Abstract
Background
Diabetes overtreatment is a frequent and severe issue in multimorbid older patients with type 2 diabetes (T2D).
Objective
This study aimed at assessing the association between diabetes overtreatment and 1-year functional decline, hospitalisation and mortality in older inpatients with multimorbidity and polypharmacy.
Methods
Ancillary study of the European multicentre OPERAM project on multimorbid patients aged ≥70 years with T2D and glucose-lowering treatment (GLT). Diabetes overtreatment was defined according to the 2019 Endocrine Society guideline using HbA1c target range individualised according to the patient’s overall health status and the use of GLT with a high risk of hypoglycaemia. Multivariable regressions were used to assess the association between diabetes overtreatment and the three outcomes.
Results
Among the 490 patients with T2D on GLT (median age: 78 years; 38% female), 168 (34.3%) had diabetes overtreatment. In patients with diabetes overtreatment as compared with those not overtreated, there was no difference in functional decline (29.3% vs 38.0%, P = 0.088) nor hospitalisation rates (107.3 vs 125.8/100 p-y, P = 0.115) but there was a higher mortality rate (32.8 vs 21.4/100 p-y, P = 0.033). In multivariable analyses, diabetes overtreatment was not associated with functional decline nor hospitalisation (hazard ratio, HR [95%CI]: 0.80 [0.63; 1.02]) but was associated with a higher mortality rate (HR [95%CI]: 1.64 [1.06; 2.52]).
Conclusions
Diabetes overtreatment was associated with a higher mortality rate but not with hospitalisation or functional decline. Interventional studies should be undertaken to test the effect of de-intensifying GLT on clinical outcomes in overtreated patients.
Keywords: type 2 diabetes, older patients, diabetes overtreatment, glucose-lowering treatment, multimorbidity, older people
Key Points
In these 490 older patients with multimorbidity, polypharmacy and type 2 diabetes, one-third had diabetes overtreatment.
In these patients, diabetes overtreatment was not associated with functional decline nor hospitalisation.
Diabetes overtreatment was independently associated with a higher mortality rate.
Background
Type 2 diabetes (T2D) is a prevalent condition in older people, reaching almost 20% of patients aged ≥65 years in European countries [1], and severely affecting their quality of life and functional status [2]. The treatment of diabetes usually includes control of glycaemia by a glucose-lowering therapy (GLT), reducing long-term complications from chronic exposure to hyperglycaemia (such as microvascular complications) [3]. However, some glucose-lowering drugs induce a high risk of hypoglycaemia (such as insulins, sulfonylureas or glinides), especially if they are used to achieve a tight glycaemic control, i.e. diabetes overtreatment [4–6].
In older patients, hypoglycaemic events are more frequent, more challenging to diagnose (due to the unawareness and their insidious clinical presentation), more severe and more frequently complicated, in particular in those with frailty or poorer health status [5, 7, 8]. These harmful hypoglycaemic events increase fall-related fractures, cardiovascular events and cerebral events (comas and seizures), whereas they reduce the cognitive status, functional status and life expectancy [9–12].
Recent clinical practice guidelines (CPGs) from major scientific societies strongly recommend avoiding hypoglycaemia and diabetes overtreatment in older patients. They suggest that GLT should be individualised, in particular by using individualised HbA1c goals according to the patients’ health status and the type of GLT used (i.e. inducing the high risk of hypoglycaemia or not) to define an individualised treatment objective [13–16]. Individualisation of treatment objective allows the benefit–risk balance of the glycaemic treatment prescribed to older patients to be more accurately adjusted to their health status and life expectancy, i.e. the balance between the potential long-term benefits of GLT versus the risks of short-term complications.
However, there are limited data on the association between clinical outcomes and overtreatment of diabetes in older patients particularly in populations usually less represented in studies, such as multimorbid older people [17, 18]. This study aimed at assessing the association of diabetes overtreatment with three outcomes at 1 year: functional decline, hospitalisations and mortality.
Methods
Study design and patients’ inclusion
This study was a substudy of a European multicentre cluster randomised controlled trial (OPtimising thERapy to prevent avoidable hospital Admissions in Multimorbid older adults (OPERAM)) [19, 20]. The OPERAM trial was designed to assess the effect of pharmacological treatment optimisation on drug-related hospital admissions in older inpatients with multimorbidity and polypharmacy. It included 2,008 patients aged ≥70 years with multimorbidity (≥ 3 conditions) and polypharmacy (≥ 5 different drugs/day) admitted to a university hospital in four countries, namely Switzerland (Bern), Netherlands (Utrecht), Belgium (Louvain) and Republic of Ireland (Cork). Patients were excluded when admitted to palliative care within 24 hours after hospital admission. Clusters were 1:1 randomised to standard of care (control group) or a structured pharmacotherapy optimisation intervention (evidence-based structured medication review using the Dutch Systematic Tool to Reduce Inappropriate Prescribing (STRIP) [21], based on the STOPP/START.v2 criteria [22]). Besides the discontinuation of long-acting sulfonylureas (STOPP J1) and the discontinuation of thiazolidinediones in the presence of cardiac failure (STOPP J2) in STOPP/START.v2 criteria and the fact that the physician-pharmacist pairs could make recommendations on GLT if thought to be relevant, no GLT intervention was required. All patients were followed up for 1 year after the inclusion.
The present substudy included all patients with T2D, a GLT prescribed before the hospitalisation and a concomitant value of HbA1c. Patients with T2D were identified by the presence of the International Classification of Disease-10 code E11 (and all subcategories of the code E11) in the list of their comorbidities recorded at index hospitalisation.
Data collection
The collected data at index hospitalisation were related to patients’ socio-demographic characteristics (age, sex, place of residency), main trial characteristics (group of allocation—intervention or control arm), clinical, biological and functional characteristics. Comorbidities were collected to compute the Charlson Comorbidity Index [23]. Functional status was assessed using the Barthel Index (score/100 [24]), scoring independence in 10 activities of daily living (ADL). Cognitive impairment was defined as a diagnosed dementia. Quality of life was assessed using the EQ-5D score [25]. Severe frailty was defined as a score of ≥7 on the Clinical Frailty Scale [26]. Hyperpolypharmacy was defined as ≥10 prescribed daily drugs at the usual place of living for at least 30 days before admission.
HbA1c, expressed in both International Federation of Clinical Chemistry nomenclature (mmol/mol) and National Glycohemoglobin Standardization Program nomenclature (%), was retrospectively collected after the inclusion of patients (data from medical records). We considered the value of HbA1c, which was closest to the enrolment date in OPERAM trial within a year. The glucose-lowering treatment (GLT) concomitant to the HbA1c value was collected. Glucose-lowering agents were encoded according to the Anatomical Therapeutic Chemical classification system [27]. Glucose-lowering agents included hypoglycaemic drugs (insulins (A10A), sulfonylureas (A10BB) and glinides (A10BX02–03–05-08)) and non-hypoglycaemic agents (biguanides (A10BA), GLP1-receptor agonists (A10BJ), DPP4-inhibitors (A10BH), alpha-glucosidase inhibitors (A10BF), thiazolidinediones (A10BG) and SGLT2-inhibitors)).
Outcomes
The occurrences of outcomes of interest were systematically collected during the year of follow-up after inclusion: functional decline (measured at 2 and 12 months), hospitalisations and all-cause mortality. Functional decline and all-cause mortality were assessed from inclusion in the study in all participants, whereas hospitalisations were assessed from hospital discharge from the index hospitalisation among patients who were still alive and followed-up at discharge.
Overtreatment
Diabetes overtreatment refers in this study to overtreatment of glycaemic control. This is defined based on the CPG of the 2019 Endocrine Society [15] on the management of diabetes in older adults, i.e. according to the patients’ health status, hypoglycaemic drugs and HbA1c. Diabetes overtreatment is defined as having a GLT including a glucose-lowering agent at high risk of hypoglycaemia (i.e. insulin, sulfonylurea or glinide) while having an HbA1c < 53 mmol/mol (7.0%) for patients in good health, < 58 mmol/mol (7.5%) for patients in intermediate health and < 64 mmol/mol (8.0%) for those in poor health [15].
The patient’s health status was assessed according to their number of comorbidities, functional status, cognitive status and place of residency, by one of the authors of this study. Each patient was classified into one of three tiers of global health status: good, intermediate or poor (see Figure S1 in Appendices).
Statistical analysis
Categorical data were expressed as the absolute frequency (n) and relative frequency (%). Continuous variables were expressed as medians and interquartile range (median [first quartile; third quartile]).
The prevalence of functional decline (defined categorically as a relative decrease of ≥10% in Barthel index [28, 29]) was compared between groups (overtreated versus not overtreated) using a Pearson’s Chi-squared test among patients with at least two measurements. The incidence rates of hospitalisation during 1 year after discharge from the index hospitalisation and of mortality during 1 year after the index hospital admission were expressed as a number of events per 100 patient-years at risk. The comparison of incidence rates between groups (overtreated versus not overtreated) was performed using a z-test.
Factors associated with functional decline (defined continuously as an absolute loss of Barthel Index) based on measures of Barthel Index at baseline, 2 months and 12 months of follow-up, were assessed using a multivariable linear mixed-effects regression.
Factors associated with hospitalisations over 1 year after the discharge of the index hospitalisation were assessed using a multivariable semi-parametric Cox Proportional Hazards frailty regression. This model allows taking into account the inter-individual variability (frailty) on the risk of hospital admission that is potentially not explained by observed covariates and the within-subject correlation between hospitalisations. The number of previous hospitalisations was considered as a time-dependent variable in the model to account for the impact of consecutive hospitalisations on the risk of hospitalisation during the follow-up. The absence of multicollinearity was checked using the variance inflation factor (VIF; a VIF value >5 indicated multicollinearity) and the conditions of validity of the model were fulfilled: the proportional hazards assumption was checked using Schoenfeld residuals, non-linearity was assessed using Martingale residuals and influential observations were examined using Deviance residuals.
Factors associated with mortality at one year were assessed using a multivariable Cox’s Proportional Hazards regression for time-dependent variables. The number of previous hospitalisations was considered as a time-dependent variable in the model to account for the impact of consecutive hospitalisations on the risk of death. Conditions of validity of the model described above were also checked and were fulfilled.
For all models, a univariable model was done as well as a multivariable model with all variables (including age ≥ 80 years, sex (male), Charlson comorbidity index, severe polypharmacy, severe frailty, GLT bi-therapy or more, number of previous hospitalisation and intervention group). For all analyses, a P-value <0.05 was considered as statistically significant. All statistical analyses were performed using R statistical software (version 4.1.2).
Ethical consideration
The OPERAM trial was approved by the independent research ethics committees at each site (lead ethics committee: Cantonal Ethics Committee Bern, Switzerland, ID 2016-01200; Medical Research Ethics Committee Utrecht, Netherlands, ID 15-522/D; Comité d’Ethique Hospitalo-Facultaire Saint-Luc-UCL: 2016/20JUL/347–Belgian registration No: B403201629175; Cork University Teaching Hospitals Clinical Ethics Committee, Cork, Republic of Ireland; ID ECM 4 (o) 07/02/17), and Swissmedic as a responsible regulatory authority [19].
Results
Of the 2,008 older patients included in the OPERAM trial, 564 (28.1%) had T2D. Among them, 490 patients (24.4%) had GLT before the index hospital admission with a concomitant HbA1c measurement and were included in the present analyses.
Baseline characteristics
These 490 older patients with T2D had a median age of 78 years and 38.0% were women (Table 1). The median number of comorbidities was 5, resulting in a median Charlson comorbidity index of 7. The median number of daily drugs was 11 and hyperpolypharmacy (≥ 10 drugs daily) was observed in 63.7% of the patients.
Table 1.
General characteristics at baseline (N = 490)
| Variable | All patients (n = 490; 100%) n (%) or median [P25; P75] |
Diabetes overtreatment (n = 168; 34.3%) n (%) or median [P25; P75] |
No diabetes overtreatment (n = 322; 65.7%) n (%) or median [P25; P75] |
|---|---|---|---|
| Age, years | 78 [74; 82] | 76 [73; 83] | 78 [74; 82] |
| Age ≥ 80 years | 197 (40.2) | 67 (39.9) | 130 (40.4) |
| Female sex | 186 (38.0) | 59 (35.1) | 127 (39.4) |
| Site | |||
| Bern | 184 (37.6) | 60 (35.7) | 124 (38.5) |
| Cork | 72 (14.7) | 11 (6.5) | 61 (18.9) |
| Louvain | 123 (25.2) | 58 (34.5) | 65 (20.2) |
| Utrecht | 111 (22.7) | 39 (23.2) | 72 (22.4) |
| Group intervention | 207 (42.2) | 73 (43.5) | 134 (41.6) |
| CLINICAL CHARACTERISTICS | |||
| Number of comorbidities | 5 [2; 7] | 5 [3; 7] | 4 [2; 7] |
| Charlson comorbidity index | 7 [5; 8] | 7 [5; 8] | 6 [5; 8] |
| Number of drugs/day | 11 [8; 14] | 11 [9; 14] | 11 [8; 14] |
| Hyperpolypharmacy (≥10 drugs/day) | 312 (63.7) | 116 (69.0) | 196 (60.9) |
| Functional status | |||
| ≥ 2 impairments in ADL | 150 (30.6) | 59 (35.1) | 91 (28.3) |
| Barthel index (12 missing values) | 90 [75; 100] | 90 [70; 100] | 95 [80; 100] |
| Cognitive impairment | 48 (9.8) | 19 (11.3) | 29 (9.0) |
| Fall (≥2 in the last year) | 54 (11.0) | 14 (8.3) | 40 (12.4) |
| Nursing home residency | 34 (6.9) | 12 (7.1) | 22 (6.8) |
| Severe frailty (CFS ≥ 7) | 100 (20.4) | 40 (23.8) | 60 (18.6) |
| Health status | |||
| Good | 77 (15.7) | 19 (11.3) | 58 (18.0) |
| Intermediate | 210 (42.9) | 68 (40.5) | 142 (44.1) |
| Poor | 203 (41.4) | 81 (48.2) | 122 (37.9) |
| GLUCOSE-LOWERING TREATMENT | |||
| Number of glucose-lowering drugs/day | 2 [1; 2] | 2 [1; 2] | 1 [1; 2] |
| Bi-therapy or more | 259 (52.9) | 119 (70.8) | 140 (43.5) |
| Glucose-lowering treatment | |||
| Monother. non-hypoglycaemic drug | 148 (30.2) | 0 (0) | 148 (46.0) |
| Monother. hypoglycaemic drug | 83 (16.9) | 49 (29.2) | 34 (10.6) |
| Bi or trither. Non-hypoglycaemic drug | 30 (6.1) | 0 (0) | 30 (9.3) |
| Bi or trither. Hypoglycaemic drug | 229 (46.7) | 119 (70.1) | 110 (34.2) |
| HbA1c, mmol/mol (%) | 53 [45; 62] (7.0 [6.3; 7.8]) |
50 [44; 55] (6.7 [6.2; 7.2]) |
58 [48; 67] (7.5 [6.5; 8.3]) |
| < 48 mmol/mol (6.5%) | 136 (27.8) | 65 (38.7) | 71 (22.0) |
| 48–57 mmol/mol (6.5–7.49%) | 167 (34.1) | 86 (51.2) | 81 (25.2) |
| 58–68 mmol/mol (7.5–8.49%) | 113 (23.1) | 17 (10.1) | 96 (29.8) |
| ≥ 69 mmol/mol (8.5%) | 74 (15.1) | 0 (0) | 74 (23.0) |
ADL: activities of daily living; CFS: clinical Frailty Scale; Monother.: monotherapy; Trither.: tritherapy; HbA1c: glycated haemoglobin. Health status was defined according to the criteria of the Endocrine Society Guidelines (2019) [15]. Patients were considered overtreated when GLT with a high risk of hypoglycaemia (i.e. including insulin, sulfonylurea or glinide) was taken and HbA1c was <53 mmol/mol (7.0%) (for patients in good health), < 58 mmol/mol (7.5%) (for patients in intermediate health) or < 64 mmol/mol (8.0%) (for patients in poor health). Group intervention: intervention used in the OPERAM trial consisted of a Systematic Tool to Reduce Inappropriate Prescribing (STRIP) described in [19].
Of the 10 patients, 3 (30.6%) had ≥2 impairments in basic ADL (bathing, dressing, eating, toileting and transferring), 9.8% had cognitive impairment and 6.9% lived in a nursing home. These features resulted in a health status being poor in 41.4%, intermediate in 42.9% and good in 15.7% of the patients. Severe frailty (clinical frailty scale ≥7) was found in 20.4% of the patients.
As far as GLT was concerned, half of the patients (52.9%) received a daily bi- or tri-therapy. The most frequent GLT regimen was a bi- or tritherapy including hypoglycaemic drug(s) (46.7%) followed by monotherapy with non-hypoglycaemic drug (30.2%). The median HbA1c was 53 mmol/mol (7.0%) [45; 62 mmol/mol (6.3; 7.8%)]. Diabetes overtreatment was found in 168 patients (34.3%) (Table 1).
Outcomes at 1-year (all-cause mortality, hospitalisation and functional decline) and associated factors
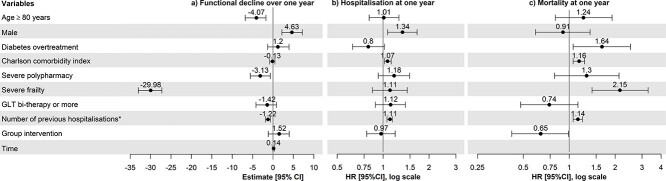
The proportion of functional decline (loss ≥10% of Barthel index) among the 407 patients with at least two measures of the Barthel index was not different between patients with and those without overtreatment and was 35.1% (Table 2). For the regression model shown in Table S1 in Appendices, 488 patients were included, representing 478 measures at baseline, 398 measures at 2 months and 335 measures available at 12 months. In the multivariable regression model, overtreatment was not associated with a functional decline during the 1-year follow-up (coefficient (95%CI): 1.20 (−1.32; 3.92); Figure 1; Table S1 in Appendices). Factors associated with a higher functional decline were age ≥ 80 years (coefficient (95%CI): −4.07 (−6.82; −1.73)), severe polypharmacy (coefficient (95%CI): −3.13 (−5.55; −0.64)) and the number of previous hospitalisations (coefficient (95%CI): −1.22 (−1.85; −0.63), whereas being a male was a protective factor (coefficient (95%CI): 4.63 (2.17; 7.21)) (Table S1 in Appendices). There was no effect of time in the model.
Table 2.
Incidence of outcomes (functional decline, hospitalisation and all-cause mortality) according to diabetes overtreatment
| Outcomes at 1 year | All patients (n = 490) IR and 95% CI (cases per 100 patients-year) or % and 95%CI |
Diabetes overtreatment (n = 168; 34.3%) IR and 95% CI (cases per 100 patients-year) or % and 95%CI |
No diabetes overtreatment (n = 322; 65.7%) IR and 95% CI (cases per 100 patients-year) or % and 95%CI |
P-value |
|---|---|---|---|---|
| Mortality, IR | 25.1 (20.4; 30.5) | 32.8 (23.7; 44.2) | 21.4 (16.2; 27.6) | 0.033 |
| Hospitalisation, IR | 119.9 (109.2; 131.1) | 107.3 (90.1; 126.8) | 125.8 (112.7; 140.0) | 0.115 |
| Functional decline, %a | 35.1 (30.5; 40.0) | 29.3 (21.9; 37.9) | 38.0 (32.2; 44.0) | 0.087 |
Defined as a loss of ≥10% of Barthel index occurring during the follow-up among patients with at least two measurements of Barthel index (n = 407); IR: incidence rate (cases per 100 patient-years); CI: confidence interval.
Figure 1.
Forest plots of characteristics associated with outcomes at 1 year, resulting from multivariable analyses: (a) functional decline; (b) hospitalisation; (c) all-cause mortality. 95%CI: confidence interval at 95%; HR: hazard ratio; severe frailty: Clinical Frailty Scale ≥7; severe polypharmacy: ≥ 10 drugs/day. Functional decline was defined as an absolute loss of Barthel index between two consecutive measures during the 1-year follow-up. Patients were considered overtreated when GLT with a high risk of hypoglycaemia (insulin, sulfonylureas and/or glinides) was taken and HbA1c was <53 mmol/mol (7.0%) (for patients in good health), < 58 mmol/mol (7.5%) (for patients in intermediate health) or < 64 mmol/mol (8.0%) (for patients in poor health). The above-mentioned health status category was defined according to the criteria of the Endocrine Society Guidelines (2019) [15]. Group intervention: intervention used in the OPERAM trial consisted of a Systematic Tool to Reduce Inappropriate Prescribing (STRIP) described in [19].*The number of previous hospitalisations was entered as a time-varying variable in the models.
Of the 490 included patients, 476 were still followed up after discharge of the index hospitalisation and analysed for hospital admission within the year. Among the 14 excluded patients, 9 patients died during the index hospitalisation and 4 patients withdrew from the study. The hospitalisation incidence rate (119.9 hospitalisations per 100 patient-years during the year after inclusion) was not different between patients with and those without overtreatment (Table 2). In multivariable analysis, overtreatment was not associated with hospitalisation (hazard ratio, HR (95%CI): 0.80 (0.63; 1.02), P = 0.066) (Figure 1; Table S2 in Appendices). Other factors associated with a higher risk of hospitalisation were male sex (HR (95%CI): 1.34 (1.07; 1.67)), higher Charlson Comorbidity Index (HR (95%CI): 1.07 (1.02; 1.13)) and higher number of previous hospitalisations (HR (95%CI): 1.11 (1.06; 1.15)) (Table S2 in Appendices). The variance of the frailty parameter in the multivariable model was 0.257 (P < 0.001).
Finally, the all-cause mortality rate (23.9 per 100 patient-years during the year after the inclusion) was higher in the 168 (34.3%) patients with diabetes overtreatment than in the 322 others (31.8 versus 20.1 per 100 person-years; P = 0.023) (Figure 2 and Table 2). In multivariable analyses, the risk of death at one year was 1.64 times higher in overtreated patients as compared with those without overtreatment (HR (95%CI): 1.64 (1.06; 2.52), P = 0.026) (Figure 1; Table S3 in Appendices). Other factors associated with a higher 1-year mortality rate were higher Charlson Comorbidity Index (HR (95%CI): 1.16 (1.06; 1.26)), severe frailty (HR (95%CI): 2.15 (1.41; 3.29)), higher number of previous hospitalisations (HR (95%CI): 1.14 (1.06; 1.22)), whereas being allocated to the intervention group was associated with a lower 1-year mortality rate (HR (95%CI): 0.65 (0.42; 0.99)) (Table S3 in Appendices).
Figure 2.
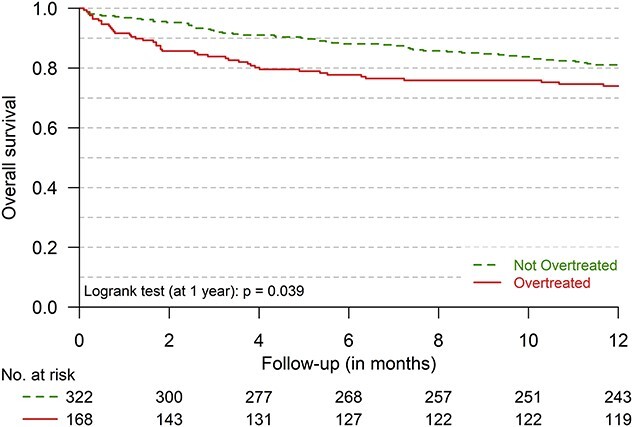
1-year survival of older multimorbid patients with T2D (n = 490) with (n = 168) and without (n = 322) diabetes overtreatment
Discussion
In this study of hospitalised multimorbid older patients with T2D, diabetes overtreatment was present in one-third of patients and associated with a 64% increase in the mortality risk within 1 year in multivariable analysis after adjusting for multiple confounders. Besides, diabetes overtreatment was not associated with hospitalisations nor with functional decline in our study.
In this study, diabetes overtreatment was not associated with a functional decline or hospitalisation at 1 year. However, these results should be interpreted with caution. Regarding functional decline, the interpretation is probably limited by the important number of missing values in the follow-up. Concerning hospitalisations at 1 year, the analysis did not distinguish elective from non-elective hospitalisations, nor, more generally, the causes of hospitalisations, which could therefore interfere with the results. Competing risk of death might have interfered with functional decline and hospitalisation. However, due to convergence issues, we were unable to consider competing risk in these analyses.
This study shows that diabetes overtreatment was independently associated with higher mortality at 1 year, after controlling for major potential confounders like clinical frailty, Charlson Comorbidity Index and age. Similar results were found in another cohort study of patients admitted to a geriatric ward [30]. No causal link between diabetes overtreatment and mortality can be established, given the potential of other confounding factors. As highlighted by this study, it is of concern that overtreatment, known to induce hypoglycaemic events, is more frequent in patients with a short life expectancy, i.e. those who have no benefit to expect from an intensive glycaemic treatment aimed at preventing long-term diabetic complications. The present results could however support the hypothesis that overtreatment is directly increasing mortality in multimorbid older patients with diabetes. Overtreatment indeed induces hypoglycaemic events that may be fatal [6, 31]. Patients in poorer health or with severe frailty are more sensitive to hypoglycaemic events and their consequences [5]. Thus, in any case, individualisation of the treatment of T2D, in particular the glycaemic control, must be implemented.
This study was limited by the secondary analysis of data collected prospectively, which did not include other important variables on diabetes (diabetes complications, age at diagnosis or presence of hypoglycaemia) or GLT use (e.g. patient’s preferences and prescriber’s profile), by the single measure of overtreatment over the time, by the inclusion limited to hospitalised patients with known diagnosis of T2D, preventing the generalisation of the results to the general older population, and by the fact that the main study was not designed for these aims [19]. This study has also strengths: the use of data collected among multimorbid patients usually poorly represented in studies, and the use of a definition of overtreatment based on an individualised definition of diabetes overtreatment, based on the 2019 Endocrine Society guideline, both patient-centred (individualisation) and safe (avoidance of hypoglycaemia), in the absence of a standardised definition.
Further studies are needed to improve the knowledge in this area. Better knowledge is required for better therapeutic management of older patients with T2D. In particular, interventional studies, conducted in representative populations including multimorbid older adults, should be undertaken on selecting the best HbA1c targets, prescribing newer oral glucose-lowering medications (such as SGLT2 inhibitors), or de-intensifying GLT.
In conclusion, avoiding diabetes overtreatment is a major medical priority in multimorbid older patients, as the harms of intensive treatment may likely exceed the benefits, regarding the high 1-year mortality rate observed in patients with glycaemic overtreatment. This can be achieved by individualising the management of GLT according to the patient’s health status and de-intensifying GLT in older patients with diabetes overtreatment.
Supplementary Material
Contributor Information
Antoine Christiaens, Fonds de la Recherche Scientifique (FNRS), Brussels, Belgium; Clinical Pharmacy Research Group, Louvain Drug Research Institute (LDRI), Université catholique de Louvain, Brussels, Belgium; Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP), INSERM, Sorbonne Université, Paris, France.
Oliver Baretella, Department of General Internal Medicine, Inselspital, Bern University Hospital, Bern, Switzerland; Institute of Primary Health Care (BIHAM), University of Bern, Bern, Switzerland.
Cinzia Del Giovane, Institute of Primary Health Care (BIHAM), University of Bern, Bern, Switzerland.
Nicolas Rodondi, Department of General Internal Medicine, Inselspital, Bern University Hospital, Bern, Switzerland; Institute of Primary Health Care (BIHAM), University of Bern, Bern, Switzerland.
Wilma Knol, Department of Geriatrics and Expertise Centre Pharmacotherapy in Old Persons (EPHOR), UMC Utrecht, Utrecht, the Netherlands.
Mike Peters, Department of Geriatrics and Expertise Centre Pharmacotherapy in Old Persons (EPHOR), UMC Utrecht, Utrecht, the Netherlands.
Emma Jennings, Department of Medicine Cork, University College Cork National University of Ireland, Munster, IE, Republic of Ireland; Department of Geriatric Medicine Cork, Cork University Hospital Group, Munster, IE, Republic of Ireland.
Denis O’Mahony, Department of Medicine Cork, University College Cork National University of Ireland, Munster, IE, Republic of Ireland; Department of Geriatric Medicine Cork, Cork University Hospital Group, Munster, IE, Republic of Ireland.
Anne Spinewine, Clinical Pharmacy Research Group, Louvain Drug Research Institute (LDRI), Université catholique de Louvain, Brussels, Belgium; Department of Pharmacy, Centre Hospitalier Universitaire UCL Namur—Godinne, Université catholique de Louvain, Brussels, Belgium.
Benoit Boland, Institute of Health and Society (IRSS), Université catholique de Louvain, Brussels, Belgium; Department of Geriatric Medicine, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Brussels, Belgium.
Séverine Henrard, Clinical Pharmacy Research Group, Louvain Drug Research Institute (LDRI), Université catholique de Louvain, Brussels, Belgium; Institute of Health and Society (IRSS), Université catholique de Louvain, Brussels, Belgium.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was supported by a Research Fellow grant [to A.C.; no. FC23595] from the Fund for Scientific Research—FNRS (Belgium). The OPERAM trial was supported by the European Union’s Horizon 2020 research and innovation program under the grant agreement no. 6342388, and the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137.
The funding bodies played no part in the design of the study and collection, analysis, interpretation of data or in writing the manuscript.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. Data will be made available for scientific purposes for researchers whose proposed use of the data has been approved by the OPERAM publication committee.
References
- 1. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65-99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2020; 162: 108078. [DOI] [PubMed] [Google Scholar]
- 2. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014; 174: 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017; 389: 2239–51. [DOI] [PubMed] [Google Scholar]
- 4. Ray KK, Seshasai SR, Wijesuriya Set al. . Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009; 373: 1765–72. [DOI] [PubMed] [Google Scholar]
- 5. Abdelhafiz AH, Rodriguez-Manas L, Morley JE, Sinclair AJ. Hypoglycemia in older people—a less well recognized risk factor for frailty. Aging Dis 2015; 6: 156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller ME, Bonds DE, Gerstein HCet al. . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010; 340: b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipska KJ, Ross JS, Wang Yet al. . National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014; 174: 1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnold SV, Lipska KJ, Wang J, Seman L, Mehta SN, Kosiborod M. Use of intensive glycemic management in older adults with diabetes mellitus. J Am Geriatr Soc 2018; 66: 1190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruce DG, Davis WA, Davis TME. Glycaemic control and mortality in older people with type 2 diabetes: the Fremantle diabetes study phase II. Diabetes Obes Metab 2018; 20: 2852–9. [DOI] [PubMed] [Google Scholar]
- 10. Zoungas S, Patel A, Chalmers Jet al. . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–8. [DOI] [PubMed] [Google Scholar]
- 11. Sircar M, Bhatia A, Munshi M. Review of hypoglycemia in the older adult: clinical implications and management. Can J Diabetes 2016; 40: 66–72. [DOI] [PubMed] [Google Scholar]
- 12. Yaffe K, Falvey CM, Hamilton Net al. . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013; 173: 1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christiaens A, Henrard S, Zerah L, Dalleur O, Bourdel-Marchasson I, Boland B. Individualisation of glycaemic management in older people with type 2 diabetes: a systematic review of clinical practice guidelines recommendations. Age Ageing 2021; 50: 1935–42. [DOI] [PubMed] [Google Scholar]
- 14. Diabetes Canada Clinical Practice Guidelines Expert Committee, Meneilly GS, Knip Aet al. . Diabetes in older people. Can J Diabetes 2018; 42: S283–95. [DOI] [PubMed] [Google Scholar]
- 15. LeRoith D, Biessels GJ, Braithwaite SSet al. . Treatment of diabetes in older adults: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab 2019; 104: 1520–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . 12. Older adults: standards of medical Care in diabetes-2021. Diabetes Care 2021; 44: S168–79. [DOI] [PubMed] [Google Scholar]
- 17. Hart HE, Rutten GE, Bontje KN, Vos RC. Overtreatment of older patients with type 2 diabetes mellitus in primary care. Diabetes Obes Metab 2018; 20: 1066–9. [DOI] [PubMed] [Google Scholar]
- 18. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015; 175: 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blum MR, Sallevelt B, Spinewine Aet al. . OPtimizing thERapy to prevent Avoidable hospital admissions in Multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ 2021; 374: n1585. 10.1136/bmj.n1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adam L, Moutzouri E, Baumgartner Cet al. . Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): a cluster randomised controlled trial. BMJ Open 2019; 9: e026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drenth-van Maanen AC, Leendertse AJ, Jansen PAFet al. . The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): combining implicit and explicit prescribing tools to improve appropriate prescribing. J Eval Clin Pract 2018; 24: 317–22. [DOI] [PubMed] [Google Scholar]
- 22. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 24. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965; 14: 61–5. [PubMed] [Google Scholar]
- 25. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–108. [DOI] [PubMed] [Google Scholar]
- 26. Theou O, Perez-Zepeda MU, Valk AM, Searle SD, Howlett SE, Rockwood K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing 2021; 50: 1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC Classification and DDD Assignment 2018. Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2017. [Google Scholar]
- 28. Andrew MK, MacDonald S, Godin Jet al. . Persistent functional decline following hospitalization with influenza or acute respiratory illness. J Am Geriatr Soc 2021; 69: 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnau A, Espaulella J, Serrarols M, Canudas J, Formiga F, Ferrer M. Risk factors for functional decline in a population aged 75 years and older without total dependence: a one-year follow-up. Arch Gerontol Geriatr 2016; 65: 239–47. [DOI] [PubMed] [Google Scholar]
- 30. Christiaens A, Boland B, Germanidis M, Dalleur O, Henrard S. Poor health status, inappropriate glucose-lowering therapy and high one-year mortality in geriatric patients with type 2 diabetes. BMC Geriatr 2020; 20: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonds DE, Miller ME, Bergenstal RMet al. . The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340: b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. Data will be made available for scientific purposes for researchers whose proposed use of the data has been approved by the OPERAM publication committee.