredar N, Razeen M, Kowalski B, et al. Comparison of confocal and non-confocal split-detection cone photoreceptor imaging. Biomed Opt Express. 2021;12:737–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tremblay M-È, Lecours C, Samson L, et al. From the Cajal alumni Achúcarro and Río-Hortega to the rediscovery of never-resting microglia. Front Neuroanat. 2015;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wolf J, Boneva S, Rosmus D-D, et al. Deciphering the molecular signature of human hyalocytes in relation to other innate immune cell populations. Investigative Ophthalmology & Visual Science. 2022;63:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Boneva S, Wolf J, Wieghofer P et al. Hyalocyte functions and immunology. Exp. Rev. Opthalmol. [In Press] (2022). [Google Scholar]
- [134].Jones CH, Gui W, Schumann R et al. Hyalocytes in proliferative vitreo-retinal diseases. Exp. Rev. Opthalmol. [In Press] (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Video showing the AOSLO time-lapse video of the two hyalocytes presented in Figure 9 and their processes movement over 2 hours. Images courtesy of authors TYP Chui and RB Rosen.


