Abstract
Objective:
Hand hygiene using either 4% chlorhexidine gluconate (CHG) or natural soap during hand rubbing, followed by alcohol-based 1% CHG sanitizer lotion in the operating room was compared to assess bacterial reduction, skin moisture, skin texture, and hand hygiene using qualitative questionnaires.
Approach:
A crossover study with 36 professional scrub nurses at two medical centers was performed to compare 4% CHG followed by alcohol-based 1% CHG sanitizer lotion, the Two-stage method with handwashing using natural soap followed by alcohol-based 1% CHG sanitizer lotion, and the Waterless method, after a period of 10 days of use. The study completely followed CONSORT, www.consort-statement.org.
Results:
There was no significant difference in bacterial reduction based on the bacterial colony-forming units between the two methods. The skin moisture and skin roughness scores were not significantly different between the two methods. The Waterless method was significantly better than the Two-stage method regarding “foaming,” “quality,” “longevity” (p < 0.0001, p < 0.0001, and p < 0.0001, respectively), but “disappearance” was significantly better by the Two-stage method (p = 0.0095) during washing and rubbing. Immediately after washing and rubbing, the Waterless method was significantly better regarding “tightness” and “moisture,” whereas the Two-stage method was significantly better regarding “stickiness” (p = 0.0114, p = <0.0001, and 0.0059, respectively)
Innovation:
The Waterless method using natural soap during handwashing followed by alcohol-based 1% CHG sanitizer lotion was as effective as the Two-stage method of 4% CHG followed by alcohol-based 1% CHG sanitizer lotion.
Conclusion:
Handwashing using natural soap is simple and superior to hand scrubbing in several aspects.
Keywords: Two-stage methods, Waterless method, hand scrubbing, hand rubbing lotion, surgical-site infection, chlorhexidine gluconate, natural soap

Sadanori Akita, MD, PhD
Introduction
Micropathogens and organisms that may cause surgical-site infections (SSIs), come from a variety of sources within the operating room, including the surgical members' hands. Members of the surgery team wear sterile gloves to prevent the transmission of bacteria from their hands to patients. Surgical gloves, however, may tear during surgery; therefore, it is necessary to keep the hands pathogen free. This is accomplished by cleansing the hands immediately before wearing sterile gloves before performing surgical procedures. Handwashing removes transient microorganisms, whereas surgical hand antisepsis is a further measure to inhibit the growth of resident microorganisms, thereby minimizing the risk of a patient developing an SSI.1 Surgical hand preparation plays an essential role in the prevention of SSIs, which are reported in 2–5% cases of inpatient surgery in the United States.2–4 Colonization of the causative pathogenic bacteria of SSIs leads to wound chronicity if the pathogens are not controlled properly.5 SSIs in patients are a direct high-risk factor for death and are considered to increase health care costs.2
A study of 3,317 patients undergoing clean and clean-contaminated surgery was performed to compare alcohol-based hand rubbing with plain soap and water. Based on follow-up data 30 days after discharge, SSIs developed in 255 patients (8.1%); 8.3% for alcohol-based hand rubbing versus 8.0% for plain soap and water (adjusted odds ratio 1.06, 95% confidence interval 0.81–1.38 odds ratio 1.03, 95% confidence interval 0.81–1.38 after adjusting for imbalances between study arms and clustering effects, which demonstrated no significant or clinical difference in SSI rates).6
In a comparative microbiological efficacy study of hand rubbing using alcohol-based solution or handwashing using nonmedical soap over 6 months in 50 health care workers, hand rubbing significantly reduced the microbiological load more than handwashing in the palms and fingertips. Hand rubbing is more efficient than handwashing for the decontamination of health care workers' hands following contact with patients and their environment. However, gloving itself may reduce microbiological hand contamination by transient pathogens during the health care procedures.7 Handwashing or hand rubbing also reduce viral contact with hands. In routine hand hygiene, soap and water or alcohol-based hand rubbing is effective against the influenza A virus on the hands.8 A povidone-iodine-containing soap was demonstrated to be superior to alcohol- or ethanol-based sanitizers against poliovirus, adenovirus, vacciniavirus, bovine viral diarrhea virus, feline calicivirus, and murine norovirus, in a finger pad test.9 Hand washing with soap is better for enteric and respiratory viral reduction than ethanol-based hand disinfectants.10 In a severe disease model, such as Ebola virus, test organisms of Escherichia coli and bacteriophage Phi 6, a nonpathogenic, biosafety-level 1 bacteriophage surrogate for the biosafety-level 4 Ebola virus, were effectively removed by soap and water handwashing with and without organic load added to simulate bodily fluids.11
An intensified hand-hygiene campaign of soap-and-water washing over 16 months in a total of 1,270 persons with 21 working designated cluster units, each including at least 50 office employees, demonstrated partial protection from gastrointestinal tract infection.12
Potassium oleate, a major component of natural soap, which is made from a salt of fatty acids generated from natural oils and fats, had bactericidal effects and removed Staphylococcus aureus biofilms while exhibiting reduced cytotoxicity toward mouse fibroblasts and human keratinocytes.13 It also inactivated human and avian viruses through exothermic interaction.14
We, therefore, performed a randomized single-blinded study to compare the effects of hand washing using natural soap followed by 1% chlorhexidine gluconate (CHG) sanitizer lotion with those of surgical scrubbing using 4% CHG followed by 1% CHG sanitizer lotion in the surgical setting. This study comprised the reduction factor (RF) of bacteria colonies using the finger pad test, clinical assessment of the hand, and questionnaires related to handwashing using natural soap and hand scrubbing with an antiseptic detergent.
Clinical Problem Addressed
Currently, various approaches are adopted for surgical hand hygiene, and they all aim to accomplish a high standard of hand hygiene practices crucial for preventing SSI, which may be the cause during surgery. Surgical hand scrubbing has been performed with scrubbing agents; and alcohol-based antiseptic agents are used to inhibit the growth of residual microorganisms and thereby decrease the hazard of SSI. Recently, the most common method, the Two-stage method, is a hand scrubbing with an antiseptic agent, followed by alcohol-based hand rubbing. Recently, the Waterless hand hygiene was introduced into the guideline for hand hygiene in health-care settings published in 2002 by the U.S. Centers for Disease Control and Prevention (CDC).15 The Waterless method is also a measure for surgical hand antisepsis using only nonantimicrobial soap and an alcohol-based hand rubbing. The Waterless hand rubbing formation contains 0.5% or 1% CHG and ethyl alcohol. Compared with conventional surgical hand scrubbing such as 4% CHG, the Waterless method may reduce the length of hand hygiene, diminish skin irritation, and reduce costs associated with hand antisepsis when the nonantimicrobial soap is a good quality for this purpose. It seems that there have yet been no studies comparing the two methods, focusing strictly on procedures in the operation room.
Materials and Methods
The study protocols
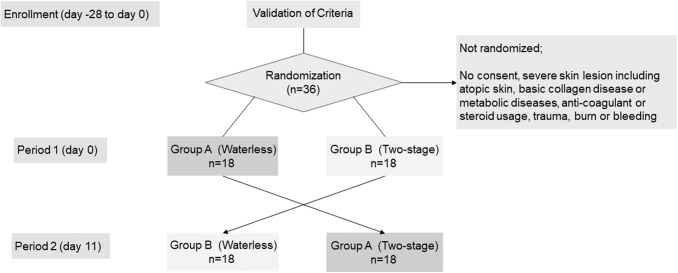
The details of the study protocol were explained to all candidate volunteers 2 weeks before registration. The inclusion and exclusion criteria are shown in Table 1. After registration, 36 registered nurses, 20 from Fukuoka University Hospital and 16 from the National Hospital Organization Nagasaki Medical Center with >6 months of experience as a scrub nurse were randomly allocated to two groups. Written informed consent was received from all participants. A crossover test was designed. In the first phase, Group A first used the Waterless method, handwashing with a natural soap followed by an alcohol-based 1% chlorhexidine sanitizer gluconate lotion, then the Two-stage method, scrubbing with antiseptic detergent composed of 4% CHG followed by alcohol-based 1% CHG sanitizer lotion and after 10 days. Group B performed the Two-stage method first and the Waterless method second after 10 days. Each group was switched to the other in 10 days (Fig. 1). The half of the participants in the Waterless method is assigned to the Two-stage method in 10 days and vice versa to the rest of the half of the participants.
Table 1.
Criteria for nurses to participate in the present study
| Inclusion criteria | |
| 1. | Written informed consent to participate in the study |
| 2. | Full-time scrub nurse |
| 3. | Engaged in surgery categorized as class I or class II in wound classification for >1 h and <4 h |
| 4. | Routinely using only chlorhexidine for surgical hand hygiene and disinfection |
| 5. | 20 Years of age or older |
| Exclusion criteria | |
| 1. | Markedly rough hands (three or more on a four-point scale judged from “Indication of severity” using Guidelines for the management of atopic dermatitis 2018 by a plastic surgeon) |
| 2. | Engaged in work with a higher risk of rough hands or hand injury than usual work and housework in daily life |
| 3. | Using hand cream containing steroids |
| 4. | Skin disease on the forearms, hands, or fingers |
| 5. | Use of oral or external medicine recently on washing areas due to injury, burn, ulcer, bleeding, collagen disease, systemic metabolic disease, etc. |
| 6. | Using immunosuppressants such as anticoagulants, antiplatelets, steroids, etc. |
| 7. | Taking medicine that may interfere with this study at the discretion of the family doctor |
Figure 1.
Flow of the study. Total 36 registered nurses from two hospitals were randomly allocated to two groups. Written informed consent was received from all participants. A crossover test was designed. In the first phase, Group A first used the Waterless method, handwashing with a natural soap, followed by an alcohol-based 1% chlorhexidine sanitizer gluconate lotion, then the Two-stage method, scrubbing with antiseptic detergent composed of 4% chlorhexidine gluconate followed by alcohol-based 1% chlorhexidine gluconate sanitizer lotion for a period of 10 days. Group B performed the Two-stage method first and the Waterless method second for 10 days. Each group was switched to the other after 10 days.
The study was approved by the Ethics Committee of the Clinical Research Network Fukuoka, Fukuoka, Japan, approval number 19-E06, and then secondarily approved by the dean of the Faculty of Medicine of Fukuoka University.
The evaluators for the bacterial colony count, skin moisture, skin roughness score, and questionnaires were blinded to the groups.
Participants engaged in surgery, which were classified as either clean wound or clean-contaminated wound surgeries, for 1 to 4 h.
Clean wound surgeries consisted of primarily closed noninflammatory and nonpenetrating wounds, and closed drainage was used if necessary. Clean-contaminated wound surgeries consisted of respiratory, digestive, or genital urinary tract organs without abnormal contamination, particularly involving the bile duct, appendix, vagina, and oral cavity unless infection or breakout was noted.
Each question was asked by a professionally trained interviewer to each participant. Bacterial colony counting was performed by an external institute, Kitakyushu Life Science Center, Public Interest Incorporated Foundation, Fukuoka, Japan.
The bacterial colony count, skin moisture, skin roughness scores, and the questionnaires were collected and securely stored at the Clinical Research Network Fukuoka, until analysis by data analyzers, T.A. and J.T., who only saw the data before statistical analysis.
Handwashing and hand scrubbing procedures
As hand hygiene products, Bubble Guard (Shabondama Soap Co., Ltd., Fukuoka, Japan), Hexizac Scrub (Yoshida Pharmaceutical Co., Ltd., Tokyo, Japan), and 3M™ Avagard™ 1% CHG Lotion (3M Japan Limited, Tokyo, Japan) were used for natural soap, 4% CHG and alcohol-based 1% CHG sanitizer lotion, respectively. Bubble Guard is a nonantibacterial soap composed only of water and fatty acid potassium. Hexizac Scrub and 3M Avagard 1% CHG Lotion are used for daily surgical hand hygiene in medical practice, and their main component is CHG.
The Waterless and Two-stage methods were strictly performed as described below, and there were observers watching during washing and disinfection.
Waterless method
-
1.
Wash the hands from the fingertips to the elbows with sterilized water for >10 s.
-
2.
Place liquid hand soap from the noncontact sensor dispenser to hands and wash gently and rigorously from the fingertips, palms, dorsum, interphalangeal spaces and webs, thumbs, forearms, and elbows for >60 s.
-
3.
Rinse soap from the hands with sterilized water for 10 to 15 s.
-
4.
Repeat the above steps three times.
-
5.
Wipe the hands with a sterilized paper towel.
-
6.
The disinfection procedure is the same as in the Waterless method.
Place and rub alcohol-based 1% CHG sanitizer lotion onto the right palm from the noncontact sensor dispenser, and rub the left fingers, left hand, left forearm, and left elbow for 1 min.
-
7.
Place and rub alcohol-based 1% CHG sanitizer lotion onto the left palm from the noncontact sensor dispenser, and rub the right fingers, right hand, right forearm, and right elbow for 1 min.
-
8.
Place and rub alcohol-based 1% CHG sanitizer lotion onto both the palms from the noncontact sensor dispenser, and rub both fingers, both hands, both forearms, and both elbows for 1 min.
Two-stage method
-
1.
Wash hands from the fingertips to the elbows with sterilized water for >10 s.
-
2.
Place scrubbing detergent with 4% CHG from the noncontact sensor dispenser on hands, and rub gently and rigorously from the fingertips, palms, dorsum, interphalangeal spaces and webs, thumbs, forearms, and elbows for >60 s.
-
3.
Rinse the detergent from the hands with sterilized water for 10 to 15 s.
-
4.
Repeat the above steps three times.
-
5.
Wipe the hands with a sterilized paper towel.
-
6.
The disinfection procedure is the same as in the Waterless method.
Place and rub alcohol-based 1% CHG sanitizer lotion onto the right palm from the noncontact sensor dispenser, and rub the left fingers, left hand, left forearm, and left elbow for 1 min.
-
7.
Place and rub alcohol-based 1% CHG sanitizer lotion onto the left palm from the noncontact sensor dispenser, and rub the right fingers, right hand, right forearm, and right elbow for 1 min.
-
8.
Place and rub alcohol-based 1% CHG sanitizer lotion onto the both palms from the noncontact sensor dispenser, and rub both fingers, both hands, both forearms and both elbows for 1 min.
Primary endpoint: bacterial count on hands
Sampling was performed according to the partially modified EN12791 method. Sampling fluid was plated on the spot, and all intervals between sampling and plating did not exceed 30 min. All plates were cultured within the same day.
For prehandwashing or hand scrubbing, each hand was soaked in 10 mL of Tryptic Soy Broth (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) for 1 min, and 100 μL of undiluted, 10 × or 100 × diluted soaked solution was dispersed and plated over Tryptic Soy Agar (TSA; Nissui Pharmaceutical Co., Ltd.) at test sites. Immediately after handwashing, the left hand was soaked in 10 mL of Soybean-Casein Digest Broth with Lecithin and Polysorbate 80 (SCDLP, DAIGO; Nihon Pharmaceutical Co., Ltd., Tokyo, Japan) for 1 min, and 1 mL of undiluted solution and 100 μL of undiluted and 10 × diluted solution were dispersed and plated over TSA at test sites. Postsurgery, the right hand was soaked, and the solution was plated in the same manner as for the left hand immediately after handwashing.
TSA plates were incubated at 37°C in a 5% CO2 incubator for 20 to 24 h and colony-forming units (CFUs), were calculated. After further incubation for 20 to 24 h, the plates were recounted, and the greater value was used. The RF, which was calculated as log(CFU/mL) (before washing and sanitizing/after washing and sanitizing on the left hand or after surgery on the right hand), was measured. To assess the culture system, before and after washing/sanitizing on the left hand and before and after surgery on the right hand were evaluated.
The log(CFU/mL) was calculated as follows:
-
1.
The bacterial count was recorded as it is.
-
2.
TSA plate count was expressed as 15 to 300 CFU and the error bar was set to 10%, thus the range of CFU was 14 to 330.
-
3.
If the CFU value was <14, it was recorded as the number.
-
4.
If the CFU value exceeded 330, it was recorded as >330.
-
5.
If suitable counts were obtained from two subsequent dilution steps, the weighted arithmetic mean from these counts was calculated using the following formula:
Z: the weighted mean CFU per mL; Ci: the CFU counted on plates retained for calculation; vi: the volume of inoculum on the plate in mL; di: the dilution factor corresponding to the sampling fluid; Subscript i means either of the two plates
Secondary endpoints
The skin moisture was measured before washing or scrubbing/sanitizing and immediately after surgery at six points
A compact and portable moisture meter was used (ASA-M2; Asahi Biomed, Yokohama, Japan). It weighed 250 g (200 g for the power supply and 50 g for the hand piece) and was able to detect Transepidermal Water Loss, using an effective contact coefficient,16 water quantity, and the thickness of the stratum corneum by formulating the value of the effective contact coefficient, evaluated by electrolytes in the stratum corneum. This machine records and analyzes conduction susceptibility using a low-frequency (160 Hz) alternate currency and detects conduction admittance using a high-frequency (143 kHz) alternate current.
The proposed formula is as follows:
Low- and high-frequency electric voltages were added to effectively enable these formula factors. The round probe of the hand piece was 5 mm in diameter, and detection was set to 5 s after probe contact with the subject to stabilize the electrodes and skin condition. The measurement of each contact point was always perpendicular to the subject, thereby avoiding unnecessary pressure or loading; it was repeated five times. The mean value of six adjacent points at least 10 mm apart and 20 mm from the edge of intact skin was assessed following the manufacturer's instructions.16 All evaluations were performed by a board-certified plastic surgeon.
The upper extremity skin roughness score
The upper extremity roughness score was evaluated before washing or scrubbing/sanitizing and immediately after surgery at six and three points, respectively, on each forearm. Four-stages from mild skin rash, score = 0, inflammatory skin rash occupying no >10% of the hand and forearm, score = 1, inflammatory skin rash occupying 10% to 30% of the hand and forearm, score = 2, inflammatory skin rash occupying >30% of the hand and forearm, score 3 were evaluated by a board-certified plastic surgeon under a dermoscope.17
Questionnaires
Each participant was independently questioned by professionally trained interviewers while washing or rubbing hands, and right after completion of handwashing or hand scrubbing/sanitization. Each degree was evaluated 1 = low to 4 = high in four-integer points.
Questionnaire during washing or scrubbing hands
-
1.
How is the “foam” of the detergent or soap?
-
2.
How fine or coarse is the “foam” of the detergent or soap?
-
3.
How long is the “foam” of the detergent or soap lasting?
-
4.
How sensitive is the skin to the detergent or soap?
-
5.
How quickly does the “foam” of the detergent or soap disappear?
Questionnaire right after completion of washing or scrubbing/sanitizing of hands
-
1.
How tight is the skin after washing or scrubbing?
-
2.
How dry is the skin after washing or scrubbing?
-
3.
How moist is the skin after washing or scrubbing?
-
4.
How sticky is the skin after washing or scrubbing?
-
5.
How itchy is the skin after washing or scrubbing?
Statistics
For the primary endpoint, we estimated needing 36 subjects to detect an RF value difference of 0.3 between the Two-stage group and Waterless group, with a standard deviation value of 0.6, power of 0.8, index of Individual difference of 1.0 and dropout rate of 55%. Repeated measure analysis of variance of RF for objective variance and group, subject, detergent, and timing for explanatory variances was used for the primary endpoint. Carryover and period effects were adjusted for detergents, either soap or scrubbing detergent. The Wilcoxon rank sum test was used for skin moisture, upper extremity roughness score, and questionnaires by measuring the difference postmethod in period 1 and then subtracting the difference in period 2.
JMP® 14 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. p-Values <0.05 were considered significant.
Results
There were no significant health problems or serious adverse events among the participants in this study, including the nurses, surgeons, other health care professionals, surgical patients, and investigators. No patients developed SSIs and there were no infections in the surgical ward for up to 2 months, postoperatively. The study was uneventful, and subject to data storage and analysis.
Primary endpoint: bacterial count on hands
The bacterial colony count on index finger pads of the left hand was compared between pre- and immediately postmethods. Sixteen of 36 participants were excluded from analysis because the CFU immediately after handwashing or hand scrubbing were significantly greater. Among the 16, 3 used both methods, 10 used only the Waterless method, and 3 used only the Two-stage method.
The RF in Group A (first Waterless and then Two-stage) was 0.93 ± 0.84 and 1.34 ± 1.29 in the Period 1 and Period 2, respectively, and there was a difference of −0.40 ± 1.79 between the Waterless and Two-stage methods. The RF in Group B (first Two-stage and then Waterless) was 1.43 ± 0.92 and 0.95 ± 1.04 in Period 1 and Period 2, respectively, and there was a difference of −0.47 ± 1.67 between the Waterless and Two-stage methods. The least squares of the mean difference were −0.44 (−1.25 to 0.39, 95% confidence interval) and there was no significant difference (p < 0.2794) (Table 2).
Table 2.
Reduction factor pre- and immediately after handwashing or hand scrubbing
| Period 1 | Period 2 | Within Subject Difference (WL − TS) | |
|---|---|---|---|
| Group A | |||
| RF (SD) | 0.93 (0.84) | 1.34 (1.29) | −0.40 (1.79) |
| Subject (number) | 11 | 11 | 11 |
| Group B | |||
| RF (SD) | 1.43 (0.92) | 0.95 (1.04) | −0.47 (1.67) |
| Subject (number) | 9 | 9 | 9 |
| Comparison of RF | |||
| Least squares of mean difference (95% confidence interval) | −0.44 (−1.25 to 0.39) | ||
| Subject (number) | 20 | ||
| Paired analysis | p = 0.2794 | ||
RF, reduction factor; SD, standard deviation; TS, Two-stage method; WL, Waterless method.
Secondary endpoints
Skin moisture
There was no significant difference between the Waterless and Two-stage methods. The mean value subtracting the Two-stage method from the Waterless method was 1.98 (95% confidence interval: 2.52–6.47, p = 0.3781) (Table 3).
Table 3.
Skin moisture pre- handwashing or hand scrubbing and postsurgery in each group
| Group A |
Group B |
Difference (WL − TS) |
||||
|---|---|---|---|---|---|---|
| Period 1 (WL) | Period 2 (TS) | Period 1 (TS) | Period 2 (WL) | Mean | p | |
| Prehandwashing | 26.8 (10.9) | 28.9 (12.6) | 25.1 (5.9) | 29.0 (11.8) | ||
| Prehand scrubbing | ||||||
| Postsurgery | 29.2 (10.4) | 23.2 (6.9) | 26.2 (10.9) | 25.8 (7.1) | ||
| Difference | 2.5 (9.8) | −5.7 (10.9) | 1.1 (11.1) | −3.2 (8.4) | 1.98 (95% CI: −2.52 to 6.47) | 0.3781 |
| Postsurgery | ||||||
| Prehandwashing or Prehand scrubbing | ||||||
CI, confidence interval.
Upper extremity skin roughness score
All subjects scored 0 and further statistical analysis was not possible.
Questionnaires
The Waterless method was significantly better than the Two-stage methods regarding “foaming,” “quality,” and “longevity” (p < 0.0001, p < 0001, and p < 0.0001, respectively), but “disappearance” was significantly better with the Two-stage method (p = 0.0095) during washing and rubbing. Immediately after washing and rubbing, the Waterless method was significantly better regarding “tightness” and “moisture,” whereas the Two-stage method was significantly greater regarding “stickiness” (p = 0.0114, <0.0001 and 0.0059, respectively) (Table 4).
Table 4.
Questionnaires
| Group A |
Group B |
Difference (WL − TS) |
||||
|---|---|---|---|---|---|---|
| Period 1 (WL) | Period 2 (TS) | Period 1 (TS) | Period 2 (WL) | p | Significance | |
| During | ||||||
| Foaming | 3.3 (0.8) | 2.1 (0.6) | 2.3 (1.1) | 3.7 (0.5) | <0.0001 | WL |
| Quality | 3.3 (0.7) | 2.3 (0.8) | 2.6 (1.0) | 3.5 (0.7) | <0.0001 | WL |
| Longevity | 2.9 (1.0) | 2.2 (0.9) | 2.6 (1.0) | 3.7 (0.5) | <0.0001 | WL |
| Sensitive | 4.0 (0.0) | 3.9 (0.2) | 3.8 (0.7) | 3.8 (0.7) | 0.6206 | No significance |
| Disappearance | 2.8 (1.0) | 3.5 (0.9) | 3.5 (0.9) | 3.1 (1.0) | 0.0095 | TS |
| Immediately after | ||||||
| Tight | 3.9 (0.2) | 3.6 (0.6) | 3.7 (0.7) | 3.9 (0.3) | 0.0114 | WL |
| Dried | 3.8 (0.4) | 3.8 (0.5) | 3.6 (0.8) | 3.9 (0.4) | 0.2192 | No significance |
| Moist | 3.3 (0.7) | 3.1 (0.8) | 2.9 (0.8) | 3.3 (0.7) | <0.0001 | WL |
| Sticky | 3.0 (0.9) | 3.1 (1.0) | 3.7 (0.7) | 3.1 (0.7) | 0.0059 | TS |
| Itchy | 3.9 (0.3) | 3.8 (0.4) | 3.8 (0.7) | 3.9 (0.3) | 0.7657 | No significance |
Discussion
Clinical comparison of the Waterless and Two-stage methods among surgical nurses is considered consistent because the surgical ward strictly manages cleanliness.18 There are few studies on the influence of operating room behaviors on the risk of infection.19 One study on pediatric surgeries, which often allow Teddy bears, demonstrated 15% to be positive for potential pathogenic bacteria and 6% for fungi.20
In a randomized controlled study investigating the effectiveness of the three antiseptic methods, conventional 10% povidone-iodine scrub, conventional 4% chlorhexidine scrub, or waterless hand rubbing (1% CHG and 61% ethyl alcohol), the CFU was reduced by 4% by conventional, chlorhexidine scrub, or waterless hand rub compared with the 10% by povidone-iodine scrub in 80 enrolled surgical staff members.21 This study is similar to ours except for the types of antiseptic agents. We used the Two-stage method, 4% chlorhexidine scrub followed by alcohol-based 1% CHG sanitizer lotion, and the Waterless method, handwashing with natural soap followed by alcohol-based 1% CHG sanitizer lotion. Sanitizer was used in both methods. In our study, after using 4% w/v CHG, as a liquid or foam, the RF 1 h posthand scrubbing was 1.99 ± 0.64. The estimated RF for the Two-stage method was 2.0, thus that for the Waterless method was set as 2.3 considering its superiority and the standard deviation was estimated to be 0.6. The required number of participants for a parallel group comparison study was 63. Considering the individual difference index obtained by the ratio intersubject variation to intrasubject variation to be 1.0 and the dropout rate to be 55%, the required number of participants in both groups was 18 or 36 for a crossover study.22
There was no significant difference between the Two-stage method and Waterless method in our analysis. In our study, both methods included the alcohol-based 1% CHG sanitizer, which was different from previous studies.6,7,21 A retrospective cohort study of orthopedic surgery demonstrated no significant difference in the incidence of SSIs between a standard method of antimicrobial chlorhexidine and the Waterless method followed by alcohol-based CHG.23 No SSIs developed for at least 2 months postoperatively and the RF after surgery in both groups was not significantly different (data not shown).
The skin moisture and skin roughness scores were not significantly different, and the condition for bacterial analysis was not affected. Based on the questionnaires, the Waterless method using natural soap was significantly better regarding “foaming,” “quality,” and “longevity” during washing or scrubbing, and “tightness” and “moisture” immediately after washing and scrubbing than the Two-stage method. On the other hand, the Two-stage method resulted in “disappearance” during washing or scrubbing and “stickiness.”
The Waterless method was as effective as the Two-stage method for reducing bacteria on the hand and more useful in practice according to the participants.
Innovation
The Waterless method is as effective as the Two-stage method in hand hygiene in an operation room. When the nonantiseptic soap in the Waterless method is a natural soap made from only natural ingredients, more subjective parameters of the questionnaires in the Waterless method is significantly favorable than those in the Two-stage method, where antiseptic detergent is used. It is recognized that a nonantiseptic soap is useful for hand hygiene in an operation room.
Limitation of the study
This crossover prospective study is relatively small even though it is statistically sound. The data of the bacterial count in this study may need to be further evaluated, a more specific method in bacteriology, such as a next-generation sequence, to clarify bacterial growth state and the community composition. A large-number study may help understand more consistently the direct data.
Key Findings
No significant difference in Two-stage method or Waterless method in a bacterial reduction factor before and after hand scrubbing or washing followed by sanitization.
During the procedure, the Waterless method is significantly superior in foaming, quality, and longevity, whereas the Two-stage method is significantly better in disappearance.
Immediately after the procedure, the Waterless method is significantly superior in tightness, and moisture, whereas the Two-stage method is significantly sticky.
Acknowledgment and Funding Sources
This study was partly supported by nonrestricted fund from the Shabondama Soap CO., Ltd.
Abbreviations and Acronyms
- CDC
Centers for Disease Control and Prevention
- CHG
chlorhexidine gluconate
- SCDLP
Soybean-Casein Digest Broth with Lecithin and Polysorbate 80
- TSA
Tryptic Soy Agar
Author Contributions
Conceptualization, A.M., T.K., and S.A.; methodology, T.A., J.T., and S.A.; validation, M.F. and J.T.; statistical analysis, T.A. and J.T; investigation, M.F. and S.A.; data curation, T.A. and J.T.; writing—original draft preparation, T.A., J.T., and S.A.; writing—review and editing, M.F. All authors read and agreed to the published version of the article.
Author Disclosure and Ghostwriting
The authors declare no conflict of interest, and ghostwriting services were not used.
About the Authors
Sadanori Akita, MD, PhD, is a professor and chief at the Department of Plastic Surgery, Wound Repair and Regeneration, School of Medicine, Fukuoka University, Fukuoka, Japan. Masaki Fujioka, MD, PhD, is a chief at the Department of Plastic and Reconstructive Surgery, National Hospital Organization, Nagasaki Medical Center, Nagasaki, Japan. Tomoyuki Akita, PhD, is an associate professor at the Department of Epidemiology, Infectious Disease Control and Prevention, Graduate School of Biomedical & Health Science, Hiroshima University, Hiroshima, Japan. Junko Tanaka, PhD, is a professor and chief at the Department of Epidemiology, Infectious Disease Control and Prevention, Graduate School of Biomedical & Health Science, Hiroshima University, Hiroshima, Japan and a vice president of Hiroshima University. Akihiro Masunaga, BS, is a senior researcher at Shabondama Soap Co., Ltd., Fukuoka, Japan, and Takayoshi Kawahara, PhD, is a chief scientific researcher at Shabondama Soap Co., Ltd., Fukuoka, Japan.
References
- 1. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. WHO Guideline Series. Geneva: World Health Organization, 2009. [Google Scholar]
- 2. Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project—statistics on hospital stays. 2013. [Google Scholar]
- 4. Scott RD. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. Atlanta: Centers for Disease Control and Prevention, 2009. [Google Scholar]
- 5. Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol 2017;73:710–721. [DOI] [PubMed] [Google Scholar]
- 6. Nthumba PM, Stepita-Poenaru E, Poenaru D, et al. Cluster-randomized, crossover trial of the efficacy of plain soap and water versus alcohol-based rub for surgical hand preparation in a rural hospital in Kenya. Br J Surg 2010;97:1621–1628. [DOI] [PubMed] [Google Scholar]
- 7. Kac G, Podglajen I, Gueneret M, Vaupré S, Bissery A, Meyer G. Microbiological evaluation of two hand hygiene procedures achieved by healthcare workers during routine patient care: a randomized study. J Hosp Infect 2005;60:32–39. [DOI] [PubMed] [Google Scholar]
- 8. Grayson ML, Melvani S, Druce J, et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis 2009;48:285–291. [DOI] [PubMed] [Google Scholar]
- 9. Steinmann J, Paulmann D, Becker B, Bischoff B, Steinmann E, Steinmann J. Comparison of virucidal activity of alcohol-based hand sanitizers versus antimicrobial hand soaps in vitro and in vivo. J Hosp Infect 2012;82:277–280. 23. [DOI] [PubMed] [Google Scholar]
- 10. Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Duizer E, Beumer RR. Reducing viral contamination from finger pads: handwashing is more effective than alcohol-based hand disinfectants. J Hosp Infect 2015;90:226–234. [DOI] [PubMed] [Google Scholar]
- 11. Wolfe MK, Gallandat K, Daniels K, Desmarais AM, Scheinman P, Lantagne D. Handwashing and Ebola virus disease outbreaks: a randomized comparison of soap, hand sanitizer, and 0.05% chlorine solutions on the inactivation and removal of model organisms Phi6 and E. coli from hands and persistence in rinse water. PLoS One 2017;12:e0172734..eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hovi T, Ollgren J, Savolainen-Kopra C. Intensified hand-hygiene campaign including soap-and-water wash may prevent acute infections in office workers, as shown by a recognized-exposure -adjusted analysis of a randomized trial. BMC Infect Dis 2017;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawahara T, Takita M, Masunaga A, et al. Fatty acid potassium had beneficial bactericidal effects and removed Staphylococcus aureus biofilms while exhibiting reduced cytotoxicity towards mouse fibroblasts and human keratinocytes. Int J Mol Sci 2019;20:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawahara T, Akiba I, Sakou M, Sakaguchi T, Taniguchi H. Inactivation of human and avian influenza viruses by potassium oleate of natural soap component through exothermic interaction. PLoS One 2018;13:e0204908..eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002;51:1–45. [PubMed] [Google Scholar]
- 16. Akita S, Hayashida K, Yoshimoto H, et al. Novel application of cultured epithelial autografts (CEA) with expanded mesh skin grafting over an artificial dermis or dermal wound bed preparation. Int J Mol Sci 2017;19. pii: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katoh N, Ooya Y, Ikeda M, et al. Clinical guidelines for atopic dermatitis. Jpn J Dermatol 2018;12812:2431–2502. [Google Scholar]
- 18. Hassen Y, Singh P, Pucher PH, Johnston MJ, Darzi A. Identifying quality markers of a safe surgical ward: an interview study of patients, clinical staff, and administrators. Surgery 2018;163:1226–1233. [DOI] [PubMed] [Google Scholar]
- 19. Birgand G, Saliou P, Lucet JC. Influence of staff behavior on infectious risk in operating rooms: what is the evidence? Infect Control Hosp Epidemiol 2015;36:93–106. [DOI] [PubMed] [Google Scholar]
- 20. Hardy A, Sabatier V, Rosello O, Salauze B, Barbut F, Vialle R. More than just teddy bears: unconventional transmission agents in the operating room. Arch Pediatr 2018;25:416–420. [DOI] [PubMed] [Google Scholar]
- 21. Tsai JC, Lin YK, Huang YJ, et al. Antiseptic effect of conventional povidone-iodine scrub, chlorhexidine scrub, and waterless hand rub in a surgical room: a randomized controlled trial. Infect Control Hosp Epidemiol 2017;38:417–422. [DOI] [PubMed] [Google Scholar]
- 22. Hosoya J, Satoh T, Shiraishi T. Effect of surgical hand-scrubbing and its impact on skin, and evaluation of use of foam scrub pharmaceutical including 4 w/v% chlorhexidine gluconate. Jpn J Environ Infect 2014;29:189–195. [Google Scholar]
- 23. Iwakiri K, Kobayashi A, Seki M, et al. Waterless hand rub versus traditional hand scrub methods for preventing the surgical site infection in orthopedic surgery. Spine (Phila Pa 1976) 2017;42:1675–1679. [DOI] [PubMed] [Google Scholar]