Abstract
Psychopathic individuals are notorious for their callous disregard for others’ emotions. Prior research has linked psychopathy to deficits in affective mechanisms underlying empathy (e.g., affective sharing), yet research relating psychopathy to cognitive mechanisms underlying empathy (e.g., affective perspective-taking and Theory of Mind) requires further clarification. To elucidate the neurobiology of cognitive mechanisms of empathy in psychopathy, we administered an fMRI task and tested for global as well as emotion-specific deficits in affective perspective-taking. Adult male incarcerated offenders (N = 94) viewed images of two people interacting, with one individual’s face obscured by a shape. Participants were cued to either identify the emotion of the obscured individual or identify the shape from one of two emotion or shape choices presented on each trial. Target emotions included anger, fear, happiness, sadness, and neutral. Contrary to predictions, psychopathy was unrelated to neural activity in the Affective Perspective-taking > Shape contrast. In line with predictions, psychopathy was negatively related to task accuracy during affective perspective-taking for fear, happiness, and sadness. Psychopathy was related to reduced hemodynamic activity exclusively during fear perspective-taking in several areas: left anterior insula extending into posterior orbitofrontal cortex, right precuneus, left superior parietal lobule, and left superior occipital cortex. Although much prior research has emphasized psychopathy-related abnormalities in affective mechanisms mediating empathy, current results add to growing evidence of psychopathy-related abnormalities in a cognitive mechanism related to empathy. These findings highlight brain regions that are hypoactive in psychopathy when explicitly processing another’s fear.
Keywords: Psychopathy, Fmri, Social cognition, Emotion, Empathy, Psychopathology
1. Introduction
Psychopathy is a personality disorder characterized by lack of empathy, grandiosity, an impulsive and irresponsible lifestyle, and habitual antisociality. Present in roughly a quarter of adult prison inmates (Hare, 2003), psychopathy is a significant predictor of violent crime and recidivism (Harris et al., 1991). The disorder has been estimated to cost the U.S. alone $460 billion dollars per year, making it one of the most costly mental health disorders (Kiehl and Hoffman, 2011). Specifying the neurobiological mechanisms underlying the core features of psychopathy could lead to more targeted and effective treatments for psychopathic offenders.
Lack of empathy is considered a core deficit in psychopathy (Blair et al., 2005; Soderstrom, 2003). Although definitions of empathy abound, there is general consensus that the process of understanding and responding to another individual’s affective state is supported by several interacting mechanisms (Decety, 2015; Decety and Jackson, 2004; Lamm and Majdandžić, 2015). Most contemporary researchers focus especially on affective and cognitive mechanisms underlying empathy. Whereas affective mechanisms (central to empathy, according to Lamm and Majdandžić, 2015) include processes such as affective sharing and physiological arousal to another’s emotion, cognitive mechanisms linked to empathy include affective perspective-taking: adopting another’s perspective to understand their emotional state. In support of this delineation, these mechanisms recruit activity in overlapping brain regions (left anterior insula) and in distinct brain regions (affective: right anterior insula, right dorsal anterior cingulate, right dorsal medial thalamus, and midbrain; cognitive: left orbitofrontal cortex, left anterior midcingulate, and left dorsal medial thalamus; Fan et al., 2011). Some researchers have also labelled Theory of Mind (ToM), which involves taking another’s perspective to understand their beliefs and mental state, as a cognitive mechanism of empathy (Cerniglia et al., 2019; Shamay-Tsoory et al., 2010). As noted below, findings for affective perspective-taking sometimes differ from those for ToM; consequently, for clarity, we distinguish affective perspective-taking from ToM.
Numerous laboratory studies have linked psychopathy to reduced capacity for affective empathy (Blair, 2005b). For example, psychopathic individuals have exhibited reduced physiological arousal (i.e., skin conductance) in response to another person’s distress (Aniskiewicz, 1979; Blair et al., 1997; House and Milligan, 1976). When confronted with facial or vocal cues of another person’s emotion, psychopathic individuals often show impaired affect recognition (especially for fear, happiness, sadness, and surprise; Dawel et al., 2012). However, it is possible that both affective and cognitive mechanisms of empathy contribute to facial affect recognition. Findings from neuroimaging studies of facial emotion and pain processing have also been interpreted as suggesting deficits in affective empathy. In several studies, psychopathic individuals have displayed attenuated neural response to facial emotion expressions in amygdala, ventromedial prefrontal cortex, dorsomedial prefrontal cortex, anterior insula, and inferior frontal gyrus (Decety et al., 2014; Dolan and Fullam, 2009; Gordon et al., 2004; Sethi et al., 2018). Similarly, psychopathic individuals show attenuated neural responses to others experiencing pain in ventromedial prefrontal cortex, midcingulate cortex, anterior insula, and inferior frontal gyrus (Decety et al., 2015, 2013; Meffert et al., 2013). Each of these regions is recruited during emotional arousal in healthy individuals (Lindquist et al., 2016). Thus, psychopathy appears to be characterized by diminished physiological arousal and neural response to others’ emotions.
Yet the extant literature on cognitive mechanisms underlying empathy in psychopathy requires further clarification. Although earlier studies suggested intact capacity for ToM in psychopathic individuals (e.g., Blair, 2005b), recent work suggests distinct impairments in affective perspective-taking (Brook and Kosson, 2013; Decety et al., 2013). More concretely, one study found that psychopathy was associated with poorer accuracy when inferring another person’s emotional state from video recordings of the person describing actual emotional life events (Brook and Kosson, 2013). In another study, psychopathy was negatively related to accuracy during affective perspective-taking, but unrelated to accuracy during ToM (Shamay-Tsoory et al., 2010). Furthermore, when adopting the perspective of another person in pain, psychopathic offenders showed significantly less neural activity than non-psychopathic offenders in regions involved in empathy (anterior insula and anterior midcingulate) and mentalizing (medial prefrontal cortex; Decety et al., 2013). These apparent discrepancies can be resolved by distinguishing between aspects of perspective-taking related to another person’s knowledge or beliefs (intact in psychopathy) versus aspects of perspective-taking related to another person’s affective state (deficient in psychopathy; e.g., Brook and Kosson, 2013; Decety et al., 2013; Shamay-Tsoory et al., 2010).
Similar to adults with psychopathy, juveniles with conduct disorder and callous-unemotional traits show impairments in affective empathy but not ToM (Jones et al., 2010; Schwenck et al., 2012). Some prior studies of youth with conduct problems and callous-unemotional traits also suggest an impairment in affective perspective-taking, with two studies reporting behavioral impairment in this domain (Anastassiou-Hadjicharalambous and Warden, 2008; Lui et al., 2016). Although two other studies found no behavioral impairment in affective perspective-taking per se (Schwenck et al., 2012; Sebastian et al., 2012), one of these studies linked callous-unemotional traits to reduced activity in right amygdala during affective perspective-taking (Sebastian et al., 2012). Despite these findings, our understanding of the neural correlates of adult psychopathy during perspective-taking for emotional experiences other than pain is quite limited.
To this end, we administered an affective perspective-taking task with five emotion categories (anger, fear, happiness, sadness, and neutral) to incarcerated offenders. Psychopathy was predicted to be negatively related to neural activity in regions involved in cognitive mechanisms underlying empathy (left anterior insula, left orbitofrontal cortex, left anterior midcingulate, and left dorsal medial thalamus; Fan et al., 2011) during the affective perspective-taking task, relative to a secondary shape-matching task. Furthermore, we sought to determine which emotions elicit reduced neural activity during affective perspective-taking. Based on prior findings (Dawel et al., 2012), psychopathy was predicted to be negatively related to task accuracy and neural activity during perspective-taking for fear, sadness, and happiness, but not anger.
For the sake of transparency, we note that this study was originally designed to also provide tests of the predictions of four etiological theories of psychopathy. However, because manipulation tests demonstrated that the preconditions for testing several theories were not met, description of these theory-based predictions has been relegated to Supplemental Materials. Thus, this report focuses on: 1) assessing the generality of the affective perspective-taking deficit in psychopathy, and 2) assessing the possibility of anomalies in brain responsiveness to conditions eliciting affective perspective-taking. Nonetheless, where theoretical perspectives on psychopathy appear useful for understanding observed findings, these perspectives are addressed briefly in the Discussion.
2. Methods and materials
2.1. Participants
Adult male inmates were recruited from two medium-security correctional facilities in Wisconsin. All participants met the following inclusion criteria: between ages 18 and 55; no history of psychosis, bipolar disorder, PTSD, epilepsy or stroke; not currently using psychotropic medications; no history of head injury with loss of consciousness >30 min; >4th grade English reading level; intact auditory and visual capabilities; IQ >70; and no MRI contraindications. One hundred participants met inclusion criteria and finished the fMRI task. Six participants were excluded from analyses (three participants did not demonstrate adequate learning of the task, two encountered technical errors, and one had excessive head motion on more than 20% of time points), leaving a final sample of N = 94. All subjects provided written informed consent. The study was approved by the IRBs at the universities of the last three authors.
2.2. Assessments
Psychopathy was assessed with the Psychopathy Checklist-Revised (PCL-R; Hare, 2003), which is scored on the basis of a semi-structured interview and file review. The 20 PCL-R items were rated on a scale of 0–2, yielding total scores between 0 and 40 (Hare et al., 1990). Although intra-class correlations (ICC) of PCL-R Total scores for the current sample were unavailable, prior PCL-R data from our lab show high inter-rater reliability (N = 129, ICC = 0.97).
IQ was estimated from the Wechsler Adult Intelligence Scale 3rd Ed. (WAIS-III; Wechsler, 1997) vocabulary and matrix reasoning scales. Lifetime substance use disorder diagnoses were determined using the Structured Clinical Interview for the DSM-IV (SCID-IV; First et al., 2012). To minimize the number of covariates used in statistical models, a single dichotomous variable was calculated for substance use disorder (present or absent), based on whether each participant met criteria for abuse or dependence on any substance (alcohol, cannabis, cocaine, opioids, stimulants, sedatives, or hallucinogens) in his lifetime (Korponay et al., 2016; Wolf et al., 2015).
2.3. Behavioral tasks
Participants viewed static images portraying two individuals interacting (e.g., one person scolding the other, one person consoling the other), with one individual’s face obscured by a shape (see Fig. 1; Haas et al., 2015). The affective perspective-taking task (half the trials) required participants to decide which of two emotional faces best matched the obscured face from the social scene. Prior research has found this affective perspective-taking task to elicit neural activity within the ‘empathy network’, including insula, medial prefrontal cortex, and precuneus (Haas et al., 2015). The shape task (the other half of trials) required participants to indicate which of two shapes matched the shape embedded within the social scene. Thus, each task presented the same visual stimuli and required participants to visually search both the social scene at the top of the screen and the response options at the bottom. All responses were recorded via response box.
Fig. 1.
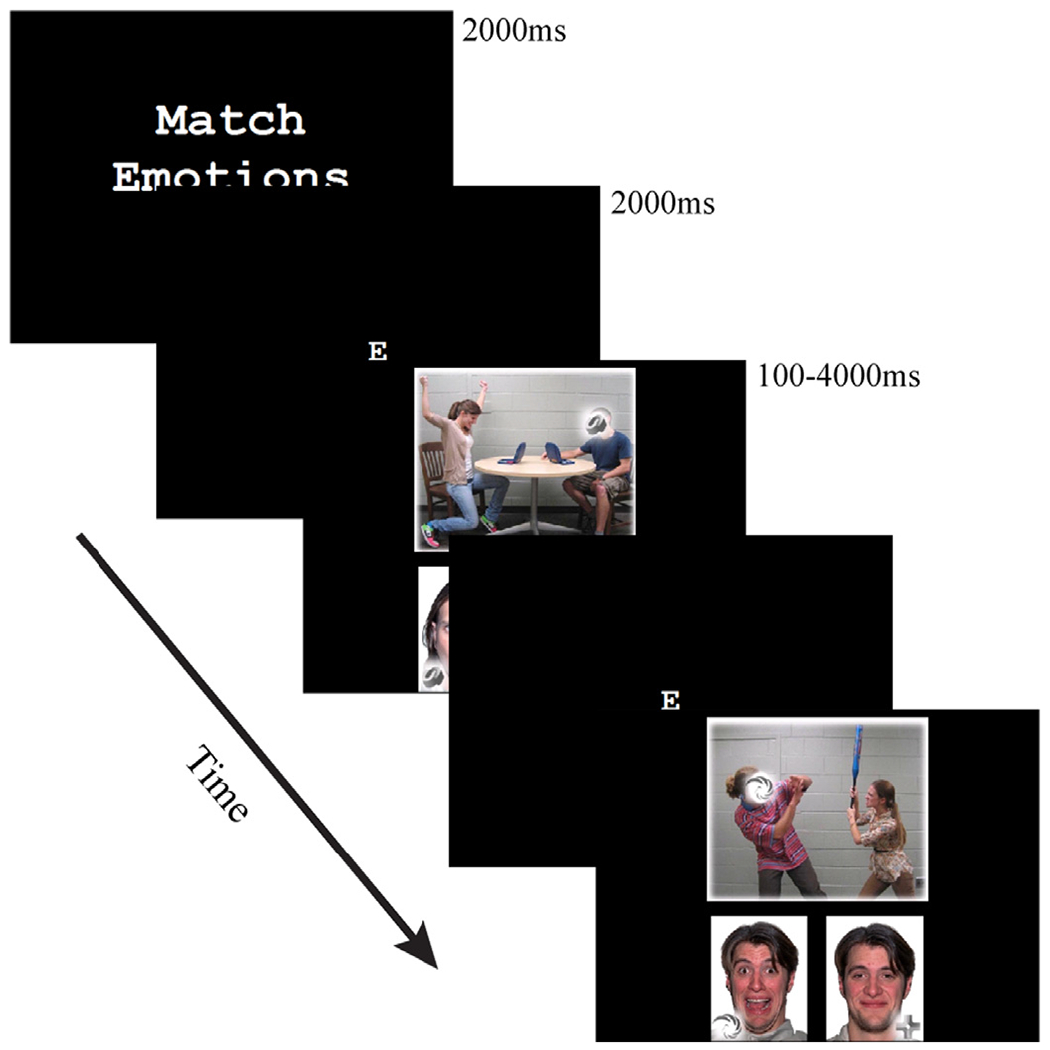
Temporal sequence of the affective perspective-taking task. Participants selected one of two emotional faces that best matched the emotion of the obscured face from the social scene. For the shape task, participants selected one of two shapes that matched the shape embedded within the social scene.
The task was administered in four runs of 35 trials, resulting in a total of 140 trials. The social scenes were divided evenly among five emotion categories (anger, fear, happiness, sadness, and neutral), with the emotions task-relevant only for 70 trials (i.e., the affective perspective-taking trials). Trials were presented in blocks of 3 or 4 trials, alternating between the affective perspective-taking and shape tasks. Each trial began with the presentation of a letter denoting the task, followed by the stimulus (mean presentation time 1.8 s, range 0.1–4.0 s), and finally a fixation cross before the next trial (2 s). A performance-based monetary reward was offered on the third and fourth runs (see Supplemental Materials for details). Our principal analyses collapsed across rewarded and non-rewarded conditions.
2.4. fMRI acquisition
Imaging data were collected on prison grounds in the Mind Research Network’s 1.5 T mobile imaging unit, using a 32-channel head coil. Multiband echo planar images (EPIs) were collected with the following parameters: TR=350 ms, TE=39.0 ms, flip angle=38°, FOV=248 × 248 mm, phase encoding direction=anterior to posterior, slice thickness=3.5 mm, voxel size=3.5 × 3.5 × 3.5mm3, 48 slices per volume and a total of 3347 vol (Feinberg and Setsompop, 2013). High-resolution T1-weighted anatomical scans were collected for each subject (TR=2400 ms, TE=1.9 ms, flip angle=8°, FOV=256 × 256mm2, slice thickness=1.00 mm, voxel size=1 × 1 × 1mm3 and 176 interleaved sagittal slices).
2.5. Task performance analysis
Correct responses were summed for each of the five emotions and two tasks. These responses were then entered into linear mixed effects models for the Affective Perspective-Taking > Shape contrast, as well as the Fear > Neutral, Sadness > Neutral, Happiness > Neutral, and Anger > Neutral contrasts within the affective perspective-taking task. We first modeled task accuracy across all participants, then modeled task accuracy in relation to PCL-R Total scores. Average response times (RTs) were modeled in the same manner.
2.6. fMRI analysis
fMRI data were processed using AFNI (16.0; Cox, 1996). Two EPI spin-echo sequences (one in the anterior-to-posterior phase encoding direction; one in the posterior-to-anterior direction) were used to correct geometric distortions due to field inhomogeneity. EPI volumes were motion corrected by rigid body alignment, using the first volume as a reference. Next, EPIs were smoothed with a 6 mm full-width-at-half-maximum Gaussian kernel and scaled to a mean of 100. The T1-weighted anatomical scans were skull-stripped and intensity normalized. EPIs were then aligned to the anatomical scans, and both were aligned to MNI-152 template space. Finally, the four EPI runs were concatenated and modeled with two regressors for each stimulus category, representing stimulus onsets and their temporal derivatives (3dDeconvolve; Calhoun et al., 2004). The first 15 TRs (5.25 s) were censored from each run during this step. Button press times, fixation times, and residual head motion after volume correction were also modeled as regressors of no interest. The resulting statistical maps were entered in general linear tests of the Affective Perspective-Taking > Shape contrast, as well as the Fear > Neutral, Sadness > Neutral, Happiness > Neutral, and Anger > Neutral contrasts within the affective perspective-taking task.
At the final analysis stage, PCL-R Total score was entered as a regressor with the contrasts of interest in separate, whole-brain general linear models. Principal analyses also included age, race/ethnicity, IQ (WAIS-III) and substance use disorder diagnosis (SCID-IV) as covariates. Additional analyses were conducted without the covariates. Hemodynamic responses were considered significant at pFWE < 0.05 (cluster size > 11 voxels, uncorrected p = .002). Monte Carlo simulations (3dFWHMx and 3dClustSim) determined the cluster extent threshold (postprocessing FWHM = 9.21; Cox et al., 2017; Eklund et al., 2016). To control for testing multiple contrasts, principal analyses were also checked at a Bonferroni-corrected level of pFWE < 0.01 (pFWE = 0.05/5 contrasts = 0.01; cluster size > 14, uncorrected p = .002).
3. Results
3.1. Participant Characteristics
Participant characteristics are displayed in Table 1. Neither age, r = 0.05, p = .62, nor IQ, r = 0.09, p = .38, was significantly correlated with PCL-R Total scores. However, non-white participants (M = 25.2, SD = 6.7) had higher PCL-R Total scores than white participants (M = 21.6, SD = 7.0), F (1, 92) = 6.27, p < .02. Furthermore, participants with a substance use disorder (M = 24.4, SD = 6.4) had higher PCL-R Total scores than participants without a substance use disorder (M = 19.7, SD = 8.0), F(1, 92) = 7.94, p < .01.
Table 1.
Participant characteristics (n = 94).
| Measure | M (SD) | Range |
|---|---|---|
| PCL-R Total | 23.3 (7.0) | 8.4–37.0 |
| Age | 32.9 (8.2) | 20.0–52.0 |
| IQ | 97.7 (13.9) | 72.0–134.0 |
| Measure | % | |
|
| ||
| Proportion Caucasian | 53.2% | |
| Proportion with Substance Use Disorder | 76.6% | |
3.2. Task performance results across participants
Participants were significantly more accurate in the shape task (mean accuracy = 88.7%) than the affective perspective-taking task (72.7%), F(1, 93) = 207.99, p < .001. During the affective perspective-taking task, participants were generally more accurate on trials with emotional compared to neutral content, a difference that was statistically significant for two of four emotions. Compared to neutral trials (mean accuracy = 71.6%), participants were significantly more accurate on sadness trials (75.4%), F(1, 93) = 4.55, p < .04, and happiness trials (82.6%), F(1, 93) = 36.54, p < .001. Participants were not significantly more accurate on fear trials (74.8%) than neutral trials, F(1, 93) = 3.19, p = .08. However, participants were less accurate on anger trials (58.9%) than on neutral trials, F(1, 93) = 36.00, p < .001.
In addition to being more accurate, participants were faster to respond to the shape task (mean RT = 1.48 s) than to the affective perspective-taking task (2.06 s), F(1, 93) = 321.89, p < .001. During the affective perspective-taking task, participants responded more quickly on happiness trials (1.92 s) than neutral trials (2.06 s), F(1, 93) = 38.97, p < .001. However, participants responded more slowly to anger trials (2.11 s), F(1, 93) = 6.85, p < .02, and sadness trials (2.15 s), F(1, 93) = 15.11, p < .001, than to neutral trials (2.06 s). Response times for fear trials (2.08 s) did not differ from those for neutral trials (2.06 s), F(1, 93) = 0.69, p = .41.
3.3. Task performance results related to psychopathy
Psychopathy was unrelated to task accuracy in the Affective Perspective-Taking > Shape contrast, F(1, 92) = 0.41, p = .52. As predicted, during the affective perspective-taking task higher PCL-R Total scores were related to poorer task accuracy in the Fear > Neutral, F(1, 92) = 6.59, p < .02, Sadness > Neutral, F(1, 92) = 4.90, p < .03, and Happiness > Neutral contrasts, F(1, 92) = 10.19, p < .01. Psychopathy was unrelated to task accuracy in the Anger > Neutral contrast, F(1, 92) = 1.92, p = .17, during the affective perspective-taking task.
Psychopathy was also unrelated to response times in the Affective Perspective-Taking > Shape contrast, F(1, 92) = 0.52, p = .47. During the affective perspective-taking task, higher PCL-R Total scores were related to slower response times in the Sadness > Neutral contrast, F(1, 92) = 4.98, p < .03. Higher PCL-R Total scores were not significantly related to slower response times in the Fear > Neutral contrast, F(1, 92) = 3.05, p = .08. Psychopathy was also unrelated to response times in the Anger > Neutral, F(1, 92) = 2.17, p = .14, and Happiness > Neutral contrasts, F(1, 92) = 1.14, p = .29.
3.4. fMRI results across participants
The task recruited neural regions (Fig. 2) consistent with previous affective perspective-taking studies (Fan et al., 2011), including studies that used the same task (Haas et al., 2015). The Affective Perspective-Taking > Shape contrast revealed greater activation in areas previously implicated in empathy and mentalizing, such as ventromedial prefrontal cortex, dorsomedial prefrontal cortex, anterior insula (AI), inferior frontal gyrus, and precuneus, all bilaterally (pFWE < 0.05; Denny et al., 2012; Fan et al., 2011). Additionally, the affective perspective-taking task elicited greater activation in visual processing regions than the shape task, including inferior occipital gyrus, inferior temporal gyrus, and fusiform gyrus. The contrast also demonstrated several regions showing greater activation in the shape task, including superior temporal gyrus, hippocampus, parahippocampal gyrus, and superior parietal lobule, areas identified in previous shape processing studies (Peelen and Caramazza, 2012).
Fig. 2.
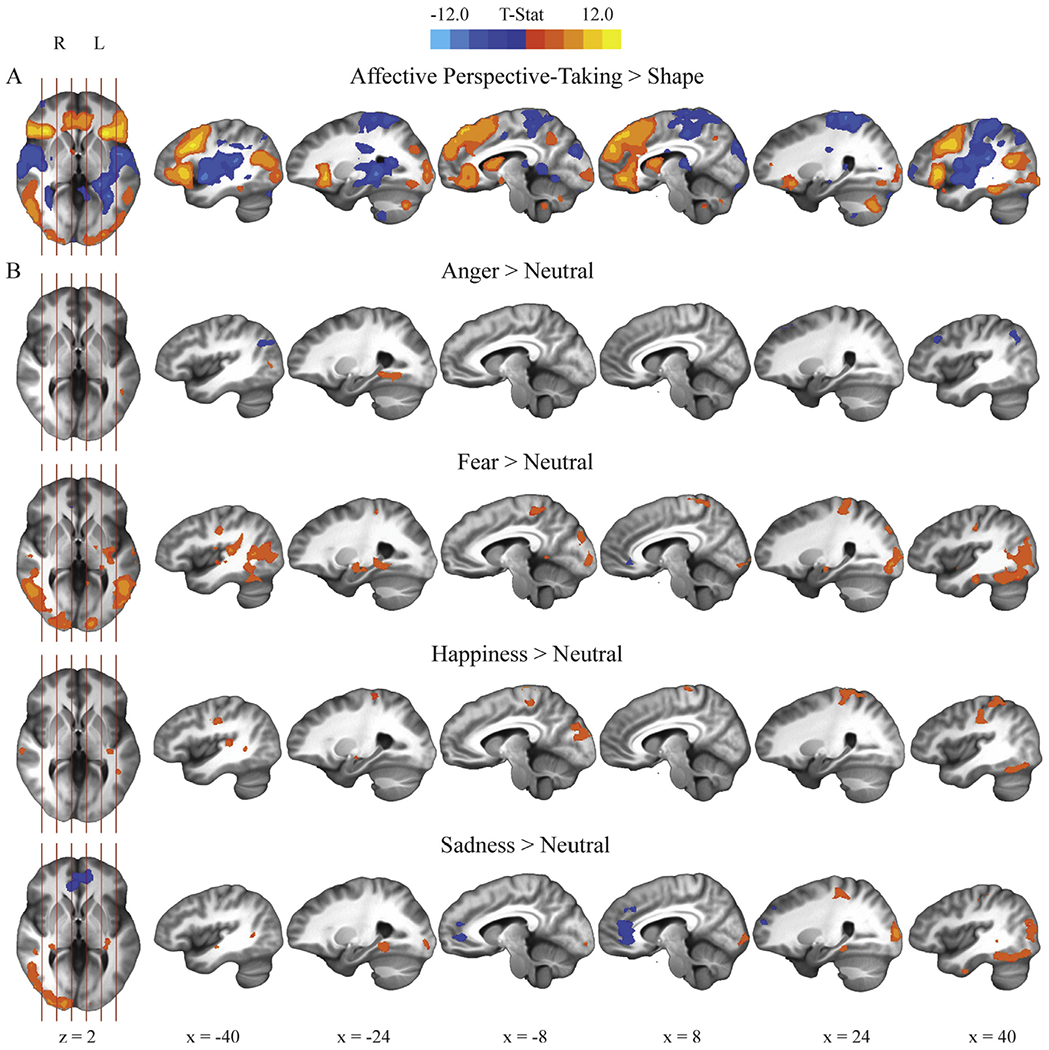
Areas of significant activation across the entire sample for A) the Affective Perspective-Taking > Shape contrast, and B) the emotion-specific contrasts within the affective perspective-taking task: Anger > Neutral, Fear > Neutral, Sadness > Neutral, and Happiness > Neutral (uncorrected p = .002, pFWE < 0.05).
Main effects across participants showed greater neural activity related to emotional, relative to neutral, content in the affective perspective-taking task, in line with previous studies of emotion processing (Fig. 2; Fusar-Poli et al., 2009; Phan, Wager, Taylor, & Liberzon, 2002). The Fear > Neutral, Sadness > Neutral, and Happiness > Neutral contrasts all indicated greater activity in left hippocampus, right fusiform gyrus, and bilateral precentral gyrus during emotional than during neutral trials. Furthermore, the Fear > Neutral and Sadness > Neutral contrasts elicited greater activity in right inferior temporal gyrus, bilateral middle temporal gyrus, left parahippocampal gyrus, and right occipital cortex during emotional than during neutral trials. The Anger > Neutral contrast likewise recruited greater activity in left parahippocampal gyrus, fusiform gyrus, and middle temporal gyrus during anger than during neutral trials. The Fear > Neutral contrast revealed greater activity in left amygdala during fear than during neutral trials. Finally, the Fear > Neutral and Sadness > Neutral contrasts revealed less activity in rostral anterior cingulate and adjacent ventromedial prefrontal cortex during fear and sadness than during neutral trials.
3.5. fMRI results related to psychopathy
Contrary to our predictions, PCL-R Total scores were unrelated to activity in any brain areas in the Affective Perspective-Taking > Shape contrast.
In the affective perspective-taking task, psychopathy was related to reduced neural activity only during fear trials. PCL-R Total scores were negatively related to Fear > Neutral activity in several regions, including left AI extending into posterior orbitofrontal cortex (pOFC; Fig. 3), right precuneus (Fig. 4), left superior occipital cortex, and left superior parietal lobule (Table 2). This pattern of findings remained after Bonferroni correction and when the covariates were removed from the model. PCL-R Total scores were not significantly related to Happiness > Neutral, Sadness > Neutral, or Anger > Neutral activity in any brain region. As a follow-up analysis, fear trials were contrasted with an aggregate of the remaining emotions [Fear > (Anger + Happiness + Sadness)] for the affective perspective-taking task. PCL-R Total scores were again negatively related to activity in right precuneus, among other regions (Table 2).
Fig. 3.
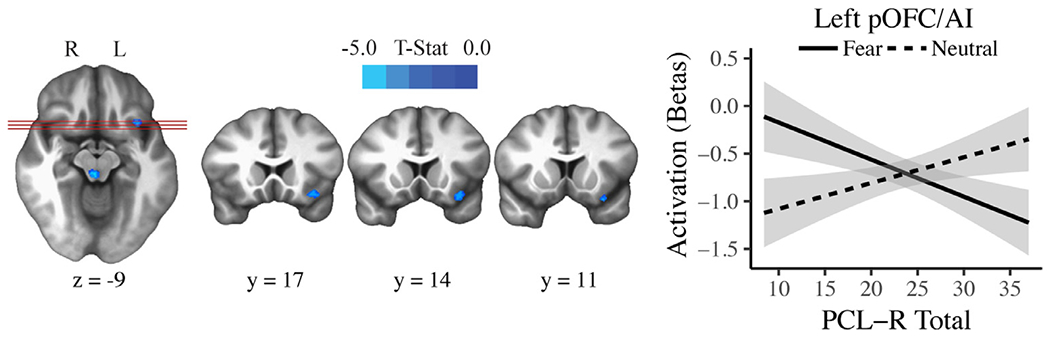
The significant association between PCL-R Total scores and Fear > Neutral activity in left anterior insula (AI) extending into posterior orbitofrontal cortex (pOFC) in the affective perspective-taking task (uncorrected p = .002, pFWE < 0.05). The activation plot shows regression lines with error bands representing 1 SE above and below the point estimate of the model.
Fig. 4.
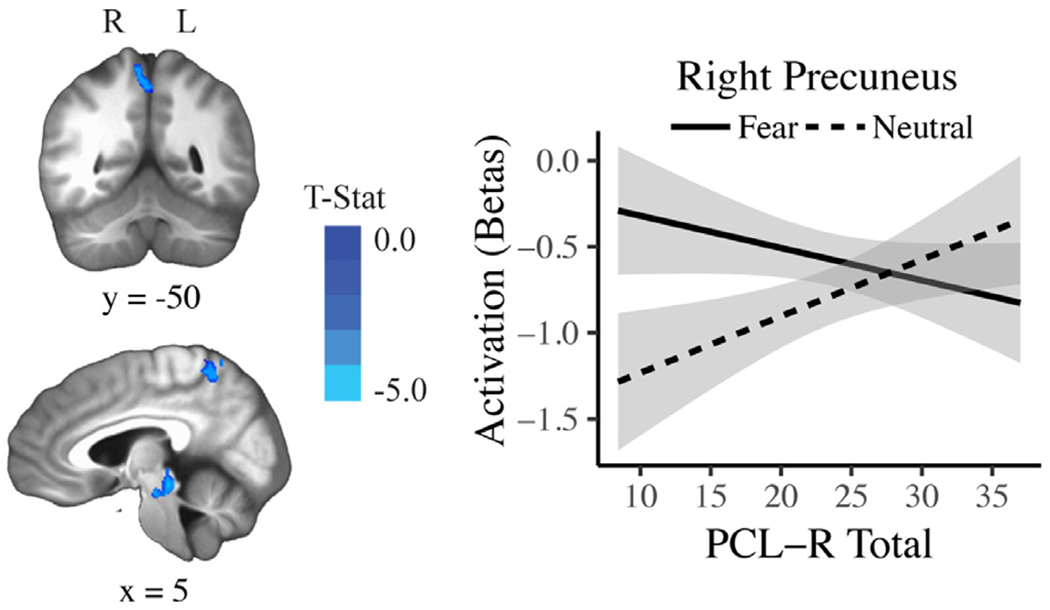
The significant association between PCL-R Total scores and Fear > Neutral activity in right precuneus in the affective perspective-taking task (uncorrected p = .002, pFWE < 0.05). The activation plot shows regression lines with error bands representing 1 SE above and below the point estimate of the model.
Table 2.
Regions showing significant negative associations between fear contrasts and PCL-R Total scores during the affective perspective-taking task in whole-brain analyses (uncorrected p = .002; pFWE < 0.05).
| Contrast | Brain Region | L/R | MNI Peak Coordinates(x, y, z) | Size (Voxels) | Peakt-value |
|---|---|---|---|---|---|
| Fear > Neutral | Superior Occipital Cortex | L | −37, −78, 31 | 36 | −3.87 |
| Superior Parietal Lobule | L | −30, −64, 52 | 21 | −3.82 | |
| Brainstem | R | 9, −19, −15 | 20 | −4.01 | |
| Precuneus | R | 5, −50, 56 | 18 | −3.78 | |
| Anterior Insula/ | L | −30, 13, −15 | 16 | −3.87 | |
| Posterior Orbitofrontal Cortex | |||||
| Fear > (Anger + Happiness + Sadness) | Superior Occipital Cortex | R | 30, −75, 28 | 21 | −4.20 |
| Precentral Gyrus | L | −37, −8, 45 | 18 | −4.38 | |
| Postcentral Gyrus | R | 33, −43, 52 | 17 | −4.29 | |
| Precuneus | R | 9, −64, 52 | 17 | −3.69 | |
| Superior Occipital Cortex | R | 30, −85, 14 | 16 | −3.60 | |
| Fusiform Gyrus | L | −26, −36, −11 | 12 | −3.33 | |
| Postcentral Gyrus | L | −40, −33, 52 | 12 | −3.39 |
All models controlled for age, race/ethnicity, substance abuse and IQ.
In the Fear > Neutral contrast, all regions were additionally significant at a Bonferroni-corrected level of PFWE < 0.01 (cluster size > 14, uncorrected p = .002).
Follow-up analyses, including results regarding PCL-R Factor scores, PCL-R groups, trait empathy, and additional analyses related to contemporary theoretical perspectives can be found in the Supplemental Materials.
4. Discussion
The current study sought to identify relations between psychopathy and neural activity under conditions demanding affective perspective-taking (a cognitive mechanism of empathy) in a sample of offenders. Although psychopathy was unrelated to neural activity during affective perspective-taking in general, further analyses revealed deficits during perspective-taking for specific emotions, particularly fear. Performance analyses showed that psychopathy was associated with reduced accuracy in the affective perspective-taking task in identifying fear, sadness, and happiness. Yet psychopathy was negatively related to neural activation in brain regions involved in empathy, perspective-taking, and visual processing only during fear trials. Here, we review each of these findings in turn.
The behavioral findings described above provide evidence consistent with diminished capacity for affective perspective-taking in psychopathy. Earlier work had argued for disrupted affective mechanisms of empathy (i.e., physiological arousal to another’s emotion) but intact ToM (another cognitive mechanism linked with empathy; Blair, 2005b). The current findings and other recent studies suggest a distinct impairment in affective perspective-taking and corroborate the distinction between these two cognitive mechanisms of empathy (Brook and Kosson, 2013; Decety et al., 2013; Shamay-Tsoory et al., 2010). Recent studies have found behavioral deficits in affective perspective-taking generally (Shamay-Tsoory et al., 2010) and specifically for fear and sadness (Brook and Kosson, 2013), as well as neural deficits during perspective-taking for pain (Decety et al., 2013). The current study extends these findings to include reduced neural activity during fear perspective-taking.
Recent neuroimaging studies have also examined psychopathic offenders’ affective perspective-taking deficits in scenarios involving pain (Decety et al., 2013), social exclusion, and love (Meffert et al., 2013). Growing evidence from these studies suggests that psychopathic individuals fail to automatically and spontaneously adopt the perspective of others. In one study, psychopathic individuals showed greater neural abnormalities while passively viewing emotional scenarios, compared to when asked to actively empathize with the actors in the scenarios (Meffert et al., 2013). Similarly, psychopathic offenders failed to automatically take another’s perspective during a non-emotional task (an avatar dot-counting task; Drayton et al., 2018). In the current study, participants were explicitly instructed to take another’s perspective in one of two tasks. Future studies might predict psychopathic offenders to exhibit more pronounced reductions in neural activity under passive viewing conditions, compared to the instructed affective perspective-taking task administered in this study. Nevertheless, the current study adds to previous evidence of reduced neural activity in key empathy regions when psychopathic individuals are explicitly instructed to adopt another’s perspective (Decety et al., 2013).
A pattern of attenuated neural response in psychopathy was observed in two key regions involved in empathy and emotion, left AI and left pOFC. The subregion of AI observed in this study appears to subserve social-emotional functions, including empathy (Fan et al., 2011; Singer et al., 2004) and the experience of emotion (Kurth et al., 2010; Lindquist et al., 2012). Furthermore, left AI, as observed in this study, appears involved in both affective and cognitive mechanisms of empathy (whereas right AI is involved primarily in affective mechanisms of empathy; Fan et al., 2011). It has been proposed that AI facilitates empathy by integrating external and visceral information in order to predict another person’s emotional state (Singer et al., 2009). Across a variety of forms of social cognition, psychopathy has been consistently related to reduced AI activity. When imagining another person in pain, psychopathic offenders show attenuated bilateral AI activity (Decety et al., 2013). Moreover, psychopathic traits are negatively related to AI activity when administering punishment to others (Molenberghs et al., 2014), anticipating guilt for moral transgressions (Seara-Cardoso et al., 2016) and responding emotionally to others’ affective faces (Seara-Cardoso et al., 2016) in community samples. The left AI in psychopathic offenders has also been reported to be thinner and less functionally connected to dorsal anterior cingulate cortex, another important node in the empathy network (Ly et al., 2012; Philippi et al., 2015). The current study adds to a growing body of literature identifying AI as an important region of neural dysfunction in psychopathy.
Alternative explanations of this study’s AI findings may be addressed in future research. For example, AI is also involved in processing uncertainty (Singer et al., 2009). Thus, the present AI findings may reflect diminished capacity to represent uncertain information in social scenes in psychopathic individuals. Future studies may use computational modeling as in Brazil et al. (2017) to determine how AI represents uncertain information during affective perspective-taking in psychopathy. Relatedly, the present findings may reflect disrupted processing of threat cues in psychopathy. Although early research suggested global deficits in fear processing in psychopathy (Lykken, 1957), recent literature suggests a more specific deficit in threat processing, rather than an attenuated conscious experience of fear (Hoppenbrouwers et al., 2016). Taking the perspective of the target individual in the fear trials of the current study required identifying the presence of threat cues in the social scene (e.g., a spider or an assailant with a bat). Reduced activity of left AI, which also plays a role in threat processing (Pichon et al., 2012), may partly reflect disrupted processing of threat cues in psychopathy. Future studies could manipulate the focus to or away from threat cues in the social scene to determine the kinds of information to which left AI is responding during affective perspective-taking in psychopathic and non-psychopathic individuals.
Posterior OFC shares dense interconnections with the amygdala, and this pathway is thought to be critical for emotion processing (Timbie and Barbas, 2014). Receiving information about emotional context from the amygdala, pOFC appears to be involved in modulating the intensity of emotional experience through feedback connections to the amygdala and autonomic structures (Barbas, 2007). Psychopathic individuals consistently show OFC dysfunction while processing emotional stimuli (Blair, 2010), and several theories of psychopathy pinpoint OFC as a key region of dysfunction, including the Integrated Emotion Systems perspective (Blair, 2005a) and Paralimbic Hypothesis (Kiehl, 2006), with the latter also predicting AI dysfunction in psychopathy. Although current OFC findings corroborate these accounts, these theories do not explain our findings of links between psychopathy and anomalies in other regions, such as precuneus and visual cortex. For example, does reduced activity in these regions reflect downstream effects of reduced activation of paralimbic regions, such as pOFC, AI, and amygdala? Or does the reduced activity arise from dysfunction within these non-paralimbic brain areas? Future studies and neurobiological accounts of psychopathy should address the relatively consistent observations of dysfunction in regions both within and outside the paralimbic cortex (Deming and Koenigs, 2020; Koenigs et al., 2011).
The following limitations should be considered when interpreting the current results. First, one of the original goals of the study was to test the predictions of four etiological theories of psychopathy. The utility of these tests was not corroborated by most of the manipulation checks we conducted. Consequently, we did not use this paradigm to test these specific theoretical perspectives. Future studies may continue to develop manipulations that contribute to better tests of these theories, as well as manipulations that provide more powerful tests of the conditions under which psychopathic individuals display affective perspective-taking deficits. Studies like the current study are important in providing evidence that is needed in order to extend psychological theories of psychopathy to the neural substrates of social processes such as empathy. In this context, the current findings provide important insight into psychopathic individuals’ behavioral and neural responses when taking another’s perspective to understand their affective state. Next, the difference in difficulty between the affective perspective-taking and shape tasks may have undermined our ability to detect a relationship between psychopathy and Affective Perspective-Taking > Shape activity. Additionally, the lack of psychopathy-related neural findings during perspective-taking for sadness and happiness was unexpected, given that psychopathy was negatively related to task accuracy during these trials. Although fMRI analyses can be more sensitive than behavioral analyses (Raemaekers et al., 2006; Whalen et al., 1998), the absence of fMRI findings related to happiness and sadness in this study may stem from our reliance on corrected whole-brain analyses, which are less powerful than region of interest analyses, or from low power as a result of the relatively small number of trials (14) per emotion. Even so, this pattern raises the possibility that some brain systems in psychopathic offenders are under at least some conditions less responsive to fear cues than to cues for other emotions.
In sum, we have identified a set of brain regions whose activity may underlie some of the social cognitive deficits observed in psychopathy. Whereas some prior concepts of empathic deficits in psychopathy have emphasized the affective mechanisms of empathy, the current study adds to recent findings of an impaired cognitive mechanism of empathy and points to specific brain regions previously linked to cognitive mechanisms of empathy and mentalizing as candidate substrates of this impairment.
Supplementary Material
Acknowledgments
We thank the many individuals at the Wisconsin Department of Corrections who made this research possible, and are especially indebted to Deputy Warden Tom Nickel, Warden Randy Hepp, and Dr. Kevin Kallas. We thank Keith Harenski for managing fMRI data collection, and Vince Calhoun and Rasmus Birn for consulting on fMRI analyses. Authors D.K., M.K. and P.D. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by grants from the National Institutes of Health R01MH090169 to David Kosson, and R01MH087525–01 and R01MH109329 to Jean Decety.
Footnotes
Financial disclosures
The authors declare no competing financial interests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2020.117342.
References
- Anastassiou-Hadjicharalambous X, Warden D, 2008. Cognitive and affective perspective-taking in conduct-disordered children high and low on callous-unemotional traits. Child Adolesc Psychiatry Ment Health 2 (16), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniskiewicz AS, 1979. Autonomic components of vicarious conditioning and psychopathy. J Clin Psychol. [DOI] [PubMed] [Google Scholar]
- Barbas H, 2007. Flow of information for emotions through temporal and orbitofrontal pathways. J. Anat 211, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, 2005a. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev. Psychopathol 17, 865–891. [DOI] [PubMed] [Google Scholar]
- Blair RJR, 2005b. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn 14, 698–718. [DOI] [PubMed] [Google Scholar]
- Blair RJR, 2010. Neuroimaging of Psychopathy and Antisocial Behavior: a Targeted Review. Curr Psychiatry Rep 12 (1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Jones L, Clark F, Smith M, 1997. The psychopathic individual: a lack of responsiveness to distress cues. Psychophysiology 34 (2), 192–198. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DGV, Blair K, 2005. The Psychopath: Emotion and the Brain. Blackwell Publishing, Oxford: . [Google Scholar]
- Brazil IA, Mathys CD, Popma A, Hoppenbrouwers SS, Cohn MD, 2017. Representational uncertainty in the brain during threat conditioning and the link with psychopathic traits. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Brook M, Kosson DS, 2013. Impaired Cognitive Empathy in Criminal Psychopathy: evidence From a Laboratory Measure of Empathic Accuracy. J Abnorm Psychol 122 (1), 156–166. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA, 2004. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage 22 (1), 252–257. [DOI] [PubMed] [Google Scholar]
- Cerniglia L, Bartolomeo L, Capobianco M, Russo Lo, Festucci F, Tambelli R, Cimino SSLM, 2019. Intersections and Divergences Between Empathizing and Mentalizing: development, Recent Advancements by Neuroimaging and the Future of Animal Modeling. Front Behav Neurosci 13 (September), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29 (3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA , 2017. FMRI Clustering in AFNI: false Positive Rates Redux. Brain Connect 7 (3), 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawel A, O’Kearney R, McKone E, Palermo R, 2012. Not just fear and sadness: meta–analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Reviews 36 (10), 2288–2304. [DOI] [PubMed] [Google Scholar]
- Decety J, 2015. The neural pathways, development and functions of empathy. Curr Opin Behav Sci 3, 1–6. [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA, 2013a. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front Hum Neurosci 7 (September), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA , 2015. Socioemotional processing of morally-laden behavior and their consequences on others in forensic psychopaths. Hum Brain Mapp 36 (6), 2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Jackson PL, 2004. The functional architecture of human empathy. Behav Cogn Neurosci Rev 3, 71–100. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Kiehl KA, 2013b. Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry 70 (6), 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Yoder KJ, Kiehl KA, 2014. Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci 9 (1), 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming P, Koenigs M, 2020. Functional neural correlates of psychopathy: a meta-analysis of MRI data. Transl Psychiatry 10 (133), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN, 2012. A Meta-analysis of Functional Neuroimaging Studies of Self- and Other Judgments Reveals a Spatial Gradient for Mentalizing in Medial Prefrontal Cortex. J Cogn Neurosci 24 (8), 1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Fullam RS, 2009. Psychopathy and Functional Magnetic Resonance Imaging Blood Oxygenation Level-Dependent Responses to Emotional Faces in Violent Patients with Schizophrenia. Biol. Psychiatry 66 (6), 570–577. [DOI] [PubMed] [Google Scholar]
- Drayton LA, Santos LR, Baskin-Sommers AR, 2018. Psychopaths fail to automatically take the perspective of others. Proceedings of the National Academy of Sciences 115 (13), 3302–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113 (28), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G, 2011. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 35 (3), 903–911. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Setsompop K, 2013. Ultra-Fast MRI of the Human Brain with Simultaneous Multi-Slice Imaging. Journal of Magnetic Resonance 229, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB , 2012. Structured Clinical Interview For DSM-IV Axis I Disorders (SCID-I), Clinical Version, Administration Booklet. American Psychiatric Pub. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F , Landi P, Allen P, Surguladze S, Politi P, 2009. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience 34 (6), 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A, 2004. Functional differences among those high and low on a trait measure of psychopathy. Biol. Psychiatry 56 (7), 516–521. [DOI] [PubMed] [Google Scholar]
- Haas BW, Brook M, Remillard L, Ishak A, Anderson IW, Filkowski MM, 2015. I know how you feel: the warm-altruistic personality profile and the empathic brain. PLoS ONE 10 (3), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD, 2003. The Hare psychopathy Checklist-Revised, 2nd ed Multi-Health Systems, Toronto. [Google Scholar]
- Hare RD , Harpur TJ, Hakstian AR, Forth AE, Hart SD , Newman JP, 1990. The revised Psychopathy Checklist: reliability and factor structure. Psychol Assess 2 (3), 338–341. [Google Scholar]
- Harris GT, Rice ME, Cormier CA, 1991. Psychopathy and violent recidivism. Law Hum Behav 15 (6), 625–637. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Bulten BH, Brazil IA, 2016. Parsing Fear: a Reassessment of the Evidence for Fear Deficits in Psychopathy. Psychol Bull 142 (6), 1–29. [DOI] [PubMed] [Google Scholar]
- House TH, Milligan WL, 1976. Autonomic responses to modeled distress in prison psychopaths. J Pers Soc Psychol 34 (4), 556–560. [DOI] [PubMed] [Google Scholar]
- Jones AP, Happé FGE, Gilbert F, Burnett S, Viding E, 2010. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry 51 (11), 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, 2006. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res 142, 107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Hoffman MB, 2011. The Criminal Psychopath: history, neuroscience, treatment, and economics. Jurimetrics 51, 355–397. [PMC free article] [PubMed] [Google Scholar]
- Koenigs MR, Baskin-Sommers AR, Zeier J, Newman JP, 2011. Investigating the neural correlates of psychopathy: a critical review. Mol. Psychiatry 16124, 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara MS, Deming P, Philippi CL, Decety J, Kosson DS, Koenigs MR, 2016. Impulsive-Antisocial Dimension of Psychopathy Linked to Enlargement and Abnormal Functional Connectivity of the Striatum. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB, 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function 214, 519–534 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Majdandžić J, 2015. The role of shared neural activations, mirror neurons, and morality in empathy - A critical comment. Neurosci. Res 90, 15–24. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB , Wager TD, Weber J, Barrett LF, 2016. The Brain Basis of Positive and Negative Affect: evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cerebral Cortex 26 (5), 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences 35, 121–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JHL, Barry CT, Sacco DF, 2016. Callous-unemotional traits and empathy deficits: mediating effects of affective perspective-taking and facial emotion recognition. Cognition and Emotion 30 (6), 1049–1062. [DOI] [PubMed] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, Koenigs MR, 2012. Cortical Thinning in Psychopathy. American Journal of Psychiatry 169 (11), 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, 1957. A study of anxiety in the sociopathic personality. J Abnorm Soc Psychol 55 (1), 6–10. [DOI] [PubMed] [Google Scholar]
- Meffert H, Gazzola V, den Boer JA, Bartels AAJ, Keysers C, 2013. Reduced spontaneous but relatively normal deliberate vicarious representations in psychopathy. Brain 136 (8), 2550–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Bosworth R, Nott Z, Louis WR, Smith JR, Amiot CE, Decety J, 2014. The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum Brain Mapp 35 (10), 4989–4999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Caramazza A, 2012. Conceptual Object Representations in Human Anterior Temporal Cortex. Journal of Neuroscience 32 (45), 15728–15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL , Wager T, Taylor SF , Liberzon I, 2002. Functional Neuroanatomy of Emotion: a Meta-Analysis of Emotion Activation Studies in PET and fMRI. Neuroimage 16, 331–348. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Koenigs MR, 2015. Altered Resting-State Functional Connectivity in Cortical Networks in Psychopathy. Journal of Neuroscience 35 (15), 6068–6078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon S, De Gelder B, Grézes J, 2012. Threat prompts defensive brain responses independently of attentional control. Cerebral Cortex 22 (2), 274–285. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Ramsey NF, Vink M , Van Den Heuvel MP, Kahn RS, 2006. Brain Activation During Antisaccades in Unaffected Relatives of Schizophrenic Patients. Biol. Psychiatry 59, 530–535 . [DOI] [PubMed] [Google Scholar]
- Schwenck C, Mergenthaler J, Keller K, Zech J, Salehi S, Taurines R , Freitag CM, 2012. Empathy in children with autism and conduct disorder: group-specific profiles and developmental aspects. Journal of Child Psychology and Psychiatry 53 (6), 651–659. [DOI] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, McCrory E, Foulkes L, Buon M, Roiser JP, Viding E, 2016a. Anticipation of guilt for everyday moral transgressions: the role of the anterior insula and the influence of interpersonal psychopathic traits. Sci Rep 6 (1), 36273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, Viding E, Roiser JP, 2016b. Affective resonance in response to others’ emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Soc Neurosci 11 (2), 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Dadds MR, Cecil CAM, Lockwood PL, De Brito SA , Viding E, 2012. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch. Gen. Psychiatry 69 (8), 814–822. [DOI] [PubMed] [Google Scholar]
- Sethi A, McCrory E, Puetz V, Hoffmann F, Knodt AR, Radtke SR, Viding E, 2018. Primary and Secondary Variants of Psychopathy in a Volunteer Sample Are Associated With Different Neurocognitive Mechanisms. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Harari H, Aharon-Peretz J, Levkovitz Y, 2010. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex 46 (5), 668–677. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K, 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. (Regul. Ed.) 13 (8), 334–340. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Dolan RJ, Kaube H, Frith CD, 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303 (5661), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, 2003. Psychopathy as a disorder of empathy. Eur Child Adolesc Psychiatry 12, 249–252. [DOI] [PubMed] [Google Scholar]
- Timbie C, Barbas H, 2014. Specialized Pathways from the Primate Amygdala to Posterior Orbitofrontal Cortex. Journal of Neuroscience 34 (24), 8106–8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Adult Intelligence Scales, 3rd ed Brace & Co., San Antonio: Harcourt. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA , 1998. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience 18 (1), 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC , Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Decety J, Koenigs MR , 2015. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp 36 (10), 4202–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


