Abstract
The current study is designed to investigate dietary guanidinoacetic acid (GAA) supplementation on the growth performance, intestinal histomorphology, and jejunum mucosal barrier function of broilers that are subjected to chronic heat stress (HS). A total of 192 male broilers (28-d old) were randomly allocated to four groups. A chronic HS model (at a temperature of 32 °C and 50%–60% relative humidity for 24 h daily) was applied in the experiment. Normal control (NC, ad libitum feeding, 22 °C), HS group (HS, ad libitum feeding, 32 °C), pair-fed group (PF, received food equivalent to that consumed by the HS group on the previous day, 22 °C), guanidinoacetic acid group (HG, ad libitum feeding, supplementing the basal diet with 0.6 g/kg GAA, 32 °C). The experiment lasted from 28 to 35 and 28 to 42 d of age of broilers. Our results showed that broilers subjected to HS had lower average daily feed intake and average daily gain (P < 0.05), higher feed-to-gain ratio and relative length of the small intestine (P < 0.05), as well as lower relative weight and weight per unit length of the small intestine (P < 0.05). HS damaged the small intestinal histomorphology by decreasing the small intestinal VH and the VH/CD (P < 0.05). Compared with the HS group, supplementation with 0.6 g/kg GAA increased jejunal VH and VH/CD (P < 0.05), but decreased relative weight and relative length of the small intestine (P < 0.05). Moreover, in comparison with NC, HS elevated intestinal permeability (D-Lactic acid concentration and diamine oxidase activity) and mRNA expression levels of interleukin-1β, interleukin-6, and tumor necrosis factor-α (P < 0.05), reduced jejunal mucus thickness, number of goblet cells, IgA + cell density, and mucin2 mRNA expression level of broilers (P < 0.05). Compared with the HS group, dietary GAA elevated jejunal mucus thickness, goblet cell number and IgA+ cell density (P < 0.05), and up-regulated jejunal mRNA expression of interleukin-1β and tumor necrosis factor-α (P < 0.05). In conclusion, HS impaired growth performance, and the intestinal mucosal barrier function of broilers. Dietary supplementation with 0.6 g/kg GAA alleviated HS-induced histomorphology changes of small intestine and jejunal mucosal barrier dysfunction.
Keywords: broiler, guanidinoacetic acid, heat stress, intestinal histomorphology, mucosal barrier
Chronic heat stress impaired the growth performance and intestinal mucosal barrier function of broilers. Dietary guanidinoacetic acid could improve intestinal barrier function of broilers that are subjected to chronic heat stress.
Introduction
In recent years, global climate is increasingly diversified, and sometimes extreme climates occur such as extremely hot and humid climates (Bayer Altın and Barak, 2017). Birds have limited ability to regulate heat loss through behavioral and physiological means. It is difficult for birds to dissipate heat under high ambient temperature, which increases body temperature and even causes heat stress (HS). Therefore, HS draws attention as a severe environmental stress source in poultry production, especially in tropical and subtropical regions (Mascarenhas et al., 2020). Broilers that subjected to HS showed poor performance and physiological disorders (Habashy et al., 2017). Chronic HS alters the physiological functions of poultry, including endocrine disruption, reduction of metabolic rate, lipid peroxidation, immune suppression, and intestinal microorganism dysbiosis (Syafwan et al., 2011; Sohail et al., 2012; Lara and Rostagno, 2013).
The small intestine is in charge of nutrient absorption and digestion, and also plays a vital role in maintaining mucosal barrier function and regulating signal transduction (Fre et al., 2005; Turner, 2009). However, the small intestine is easily affected by HS (Varasteh et al., 2015). Broilers that experienced chronic HS showed decreased transmembrane resistance and increased intestinal permeability in intestinal epithelial cells, resulting in poor intestinal integrity (Pearce et al., 2012). A disruption in the integrity of the intestinal epithelial barrier results in toxic substances invasion in the intestinal mucosa and triggers the increase of inflammatory cytokines, which cause immune function imbalances (Mohammad et al., 2019). Several reports have studied strategies for the mitigation of HS, in which dietary supplementation with additives is considered as an effective and relatively economical solution (Lin et al., 2006; Renaudeau et al., 2012). Thus, the interest of nutritionists focuses on the nutritional interventions to mitigate the HS responses of birds (Mohammed et al., 2018; He et al., 2019; Chen et al., 2020).
GAA is first isolated by Weber from human and dog urine, which has the ability to conserve arginine, affect energy metabolism, and improve performance (Kodambashi Emami et al., 2017; Majdeddin et al., 2020). During chronic HS, mitochondrial ATP generation is reduced, leading to energy metabolism imbalance (Azad et al., 2010). On this note, dietary supplementation with GAA could improve energy metabolism by enhancing the synthesis of creatine and phosphocreatine, which might offer benefits for the broilers that are subjected to HS (Murakami et al., 2014; Majdeddin et al., 2020). In addition, dietary supplementation with GAA could spare arginine (Arg) in broilers, owing to the fact that less endogenous GAA is synthesized, and Arg could improve intestinal mucosal barrier function subjected to HS (Basoo et al., 2012; Michiels et al., 2012; DeGroot et al., 2018). To date, the alleviating effect of dietary GAA on the intestinal mucosal barrier of broilers that subjected to HS is still unclear. Consequently, this study was conducted to evaluate the influence of dietary supplementation with GAA on growth performance, intestine histomorphology, and jejunal mucosal barrier of broilers subjected to chronic HS.
Materials and Methods
Experimental design
All experimental procedures and bird managements were approved by Nanjing Agricultural University Institutional Animal Care and Use Committee under protocol number SYXK 2021-0014. A total of 192 28-d-old Arbor Acres male birds (body weight, 1457.0 ± 5.0 g) were divided into two environment-controlled chicken rooms: normal control (NC, ad libitum feeding, 22 °C), heat stress group (HS, ad libitum feeding, 32 °C), pair-fed group (PF, received food equivalent to that consumed by the HS group on the previous day, 22 °C), GAA group (HG, ad libitum feeding, supplementing the basal diet with 0.6 g/kg GAA, 32 °C). GAA was acquired from Tianjin Tiancheng Pharmaceutical Co., Ltd. (Tianjin, China). Each treatment consisted of six replicates (1.2 m × 0.8 m × 0.45 m) with 8 birds per cage. The trial lasted for 7 and 14 d. The basal diet composition and nutrition levels are summarized in Table 1. Broilers were weighed at 28, 35, and 42 d, respectively, and feed consumption was also recorded for further analysis.
Table 1.
Ingredients and nutrient composition of basal diets (as fed basis)
| Ingredients (%) | |
|---|---|
| Corn | 59.37 |
| Soybean meal1 | 31.90 |
| Soybean oil | 5.00 |
| Limestone | 1.23 |
| Dicalcium phosphate | 1.50 |
| L-lysine·HCl | 0.11 |
| DL-methionine | 0.27 |
| Salt | 0.30 |
| Vitamin premix2 | 0.03 |
| Mineral premix3 | 0.20 |
| 70% Choline chloride | 0.09 |
| Calculated nutrients | |
| Metabolizable energy (MJ/kg) | 12.98 |
| Crude protein | 19.00 |
| Calcium | 0.90 |
| Total phosphorus | 0.56 |
| Nonphytate phosphorus | 0.35 |
| Lysine | 1.00 |
| Methionine | 0.46 |
| Methionine + cystine | 0.80 |
| Threonine | 0.60 |
| Tryptophan | 0.20 |
1 Crude protein level of soybean meal: 44.2%.
2 Vitamin premix provided per kilogram of diet: Vitamin A, 12,000 IU; Vitamin D3, 2,500 IU; Vitamin E, 20 IU; menadione, 1.3 mg; thiamin, 2.21 mg; riboflavin, 7.8 mg; nicotinamide, 40 mg; calcium pantothenate, 16.5 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1.2 mg; Vitamin B12, 0.015 mg.
3 Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8.0 mg; manganese, 110 mg, zinc 65 mg; iodine, 1.1 mg; selenium, 0.3 mg.
Sample collection
On 35 and 42 d of age, 2 broilers per cage closest to the mean body weight were electric stunned (400 Hz for 5 s; 50 V, alternating current) and immediately exsanguinated via the left carotid artery. Blood was loaded into heparinized tubes and immediately centrifuged for 10 min at 825 g. After dissecting the abdominal cavity, the proventriculus and gizzard were emptied, and all the fillings and attached materials were weighted. The small intestine of broilers was separated into three sections: duodenum (from ventriculus to the pancreo-biliary duct), jejunum (from pancreo-biliary duct to yolk stalk), and ileum (from yolk stalk to ileocecal junction). About 1-cm middle portion of the duodenum, jejunum, and ileum were collected, flushed with 0.75% (w/v) sterile saline, and fixed in 4% paraformaldehyde for morphological analysis. After squeezing out the contents, the remaining small intestine was cut open. The mucosa was gently scraped off with a clean slide, frozen in liquid nitrogen, and immediately stored at −80 °C for the following analysis.
Intestinal histomorphology examination
A cross-section (6 μm) was cut for each intestinal sample. Then intestinal samples were stained with hematoxylin-eosin staining. Images were captured using an Olympus DP12 CCD digital camera (Olympus Optical Co. Ltd, Tokyo, Japan). Images were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD) to measure the villus height (VH, from the tip of the villus to the villus-crypt junction level) and crypt depth (CD, the vertical distance from the villus-crypt junction to the lower limit of the crypt). Six views from each gut sample were selected for measurement.
Intestinal index calculation formula: relative intestinal length (cm/kg) = length of each intestinal segment (cm)/ the corresponding live weight of bird (kg). Relative intestinal weight (g/kg) = weight of each intestinal segment (cm)/ the corresponding live weight of bird (kg). Weight per unit length (g/cm) = weight of each small intestine segment (g)/ length of each small intestine segment (cm).
D-lactic acid and diamine oxidase concentration determination
Plasma diamine oxidase activity (DAO) was spectrophotometrically assessed based on the instruction of the colorimetric kit. The plasma D-Lactic acid (D-Lac) concentration was measured by a commercial ELISA kit. Both kits were purchased from Jiancheng Bioengineering Co., Ltd. (Nanjing, China).
Total RNA extraction and real-time PCR
Total RNA was separated from jejunal mucosa samples by Trizol reagent (Takara Biotechnology Co. Ltd., Dalian, China). The purity and quantity of RNA were measured by a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). The A260/280 ratios of all samples’ RNA were ranged between 1.8 and 2.0. Reverse transcription of total RNA was completed using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd.) to generate cDNA. With the SYBR Premix Ex Taq kit (Applied Biosystems, Foster City, CA), the RT-qPCR was carried out in the QuanStudio6 RT-qPCR detection system (Applied Biosystems, Foster City, CA). The total reaction volume was 20 µL, including 0.4 µL of a forward primer (10 µM), 0.4 µL of a reverse primer (10 µM), 1 µL of cDNA, 8.2 µL sterilized double distilled water, and 10 µL of SYBR Premix Ex Taq II (2×). The following cycling conditions were used: 1 cycle at 95 °C for 30 s and 40 cycles at 95 °C for 5 s, 60 °C for 30 s, 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. The specific primer sequences are listed in Table 2. The relative levels of mRNA expression were calculated using the 2–ΔΔCT method with β-actin as a reference gene (Livak and Schmittgen, 2001).
Table 2.
Primer sequences for real-time quantitative PCR analysis
| Genes1 | Accession number | Primer sequence | Product size, bp |
|---|---|---|---|
| Claudin1 | NM_001013611.2 | F: GACCAGGTGAAGAAGATGCGGATG | 107 |
| R: CGAGCCACTCTGTTGCCATACC | |||
| Occludin | XM_025144248.1 | F: TCATCGCCTCCATCGTCTAC | 240 |
| R: TCTTACTGCGCGTCTTCTGG | |||
| ZO-1 | XM_015278981.2 | F: CTTCAGGTGTTTCTCTTCCTCCTC | 131 |
| R: CTGTGGTTTCATGGCTGGATC | |||
| ZO-2 | NM_204918.1 | F: GAGAGCACAACCGAAGCAGAGG | 157 |
| R: TAGTCCTGTCCATAGCCACCATCC | |||
| Mucin-2 | NM_001318434.1 | F: ACTGGACTTCACGGACACCT | 121 |
| R: CCCCCTCTACCATCATCAAA | |||
| IL-1β | XM_015297469.1 | F: GTACCGAGTACAACCCCTGC | 112 |
| R: AGCAACGGGACGGTAATGAA | |||
| IL-6 | NM_204628.1 | F: TGTGCAAGAAGTTCACCGTG | 213 |
| R: ACTCGACGTTCTGCTTTTCG | |||
| TNF-α | NC_006101.4 | F: GCACTCCGTTCAGACATCCA | 112 |
| R: CGCACCTGTCCTGTATCTGC | |||
| β-actin | NM_205518.1 | F: ATCCGGACCCTCCATTGTC | 120 |
| R: AGCCATGCCAATCTCGTCTT |
1ZO-1, zonula occludens-1; ZO-2, zonula occludens-2; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Mucus thickness, goblet cell and immunoglobulin A-producing cell counts
Images were captured using an Olympus BX51 light microscope (Olympus Optical Co. Ltd). Images were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD) to measure the mucus thickness. Jejunal cross section embedded in paraffin was stained with Periodic Acid–Schiff reagent (Sigma, St. Louis, MO) to count the goblet cells. The average number of goblet cells in the stained tissue slices was calculated under an Olympus BX51 light microscope with a final magnification of 200×. The number of five villi per sample was counted for statistical analysis, and the results were defined as the number of goblet cells per 100 columnar epithelial cells (n/100 epithelial cells). For immunoglobulin A (IgA+) cell counts, paraffin sections were prepared using the method of Wang et al. (2009). The mouse antirabbit monoclonal antibody was used (#8330-01; Southern Biotechnology Inc., Birmingham, AL) for immunohistochemistry staining. Five intact villi were randomly selected from each sample. The Image Pro Plus 6.0 software was used to calculate IgA + cells density as described by Wang et al. (2009).
Statistical analysis
Statistical Analysis System with the SPSS (version 25.0, SPSS Inc., USA) was used to analyze the data for the NC, HS, and PF groups using one-way analysis of variance and a Tukey multiple range test. T-tests for independent samples were performed between the HS and HG groups. Results are presented as mean ± standard error and the statistical significance was considered at P < 0.05.
Results
Growth performance
As shown in Table 3, after thermal exposure for 7 and 14 d, birds in the HS group exhibited a reduction in average daily gain (ADG), average daily feed intake (ADFI), and an elevation in feed to gain ratio (F/G) compared with those in the NC group (P < 0.05). The birds from the HS group had a lower ADG and a higher F/G compared with those in the PF group (P < 0.05). No substantial difference in growth performance was observed between HG and HS groups.
Table 3.
Effect of heat stress on the performance of broilers and guanidinoacetic (GAA) acid intervention effect
| Items2 | Treatment1 | P-value | ||||
|---|---|---|---|---|---|---|
| NC | HS | PF | HG | ANOVA | HS vs. HG | |
| BW at d 28, g/bird | 1458.3 ± 5.4 | 1452.5 ± 12.0 | 1452.5 ± 6.9 | 1464.4 ± 3.4 | 0.858 | 0.365 |
| BW at d 35, g/bird | 2077.9 ± 21.6a | 1855.7 ± 31.8b | 2017.9 ± 22.4a | 1957.9 ± 35.1 | <0.001 | 0.068 |
| BW at d 42, g/bird | 2637.3 ± 50.0a | 2149.4 ± 65.9b | 2457.7 ± 68.8a | 2326.5 ± 63.9 | <0.001 | 0.083 |
| Heat exposure for 7 d | ||||||
| ADG, g/bird/day | 88.5 ± 3.1a | 57.6 ± 4.0b | 80.8 ± 3.9a | 69.3 ± 4.4 | <0.001 | 0.077 |
| ADFI, g/bird/day | 152.3 ± 5.7a | 125.7 ± 4.4b | 131.6 ± 4.8b | 133.7 ± 3.5 | 0.005 | 0.186 |
| F/G, g/g | 1.72 ± 0.04b | 2.21 ± 0.10a | 1.64 ± 0.04b | 1.95 ± 0.09 | <0.001 | 0.075 |
| Heat exposure for 14 d | ||||||
| ADG, g/bird/day | 84.2 ± 3.8a | 49.8 ± 5.4b | 71.8 ± 5.0a | 61.6 ± 4.3 | <0.001 | 0.119 |
| ADFI, g/bird/day | 163.2 ± 5.1a | 127.1 ± 6.5b | 131.7 ± 5.3b | 141.5 ± 4.3 | 0.001 | 0.095 |
| F/G, g/g | 1.95 ± 0.09b | 2.63 ± 0.37a | 1.86 ± 0.17b | 2.33 ± 0.22 | <0.001 | 0.112 |
1NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
2F/G, feed to gain ratio.
a,b Means within a row without a common superscript differ significantly among the NC, HS, and PF groups (P < 0.05). The results were expressed as the means ± SE.
Intestinal index
As shown in Table 4, after thermal exposure for 7 d, the relative length of the jejunum and ileum increased in the HS group compared with the NC group, while the relative weight of the ileum and the weight per unit length of the jejunum and ileum decreased (P < 0.05). The relative length of ileum and jejunum, as well as the relative weight of ileum increased in the HS group compared with the PF group (P < 0.05). The relative length of jejunum, relative length and relative weight of ileum were higher in the HG group than those in the HS group (P < 0.05).
Table 4.
Effects of heat stress on the relative length and weight of the small intestine of broilers and guanidinoacetic acid (GAA) intervention effect
| Items | Treatment1 | P-value | ||||
|---|---|---|---|---|---|---|
| NC | HS | PF | HG | ANOVA | HS vs. HG | |
| Heat exposure for 7 d | ||||||
| Relative length, cm/kg·BW | ||||||
| Duodenum | 15.4 ± 0.6 | 15.4 ± 0.7 | 14.4 ± 0.3 | 15.0 ± 0.4 | 0.330 | 0.545 |
| Jejunum | 34.7 ± 1.3b | 43.7 ± 2.0a | 34.5 ± 1.9b | 36.9 ± 1.5* | 0.003 | 0.023 |
| Ileum | 33.9 ± 1.1b | 45.5 ± 2.3a | 33.2 ± 2.2b | 37.7 ± 0.8* | 0.001 | 0.010 |
| Relative weight, g/kg·BW | ||||||
| Duodenum | 5.7 ± 0.3 | 5.1 ± 0.2 | 5.1 ± 0.3 | 5.3 ± 0.2 | 0.135 | 0.502 |
| Jejunum | 14.1 ± 0.5a | 12.7 ± 0.4a,b | 11.8 ± 0.6b | 12.4 ± 0.2 | 0.016 | 0.578 |
| Ileum | 11.6 ± 0.5a | 10.1 ± 0.2b | 8.7 ± 0.6c | 9.1 ± 0.2* | 0.001 | 0.005 |
| Relative weight/relative length, g/cm | ||||||
| Duodenum | 0.38 ± 0.02 | 0.33 ± 0.02 | 0.36 ± 0.02 | 0.36 ± 0.01 | 0.384 | 0.342 |
| Jejunum | 0.39 ± 0.01a | 0.30 ± 0.02b | 0.35 ± 0.02a,b | 0.34 ± 0.02 | 0.012 | 0.141 |
| Ileum | 0.32 ± 0.01a | 0.22 ± 0.01b | 0.27 ± 0.02b | 0.24 ± 0.01 | 0.001 | 0.173 |
| Heat exposure for 14 d | ||||||
| Relative length, cm/kg·BW | ||||||
| Duodenum | 11.9 ± 0.4 | 12.4 ± 0.3 | 11.8 ± 0.1 | 12.1 ± 0.4 | 0.342 | 0.492 |
| Jejunum | 32.6 ± 1.1a | 32.8 ± 0.8a | 28.5 ± 0.9b | 32.4 ± 1.7 | 0.009 | 0.867 |
| Ileum | 32.4 ± 1.4a | 33.8 ± 1.4a | 28.0 ± 1.0b | 30.6 ± 0.8 | 0.014 | 0.072 |
| Relative weight, g/kg·BW | ||||||
| Duodenum | 5.2 ± 0.4 | 4.7 ± 0.3 | 4.5 ± 0.3 | 4.4 ± 0.2 | 0.291 | 0.446 |
| Jejunum | 12.4 ± 0.4a | 9.2 ± 0.4b | 8.5 ± 0.3b | 9.5 ± 0.2 | <0.001 | 0.518 |
| Ileum | 9.6 ± 0.3a | 7.9 ± 0.4b | 7.6 ± 0.4b | 8.2 ± 0.2 | 0.001 | 0.545 |
| Relative weight/relative length, g/cm | ||||||
| Duodenum | 0.42 ± 0.02 | 0.39 ± 0.02 | 0.38 ± 0.01 | 0.36 ± 0.02 | 0.202 | 0.257 |
| Jejunum | 0.36 ± 0.01a | 0.29 ± 0.01b | 0.31 ± 0.01b | 0.30 ± 0.02 | 0.001 | 0.451 |
| Ileum | 0.30 ± 0.01a | 0.24 ± 0.01b | 0.28 ± 0.02a,b | 0.27 ± 0.02 | 0.017 | 0.216 |
1NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
a,bMeans within a row without a common superscript differ significantly among the NC, HS, and PF groups (P < 0.05).
*Indicates a significant difference between HS vs. HG (P < 0.05). The results were expressed as the means ± SE.
After thermal exposure for 14 d, birds in the HS group showed reduction in the relative weight and weight per unit length of jejunum and ileum compared with the NC group (P < 0.05). The relative lengths of jejunum and ileum were higher in the HS group than those in the PF group (P < 0.05). No obvious differences in intestinal indexes were found between groups HS and HG.
Intestinal morphology
As exhibited in Table 5, after thermal exposure for 7 d, VH of duodenum was decreased in HS group compared with NC group. VH and the ratio of VH to CD (VH/CD) were lower in the jejunum and ileum of birds from the HS group than those in the NC group (P < 0.05). Both VH and VH/CD in the jejunum and ileum of the HS group were lower than those in the PF group (P < 0.05). In the jejunum and ileum, VH and VH/CD were higher in the HG group than those in the HS group (P < 0.05).
Table 5.
Effects of heat stress on the small intestinal morphology of broilers and guanidinoacetic acid (GAA) intervention effect
| Items2 | Treatment1 | P-value | ||||
|---|---|---|---|---|---|---|
| NC | HS | PF | HG | ANOVA | HS vs. HG | |
| Heat exposure for 7 d | ||||||
| Duodenum | ||||||
| VH, μm | 1606.5 ± 25.2a | 1373.9 ± 37.0b | 1337.1 ± 45.3b | 1469.7 ± 96.1 | <0.001 | 0.374 |
| CD, μm | 247.8 ± 5.9 | 255.3 ± 9.8 | 238.1 ± 20.7 | 241.4 ± 9.3 | 0.678 | 0.328 |
| VH/CD | 6.5 ± 0.2 | 5.4 ± 0.3 | 5.8 ± 0.4 | 6.2 ± 0.5 | 0.073 | 0.236 |
| Jejunum | ||||||
| VH, μm | 1218.2 ± 60.4a | 846.8 ± 74.5b | 1230.4 ± 21.4a | 1064.9 ± 60.4* | <0.001 | 0.046 |
| CD, μm | 192.3 ± 15.7 | 202.6 ± 13.9 | 191.8 ± 15.3 | 186.3 ± 5.7 | 0.849 | 0.303 |
| VH/CD | 6.4 ± 0.3a | 4.3 ± 0.5b | 6.6 ± 0.6a | 5.7 ± 0.5* | 0.006 | 0.026 |
| Ileum | ||||||
| VH, μm | 996.6 ± 71.7a | 590.7 ± 40.7b | 875.8 ± 59.4a | 758.0 ± 31.5* | 0.001 | 0.009 |
| CD, μm | 195.5 ± 11.9 | 176.9 ± 10.0 | 175.5 ± 12.5 | 156.9 ± 13.1 | 0.410 | 0.255 |
| VH/CD | 5.1 ± 0.3a | 3.5 ± 0.4b | 5.0 ± 0.3a | 4.9 ± 0.2* | 0.006 | 0.016 |
| Heat exposure for 14 d | ||||||
| Duodenum | ||||||
| VH, μm | 1485.3 ± 69.0 | 1211.0 ± 79.5 | 1338.4 ± 74.2 | 1381.3 ± 115.7 | 0.060 | 0.253 |
| CD, μm | 244.9 ± 16.9 | 218.4 ± 11.1 | 222.8 ± 16.6 | 236.2 ± 11.6 | 0.433 | 0.295 |
| VH/CD | 6.1 ± 0.3 | 5.6 ± 0.5 | 6.2 ± 0.6 | 5.8 ± 0.4 | 0.627 | 0.701 |
| Jejunum | ||||||
| VH, μm | 1290.0 ± 93.3a | 871.2 ± 90.4b | 1144.6 ± 83.3a,b | 885.2 ± 29.6 | 0.014 | 0.886 |
| CD, μm | 211.0 ± 17.3 | 195.1 ± 5.3 | 213.7 ± 18.0 | 221.2 ± 22.2 | 0.637 | 0.280 |
| VH/CD | 6.2 ± 0.3a | 4.5 ± 0.5b | 5.4 ± 0.4a,b | 4.2 ± 0.9 | 0.022 | 0.635 |
| Ileum | ||||||
| VH, μm | 769.8 ± 89.4 | 683.1 ± 79.0 | 883.9 ± 36.6 | 710.9 ± 79.0 | 0.176 | 0.808 |
| CD, μm | 159.1 ± 18.7 | 183.0 ± 21.6 | 183.0 ± 8.8 | 166.8 ± 23.5 | 0.541 | 0.623 |
| VH/CD | 4.9 ± 0.4a | 3.8 ± 0.3b | 4.9 ± 0.2a | 4.4 ± 0.3 | 0.036 | 0.218 |
1NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22°C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
2VH, villus height; CD, crypt depth; VH/CD, the ratio of VH to CD.
a,bMeans within a row without a common superscript differ significantly among the NC, HS, and PF groups (P < 0.05).
*Indicates a significant difference between HS vs. HG (P < 0.05). The results were expressed as the means ± SE.
After thermal exposure for 14 d, VH and VH/CD of jejunum were reduced in the HS group than NC group (P < 0.05). Lower VH/CD of ileum was found in HS group, compared with those in NC and PF groups (P < 0.05). No significant difference was exhibited in intestinal morphology between the HG and HS groups.
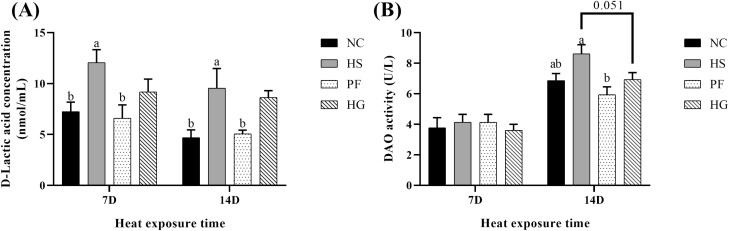
Plasma D-lactic acid concentration and diamine oxidase activity
As seen in Figure 1, after thermal exposure for 7 d, the HS group exhibited higher D-Lac concentration than that in the NC and PF groups (P < 0.05). No obvious differences in concentration of plasma D-Lac and DAO activity were observed between HG and HS groups.
Figure 1.
Effect of chronic heat stress on the plasma concentration of (A) D-Lactic acid and activity of (B) DAO (diamine oxidase) in broilers and the intervention effect of guanidinoacetic acid (GAA) supplementation in diets. Data were represented as the means with SE. a,bMeans within a row without a common superscript differ significantly among the NC, HS and PF groups (P < 0.05). *Indicates a significant difference between HS vs. HG (P < 0.05). NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
After thermal exposure for 14 d, the plasma D-Lac concentration in the HS group was elevated compared with that in the NC and PF groups (P < 0.05). In HS group, birds had higher DAO activity compared with the PF group (P < 0.05). In the HG group, there was a decreasing trend of DAO activity compared with the HS group (P = 0.051).
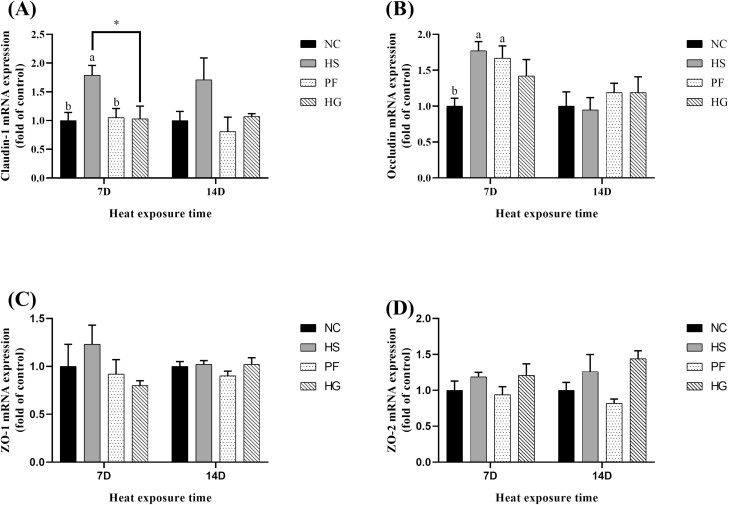
Relative mRNA expression of jejunal tight junction-related proteins
As seen in Figure 2, after thermal exposure for 7 d, birds in the HS group exhibited a higher mRNA expression level of jejunal claudin1 than that of the other three groups (P < 0.05). The mRNA expression level of jejunal occludin was higher in the HS group than that in the NC group (P < 0.05). No significant variation in mRNA expression levels of jejunal claudin1 and occludin were found between the HS and PF groups.
Figure 2.
Effect of chronic heat stress on the mRNA expression levels of jejunum (A) claudin-1, (B) occludin, (C) ZO-1 and (D) ZO-2 in broilers and the intervention effect of guanidinoacetic acid (GAA) supplementation in diets. Data were represented as the means with SE. a,b Means within a row without a common superscript differ significantly among the NC, HS, and PF groups (P < 0.05). *Indicates a significant difference between HS vs. HG (P < 0.05). NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
No significant difference in mRNA expression levels of jejunal tight junction protein-related gene was observed among all groups during thermal exposure for 14 d.
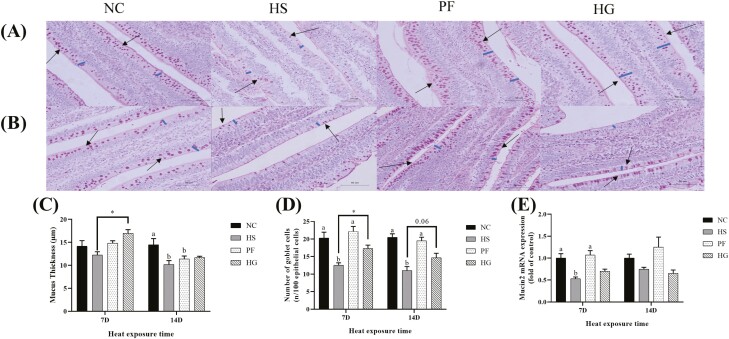
Mucus thickness, the number of goblet cells and relative mRNA expression level of mucin2 in jejunum
As illustrated in Figure 3 (A, B), after thermal exposure for 7 d, the number of jejunal goblet cells and the mRNA expression level of mucin2 were reduced in the HS group compared with the NC and PF groups (P < 0.05). Mucus thickness and the number of jejunal goblet cells were higher in the HG group compared with the HS group (P < 0.05). No difference in the mRNA expression level of mucin2 was found between HG and HS groups.
Figure 3.
Guanidinoacetic acid (GAA) attenuated heat stress (HS)-induced damage of the mucous layer. (A) Hematoxylin and eosin staining after 7 d thermal exposure. (B) Hematoxylin and eosin staining after 14 d thermal exposure. The blue marks in (A) and (B) indicate intestinal mucus thickness; the arrows in (A) and (B) indicate the stained goblet cells. Mucus thickness (C), the number of jejunal goblet cells (D) and the mRNA expression level of Mucin2 (E). Data were represented as the means ± SE. a,bMeans within a row without a common superscript differ remarkably among the NC, HS, and PF groups (P < 0.05). *Indicates a significant difference between HS vs. HG (P < 0.05). NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
As shown in Figure 3C and D, after thermal exposure for 14 d, mucus thickness was reduced in the HS group compared with the NC group (P < 0.05). The number of jejunal goblet cells was reduced in the HS group compared with the NC and PF groups (P < 0.05). The number of jejunal goblet cells in the HG group exhibited an increasing trend compared with the HS group (P = 0.06).
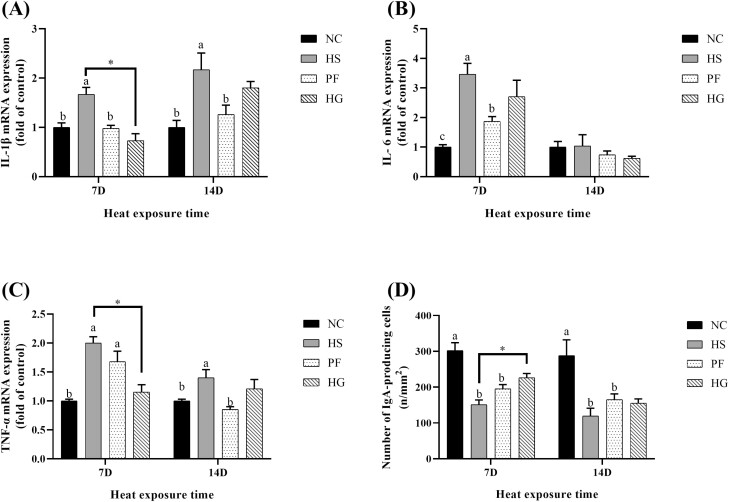
Jejunal relative mRNA expression levels of IL-1β, IL-6, TNF-α, and number of IgA + cells
As shown in Figure 4, after thermal exposure for 7 d, the mRNA expression levels of jejunum interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) were higher and the number of jejunal IgA + cells was lower in the HS group compared with the NC group (P < 0.05). HS group showed higher IL-1β and IL-6 mRNA expression levels compared with the PF group (P < 0.05). The mRNA expression levels of jejunal IL-1β and TNF-α were lower and the number of IgA + cells was higher in the HG group compared with the HS group (P < 0.05).
Figure 4.
Effect of chronic heat stress on the mRNA expression levels of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) IgA + cells density in broiler jejunum and the intervention effect of guanidinoacetic acid (GAA) supplementation in diets. Data were represented as the means with SE. a,b,cMeans within a row without a common superscript differ significantly among the NC, HS, and PF groups (P < 0.05). *Indicates a significant difference between HS vs. HG (P < 0.05). NC, normal control group, 22 °C, ad libitum feed basal diet; HS, heat stress group, 32 °C, ad libitum feed basal diet; PF, pair-fed group, 22 °C, received food equivalent to that consumed by the HS group on the previous day; HG, guanidinoacetic acid group, 32 °C, ad libitum feed basal diet with 0.6 g/kg guanidinoacetic acid.
After thermal exposure for 14 d, the mRNA expression of IL-1β and TNF-α were higher as well as the number of IgA + cells was lower in jejunum of birds from the HS group compared with the NC group (P < 0.05). IL-1β and TNF-α mRNA expressions were higher in the HS group compared with the PF group (P < 0.05). No differences were observed in mRNA expression levels of jejunal IL-1β, IL-6 and TNF-α, and amount of IgA + cell between HS and HG groups.
Discussion
The markedly increased occurrence of HS in the global poultry industry results in negative impacts on birds performance and intestinal histomorphology, causing substantial economic loss (St-Pierre et al., 2003; Ma et al., 2021). GAA has been considered as a functional additive to alleviate the negative effects of HS on broiler performance (Amiri et al., 2019; Majdeddin et al., 2020). In the present study, after thermal exposure for 7 and 14 d at the duration of 28 to 35 and 28 to 42 d, the birds of the HS group had lower ADG and feed conversion efficiency than birds of the NC group and PF group, which was consistent with the results of Lu et al. (2018). These results suggested that the impairment of growth performance caused by HS was independent of the reduction in feed intake. Under thermal exposure, poor growth performance may associate with decreasing feed efficiency resulted from changes in absorption and metabolism. The benefits of dietary addition with GAA have been controversially discussed for uncertain effects on performance of broilers. Some studies reported that dietary supplementation with 0.6 and 1.2 g/kg GAA improved broiler growth performance, and increased creatine levels in serum and muscle (Carvalho et al., 2013; Majdeddin et al., 2018). Whereas, DeGroot et al. (2018) and Córdova-Noboa et al. (2018) indicated that GAA did not affect broiler body weight gain and ADFI. Our current results showed that dietary addition of 0.6 g/kg of GAA did not affect broiler performance under thermal exposure. These inconsistent results might be ascribed to the differences in experiment duration, GAA dosage and bioavailability.
As one of the main target organs, the development of intestine is easily affected by HS. In current study, thermal exposure 7 and 14 d reduced the relative weight and the weight per unit length of small intestine, resulted in impaired intestinal development. However, both the relative lengths of the jejunum and ileum increased after 7 and 14 d of thermal exposure. It was reasonable to assume that this phenomenon was due to a lower body weight gain and the compensatory intestinal growth in heat-stressed broilers. The observed results indicated that after 7 d of thermal exposure, the relative weight of ileum was reduced with GAA supplementation. Hashemipour et al. (2016) and Ma et al. (2018) confirmed that dietary addition of some functional additives improved digestion and absorption function, but it might weaken intestinal secretory function and thereby lead to a reduction in the weight of broiler organs.
Critical functions of the intestine include mucosal barrier function, signal recognition and immune function modification (Pietrzak et al., 2020; Zhang et al., 2020). Intestinal VH and CD are critical structural features in the intestinal epithelium, which are used to judge intestinal health status (Zhang et al., 2019). We found that thermal exposure for 7 d significantly reduced VH and V/C of the small intestine, which were matched with findings observed by He et al. (2018). The current study indicated that intestine development was particularly susceptible to HS. In addition, VH and V/C were higher in PF group compared with HS group. The current study implied that heat-induced lower VH and V/C has no relation to decreased feed intake. However, dietary supplementation with GAA improved morphology of jejunum and ileum after thermal exposure for 7 d. The increase of the VH could effectively promote the absorption of nutrients (Wijtten et al., 2012). The results suggested that GAA might improve the absorption ability of nutrients in the jejunum and ileum in broilers subjected to thermal exposure.
HS caused gastrointestinal dysfunction, especially in the damage to intestinal morphology and changed in intestinal permeability (Yang et al., 2007). Some blood indicators, such as D-Lac and DAO, can be used to assess intestinal permeability. The D-Lac is produced by intestinal flora (Vella and Farrugia, 1998), while DAO is primarily produced by cells of the small intestinal mucosa and gastric mucosa (Thompson et al., 1987). Both D-Lac and DAO were commonly used as indicators of intestinal permeability because they could enter the blood in large quantities after intestinal mucosal damage (Fukudome et al., 2014; Cheng et al., 2019). In accordance with previous results by Lan et al. (2020), we noted that thermal exposure significantly elevated plasma D-Lac and DAO levels in the present study. Compared with HS group, PF group had lower D-Lac concentration. It suggested that heat-induced higher D-Lac concentration was not connected with feed restriction. There was a trend of reduction in plasma DAO content after dietary GAA addition. Together with the results on small intestinal histomorphology, it was suggested that thermal exposure impaired the integrity of the intestinal barrier but dietary additional with GAA mitigated heat-induced damage to intestinal barrier integrity.
The tight junction proteins included membrane proteins claudin1 and occludin, as well as the cytoplasmic adaptor proteins such as zonula occludens-1 (ZO-1) and zonula occludens-2 (ZO-2), contributed to the establishment and the regulation of intestinal barrier function (Schneeberger and Lynch, 2004). Lower protein levels of occludin and ZO-1 were found in the intestinal mucosa of broilers experienced HS (Song et al., 2014). However, Goo et al. (2019) concluded that HS had no significant effects on mRNA expression levels of tight junction protein-related genes in jejunal mucosal of broilers. Dokladny et al. (2006) found that occludin protein expression in Caco-2 intestinal epithelial cells was increased compensatory after 24 h of thermal exposure at 42 °C. Higher mRNA expression levels of jejunal claudin1 and occludin in birds after 7 d of thermal exposure might also be a compensatory increase in present results.
Goblet cell produced mucus that covered the intestinal epithelium, which protected the mucosa of the digestive tract from pathogens and environmental toxins. Moreover, goblet cell was involved in the repair of mucosa upon damage (Liu et al., 2016). Hence, intestinal mucus and goblet cell were critical to maintain the normal physiological functions of the intestinal barrier. Tsirtsikos et al. (2012) reported that increasing in mucus layer thickness was beneficial to intestinal health. After 7 d thermal exposure, higher mucus thickness was found in HG group, indicating that dietary supplementation with GAA was beneficial to the intestinal health. In the present study, chronic HS significantly decreased the number of jejunal goblet cells and downregulated the mRNA expression of mucin2. Whereas, birds of PF group had higher number of jejunal goblet cells compared with the HS group, suggesting that the number of jejunal goblet cells had no relation with heat-induced lower feed intake. The present study attempted to alleviate thermal exposure induced intestinal damage by means of dietary addition of GAA. The current results showed an increase in the number of broilers jejunal mucus thickness and goblet cell, indicating that dietary GAA improved jejunal barrier function via repairing jejunal chemical barrier function of heat-stressed birds.
Secretory immunoglobulin A (SIgA), the main type of antibody secreted by the intestinal mucosa, prevented toxic and harmful substances from adhering to and entering the intestinal barrier. SIgA is mainly secreted by IgA + cells in the intestinal epithelium and its secretion is positively correlated with the number of IgA + cells in the lamina propria of the intestine (Pietrzak et al., 2020). As organisms undergo HS, pro-inflammatory cytokines are overexpressed in intestinal tissue, which contribute to intestinal mucosal injury. The current study demonstrated that thermal exposure up-regulated the gene expression of pro-inflammatory factors (IL-1β, TNF-α, IL-6) in jejunal and decreased the density of jejunal IgA + cells. The results of Alhotan et al. (2021) and Yang et al. (2019) were in similarity to our findings, suggesting that chronic HS impaired the gut immune functions by promoting the initiation of inflammation and reduced the secretion of IgA. Another study indicated that TNF-α increased intestinal permeability (Su et al., 2013), which might be one of the reasons for the increased permeability of the HS group in the current research. Aziza et al. (2020) observed that supplementation with 0.18% GAA in Nile Tilapia fish reduced the mRNA expression levels of IL-1β and TNF-α in the liver, which suggested that GAA might have an anti-inflammatory effect. The present results also exhibited that the dietary GAA down-regulated the mRNA expression levels of jejunal pro-inflammatory factors, increased the number of jejunal IgA + cells, and enhanced jejunal immunity of birds subjected to thermal exposure for 7 d. These results indicated that GAA also could exert anti-inflammatory effects in broilers under thermal exposure condition.
In conclusion, HS damaged intestinal histomorphology, intestinal integrity, and jejunal mucosal barrier function. The current results demonstrated that GAA, as a functional additive, was effective in alleviating intestinal injury by improving small intestinal histomorphology and jejunal mucosal barrier function.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32072780), the National Key Research and Development Program of China (2016YFD0500501), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2022]460).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- Arg
arginine
- ATP
adenosine triphosphate
- CD
crypt depth
- Ct
cycle threshold
- DAO
diamine oxidase activity
- F/G
feed to gain ratio
- GAA
guanidinoacetic acid
- IgA
immunoglobulin A
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- qPCR
quantitative PCR
- SIgA
Secretory immunoglobulin A
- TNF-α
tumor necrosis factor-α
- VH
villus height
- VH/CD
ratio of VH to CD
- ZO-1
zonula occludens-1
- ZO-2
zonula occludens-2
Contributor Information
Xu Y Peng, College of Animal Science and Technology, Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Tong Xing, College of Animal Science and Technology, Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Jiao L Li, Institute of Agricultural Products Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, People’s Republic of China.
Lin Zhang, College of Animal Science and Technology, Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Yun Jiang, School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Nanjing 210023, People’s Republic of China.
Feng Gao, College of Animal Science and Technology, Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Conflict of Interest Statement
The authors declare that they have no financial interests.
Literature Cited
- Alhotan, R. A., A. R. A. Sulaiman, A. S. Alharthi, A. M. Abudabos. 2021. Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult Sci. 100: 101337. doi: 10.1016/j.psj.2021.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, M., H. A. Ghasemi, I. Hajkhodadadi, A. H. Khaltabadi Farahani. 2019. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim. Feed Sci. Technol. 254: 114208. doi: 10.1016/j.anifeedsci.2019.114208 [DOI] [Google Scholar]
- Azad M. A. K., M. Kikusato, T. Maekawa, H. Shirakawa, M. Toyomizu. 2010. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp Biochem Phys A. 155(3): 401–6. doi: 10.1016/j.cbpa.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Aziza, A., R. Mahmoud, E. Zahran, H. Gadalla. 2020. Dietary supplementation of guanidinoacetic acid improves growth, biochemical parameters, antioxidant capacity and cytokine responses in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immun. 97: 367–74. doi: 10.1016/j.fsi.2019.12.052 [DOI] [PubMed] [Google Scholar]
- Basoo, H., F. Khajali, E. A. Khoshoui, M. Faraji, F. R. Wideman. 2012. Re-evaluation of arginine requirements for broilers exposed to hypobaric condition during the 3-to 6-week period. Poult Sci. 49: 303–7. doi: 10.2141/jpsa.0110133 [DOI] [Google Scholar]
- Bayer, A. T., B. Barak. 2017. Trends and changes in tropical and summer days at the Adana Sub-Region of the Mediterranean Region, Southern Turkey. Atmospheric Research. 196: 182–99. doi: 10.1016/j.atmosres.2017.06.017 [DOI] [Google Scholar]
- Carvalho, C., E. Fernandes, A de Carvalho, M. Maciel, R. Caires, N. Fagundes. 2013. Effect of creatine addition in feeds containing animal meals on the performance and carcass yield of broilers. Braz J Poultry Sci. 15: 269–75. doi: 10.1590/S1516-635X2013000300015 [DOI] [Google Scholar]
- Chen, Y., Y. Cheng, C. Wen, Y. Zhou. 2020. Protective effects of dietary mannan oligosaccharide on heat stress–induced hepatic damage in broilers. Environ Sci Pollut Res Int. 27: 29000–008. doi: 10.1007/s11356-020-09212-2 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. F., Y. P. Chen, R. Chen, Y Su, R. Q. Zhang, Q. F. He, Y. M. Zhou. 2019. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult Sci. 98: 4767–76. doi: 10.3382/ps/pez192 [DOI] [PubMed] [Google Scholar]
- Córdova-Noboa, H. A., E. O. Oviedo-Rondón, A. H. Sarsour, J. Barnes, P. Ferzola, M. Rademacher-Heilshorn, U. Braun. 2018. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poult Sci. 97: 2479–93. doi: 10.3382/ps/pey096 [DOI] [PubMed] [Google Scholar]
- DeGroot, A. A., U. Braun, R. N. Dilger. 2018. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poult Sci. 97: 890–900. doi: 10.3382/ps/pez036 [DOI] [PubMed] [Google Scholar]
- Dokladny, K., P. L. Moseley, T. Y. Ma. 2006. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol-Gastr L. 290: G204–12. doi: 10.1152/ajpgi.00401.2005 [DOI] [PubMed] [Google Scholar]
- Fre, S., M. Huyghe, P. Mourikis, S. Robine, D. Louvard, S. Artavanis-Tsakonas. 2005. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 435: 964–68. doi: 10.1038/nature03589 [DOI] [PubMed] [Google Scholar]
- Fukudome, I., M. Kobayashi, K. Dabanaka, H. Maeda, K. Okamoto, T. Okabayashi, K. Hanazaki. 2014. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol. 47: 100–07. doi: 10.1007/s00795-013-0055-7 [DOI] [PubMed] [Google Scholar]
- Goo, D., J. H. Kim, G. H. Park, J. B. D. Reyes, D. Y. Kil. 2019. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 9: 107. doi: 10.3390/ani9030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashy, W. S., M. C. Milfort, A. L. Fuller, Y. A. Attia, R. Rekaya, S. E. Aggrey. 2017. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int J Biometeorol. 61: 2111–18. doi: 10.1007/s00484-017-1414-1 [DOI] [PubMed] [Google Scholar]
- Hashemipour, H., V. Khaksar, L. A. Rubio, T. Veldkamp, M. M. van Krimpen. 2016. Effect of feed supplementation with a thymol plus carvacrol mixture, in combination or not with an NSP-degrading enzyme, on productive and physiological parameters of broilers fed on wheat-based diets. Anim Feed Sci Technol. 211: 117–31. doi: 10.1016/j.anifeedsci.2015.09.023 [DOI] [Google Scholar]
- He, X., Z. Lu, B. Ma, L. Zhang, J. Li, Y. Jiang, F. Gao. 2018. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers: Effects of chronic heat exposure on growth appetite. J Sci Food Agr. 98: 4471–78. doi: 10.1002/jsfa.8971 [DOI] [PubMed] [Google Scholar]
- He, X., Z. Lu, B. Ma, L. Zhang, J. Li, Y. Jiang, F. Gao. 2019. Effects of dietary taurine supplementation on growth performance, jejunal morphology, appetite-related hormones, and genes expression in broilers subjected to chronic heat stress. Poult Sci. 98: 2719–28. doi: 10.3382/ps/pez054 [DOI] [PubMed] [Google Scholar]
- Kodambashi Emami, N., A. Golian, D. D. Rhoads, M. D. Mesgaran. 2017. Interactive effects of temperature and dietary supplementation of arginine or guanidinoacetic acid on nutritional and physiological responses in male broiler chickens. Br Poultry Sci. 58: 87–94. doi: 10.1080/00071668.2016.1257779 [DOI] [PubMed] [Google Scholar]
- Lan, R., Y. Li, Q. Chang, Z. Zhao. 2020. Dietary chitosan oligosaccharides alleviate heat stress–induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult Sci. 99: 6745–52. doi: 10.1016/j.psj.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara, L., M. Rostagno. 2013. Impact of Heat Stress on Poultry Production. Animals. 3: 356–69. doi: 10.3390/ani3020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H., H. C. Jiao, J. Buyse. 2006. Strategies for preventing heat stress in poultry. World Poultry Sci J. 62: 71–86. doi: 10.1079/WPS200585 [DOI] [Google Scholar]
- Liu, L., C. Fu, M. Yan, H. Xie, S. Li, Q. Yu. 2016. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 7: 1329–38. doi: 10.1039/c5fo01338k [DOI] [PubMed] [Google Scholar]
- Livak, K. J., T. D. Schmittgen, 2001. Analysis of relative gene expression data using real- time quantitative PCR and the 2−ΔΔCT method. Methods. 25: 402– 408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu, Z., X. He, B. Ma, L. Zhang, J. Li, Y. Jiang, F. Gao. 2018. Serum metabolomics study of nutrient metabolic variations in chronic heat-stressed broilers. Brit J Nutr. 119: 771–81. doi: 10.1017/S0007114518000247 [DOI] [PubMed] [Google Scholar]
- Ma, Y., W. Wang, H. Zhang, J. Wang, W. Zhang, J. Gao. 2018. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci Rep. 8: 15358. doi: 10.1038/s41598-018-33762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, B., L. Zhang, J. Li, T. Xing, Y. Jiang, F. Gao. 2021. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult Sci. 100: 215–23. doi: 10.1016/j.psj.2020.09.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdeddin, M., A. Golian, H. Kermanshahi, S. D. Smet, J. Michiels. 2018. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Brit Poultry Sci. 59: 443–51. doi: 10.1080/00071668.2018.1476678 [DOI] [PubMed] [Google Scholar]
- Majdeddin, M., U. Braun, A. Lemme, A. Golian, H. Kermanshahi, S. D. Smet. 2020. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to heat stress associated with muscle creatine loading and arginine sparing. Poult Sci. 99: 4442–53. doi: 10.1016/j.psj.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas, N. M. H., A. N. L. D. Costa, M. L. L. Pereira. 2020. Thermal conditioning in the broiler production: challenges and possibilities. J Anim Behav Biometeorol. 6: 52–55. doi: 10.31893/2318-1265jabb.v6n2p52-55 [DOI] [Google Scholar]
- Michiels, J., L. Maertens, J. Buyse, A. Lemme, M. Rademacher, N. A. Dierick. Supplementation of guanidinoacetic acid to broiler diets: 2012. Effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult Sci. 91: 402–12. doi: 10.3382/ps.2011-01585 [DOI] [PubMed] [Google Scholar]
- Mohammad, B. A., K. M. Saleh, M. M. K. Ababneh. 2019. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult Sci. 98: 1805–19. doi: 10.3382/ps/pey499 [DOI] [PubMed] [Google Scholar]
- Mohammed, A. A., J. A. Jacobs, G. R. Murugesan, H. W. Cheng. 2018. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult Sci. 97(4): 1101–08. doi: 10.3382/ps/pex421 [DOI] [PubMed] [Google Scholar]
- Murakami, A. E., R. J. B. Rodrigueiro, T. C. Santos, I. C. Ospina-Rojas, M. Rademacher. 2014. Effects of dietary supplementation of meat-type quail breeders with guanidinoacetic acid on their reproductive parameters and progeny performance. Poult Sci. 93: 2237–44. doi: 10.3382/ps.2014-03894 [DOI] [PubMed] [Google Scholar]
- Pearce, S. C., V. Mani, R. L. Boddicker, J. S. Johnson, T. E. Weber, J. W. Ross. 2012. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs1. J Anim Sci. 90: 257–59. doi: 10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Pietrzak, B., K. Tomela, A. Olejnik-Schmidt, A. Mackiewicz, M. Schmidt. 2020. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against bicrobial cells. Int J Mol Sci. 21: 9254. doi: 10.3390/ijms21239254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau, D., A. Collin, S. Yahav. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6: 707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Schneeberger, E. E., R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am J Physiol-Cell Ph. 286: C1213–28. doi: 10.1152/ajpcell.00558.2003 [DOI] [PubMed] [Google Scholar]
- Sohail, M. U., M. E. Hume, J. A. Byrd, D. J. Nisbet, A. Ijaz, A. Sohail. 2012. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 91: 2235–40. doi: 10.3382/ps.2012-02182 [DOI] [PubMed] [Google Scholar]
- Song, J., K. Xiao, Y. L. Ke, L. F. Jiao, C. H. Hu, Q. Y. Diao. 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci. 93: 581–8. doi: 10.3382/ps.2013-03455 [DOI] [PubMed] [Google Scholar]
- St-Pierre, N. R., B. Cobanov, G. Schnitkey. 2003. Economic losses from heat stress by US Livestock Industries. J Dairy Sci. 86: E52–77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Su, L., S. C. Nalle, L. Shen, E. S. Turner, G. Singh, L. A. Breskin. 2013. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 145: 407–15. doi: 10.1053/j.gastro.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafwan, S., R. P. Kwakkel, M. W. A. Verstegen. 2011. Heat stress and feeding strategies in meat-type chickens. World Poultry Sci J. 67: 653–674. doi: 10.1017/S0043933911000742 [DOI] [Google Scholar]
- Thompson, J., W. Vaughan, C. Forst, D. Jacobs, J. Weekly, L. Rikkers. 1987. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. Jpen-Parenter Enter. 11: 28–32. doi: 10.1177/014860718701100128 [DOI] [PubMed] [Google Scholar]
- Tsirtsikos, P., K. Fegeros, C. Balaskas. 2012. Dietary probiotic inclusion level modulates intestinal mucin composition and mucosal morphology in broilers. Poult Sci. 91: 1860–1868. doi: 10.3382/ps.2011-02005 [DOI] [PubMed] [Google Scholar]
- Turner, J. R. 2009. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 9:799–809. doi: 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- Varasteh, S., S. Braber, P. Akbari, J. Garssen, J. Fink-Gremmels. 2015. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of Galacto-Oligosaccharides. PLoS One. 10: e0138975. doi: 10.1371/journal.pone.0138975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella, A., G. Farrugia. 1998. D-lactic acidosis: pathologic consequence of saprophytism. Mayo Clin Proc. 73: 451–6. doi: 10.1016/S0025-6196(11)63729-4 [DOI] [PubMed] [Google Scholar]
- Wang, D., W. Ma, R. She, Qu. Sun, Y. Liu, Y. Hu. 2009. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult Sci. 88: 967–74. doi: 10.3382/ps.2008-00533 [DOI] [PubMed] [Google Scholar]
- Wijtten, P. J. A., D. J. Langhout, M. W. A. Verstegen. 2012. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: A review. Acta Agr Scand A-An. 62: 1–12. doi: 10.1080/09064702.2012.676061 [DOI] [Google Scholar]
- Yang, P. C., S. H. He, P. Y. Zheng. 2007. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J Gastroen Hepatol. 22: 1823–31. doi: 10.1111/j.1440-1746.2006.04710.x [DOI] [PubMed] [Google Scholar]
- Yang, Q., Z. Wang, Y. Cui, R. Sun, W. Liang, L. Wang. 2019. Effects of taurine on bowel inflammatory factor of small intestinal mucosa impaired by heat stress in broilers. Adv Exp Med Biol. 86: 1049–56. doi: 10.1007/978-981-13-8023-5_86 [DOI] [PubMed] [Google Scholar]
- Zhang, C., K. K. Chen, X. H. Zhao, C. Wang, Z. Y. Geng. 2019. Effect of l-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal 13: 1145–53. doi: 10.1017/S1751731118002884 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Y. Liu, J. Li, T. Xing, L. Zhang, F. Gao. 2020. Dietary corn resistant starch regulates intestinal morphology and barrier functions by activating the Notch signaling pathway of broilers. Asian Austral J Anim. 33: 2008. doi: 10.5713/ajas.19.0967 [DOI] [PMC free article] [PubMed] [Google Scholar]