Abstract
Adoptive immunotherapy with virus-specific cytotoxic T cells (VSTs) has evolved over the last three decades as a strategy to rapidly restore virus-specific immunity to prevent or treat viral diseases after solid organ or allogeneic hematopoietic cell-transplantation (allo-HCT). Since the early proof-of-principle studies demonstrating that seropositive donor-derived T cells, specific for the commonest pathogens post transplantation, namely cytomegalovirus or Epstein-Barr virus (EBV) and generated by time- and labor-intensive protocols, could effectively control viral infections, major breakthroughs have then streamlined the manufacturing process of pathogen-specific T cells (pSTs), broadened the breadth of target recognition to even include novel emerging pathogens and enabled off-the-shelf administration or pathogen-naive donor pST production. We herein review the journey of evolution of adoptive immunotherapy with nonengineered, natural pSTs against infections and virus-associated malignancies in the transplant setting and briefly touch upon recent achievements using pSTs outside this context.
INTRODUCTION
Opportunistic infections/reactivations remain the Achilles heel of allogeneic hematopoietic cell transplantation (allo-HCT) significantly increasing morbidity and mortality among allograft recipients.1–4 Despite routine molecular viral monitoring and the extensive application of antiviral prophylaxis and preemptive therapy with pharmacological agents, common viruses such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus (AdV), BK virus, as well as fungi, mainly Aspergillus fumigatus (AF) are major clinical concerns. Prolonged immune deficiency and especially delayed T-cell reconstitution posttransplant plays an important role in the outcome of antiviral prophylaxis and treatment, affecting the transplant-related mortality, all-cause mortality, and even relapse.3,4 To address this major limitation, sophisticated antiviral approaches have been explored, including adoptive transfer of T cells. In the current review, we describe the preclinical and clinical achievements of adoptive immunotherapy with pathogen-specific T cells (pSTs) in the allo-HCT setting and discuss the challenges and striking developments toward broader application. We also summarize recent achievements using nonengineered and genetically engineered pSTs against infections and virus-associated malignancies, beyond the transplant setting.
SINGLE-TARGETING PSTS AGAINST THE MOST COMMON PATHOGENS IN THE TRANSPLANT SETTING
In 1994, 5 patients with monoclonal EBV-post-transplant lymphoproliferative disease (EBV-PTLD) achieved complete remission after the infusion of peripheral blood mononuclear cells (PBMCs) from their EBV-seropositive transplant donors, demonstrating that donor lymphocyte infusions (DLIs) are effective in restoring virus-specific immunity and controlling viral infection.5 Nevertheless, broader application of DLIs in this context has been limited mainly due to their low content in virus-specific T cells compared with the higher frequency of alloreactive T cells that can cause severe graft-versus-host-disease (GvHD).6 To circumvent such an unwanted event, the use of conditionally eliminated DLIs being genetically modified to express suicide genes has been explored providing promising results.7
Later on, Rooney et al prepared and administered the first donor-derived, EBV-specific cytotoxic T-lymphocyte lines (EBV-CTLs), successfully preventing or controlling EBV reactivation in ten allograft recipients, without evidence of GvHD.8 In a multicenter study, Heslop et al. demonstrated high efficacy of EBV-CTLs as prophylaxis (0/101 patients developed PTLD) or treatment of PTLD (11/13 patients controlled PTLD) in allograft recipients and also persistence of functional CTLs for up to 9 years.9
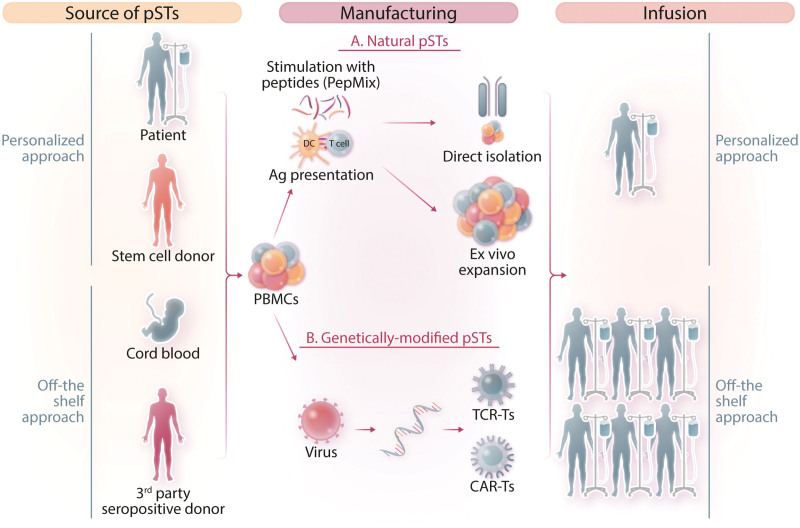
Despite efficacy, the widespread use of EBV-CTLs was severely limited by the 8–12 weeks manufacturing time and the process complexity,10 major obstacles for timely delivery to a patient developing PTLD. Since then, several groups have explored the development of faster and less sophisticated approaches and the manufacturing process has been significantly shortened and simplified. Such approaches involve (i) direct selection of donor VSTs with multimers that select T cells directed against specific viral peptides in the context of a specific HLA class I molecule11 or IFN-γ capture, where specific T cells are selected on the basis of IFN-γ secretion upon viral stimulation12 and (ii) ex vivo expansion of the endogenous antigen-specific T cells by specific T-cell stimulation with mixtures of overlapping peptides13 (Figure 1).
Figure 1.
Strategies for adoptive immunotherapy with pSTs. The starting material for the generation of pSTs is the mononuclear cell population isolated from different donor sources. In personalized adoptive immunotherapy, either the patient or the HLA-matched stem cell donor serves as the cell source (autologous or allogeneic, respectively). In off-the-shelf adoptive immunotherapy, pSTs are derived either from cord blood or selected sepositive donor banked blood. T cells can then be stimulated via viral peptide/protein/lysate or antigen-loaded antigen-presenting cells and pSTs are either directly isolated or ex vivo expanded, as natural pSTs (A) or T cells are transduced with a viral vector to express either a transgenic TCR of specific targeting or a CAR redirecting the specificity of T cells against a certain target pathogen. (B) Each either natural or genetically engineered pST cell product can be administered in the transplantation setting to a single, HLA-matched patient (personalized therapy, one donor-one recipient) or multiple, partially HLA-matched patients, within or outside the transplantation context (off-the-shelf therapy, one donor-multiple recipients). CAR = chimeric antigen receptor; pSTs = pathogen-specific T cells; TCR = T-cell receptor.
CMV-specific T-cell generation went also through various stages of protocol refinements. In the first proof-of-principle study by Riddell et al, virus-specific T-cell clones from a healthy donor generated ex vivo from autologous CMV-infected fibroblasts and adoptively transferred into an allo-HCT recipient, prevented CMV infection without causing GvHD.14 In another early study, Walter et al used fibroblasts infected with the AD169 strain of CMV to stimulate donor T cells followed by limiting-dilution cloning to isolate cytolytic CD8+ CMV-directed T cells for adoptive transfer. The CMV-specific clones were administered prophylactically to 14 recipients of HLA-matched sibling donor grafts in weekly escalated doses. All patients developed early on, CMV-specific T-cell reconstitution showing in their majority (11/14) significantly increased cytotoxic activity against CMV in vitro. GvHD developed in 7 patients before the first infusion, in 3 patients during the T-cell therapy course or after the last dose, while 4 patients did not develop GvHD. Neither CMV viremia nor disease developed in any treated patient.15
Also, Perruccio et al generated CD4+CMV-specific T-cell clones using donor PBMCs stimulated with CMV lysate, which were infused in 25 haploidentical HCT recipients. Cellular therapy proved to be safe and efficient in accelerating the recovery of endogenous CD8+CMV-specific responses, clinically translating into fewer CMV reactivation episodes and lower antigenemia levels.16
Alternatively, Einsele et al generated both CD4+- CD8+-CMV-specific CTLs using donor PBMCs pulsed with CMV lysate. The cellular products were safely infused to 8 HCT recipients who presented ganciclovir-resistant CMV viremia. Significant viral load reduction was observed in 7 patients, whereas in the 2 patients with the highest viral loads, the antiviral effect was only transient. A second T-cell infusion in one of the 2 patients resulted in complete response.17 Similar results with excellent response rates were reported by other groups as well.12,18
Many studies targeted AdV, another common pathogen, especially in the pediatric HCT field. Feuchtinger et al isolated AdV-specific donor T cells based on IFN-γ secretion after in vitro antigenic stimulation. The cellular products which combined both CD4+ and CD8+ T cells were infused into 9 children with systemic AdV infection post-HCT. Five of 6 evaluable patients managed to successfully clear viremia. T-cell infusion was well tolerated in all patients, except one case with skin GvHD grade II.19 In another phase I/II multicenter study, Ip et al demonstrated safety and feasibility of the preemptive administration of AdV-specific T cells in high-risk pediatric patients after allo-HCT to control viremia. Eight children received the cellular products and all successfully controlled the infection, while 1 patient developed stage II GvHD 2 months postinfusion.20 Feucht et al showed that adoptive transfer of TH1 cells is a safe and effective alternative therapeutic approach for refractory AdV disease or viremia after HCT, resulting, in patients with antigen-specific T-cell responses (14/23), in 86% (12/14) complete remissions and 100% survival (versus 9.5% in nonresponders).21 Clinical trials and preclinical studies with pSTs are summarized in Tables 1, 2.
Table 1.
Clinical Trials With Single- and Multitargeting Pathogen-specific T Cells
| Targeted Pathogen(s) | Manufacturing | Pts | Prophylaxis/Treatment | Dose | Safety | Clinical Response | References | |
|---|---|---|---|---|---|---|---|---|
| Donor-derived | CMV | Ex vivo expansion | 14 | Prophylaxis | 33 × 106–1 × 109/m2 | 3/14 GvHD grade I-II | 14/14 CR | Walter et al15 |
| CMV | Ex vivo expansion | 8 | Treatment | 1 × 107/m2 | No toxicity | 6/8 CR, 1/8 PR, 1/8 NR | Einsele et al17 | |
| CMV | Ex vivo expansion | 30 | Prophylaxis | 0.6–1 × 105/kg | 7/30 aGvHD grade I, 4/30 aGvHD grade II-III, 12/28 cGvHD | 27/27 CR, 20/27 required antivirals | Peggs et al18 | |
| CMV | Ex vivo expansion | 50 | Prophylaxis | 2 × 107/m2 | 7/50 aGvHD, 1 CMV-related death | 26/50 reactivations, 5/26 reactivations post infusion, 1/5 required antivirals | Blyth et al22 | |
| CMV | Ex vivo expansion | 32 | Treatment | CD8+: 0.66–15.41 × 107, CD4+: 0.68–9.25 × 105 | 1/32 GvHD grade II | 27/32 CR, 5/32 NR | Pei et al23 | |
| CMV | Ex vivo expansion and DC vaccine | 4 | Prophylaxis | 2 × 107/m2, DC 19 × 106 | 1/4 aGvHD grade III, 3/4 cGvHD | 3/4 CR | Ma et al24 | |
| CMV | IFN-gamma capture | 18 | Prophylaxis/ preemptive | 1 × 104/kg | 7/18 aGVHD grade I-II, 1/18 aGVHD grade III, 3/18 cGvHD | 1/7 pts treated prophylactically reactivated - 11/11 pts treated preemptively cleared CMV | Peggs et al12 | |
| CMV | Streptamer selection | 2 | Treatment | 0.37–2.2 × 105/kg | No toxicity | 2/2 CR | Schmitt et al25 | |
| CMV | Streptamer selection | 2 | Treatment | 3.7–5.1 × 103/kg | No toxicity | 2/2 CR | Stemberger et al26 | |
| CMV | Tetramer selection | 9 | Treatment | 1.2 × 103–3.3 × 104/kg | No toxicity | 8/9 CR, 1/9 PR | Cobbold et al27 | |
| CMV | Ex vivo expansion | 9 | Prophylaxis | 2 × 107/m2 | 3/9 aGvHD grade I-II, 2/9 cGvHD | 7/9 CR, 2/9 CMV reactivations | Mickleithwaite et al28 | |
| CMV | Ex vivo expansion | 12 | Prophylaxis | 2 × 107/m2 | 4/12 GvHD | 4/12 CMV reactivations | Micklethwaite et al29 | |
| CMV | Ex vivo expansion | 16 | Prophylaxis | 0.5–1 × 105 | 3/16 GvHD grade I | 2/16 CMV reactivations | Peggs et al30 | |
| CMV | Ex vivo expansion | 7 | Treatment | 2.5–5 × 105/kg | No toxicity | 4/7 CR, 2/7 PR, 1/7 NR | Bao et al31 | |
| CMV | IFN-gamma capture | 6 | Treatment | 0.9 × 104–3.1 × 105/kg | No toxicity | 6/6 CR | Meij et al32 | |
| CMV or EBV | Ex vivo expansion | 3 | Treatment | 106 cells/kg (3–8 infusions) | 1/3 aGvHD grade II | CMV: 3/3 CR; EBV: 1/1 CR | Dong et al33 | |
| CMV or AdV | IFN-gamma capture | 15 | Treatment | 3.5–3.7 × 103/kg | 1/15 GvHD grade III, 2/15 septic shock, 4/15 worsening of respiratory symptoms or liver cytolysis | CMV 3/8 CR, 5/8 NR; AdV 3/5 CR, 1/5 PR, 1/5 NR | Creidy et al34 | |
| EBV | Ex vivo expansion | 114 | Prophylaxis/ treatment | 2–5 × 107/m2 | 8/114 aGvHD, 13/114 cGvHD | Prophylaxis: 101/101 CR, treatment: 11/13 CR, 2/NR | Rooney, Heslop8,9,35–37 | |
| EBV | Ex vivo expansion | 6 | Treatment | 1 × 107/m2 (2–4 infusions) | 1/6 aGvhD limited reactivation | 5/6 PR, 1/6 NR | Gustafsson et al38 | |
| EBV | Ex vivo expansion | 4 | Treatment | 35 × 106/m2 | No toxicity | 3/4 CR | Comoli et al39 | |
| EBV | Ex vivo expansion | 6 | Treatment | 0.5 × 106/kg | No toxicity | 6/6 CR | Comoli et al40 | |
| EBV | IFN-gamma capture | 6 | Treatment | 0.4–9.7 × 104/kg | No toxicity | 3/6 CR, 3/6 NR | Moosman et al41 | |
| EBV | IFN-gamma capture | 10 | Treatment | 1.5 × 102–5.4 × 104/kg | 1/10 aGvhD grade I-II | 6/10 CR, 1/10 PR, 3/10 NR | Icheva et al42 | |
| EBV | n/a | 1 | Treatment | 8.2 × 106–1 × 107 | No toxicity | 1/1 NR | Imashuku et al43 | |
| AdV | Ex vivo expansion | 8 | Preemptive | 1 × 104/kg | 1/8 aGvHD | 8/8 CR | Ip et al20 | |
| AdV | IFN-gamma capture | 9 | Treatment | 1.2 × 103–5 × 104/kg | 1/6 aGvHD reactivation | 3/6 CR, 2/6 PR, 1/6 NR | Feuchtinger et al19 | |
| AdV | IFN-gamma capture | 30 | Treatment | 0.3–24 × 10e3 cells/kg | 2/30 aGvHD | 18/29 CR, 3/29 PR, 8/29 NR | Feucht et al44 | |
| AdV | Ex vivo expansion | 2 | Treatment | 104/kg | 1/2 GvHD | 1/2 CR, 1/2 NR | Geyqerreger et al45 | |
| AdV | IFN-gamma capture | 1 | Treatment | 34 × 103 CD3+/kg 73 × 103 CD8+/kg | No toxicity | 1/1 CR | Di Nardo et al46 | |
| AF or CMV (HCT) | Ex vivo expansion | 35 | Prophylaxis (CMV) or treatment (AF) | 105–3 × 106 cells/kg | 1/35 aGvHD grade II | AF: 9/10 CR; CMV: 7/25 reactivations | Perrucio et al16 | |
| BKV (HCT) | IFN-γ capture | 1 | Treatment | 0.34 × 104 cells/kg | No toxicity | 1/1 CR | Pello et al47 | |
| JC (PML post-HCT) | Ex vivo expansion | 1 | Treatment | 0.5 × 106 and 106 cells/kg | No toxicity | 1/1 PR | Balduzzi et al48 | |
| JC (PML post-HCT) | IFN-γ capture | 1 | Treatment | 2 × 104 total | No toxicity | 1/1 CR | Steinhardt et al49 | |
| EBV, AdV | Ex vivo expansion | 13 | Prophylaxis/ treatment | 0.5–13.5 × 107 cells/m2 | No toxicity | EBV: 13/13 prophylaxis; AdV: 2/2 CR | Leen et al50 | |
| CMV, EBV, AdV | Ex vivo expansion | 11 | Prophylaxis/ treatment | 0.5–10 × 107 cells/m2 | No toxicity | CMV: 3/3; EBV: 3/3; AdV: 6/6 | Leen et al51 | |
| CMV, EBV, AdV | Ex vivo expansion | 14 | Prophylaxis/ treatment | 0.5–2.5 × 107 cells/m2 | 1/14 GvHD reactivation | CMV: 4/4; EBV: 1/2; Adv: 1/1 | Abraham et al52 | |
| CMV, EBV, AdV | Ex vivo expansion | 10 | Treatment | 0.5–2 × 107 cells/m2 | 1/10 possible GvHD grade II (skin) | CMV: 4/5 CR; EBV: ¾ CR; AdV: 5/5 CR | Gerdeman et al53 | |
| CMV, EBV, AdV, VZV | Ex vivo expansion | 10 | Prophylaxis | 2 × 107 cells/m2 | No toxicity | CMV: 2/2; EBV, AdV, VZV: no reactivation | Ma et al54 | |
| CMV, EBV, AdV, BKV | Ex vivo expansion | 38 HCT; 3 SOT | Treatment | 5 × 107 VSTs/m2 (up to 10 infusions) |
No toxicity | HCT donor-derived: 17/19 CR; HCT-3rd party-derived: 17/18 CR; SOT-3rd party-derived: EBV: 2/2 CR; BK: 3/3 CR | Nelson et al55 | |
| CMV, EBV, AdV, BKV | Ex vivo expansion | 23 | Prophylaxis | 2 × 107 cells/m2 | 2/23 GvHD grade II-III | 11/23 (CMV: 3/4; EBV: 6/7; BK: 5/5); 7/23 no viral load | Rubinsten et al56 | |
| CMV, EBV, AdV, BKV, HHV6 | Ex vivo expansion | 11 | Prophylaxis/treatment | 0.5–2 × 107 cells/m2 | 1/11 GvHD grade II | CMV: 3/3; EBV: 5/5; AdV: 1/1; BKV: 6/7; HHV6: 2/2 | Papadopoulou et al57 | |
| CMV, EBV, AdV, BKV, VZV, AF, Influenza | Ex vivo expansion | 11 | Prophylaxis | 2 × 107 cells/m2 | 2/11 aGvHD grade I-II, 4/11 aGvHD grade III-IV | CMV: mixed; EBV: mixed; BKV: 10/10 CR; Influenza: 2/2 CR; AdV-AF-VZV: 0 developed | Gottllieb et al58 | |
| Donor- & third party-derived | CMV | Streptamer selection | 16 | Treatment | 6.34–14.6 × 106 | 1/16 aGvHD; 1/16 cGvHD | 9/16 CR, 3/16 PR, 4/16 NR | Neuenhahn et al59 |
| CMV | Ex vivo expansion | 17 | Treatment | 0.5–2 × 106/kg | No toxicity | 15/17 CR, 2/17 NR | Koehne et al60 | |
| CMV | IFN-gamma capture | 18 | Treatment | 1.2 × 103–1.7 × 104/kg | 1/18 upper GI Bleeding | 9/18 CR, 6/18 PR, 3/18 NR | Feuchtinger et al61 | |
| CMV or EBV or AdV | Pentamer selection | 8 | Treatment | 0.8–24.6 × 104/kg | N/a | EBV-1/1 CR, ADV-1/1 NR, CMV-4/6 CR, 1/6 PR, 1/6 NR | Uhlin et al62 | |
| EBV | Ex vivo expansion | 19 | Treatment | 0.5–3.5 × 106/kg | No toxicity | 13/19 CR, 6/19 NR | Doubrovina et al63 | |
| AdV | IFN-gamma capture | 5 | Treatment | 104–105/kg | No toxicity | 3/5 CR, 2/5 NR | Qasim et al64 | |
| AdV | IFN-gamma capture | 11 | Treatment | 0.25–9.38 × 103/kg | 3/11 GvHD reactivation | 9/11 CR, 2/11 NR | Gerdemann et al53 | |
| JC (PML; 5 post-HCT) | Ex vivo expansion | 9 | Treatment | 1–2 × 105 cells/kg (up to 6 infusions) | 1/9 died VZV infection | 4/9 PR; 1/9 SD; 3/9 PD; 1/9 died VZV infection | Berzero et al65 | |
| Third party-derived | CMV | Ex vivo expansion | 10 | Treatment | 2 × 107/m2 | No toxicity | 7/10 CR, 3/10 PR | Tzannou et al66 |
| CMV | IFN-gamma capture | 2 | Treatment | 0.8–4.4 × 104/kg | No toxicity | 1/2 CR, 1/2 PR | Alonso et al67 | |
| EBV | Ex vivo expansion | 33 | Treatment | 9–18 × 106/kg | 1/33 aGvHD grade I | 19/33 CR, 3/33 PR, 1/33 SD, 9/33 PD | Prockop et al68 | |
| EBV | Ex vivo expansion | 11 | Treatment | 1–2 × 106/kg | 1/11 aGvHD | 8/11 CR, 1/11 PR, 2/11 NR | Vickers et al69 | |
| EBV | Ex vivo expansion | 11 | Treatment | 5 × 106/kg | No toxicity | 3/11 CR, 1/11 PR, 7/11 NR | Gallot et al70 | |
| EBV (1 HCT–7 SOT) | Ex vivo expansion | 8 | Treatment | 106 cells/kg (up to 6 infusions) | No toxicity | 3/6 CR; 1/6 PR; 2/6 NR; 2 passed away before evaluation (unrelated to VSTs) | Haque et al71 | |
| EBV (2 HCT–31 SOT) | Ex vivo expansion | 33 | Treatment | 2 × 106 cells/kg (up to 8 infusions) | No toxicity | 12/33 CR; 9/33 PR; 12/33 NR | Haque et al72 | |
| EBV (HCT) | Ex vivo expansion | 2 | Treatment | 106 cells/kg (5–6 infusions) | No toxicity | 2/2 CR | Barker et al73 | |
| BKV (renal transplant) | Ex vivo expansion | 1 | Treatment | 3.6 × 107 cells total (10 infusions) | No toxicity | 1/1 CR | Jahan et al74 | |
| BKV (HCT) | Ex vivo expansion | 59 | Treatment | 1–2 × 105 cells/kg (up to 5 infusions) | 1/59 aGvHD Grade II (skin), 1/59 aGrade III GvHG (GI), 9/59 cGvHD | 34/49 CR; 6/49 PR; 9/49 NR | Olson et al75 | |
| BK (JC-PML) –not HCT | IFN-γ capture | 2 | Treatment | 2.5 × 104 cells/kg (3–4 infusions) | N/a | 2/2 PR | Hopfner et al76 | |
| BK (JC (PML) (1 HCT pt–2 not HCT) | Ex vivo expansion | 3 | Treatment | 2 × 105 cells/kg (up to 3 additional infusions) | No toxicity | 2/3 PR, 1/3 SD | Muftuoglu et al77 | |
| BK (JC (PML) –not HCT | Ex vivo expansion | 12 | Treatment | 106 cells/kg (plus up to 2 doses of 2 × 106 cells/kg) | No toxicity | 7/12 survived beyond 12 m | Cortese et al78 | |
| SARS-CoV-2 | Ex vivo expansion | 57 | Treatment | 2 × 107 cells/m2 | No toxicity | Increased likelihood of recovery, lower risk of mortality than SoC alone | Papadopoulou et al79 | |
| SARS-CoV-2 | Memory T-cell selection | 9 | Treatment | 0.1–1 × 106 cells/kg | No toxicity | 9/9 improvement | Perez-Martinez et al80 | |
| SARS-CoV-2 | Ex vivo expansion | 1 | Treatment | 2 × 107 cells (3 infusions) | No toxicity | 1/1 CR | Martits-Chalangari et al81 | |
| CMV, AdV or CMV, EBV or CMV or EBV or AdV | IFN-γ capture | 9 | Treatment | 32–204.8 × 103 cells/kg | 1/9 cytokine storm | CMV: 4/5 CR; AdV: 3/3 CR; EBV: 3/3 CR | Kallay et al82 | |
| CMV, EBV, AdV | Ex vivo expansion | 50 | Treatment | 2 × 107 cells/m2 (up to 6 infusions) | 8/50 aGvHD (2 de novo) | CMV: 14/18 CR; EBV: 6/9 CR; AdV: 17/23 CR | Leen et al83 | |
| CMV, EBV, AdV | Ex vivo expansion | 30 | Treatment | 2–5 × 107 cells/m2 (up to 3 infusions) | 2/30 aGvHD grade II-IV; 5/30 cGvHD | CMV: 27/28 CR; EBV: 0/1 CR; AdV: 1/1 CR | Whithers et al84 | |
| CMV, EBV, AdV, BKV | Ex vivo expansion | 1 | Treatment | 5 × 107 cells/m2 (2 infusions) | 1/1 CRS | 1/1 NR | Holland et al85 | |
| CMV, EBV, AdV, BKV | Ex vivo expansion | 4 | Treatment PML (HCT setting) | 5 × 107 cells/m2 (up to 6 infusions) | No GvHD | 1/4 SD; 3/4 PD | Rubinstein et al86 | |
| CMV, EBV, AdV, BKV, HHV6 | Ex vivo expansion | 38 | Treatment | 2 × 107 cells/m2 (up to 3 infusions) | 6/38 aGvHD (3 de novo) | BKV: 20/20 CR; HHV6: 3/4 CR, 1/4 NE (not evaluated); CMV: 18/19 CR; EBV: 2/2 CR; ADV: 6/8 CR | Tzannou et al87 | |
| Autologous | MCPyV | Ex vivo expansion | 1 | Treatment | 1010/m2 (3 infusions) | No toxicity | tumor regression in 2 of 3 detectable metastases | Chapuis et al88 |
| MCPyV | Ex vivo expansion | 2 | Treatment | 1010/m2 (2–4 infusions) | Transient fevers lasting <24 h | 2/2 CR, 2/2 late relapse | Paulson et al89 | |
| HPV (no hct) | Ex vivo expansion from fragments of metastatic tumors (tumor-infiltrating T cells) | 29 | Treatment | 9–152 × 109 total | No toxicity | Cervidal cancer: 3/18 PR, 2/18 CR - noncervical cancer: 2/11 PR | Stevanović et al90 | |
| HIV | Ex vivo expansion | 1 | Treatment | 12–13 × 109 (2 infusions) | N/a | 1/1 PD | Koenig et al91 | |
| HIV | Ex vivo expansion | 6 | Treatment | 1 × 109 total | No toxicity | 3/6 PR | Lieberman et al92 | |
| HIV | Ex vivo expansion | 3 | Treatment | 3.3–33 × 108 cells/m2 (5 infusions) | No toxicity | 0/3 NR | Brodie et al93 | |
| HIV | Ex vivo expansion | 1 | Treatment | 1–1.7 × 109 total (2 infusions) | No toxicity | 1/1 SD | Tan et al94 | |
| HIV | Ex vivo expansion | 7 | Treatment | 3.3 × 109 total | N/a | 7/7 SD | Chapuis et al95 | |
| HIV | Ex vivo expansion | 6 | Treatment | 2 × 107 cells/m2 (2 infusions) | No toxicity | 6/6 SD (No detectable impact on the latent reservoir) | Sung et al96 |
AdV = adenovirus; AF = Aspergillus fumigatus; aGvHD = acute graft-versus-host-disease; BKV = BK virus; CMV = cytomegalovirus; CR = complete response; CRS = cytokine release syndrome; EBV = Epstein-Barr virus; GvHD = graft-versus-host-disease; HCT = hematopoietic cell transplantation; HHV6 = human herpesvirus 6; HIV = human immunodeficiency virus; HPV = human papillomavirus; IFN-γ = interferon-γ; MCPyV = Merkel cell polyomavirus; NR = no response; PBMCs = peripheral blood mononuclear cells; PD = progressive disease; PR = partial response; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SD = stable disease; VSTs = virus-specific T cells; VZV = varicella zoster.
Table 2.
Preclinical Studies With Single- and Multitargeting Pathogen-specific T Cells
| Targeted Pathogen(s) | Manufacturing | References |
|---|---|---|
| AF | Ex vivo expansion | Ramadan et al97 |
| AF | IFN-γ capture | Beck et al98 |
| AF | Ex vivo expansion | Zhu et al99 |
| AF | IFN-γ capture and ex vivo expansion | Tramsen et al100 |
| AF | Ex vivo expansion | Gaundar et al101 |
| AF | CD137 selection and ex vivo expansion | Jolink et al102 |
| AF | CD137 selection | Bacher et al103 |
| AF | CD154 or CD137 selection and ex vivo expansion | Stuehler et al104 |
| AF | Ex vivo expansion | Papadopoulou et al123 |
| AF or Aspergillus flavus or Aspergillus terreus or Candida albicans or Candida krusei or Fusarium solani or Fusarium oxysporum or Rhizopus oryzae or Lomentospora prolificans | Ex vivo expansion | Deo et al124 |
| BKV | Ex vivo expansion | Blyth et al108 |
| BKV | Ex vivo expansion | Lamarche et al110 |
| BKV | Ex vivo expansion | Wilhelm et al109 |
| C. albicans | IFN-γ capture and ex vivo expansion | Tramsen et al105 |
| HIV | Ex vivo expansion | Lam et al128 |
| HIV | Ex vivo expansion | Patel et al129 |
| HIV | Ex vivo expansion | Sung et al96 |
| HIV | Ex vivo expansion | Patel et al130 |
| HHV6 | Ex vivo expansion | Gerdemann et al112 |
| hMPV | Ex vivo expansion | Tzannou et al116 |
| HPV | Ex vivo expansion | McCormack et al120 |
| HPV | Ex vivo expansion | Van Poelgeest et al119 |
| HPV | Ex vivo expansion | Ramos et al118 |
| HPIV3 | Ex vivo expansion | McLaughlin et al115 |
| HPIV3 | Ex vivo expansion | Harris et al114 |
| HSV-1 | Ex vivo expansion | Ma et al117 |
| Influenza | Ex vivo expansion | Gaundar et al113 |
| MCPyV | Ex vivo expansion | Davies et al131 |
| Mycobacteria spp. | Ex vivo expansion | Patel et al127 |
| Norovirus | Ex vivo expansion | Hanajiri et al122 |
| R. oryzae | CD154 selection, ex vivo expansion, and IFN-γ enrichment | Schmidt et al126 |
| R. oryzae | Ex vivo expansion | Castillo et al125 |
| SARS-CoV-2 | Ex vivo expansion | Keller et al132 |
| SARS-CoV-2 | Ex vivo expansion | Papayanni et al133 |
| SARS-CoV-2 | IFN-γ capture and ex vivo expansion | Cooper et al134 |
| SARS-CoV-2 | Ex vivo expansion | Kim et al135 |
| SARS-CoV-2 | Ex vivo expansion | Guerreiro et al136 |
| SARS-CoV-2 | Ex vivo expansion | Ferreras et al80 |
| SARS-CoV-2 | IFN-γ capture and ex vivo expansion | Garcia-Rios et al137 |
| SARS-CoV-2 | Ex vivo expansion | Pannikar et al138 |
| VZV | Ex vivo expansion | Blyth et al111 |
| Zika virus | Ex vivo expansion | Hanajiri et al121 |
| CMV, EBV, AdV | Ex vivo expansion | Hanley et al139 |
| CMV, EBV, AdV | Ex vivo expansion | Hanley et al140 |
| CMV, EBV, AdV, BKV | Ex vivo expansion | Dasari et al141 |
| CMV, EBV, AdV, BKV | Ex vivo expansion | Dave et al142 |
| RSV, influenza, PIV, and hMPV | Ex vivo expansion | Vasileiou et al143 |
| CMV, EBV, AdV, BKV, HHV6, VZV, JCV | Ex vivo expansion | Gerdeman et al13 |
| AF, C. albicans, and R. oryzae | IFN-γ capture and ex vivo expansion post | Tramsen et al144 |
| A. terreus, C. krusei, and R. oryzae | Ex vivo expansion ± TNF-α selection | Deo et al124 |
| C. krusei and A. terreus | CD137 selection and ex vivo expansion | Castellano-Gonzalez et al145 |
| AdV, EBV, CMV, C. albicans, and/or AF or AdV, EBV, C. albicans, and AF | CD154 selection and ex vivo expansion | Khanna et al146 |
| EBV, CMV, BKV, and AF | Ex vivo expansion | Papadopoulou et al147 |
AdV = adenovirus; AF = Aspergillus fumigatus; BKV = BK virus; CMV = cytomegalovirus; EBV = Epstein-Barr virus; HHV6 = human herpesvirus 6; HIV = human immunodeficiency virus; hMPV = human metapneumovirus; HPV = human papillomavirus; HPIV3 = human parainfluenza virus-3; HSV-1 = herpes simplex virus type 1; IFN-γ = interferon-γ; MCPyV = Merkel cell polyomavirus; PIV = parainfluenza virus; RSV = respiratory syncytial virus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VZV = varicella zoster.
SINGLE-TARGETING PSTS AGAINST LESS COMMON PATHOGENS IN THE TRANSPLANT SETTING
The high response rates, along with the low toxicity observed in numerous early phase clinical trials of adoptively transferring VSTs (Table 1)
as prophylaxis or treatment against the main pathogens causing morbidity and mortality in immunocompromised hosts—namely CMV, EBV, and AdV—set the rationale for developing pSTs targeting less common, albeit well-recognized and potentially lethal for this particular population, opportunistic pathogens. In particular, since mid-2000s, manufacturing by ex vivo expansion or direct isolation, were separately employed or combined to enable the generation of AF- or Candida albicans-specific T-cell products.97–105 Nevertheless, clinical use of pSTs against mold infections was at that time hampered, due to the complex, lengthy, labor-intense, and costly manufacturing procedures.106,107
Major advances in synthetic overlapping peptide pools, use of cytokines and culture vessels/bioreactors, along with the in-depth characterization of immunogenic epitopes/antigens presented through human leukocyte antigen (HLA) molecules, have since then facilitated the production process and provided a springboard to extend the breadth of pathogens that can be targeted by T cells. Indeed, over the last years, the pST cell therapy platform has been simplified and adapted to an array of pathogens which complicate immune-deficient states, including viruses such as BK polyomavirus (BKV),108–110 human herpesviruses [varicella zoster virus (VZV),111 human herpesvirus 6 (HHV6)112], respiratory viruses [influenza,113 human parainfluenza virus-3 (HPIV3),114,115 human metapneumovirus (hMPV)116], herpes simplex virus type 1 (HSV-1),117 human papillomavirus (HPV),118–120 Zika virus,121 norovirus,122 fungi such as AF, Aspergillus flavus, Aspergillus terreus, C. albicans, Candida krusei, Fusarium solani, Fusarium oxysporum, Rhizopus oryzae, Lomentospora prolificans123–126 and recently the more complex Mycobacteria127 (Table 2).
In the transplant setting, Perrucio et al16 reported that 9 of 10 haploidentical HCT patients treated with AF-specific T-cell clones survived invasive aspergillosis versus 7 of 13 individuals receiving conventional antifungal therapy. Other cases of transplanted patients treated with adoptive immunotherapy with donor-derived, monovalent virus-specific T cells (VSTs) against less common pathogens, included three patients who received in different studies, donor-derived JC-VSTs as treatment for the JC virus-associated progressive multifocal leukoencephalopathy (PML) and managed to clear JC achieving remarkable clinical improvement.48,49,65 In an another patient who was treated with IFN-γ capture-enriched BKV-STs from his HLA-haploidentical donor for drug-resistant BKV hemorrhagic cystitis, the treatment led to full resolution of symptoms and viremia.47 Those cases affirmed that the adoptive transfer of either isolated or ex vivo expanded T cells against less common pathogens, is safe and can restore pathogen-specific immune competence and control of opportunistic pathogen-associated diseases.
MULTIVALENT PSTS AGAINST VARIOUS PATHOGENS IN THE TRANSPLANT SETTING
Despite the compelling results of single-targeting VSTs, transplanted patients usually experience reactivations from more than one opportunistic pathogens, thus making the generation of monovalent pSTs through multiple, time- labor-, and cost-intensive manufacturing processes, each time a new pathogen is being reactivated, rather impractical.
Leen et al pioneered the generation of bivalent and trivalent VSTs.50,51 By stimulating PBMCs with either a null adenoviral vector (Ad5f35null)50 or the Ad5f35pp65 vector encoding the CMV-pp65 antigen,51 and subsequently with autologous, EBV-transformed cell lines (EBV-LCLs) modified with the same vector(s) to artificially present adenoviral and/or (in the case of Ad5f35pp65) CMV-pp65 antigens, along with the constitutively expressed EBV antigens, demonstrated that the production of a single-cell product simultaneously targeting 2 or 3 viruses was feasible. Moreover, bi- or tri-VSTs presented clinically measurable antiviral activity after their administration to immunocompromised individuals, however, the long manufacturing procedure of 10 weeks limited wider applicability. By a similar approach, Hanley et al expanded tri-VSTs from virus-naive cord blood units (CBUs) using monocyte-derived dendritic cells (DCs) and Ad5f35pp65-transduced LCLs.139 The administration of tri-VSTs derived from a 20% fraction of the CBU to patients receiving a single CBU transplantation was safe and effective in preventing viral disease in the highest-risk patients.52 To simplify and accelerate the tri-VST production from seropositive donors, DCs were nucleofected with plasmids encoding CMV, EBV, and AdV antigens and used as antigen-presenting cells (APCs) for T-cell stimulation.53 This approach considerably shortened the manufacturing of tri-VSTs to 9–11 days.
Following the clinical success of tri-VSTs, expansion protocols for the production of tetravalent, pentavalent, and even heptavalent VSTs have been developed (Table 2), employing various APCs, including (i) DCs exposed to Ad5F35 encoding selected EBV epitopes and to VZV vaccine54; (ii) PBMCs infected with an AdV vector-based antigen presentation platform141; (iii) DCs pulsed with overlapping peptide pools of immunogenic viral proteins13,57,143; and (iv) phytohemagglutinin (PHA) blasts and K562 cells genetically modified to express co-stimulatory molecules.142 Among the various multi-VST generation methods, DCs-based approaches have been clinically tested, providing substantial responses as prophylaxis/treatment against VZV,54 EBV, CMV, AdV, BKV, and HHV6.55–57
Single- or multitargeting T-cell therapies against fungi have lagged well behind VSTs, mainly due to the complexity and length of the manufacturing procedures.106,107 Preclinical efforts, including ours, have been made towards the development of fungus-specific T-cell products,124,144,145 yet not clinically translated. To further unleash the potential of adoptive immunotherapy with antigen-specific T cells and enable the simultaneous treatment of multiple viral and fungal infections by an “all-in-one” single T-cell product, we and others, have developed refined strategies for manufacturing multipathogen-specific T-cell products (multi-pSTs). By either ex vivo expansion or magnetic isolation, T-cell products with specificity against a plethora of viruses and the main fungi affecting severely immunocompromised patients (AF and Candida)148–151 have been generated146,147 (Figure 1). In a clinical trial, multi-pSTs against 7 infectious pathogens, produced after combining and then expanding individually-primed cell products (AF-primed T cells combined with VZV-Influenza- and CMV-AdV-EBV-BKV-stimulated cultures) were administered prophylactically to allo-HCT recipients. This study provided the feasibility of multitargeting by a single-cell product; however, data interpretation could not be clear-cut, as the lack of a comparator group, the small sample size, and the prophylactic over therapeutic administration, did not allow firm conclusions about efficacy and safety. Indeed, it was impossible to distinguish whether GvHD occurrence in these patients was a de novo or multi-pST-induced event whereas the high-dose steroids needed to control GvHD in those patients, probably had rendered the infused multi-pSTs nonfunctional against the targeted-pathogens.58 We are currently evaluating in a clinical trial, the safety and efficacy of multi-pSTs simultaneously targeting AdV, CMV, EBV, BKV, and AF as treatment of corresponding infections post-allogeneic-HCT (EudraCT #2020-004725-23; NCT05471661).
There is accumulating evidence that AF and C. albicans, despite being profoundly different fungal pathogens, may induce CD4+ memory Th1 cells cross-protective against both pathogens, probably through molecular mimicry; the epitope p41 of the AF’s cell wall glucanase Crf1 has high homology to Chr1 epitope of Candida albicans, both being recognized by the same T-cell receptor (TCR).152,153 We and others have shown that such cross-reactivity may extend also to other Aspergillus, Candida, Fusarium, and Mucorales species,101,104,123 an important feature that will further augment the efficacy of multi-pSTs, induce a rapid restoration of a “broad repertoire” immunity, broaden the target patient population and reduce the manufacturing costs.
CONFRONTING THE CHALLENGES TOWARD BROADER APPLICATION OF PSTS
Donor unavailability and urgent need: the “third-party” approach
Multi-pSTs is an individualized product that for the time being can only be manufactured in specialized centers. Refined manufacturing procedures require at least 15 days for release, making these products unavailable for urgent administration, as in cases where an infection rapidly evolves or/and the stem cell donor is not accessible. The establishment of “off-the shelf,” third-party banks can help overcome such issues. With this strategy, pSTs are manufactured in advance from seropositive, transplant-eligible healthy donors, then being readily available for urgent use in partially HLA-matched patients with active infections (Figure 1).
The third-party bank approach was first studied in solid organ transplant (SOT) recipients where donor blood is not generally available for pST manufacture. Haque et al72 prepared EBV-STs from 100 healthy donors and treated 31 solid organ- and 2 HCT recipients with biopsy proven PTLD, refractory to conventional treatment. The patients received 1–6 infusions of 2 × 106 VST cells/kg matched at 2–5 of 6 ΗLA loci with the patient. Despite the HLA disparity, infusions were well-tolerated without associated acute reactions, adverse effect on the transplanted organ or GvHD. Sixty-four percent of patients responded to treatment and 12 of 33 (36%) had a complete response. The investigators found an association between the number of matches and patient outcome. TCR spectratyping for in vivo tracking of the infused VSTs showed either a gradual increase in the peak signal with each infusion or a maximum signal, 4 or 24 hours after each infusion. The cells were detected until 7 days postinfusion. These results were recapitulated by several other groups.68–70,73 In a most recent study by the Memorial Sloan Kettering group, 33 HCT- and 13 SOT-individuals with rituximab-refractory PTLD, received 3 weekly VST infusions, with minimum 2 HLA allele-matching, followed by a 3-week observational period. The infusions were well-tolerated, but additional cycles were required for response (68% and 54% in the HCT and SOT recipients). In contrast to the study by Haque et al, this study showed no correlation between HLA matching and response.68
The use of multitargeting, off-the-shelf VSTs was first investigated by the Baylor group to treat patients with persistent CMV (n = 19), EBV (n = 18), and AdV (n = 9) infections, reaching objective response rates 74%, 79%, and 67%, respectively.83 The same group later reported the generation of a pentavalent product additionally targeting BKV and HHV6. The cells were administered to 38 HCT recipients with drug refractory CMV (n = 17), EBV (n = 2), AdV (n = 7), BKV (n = 16), and HHV6 (n = 3) infections. While VST products and patients matched at 1–7 HLA alleles, there were only 3 cases of grade-I, de novo GvHD. Ninety-two percent of patients responded to therapy, including patients with viral diseases; EBV-PTLD (n = 1), CMV colitis (n = 2), encephalitis (n = 1), AdV enteritis (n = 11), respiratory tract infection (n = 2), HHV6 encephalitis (n = 1), BKV-hemorrhagic cystitis (n = 14), and nephritis (n = 2). VST responses were confirmed to be of third-party donor origin in 11 cases and shown to persist in vivo for up to 12 weeks postinfusion.87
To facilitate the establishment of third-party cell banks and make pSTs broadly available, investigators sought to use donors with HLA types found at high frequency in the respective population (Figure 1). Given the multiple doses generated per each donor cell production, such banks established from as little as 25–30 carefully selected donors, could supply VSTs to all patients in need for urgent use based on HLA matching and provided 80% and 93% overall responses in refractory infections.69,154
More recently, the Baylor group generated a bank of CMV-STs from just 8 healthy donors. Donors were carefully selected based on an algorithm that compared 666 HCT recipient HLA types with the HLA types of a pool of diverse, healthy donors. This minibank was sufficient to provide VST matching at ≥2 HLA antigens to 28 of 29 patients referred.66 In addition, using a scale-up manufacturing protocol, by starting with a unit of blood it was feasible to generate >2000 VST doses for infusion.
As adoptive therapy with either third-party VSTs or transplant donor VSTs seems to trigger comparable antiviral responses that might be mediated by restoration of endogenous virus-specific immunity,155 off-the-shelf cell products are attracting increased interest. Currently, ElevateBio and Allovir biotech companies have joined forces to explore the use of off-the-shelf multi-VSTs as prophylaxis or treatment of CMV, AdV, EBV, BKV or HHV6 viral infections post-HCT (phase-2 or -3 trials, NCT04693637, NCT05305040, NCT05179057), as treatment of BKV infection post kidney transplant (phase-2, NCT04605484), as treatment of BKV-associated hemorrhagic cystitis post-HCT (phase-3, NCT04390113), or as treatment of respiratory viral infections from community acquired respiratory viruses (RSV, influenza, hMPV, and/or PIV) post-HCT (phase I/II NCT04933968).
An attractive alternative cell source for adoptive immunotherapy, especially as third-party, off-the-shelf approach, are the unconventional γδ T cells, an early innate arm of the immune system and key players in the fight against viruses. Their unique property of major histocompatibility complex (MHC)-independent recognition of their targets allows effective antitumor and antiviral control without GvHD. Their potential has mainly been explored within the context of cancer treatment, using either unmanipulated or genetically engineered γδ T cells by the introduction of αβ TCRs, chimeric antigen receptors (CARs), and drug-resistance genes.156,157 Gamma-delta T cells represent also an interesting treatment option for viral reactivations, yet overall understudied as a platform for antiviral immunotherapy.
Pathogen-naive donors
pSTs can only be expanded from donors who have previously been exposed to pathogens thus having already shaped their immunological memory; given this, pST generation from antigen-seronegative donors, often CBU or younger donors, becomes highly challenging. Beyond the aforementioned third-party pST banks, several other strategies have also been employed to tackle this problem. First, by ex vivo priming of T cells, where T cells from pathogen-naive sources are ex vivo “educated” to recognize the targeted antigen(s) by priming and repeated stimulations with APCs in the presence of various cytokines.139,142,158–161 First results of the safety and efficacy profile of CBU-derived tri-VSTs look promising.52 A second approach is the donor vaccination. The rationale lies in the establishment of specific immunity by vaccination of a seronegative donor and subsequent generation and adoptive transfer of pSTs to the transplant recipient in cases of refractory and persistent viremia posttransplant.162 CMV-pp65 specific CTLs ex vivo expanded from a vaccinated CMV-sero-negative stem cell donor (Towne strain CMV vaccine) were administered in a haploidentical HCT recipient with a refractory CMV disease; CMV DNA decreased after the CTL infusion while CMV-specific cytotoxicity increased.162 However, this strategy has the limitations of usually not timely identification before transplantation of the most appropriate donor to be vaccinated early enough to develop immunological memory, the lack of effective vaccines against the commonest pathogens posttransplant and the logistical, ethical, and medical concerns on donor vaccination for the benefit of the recipient.163 A third approach to overcome the “pathogen-naive” donor issue is to generate genetically engineered pSTs with a transgenic TCR to redirect patient’s (autologous) or seronegative donor’s T cells against a desirable pathogen164–175 (Figure 1; Table 3).
Table 3.
Genetically Modified pSTs
| Advanced Therapy Medicinal Product | Method | Reference | |
|---|---|---|---|
| TCR-redirected | TCR-transgenic single-targeting VSTs (CMV-STs) | Vector-mediated knock in of a transgenic CMV TCR | Shub et al169 |
| TCR-transgenic single-targeting VSTs (CMV-STs) | Vector-mediated knock in of a transgenic CMV TCR | Wang et al168 | |
| TCR-transgenic single-targeting VSTs (HBV-STs) | Vector-mediated knock in of a transgenic HBV TCR | Gehring et al174 | |
| TCR-transgenic single-targeting VSTs (HBV-STs) | Knock in of a transgenic HBV TCR by electroporation | Meng et al172 | |
| TCR-transgenic single-targeting VSTs (HBV-STs) | Knock in of a transgenic HBV TCR | Qasim et al170 | |
| TCR-transgenic single-targeting VSTs (HCV-STs) | Vector-mediated knock in of a transgenic HCV TCR | Zhang et al164 | |
| TCR-transgenic single-targeting VSTs (HIV-STs) | Vector-mediated knock in of a transgenic HIV TCR | Varela-Rohena et al167 | |
| TCR-transgenic single-targeting VSTs (HIV-STs) | Vector-mediated knock in of a transgenic HIV TCR | Joseph et al166 | |
| TCR-transgenic single-targeting VSTs (HPV-STs) | Vector-mediated knock in of a transgenic HPV TCR | Doran et al175 | |
| TCR-transgenic single-targeting VSTs (HPV-STs) | Vector-mediated knock in of a transgenic HPV TCR | Jin et al173 | |
| TCR-transgenic single-targeting VSTs (HPV-STs) | Vector-mediated knock in of a transgenic HPV TCR | Nagarsheth et al171 | |
| TCR-transgenic single-targeting VSTs (EBV-STs) | Vector-mediated knock in of a transgenic EBV TCR | Zhang et al176 | |
| Drug-resistant | Tacrolimus-resistant single-targeting VSTs (EBV-STs) | FKBP12 knock down using small interfering RNA (siRNA) | De Angelis et al177 |
| Calcineurin inhibitors-resistant single-targeting VSTs (EBV-STs) | Retroviral transduction with a calcineurin mutant | Brewin et al178 | |
| Calcineurin inhibitors-resistant single-targeting VSTs (EBV-STs) | IFN-γ secreting EBV-CTLs and retroviral transduction with a calcineurin mutant | Richiardeli et al179 | |
| Glucocorticoid-resistant single-targeting VSTs (CMV-STs) | TALEN-mediated glucocorticoid receptor inactivation | Menger et al180 | |
| Glucocorticoid-resistant single-targeting VSTs (CMV-STs) | CRISPR-mediated glucocorticoid receptor inactivation | Kaeufferle et al181 | |
| Glucocorticoid-resistant multitargeting VSTs (CMV-, BKV-, AdV-STs) | CRISPR-mediated glucocorticoid receptor inactivation | Basar et al182 | |
| Glucocorticoid-resistant multitargeting pSTs (CMV-, EBV-, AdV-, BKV-, AF-STs) | CRISPR-mediated glucocorticoid receptor inactivation | Koukoulias et al183 | |
| Tacrolimus-resistant single-targeting VSTs (CMV-STs) | CRISPR-mediated FKBP12 knock out | Amini et al184 | |
| Tacrolimus and MMF- transiently resistant TCR-transgenic single-targeting VSTs (HBV-STs or EBV-STs) | Electroporation of HBV-TCR or EBV-TCR mRNA and siRNA to knockdown FKPB1A | Hafezi et al185 | |
| Glucocorticoid-resistant single-targeting VSTs (CoV-2-STs) | CRISPR-mediated GR inactivation | Basar et al186 | |
| Tacrolimus-resistant single-targeting VSTs (CoV-2-STs) | CRISPR-mediated FKBP12 knock | Peter et al187 | |
| CAR-Ts specific for pathogens | AF-CAR-Ts | T cells redirected through CAR toward carbohydrate | Kumaresan et al188 |
| CMV-CAR-Ts | T cells redirected through CAR toward CMV glycoprotein B | Full et al189 | |
| CMV-CAR-Ts | T cells redirected through CAR toward several proteins of CMV | Ali et al190 | |
| EBV-CAR-Ts | T cells redirected through CAR toward EBV LMP1 | Tang et al191 | |
| HBV-CAR-Ts | T cells redirected through CAR toward HBV S or L protein | Bohne et al192 | |
| HBV-CAR-Ts | T cells redirected through CAR toward HBV S protein | Krebs et al193 | |
| HCV-CAR-Ts | T cells redirected through CAR toward HCV E2 glycoprotein | Sautto et al194 | |
| HIV-CAR-Ts | HIV-specific T cells | Walker et al195 | |
| HIV-CAR-Ts | T cells redirected through CAR toward HIV env | Mitsuyasu et al196 | |
| HIV-CAR-Ts | T cells redirected through CAR toward HIVgp120 | Deeks et al197 | |
| HIV-CAR-Ts | HIV-resistant T cells redirected through CAR toward HIV | Hale et al198 | |
| HIV-CAR-Ts | Broadly neutralizing antibody-derived (bNAb-derived) CAR T-cell therapy | Liu et al199 | |
| HIV-CAR-Ts | Multi-specific anti-HIV duoCARs | Anthony-Gonda et al200 | |
| HIV-CAR-Ts | Re-engineered CD4-based | Leibman et al201 | |
| pSTs coupled with a CAR | EBV-STs armed with CAR-Ts | Proof of principle for ex vivo expanded EBV-STS armed with a 1st generation GD2 (disialoganglioside)-CAR | Rossig et al202 |
| EBV-STs armed with CAR-Ts | Ex vivo expanded EBV-STS armed with a 1st generation CD30-CAR | Savoldo et al203 | |
| Patient-derived tri-VSTs armed with CAR-Ts | Ex vivo expanded tri-VSTs (EBV-, CMV-, AdV-STs) armed with a 1st generation GD2 (disialoganglioside)-CAR | Pule et al204 | |
| HCT donor-derived tri-VSTs armed with CAR-Ts | Ex vivo expanded tri-VSTs (EBV-, CMV-, AdV-STs) armed with a 2nd generation CD19-CAR | Cruz et al205 | |
| tri-VSTs armed with CAR-Ts | Ex vivo expanded tri-VSTs (EBV-, CMV-, AdV-STs) armed with a 1st generation GD2 (disialoganglioside)-CAR | Sun et al206 | |
| CMV-STs armed with CAR-Ts | IFN-γ capture-enriched CMV-STs armed with a 2nd generation CD19-CAR and combined with a CMV vaccine | Wang et al207 | |
| HCT donor-EBV-STs armed with CAR-Ts | Ex vivo expanded EBV-STS armed with a 1st generation CD30-CAR | Rossig et al208 | |
| HCT donor-derived tri-VSTs armed with CAR-Ts | Ex vivo expanded tri-VSTs (EBV-, CMV-, AdV-STs) armed with a 2nd generation CD19-CAR | Lapteva et al209 | |
| CMV-CD19 CAR-Ts | IFN-γ capture enriched CMV-STs armed with a 2nd generation CD19-CAR | Wang et al210 |
AdV = adenovirus; AF = Aspergillus fumigatus; BKV = BK virus; CAR-Ts = chimeric antigen receptor T cells; CMV = cytomegalovirus; EBV = Epstein-Barr virus; HHV6 = human herpesvirus 6; HIV = human immunodeficiency virus; hMPV = human metapneumovirus; HPV = human papillomavirus; HPIV3 = human parainfluenza virus-3; HSV-1 = herpes simplex virus type 1; IFN-γ = interferon-γ; MCPyV = Merkel cell polyomavirus; PIV = parainfluenza virus; RSV = respiratory syncytial virus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VZV = varicella zoster.
However, this approach entails several challenges, including (i) limited access to patients expressing specific HLAs; (ii) epitope restriction potentially increasing the risk of immune escape; (iii) risk for TCR mispairing between the endogenous and the transgenic TCR, mounting a potential harmful specificity211,212; and (iv) the absence of normal negative regulation, such as TCR downregulation in proportion to the strength of initial antigen recognition, stemming from the extrinsic promoter-driven expression of transgenic TCR.213 Orthotopic TCR replacement by a transgenic one may resolve some of the above issues and enable TCR-based therapies.214
HLA restriction
The HLA restriction is another brake to broader pSTs applicability, limiting the administration of pSTs to HLA-matched recipients. To overcome this hurdle, the MHC-independent chimeric antigen receptor T cells (CAR-Ts) have also been proposed against infections and infectious diseases. To date, few anti-infectious CARs against CMV,189,190 EBV,191 hepatitis B virus (HBV),25,192 HCV,194 and AF188 have been described (Figure 1; Table 3). These proof-of-principle studies suggest that the application of CAR-Ts might extend beyond cancer to both viral and fungal infections or even couple the antipathogen with the antitumor response by generating bi-specific T cells recognizing both a pathogen and a tumor marker (as discussed later below)202,203,210,215–217; yet further studies are required to overcome technical challenges, identify optimal surface proteins as targets, and improve safety and efficacy.
Sensitivity of pSTs to immunosuppressive drugs
In patients receiving high-dose and long-term immunosuppression, such as HCT patients with GvHD or SOT patients, who are in fact, the most vulnerable to opportunistic infections, pSTs become functionally impaired in vivo.218–222 This creates the paradox of precluding from the potential benefits of adoptive immunotherapy, the patients who are most likely to experience severe infections and are those with an unmet need for effective treatment of opportunistic infections.
To this end, several groups have been exploring mechanisms to genetically engineer pSTs and render them resistant to immunosuppressants (Table 3). Two teams have simultaneously introduced the generation of EBV-STs with resistance to calcineurin inhibitors (tacrolimus, FK506, or/and cyclosporin A), the most widely used immunosuppressive agents in the transplant setting, using either a specific small interference RNA (siRNA) to knock down the FK506-binding protein (FKBP12)177 or calcineurin mutants to disrupt its docking to calcineurin inhibitors.178,179 The latter approach is being currently evaluated in an open-label, nonrandomized, multicenter phase I trial assessing the safety of tacrolimus-resistant autologous EBV-STs in SOT patients with PTLD (NCT03131934).
The recent emergence of highly versatile genome-editing technologies has provided additional tools for rapid and affordable manufacturing of gene manipulated pSTs. In addition to FKBP12, the glucocorticoid receptor has also been targeted for inactivation by both transcription activator–like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9). We and others have generated either glucocorticoid-resistant single-targeting VSTs,180,181 multitargeting VSTs,182 multi-pSTs,183 or tacrolimus-resistant single-targeting VSTs184 (Table 3), being able to expand and actively function even in the presence of intense immunosuppression posttransplant. Clinical studies are underway to assess their safety and preliminary efficacy (NCT05101213).
Antigenic competition
Antigenic competition represents the major challenge for the generation of a single T-cell product targeting a broad spectrum of pathogens that arises from shared antigen-bearing APCs, as well as from the varying (high or low) frequencies of subsets of antigen-specific T cells in the starting PBMC population.223,224 Antigenic competition may favor the preferential outgrowth of high frequency VSTs (such as those directed against EBV and CMV antigens) over the low frequency antigen-specific T cells (such as those directed against AF and BKV), resulting in products dominated by responses to a single or a restricted repertoire of pathogens. Although in several reports, antigenic competition noticeably decreased the frequency of antigen-specific T cells in a multi-pST product as compared to single-targeting pSTs,50,144,146,225 other reports have shown that antigenic competition could be mitigated by the direct stimulation of PBMCs with pepmixes and culture conditions.13,53,57 Indeed, multivalent cell products simultaneously targeting a broad spectrum of pathogens (up to 7)58 could expand in vivo, in response to viral challenge and produce clinically beneficial effects against multiple pathogens (Table 1). However, the number of antigens to be targeted by a single-cell product could not be unlimited and should be determined on the basis of the clinical need, balancing between a broad, as possible, coverage against pathogens, manufacturing costs, and possible antigenic competition.
pSTs as prophylaxis or treatment?
Recently, letermovir as primary prophylaxis to prevent clinically significant CMV infection in HCT seropositive patients has been associated with significant clinical benefit, leading to decreased clinically significant CMV reactivation both in a phase III trial226 as well as in the real world.227 Therefore, prophylactic administration of pSTs for CMV reactivation prevention may not be necessary. However, mutations in the genes encoding for the CMV terminase complex may confer resistance of the virus to letermovir, and in this context, VSTs remain important in limiting drug-refractory CMV disease. Moreover, letermovir prophylaxis may be associated with impaired CMV-specific immunity.228 In patients who lack effective CMV-specific T-cell immunity, delayed-onset CMV infection has been shown to occur at higher frequency compared to patients with reactivation following letermovir229; thus, there may be room for pST therapy in those patients. As far as other dsDNA viruses are concerned (ie, AdV, EBV, HHV6, BKV, JC virus), there are limited if any, antiviral drugs available. Current medical prophylaxis regimens are ineffective at preventing viral reactivation and often associated with toxicities. Therefore, the prophylactic administration of pSTs against these viruses in high-risk patients early post-HCT, remains rational. However, given as treatment, pSTs may be more cost-effective as some patients may not develop infections or acute GvHD requiring high-dose steroids may occur before infection, thus abrogating the pSTs potential.
EXTENDING THE USE OF PSTS BEYOND THE TRANSPLANT SETTING
The compelling results on the management of viral complications posttransplant with adoptive immunotherapy has stimulated research with the use of pSTs in other immunocompromised patient populations, outside the context of transplantation.
Less-immunogenic EBV-associated diseases
The impressive results of EBV-STs in type II latency tumors posttransplant, has attracted researchers’ interest to the evaluation of adoptive transfer of EBV-STs as treatment of less-immunogenic EBV-related malignancies, occurring outside the transplantation context. Despite the low immunogenicity of the targeted antigens, autologous EBV-STs, either nonengineered230–232 or genetically modified with a dominant-negative TGF-β receptor to become resistant to otherwise inhibitory concentrations of TGF-β in the tumor microenvironment,233 could be generated from patients with type 2 latency tumors. Upon administration, EBV-STs elicited objective clinical benefit in patients with Hodgkin- and non-Hodgkin lymphomas230–233 or nasopharyngeal carcinoma (NPC)234–237 without major toxicities.
EBV-STs have also been proposed as an alternative cell-based approach for the management of multiple sclerosis (MS). Indeed, EBV has been very recently proposed as the leading cause of MS.238 Preliminary results from a phase I study have reported sustained clinical improvement in 7 of 10 patients with MS treated with autologous EBV-STs, without experiencing any serious adverse event, including disease exacerbation.239,240 Therefore, an academic phase-I (Nantes University Hospital) and a commercial phase-I/II (Atara Biotherapeutics: product ATA188) clinical trial, assessing the safety and feasibility or/and efficacy of adoptive cell therapy with autologous or allogeneic EBV-STs in patients with a first clinical episode highly suggestive of MS or progressive MS, respectively, are currently running (NCT02912897 and NCT03283826).
Primary immunodeficiency disorders
Patients with primary immunodeficiency disorders (PIDs), a group of congenital defects of immunity such as severe combined immunodeficiency (SCID), are susceptible to opportunistic infections frequently caused by common pathogens such as CMV, EBV, AdV, VZV, and respiratory viruses, even before HCT.241,242 Theoretically, in this patient population, pSTs could serve as a bridging therapy before HCT. So far, only 6 of 63 patients with PID treated with pST therapy, received the cell products before HCT.243 Notwithstanding the very small number of patients, this study provided the first evidence that individuals with PID could benefit, albeit at higher doses, from adoptive immunotherapy with pSTs, and proceed to transplant expeditiously.
HPV-associated cancer
In a similar context, Hinrichs’s group developed autologous genetically engineered TCR-Ts directed against the HPV-E6175, and recently -E7 antigen,171,173, demonstrating robust tumor regression and objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease in a first-in-human phase I trial in collaboration with Kite-Gilead. E7 TCR-T cells are currently being tested in the ph-II arm of that trial (NCT02858310) in patients with metastatic HPV-associated epithelial cancers. In an academic setting, the Baylor group is clinically investigating the safety and efficacy of patient-derived HPV-STs, which are being ex vivo expanded to target both the E6- and E7- antigens through their native TCR, in patients with relapsed HPV-associated cancers. HPV-STs are also genetically modified to become TGF-beta resistant and combined with the immune-checkpoint inhibitor Nivolumab, to reduce antigen escape-mediated relapses and T-cell exhaustion (NCT02379520).
Merkel cell carcinoma
There are limited data on adoptive transfer of VSTs in Merkel cell carcinoma (MCC), an aggressive skin cancer, typically caused by the Merkel cell polyomavirus (MCPyV). Chapuis’s group first observed a complete response in 2 of 3 metastatic lesions of a 67-year-old man with metastatic MCPyV-expressing MCC who was treated with autologous ex vivo expanded, HLA-A*2402-epitope-restricted specific T cells (MCPyV-STs).88 They later treated 2 more patients with autologous HLA B*3502- and HLA-A*0201-epitope-restricted MCPyV-STs.89 Notwithstanding the observed tumor lesion regressions, both patients relapsed, thus confirming the risk of tumor escape from single-epitope-targeting T cells. In the gene-engineered T-cell field, Bluebird bio and Fred Hutchinson Cancer Research Center are actively recruiting patients with metastatic or unresectable MCPyV-associated MCC to be treated with autologous A2-MCC1-expressing TCR-Ts, specific for an HLA-A02-restricted viral oncoprotein (NCT03747484).
HBV-related hepatocellular carcinoma
The proof-of-concept of using TCR-Ts redirected to target HBV antigen was assessed in a patient who developed hepatocellular carcinoma (HCC) relapse 10 years following liver transplantation for HBV+ HCC.170 A single infusion of only 104 per kg HBsAg-specific TCR-Ts led to a reduction of serum HBsAg levels by >90% within 30 days, without any detectable on/off-target toxicities. Importantly, the risk of inducing a severe liver injury due to on-target, off-tumor lysis of normal infected hepatocytes was minimized in this patient in whom HBsAg was exclusively produced by HCC extrahepatic metastasis and not by the transplanted liver; yet a possibility that it should always be taken into consideration. Lion TCR biotechnology company reported the first results of this approach on 8 patients who had not met the criteria for liver transplantation, showing a reduction or stabilization of circulating HBsAg and HBV DNA levels in most of the patients after HBV-TCR-T-cell infusion,172 while post-liver transplantation data are still pending (NCT02719782). Importantly, immunosuppressive drug-resistant HBV-TCR-Ts have been recently developed for immune therapy of HCC in liver transplant patients.185
Progressive multifocal leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is a devastating and generally fatal neurologic disease occurring in profoundly immunocompromised patients due to brain infection with JC polyomavirus and lacking effective treatment, unless cellular immunity is restored.244 As BKV and JC virus are closely related and share several immunogenic proteins, adoptive transfer of allogeneic JC-STs or BKV-STs has also been explored as treatment of PML.49,65,76–78,86 Preliminary, albeit very promising, results of those trials demonstrated safety of the infusion of partially matched VSTs as well as alleviation of clinical symptoms, progressive decline of viral load in cerebrospinal fluid and an unprecedented survival beyond 1 year in a number of PML patients with poor expectation for survival.49,65,76–78,86
Human immunodeficiency virus
Although antiretroviral therapy (ART) is successfully used to suppress HIV-1 replication, viral latency is responsible for the persistence of a reservoir of infected CD4+ T cells which lead to systemic viremia should ART is ceased, thus constituting major barrier to HIV cure.245 Functional HIV-specific T cells (HIV-STs), both natural91–96,128,129,246 or genetically redirected,166,167,194–200 have been manufactured from HIV+ or HIV-seronegative donors (Table 3) and are currently being evaluated in early-stage immunotherapy trials as a strategy to eradicate the HIV reservoir and prevent HIV rebound.
Coronavirus disease
The urgency of the pandemic has prompted scientists from different fields (pharmaceuticals, vaccines, cellular therapies) to address the problem. Leveraging the VST platform has been recently proposed as treatment against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated life-threatening coronavirus disease (COVID-19)80,132–138 (Table 2). In the clinical setting, 7 groups including ours, have been evaluating off-the-shelf SARS-CoV-2-specific T cells (CoV-2-STs) derived from convalescent donors as treatment for high-risk COVID-19 patients (EudraCT:2021-001022-22/NCT05447013, NCT04351659/NCT04457726, NCT04578210, NCT04762186, NCT04896606, NCT04742595, NCT04765449), whereas 1 group uses stem cell donor-derived CoV-2-STs as prophylaxis against SARS-CoV-2 in individuals receiving allo-HCT (NCT05141058). Thus far, the Madrid team has published the phase I results showing that infusion of memory T cells from convalescent donors was safe at all tested doses and resulted in lymphocyte recovery 2 weeks postinfusion in 9 treated patients.247 Repeated off-the-shelf CoV-2-ST infusions (Allovir’s product ALVR109) also resulted in clinical and virologic improvement when administered to treat refractory severe COVID-19 pneumonia in a heart transplant patient.81 Our group assessed in a randomized 2:1 phase I/II trial, the safety and efficacy of the administration of ex vivo expanded, partially HLA-matched CoV-2-STs in addition to standard of care (SoC) in 57 patients with severe COVID-19 as compared to 30 patients who received SoC-only (control arm) (NCT05447013). The add-on treatment with CoV-2-STs was well tolerated and increased the likelihood of recovery by day 30, shortened the time to recovery and lowered by 51% the risk of mortality than SoC-alone, suggesting that the off-the-shelf immunotherapy with CoV-2-STs can serve as a safe and effective treatment in a real-world environment for severe COVID-19.79
To optimize CoV-2-ST performance in the presence of immunosuppressants which are used for the management of severe COVID-19, genetically modified CoV-2-STs resistant to glucocorticoids186 or tacrolimus187 have also been proposed (Table 1).
UNLEASHING THE POTENTIAL OF PSTS
CAR-Ts have achieved unprecedented success in patients with previously incurable relapsed/refractory B-cell malignancies. Nonetheless, their poor persistence attributed to the frail fitness of patient-derived T cells, the phenotype (proportion of CD4+ versus CD8+ T cells, less memory-like cell phenotype), and the exhaustion due to sustained antigenic stimulation in vivo, represents the main reason for antigen-positive relapse and a major limitation.248–251
Unlike CAR-Ts, pSTs have been shown to persist for up to 9 years.9 The remarkable difference in their lifespan mainly stems from the fact that pSTs are nonengineered cells “per se” and as “natural” pSTs, their transmitting signaling via native TCR involved in cognate antigen recognition and T-cell activation, remains unadulterated. In contrast, artificial CARs result in an abnormal or rewired TCR signaling252,253 as well as an unconstrained ligand-independent constitutive signaling, known as tonic signaling,254 being incriminated in the exhaustion, poor persistence or the toxicities associated with CAR-Ts255. Indeed, CD19-CAR-T cells with disrupted endogenous TCR, although similarly functional to TCR+/CAR-Ts, presented only limited persistence in vivo.256 Another important factor on the difference in persistence between the engineered and nonengineered, antigen-specific T-cell products, is the starting source of T cells; healthy donor- and quiescent memory pool-derived pSTs have a clear intrinsic survival advantage over CAR-Ts, which mainly derive from lymphopenic, usually heavily pretreated patients and contain higher frequencies of effector memory cells. Numerous studies have shown that T-cell products enriched for early lineage cells expand better and may delay CAR T-cell exhaustion, leading to improved persistence of the CAR-Ts.251
To overcome the significant hindrance of limited CAR-T function and persistence, the Baylor group proposed the introduction of CARs (CD30-, GD2- or CD19-CAR) into EBV- or tri-VSTs (either patient- or HCT donor-derived), as a platform to generate CAR-equipped pSTs202–205,209,257 (Table 3) presenting the ability to expand in the presence of viral antigens through their native TCR, while producing some objective clinical responses through CAR204,209,258 or/and after boosting the antitumor activity with vaccination.207,210 However, the latter was not confirmed in a clinical trial with 11 pediatric patients with relapsed acute lymphoblastic leukemia who received EBV-STs bearing CD19-CAR and were boosted with vaccination; despite the enhanced persistence of adoptively transferred T cells in vivo, their expansion was poor and antileukemic activity limited.208 Nonetheless, it has become clear that the transfer of pSTs coupled with a CAR is safe, without inducing GvHD, cytokine release syndrome or neurotoxicity, thus confirming the more “physiological” function of nonengineered pSTs. Importantly, the minimized risk for alloreactivity, associated with native TCR engagement to latent virus antigens, makes the dual, specific TCR- and CAR- T cells ideal products for off-the-shelf therapy with CAR-Ts. To date, ongoing clinical trials assess the safety and efficacy of autologous dual TCR- and CAR-specific T cells with (NCT01192464, NCT00709033) or without vaccination boosting (NCT05432635) or allogeneic dual TCR/CAR-specific T cells (NCT01430390, NCT04288726). In parallel, Atara Bio expects IND for off-the-shelf, allogeneic CD19-CAR EBV-STs (product A3219) in the last quarter of 2022. If proved safe and effective, this platform will improve longevity of remissions after CAR-T-cell therapy in the future.
CONCLUDING REMARKS
The ability of adoptive immunotherapy to restore antiviral immunity and clear viral infections as a “living drug” platform, has been unequivocally shown and over the last three decades, the manufacture and clinical application of antigen-specific T cells has greatly evolved. The high and durable responses of pathogen-specific T cells, the broad coverage of antiviral spectrum, the good safety profile, the fast manufacturing, the increasing applicability due to third-party cell banks, have introduced a new era in the management of opportunistic infections. pSTs, eventually become part of the conventional antiviral treatment, falling under ECIL’s recommendations as regards EBV-PTLD and refractory CMV infection or disease post-HCT. Nevertheless, for cellular therapies, the strength of recommendation in combination with the level of evidence does not exceed level BIIu in refractory CMV disease259 or BIII as the second-line therapy for EBV-PTLD,260 implicating that such approaches, albeit a major breakthrough, have not yet become a standard treatment. This could be attributed to the fact that the majority of clinical trials conducted so far were early phase clinical trials, under academic grant funding, including in their majority limited number of patients. However, the progress made the last decade on simplifying the manufacturing process and speeding up the production along with the extended applications beyond the transplant setting, have attracted the interest of biotech and pharmaceutical companies and paved the way for late phase, controlled, prospective clinical trials studying the efficacy of adoptive immunotherapy. The results from Trace (“TRansfer of Adenovirus, Cytomegalovirus and Epstein-Barr virus-specific T cells”—NCT04832607), the first, multinational, ph-III trial, sponsored by the European Commission, and other nonrandomized (NCT03394365) or randomized ph-III trials sponsored by Atara Biotherapeutics, Allovir (NCT05305040, NCT04390113, NCT05179057) and Tessa Therapeutics (NCT02578641) are expected to generate strong clinical evidence on safety and efficacy of adoptive immunotherapy and enable the industrial investment and ultimately, regulatory approval. To facilitate academic medicine developers targeting an unmet medical need to optimize their development plans, European Medicines Agency (EMA) has recently launched the “PRIME” project, offering early and proactive support at improving clinical trial designs and enabling accelerated assessment of medicines application (https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines).
By the future incorporation of adoptive transfer of pSTs into clinical practice and acquisition of real world data, the adoptive transfer of pSTs may obtain a higher level of recommendation and evidence in the treatment’s guidelines. Moreover, patient risk stratification by monitoring the pathogen-specific immune reconstitution posttransplant by functional assays can guide the clinical management of patients at high-risk for morbidity and mortality from opportunistic reactivations and select the best candidates for pathogen-specific immunotherapy.261 Adoptive immunotherapy with pSTs may ultimately become a standard treatment for opportunistic infections post-HCT while serve as a platform for targeting novel emerging pathogens in the future.
AUTHOR CONTRIBUTIONS
Conceptualization, writing, review and editing, AP, MA, GK, IT and EY.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
Funding for this project was provided by the T2EDK-02437 Research, Technology Development and Innovation (RTDI) State Aid Action “RESEARCH - CREATE -INNOVATE.”
Footnotes
Anastasia Papadopoulou and Maria Alvanou contributed equally.
REFERENCES
- 1.Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36:757–769. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Kurosawa S, Tajima K, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51:553–559. [DOI] [PubMed] [Google Scholar]
- 3.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young JAH, Logan BR, Wu J, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. [DOI] [PubMed] [Google Scholar]
- 6.Leen AM, Heslop HE, Brenner MK. Antiviral T-cell therapy. Immunol Rev. 2014;258:12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125:4103–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet (London, England). 1995;345:9–13. [DOI] [PubMed] [Google Scholar]
- 9.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CA, Ng CYC, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995;4:73–79. [DOI] [PubMed] [Google Scholar]
- 11.Knabel M, Franz TJ, Schiemann M, et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8:631–637. [DOI] [PubMed] [Google Scholar]
- 12.Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52:49–57. [DOI] [PubMed] [Google Scholar]
- 13.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. [DOI] [PubMed] [Google Scholar]
- 15.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. [DOI] [PubMed] [Google Scholar]
- 16.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. [DOI] [PubMed] [Google Scholar]
- 18.Peggs KS, Verfuerth S, Pizzey A, et al. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis. 2009;49:1851–1860. [DOI] [PubMed] [Google Scholar]
- 19.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. [DOI] [PubMed] [Google Scholar]
- 20.Ip W, Silva JMF, Gaspar H, et al. Multicenter phase 1/2 application of adenovirus-specific T cells in high-risk pediatric patients after allogeneic stem cell transplantation. Cytotherapy. 2018;20:830–838. [DOI] [PubMed] [Google Scholar]
- 21.Feucht J, Opherk K, Lang P, et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125:1986–1994. [DOI] [PubMed] [Google Scholar]
- 22.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121:3745–3758. [DOI] [PubMed] [Google Scholar]
- 23.Pei X-Y, Zhao X-Y, Chang Y-J, et al. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis. 2017;216:945–956. [DOI] [PubMed] [Google Scholar]
- 24.Ma CKK, Clancy L, Simms R, et al. Adjuvant peptide pulsed dendritic cell vaccination in addition to t cell adoptive immunotherapy for cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2018;24:71–77. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective recon- stitution of streptamer-selected cytomegalovirus-specific CD8þ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–599. [DOI] [PubMed] [Google Scholar]
- 26.Stemberger C, Graef P, Odendahl M, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124:628–637. [DOI] [PubMed] [Google Scholar]
- 27.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:707–714. [DOI] [PubMed] [Google Scholar]
- 29.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. [DOI] [PubMed] [Google Scholar]
- 30.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. [DOI] [PubMed] [Google Scholar]
- 31.Bao L, Cowan MJ, Dunham K, et al. Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother. 2012;35:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meij P, Jedema I, Zandvliet ML, et al. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. J Immunother. 2012;35:621–628. [DOI] [PubMed] [Google Scholar]
- 33.Dong L, Gao Z-Y, Chang L-J, et al. Adoptive transfer of cytomegalovirus/Epstein-Barr virus-specific immune effector cells for therapeutic and preventive/preemptive treatment of pediatric allogeneic cell transplant recipients. J Pediatr Hematol Oncol. 2010;32:e31–e37. [DOI] [PubMed] [Google Scholar]
- 34.Creidy R, Moshous D, Touzot F, et al. Specific T cells for the treatment of cytomegalovirus and/or adenovirus in the context of hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2016;138:920–924.e3. [DOI] [PubMed] [Google Scholar]
- 35.Heslop HE, Sharma S, Rooney CM. Adoptive T-cell therapy for Epstein-Barr virus-related lymphomas. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. [DOI] [PubMed] [Google Scholar]
- 37.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 38.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 39.Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7:1648–1655. [DOI] [PubMed] [Google Scholar]
- 40.Comoli P, Basso S, Labirio M, et al. T cell therapy of Epstein–Barr virus and adenovirus infections after hemopoietic stem cell transplant. Blood Cells Mol Dis. 2008;40:68–70. [DOI] [PubMed] [Google Scholar]
- 41.Moosmann A, Bigalke I, Tischer J, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–2970. [DOI] [PubMed] [Google Scholar]
- 42.Icheva V, Kayser S, Wolff D, et al. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1–specific T cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2012;31:39–48. [DOI] [PubMed] [Google Scholar]
- 43.Imashuku S, Goto T, Matsumura T, et al. Unsuccessful CTL transfusion in a case of post-BMT Epstein–Barr virus-associated lymphoproliferative disorder (EBV-LPD). Bone Marrow Transplant. 1997;20:337–340. [DOI] [PubMed] [Google Scholar]
- 44.Feucht J, Opherk K, Lang P, et al. Adoptive T-cell therapy with hexon-speci fi c Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125:1986–1994. [DOI] [PubMed] [Google Scholar]
- 45.Geyeregger R, Freimüller C, Stemberger J, et al. First-in-man clinical results with good manufacturing practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother. 2014;37:245–249. [DOI] [PubMed] [Google Scholar]
- 46.Di Nardo M, Li Pira G, Amodeo A, et al. Adoptive immunotherapy with antigen-specific T cells during extracorporeal membrane oxygenation (ECMO) for adenovirus-related respiratory failure in a child given haploidentical stem cell transplantation. Pediatr Blood Cancer. 2014;61:376–379. [DOI] [PubMed] [Google Scholar]
- 47.Pello OM, Innes AJ, Bradshaw A, et al. BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur J Haematol. 2017;98:632–634. [DOI] [PubMed] [Google Scholar]
- 48.Balduzzi A, Lucchini G, Hirsch HH, et al. Polyomavirus JC-targeted T-cell therapy for progressive multiple leukoencephalopathy in a hematopoietic cell transplantation recipient. Bone Marrow Transplant. 2011;46:987–992. [DOI] [PubMed] [Google Scholar]
- 49.Steinhardt MJ, Wiercinska E, Pham M, et al. Progressive multifocal leukoencephalopathy in a patient post allo-HCT successfully treated with JC virus specific donor lymphocytes. J Transl Med. 2020;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. [DOI] [PubMed] [Google Scholar]
- 52.Abraham AA, John TD, Keller MD, et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients. Blood Adv. 2019;3:2057–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21:2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma CKK, Blyth E, Clancy L, et al. Addition of varicella zoster virus-specific T cells to cytomegalovirus, Epstein-Barr virus and adenovirus tri-specific T cells as adoptive immunotherapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Cytotherapy. 2015;17:1406–1420. [DOI] [PubMed] [Google Scholar]
- 55.Nelson AS, Heyenbruch D, Rubinstein JD, et al. Virus-specific T-cell therapy to treat BK polyomavirus infection in bone marrow and solid organ transplant recipients. Blood Adv. 2020;4:5745–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubinstein JD, Lutzko C, Leemhuis T, et al. Scheduled administration of virus-specific T cells for viral prophylaxis after pediatric allogeneic stem cell transplant. Blood Adv. 2022;6:2897–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6:242ra83–242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottlieb DJ, Clancy LE, Withers B, et al. Prophylactic antigen-specific T-cells targeting seven viral and fungal pathogens after allogeneic haemopoietic stem cell transplant. Clin Transl Immunol. 2021;10:e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuenhahn M, Albrecht J, Odendahl M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–2171. [DOI] [PubMed] [Google Scholar]
- 60.Koehne G, Hasan A, Doubrovina E, et al. Immunotherapy with donor T cells sensitized with overlapping pentadecapeptides for treatment of persistent cytomegalovirus infection or viremia. Biol Blood Marrow Transplant. 2015;21:1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. [DOI] [PubMed] [Google Scholar]
- 62.Uhlin M, Gertow J, Uzunel M, et al. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis. 2012;55:1064–1073. [DOI] [PubMed] [Google Scholar]
- 63.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qasim W, Derniame S, Gilmour K, et al. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol. 2011;154:150–153. [DOI] [PubMed] [Google Scholar]
- 65.Berzero G, Basso S, Stoppini L, et al. Adoptive transfer of JC virus-specific T lymphocytes for the treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;89:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzannou I, Watanabe A, Naik S, et al. “Mini” bank of only 8 donors supplies CMV-directed T cells to diverse recipients. Blood Adv. 2019;3:2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alonso L, Rudilla F, Gimeno R, et al. Successful treatment of post-transplant CMV meningoencephalitis with third-party CMV virus-specific T cells: lessons learned. Pediatr Transplant. 2019;23:e13584. [DOI] [PubMed] [Google Scholar]
- 68.Prockop S, Doubrovina E, Suser S, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest. 2020;130:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol. 2014;167:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallot G, Vollant S, Saïagh S, et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: Lessons from a phase I/II feasibility and safety study. J Immunother. 2014;37:170–179. [DOI] [PubMed] [Google Scholar]
- 71.Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. [DOI] [PubMed] [Google Scholar]
- 72.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. [DOI] [PubMed] [Google Scholar]
- 73.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jahan S, Scuderi C, Francis L, et al. T-cell adoptive immunotherapy for BK nephropathy in renal transplantation. Transpl Infect Dis an Off J Transplant Soc. 2020;22:e13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olson A, Lin R, Marin D, et al. Third-party BK virus-specific cytotoxic T lymphocyte therapy for hemorrhagic cystitis following allotransplantation. J Clin Oncol. 2021;39:2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopfner F, Möhn N, Eiz-Vesper B, et al. Allogeneic BK virus-specific T-cell treatment in 2 patients with progressive multifocal leukoencephalopathy. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK virus–specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379:1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortese I, Beck ES, Al-Louzi O, et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papadopoulou A, Karavalakis G, Papadopoulou E, et al. Immunotherapy: safety and efficacy of SARS-CoV-2-specific T cells as adoptive immunotherapy for high-risk COVID-19 patients: a phase I/II, randomized clinical trial. Cytotherapy. 2022;24:S37–S38. [Google Scholar]
- 80.Perez-Martinez A, Mora-Rillo M, Ferreras C, et al. Phase I dose-escalation single centre clinical trial to evaluate the safety of infusion of memory T cells as adoptive therapy in COVID-19 (RELEASE). E Clinical Medicine. 2021;39:101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martits-Chalangari K, Spak CW, Askar M, et al. ALVR109, an off-the-shelf partially HLA matched SARS-CoV-2-specific T cell therapy, to treat refractory severe COVID-19 pneumonia in a heart transplant patient: case report. Am J Transplant. 2022;22:1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kállay K, Kassa C, Réti M, et al. Early experience with CliniMACS prodigy CCS (IFN-gamma) system in selection of virus-specific T cells from third-party donors for pediatric patients with severe viral infections after hematopoietic stem cell transplantation. J Immunother. 2018;41:158–163. [DOI] [PubMed] [Google Scholar]
- 83.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Withers B, Blyth E, Clancy LE, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017;1:2193–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holland EM, Gonzalez C, Levy E, et al. Case report: fatal complications of BK virus-hemorrhagic cystitis and severe cytokine release syndrome following BK virus-specific T-cells. Front Immunol. 2021;12:801281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubinstein JD, Jodele S, Heyenbruch D, et al. Off-the-shelf third-party virus-specific T cell therapy to treat JC polyomavirus infection in hematopoietic stem cell transplantation recipients. Transplant Cell Ther. 2022;28:116.e1–116.e7. [DOI] [PubMed] [Google Scholar]
- 87.Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35:3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chapuis AG, Afanasiev OK, Iyer JG, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res. 2014;2:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paulson KG, Voillet V, McAfee MS, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun. 2018;9:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevanovic S, Helman SR, Wunderlich JR, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koenig S, Conley AJ, Brewah YA, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. [DOI] [PubMed] [Google Scholar]
- 92.Lieberman J, Skolnik PR, Parkerson GR, et al. Safety of autologous, ex vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte infusion in HIV-infected patients. Blood. 1997;90:2196–2206. [PubMed] [Google Scholar]
- 93.Brodie SJ, Lewinsohn DA, Patterson BK, et al. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. [DOI] [PubMed] [Google Scholar]
- 94.Tan R, Xu X, Ogg GS, et al. Rapid death of adoptively transferred T cells in acquired immunodeficiency syndrome. Blood. 1999;93:1506–1510. [PubMed] [Google Scholar]
- 95.Chapuis AG, Casper C, Kuntz S, et al. HIV-specific CD8+ T cells from HIV+ individuals receiving HAART can be expanded ex vivo to augment systemic and mucosal immunity in vivo. Blood. 2011;117:5391–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sung JA, Patel S, Clohosey ML, et al. HIV-specific, ex vivo expanded T cell therapy: feasibility, safety, and efficacy in ART-suppressed HIV-infected individuals. Mol Ther. 2018;26:2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramadan G, Konings S, Kurup VP, et al. Generation of Aspergillus- and CMV- specific T-cell responses using autologous fast DC. Cytotherapy. 2004;6:223–234. [DOI] [PubMed] [Google Scholar]
- 98.Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–2569. [DOI] [PubMed] [Google Scholar]
- 99.Zhu F, Ramadan G, Davies B, et al. Stimulation by means of dendritic cells followed by Epstein-Barr virus-transformed B cells as antigen-presenting cells is more efficient than dendritic cells alone in inducing Aspergillus f16-specific cytotoxic T cell responses. Clin Exp Immunol. 2008;151:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tramsen L, Koehl U, Tonn T, et al. Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy. Bone Marrow Transplant. 2009;43:13–19. [DOI] [PubMed] [Google Scholar]
- 101.Gaundar SS, Clancy L, Blyth E, et al. Robust polyfunctional T-helper 1 responses to multiple fungal antigens from a cell population generated using an environmental strain of Aspergillus fumigatus. Cytotherapy. 2012;14:1119–1130. [DOI] [PubMed] [Google Scholar]
- 102.Jolink H, Meijssen IC, Hagedoorn RS, et al. Characterization of the T-cell-mediated immune response against the Aspergillus fumigatus proteins Crf1 and catalase 1 in healthy individuals. J Infect Dis. 2013;208:847–856. [DOI] [PubMed] [Google Scholar]
- 103.Bacher P, Jochheim-Richter A, Mockel-Tenbrink N, et al. Clinical-scale isolation of the total Aspergillus fumigatus-reactive T-helper cell repertoire for adoptive transfer. Cytotherapy. 2015;17:1396–1405. [DOI] [PubMed] [Google Scholar]
- 104.Stuehler C, Nowakowska J, Bernardini C, et al. Multispecific Aspergillus T cells selected by CD137 or CD154 induce protective immune responses against the most relevant mold infections. J Infect Dis. 2015;211:1251–1261. [DOI] [PubMed] [Google Scholar]
- 105.Tramsen L, Beck O, Schuster FR, et al. Generation and characterization of anti-Candida T cells as potential immunotherapy in patients with Candida infection after allogeneic hematopoietic stem-cell transplant. J Infect Dis. 2007;196:485–492. [DOI] [PubMed] [Google Scholar]
- 106.Gottlieb D. Antifungal T cells-progress in manufacture and prospects for the clinic. Cytotherapy. 2015;17:1329–1331. [DOI] [PubMed] [Google Scholar]
- 107.Papadopoulou A, Kaloyannidis P, Yannaki E, et al. Adoptive transfer of Aspergillus-specific T cells as a novel anti-fungal therapy for hematopoietic stem cell transplant recipients: progress and challenges. Crit Rev Oncol Hematol. 2016;98:62–72. [DOI] [PubMed] [Google Scholar]
- 108.Blyth E, Clancy L, Simms R, et al. BK virus-specific T cells for use in cellular therapy show specificity to multiple antigens and polyfunctional cytokine responses. Transplantation. 2011;92:1077–1084. [DOI] [PubMed] [Google Scholar]
- 109.Wilhelm M, Kaur A, Wernli M, et al. BK Polyomavirus-specific CD8 T-Cell expansion in vitro using 27mer peptide antigens for developing adoptive T-Cell transfer and vaccination. J Infect Dis. 2021;223:1410–1422. [DOI] [PubMed] [Google Scholar]
- 110.Lamarche C, Orio J, Georges-Tobar V, et al. Clinical-scale rapid autologous BK virus-specific T cell line generation from kidney transplant recipients with active viremia for adoptive immunotherapy. Transplantation. 2017;101:2713–2721. [DOI] [PubMed] [Google Scholar]
- 111.Blyth E, Gaundar SS, Clancy L, et al. Clinical-grade varicella zoster virus-specific T cells produced for adoptive immunotherapy in hemopoietic stem cell transplant recipients. Cytotherapy. 2012;14:724–732. [DOI] [PubMed] [Google Scholar]
- 112.Gerdemann U, Keukens L, Keirnan JM, et al. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood. 2013;121:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaundar SS, Blyth E, Clancy L, et al. In vitro generation of influenza-specific polyfunctional CD4+ T cells suitable for adoptive immunotherapy. Cytotherapy. 2012;14:182–193. [DOI] [PubMed] [Google Scholar]
- 114.Harris KM, Horn SE, Grant ML, et al. T-cell therapeutics targeting human parainfluenza virus 3 are broadly epitope specific and are cross reactive with human parainfluenza virus 1. Front Immunol. 2020;11:2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McLaughlin LP, Lang H, Williams E, et al. Human parainfluenza virus-3 can be targeted by rapidly ex vivo expanded T lymphocytes. Cytotherapy. 2016;18:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tzannou I, Nicholas SK, Lulla P, et al. Immunologic profiling of human metapneumovirus for the development of targeted immunotherapy. J Infect Dis. 2017;216:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma CKK, Clancy L, Deo S, et al. Herpes simplex virus type 1 (HSV-1) specific T-cell generation from HLA-A1- and HLA-A2-positive donors for adoptive immunotherapy. Cytotherapy. 2017;19:107–118. [DOI] [PubMed] [Google Scholar]
- 118.Ramos CA, Narala N, Vyas GM, et al. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes for adoptive immunotherapy of HPV-associated malignancies. J Immunother. 2013;36:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Poelgeest MIE, Visconti VV, Aghai Z, et al. Potential use of lymph node-derived HPV-specific T cells for adoptive cell therapy of cervical cancer. Cancer Immunol Immunother. 2016;65:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCormack SE, Cruz CRY, Wright KE, et al. Human papilloma virus–specific T cells can be generated from naïve T cells for use as an immunotherapeutic strategy for immunocompromised patients. Cytotherapy. 2018;20:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanajiri RYO, Sani GM, Hanley PJ, et al. Generation of Zika virus–specific T cells from seropositive and virus-naïve donors for potential use as an autologous or “off-the-shelf” immunotherapeutic. Cytotherapy. 2019;21:840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanajiri R, Sani GM, Saunders D, et al. Generation of norovirus-specific T cells from human donors with extensive cross-reactivity to variant sequences: implications for immunotherapy. J Infect Dis. 2020;221:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Papadopoulou A, Alvanou M, Koukoulias K, et al. Clinical-scale production of Aspergillus-specific T cells for the treatment of invasive aspergillosis in the immunocompromised host. Bone Marrow Transplant. 2019;54:1963–1972. [DOI] [PubMed] [Google Scholar]
- 124.Deo SS, Virassamy B, Halliday C, et al. Stimulation with lysates of Aspergillus terreus, Candida krusei and Rhizopus oryzae maximizes cross-reactivity of anti-fungal T cells. Cytotherapy. 2016;18:65–79. [DOI] [PubMed] [Google Scholar]
- 125.Castillo P, Wright KE, Kontoyiannis DP, et al. A new method for reactivating and expanding T cells specific for Rhizopus oryzae. Mol Ther Methods Clin Dev. 2018;9:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schmidt S, Tramsen L, Perkhofer S, et al. Characterization of the cellular immune responses to Rhizopus oryzae with potential impact on immunotherapeutic strategies in hematopoietic stem cell transplantation. J Infect Dis. 2012;206:135–139. [DOI] [PubMed] [Google Scholar]
- 127.Patel S, Lang H, Sani G, et al. Mycobacteria-specific T cells may be expanded from healthy donors and are near absent in primary immunodeficiency disorders. Front Immunol. 2019;10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lam S, Sung J, Cruz C, et al. Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy. Mol Ther. 2015;23:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel S, Lam S, Cruz CR, et al. Functionally active HIV-specific T cells that target gag and nef can be expanded from virus-naive donors and target a range of viral epitopes: implications for a cure strategy after allogeneic HSCT. Biol Blood Marrow Transplant. 2016;22:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patel S, Hanajiri R, Grant M, et al. HIV-specific T cells can be generated against non-escaped T cell epitopes with a GMP-compliant manufacturing platform. Mol Ther Methods Clin Dev. 2019;16:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davies SI, Muranski P. T cell therapies for human polyomavirus diseases. Cytotherapy. 2017;19:1302–1316. [DOI] [PubMed] [Google Scholar]
- 132.Keller MD, Harris KM, Jensen-Wachspress MA, et al. SARS-CoV-2 specific T-cells are rapidly expanded for therapeutic use and target conserved regions of membrane protein. Blood. 2020;136:2905–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Papayanni P-G, Chasiotis D, Koukoulias K, et al. Vaccinated and convalescent donor–derived severe acute respiratory syndrome coronavirus 2–specific T cells as adoptive immunotherapy for high-risk coronavirus disease 2019 patients. Clin Infect Dis. 2021;73:ciab371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cooper RS, Fraser AR, Smith L, et al. Rapid GMP-compliant expansion of SARS-CoV-2-specific T cells from convalescent donors for use as an allogeneic cell therapy for COVID-19. Front Immunol. 2021;11:598402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim N, Lee JM, Oh EJ, et al. Off-the-shelf partial HLA matching SARS-CoV-2 antigen specific T cell therapy: a new possibility for COVID-19 treatment. Front Immunol. 2021;12:5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guerreiro M, Aguilar-Gallardo C, Montoro J, et al. Adoptive transfer of ex vivo expanded SARS-CoV-2-specific cytotoxic lymphocytes: a viable strategy for COVID-19 immunosuppressed patients? Transpl Infect Dis. 2021;23:e13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.García-Ríos E, Leivas A, Mancebo FJ, et al. Isolation of functional SARS-CoV-2 antigen-specific T-cells with specific viral cytotoxic activity for adoptive therapy of COVID-19. Biomedicines. 2022;10:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panikkar A, Lineburg KE, Raju J, et al. SARS-CoV-2-specific T cells generated for adoptive immunotherapy are capable of recognizing multiple SARS-CoV-2 variants. PLoS Pathog. 2022;18:e1010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hanley PJ, Cruz CRY, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanley PJ, Shaffer DR, Cruz CRY, et al. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein–Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011;13:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dasari V, Schuessler A, Smith C, et al. Prophylactic and therapeutic adenoviral vector-based multivirus-specific T-cellimmunotherapy for transplant patients. Mol Ther Methods Clin Dev. 2016;3:16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dave H, Luo M, Blaney JW, et al. Toward a rapid production of multivirus-specific T cells targeting BKV, adenovirus, CMV, and EBV from umbilical cord blood. Mol Ther Methods Clin Dev. 2017;5:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vasileiou S, Turney AM, Kuvalekar M, et al. Rapid generation of multivirus-specific T lymphocytes for the prevention and treatment of respiratory viral infections. Haematologica. 2020;105:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tramsen L, Schmidt S, Boenig H, et al. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy. 2013;15:344–351. [DOI] [PubMed] [Google Scholar]
- 145.Castellano-González G, McGuire HM, Luciani F, et al. Rapidly expanded partially HLA DRB1-matched fungus-specific T cells mediate in vitro and in vivo antifungal activity. Blood Adv. 2020;4:3443–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khanna N, Stuehler C, Conrad B, et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood. 2011;118:1121–1131. [DOI] [PubMed] [Google Scholar]
- 147.Papadopoulou A, Koukoulias K, Alvanou M, et al. Multipathogen-specific T cells against viral and fungal infections. Bone Marrow Transplant. 2021;156:1445–1448. [DOI] [PubMed] [Google Scholar]
- 148.Esquirol A, Pascual MJ, kwon M, et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant. 2021;56:2432–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. [DOI] [PubMed] [Google Scholar]
- 150.Lindsay J, Kerridge I, Wilcox L, et al. Infection-related mortality in adults and children undergoing allogeneic hematopoietic cell transplantation: an Australian registry report. Transplant Cell Ther. 2021;27:798.e1–798.e10. [DOI] [PubMed] [Google Scholar]
- 151.Linke C, Ehlert K, Ahlmann M, et al. Epidemiology, utilisation of healthcare resources and outcome of invasive fungal diseases following paediatric allogeneic haematopoietic stem cell transplantation. Mycoses. 2020;63:172–180. [DOI] [PubMed] [Google Scholar]
- 152.Bacher P, Hohnstein T, Beerbaum E, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. 2019;176:1340–1355.e15. [DOI] [PubMed] [Google Scholar]
- 153.Stuehler C, Khanna N, Bozza S, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood. 2011;117:5881–5891. [DOI] [PubMed] [Google Scholar]
- 154.Withers B, Clancy L, Burgess J, et al. Establishment and operation of a third-party virus-specific T cell bank within an allogeneic stem cell transplant program. Biol Blood Marrow Transplant. 2018;24:2433–2442. [DOI] [PubMed] [Google Scholar]
- 155.Pei XY, Liu XF, Zhao XY, et al. Comparable anti-CMV responses of transplant donor and third-party CMV-specific T cells for treatment of CMV infection after allogeneic stem cell transplantation. Cell Mol Immunol. 2022;19:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhat J, Placek K, Faissner S. Contemplating dichotomous nature of gamma delta T cells for immunotherapy. Front Immunol. 2022;13:2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Janssen A, van Diest E, Vyborova A, et al. The role of γδ T cells as a line of defense in viral infections after allogeneic stem cell transplantation: opportunities and challenges. Viruses. 2022;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Savoldo B, Cubbage ML, Durett AG, et al. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168:909–918. [DOI] [PubMed] [Google Scholar]
- 159.Popescu I, Macedo C, Zeevi A, et al. Ex vivo priming of naïve T cells into EBV-specific Th1/Tc1 effector cells by mature autologous DC loaded with apoptotic/necrotic LCL. Am J Transplant. 2003;3:1369–1377. [DOI] [PubMed] [Google Scholar]
- 160.Hanajiri R, Sani GM, Hanley PJ, et al. Generation of Zika virus-specific T cells from seropositive and virus-naïve donors for potential use as an autologous or “off-the-shelf” immunotherapeutic. Cytotherapy. 2019;21:840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Comoli P, Ginevri F, Maccario R, et al. Successful in vitro priming of EBV-specific CD8+ T cells endowed with strong cytotoxic function from T cells of EBV-seronegative children. Am J Transplant. 2006;6:2169–2176. [DOI] [PubMed] [Google Scholar]
- 162.Horn B, Bao L, Dunham K, et al. Infusion of cytomegalovirus specific cytotoxic T lymphocytes from a sero-negative donor can facilitate resolution of infection and immune reconstitution. Pediatr Infect Dis J. 2009;28:65–67. [DOI] [PubMed] [Google Scholar]
- 163.Harris AE, Styczynski J, Bodge M, et al. Pretransplant vaccinations in allogeneic stem cell transplantation donors and recipients: an often-missed opportunity for immunoprotection? Bone Marrow Transplant. 2015;50:899–903. [DOI] [PubMed] [Google Scholar]
- 164.Zhang Y, Liu Y, Moxley KM, et al. Transduction of human T cells with a novel T-cell receptor confers anti-HCV reactivity. PLoS Pathog. 2010;6:e1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang C, Tan Q, Li S, et al. Original research: induction of EBV latent membrane protein-2A (LMP2A)-specific T cells and construction of individualized TCR-engineered T cells for EBV-associated malignancies. J ImmunoTher Cancer. 2021;9:e002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Joseph A, Zheng JH, Follenzi A, et al. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo hiv-1-specific inhibitory activity. J Virol. 2008;82:3078–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang F, Xu Q, Zhuang Z, et al. A single-cell approach to engineer CD8+ T cells targeting cytomegalovirus. Cell Mol Immunol. 2020;18:1326–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schub A, Schuster IG, Hammerschmidt W, et al. CMV-specific TCR-transgenic T cells for Immunotherapy. J Immunol. 2009;183:6819–6830. [DOI] [PubMed] [Google Scholar]
- 170.Qasim W, Brunetto M, Gehring AJ, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486–491. [DOI] [PubMed] [Google Scholar]
- 171.Nagarsheth NB, Norberg SM, Sinkoe AL, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meng F, Zhao J, Tan AT, et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021;15:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jin BY, Campbell TE, Draper LM, et al. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight. 2018;3:e99488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gehring AJ, Xue SA, Ho ZZ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55:103–110. [DOI] [PubMed] [Google Scholar]
- 175.Doran SL, Stevanović S, Adhikary S, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37:2759–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang C, Tan Q, Li S, et al. Induction of EBV latent membrane protein-2A (LMP2A)-specific T cells and construction of individualized TCR-engineered T cells for EBV-associated malignancies. J ImmunoTher Cancer. 2021;9:e002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Angelis B, Dotti G, Quintarelli C, et al. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506). Blood. 2009;114:4784–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brewin J, Mancao C, Straathof K, et al. Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood. 2009;114:4792–4803. [DOI] [PubMed] [Google Scholar]
- 179.Ricciardelli I, Brewin J, Lugthart G, et al. Rapid generation of EBV-specific cytotoxic T lymphocytes resistant to calcineurin inhibitors for adoptive immunotherapy. Am J Transplant. 2013;13:3244–3252. [DOI] [PubMed] [Google Scholar]
- 180.Menger L, Gouble A, Marzolini MV, et al. TALEN-mediated genetic inactivation of the glucocorticoid receptor in cytomegalovirus-specific T cells. Blood. 2015;126:2781–2789. [DOI] [PubMed] [Google Scholar]
- 181.Kaeuferle T, Deisenberger L, Jablonowski L, et al. CRISPR-Cas9-mediated glucocorticoid resistance in virus-specific T cells for adoptive T cell therapy posttransplantation. Mol Ther. 2020;28:1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Basar R, Daher M, Uprety N, et al. Large-scale GMP-compliant CRISPR-Cas9 – mediated deletion of the glucocorticoid receptor in multivirus-speci fi c T cells. Blood Adv. 2020;4:3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koukoulias K, Papayanni P-G, Georgakopoulou A, et al. “Cerberus” T cells: a glucocorticoid-resistant, multi-pathogen specific T cell product to fight infections in severely immunocompromised patients. Front Immunol. 2021;11:608701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Amini L, Wagner DL, Rössler U, et al. CRISPR-Cas9-edited tacrolimus-resistant antiviral T cells for advanced adoptive immunotherapy in transplant recipients. Mol Ther. 2021;29:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hafezi M, Lin M, Chia A, et al. Immunosuppressive drug-resistant armored T-cell receptor T cells for immune therapy of HCC in liver transplant patients. Hepatology. 2021;74:200–213. [DOI] [PubMed] [Google Scholar]
- 186.Basar R, Uprety N, Ensley E, et al. Generation of glucocorticoid-resistant SARS-CoV-2 T cells for adoptive cell therapy. Cell Rep. 2021;36:109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peter L, Wendering DJ, Schlickeiser S, et al. Tacrolimus-resistant SARS-CoV-2-specific T cell products to prevent and treat severe COVID-19 in immunosuppressed patients. Mol Ther Methods Clin Dev. 2022;25:52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kumaresan PR, Manuri PR, Albert ND, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci USA. 2014;111:10660–10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Full F, Lehner M, Thonn V, et al. T cells engineered with a cytomegalovirus-specific chimeric immunoreceptor. J Virol. 2010;84:4083–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ali A, Chiuppesi F, Nguyen M, et al. Chimeric antigen receptors targeting human cytomegalovirus. J Infect Dis. 2020;222:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang X, Zhou Y, Li W, et al. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bohne F, Chmielewski M, Ebert G, et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134:239–247. [DOI] [PubMed] [Google Scholar]
- 193.Krebs K, Böttinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145:456–465. [DOI] [PubMed] [Google Scholar]
- 194.Sautto GA, Wisskirchen K, Clementi N, et al. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut. 2016;65:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Walker R, Bechtel C, Natarajan V, et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96:467–474. [PubMed] [Google Scholar]
- 196.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus–infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- 197.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5:788–797. [DOI] [PubMed] [Google Scholar]
- 198.Hale M, Mesojednik T, Romano Ibarra GS, et al. Engineering HIV-resistant, anti-HIV chimeric antigen receptor T cells. Mol Ther. 2017;25:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu B, Zhang W, Xia B, et al. Broadly neutralizing antibody–derived CAR T cells reduce viral reservoir in individuals infected with HIV-1. J Clin Invest. 2021;131:e150211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Anthony-Gonda K, Bardhi A, Ray A, et al. Multi-specific anti-HIV duoCAR-T cells display broad antiviral activity and potent elimination of HIV-infected cells in vivo. Sci Transl Med. 2019;11:eaav5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leibman RS, Richardson MW, Ellebrecht CT, et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 2017;13:e1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rossig C, Bollard CM, Nuchtern JG, et al. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. [DOI] [PubMed] [Google Scholar]
- 203.Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cruz CRY, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sun Y, Meng F, Han M, et al. Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in china: a multicenter prospective observational study. Biol Blood Marrow Transplant. 2015;21:1117–1126. [DOI] [PubMed] [Google Scholar]
- 207.Wang X, Wong CW, Urak R, et al. CMVpp65 vaccine enhances the antitumor efficacy of adoptively transferred CD19-redirected CMV-specific T cells. Clin Cancer Res. 2015;21:2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rossig C, Pule M, Altvater B, et al. Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia. 2017;31:1087–1095. [DOI] [PubMed] [Google Scholar]
- 209.Lapteva N, Gilbert M, Diaconu I, et al. T cell receptor stimulation enhances the expansion and function of CD19 chimeric antigen receptor-expressing T cells. Clin Cancer Res. 2019;25:7340–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang X, Urak R, Walter M, et al. Large-scale manufacturing and characterization of CMV-CD19CAR T cells. J ImmunoTher Cancer. 2022;10:e003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570, 1p following 570. [DOI] [PubMed] [Google Scholar]
- 212.Bunse M, Bendle GM, Linnemann C, et al. RNAi-mediated TCR knockdown prevents autoimmunity in mice caused by mixed TCR dimers following TCR gene transfer. Mol Ther. 2014;22:1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gallegos AM, Xiong H, Leiner IM, et al. Control of T cell antigen reactivity via programmed TCR downregulation. Nat Immunol. 2016;17:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schober K, Müller TR, Busch DH. Orthotopic T-cell receptor replacement-an “Enabler” for TCR-based therapies. Cells. 2020;9:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Quach DH, Ramos CA, Lulla PD, et al. Safety and efficacy of off-the-shelf CD30.CAR-modified Epstein-Barr virus-specific T cells in patients with CD30-positive lymphoma. Blood. 2021;138(suppl 1):1763. [Google Scholar]
- 216.Curran KJ, Sauter CS, Kernan NA, et al. Durable remission following “Off-the-Shelf” chimeric antigen receptor (CAR) T-cells in patients with relapse/refractory (R/R) B-cell malignancies. Biol Blood Marrow Transplant. 2020;26:S89. [Google Scholar]
- 217.Nakazawa Y, Huye LE, Salsman VS, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol Ther. 2011;19:2133–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–3067. [DOI] [PubMed] [Google Scholar]
- 219.Savoldo B, Goss J, Liu Z, et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72:1078–1086. [DOI] [PubMed] [Google Scholar]
- 220.Vafadari R, Kraaijeveld R, Weimar W, et al. Tacrolimus inhibits NF-κB activation in peripheral human T cells. PLoS One. 2013;8:e60784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Widmann T, Sester U, Gärtner BC, et al. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. Stevenson PG, ed. PLoS One. 2008;3:e3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhan X, Brown B, Slobod KS, et al. Inhibition of ex vivo-expanded cytotoxic T-lymphocyte function by high-dose cyclosporine. Transplantation. 2003;76:739–740. [DOI] [PubMed] [Google Scholar]
- 223.Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kedl RM, Schaefer BC, Kappler JW, et al. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. [DOI] [PubMed] [Google Scholar]
- 225.Roubalová K, Němečková S, Kryštofová J, et al. Antigenic competition in the generation of multi-virus-specific cell lines for immunotherapy of human cytomegalovirus, polyomavirus BK, Epstein-Barr virus and adenovirus infection in haematopoietic stem cell transplant recipients. Immunol Lett. 2020;228:64–69. [DOI] [PubMed] [Google Scholar]
- 226.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–2444. [DOI] [PubMed] [Google Scholar]
- 227.The 48th Annual Meeting of the European Society for Blood and Marrow Transplantation: Physicians - Oral Sessions (O009-O155). Bone Marrow Transplant 2022;57:16–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zamora D, Duke ER, Xie H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chemaly RF, El Haddad L, Winston DJ, et al. Cytomegalovirus (CMV) cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: the REACT study. Clin Infect Dis. 2020;71:2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cho SG, Kim N, Sohn HJ, et al. Long-term outcome of extranodal NK/T Cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mol Ther. 2015;23:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Roskrow MA, Suzuki N, Gan YJ, et al. Epstein-Barr Virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood. 1998;91:2925–2934. [PubMed] [Google Scholar]
- 233.Bollard CM, Tripic T, Cruz CR, et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J Clin Oncol. 2018;36:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smith C, Lee V, Schuessler A, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: phenotype and effector function of T cells impact on clinical response. Oncoimmunology. 2017;6:e1273311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. [DOI] [PubMed] [Google Scholar]
- 237.Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. [DOI] [PubMed] [Google Scholar]
- 239.Pender MP, Csurhes PA, Smith C, et al. Epstein-Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight. 2018;3:e124714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ioannides ZA, Csurhes PA, Douglas NL, et al. Sustained clinical improvement in a subset of patients with progressive multiple sclerosis treated with Epstein–Barr virus-specific T cell therapy. Front Neurol. 2021;12:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Al-Herz W, Essa S. Spectrum of viral infections among primary immunodeficient children: report from a national registry. Front Immunol. 2019;10:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lehman HK, Yu KOA, Towe CT, et al. Respiratory infections in patients with primary immunodeficiency. J Allergy Clin Immunol Pract. 2022;10:683–691.e1. [DOI] [PubMed] [Google Scholar]
- 243.Keller MD, Bollard CM. Virus-specific T-cell therapies for patients with primary immune deficiency. Blood. 2020;135:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pavlovic D, Patera AC, Nyberg F, et al. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8:255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. [DOI] [PubMed] [Google Scholar]
- 246.Patel S, Hanajiri R, Grant M, et al. HIV-specific T cells can be generated against non-escaped T cell epitopes with a GMP-compliant manufacturing platform. Mol Ther Methods Clin Dev. 2020;16:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pérez-Martínez A, Mora-Rillo M, Ferreras C, et al. Phase I dose-escalation single centre clinical trial to evaluate the safety of infusion of memory T cells as adoptive therapy in COVID-19 (RELEASE). EClinicalMedicine. 2021;39:101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.McLellan AD, Ali Hosseini Rad SM, Alexander McLellan CD. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol. 2019;97:664–674. [DOI] [PubMed] [Google Scholar]
- 249.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Busch DH, Fräßle SP, Sommermeyer D, et al. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pietrobon V, Todd LA, Goswami A, et al. Improving CAR T-cell persistence. Int J Mol Sci. 2021;22:10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Salter AI, Rajan A, Kennedy JJ, et al. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci Signal. 2021;14:eabe2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dong R, Libby KA, Blaeschke F, et al. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR). EMBO J. 2020;39:e104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signalling. Mol Cancer Ther. 2018;17:1795–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Stenger D, Stief TA, Kaeuferle T, et al. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020;136:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sun J, Huye LE, Lapteva N, et al. Early transduction produces highly functional chimeric antigen receptor-modified virus-specific T-cells with central memory markers: a Production Assistant for Cell Therapy (PACT) translational application. J ImmunoTher Cancer. 2015;3:5. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cruz CRY, Lam S, Hanley PJ, et al. Robust T cell responses to aspergillosis in chronic granulomatous disease: implications for immunotherapy. Clin Exp Immunol. 2013;174:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e260–e272. [DOI] [PubMed] [Google Scholar]
- 260.Styczynski J, Van Der Velden W, Fox CP, et al. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Papadopoulou A, Koukoulias K, Alvanou M, et al. Patient risk stratification and tailored clinical management of post-transplant CMV-, EBV-, and BKV-infections by monitoring virus-specific T-cell immunity. EJHaem. 2021;2:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]