Abstract
The frailty index (FI) quantifies frailty as deficit accumulation. It has been adapted to employ laboratory test data (FI-Lab). Our objective was to systematically review and meta-analyse the FI-Lab’s ability to predict mortality. Secondary objectives were to review the FI-Lab’s association with adverse health outcomes and whether FI-Lab scores differed between the sexes. A systematic literature search was carried out using six online databases to identify studies that measured the FI-Lab in humans. Hazard ratios (HRs) were combined in a meta-analysis to create a pooled risk estimate for mortality. Of the 1,201 papers identified, spanning January 2010 until 11 July 2022, 38 were included. FI-Lab scores per 0.01 unit increase predicted mortality overall (HR = 1.04; 95% confidence interval (CI) = 1.03–1.05) and for studies with a mean age of 81+ years (HR = 1.04; 95% CI = 1.03–1.05). The quality of evidence for these meta-analyses are moderate and high, respectively. Further, higher FI-Lab scores were associated with more frequent adverse health outcomes. Sex differences in FI-Lab scores varied, with no consistent indication of a sex effect. The FI-Lab is associated with mortality and with a variety of adverse health outcomes. No consistent sex differences in FI-Lab scores were observed, with several studies in disagreement. Notably, these conclusions were most relevant to older (65+ years old) individuals; further evidence in younger people is needed in both clinical and population representative studies.
Keywords: sex differences, gerontology, frailty, frailty index-lab (FI-Lab), systematic review, older people
Key Points
An FI based on laboratory measures relates to mortality risk in a variety of populations.
Frailty scores using an FI based on laboratory measures relate to higher risks for diverse adverse health events.
Sex differences may not be as prominent in frailty indices based on laboratory measures compared to clinical frailty indices.
Introduction
The frailty index (FI) is an instrument used to quantify frailty and is based on a deficit accumulation model [1]. To achieve this end, various clinical health measures across physiological systems are assessed dichotomously either as deficient or not, which are then summed and divided by the total number of assessments. This yields a score ranging from 0 (no deficits) to 1 (all deficits). The resultant FI score is a macroscopic variable that reflects the state of an individual’s health irrespective of how chronologically old they are, sometimes referred to as ‘biological age’ [2]. In this way, the FI reduces dozens of dimensions into a single variable [1].
A clinical FI is often constructed from a comprehensive geriatric assessment [3–6]. A more recent FI method constructs an FI using laboratory data (FI-Lab), which employs laboratory data to substitute for, or complement the count of deficits [6, 7]. Laboratory derived components are employed as non-arbitrary physiologic measures that count as deficits when deviating from an acceptable range. The first FI-Lab was in a murine ageing model [8], although not presented as such. In 2014, Howlett et al. [7] developed the first formal FI-Lab using standard laboratory tests in humans. This approach has subsequently been used in various human ageing studies, as reviewed here. The FI-Lab can be calculated readily, and its components can usually be obtained from commonly measured hospital tests. Indeed, basing an FI-Lab on routinely collected data was part of its inception [7]. Thus, operationalising standard laboratory data into an FI-Lab may be a convenient and accessible way to assess frailty in a clinical setting. The subsequent FI-Lab score could then be used as a screening tool, as has been suggested [9].
Likely due to its relatively recent origins, we found no systematic reviews or meta-analyses that focus on the FI-Lab. To summarise the available evidence on the FI-Lab, we performed a systematic review and meta-analysis on studies involving the FI-Lab. Our primary objective was to assess the relationship between the FI-Lab and mortality in humans. Secondary objectives were to assess the FI-Lab in relation to other adverse health outcomes and to examine sex differences in FI-Lab scores.
Methods
PRISMA guidelines
This systematic review and meta-analysis followed the PRISMA 2020 guidelines. The protocol was published on Open Science Framework. The most recent protocol is publicly available at: https://doi.org/10.17605/OSF.IO/2ASF9. All amendments are dated and explained in the protocol. The PRISMA 2020 checklist for this systematic review and meta-analysis is available (Supplemental Appendix A).
Data source and search strategy
Three electronic literature searches were conducted in July 2020, May 2021 and July 2022 by author D.G.S. Papers published in English from January 2010–11 July 2022 were searched for on electronic databases (PubMed, CINAHL, MEDLINE, EMBASED, Scopus, Web of Science and AgeLine). Specifically, we searched for ((‘Frailty Index’ AND Laboratory) OR (FI-LAB AND Frailty)) using Boolean-based terms. The searches were full text unless there were over 500 results from a single database.
Inclusion criteria:
FI of at least 10 measures (below which the FI is unstable) [10, 11], where 70% of the deficits measured must be laboratory data, defined as any non-arbitrary diagnostic measure including clinical measures (e.g. hemodynamic measures).
Exclusion criteria:
Papers were excluded if they were case studies, reviews, conference reports/presentations/abstracts, opinion pieces or unpublished data.
Data collection and management
Author D.G.S. compiled a list of articles from all databases, removed duplicates and completed a primary screening. Subsequently, any two of authors D.G.S., S.E.H. and B.M.C. independently screened the remaining article titles and abstracts. Reviewer S.E.H. or S.S.H. arbitrated conflicts in screening; inconsistencies were resolved by discussion. For studies that calculated hazard ratios (HRs) using multiple models, the following model/follow-up criteria were used to select the HR in order of priority: age-adjusted, sex-adjusted and closest to 1-year follow-up time. For studies that calculated the FI-Lab with varying deficit numbers, the HR for the FI-Lab using the most items measured was used for the meta-analysis. HRs were collected at the 0.01 or 0.1 decimal place but were always reported at the 0.01 level.
Subgroupings
Three subgroupings were created to categorise papers based on their findings, including mortality, adverse health outcomes and sex differences. Subgroup inclusion criteria and sorting of studies are depicted in Supplemental Figure 1. All papers were considered for subgroup analysis; however, three studies [12–14] did not fit any of the criteria and were not included in further subgroup analysis. The ‘mortality’ subgroup was further divided for dichotomous statistical comparisons considering study populations and design, including sample size, sex, mean age, items measured and follow-up time (Supplemental Figure 2). Sub-subgroup analyses were exploratory, and credibility was assessed using related criteria (Supplemental Table 1) [15].
Risk of bias and certainty of evidence
Risk of bias assessment used a modified Newcastle-Ottawa scale (Supplemental Appendix B). Studies were excluded from the mortality meta-analysis if they had four or fewer ‘stars’ across all categories (Supplemental Table 2). The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) scale was used to determine the certainty of evidence. A detailed description of the publication bias assessment and certainty of evidence can be found in the Supplemental Data.
Statistics
The inverse variance method was used to calculate effects using log-transformed HRs based on a 0.01 FI-Lab unit change. Study heterogeneity was assessed using cautious interpretation of the I2 test statistic, given the narrow confidence intervals (CIs) of the HRs [16]. If heterogeneity was present, study populations were assessed using a random effects model rather than a fixed effects model. Detailed descriptions of the statistical approach can be found in the Supplemental Data.
Results
Search and selection
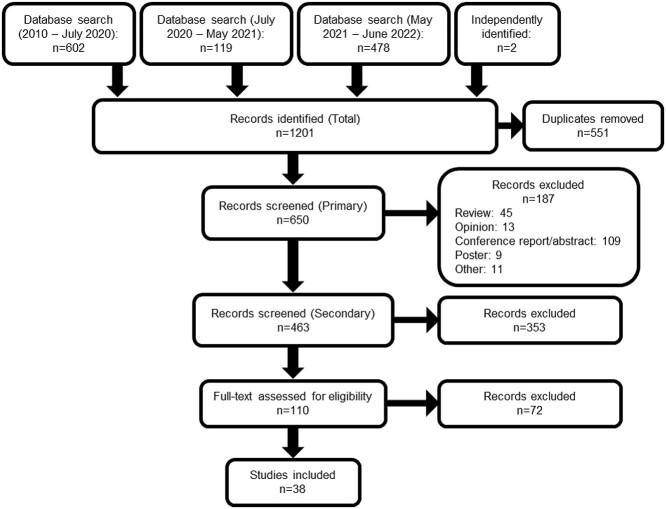
Three systematic searches across seven electronic databases (PubMed, CINAHL, MEDLINE, EMBASE, Scopus, Web of Science and AgeLine) identified 1,201 articles for the systematic review. The searches occurred in July 2020, May 2021 and July 2022. Figure 1 depicts the screening process.
Figure 1.
Search and screening results flow diagram of systematic review.
Characteristics of studies
Of 38 studies (Table 1), the first was published in 2014 [7] and 15 were published since 2021. Studies came about equally from Asia-Pacific (seven from China [17–23]; four from Australia [12, 13, 24, 25], four from South Korea [26–29] and one from Northern Taiwan [14]), Europe [30–40] and North America (six from the USA [41–46] and six from Canada [7, 9, 46–49]). The sample sizes ranged from 14 clinical trial participants [44] to 25,253 in a retrospective analysis of a Canadian national cohort [49]. The mean age range was from 49.4 years [42] to 101.3 years [38]; four studies did not disclose a mean age of participants [13, 21, 34, 43]. The average percent female population across all studies was 47.0%.
Table 1.
Summary of study characteristics of 38 included studies in the systematic review
| Author | Study details/location | Number of participants | Female (%) | Age (mean) (range) | Follow-up period | Number of items in FI-Lab | Acquisition of FI-Lab data |
|---|---|---|---|---|---|---|---|
| Arosio et al., 2022 | Centenarians Northern Italy |
65 | 70.8 | 101.3 ≥100 |
– | 42 | Blood tests from another study |
| Bello et al., 2018 | World Trade Center Health Program NY, USA |
7,346 | 16.7 | 51.0 40–85 |
– | 33 | Blood tests from another study |
| Blodgett et al., 2016 | European Male Ageing Study Europe |
Initial: 3,369 At follow-up: 2,933 |
0.0 | 60.2 40–79 |
4.4 years | 23 | Blood tests from another study |
| Blodgett et al., 2017 | National Health and Nutritional Examination Survey (NHANES) USA |
8,888 | 51.7 | 49.4 ≥20 |
– | 32 | Blood tests from another study |
| Blodgett et al., 2019 | NHANES USA |
8,898 | 51.7 | ≥20 | – | 32 | Blood tests from another study |
| Blodgett et al., 2022 | Canadian Longitudinal Study on Ageing Canada |
25,253 | 51.8 | 45–85 | – | 23 | Blood tests from another study |
| Chao et al., 2020 | Northern Taiwan | 33 | 55.0 | 69.5 | 2–3 years | LFI-1: 23 LFI-2: 32 |
Pre-dialysis blood test |
| Cheung et al., 2017 | Canada | 221 | 47.5 | 76.8 ≥65 |
– | 23 | Routine hospital admissions blood test |
| Ellis et al., 2020 | UK | 1,580 | 55.3 | 84.8 | 21 months | 27 | Routine hospital admissions blood test |
| Engvig et al., 2022 | Norway | 195 | 63 | 86.3 75–100 |
19.3 months | 14 | Routine hospital admissions blood test |
| Gu et al., 2021 | China | 154 | 29.2 | 79.7 | – | 23 | Blood tests from medical records |
| Guan et al., 2022 | Restoring Health of Acutely Unwell Adults (RESORT) Melbourne, Australia |
1,819 | 56.6 | 83.3 (median) 77.6–88.3 |
3 months | 77 | Routine hospital admissions blood test |
| Hao et al., 2019 | Project of Longevity and Ageing Sichuan Province, China |
736 | 67.5 | 93.6 90–108 |
4 years | 22 | Blood tests to assess FI-Lab |
| Heikkila et al., 2021 | Leito, Finland | 1,153 | 58.0 | 73.6 64–100 |
10 and 18 years | 14 | Blood tests from another study |
| Howlett et al., 2014 | Canadian Study of Health and Aging (CSHA) Canada |
Initial: 1,013 Follow-up: 986 |
60.7 | 81.1 ≥65 |
5 years | 23 | Blood tests from another study |
| Huang et al., 2022 | Western China | 627 | 39.23 | 80 (median) ≥60 |
– | 44 | Routine blood test |
| Jäger et al., 2019 | Erlangen, Germany | Initial: 500 Follow-up: 494 |
67.4 | 82.8 | 6 months and 1 year | 21 | Routine blood test at study admission |
| Justice et al., 2019 | Texas, USA | 14 | 14.3 | 70.8 55–85 |
1 month | 34 | Routine blood test |
| Kim CH et al., 2022 | Seoul, South Korea | 508 | 22.2 | 67.3 | 18.2 months | 32 | Pre-operative blood test |
| Kim Y et al., 2022 | Seoul, South Korea | 9,015 | 34.5 | 72.3 | 34.7 months | 32 | Pre-operative blood test |
| King et al., 2017 | Duke Established Populations for Epidemiological Studies of the Elderly NC, USA |
1,740 | 67.0 | 78.0 65–105 |
14 years | 28 | Blood tests from another study |
| Klausen et al., 2017 | Hvidovre, Denmark | 4,005 | 49.7 | ≥65 | 3 years | 17 | Routine hospital admissions blood test |
| Lim et al., 2022 | South Korea | 896 | 22 | 66 | 1 month | 30 | Pre-operative blood test |
| Ma, Liu et al., 2018 | Rugao Longevity and Ageing Study (RuLas) Jiangsu Province, China |
1,463 | 57.8 | 77.4 70–84 |
– | 23 | – |
| Ma, Cai et al., 2018 | RuLas Jiangsu Province, China |
1,780 | 52.8 | 77.0 70–87 |
– | 23 | Blood tests from another study |
| McMillan et al., 2021 | Alberta, Canada | 143 | 12 | 57.7 50–79 |
– | 29 | Routine blood test |
| Mitnitski et al., 2015 | Newcastle 85+ Study Newcastle, UK |
777 | 60.9 | 85.5 ≥85 |
7 years | 40 | Blood tests to assess FI-Lab |
| Naimimohasses et al., 2022 | Dublin, Ireland | 109 | 50.5 | 56 | – | 35 | Routine blood test for study |
| Nixon et al., 2019 | UK | 90 | 50.0 | 69.0 | – | 27 | – |
| Reid et al., 2022 | Melbourne, Australia | 214 | 69.2 | 84.7 | – | 27 | – |
| Ritt et al., 2017 | Erlangen, Germany | Initial: 306 1-year follow-up: 304 |
67.6 | 82.9 | 6 months and 1 year | 23 | Routine blood test at study admission |
| Rockwood et al., 2015 | CSHA Canada |
595 | 67.9 | 82.7 | 6 years | 23 | Blood tests from another study |
| Soh et al., 2022 | RESORT Melbourne, Australia |
1,819 | 56.5 | 83.3 (median) 77.5–88.3 |
1 year | 77 | Routine hospital admissions blood test |
| Sohn et al., 2019 | South Korea | 154 | 49.3 | 78.7 | 40 months | 32 | – |
| Stubbings et al., 2021 | Multiple cohorts USA and Canada |
9,854 | Varied | Varied | 5 years | Varied | Blood tests from another study |
| Theou et al., 2016 | South Australia, Victoria, New South Wales | 53 6-month follow-up: 44 1-year follow-up: 36 |
- | ≥65 | 3 months, 6 months and 1 year | 22 | – |
| Wang et al., 2019 | Chengdu, China | 1,020 | 28.6 | 65 (median) ≥60 |
3.9 years | 44 | Routine blood test |
| Yang et al., 2019 | Chengdu, China | 329 | 68.1 | 85.2 | 1 year | 30 | Blood tests to assess FI-Lab |
Follow-up periods ranged from 1 month [44] to 18 years [32]. Four studies included multiple follow-up periods [13, 32, 33, 37]; 2 studies were cross-sectional with mortality follow-up [42, 43]; 11 were cohort studies [19, 20, 23–25, 36, 38, 39, 41, 47, 49] and 1 was a retrospective observational study [17].
The number of items measured per FI-Lab ranged from 14 deficits [32] to 77 deficits [24, 25]. The average number of deficits across the 37 reporting studies was 30.1 (±13.2 as standard deviation). The items measured varied across studies and have been summarised in Supplemental Figure 3 and Supplemental Table 3. Of the items used, 93% were from blood/urine tests and 7% were from physical measures (especially vital signs; e.g. blood pressure, heart rate and oxygen saturation).
Normal ranges for FI-Lab items were sourced from other works in 10 studies [7, 9, 17, 19, 20, 28, 34, 42, 43]. Henry, 1991 [50] was most cited, while values from other works, such as Blodgett et al., 2015 [51]; Jones et al., 2012 [52] and Pickering et al., 2005 [53], were each used a few times. Six studies used local hospital ranges [21, 31, 39–41, 48] and two calculated their own ranges [35, 38]. The rest of the studies did not provide the source of the normal ranges for the FI-Lab.
Blood test data were acquired (Table 1) from previous studies in 12 of the FI-Lab papers, which were often following large cohorts [30, 42, 43]. Thirteen studies used blood test values obtained upon hospital admission or from routine blood tests [21, 23–25, 31, 33, 34, 37, 39, 40, 44, 47, 48]. Four studies used pre-operative data [14, 27–29], while only three took blood tests specifically to measure the FI-Lab [18, 22, 35].
Certainty of evidence
Risk of bias was assessed using a modified eight-item Newcastle-Ottawa scale (Appendix B). The GRADE approach was used to assess the quality of evidence for each of the subgroups. Mortality was also assessed separately in studies with a mean age of 81+ years subgroup due to greater availability of data in this age bracket. Evidence per subgroup ranged from very low to high (Table 2).
Table 2.
Summary of findings with GRADE.
| Outcomes | Results | No of participants (Studies) Follow-up range |
Quality of the evidence (GRADE)e |
|---|---|---|---|
| Mortality (meta-analysis) | HR: 1.04 (1.03–1.05) for 0.01 change in FI-Lab | 11,032 (11 studies) 6 months–7 years |
⊕ ⊕ ⊕ ⊖
MODERATE Due to indirectnessa |
| Mortality (meta-analysis—81+ years of age) | HR: 1.04 (1.03–1.05) for 0.01 change in FI-Lab for age 81+ years old | 7,079 (9 studies) 6 months–7 years |
⊕ ⊕ ⊕ ⊕
HIGH |
| Mortality (whole subgroup) | High FI-Lab scores were associated with increased risk of mortality in all studies | 69,691 (23 studies) 6 months–18 years |
⊕ ⊕ ⊕ ⊖
MODERATE Due to indirectnessa |
| Adverse health outcomes | High FI-Lab scores were associated with increased risk of at least one adverse health outcome in 94% of studies | 36,526 (18 studies) 1 month–18 years |
⊕ ⊕ ⊕ ⊖
MODERATE Due to indirectnessb |
| Sex differences | No clear association between FI-Lab and sex was found across all studies; 5 studies found no sex difference in FI-Lab scores, 3 found higher FI-Lab scores in males, 2 found higher FI-Lab scores in females and 1 found an age dependent sex effect | 48,274 (13 studies) N/A |
⊕ ⊖ ⊖ ⊖
VERY LOW Due to indirectnessa Risk of biasc Inconsistencyd |
aThe studies are not wholly representative of our target age range of 20+ years.
bThe studies are not wholly representative of our target age range of 20+ years. Each study identified a different adverse outcome.
cRisk of bias due to studies including men and women but not reporting sex-based analyses.
dInconsistency in results across studies. More evidence is needed to make conclusions about sex differences.
eScores out of 4. ⊕ indicates a point. ⊖ indicates the absence of a point.
The FI-Lab as a predictor of mortality
Higher FI-Lab scores related to increased mortality risk in all included studies (Table 3). The relationship with mortality was assessed by a meta-analysis of studies that reported a HR based on a continuous 0.01 increase in FI-Lab scores. These effect sizes were heterogenous, as indicated by the I2 (Figure 2A), although this is expected and not concerning when including larger studies with narrow CIs [16]. Egger’s (P = 0.2414) and Begg-Mazumdar’s (P = 0.1857) tests for funnel plot asymmetry did not show significant risk of publication bias (Supplemental Figure 4A–C). The possibility of different true effect sizes between studies due to differing populations and FI-Labs supported the use of a random effects model for the meta-analysis.
Table 3.
Subgroup summary data from studies included in each subgroup in the systematic review.
| Mortality | ||
|---|---|---|
| Study | Mortality HRs (95% CI) | Conclusions |
| Blodgett et al., 2016 | 1.04 (1.03–1.06) 0.01 change in FI-Lab score Adjustments: age |
High FI-Lab scores associated with mortality |
| Blodgett et al., 2017 | 1.63, 2.59, 3.62, 6.35 Groupings: 0.1–0.2, 0.2–.03, 0.3–0.4, ˃0.4 Adjustments: age, sex |
High FI-Lab scores associated with mortality |
| Blodgett et al., 2022 | OR: 1.05 (1.04–1.06) 0.01 change in FI-Lab score Adjustments: age, sex |
High FI-Lab scores associated with mortality |
| Ellis et al., 2020 | Unadjusted: 1.51 (1.43–1.60) Adjusted: 1.45 (1.37–1.54) change in FI-Lab score Adjustments: age, sex, clinical frailty score, dementia, delirium, falls, residence at admission |
High FI-Lab scores associated with mortality Higher mortality risk in females |
| Engvig et al., 2022 | Unadjusted: 1.04 (1.02–1.05) Adjusted: 1.03 (1.00–1.05) 0.01 change in FI-Lab score Adjustments: age, ward placement, CCI, CFS, NEWS2 |
High FI-Lab scores associated with mortality in older medical inpatients |
| Gu et al., 2021 | OR: 8.705 (3.646–20.782) Ranked FI-Lab scores |
High FI-Lab scores predict in-hospital mortality in AECOPD patients |
| Guan et al., 2022 | Unadjusted: 1.44 (1.23–1.70) Adjusted: 1.32 (1.11–1.58) 0.1 change in FI-Lab score Adjustments: age, sex, CCI, primary reason for hospital admission |
High FI-Lab scores associated with mortality in geriatric rehabilitation inpatients |
| Hao et al., 2019 | 1.33 (1.09–1.63) Adjustments: age, sex, educational levels |
Higher frailty proportions in mortality group 53.4% rate of 4-year mortality |
| Heikkila et al., 2021 | 1.69–3.75 Groupings: ≤0.08, 0.09–0.42, ≥0.43 Adjustments: age, sex |
High FI-Lab scores predict increase in mortality |
| Howlett et al., 2014 | (1.02–1.04) 0.01 change in FI-Lab score Adjustments: age, sex |
High FI-Lab scores associated with mortality |
| Huang et al., 2022 | Unadjusted: 2.955 (2.172–4.021); 4.997 (3.656–6.831) Adjusted: 2.173 (1.576–2.996); 2.877 (2.026–4.083) Groupings: <0.2 (ref), 0.2–0.35, >0.35 Adjustments: age, sex, smoking history, drinking history, state of consciousness, diabetes, hypertension, CHD, COPD, tumour, stroke history, dementia, respiratory failure, septic shock |
High FI-Lab scores associated with mortality in older community-acquired pneumonia patients |
| Jäger et al., 2019 | 1.066 (1.051–1.081) 0.01 change in FI-Lab score Adjustments: age, sex |
High FI-Lab scores associated with increased mortality rates for 6 months and 1 year after hospital readmission |
| Kim CH et al., 2022 | 1.042 (1.010–1.076) Groupings: 0.32 cut-off in FI-Lab score Adjustments: age, EuroSCORE II, peripheral vascular disease |
High FI-Lab scores predict in-hospital mortality in patients after coronary artery bypass grafting |
| Kim Y et al., 2022 | 1.75 (1.49–2.06); 4.29 (3.41–5.40) Groupings: <0.25 (ref); 0.25–0.4; >0.4 Adjustments: age, sex, number of co-morbidities, operating room duration, cancer stage |
High FI-Lab scores associated with mortality in older surgical patients with cancer |
| Klausen et al., 2017 | 1.94 (1.57–2.40); 2.84 (2.31–3.49); 3.66 (3.00–4.48) Quartiles: 1 (ref); 2; 3; 4 Adjustments: age |
Post-discharge mortality associated with FI-Lab scores |
| Mitnitski et al., 2015 | 1.05 (1.04–1.07) 0.01 change in FI-Lab score Adjustments: sex |
FI-Lab scores strongly associated with mortality |
| Ritt et al., 2017 | 1.071 (1.05–1.093) 0.01 change in FI-Lab score Adjustments: age, sex |
FI-Lab scores predict 6-month and 1-year mortality risk |
| Rockwood et al., 2015 | 1.016 (1.007–1.025) 0.01 change in FI-Lab score Adjustments: age, sex |
High FI-Lab scores associated with mortality |
| Soh et al., 2022 | Unadjusted: 1.351 (1.195–1.528) Adjusted: 1.180 (1.037–1.343) 0.1 change in FI-Lab score Adjustments: age, sex, CCI |
High FI-Lab scores associated with 1-year mortality in geriatric rehabilitation inpatients |
| Sohn et al., 2019 | 1.075 (1.040–1.111) Unadjusted |
High FI-Lab scores associated with early mortality in SAVR patients |
| Stubbings et al., 2021 | – | A quantile FI improves the predictive value of the FI-Lab |
| Wang et al., 2019 | 1.02 (1.01–1.03) 0.01 change in FI-Lab score Adjustments: age, sex |
FI-Lab scores can predict mortality in lung cancer patients |
| Yang et al., 2019 | 1.07 (1.05–1.09) 0.01 change in FI-Lab score Adjustments: age, sex |
FI-Lab scores can predict 1-year mortality |
| Adverse health outcomes | ||
| Study | Risk of adverse outcome | Conclusions |
| Bello et al., 2018 | OR: 1.06 (1.027–1.086) for short-term memory problems OR measured per deficit in FI-Lab |
FI-Lab scores are inversely associated with mental and physical health |
| Blodgett et al., 2016 | OR: 1.02 (1.00–1.04); 1.03 (1.02–1.04); 1.04 (1.02–1.05); 1.03 (1.02–1.05); 1.00 (0.99–1.02); 1.01 (1.00–1.02) for institutionalisation, frequency of doctor visits, high number of medications, poor self-reported health, fractures and falls, respectively 0.01 change in FI-Lab |
High FI-Lab scores are associated with institutionalisation, frequent doctor visits, high number of medications, poor self-reported health and falls |
| Blodgett et al., 2019 | OR: 1.46 (1.39–1.54); 1.41 (1.32–1.50); 1.35 (1.29–1.42) for self-reported health, ADL disability and heath care use 0.10 change in FI-Lab score |
High FI-Lab scores are associated with poor self-reported health, ADL disability and health care use |
| Cheung et al., 2017 | OR: 0.8 (0.3–1.8); 1.6 (0.7–3.7) for adverse discharge destination Groupings: FI-Lab between 0.25–0.4 versus <0.25 and FI-Lab >0.4 versus <0.25 Adjustments: age, total number of co-morbidities and ISS |
Severe frailty based on the FI-Lab was not associated with adverse outcomes |
| Ellis et al., 2020 | Unadjusted OR: 1.61 (1.54–1.69); 1.40 (1.29–1.53); 1.20 (1.12–1.28) Adjusted OR: 1.47 (1.41–1.54); 1.39 (1.27–1.52); 1.18 (1.11–1.26) 0.10 change in FI-Lab score For inpatient days with follow-up as offset, discharge to a higher level of care and readmission, respectively |
FI-Lab scores are associated with adverse outcomes, rates of hospital readmission and discharge location |
| Guan et al., 2022 | Unadjusted OR: 0.98 (0.87–1.11); 0.96 (0.86–1.07) Adjusted OR: 0.98 (0.86–1.12); 1.00 (0.88–1.12) 0.10 change in FI-Lab score Adjustments: age, sex CCI, primary reason for hospital admission For functional decline and institutionalisation, respectively |
FI-Lab scores are associated with functional decline and institutionalisation in geriatric rehabilitation inpatients |
| Heikkila et al., 2021 | – | Laboratory index scores do not significantly predict institutionalisation |
| Huang et al., 2022 | Unadjusted OR-respiratory failure: 3.797 (2.101–6.862); 6.113 (3.358–11.128) Unadjusted OR-septic shock: 4.385 (2.101–9.148); 16.8 (8.272–34.119) Adjusted OR-respiratory failure: 3.326 (1.799–6.15); 5.353 (2.835–10.107) Adjusted OR-septic shock: 3.701 (1.736–7.889); 12.713 (6.112–26.445) Groupings: <0.2 (ref), 0.2–0.35, ≥0.35 Adjustments: age, sex, BMI, smoking history, drinking history, state of consciousness, diabetes, hypertension, CHD, COPD, tumour, stroke history, dementia |
FI-Lab scores are associated with respiratory failure and septic shock in older community-acquired pneumonia patients |
| Justice et al., 2019 | – | FI-Lab scores are associated with pro-inflammatory cytokines |
| Kim CH et al., 2022 | OR: 1.02 (1.002–1.039); 1.06 (1.014–1.039); 1.09 (1.032–1.152) Cut-off: 0.32 FI-Lab score For atrial fibrillation, acute kidney injury and reoperation for bleeding, respectively |
FI-Lab scores associated with atrial fibrillation, acute kidney injury and reoperation for bleeding following coronary artery bypass grafting |
| Kim Y et al., 2022 | OR-readmission within 30 days of surgery: 1.20 (1.04–1.38); 1.49 (1.12–1.98) OR-ICU admission within 30 days of surgery: 1.70 (1.47–1.97); 3.58 (2.77–4.63) Groupings: <0.25 (ref), 0.25–0.4, >0.4 Adjustments: age, sex number of co-morbidity, operating room duration, cancer stage |
FI-Lab scores associated with longer length of stay, readmission after surgery and post-operative ICU admission in older surgical patients with cancer |
| Lim et al., 2022 | OR: 1.51 (0.76–2.99); 2.58 (1.15–5.80) Groupings: <0.25 (ref), 0.25–0.4, >0.4 For readmission within 30 days |
FI-Lab scores associated with longer length of hospital stay, ICU stay and hospital readmission in patients undergoing coronary artery bypass graft surgery |
| Ma, Cai et al., 2018 | Unadjusted OR: 1.33 (1.07–1.64) Adjusted OR: 1.33 (1.08–1.65) 0.10 change in FI-Lab score Adjustments: age groups and gender For QTc interval |
FI-Lab scores are associated with QTc prolongation |
| McMillan et al., 2021 | – | FI-Lab scores inversely associated with fluency, fine motor skills and attention/concentration |
| Naimimohasses et al., 2022 | – | FI-Lab scores increased with severity of non-alcoholic fatty liver disease |
| Nixon et al., 2019 | – | FI-Lab scores are associated with worsening kidney function in CKD patients |
| Sohn et al., 2019 | – | FI-Lab scores are associated with short- and long-term outcomes after SAVR in older patients |
| Wang et al., 2019 | Unadjusted OR: 1.83 (1.25–2.67); 3.19 (1.67–6.09) Adjusted OR: 1.85 (1.26–2.72); 3.19 (1.67–6.12) Groupings: <0.2 versus 0.2–0.35 and < 0.2 versus >0.35 Adjustments: age, sex, occupation, health insurance, BMI, pack-years of cigarettes and drinking history For all adverse reactions |
FI-Lab scores are associated with uncontrolled diseases FI-Lab scores can predict adverse outcomes in cancer patients |
| Sex differences | ||
| Study | Conclusions | |
| Arosio et al., 2022 | No sex differences in FI-Lab scores | |
| Bello et al., 2018 | Higher FI-Lab scores in males | |
| Blodgett et al., 2019 | Higher FI-Lab scores in females aged 20–39, males aged 60+ years | |
| Blodgett et al., 2022 | No sex differences in FI-Lab mortality HRs | |
| Cheung et al., 2017 | No sex differences in FI-Lab scores | |
| Hao et al., 2019 | Higher FI-Lab scores in males | |
| Huang et al., 2022 | No sex difference in FI-Lab scores | |
| King et al., 2017 | Higher FI-Lab scores in males | |
| Lim et al., 2022 | No sex difference in FI-Lab scores | |
| Ma, Liu et al., 2018 | No sex difference in FI-Lab scores | |
| McMillan et al., 2021 | Sex did not confound the association between FI-Lab and cognition | |
| Mitnitski et al., 2015 | Higher FI-Lab scores in females | |
| Naimimohasses et al., 2022 | Higher FI-Lab scores in females | |
Note: CCI, Charlson Co-morbidity Index; CFS, clinical FI; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; ISS, injury severity score; NEWS2, new early warning score 2; OR, odds ratio; QTc, corrected QT interval.
Figure 2.
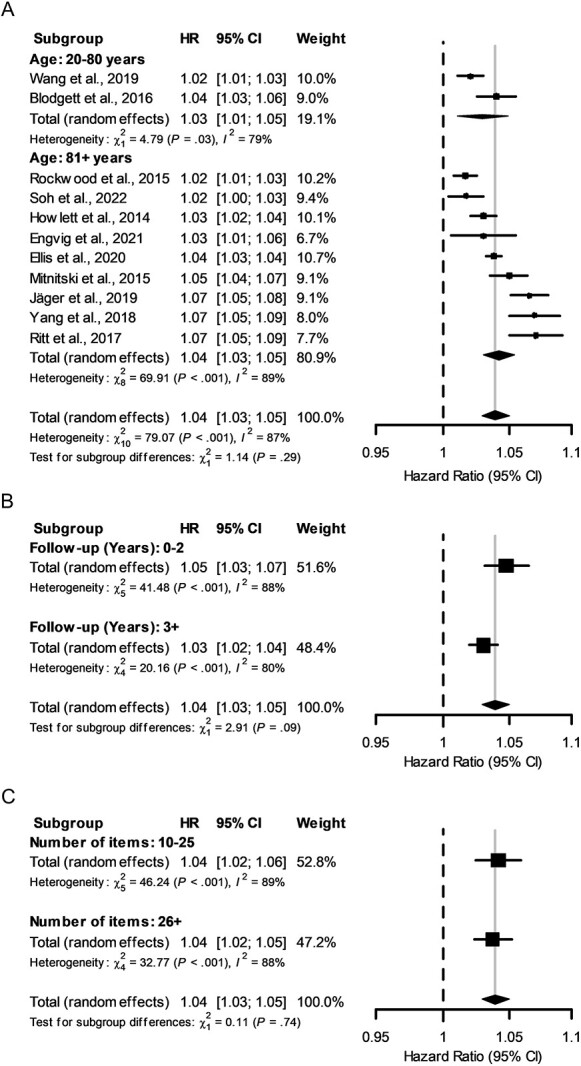
Forest plots of mortality risk by HR according to a 0.01 increase in frailty measured by the FI-Lab. All forest plots used HRs from all studies included in the mortality subgroup meta-analysis. Data are presented on a log10 scale, the dashed-vertical line represents no effect, and the solid-vertical line indicates the overall effect of all studies. (A) HR for all studies in the mortality subgroup. The mean age analysis is also represented with the respective effects of each age grouping. (B) Analysis separated by follow-up time. (C) Analysis separated by number of items measured.
Using a random effects model, the FI-Lab predicted mortality across all ages (Figure 2A). When only studies with a mean age of 81+ years were included, the GRADE score increased from moderate to high, while the HR remained equivalent. Additionally, a subgroup analysis was performed that separated studies at a mean age of 80 years. The mean age (or median when mean was not available) of a study and the HR per 0.01 unit increase in FI-Lab scores were not significantly related (Figure 2A). We also separated studies by follow-up time (>/<2 years); shorter follow-up times yielded a non-significantly higher HR (Figure 2B).
The number of FI-Lab items assessed (< or >25) had no effect on the mortality risk (Figure 2C). Likewise, the proportion who were community dwelling (Supplemental Figure 5A), the sample size (Supplemental Figure 5B) and percent female sex (data not shown) or age or sex adjustment (data not shown) demonstrated no significant differences between groups. Each mortality subgroup analysis demonstrated high heterogeneity, save for the community dwelling group that only included two studies. All exploratory subgroup analyses were considered to have very low credibility for subgroup interactions (Supplemental Table 1).
Adverse health outcomes
Eighteen studies investigated the relationship between FI-Lab scores and adverse health outcomes (Table 3). Of these, 16 showed that FI-Lab scores were related to adverse health outcomes. The FI-Lab predicted adverse events both in a general population (e.g. Blodgett and colleagues [30, 43]) and in clinical groups (e.g. cancer patients [21] and chronic kidney disease patients [36]). Variations in statistical approaches to risk assessment, participant demographics and events makes comparing risks difficult between studies. However, two studies used similar FI-Labs and risk assessment methods by binning FI-Lab scores into low (<0.25), medium (0.25–0.4) and higher (>0.4) scores and adjusting for age and sex in either cancer surgery patients [28] or coronary artery bypass surgery patients [29]. The odds ratio of readmission to hospital within 30 days for high FI-Lab scores compared to low scores had overlapping CIs (1.15–5.80 versus 1.12–1.98). However, cardiac surgery participants with high FI-Lab scores stayed 2.20 days longer in hospital than those with low FI-Lab scores [29], whereas those who underwent cancer resection surgery and had high scores stayed 9.45 days longer than those with low scores [28]. Thus, even when FI-Labs are similarly constructed, measured risks may differ based on the population.
Sex differences in FI-Lab scores
Of 13 studies that assessed how FI-Lab scores differ by sex, 3 concluded that men had higher FI-Lab scores than women [18, 41, 45], while 2 concluded that women had higher FI-Lab scores than men [35, 40]. Five studies observed no sex differences in FI-Lab scores [19, 23, 29, 38, 47]. One study [43] concluded that FI-Lab scores were higher in women aged 20–39 but were higher in men aged 60+ years.
Discussion
This systematic review of the FI-Lab, which was introduced in 2014, identified 38 studies that used this assessment.
Mortality and adverse health outcomes
Our meta-analysis of the FI-Lab as a predictor of mortality in humans combined the HR per 0.01 change in FI-Lab scores across 11 studies. A 0.01 change in FI-Lab score is roughly equivalent to a person gaining a quarter of a deficit, if 25 items were measured (or 1 deficit in 100 measures). Consequently, as in the overall deficit accumulation FI, a small change in risk is expected for a 0.01 change and becomes larger when more deficits are present. Meta-analysis of 11 studies predicting mortality from an FI-Lab yielded an effect size as a HR of 1.04 (95% CI = 1.03–1.05) per 0.01 increase in the FI score. Interestingly, this effect size is nearly identical to a meta-analysis of clinical FIs, which sometimes included some laboratory measures, to predict mortality, where the comparable HR was 1.04 (95% CI = 1.03–1.04; [54]). Together, this suggests that the FI-Lab can predict mortality, and under some circumstances, this effect may be comparable to FIs not incorporating laboratory measures.
The relationship between the FI and mortality appears to hold in older adults. Our review did not identify much research using younger adults. One study suggested the prognostic value of an FI-Lab is not as strong a predictor of mortality in younger adults compared to older adults [42]. The improved predictive value in older adults might make sense, given the original criteria FIs are based upon [10], which suggests that deficits should increase with age and should cover a range of systems. As suggested by Blodgett and colleagues [42], investigating the FI-Lab’s relationship with adverse health outcomes in younger people, instead of mortality, may be more fruitful. The current state of evidence cannot answer this question, however.
Despite most study populations having a mean age of 81+ years, the lack of association between mean age and HR at the 0.01 level using weighted means is intriguing. At the least, this suggests an FI-Lab predicts mortality similarly for older populations, such as those analysed here. In addition, the fact that the FI-Lab was associated with incident mortality in both presentative population samples and in clinical/institutional samples suggests that it is a useful tool for diverse health populations.
We also examined how the number of deficits measured per FI-Lab affected its relationship with mortality, which demonstrated no differences in HRs for studies using 20–25 items versus those with 26–77 items. This supports the notion that the number and exact items measured are not important when comparing between samples as long as they are ample enough and relate to a variety of physiological systems [10]. Sample size also did not seem to affect the FI-Lab’s ability to predict mortality, suggesting that the included studies were powered appropriately.
Regarding non-fatal health issues, every study showed that the FI-Lab was associated with adverse health outcomes, except institutionalisation in one study [32] and adverse discharge destination in another [47]. It is unknown if this trend holds up to most adverse health outcomes, but it is intriguing to think of the FI-Lab serving as a robust holistic health risk metric for non-fatal health issues.
Sex differences in the FI-Lab
There was no clear indication of a sex difference in FI-Lab score between sexes. Three of 11 studies that evaluated sex differences concluded that men have higher FI-Lab scores than women, while 2 found the opposite and another 5 found no difference. Notably, Bello and colleagues [41] found an age-frailty interaction, where women were frailer at younger ages but then became less frail compared to men at older ages. These findings are inconsistent with the male–female health survival paradox, which suggests that women have increased frailty, but they are more resilient than men and live longer [55, 56]. With the FI-Lab, there is no clear indication of a sex effect, so the paradox is not present. More dedicated studies, with less heterogeneity, are needed to establish whether there is a bona fide sex difference when using an FI-Lab. Whether the answer agrees with the male–female health survival paradox is unknown, although current evidence suggests it does not. Further, it will be important to identify whether the relationship between mortality and the FI-Lab is equal between men and women.
FI-Lab components
The deficits that make up the FI-Lab distinguish it from FIs based on clinical assessments. While both these tools function similarly, the FI-Lab is likely easier to automate and looks at frailty from a different perspective. In fact, the FI-Lab was able to improve the predictive power of a clinical FI through their combination or addition to a proportional hazard model [9]. Even so, it is not clear whether this simply reflects the nature of the additional items, or that more items typically make for more informative FIs, especially after age 65 [11]. Standard laboratory tests can be core measures used to create an FI-Lab, as was suggested [7], which operationalizes routinely collected data.
Quality of evidence
From the outset, we decided to focus on breadth for this systematic review and meta-analysis. This style has inherent benefits and limitations. We were able to collect all available information on the FI-Lab. However, the studies were quite diverse in nature, ranging widely in study populations. Our quality of evidence table, assessed by GRADE, reflects the evolving literature and our broad inclusion criteria. Our meta-analysis for the overall mortality subgroup included moderate-quality evidence due to the distribution of ages across studies. This portion of the meta-analysis lost quality because we sought to examine the FI-Lab’s association with mortality in adults for all ages of ≥20 years. However, the papers we identified mostly included older adults. In this way, the quality of evidence improved by reframing our question to older adults, but it identified that little is known about how the FI-Lab works in younger adults.
Conclusions
This systematic review and meta-analysis suggest that FI-Labs, made of diverse deficits, predict mortality and other adverse health outcomes in a variety of populations. FI-Lab scores did not show a consistent difference between sexes. This does not align with what is found with clinical FIs, which typically find females to be frailer [56].
Future research utilising an FI-Lab may benefit from investigating the relationship between frailty in younger populations and the subsequent health status changes. Additionally, there is emerging evidence that a more granular approach to health variable categorisation (i.e. a non-binary quantile approach) using an FI-Lab improves the model’s accuracy [46] relative to dichotomizing variables. Both avenues deserve further attention.
Supplementary Material
Contributor Information
David G Sapp, Department of Pathology, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
Brianna M Cormier, Department of Pharmacology, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
Kenneth Rockwood, Department of Medicine (Geriatric Medicine), Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
Susan E Howlett, Department of Pharmacology, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada; Department of Medicine (Geriatric Medicine), Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
Stefan S Heinze, Department of Pharmacology, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
Declaration of Conflicts of Interest
K.R. is supported by the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research. K.R. has asserted copyright of the Clinical Frailty Scale through Dalhousie University’s Industry, Liaison and Innovation Office. Use is free for education, research and not-for-profit health care. Users agree not to change or commercialise the scale. In addition to academic and hospital appointments, K.R. is the co-founder of Ardea Outcomes, which (as DGI Clinical), in the last 3 years, has contracts with pharma and device manufacturers (Danone, Hollister, INmune, Novartis and Takeda) on individualised outcome measurement. In 2020, he attended an advisory board meeting with Nutricia on dementia and chaired a Scientific Workshop & Technical Review Panel on frailty for the Singapore National Research Foundation. He is an associate director of the Canadian Consortium on Neurodegenerataion in Aging, itself funded by the Canadian Institutes for Health Research, the Alzheimer Society of Canada and several other charities. Otherwise, the authors do not declare any competing interests.
Declaration of Sources of Funding
This work was supported the Canadian Institutes for Health Research (SEH. PGT 155961) and the Heart and Stroke Foundation of Canada (SEH G-22-0031992). S.S.H. is supported by the Nova Scotia Health Research Foundation’s Scotia Scholars Award, a Level II Killam Predoctoral Scholarship, the Canadian Institutes of Health Research Doctoral Research Award and Dalhousie University’s President’s Award. This review was undertaken independently by the authors and the funding bodies had no role in the design, execution, analysis or interpretation and writing of this study.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002; 2: 1. 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004; 52: 1929–33. [DOI] [PubMed] [Google Scholar]
- 4. Cohen HJ, Smith D, Sun CLet al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 2016; 122: 3865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper L, Loewenthal J, Frain LNet al. From research to bedside: incorporation of a CGA-based frailty index among multiple comanagement services. J Am Geriatr Soc 2022; 70: 90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging 2021; 1: 651–65. [DOI] [PubMed] [Google Scholar]
- 7. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med 2014; 12: 171. 10.1186/s12916-014-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parks RJ, Fares E, Macdonald JKet al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci 2012; 67: 217–27. [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, McMillan M, Mitnitski A, Howlett SE. A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc 2015; 16: 842–7. [DOI] [PubMed] [Google Scholar]
- 10. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farrell SG, Mitnitski AB, Rockwood K, Rutenberg AD. Network model of human aging: frailty limits and information measures. Phys Rev E 2016; 94: 052409. 10.1103/PhysRevE.94.052409. [DOI] [PubMed] [Google Scholar]
- 12. Reid O, Ngo J, Lalic S, Su E, Elliott RA. Paracetamol dosing in hospital and on discharge for older people who are frail or have low body weight. Br J Clin Pharmacol 2022; 88: 4565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Theou O, Chapman I, Wijeyaratne Let al. Can an intervention with testosterone and nutritional supplement improve the frailty level of under-nourished older people? J Frailty Aging 2016; 5: 247–52. [DOI] [PubMed] [Google Scholar]
- 14. Chao CT, Huang JW, Chiang CK, Hung KY. COhort of GEriatric nephrology in NTUH (COGENT) Study Group. Applicability of laboratory deficit-based frailty index in predominantly older patients with end-stage renal disease under chronic dialysis: a pilot test of its correlation with survival and self-reported instruments. Nephrology (Carlton) 2020; 25: 73–81. [DOI] [PubMed] [Google Scholar]
- 15. Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010; 340: c117. 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 16. Iorio A, Spencer FA, Falavigna Met al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350: h870. 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 17. Gu JJ, Liu Q, Zheng LJ. A frailty assessment tool to predict in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2021; 16: 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao Q, Sun X, Yang M, Dong B, Dong B, Wei Y. Prediction of mortality in Chinese very old people through the frailty index based on routine laboratory data. Sci Rep 2019; 9: 221. 10.1038/s41598-018-36569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma T, Lu D, Zhu YSet al. ACTN3 genotype and physical function and frailty in an elderly Chinese population: the Rugao Longevity and Ageing Study. Age Ageing 2018; 47: 416–22. [DOI] [PubMed] [Google Scholar]
- 20. Ma T, Cai J, Zhu YSet al. Association between a frailty index based on common laboratory tests and QTc prolongation in older adults: the Rugao Longevity and Ageing Study. Clin Interv Aging 2018; 13: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Zhang R, Shen Y, Su L, Dong B, Hao Q. Prediction of chemotherapy adverse reactions and mortality in older patients with primary lung cancer through frailty index based on routine laboratory data. Clin Interv Aging 2019; 14: 1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang M, Zhuo Y, Hu X, Xie L. Predictive validity of two frailty tools for mortality in Chinese nursing home residents: frailty index based on common laboratory tests (FI-Lab) versus FRAIL-NH. Aging Clin Exp Res 2018; 30: 1445–52. [DOI] [PubMed] [Google Scholar]
- 23. Huang S, Wang Y, Chen L, Chen X. Use of a frailty index based upon routine laboratory data to predict complication and mortality in older community-acquired pneumonia patients. Arch Gerontol Geriatr 2022; 101: 104692. 10.1016/j.archger.2022.104692. [DOI] [PubMed] [Google Scholar]
- 24. Guan L, Soh CH, Reijnierse EM, Lim WK, Maier AB. Association of a modified laboratory frailty index with adverse outcomes in geriatric rehabilitation inpatients: RESORT. Mech Ageing Dev 2022; 203: 111648. 10.1016/j.mad.2022.111648. [DOI] [PubMed] [Google Scholar]
- 25. Soh CH, Guan L, Reijnierse EM, Lim WK, Maier AB. Comparison of the modified frailty-index based on laboratory tests and the Clinical Frailty Scale in predicting mortality among geriatric rehabilitation inpatients: RESORT. Arch Gerontol Geriatr 2022; 100: 104667. 10.1016/j.archger.2022.104667. [DOI] [PubMed] [Google Scholar]
- 26. Sohn B, Choi JW, Hwang HY, Jang MJ, Kim KH, Kim KB. Frailty index is associated with adverse outcomes after aortic valve replacement in elderly patients. J Korean Med Sci 2019; 34: e205. 10.3346/jkms.2019.34.e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim CH, Kang Y, Kim JS, Sohn SH, Hwang HY. Association between the frailty index and clinical outcomes after coronary artery bypass grafting. J Chest Surg 2022; 55: 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim Y, Song K, Kang CM, Lee H. Impact of preoperative laboratory frailty index on mortality and clinical outcomes in older surgical patients with cancer. Sci Rep 2022; 12: 9200. 10.1038/s41598-022-13426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim A, Choi M, Jang Y, Lee H. Preoperative frailty based on laboratory data and postoperative health outcomes in patients undergoing coronary artery bypass graft surgery [published online ahead of print, 2022 May 19]. Heart Lung 2022; 56: 1–7. 10.1016/j.hrtlng.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 30. Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing 2016; 45: 463–8. [DOI] [PubMed] [Google Scholar]
- 31. Ellis HL, Wan B, Yeung Met al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ 2020; 192: E3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heikkilä E, Salminen M, Viljanen Aet al. A practical laboratory index to predict institutionalization and mortality - an 18-year population-based follow-up study. BMC Geriatr 2021; 21: 139. 10.1186/s12877-021-02077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jäger J, Sieber CC, Gaßmann KG, Ritt M. Changes of a frailty index based on common blood and urine tests during a hospital stay on geriatric wards predict 6-month and 1-year mortality in older people. Clin Interv Aging 2019; 14: 473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klausen HH, Petersen J, Bandholm Tet al. Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study [published correction appears in BMC Geriatr. 2017 Mar 15;17 (1):67]. BMC Geriatr 2017; 17: 62. 10.1186/s12877-017-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitnitski A, Collerton J, Martin-Ruiz Cet al. Age-related frailty and its association with biological markers of ageing. BMC Med 2015; 13: 161. 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nixon AC, Bampouras TM, Pendleton N, Mitra S, Dhaygude AP. Diagnostic accuracy of frailty screening methods in advanced chronic kidney disease. Nephron 2019; 141: 147–55. 10.1159/000494223. [DOI] [PubMed] [Google Scholar]
- 37. Ritt M, Jäger J, Ritt JI, Sieber CC, Gaßmann KG. Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging 2017; 12: 1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arosio B, Geraci A, Ferri E, Mari D, Cesari M. Biological frailty index in centenarians. Aging Clin Exp Res 2022; 34: 687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Engvig A, Wyller TB, Skovlund Eet al. Association between clinical frailty, illness severity and post-discharge survival: a prospective cohort study of older medical inpatients in Norway. Eur Geriatr Med 2022; 13: 453–61. 10.1007/s41999-021-00555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naimimohasses S, O'Gorman P, McCormick Eet al. Prevalence of frailty in patients with non-cirrhotic non-alcoholic fatty liver disease. BMJ Open Gastroenterol 2022; 9: e000861. 10.1136/bmjgast-2021-000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bello GA, Lucchini RG, Teitelbaum SL, Shapiro M, Crane MA, Todd AC. Development of a physiological frailty index for the World Trade Center General Responder Cohort. Curr Gerontol Geriatr Res 2018; 2018: 3725926. 10.1155/2018/3725926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience 2017; 39: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blodgett JM, Theou O, Mitnitski A, Howlett SE, Rockwood K. Associations between a laboratory frailty index and adverse health outcomes across age and sex. Aging Med (Milton) 2019; 2: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Justice JN, Nambiar AM, Tchkonia Tet al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019; 40: 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King KE, Fillenbaum GG, Cohen HJ. A cumulative deficit laboratory test-based frailty index: personal and neighborhood associations. J Am Geriatr Soc 2017; 65: 1981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stubbings G, Rockwood K, Mitnitski A, Rutenberg A. A quantile frailty index without dichotomization. Mech Ageing Dev 2021; 199: 111570. 10.1016/j.mad.2021.111570. [DOI] [PubMed] [Google Scholar]
- 47. Cheung A, Haas B, Ringer TJ, McFarlan A, Wong CL. Canadian study of health and aging clinical frailty scale: Does it predict adverse outcomes among geriatric trauma patients? J Am Coll Surg 2017; 225: 658–65.e3. [DOI] [PubMed] [Google Scholar]
- 48. McMillan JM, Gill MJ, Power C, Fujiwara E, Hogan DB, Rubin LH. Construct and criterion-related validity of the Clinical Frailty Scale in persons with HIV. J Acquir Immune Defic Syndr 2021; 88: 110–6. [DOI] [PubMed] [Google Scholar]
- 49. Blodgett JM, Pérez-Zepeda MU, Godin Jet al. Frailty indices based on self-report, blood-based biomarkers and examination-based data in the Canadian Longitudinal Study on Aging. Age Ageing 2022; 51: afac075. 10.1093/ageing/afac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henry JB. Clinical & Diagnosis Management by Laboratory Methods. 18th edition. Philadelphia: W.B. Saunders, 1991. [Google Scholar]
- 51. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr 2015; 60: 464–70. [DOI] [PubMed] [Google Scholar]
- 52. Jones D, Kho A, Francesco L. Hypotension in Principles and Practice of Hospital Medicine. Chapter 91. New York: McGraw-Hill, 2012. [Google Scholar]
- 53. Pickering TG, Hall JE, Appel LJet al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Circulation 2005; 111: 697–716. [DOI] [PubMed] [Google Scholar]
- 54. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 2018; 47: 193–200. 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 55. Kane AE, Howlett SE. Sex differences in frailty: comparisons between humans and preclinical models. Mech Ageing Dev 2021; 198: 111546. 10.1016/j.mad.2021.111546. [DOI] [PubMed] [Google Scholar]
- 56. Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust 2020; 212: 183–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.