Abstract
Objective:
MiRNAs are important regulators of inflammation and wound healing. However, the mechanisms through which miRNAs regulate wound healing under normal and diabetic conditions are poorly understood. We aimed to determine the effects of miR-146a on the pathogenesis of wound healing in normal and streptozotocin (STZ)-induced diabetic mice.
Approach:
Wild-type (WT) and miR-146a knockout (KO) mice were induced to develop diabetes with STZ. Next, skin and corneal wounds were produced and measured. Percent wound closure and histology were evaluated. Inflammation at wound sites was analyzed using flow cytometry, reverse-transcription PCR, and western blot.
Results:
Healing of wounded skin was significantly delayed in miR-146a KO compared with WT mice. However, corneal epithelial wound healing did not differ significantly in the mice with normal blood glucose, whereas corneal and skin wound healing was significantly delayed in KO mice with diabetes. Neutrophil infiltration increased in skin wounds of KO compared with normal mice. The potential mechanisms were associated with dysregulated interleukin 1β, tumor necrosis factor alpha (TNF-α), IRAK1 (interleukin-1 receptor-associated kinase 1), TRAF6 (TNF receptor-associated factor 6), and nuclear factor kappa B (NF-κB) signaling induced by miR-146a KO.
Innovation:
Skin wound healing was delayed in miR-146a KO mice and enhanced inflammatory responses were mediated by the NF-κB signaling pathway.
Conclusions:
Deficiency in miR-146a delayed skin wound healing by enhancing inflammatory responses in normal and diabetic mice. Therefore, miR-146a may be a potential target for modulation to accelerate skin wound healing.
Keywords: microRNA, miR-146a, inflammation, diabetes, wound healing

Jun Gu, MS

Qing-Sheng Mi, MD, PhD
Introduction
Chronic wounds impose a substantial social and economic burden worldwide. An estimated US $25 billion is spent annually on treating chronic wounds in the United States. The situation is gradually being aggravated owing to an increase in the elderly population, and a corresponding rise in the incidence of diabetes and impaired venous and arterial circulation.1–3 Delayed wound closure is common in the skin and corneas of patients with diabetes. Wound healing comprises inflammatory, proliferative, and remodeling stages, in which keratinocytes, fibroblasts, endothelial cells, neutrophils, macrophages, and platelets play significant roles.4–6 Dysregulation of these cell interactions and prolonged inflammation results in delayed wound healing.
Key roles played by microRNA (miRNA) in human pathological and physiological process have recently been confirmed. Changes in specific miRNA expression at different stages of the wound healing process can be associated with aberrant wound healing.7–11 Xu et al. reported that wound healing is impaired and delayed in diabetic, compared with nondiabetic mice. The significantly downregulated expression of miR-146a in wounds in diabetic mice correlates with the increased expression of proinflammatory target genes.12 The regulatory functions of miR-146a in wound healing are also explored in the field of ophthalmology. Funari reported that miR-146a expression was upregulated 3.5-folds in diabetic central corneas compared with normal ones through miRNAs microarray analysis. The abnormal expression contributes to the impaired diabetic corneal ulcer.13–15 The investigation miR-146a has shown that it is extensively involved in the innate immune system as a negative regulator of inflammation. Macrophages are regulated by miR-146a, which promotes the production of cytokines and growth factors necessary for monocyte differentiation into macrophages.16,17 Some evidence indicates that miR-146a upregulation exerts a protective effect by downregulating inflammation-related genes, resulting in the suppression of proinflammatory and inflammatory target gene activation.
However, the effects of miR-146a on wound healing remain controversial, and the mechanisms of miR-146a in diabetic chronic wound healing require further investigation.15 We speculated that miR-146a deficiency might be responsible for the abnormal inflammation in skin wounds of miR-146a knockout (KO) mice and subsequently delayed wound healing. Therefore, we assessed the expression of interleukin (IL)-1β and tumor necrosis factor alpha (TNF-α) in miR-146a KO and wild-type (WT) mice with or without diabetes mellitus (DM). We also investigated the expression of TNF receptor-associated factor 6 (TRAF6), interleukin-1 receptor-associated kinase 1 (IRAK1), and nuclear factor kappa B (NF-κB), which are important signaling proteins in the inflammatory response.
Clinical Problem Addressed
Inflammation plays a key role in chronic wounds, especially in patients with DM. So it is of great clinical significance to explore whether miR-146a is a potential target that could be a new treatment methods.
Materials and Methods
Mice generation and induction of diabetes
The miR-146a KO and C57BL/6 WT mice were purchased from Jackson Laboratory (Bar Harbor, ME) were housed under standard conditions with water and chow ad libitum. The offspring were genotyped and confirmed using specific PCR primer pairs. Handling of mice and experimental procedures were approved by the Institutional Animal Care and Use Committee. Diabetes was induced in the miR-146a KO and WT by the repeated intraperitoneal administration of low doses of streptozotocin (STZ) as described.18,19 Ten weeks later, mice with blood glucose >350 mg/dL were considered diabetic and age-matched mice with normal blood glucose levels were the controls.
Skin punch wound model
We used the excisional punch wound model as described.20 In brief, 8-week-old KO and WT mice were anesthetized with an intraperitoneal injection of ketamine and xylazine. The fur on the dorsal surface was shaved with animal clippers and full-thickness dermal excisional wounds (6-mm diameter) were produced on the dorsum of each mouse by excising the epidermis, dermis, and panniculus with a biopsy punch. The diameter of the wound was measured three times at each time points using an Absolute Digimatic Caliper Series 500 (Mitutoyo, Japan).
Wounds were photographed using a digital camera (Nikon Corp., Tokyo, Japan) on a fixed bracket on the day of the punch and every 2 days thereafter. Wound areas were calculated as ratios (%) of the areas of original wounds. Wounds were considered completely closed when the wound area was zero. The amount of wound closure at each time point was calculated as:
Wound Closure % = 100 × (Wound Area on Day 0-Area of Inner circumference on Day X)/Wound Area on Day 0.
Corneal epithelial debridement wound
Corneal epithelial debridement wounds were created as described.21 Central corneas in anesthetized mice were demarcated with a trephine (diameter, 2.0 mm), then corneal epithelial cells (CECs) were removed using a brush or narrow blunt scalpel blade under a dissecting microscope with a photographic system (Carl Zeiss Microscopy GmbH, Jena, Germany). We avoided injuring the epithelial basement membrane, corneal stroma, and limbus. Wounded corneas were stained with 0.1% fluorescein sodium immediately or at different time points, washed with 1 × PBS, and photographed.
Histological analysis
Full-thickness excisional skin wounds were isolated, bisected, fixed overnight in 10% neutral-buffered formalin, and embedded in paraffin wax. Sections (5 mm) from the middle of the wound were stained with hematoxylin and eosin (H&E) for morphological analysis. Inflammatory cell infiltration of wounds was evaluated and the sections were photographed.
Flow cytometry
Single cell skin suspensions were obtained using a modification of the described method.22 Wound tissues were harvested, cut into 2-mm2 pieces incubated, and shaken for 2 h at 37°C in 20 mL RPMI medium containing 1 mg/mL hyaluronidase I (Sigma-Aldrich Corp., St. Louis, MO), 1 mg/mL collagenase D, and 1 U/mL DNase I (Sigma). Suspensions were agitated with a 10 mL pipette and passed through a 70 μm cell strainer, centrifuged at 250 g for 10 min at 4°C, washed twice in PBS, then resuspended in 1 mL PBS containing 2% FBS and 0.1% sodium azide. The suspensions were stained with antibodies against cluster of differentiation (CD)11b and granulocyte receptor-1 (Gr1; BD Biosciences, San Jose, CA) by rocking them overnight at 4°C. The cells were washed twice and resuspended in 0.5 mL PBS for flow cytometry using an FACS AriaII (BD Biosciences) and analyzed using FlowJo 7.6 software (TreeStar, Ashland, OR).
RNA extraction and quantitative reverse transcription PCR
Total RNA from skin wounds was extracted using Trizol (Invitrogen, Carlsbad, CA) and run on a Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA) to ensure an A260/A280 ratio in the range of 1.8–2.2. The RNA was reverse transcribed using cDNA Reverse Transcription Kits (Applied Biosystems, Carlsbad, CA). After preamplification, gene expression was assessed on a 7900HT Fast Real-Time PCR System (Applied Biosystems) as described by the manufacturer. Gene expression of IL-1β, TNF-α, TRAF6, IRAK1, NF-κB, and GAPDH was assayed using SYBR green and quantitative reverse transcription PCR (qRT-PCR), and the results were analyzed using the 2−△△Ct method. The primer sequences are given in Table 1. Meanwhile, the total RNA including miRNA fraction was also analyzed using miRNA qRT-PCR. First, miRNA were reverse transcribed to complementary DNA (cDNA) using TaqMan miRNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA) and then were amplified. Signals were normalized to U6 small-nuclear RNA.
Table 1.
Primer sequences used for quantitative reverse transcription PCR
| Gene | Forward sequences (5′–3′) | Reverse sequences (5′–3′) |
|---|---|---|
| IL-1β | GGGCCTCAAAGGAAAGAATC | CTCTGCTTGTGAGGTGCTGA |
| TNF-α | ACAAAGGTGCCGCTAACCACATGT | ATGCTGCTGTTTCAGTCGAAGGCA |
| IRAK1 | GAGACCCTTGCTGGTCAGAG | GCTACACCCACCCACAGAGT |
| TRAF6 | ATTTCATTGTCAACTGGGCA | TGAGTGTCCCATCTGCTTGA |
| NF-κB | ACAGCAGATGGCCCATACCT | CATACATAACGGAAACGAAATCCTCT |
| GAPDH | GGTGAAGGTCGGTGTGAACG | TGTAGACCATGTAGTTGAGGTCA |
Western Blot analysis
Wound tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer, and protein concentrations were determined using protein assay kits (Thermo Fisher Scientific, Inc., Waltham, MA). Protein samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Nonspecific protein binding was blocked in I-Block™ Protein-based Blocking Reagent (Thermo Fisher Scientific, Inc.) for 30 min at room temperature, then the membranes were incubated with primary IL-1β, TNF-α, TRAF6, IRAK1, and NF-κB antibodies (Santa Cruz Biotechnology, Dallas, TX) overnight at 4°C. The membranes were extensively washed in TBST and incubated with secondary antibodies for 2 h at room temperature. Bands were visualized using enhanced chemiluminescence (ECL; SuperSignal; Thermo Fisher Scientific, Inc.), and images were acquired using Kodak Image Station 4000R Pro (Carestream Health, Inc., Rochester, NY). Luminescence intensity was analyzed using ImageJ software (Rawak Software, Inc., Stuttgart, Germany).
Statistical analysis
Data were presented as mean ± SEM. Electronic laboratory notebook was not used to collect the data. Statistical analysis was performed using the Prism 5 software (GraphPad Software, La Jolla, CA). We used Student's t test to compare two groups of data and one-way or two-way analysis of variance (ANOVA) was used to compare multiple groups. For all statistical tests, p < 0.05 was considered to be statistically significant and *p < 0.05, ** p < 0.01, ***p < 0.001.
Results
Delayed skin wound healing in miR-146a KO mice compared with WT mice
Skin wound healing was significantly delayed in the miR-146a KO, compared with controls, and in diabetic miR-146a KO, compared with WT mice (Fig. 1A). The incidences of diabetes and blood glucose levels in miR-146a KO and WT control mice were comparable as we previously found.23 The speed of wound closure was WT > miR-146a KO > DM-WT > DM-miR-146a KO (Fig. 1B). Figure 1C shows the miR-146a expression levels in the four animal models.
Figure 1.
MiR-146a deficiency delayed the skin wounds healing in mice model. (A) Representative photographs of wound healing in different groups after wounding from days 0, 2, 4, 8, 10, and 12. (B) The percent of the wound healing at each time point after the injury in miR-146a WT (n = 3), KO mice (n = 4), diabetic miR-146a KO (n = 4) and WT mice (n = 4). (C) The gene expression of miR-146a in the four different group animal models (n = 3 separately). Wound measurements results indicated average of three experiments. Statistical determinations were made by two-way ANOVA (B) and by one-way ANOVA (C). Data are presented as mean ± SD. Statistical significance was determined as *p < 0.05, **p < 0.01. ANOVA, analysis of variance; KO, knockout; WT, wild type.
Corneal epithelium wound healing in miR-146a KO mice
The structure of the cornea is analogous to that of the epidermis,24 and miR-146a expression is similar in these tissues. Epithelial proliferation plays a significant role in corneal wound healing, but we did not find a significant difference in corneal epithelial wound healing between miR-146a KO and WT mice. However, wound healing in diabetic mice was delayed, and significantly differed between diabetic miR-146a KO and WT mice (Fig. 2).
Figure 2.
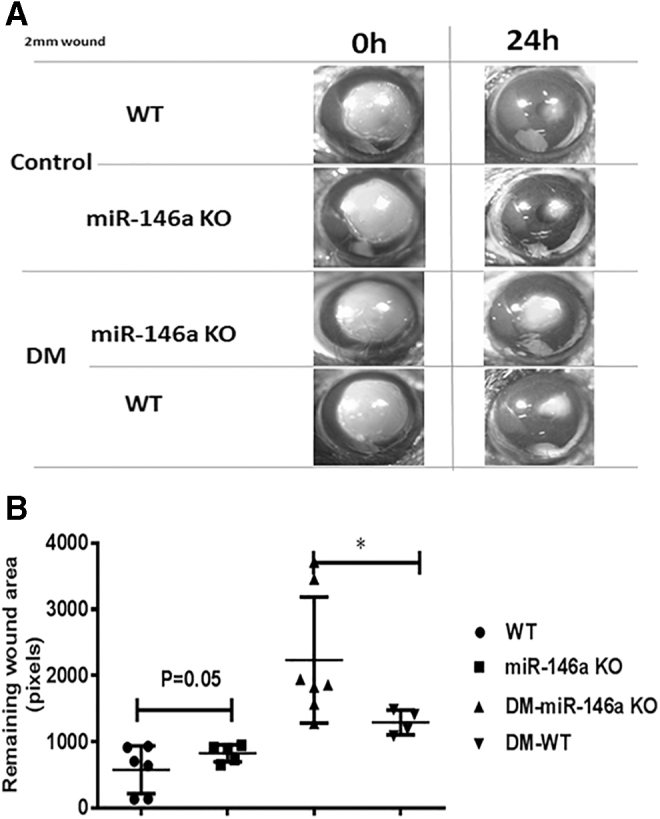
Effect of miR-146a KO and diabetic condition on corneal wound healing. (A) Photographs of corneal wounds in miR-146a WT, KO mice, diabetic miR-146a KO and WT mice at 0 and 18 h after wounding. (B) The percent of the wound healing area at 0 and 18 h after the injury in miR-146a WT (n = 6), KO mice (n = 5), diabetic miR-146a KO (n = 7) and WT mice (n = 4). Wound measurement results indicated an average of three experiments. Statistical determinations were made by Student's two-tailed t-test. Statistical significance was determined as *p < 0.05.
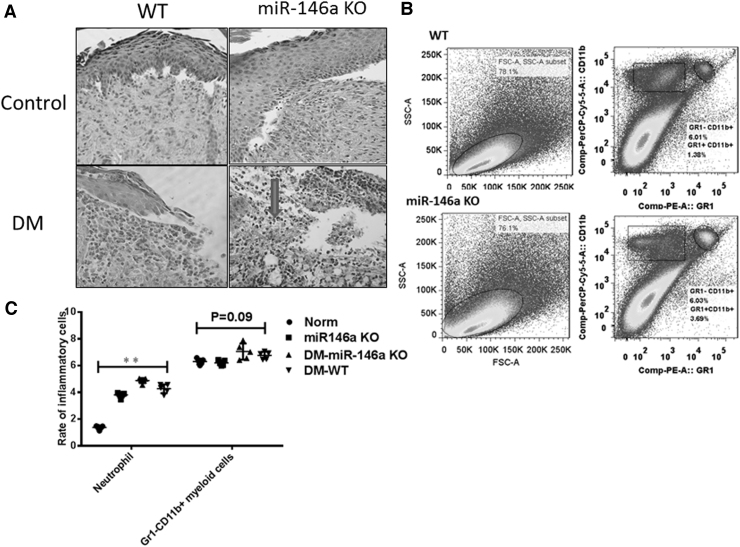
More inflammatory cells infiltrated in skin wounds of miR-146a KO and diabetic mice
The skin layers involved in our skin wound model comprised the epidermal, dermal, and subcutaneous layers. Therefore, the mechanisms of delayed wound closure could be owing to dysregulated epidermal cell proliferation, migration, or dermal inflammation. We found more serious inflammatory reactions in wounds of miR-146a KO and diabetic mice. Neutrophils usually infiltrate cutaneous wound sites for 0–3 days during normal healing, whereas macrophages can remain in wounds for 3–10 days. Our histological findings revealed obvious neutrophil and macrophage cell infiltration in skin wounds of miR-146a KO and diabetic mice at 5 days (Fig. 3A). Wound tissues were harvested, digested, and single skin wound cells were stained with various antibodies. Although ratios of Gr1-CD11b+ myeloid cells did not significantly differ in wounds between miR-146a KO and WT mice, flow cytometry showed that ratios of neutrophils increased in the wounds of miR-146a KO mice with delayed healing (Fig. 3B, C).
Figure 3.
Increased inflammatory cell infiltration in the wounds of miR-146a KO and diabetic mice. (A) Neutrophils (arrowed) and macrophage infiltration are increased in the wounds of miR-146a-deficient mice, especially neutrophils in diabetic group on the fifth day (HE × 200). (B) FACS analysis of neutrophils and CD11b + Gr1- myeloid cells showed the ratio of inflammatory cells increased at wound Day 5 in miR-146a KO mice. (C) Comparison of inflammatory cell infiltrations was made between miR-146a KO and WT mice (n = 5), **p < 0.01 by two-way ANOVA. Three independent experiments were performed.
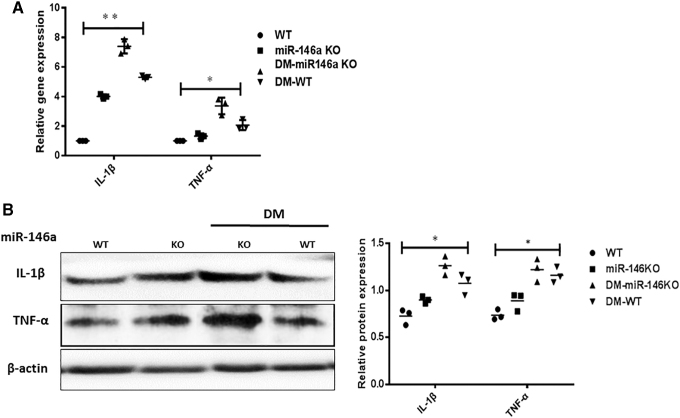
MiR-146a KO promoted inflammatory cytokine expression in mouse skin wounds
We measured levels of the representative proinflammatory cytokines IL-1β and TNF-α in skin wounds of miR-146a KO and diabetic mice to determine whether the delayed skin wound healing is owing to prolonged, aggravated inflammation. The gene expression of IL-1β and TNF-α was increased in wound tissues from miR-146a KO mice, particularly in those with DM (Fig. 4A). Consistent with the qRT-PCR results, western blotting also revealed increased protein levels of IL-1β and TNF-α in wounds with delayed healing (Fig. 4B).
Figure 4.
MiR-146a KO promotes the synthesis of proinflammatory cytokines in skin wounds. (A) The IL-1β, TNF-α gene expression was tested by qRT-PCR in miR-146a WT, KO mice, diabetic miR-146a KO and WT mice (n = 3). (B) Protein levels of IL-1β, TNF-α were detected by western blot in different groups (n = 3). Values are given as mean ± SD of three individual experiments. Two-way ANOVA analysis was used to compare multiple groups (A, B). Statistical significance was determined as *p < 0.05. TNF-α, tumor necrosis factor alpha; qRT-PCR, quantitative reverse transcription PCR.
Inflammation exacerbated the delayed healing of wounds in miR-146a KO mice owing to dysregulated NF-κB signaling
Interleukin-1β/NF-κB signaling plays a significant role in chronic inflammation. Therefore, we used RT-PCR to analyze the expression of TRAF6, IRAK1, and NF-κB, the key components of the IL-1β/NF-κB signaling pathway. Figure 5A provides obviously increased expression of these genes in wounded tissues of miR-146a KO and diabetic mice. Protein levels of TRAF6 and IRAK1 were increased in miR-146a KO mice compared with WT mice, which was consistent with changes in gene expression (Fig. 5B). Previous studies have also reported that TRAF6 and IRAK1 are direct targets of miR-146a. Collectively, our results provided evidence that deficiency of miR-146a increased the inflammatory response, delayed wound healing through the IL-1β/NF-κB signaling pathway (Fig. 5C).
Figure 5.
Dysregulation expression of TRAF6, IRAK1, NF-κB in skin wounds of miR-146a KO mice. (A) TRAF6, IRAK1, and NF-κB gene expression in skin wounds tissue were measured by qRT-PCR. (B) Western blot showed the protein levels of TRAF61, IRAK, and NF-κB were upregulated in the delayed skin wound tissue of miR-146a KO mice (n = 3), especially in miR-146a KO diabetic mice (n = 3). (C) The schematic picture shows the potential mechanisms of miR-146a in wound healing. Lack of miR-146a might promote IL-1β, TNF-α production through activation of NF-κB pathway mediated by TRAF6 and IRAK1. Values are given as mean ± SD of three individual experiments. Two-way ANOVA was used to compare multiple groups. Statistical significance was determined as *p < 0.05, **p < 0.01.
Discussion
Wound healing requires the precise and optimal responses of various inflammatory cytokines and chemokines.25–27 Wound inflammation plays a significant role in tissue regeneration, and appropriate resolution of inflammation is equally important for functional tissue repair. Excessive inflammation could be harmful and lead to chronic wounds, which occurs in patients with diabetes. Histopathological findings have shown that chronic wounds in diabetes remain a prolonged, exacerbated inflammatory stage. Key regulators of inflammation are important for the regulation of wound healing.28,29 Here, we revealed that miR-146a deficiency leads to an extensive and prolonged inflammatory response, which might be the mechanism through which miR-146a was involved in delayed wound healing.
Skin wound healing was delayed in miR-146a KO mice compared with WT mice, and was the slowest in diabetic miR-146a KO mice. Our skin punch wound model included the epidermal, dermal, and subcutaneous layers. Therefore, the potential mechanisms of delayed wound enclosure could be owing to epidermal cell proliferation, migration, and dermal inflammation. In our study, the delayed corneal epithelium wound healing of miR-146a KO mice did not reach significant difference compared with WT mice in nondiabetic group. However, the results showed statistical significance in diabetic group. Based on the similarity of epidermal and corneal structures, we considered that CEC proliferation and migration might play key roles in corneal wound healing. However, although the corneal stroma was not injured, miR-146a KO enhanced the local inflammatory response in corneal wounds in mice with DM. In the future, studies of the effects of an epidermal-specific miR-146a KO deletion on wound healing will provide more evidence regarding the role of miR-146a. A deficiency in miR-146a results in hyper-responsiveness of macrophages to bacterial LPS and leads to an exaggerated inflammatory response, whereas miR-155 and miR-146a coordinately contribute to the regulation of this response.30,31 We found more neutrophil, lymphocyte, and macrophage infiltration around the capillaries in the wounded dermis of miR-146a KO and diabetic mice. The ratios of neutrophils were higher in the wounds with delayed healing in miR-146a KO, than in WT mice. Neutrophils are usually recruited from the circulation and appear at wound sites during the early stage of inflammation.26,32 If neutrophils persist in wounds, their proteases can degrade extracellular matrix (ECM) proteins that are important for wound healing.32–34 Macrophages play a significant role in the inflammatory to proliferative phase transition during wound healing and are responsible for removing neutrophils from wounds.35 The ratio of Gr1-CD11b+ myeloid cells in the wounds did not significantly differ in this study. Excessive neutrophils persisted in wounded tissues beyond 5 days, indicating that delayed wound healing is associated with a conspicuous and prolonged inflammatory stage. We analyzed the key inflammatory cytokines IL-1β and TNF-α in our models. The mRNA and protein expression levels of IL-1β and TNF-α were upregulated in the wounds of miR-146a KO mice. The function of miR-146a in the inflammatory response might be to downregulate the expression of IRAK1 and TRAF6 that are signal transducers in the NF-κB activation pathway.36,37 We further explored the expression of IRAK1, TRAF6, and NF-κB to determine how miR-146a KO mediates skin wound healing. We found elevated IRAK1 and TRAF6 levels and activated NF-κB signaling in the nucleus, accompanied by inflammatory response exacerbation.
Regarding whether miR-146a and its target genes play significant roles in diabetic wounds, Xu also found that miR-146a expression level was significantly reduced in diabetic wounds compared with nondiabetic wounds at 1, 3, and 7 days after wounding. Meanwhile, diabetic wounds had significantly increasing expression of IRAK1 and TRAF6 at 3 and 7 days after injuring. The upregulated expression of IRAK1 and TRAF6 correlates with the downregulation of miRNA-146a in diabetic wounds.12 Wound healing is a complex process that includes cell recruitment, proliferation, and ECM deposition.38,39 Our findings in the miR-146a KO model mice provide direct evidence and support the opinion that miR-146a is involved in wound healing by affecting inflammatory processes. Of note, an effect of delayed wound healing in diabetic miR-146a KO mice was superimposed. We consider that miR-146a is not only a useful marker of inflammation, but also a potential target to treat wounds in patients with diabetes.
Innovation
We compared the effects of miR-146a in the pathogenesis of wound healing in healthy and STZ-induced diabetes mice. Skin wound healing was delayed in miR-146a KO mice, whereas corneal epithelial wound healing did not significantly differ between miR-146a KO and healthy mice. However, skin and corneal wound healing was delayed in the diabetic mice, especially in diabetic miR-146a KO mice. We also found more neutrophil infiltration in the skin wounds of miR-146a KO, than in healthy mice. The mechanisms were related to dysregulated IL-1β, TNF-α, IRAK1, TRAF6, and NF-κB signaling induced by miR-146a KO.
KEY FINDINGS
We identified the role of miR-146a in wound healing.
We found corneal and skin wound healing was significantly delayed in KO mice with diabetes.
Deficiency in miR-146a delayed skin wound healing by enhancing inflammatory responses in normal and diabetic mice.
Abbreviations and Acronyms
- CEC
corneal epithelial cell
- DM
diabetes mellitus
- ECM
extracellular matrix
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
hematoxylin–eosin
- IRAK1
interleukin-1 receptor-associated kinase 1
- KO
knockout
- IL-1
interleukin-1
- MiRNAs
microRNAs
- NF-κB
nuclear factor kappa B
- RIPA
radioimmunoprecipitation assay
- RT-PCR
reverse transcription PCR
- STZ
streptozotocin
- TNF-α
tumor necrosis factor-alpha
- TRAF6
TNF receptor associated factor 6
- WT
wild type
Acknowledgments and Funding Sources
The authors thank Haijing Sun and Cong-Cong Yin for preparing the wound model in mice. The authors also appreciate Xilin Zhang’ s contribution in FACS data analyses. The authors thank Editage for English language editing.
This study was supported by Shanghai Pujiang Program (18PJD053), the National Key Research and Development Program of China (2018YFC1705305), and the Clinical discipline innovation project (2019YXK028).
Author Disclosure and Ghostwriting
The article was designed and written by all the authors listed. No competing financial interests existed. No ghostwriters were used to write the article.
About the Authors
Xinling Bi, MD, is an associate professor in the Department of Dermatology, Changhai Hospital, Naval Medical University (Shanghai, China). Qing-Sheng Mi, MD, PhD, is professor and director of Henry Ford Hospital Immunology Program, Department of Dermatology and Department of Internal Medicine. He is leading study on diabetes, miRNAs, and wound healing at Henry Ford Hospital (Detroit, MI). Li Zhou, MD, PhD, is the professor of Department of Dermatology at Henry Ford Hospital. Yanfang Liu, MS, is supervisor nurse at Wound Care Center of Outpatient Department, Changhai Hospital. Jun Gu, MS, is professor of Department of Dermatology at Changhai Hospital.
References
- 1. Sen CK, Gordillo GM, Roy S, et al. . Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenhalgh DG. Management of the skin and soft tissue in the geriatric surgical patient. Surg Clin North Am 2015;95:103–114. [DOI] [PubMed] [Google Scholar]
- 3. Sullivan SR, Underwood RA, Sigle RO, et al. . Topical application of laminin-322 to diabetic mouse wounds. J Dermatol Sci 2007;48:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalay Z, Cevher SC. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int Wound J 2012;9:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pastar I, Stojadinovic O, Yin NC, et al. . Epithelialization in wound healing: A comprehensive review. Adv Wound Care (New Rochelle) 2014;3:445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fahs F, Bi X, Yu FS, Zhou L, Mi QS. New insights into micronas in skin wound healing. IUBMB Life 2015;67:889–896. [DOI] [PubMed] [Google Scholar]
- 8. Mori R, Tanaka K, Shimokawa I. Identification and functional analysis of inflammation-related mirnas in skin wound repair. Dev Growth Differ 2018;5:12542. [DOI] [PubMed] [Google Scholar]
- 9. Pizzino G, Irrera N, Galfo F, et al. . Effects of the antagomirs 15b and 200b on the altered healing pattern of diabetic mice. Br J Pharmacol 2018;175:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miscianinov V, Martello A, Rose L, et al. . Microrna-148b targets the tgf-beta pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther 2018;8:30205–30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng Z, Zhou D, Gao Y, Zeng M, Wang W. Mirna delivery for skin wound healing. Adv Drug Deliv Rev 2018;129:308–318. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Wu W, Zhang L, et al. . The role of microrna-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes 2012;61:2906–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funari VA, Winkler M, Brown J, et al. . Differentially expressed wound healing-related micrornas in the human diabetic cornea. PLoS One 2013;8:e84425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drewry M, Helwa I, Allingham RR, Hauser MA, Liu Y. Mirna profile in three different normal human ocular tissues by mirna-seq. Invest Ophthalmol Vis Sci 2016;57:3731–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winkler MA, Dib C, Ljubimov AV, Saghizadeh M. Targeting mir-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One 2014;9:e114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Essandoh K, Li Y, Huo J, Fan GC. Mirna-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock 2016;46:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann M, Mehta A, Zhao JL, et al. . An nf-kappab-microrna regulatory network tunes macrophage inflammatory responses. Nat Commun 2017;8:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen WB, Gao L, Wang J, et al. . Conditional ablation of hdac3 in islet beta cells results in glucose intolerance and enhanced susceptibility to stz-induced diabetes. Oncotarget 2016;7:57485–57497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70:5 47 41–20. [DOI] [PubMed] [Google Scholar]
- 20. Park SA, Covert J, Teixeira L, et al. . Importance of defining experimental conditions in a mouse excisional wound model. Wound Repair Regen 2015;23:251–261. [DOI] [PubMed] [Google Scholar]
- 21. Sun H, Mi X, Gao N, Yan C, Yu FS. Hyperglycemia-suppressed expression of serpine1 contributes to delayed epithelial wound healing in diabetic mouse corneas. Invest Ophthalmol Vis Sci 2015;56:3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dyer DP, Nebot JB, Kelly CJ, et al. . The chemokine receptor CXCR2 contributes to murine adipocyte development. J Leukoc Biol 2019;105:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin C, Weiland M, Miao ZM, et al. . Deletion of microrna mir-146a does not prevent streptozotocin-induced murine autoimmune type 1 diabetes. Diabetes Metab 2016;42:372–373. [DOI] [PubMed] [Google Scholar]
- 24. Schofield OM, McDonald JN, Fredj-Reygrobellet D, et al. . Common antigen expression between human periderm and other tissues identified by gb1-monoclonal antibody. Arch Dermatol Res 1990;282:143–148. [DOI] [PubMed] [Google Scholar]
- 25. Kroeze KL, Vink L, de Boer EM, et al. . Simple wound exudate collection method identifies bioactive cytokines and chemokines in (arterio) venous ulcers. Wound Repair Regen 2012;20:294–303. [DOI] [PubMed] [Google Scholar]
- 26. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv Wound Care (New Rochelle) 2018;7:209–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridiandries A, Tan JTM, Bursill CA. The role of chemokines in wound healing. Int J Mol Sci 2018;19:3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh K, Agrawal NK, Gupta SK, Sinha P. Increased expression of tlr9 associated with pro-inflammatory s100a8 and il-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (t2dm) cases with impaired wound healing. J Diabetes Complications 2016;30:99–108. [DOI] [PubMed] [Google Scholar]
- 29. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol Life Sci 2016;73:3861–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doxaki C, Kampranis SC, Eliopoulos AG, Spilianakis C, Tsatsanis C. Coordinated regulation of mir-155 and mir-146a genes during induction of endotoxin tolerance in macrophages. J Immunol 2015;195:5750–5761. [DOI] [PubMed] [Google Scholar]
- 31. Dai Y, Jia P, Fang Y, et al. . Mir-146a is essential for lipopolysaccharide (lps)-induced cross-tolerance against kidney ischemia/reperfusion injury in mice. Sci Rep 2016;6:27091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jun JI, Kim KH, Lau LF. The matricellular protein ccn1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun 2015;6:7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: Positive actions and negative reactions. Adv Wound Care (New Rochelle) 2013;2:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 2017;18:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park H, Huang X, Lu C, Cairo MS, Zhou X. Microrna-146a and microrna-146b regulate human dendritic cell apoptosis and cytokine production by targeting traf6 and irak1 proteins. J Biol Chem 2015;290:2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao M, Wang X, Zhang X, et al. . Attenuation of cardiac dysfunction in polymicrobial sepsis by microrna-146a is mediated via targeting of irak1 and traf6 expression. J Immunol 2015;195:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shook BA, Wasko RR, Rivera-Gonzalez GC, et al. . Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018;362:eaar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chandler LA, Alvarez OM, Blume PA, et al. . Wound conforming matrix containing purified homogenate of dermal collagen promotes healing of diabetic neuropathic foot ulcers: Comparative analysis versus standard of care. Adv Wound Care (New Rochelle) 2020;9:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]