Abstract
Significance
: Platelet-rich plasma (PRP) may be a potential drug for treatment of chronic refractory ulcers, which increase the risk of systemic infection and local canceration. However, the efficacy and safety of clinical application of PRP are still controversial. Thus, this study was aimed to assess the efficacy and safety of PRP in patients with chronic ulcers.
Recent Advances:
For this meta-analysis, Cochrane's Library, MEDLINE, EMBASE, PubMed, Web of Science, and CINAHL (Cumulate Index to Nursing and Allied Health Literature) databases were searched. Results were pooled using a random-effects model. The primary outcome was the proportion of completely healed chronic ulcers.
Critical Issues:
Seventeen randomized controlled trials were included. Compared with the control group, PRP significantly increased the fraction of healed ulcers (pooled risk ratio [RR] = 1.50; 95% confidence interval [CI] = 1.20 to 1.87; I2 = 47.8%). In autologous PRP (APRP) and homologous PRP (HPRP) subgroups, there were statistical differences between the control group versus treatment subgroup (pooled RR = 1.30, 95% CI = 1.10 to 1.54, I2 = 25.7%; pooled RR = 3.53, 95% CI = 1.94 to 6.43, I2 = 0.0%, respectively). In terms of percent of chronic ulcers area healed, there was a statistically significant difference between the PRP-treated group versus the control group (standard mean difference [SMD] = 1.37, 95% CI = 0.91 to 1.82, I2 = 22.1%). As for PRP safety, there existed a statistically significant difference between the APRP subgroup and the HPRP subgroup, respectively (pooled RR = 0.58; 95% CI = 0.35 to 0.98; I2 = 0.0%) and (pooled RR = 4.12; 95% CI = 1.55 to 10.96; I2 = 6.8%).
Future Directions
: Our findings shows that PRP may be a beneficial treatment of chronic skin ulcers and that APRP may be much safer than HPRP.
Keywords: platelet-rich plasma, chronic cutaneous ulcers, meta-analysis, wound healing

Jiayuan Zhu, MD, PhD

Bing Tang, MD, PhD
Scope and Significance
The healing of chronic cutaneous ulcer remains a major troublesome clinical problem. It was confirmed that growth factors and cytokines promote tissue regeneration, and among them, platelet-derived growth factor was considered to accelerate wound healing.
In this review, the data showed that platelet-rich plasma (PRP) may increase the incidence of complete wound healing compared with control group. Compared with previous reviews, our data showed some significant differences between two groups. The result may also have certain implications in the treatment of chronic cutaneous wounds. The treatment of autologous PRP (APRP) may challenge the classic view of treating chronic wounds.
Introduction
Rationale
Chronic cutaneous ulcers are open refractory wounds on the skin surface, which are caused by different pathogenic factors. They could be classified as diabetic ulcers, venous ulcers, pressure ulcers, arterial ulcers, neurotrophic ulcers, and so on. Importantly, they are not a rare occurrence, having a reported prevalence of 1.9% to 13.1% for the general population in various countries. As such, they have become an important challenge for contemporary health care professionals.1 Reports from the United States and Europe indicate that chronic cutaneous ulcers affect more than 9 million people.2 Nearly 10% of the people may suffer from a chronic ulcer within their lifetime, which may justify a related mortality rate of about 2.5%.3 Moreover, the economic burden of chronic cutaneous ulcers is also high: the estimated annual cost for the treatment of chronic wounds exceeds $39 billion USD.4,5 However, the effective management of patients with chronic cutaneous ulcers remains a challenging clinical problem. According to a recent guideline,6 the standard treatment strategies currently available involve mainly the debridement of necrotic tissue, wound bed preparation,7 and the creation of a moist wound microenvironment, as well as management of infections using antibiotics. However, notwithstanding that other potential treatments may also be used such as skin grafting, negative pressure wound therapy, and surgical management, a number of patients with chronic cutaneous ulcers still experience treatment failure. Therefore, the development of novel strategies to treat chronic cutaneous ulcers is of fundamental clinical consequence.8
Objectives
Multiple growth factors and cytokines promote tissue regeneration. Their interactions play important roles during the pathophysiological processes involved in wound healing.9 Reportedly, platelet-derived growth factor can accelerate wound healing by advancing cell proliferation and vascularization.10 PRP is a blood-derived product enriched with a high concentration of different growth factors and at least 1 Mio platelet/uL, which has been suggested to accelerate the healing process of chronic wounds.11,12 Moreover, the preparation of PRP is simple and easily applicable. First, blood, including autologous blood or homologous, is collected (5–10 mL). Second, the collected blood is centrifuged to separate the platelet from the rest of the blood components (1,500 g, 5 min). Third, PRP is extracted into a sterile small tube. The platelets should be active before the growth factors are released from PRP. There are mainly two methods to activate the platelets. For the first method, a platelet activator is added, which is a combination of thrombin and 10% calcium chloride. This would allow the platelet to release growth factors (platelet release). For the second method, platelet lyses (lysate) is activated by freezing. The final product is applied locally to the ulcers as a gel or solution, and then dressed with a nonabsorbent dressing (e.g., Vaseline gauze) changed every 3 days. As the mean lifetime of platelets is 7–10 days, it was suggested to repeat the PRP application once weekly.
However, the benefits of this treatment to wound healing were unclear.13–15 Some studies have claimed that PRP improved wound healing over standard care,16 while others have alleged that there was no significant difference in wound healing rates between PRP treatment and standard care.17 In particular, whether APRP is safer and more effective than homologous PRP remains to be determined. A new systematic review and meta-analysis were necessary to provide a reference for shared decision making between medical practitioners and patients. Therefore, the purpose of our meta-analysis was to systematically evaluate the potential efficacy and safety of PRP in patients with chronic cutaneous ulcers.
Materials and Methods
No review protocol exists.
Inclusion and exclusion criteria
We adopted the following inclusion criteria: (1) studies designed as randomized controlled trials (RCT); (2) works including adults (≥18 years of age) with at least one chronic wound of any etiology; and (3) investigations comparing the efficacy and safety of PRP with the outcomes of traditional treatment. Reviews, non-human studies, observational studies, or investigations that did not report the outcomes of interest were excluded from this analysis.
Information sources and search strategy
We searched all references in the Cochrane Library, Medline, EMBASE, PubMed, Web of Science, and CINAHL (Cumulate Index to Nursing and Allied Health Literature) databases up to September 2020. The Clinical Trials and the International Clinical Trials Registry Platform websites were also searched, in addition to gray references. The reference lists of selected articles, other related studies, and review articles were examined for eligible studies. No restrictions on language, date of publication, or study setting were applied during the database search.
Study selection, data collection process and data items
Two authors (S.Q.Q. and Z.C.H.) independently extracted the following data: first author, publication year, design characteristics, number of subjects, ulcer etiology, APRP/homologous PRP (HPRP), interventions, treatment duration, follow-up, proportion of completely healed chronic cutaneous ulcers, percent fraction of ulcer's area healed, total ulcer area covered by epithelium, and incidence of adverse events. Consensus with a senior researcher (B.T.) was achieved when any disagreement emerged. If data were not directly accessible, the corresponding author of the article was contacted through e-mail.
Risk of bias in individual studies
The quality of evidence of the elected studies was independently evaluated by two reviewers who used the modified Jadad scale (seven point),18,19 which included the following domains: randomization, concealment of allocation, double blinding, and withdrawal/dropout conditions. Each study was reviewed and scored as being of low or high quality. A score of 1–3 reflected low quality, while a score of 4–7 denoted high quality. Any divergent evaluations of the reviewers were settled by a senior researcher (B.T.).
Summary measures, synthesis of results, and statistical methods
All statistical analyses were performed using the STATA version 12.0 (Statacorp LP, College Station, TX) software package. Continuous outcomes were calculated and expressed as standard mean difference (SMD) with 95% confidence intervals (CIs), whereas dichotomous outcomes were calculated and expressed as risk ratio (RR) with 95% CI. Pooled estimates of SMD or RR were calculated using a random-effects model.17 Heterogeneity among these studies was evaluated through the Cochrane's Q test by calculating I2 statistic. For the Q statistic, p < 0.10 was considered to indicate significant heterogeneity. The I2 statistic denoted the percentage of observed between-study variability caused by heterogeneity. The degree of heterogeneity determined using the I2 statistic was defined as follows: 0–24%: no heterogeneity; 25–49%: moderate heterogeneity; 50–74%: sizeable heterogeneity; and 75–100%: extreme heterogeneity.20 Subgroup analyses and sensitivity analyses were also conducted. Meta-regression analysis with restricted maximum likelihood estimation was performed to assess potentially important covariates that might substantially impact on between-study heterogeneity.19 Publication bias was estimated by visual inspection of the funnel plot symmetry, and quantitatively evaluated by Egger's test.21 A value of p < 0.05 was considered statistically significant.
Risk of bias across studies—trial sequential analysis
In meta-analyses, repeated test of accumulating data may result in a false-positive or false-negative conclusion. To assess reliability and conclusiveness of the available evidence, trial sequential analysis (TSA) was used.22 TSA mainly depends on diversity-adjusted required information size (RIS) with adjusted threshold for statistical significance, and constructs trial sequential monitoring boundaries. When the cumulative Z-curve surpasses the trial monitoring boundary or the futility zone, a sufficient level of evidence for the anticipated therapeutic effect may be reached, and further trials will not be needed. If the trial sequential monitoring boundary is not crossed, we need more RCTs to confirm the reliability of the conclusions. We calculated diversity-adjusted RIS with an overall 5% risk of a type I error and a power of 80% (two sided). The relative risk reduction is a parameter calculated by dividing the absolute risk reduction by the control event rate. TSA was conducted using TSA Version 0.9 Beta.
Results
Study selection
After a comprehensive search, 901 studies were identified (Fig. 1). After screening by title and abstract, 823 studies were excluded. The remaining 78 studies were reviewed for further details. Of the latter, 58 studies were left out for various reasons. No data were available for another three of the remaining 20 studies. Finally, 17 studies were considered for meta-analysis.13,14,16,17,23–35
Figure 1.
The flow diagram of the literature search process.
Study characteristics of included studies
This meta-analysis included 17 RCTs13,14,16,17,23–35 with a total of 754 subjects (Table 1). The sample size of them ranged from 13 to 117 with a median value of 36. Four trials14,30,34,35 included patients with ulcers caused by different etiologies (mixed ulcers). Four more trials comprised patients with only venous ulcers.17,28,29,32 Finally, nine trials included patients with only diabetic ulcers.13,16,23–27,31,33 The therapeutic interventions were either APRP or HPRP. The patients of 13 trials (76.47%) received APRP13,14,16,17,24,26,27,29,30,32–35 and those of four trials (23.53%) received HPRP.23,25,28,31 The treatment time ranged from 5 to 36 weeks with a median of 12 weeks. Follow-up durations ranged from 5 to 120 weeks, their median being 16 weeks.
Table 1.
Characteristics of trials included in the meta-analysis
| Reference | Design | n | Mean Age (SD) | Ulcer Etiology | Duration, Mean (SD) |
|---|---|---|---|---|---|
| Driver16 | RCT | 72 | PG:56.4 (10.2) CG:57.5 (9.1) |
Diabetic | Not specified |
| Saldalamacchia et al.24 | RCT | 14 | PG:61.1 (9.4) CG:58.1 (7.8) |
Diabetic | Not specified |
| Steed et al.23 | RCT | 13 | PG:58.7 (12.4) CG:54.2 (12.9) |
Diabetic | PG:17.08 (15.87) CG:13 (14.37) (months) |
| Li et al.13 | RCT | 117 | PG:61.4 (13.1) CG:64.1 (9.4) |
Diabetic | PG:30 (55.56) CG:120 (80) (days) |
| Steed et al.31 | RCT | 36 | Not specified | Diabetic | PG/CG:20 (13.75) (months) |
| Ahmed et al.27 | RCT | 56 | PG:49.8 (15.4) CG:43.2 (18.2) |
Diabetic | PG:11.5 (2.8) CG:12.5 (1) (weeks) |
| Holloway et al.25 | RCT | 81 | Not specified | Diabetic | Not specified |
| Friese et al.26 | RCT | 42 | Not specified | Diabetic | Not specified |
| Weed et al.30 | RCT | 26 | PG:67.6 (11.9) CG:57.8 (18.2) |
Mixed: 9 multifactorial, 7 neurotrophic, 5 venous ulcers, 3 traumatic, 1 idiopathic, and 1 pressure ulcer | Not specified |
| Ban et al.33 | RCT | 62 | PG:58 (11) CG:60 (9) |
Diabetic | PG:90 (65.19) CG:60 (42.22) (days) |
| Stacey et al.17 | RCT | 86 | PG:70 (48.89) CG:72 (40.74) |
Venous disease | PG:60 (434.8) CG:69 (523.7) (months) |
| Knighton et al.35 | RCT | 32 | PG:64 (8) CG:62 (10) |
Mixed: 10 venous disease, 9 diabetic, 4 occlusive peripheral vascular disease, and 1 vasculitis | Not specified |
| Anitua et al.34 | RCT | 15 | PG:45 (20) CG:61 (16) |
Mixed: 10 venous disease, 4 pressure sore, and 1 other | PG:68 (61) CG:110 (164) (weeks) |
| Krupski et al.14 | RCT | 26 | PG:66 (5) CG:67 (4.5) |
Mixed: 78% diabetic, 72% occlusive peripheral vascular disease, and 28% venous disease | PG:6.2 (4.4) CG:4.3 (4.1) (months) |
| Senet et al.32 | RCT | 15 | PG:72.3 (31.8) CG:72.3 (24.4) |
Venous disease | PG:50.6 (174.8) CG:70 (71.11) (months) |
| Moneib et al.29 | RCT | 40 | PG:36.4 (10.2) CG:32.5 (7.5) |
Venous disease | Not specified |
| Oliveira et al.28 | RCT | 21 | PG:55 (18) CG:79 (8) |
Venous disease | PG:24 (47.41) CG:21 (78.52) (months) |
| Reference | APRP/HPRP | Interventions | Treatment Duration | Follow-up | Jadad Score |
|---|---|---|---|---|---|
| Driver16 |
APRP |
PRP group: application of APG twice a week. Control group: application of Saline. Co-treatment: the gel was covered with a contact layer dressing. |
Until ulcer healing or for a 12-week period |
24 weeks |
7 |
| Saldalamacchia et al.24 |
APRP |
PRP group: APG once a week plus standard care. Control group: standard care. Co-treatment: self-glucose control. |
Until ulcer healing or for a 5-week period |
5 weeks |
2 |
| Steed et al.23 |
HPRP |
PRP group: application of CT-102 once a week. Control group: application of saline gel. |
Until ulcer healing or for a 20-week period |
20 weeks |
3 |
| Li et al.13 |
APRP |
PRP group: APRP plus standard care every 2 weeks Control group: standard care. Co-treatment: all ulcers were covered with sterile wound dressings, which were changed every 3 days. |
Until ulcer healing or for a 12-week period |
12 weeks |
5 |
| Steed et al.31 |
HPRP |
PRP group: application of CT-102 once per day. Control group: application of saline gauze. |
Until ulcer healing or for a 20-week period |
120 weeks |
3 |
| Ahmed et al.27 |
APRP |
PRP group: application of PG twice a week. Control group: standard care. |
Until ulcer healing or for a 12-week period |
12 weeks |
2 |
| Holloway et al.25 |
HPRP |
PRP group: topical application of CT-102 once daily. Control group: placebo. |
Until ulcer healing or for a 20-week period |
20 weeks |
5 |
| Friese et al.26 |
APRP |
PRP group: application of PRP every 2 weeks. Control group: cleaning, polyurethane foam dressing applied and changed as needed, debridement and off- loading. |
Until ulcer healing or for a 12-week period |
25 weeks |
4 |
| Weed et al.30 |
APRP |
PRP group: autologous platelet lysate. Control group: platelet-poor plasma. Co-treatment: they both combined with collagen for the first 12 weeks of therapy. |
Until ulcer healing or for a 12-week period |
24 weeks |
5 |
| Ban et al.33 |
APRP |
PRP group: APG plus usual care. Control group: usual care. |
Until ulcer healing or for a 12-week period |
12 weeks |
4 |
| Stacey et al.17 |
APRP |
PRP group: autologous platelet-rich lysate. Control group: placebo. Co-treatment: topical application two times a week associated with gauze and pressure dressing. |
Until ulcer healing or for a 36-week period |
36 weeks |
5 |
| Knighton et al.35 |
APRP |
PRP group: application of APRP. Control group: placebo. Co-treatment: debridement before treatment. |
Until ulcer healing or for an 8-week period |
16 weeks |
6 |
| Anitua et al.34 |
APRP |
PRP group: autologous PRGF plus conventional treatment. Control group: conventional treatment. |
Until ulcer healing or for an 8-week period |
8 weeks |
4 |
| Krupski et al.14 |
APRP |
PRP group: application of APRP. Control group: saline. Co-treatment: all ulcers were debridement before treatment, and then were covered by one layer petrolatum-impregnated gauze after treatment. |
Until ulcer healing or for a 12-week period |
12 weeks |
6 |
| Senet et al.32 |
APRP |
PRP group: frozen autologous platelet suspension in saline solution. Control group: saline. Co-treatment: patients received standard topical and pressure treatment, three times per week. |
Until ulcer healing or for a 12-week period |
12 weeks |
5 |
| Moneib et al.29 |
APRP |
PRP group: application of APRP weekly Control group: conservatively by compression using graduated elastic stockings below the knee and dressing using saline and Vaseline gauze weekly |
Until ulcer healing or for a 6-week period |
6 weeks |
3 |
| Oliveira et al.28 | HPRP | PRP group: application of HPRP every 1 week Control group: application of gauze soaked in saline with a second cover of dry gauze and single-layer pressure bandage. |
Until ulcer healing or for a 90-day period | 90 days | 4 |
APG, autologous platelet gel; APRP, autologous platelet-rich plasma; CG, control group; HPRP, homologous platelet-rich plasma; PG: plate-rich plasma group; PRGF, preparation rich in growth factors; PRP, platelet-rich plasma; RCT, randomized controlled trial.
Risk of bias within studies
The quality of the evidence of all the included trials' was analyzed using the modified Jadad Rating Scales (seven points).19 Twelve trials (70.59%) were rated as of high evidence quality (scores ≥4),13,14,16,17,25,26,28,30,32–35 whereas for five trials (29.41%), the quality of the evidence was low (scores ≤3)23,24,27,29,31 (Table 1 and Supplementary Data).
Efficacy outcomes
Proportion of completely healed chronic cutaneous ulcers (primary efficacy outcome)
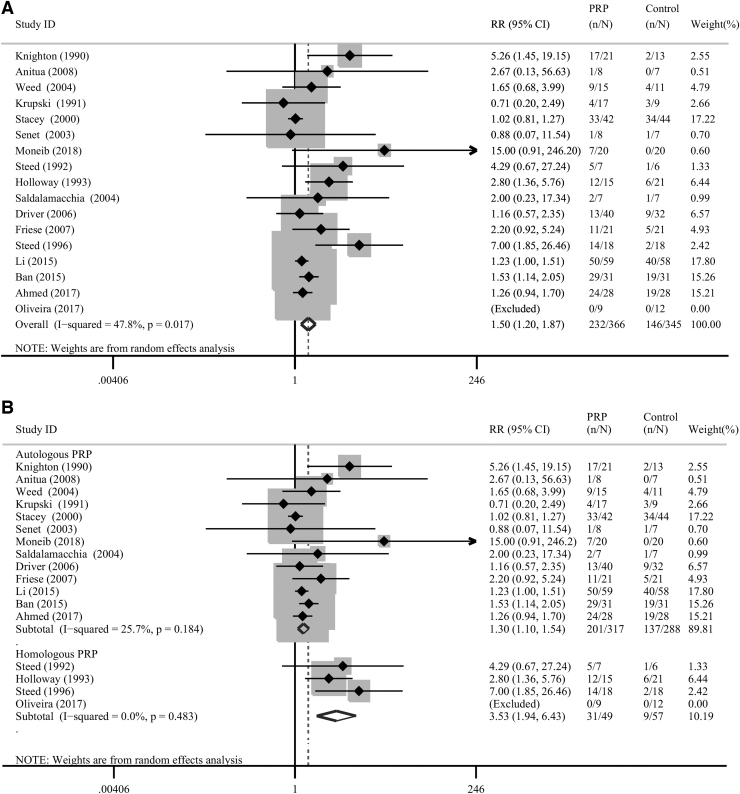
Seventeen trials involving 754 subjects reported the complete healing of the chronic cutaneous ulcers.13,14,16,17,23–35 In four of these trials,23,25,28,31 the therapy used was HPRP, and APRP was used in the remaining 13 trials.13,14,16,17,24,26,27,29,30,32–35 Compared with the untreated control group, on the whole, PRP administration significantly increased the proportion of healed ulcers (pooled RR = 1.50; 95% CI = 1.20 to 1.87; I2 = 47.8%; Fig. 2a).
Figure 2.
(A) Forest plot of the meta-analysis of the proportions of completely healed chronic cutaneous ulcers in patients allocated to the PRP group and the control group, respectively. (B) Subgroup analysis of the proportions of completely healed chronic cutaneous ulcers in patients allocated to the PRP group and the control group according to the type of PRP used. PRP, platelet-rich plasma.
Subgroup analysis
Subgroup analysis was performed to investigate the source of heterogeneity. Our results showed that the upshots of the APRP subgroup differed significantly from those of the control group (pooled RR = 1.30; 95% CI = 1.10 to 1.54; I2 = 25.7%; Fig. 2b). Moreover, the results of the HPRP subgroup also differed significantly from those of the control group (pooled RR = 3.53; 95% CI = 1.94 to 6.43; I2 = 0.0%; Fig. 2b). Besides, the subgroup analysis of ulcers with different etiologies (four mixed ulcers, four venous ulcers, and nine diabetic ulcers) revealed no significant reduction in heterogeneity (Supplementary Fig. S1).
Sensitivity analysis
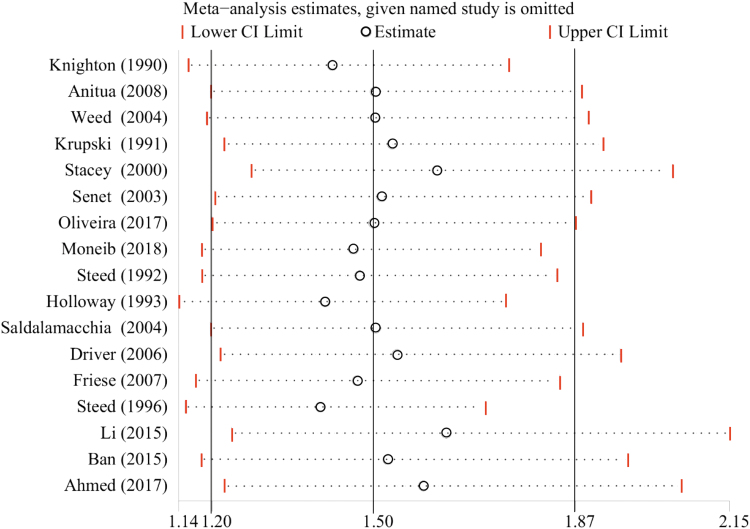
The results of the sensitivity analysis for the proportion of completely healed chronic cutaneous ulcers in patients allocated to the whole PRP group and the control group showed that omitting either of the included 17 studies one at a time did not entail any significant change (Fig. 3).
Figure 3.
Sensitivity analysis of the proportions of completely healed chronic cutaneous ulcers in patients allocated to the PRP group and the control group. Color images are available online.
Publication bias
Egger's test detected a marginal evidence of significant (p = 0.010) publication bias among the presently included trials as regard the meta-analysis of the proportion of completely healed chronic cutaneous ulcers. However, the same test did not reveal any evidence of significant publication bias (p = 0.079 and p = 0.457, respectively) between the APRP and HPRP subgroups (Fig. 4).
Figure 4.
Funnel plots of the publication bias of each subgroup (autologous PRP, A; homologous PRP, B) and of publication bias of the pooled included trials (C). Color images are available online.
Risk of bias across studies—TSA
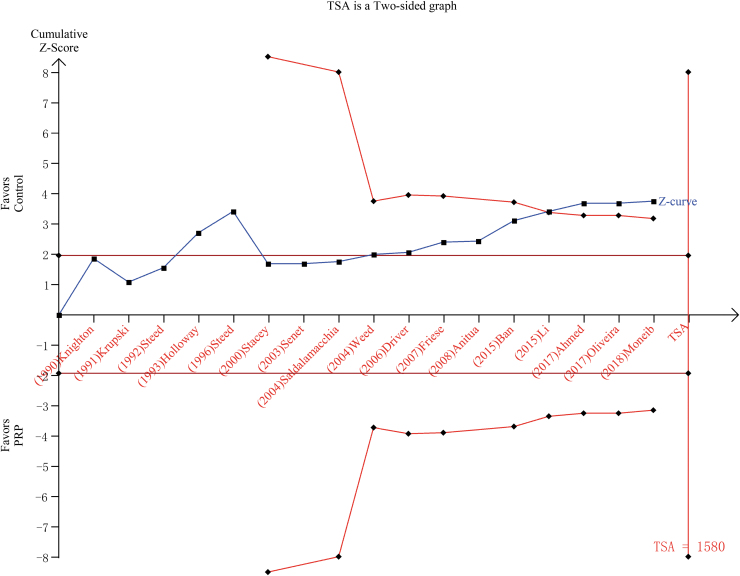
TSA was conducted by analyzing random effects by Biggerstaff-Tweedie's method. The cumulative Z-curve crossed the conventional boundary as regard benefits, but did not traverse the RIS boundary (Fig. 5).
Figure 5.
TSA of the presently included studies. A two-sided graph is plotted by TSA where the blue etched lines represent conventional significance boundaries, the blue line indicates the cumulative Z-score, and the red lines show the α-spending boundary and the required information size. TSA, trial sequential analysis. Color images are available online.
Secondary outcomes
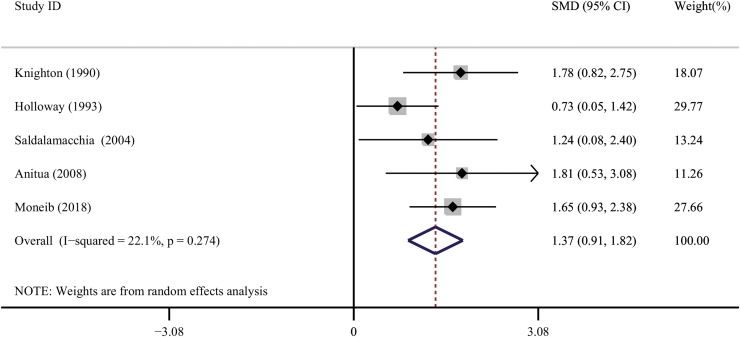
Mean percentage of chronic cutaneous ulcers area healed
Five trials specified the average reduction in chronic cutaneous ulcer area.24,25,29,34,35 Compared with controls, the treatment with PRP significantly decreased the area of diabetic ulcers (SMD = 1.37, 95% CI = 0.91 to 1.82, I2 = 22.1%; Fig. 6).
Figure 6.
Forest plot for the meta-analyses of the secondary outcomes: percentage area healed of the chronic cutaneous ulcers. Color images are available online.
Total area of chronic cutaneous ulcers newly covered by epithelium
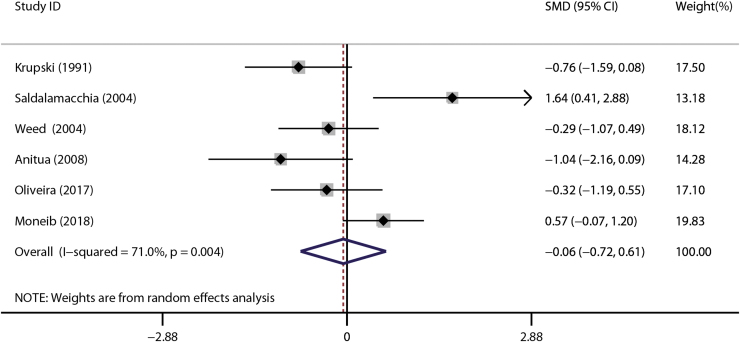
Six trials reported data of this outcome.14,24,28–30,34 There was no detectable statistically significant difference between the experiment group and control groups (SMD = −0.06, 95% CI = −0.72 to 0.61, I2 = 71.0%; Fig. 7).
Figure 7.
Forest plot for the meta-analyses of the secondary outcomes: Total epithelium-covered area of chronic cutaneous ulcers. Color images are available online.
Adverse effects related to the therapies
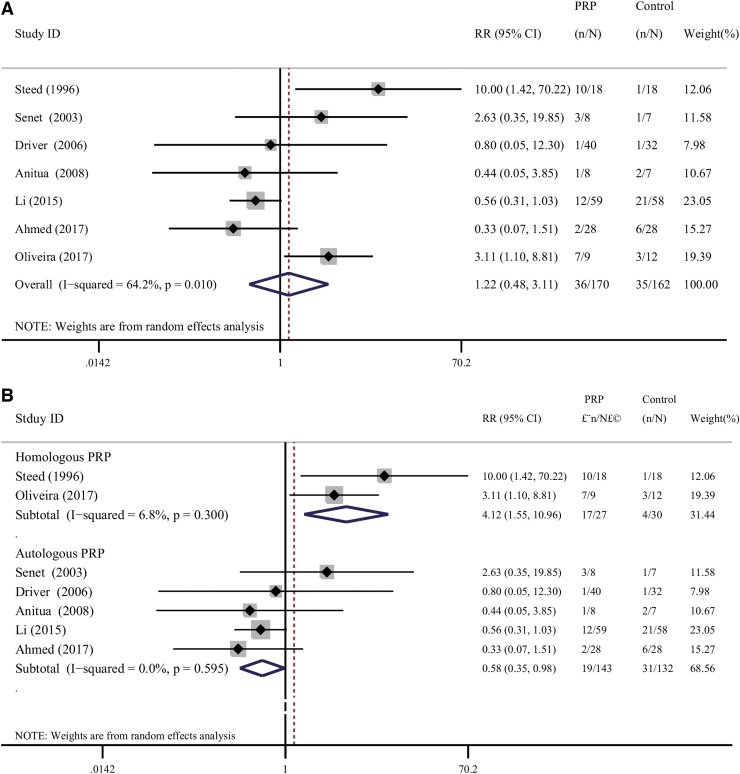
Seven trials13,16,27,28,31,32,34 reported data of adverse effects. Five of these trials (71.42%) administered APRP,13,16,27,32,34 while the remaining two trials28,31 (28.58%) applied HPRP. Overall, no significant difference regarding the occurrence of adverse effect was detected between the two groups (pooled RR = 1.22; 95% CI = 0.48 to 3.11; I2 = 64.2%; Fig. 8a). The subgroup analysis was performed to explore the sources of heterogeneity. There was a statistically significant difference between the APRP subgroup (pooled RR = 0.58; 95% CI = 0.35 to 0.98; I2 = 0.0%; Fig. 8b) and HPRP subgroup (pooled RR = 4.12; 95% CI = 1.55 to 10.96; I2 = 6.8%; Fig. 8b), respectively.
Figure 8.
(A) Forest plot for meta-analysis of the adverse effects related to the therapies in patients allocated to the PRP group and the control group. (B) Subgroup analysis of the adverse effects related to the therapies in patients allocated to the PRP group and the control group according to the type of PRP used. Color images are available online.
Discussion
Summary of evidence
This review was performed to provide evidence-based validation for the potential efficacy and safety of PRP for the treatment of chronic cutaneous ulcers. Overall, our results showed that PRP significantly improved the rate of complete healing of chronic cutaneous ulcers compared to the control (PRP untreated) group. In terms of adverse effects related to the treatment, APRP appeared to be much safer and HPRP may be a potential risk, compared with the traditional treatment. However, more high-quality RCTs are needed to verify these results.
In the 1960s, hematologists used PRP as a transfusion product for patients having coagulation problems. However, when Knighton et al. used PRP to repair chronic nonhealing cutaneous ulcers, they gave the patients a new hope.36 Two recent meta-analysis on platelet therapies for ulcer healing were available. The first meta-analysis performed by Carter et al.37 included prospective and retrospective trials. They reached the conclusions that PRP favored healing of chronic cutaneous ulcers and the occurrence of infections was reduced in acute wounds treated with PRP.37 Martinez-Zapata et al.15 performed a meta-analysis of six RCTs (of which three RCTs involved diabetic foot ulcers and the other three involved venous ulcers) investigating the efficacy of APRP for chronic wounds. Taken as a whole, the results of their meta-analysis indicated that no difference existed in efficacy and safety between the two groups.15 However, as regard the diabetic foot ulcer subgroup, APRP did shed some light on potential PRP efficacy. Owing to the small sample sizes of the six RCTs, the power of the meta-analysis may have been too low to allow significance between the two groups to show up. At any rate, these conclusions were based on limited studies with low-quality evidence. In contrast, the results of our work showed that PRP significantly improved the healing rate of chronic cutaneous ulcers and that APRP had fewer side effects than HPRP.
Previous evidences showed that the concentrations of various growth factors in ulcers increased after topical applications of PRP.38,39 Growth factors are naturally occurring compounds capable of stimulating cell growth, proliferation, and differentiation and wound healing. Basic science also indicates that PRP could enhance cell migration, proliferation, angiogenesis, and tissue anabolism.40 Platelets not only play the role in tissue repair but also partake actively in the inflammatory and immune responses.41–43 PRP might also exert antibacterial effects against Gram-positive bacteria.44 However, risks of administering HPRP are immune rejection and the spread of microbial and/or viral infections. Based on the current medical trials, PRP is endowed with some valuable degree of potential clinical application. It may be used as a new type of weapon to treat cutaneous ulcers. Besides, it may also constitute a tool for basic research and for applications in clinical and other medicine-related domains.
According to previous reports, the possible adverse effects include pain, itching, and burning sensation associated with the ulcer after PRP therapy. In our study, due to adverse effects related to the treatment, there was no significant difference between APRP and HPRP. However, the subgroup analysis prompted that APRP appeared to be much safer and HPRP may be a potential risk, compared with the traditional treatment.
Limitations
Our systematic review has some limitations. The principal limitation is the low-quality evidence. We wish to discuss here the reasons for the low-quality evidence in the following context. The first important reasons are the various risks of bias. Trials with negative results are always difficult to publish, and the lack of these trials would overestimate the effective values and reduce the quality of evidence.45 The pooled funnel plot shows that there exists concealed publication bias. However, we performed the analysis of the publication bias concerning each subgroup and Egger's test provided values of p > 0.05 for each subgroup. This result due to the low-quality evidence threatens the validity of the conclusions drawn from meta-analyses of published scientific researches. Therefore, more high-quality RCTs are needed to validate our present results.
Conclusions
Currently, evidence exists showing that autologous and HPRP could be an effective tool for treating chronic cutaneous ulcers. With respect to safety, APRP may be much safer than the homologous product. However, we do not take the results of our review as being conclusive. Additional high-quality RCTs are needed to confirm or refute our present conclusions.
Take-Home Messages
The healing of chronic cutaneous ulcer remains a major clinical problem.
PRP may improve ulcer healing without significant adverse effects for patients with chronic cutaneous ulcers.
Autologous platelet-rich plasma may be much safer than homologous platelet-rich plasma used in the chronic cutaneous ulcers.
Supplementary Material
Acknowledgments and Funding Sources
This work was supported by research grant 82072180 (J.Y.Z.), 81471875 (J.Y.Z.), 82072181 (B.T.), 81871565(B.T.), and 81501675 (Z.C.H.) from National Natural Science Foundation of China, research grant 2016B090916001 (J.Y.Z.) from Science and Technology Planning Project of Guangdong Province, research grant 2019A1515012208 (Z.C.H.) from Guangdong Basic and Applied Basic Research Foundation, research grant 19ykpy66 (Z.C.H.) from the Fundamental Research Funds for the Central Universities of Sun Yat-sen University, and research grant 2013001 (J.Y.Z.) and 2018003 (B.T.) from the Sun Yat-sen University Clinical Research 5010 Program.
Abbreviations and Acronyms
- APG
autologous platelet gel
- APRP
autologous platelet-rich plasma
- CG
control group
- CI
confidence intervals
- CINAHL
Cumulate Index to Nursing and Allied Health Literature
- HPRP
homologous platelet-rich plasma
- PG
platelet-rich plasma group
- PRGF
preparation rich in growth factors
- PRP
platelet-rich plasma
- RCTs
randomized controlled trials
- RIS
required information size
- RR
risk ratio
- SMD
standard mean difference
- TSA
trial sequential analysis
Authors' Contributions
J.Z. and B.T. contributed to design of this review. S.Q., Z.H., and Y.Z. collect all data and analyze data. S.Q., Y.Z., P.W., S.L., S.H., Y.D., Y.R., H.X., and W.Z. contributed to data verification and statistical analysis, and made all figures and tables. S.Q. and Z.H. contributed to article writing. J.Z. and B.T. revised the article. All listed authors checked and agreed to the final article.
Author Disclosure and Ghostwriting
No competing financial interests exist.
About the Authors
Shanqiang Qu, a postdoctoral of the Nanfang Hospital of Southern Medical University, who had published 1 SCI article in Adv Wound Care.
Zhicheng Hu, an associate chief physician in the First Affiliated Hospital of Sun Yat-sen University, who published 11 SCI articles until now, including 2 articles of J Invest Dermatol, 2 articles of Brit J Surg, 1 article of Acta Biomater, and so on.
Yi Zhang is a professor, chief physician in Affiliated Hospital of Nantong University.
Peng Wang is the resident doctor of the First Affiliated Hospital of Sun Yat-sen University.
Shuting Li is a nurse in the First Affiliated Hospital of Sun Yat-sen University.
Shaobin Huang, Yunxian Dong, Hailin Xu, and Yanchao Rong are all PhD candidates of the First Affiliated Hospital of Sun Yat-sen University.
Wenkai Zhu now is a student of Department of Chemistry, Portland State University, Portland, the United States.
Bing Tang, a professor and chief physician in the First Affiliated Hospital of Sun Yat-sen University. He published 16 SCI articles, including J Exp Med, J Invest Dermatol, Cell Death Dis, Brit J Surg, Acta Biomater, and so on.
Jiayuan Zhu, a professor and chief physician and vice director of Surgery in the First Affiliated Hospital of Sun Yat-sen University, the Chairman of Chinese Burn Rehabilitation Association. He published 15 SCI articles, including J Invest Dermatol, Brit J Surg, J Am Coll Surg, J Biol Chem, Plast Reconstr Surg, Adv Wound Care, and so on. He also hosts 3 projects on the Natural Science Foundation of China and 4 major projects at the provincial foundation, and so on.
Supplementary Material
References
- 1. Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci 2017;24:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farooqui MF, Shamim A. Low cost inkjet printed smart bandage for wireless monitoring of chronic wounds. Sci Rep 2016;6:28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013;2013:9. [Google Scholar]
- 4. Sen CK, Gordillo GM, Roy S, et al. . Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care 2009;18:154–161. [DOI] [PubMed] [Google Scholar]
- 6. Lavery LA, Davis KE, Berriman SJ, et al. . WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen 2016;24:112–126. [DOI] [PubMed] [Google Scholar]
- 7. Harries RL, Bosanquet DC, Harding KG. Wound bed preparation: TIME for an update. Int Wound J 2016;13:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jose Martinez-Zapata M, Marti-Carvajal AJ, Sola I, et al. . Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2012;10:CD006899. [DOI] [PubMed] [Google Scholar]
- 9. Weibrich G, Buch RSR, Kleis WKG, Hafner G, Hitzler WE, Wagner W. Quantification of thrombocyte growth factors in platelet concentrates produced by discontinuous cell separation. Growth Factors 2002;20:93–97. [DOI] [PubMed] [Google Scholar]
- 10. Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017;22:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pietramaggiori G, Kaipainen A, Czeczuga JM, Wagner CT, Orgill DP. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen 2006;14:573–580. [DOI] [PubMed] [Google Scholar]
- 12. Duran A, Yasar S, Aytekin S, Gunes P, Adaleti R, Duran A. Clinical and histopathological evaluation of the effects of platelet rich plasma, platelet poor plasma and topical serum physiologic treatment on wound healing caused by radiofrequency electrosurgery in rats. Turkderm-Turk Arch Dermatol Venereol 2018;52:44–50. [Google Scholar]
- 13. Li L, Chen D, Wang C, et al. . Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen 2015;23:495–505. [DOI] [PubMed] [Google Scholar]
- 14. Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg 1991;14:526–536. [PubMed] [Google Scholar]
- 15. Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, et al. . Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;66:CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Driver VR, Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled, blinded, multicenter pivotal trial of a Platelet Rich Plasma Gel* versus control when added to the standard of care in the treatment of non-healing diabetic foot ulcers. Diabetes 2006;55:A25. [Google Scholar]
- 17. Stacey MC, Mata SD, Trengove NJ, Mather CA. Randomised double-blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg 2000;20:296–301. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Pham B, Jones A, et al. . Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–613. [DOI] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D, et al. . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 20. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–826. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 23. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15:1598–1604. [DOI] [PubMed] [Google Scholar]
- 24. Saldalamacchia G, Lapice E, Cuomo L, et al. . A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis 2004;14:395–396. [DOI] [PubMed] [Google Scholar]
- 25. Holloway GA, Steed DL, Demarco MJ, et al. . A randomized, controlled dose response trial of activated platelet supernatant, topicalCT-102 in chronic, nonhealing diabetic wounds. Wounds 1993;5:198–206. [Google Scholar]
- 26. Friese G, Herten M, Scherbaum WA. The use of autologous platelet concentrate activated by autologous thrombin (APC+) is effective and safe in the treatment of chronic diabetic foot ulcers: a randomized controlled trial. Fifth Int Symp Diabet Foot, 9–12 May 2007, Noordwijkerhout, The Netherlands. https://onlinelibrary.wiley.com/doi/abs/10.1002/dmrr.824.
- 27. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg 2017;38:206–211. [DOI] [PubMed] [Google Scholar]
- 28. Oliveira MG, Abbade LPF, Miot HA, Ferreira RR, Deffune E. Pilot study of homologous platelet gel in venous ulcers. An Bras Dermatol 2017;92:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moneib HA, Youssef SS, Aly DG, Rizk MA, Abdelhakeem YI. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: a comparative study. J Cosmet Dermatol 2018;17:495–501. [DOI] [PubMed] [Google Scholar]
- 30. Weed B, Davis MDP, Felty CL, et al. . Autologous platelet lysate product versus placebo in patients with chronic leg ulcerations: a pilot study using a randomized, double-blind, placebo-controlled trial. Wounds 2004;16:273–282. [Google Scholar]
- 31. Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair Regen 1996;4:230–233. [DOI] [PubMed] [Google Scholar]
- 32. Senet P, Bon F-X, Benbunan M, et al. . Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg 2003;38:1342–1348. [DOI] [PubMed] [Google Scholar]
- 33. Ban Y, Wang A, Xu Z. A randomized controlled study of platelet—rich gel in treatment of refractory diabetic foot ulcer. Chin J Diabetes Mellitus 2015;7:306–310. [Google Scholar]
- 34. Anitua E, Aguirre JJ, Algorta J, et al. . Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater 2008;84:415–421. [DOI] [PubMed] [Google Scholar]
- 35. Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet 1990;170:56–60. [PubMed] [Google Scholar]
- 36. Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg 1986;204:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carter MJ, Fylling CP, Parnell LKS. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 38. Pietramaggiori G, Scherer SS, Mathews JC, et al. . Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen 2008;16:218–225. [DOI] [PubMed] [Google Scholar]
- 39. Crovetti G, Martinelli G, Issi M, et al. . Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–151. [DOI] [PubMed] [Google Scholar]
- 40. Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci 2008;13:3532–3548. [DOI] [PubMed] [Google Scholar]
- 41. von Hundelshausen P, Weber C. Platelets as immune cells—bridging inflammation and cardiovascular disease. Circ Res 2007;100:27–40. [DOI] [PubMed] [Google Scholar]
- 42. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- 43. Weyrich AS, Prescott SA, Zimmerman GA. Platelets, endothelial cells, inflammatory chemokines, and restenosis—complex signaling in the vascular play book. Circulation 2002;106:1433–1435. [DOI] [PubMed] [Google Scholar]
- 44. Drago L, Bortolin M, Vassena C, Romano CL, Taschieri S, Fabbro MD. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One 2014;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ioannidis JPA. How to make more published research true. PLos Med 2014;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.