Abstract
In contrast to normal type I collagen (Col1) heterotrimer (α1/α2/α1) produced by fibroblasts, pancreatic cancer cells specifically produce unique abnormal Col1 homotrimer (α1/α1/α1). Col1 homotrimer results from epigenetic suppression of the Col1a2 gene and promotes oncogenic signaling, cancer cell proliferation, tumor organoid formation and growth via α3β1 integrin on cancer cells, associated with tumor microbiome enriched in anaerobic Bacteroidales in the hypoxic and immunosuppressive tumors. Deletion of Col1 homotrimers increases overall survival of mice with PDAC, associated with reprograming of the tumor microbiome with increased microaerophilic Campylobacterales, which can be reversed with broad spectrum antibiotics. Deletion of Col1 homotrimers enhances T cell infiltration and enables efficacy of anti-PD-1 immunotherapy. This study identifies the functional impact of Col1 homotrimers on tumor microbiome and tumor immunity, implicating Col1 homotrimer-α3β1 integrin signaling axis as a cancer specific therapeutic target.
eTOC blurb
Chen et al. identify that type I collagen (Col1) produced by pancreatic cancer cells is the abnormal homotrimer variant with oncogenic properties. Deletion of Col1 homotrimer in cancer cells inhibits tumor progression and reshapes the tumor microbiome. Cancer-Col1 homotrimer deletion enhances T cell infiltration and enables efficacy of anti-PD-1 immunotherapy.
Graphical Abstract
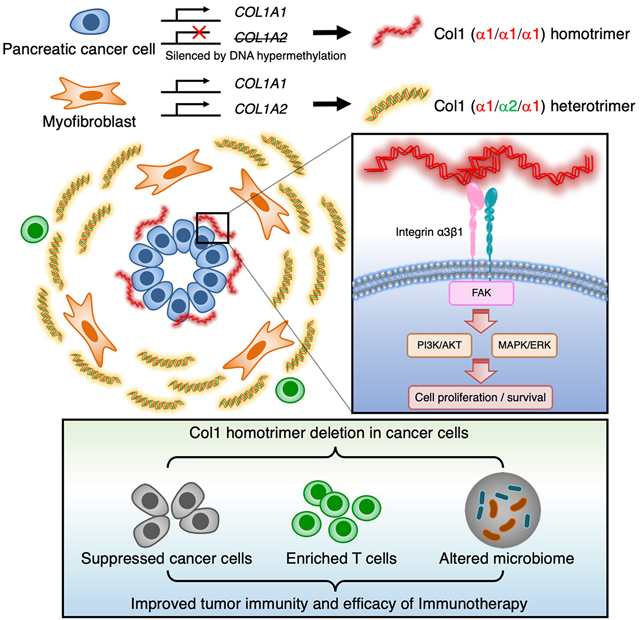
Introduction
Type I collagen (Col1) is the most abundant protein in the human body and accumulates extensively in the microenvironment of pancreatic ductal adenocarcinoma (PDAC) (Mollenhauer et al., 1987; Tian et al., 2019). Recent studies suggest that Col1 produced by myofibroblasts restrains PDAC (Bhattacharjee et al., 2021; Chen et al., 2021), but the structure and function of Col1 produced by cancer cells (cancer-Col1) in PDAC remain unknown. Previous studies in the last few decades highlight the capacity of cancer cells to generate Col1 within the tumor stroma (Egeblad et al., 2010; Han et al., 2010; Han et al., 2008; Makareeva et al., 2010; Minafra et al., 1988; Moro and Smith, 1977; Rupard et al., 1988; Sengupta et al., 2003), albeit at lower synthesis rate compared to fibroblasts (Chen et al., 2021). Questions still remain as to whether the biochemistry and biology of cancer-Col1 are different from those of fibroblast-derived Col1, including its capacity to form complex fibers and engage in outside-in signaling via several putative Col1-binding cell surface receptors (Egeblad et al., 2010; Leitinger, 2011; Yeh et al., 2012). In order to probe the role of cancer-Col1 in PDAC initiation and progression, we generated several genetically engineered mouse models (GEMMs) to delete Col1 specifically in pancreatic cancer cells.
Results
Type I collagen (Col1) produced by PDAC cells is composed of abnormal homotrimers (α1/α1/α1)
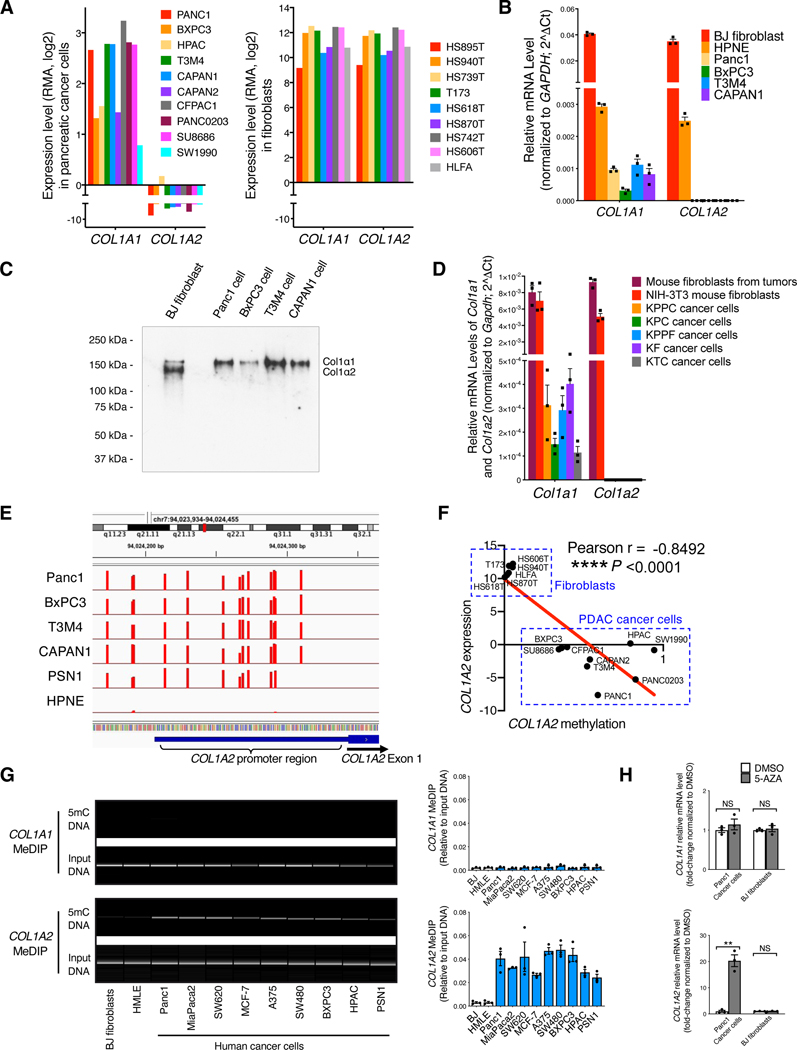
Assessment of functional Col1 expression involves the detection of both Col1α1 and Col1α2 chains (encoded by COL1A1 and COL1A2 genes, respectively), which form the functional unit of Col1. We evaluated the expression of Col1 in human cell lines from the CCLE dataset (Barretina et al., 2012; Ghandi et al., 2019). Human pancreatic cancer cell lines express only Col1α1 chain (Figure 1A), in contrast to human fibroblast lines that express both α1 and α2 chains (Figure 1A). Such transcriptional expression differences in the Col1α1 and Col1α2 chains were further confirmed by qRT-PCR analysis (Figure 1B). Western blot assay further validated that human pancreatic cancer cells produced only the αl chain of Col1 at the protein level, whereas human fibroblasts produced both αl and α2 chains of Col1 (Figure 1C).
Figure 1. Pancreatic cancer cells express only COL1A1 (forming α1/α1/α1 homotrimer) but not COL1A2 due to COL1A2 promoter hypermethylation.
(A) The expression levels of type I collagen (Col1) genes: COL1A1 (encoding Col1 α1 chain) and COL1A2 (encoding Col1 α2 chain) in human pancreatic cancer cell and fibroblast lines based on RNA-seq data from the CCLE database. RMA (Robust Multi-array Average algorithm) normalization was used and data were presented as the log2 gene expression.
(B) The expression levels of COL1A1 and COL1A2 in human cell lines by qRT-PCR. Human BJ fibroblast, normal human pancreatic epithelial cell line (HPNE), and pancreatic cancer cell lines were examined (n = 3).
(C) Detection of purified Col1 (human Col1α1 and/or Col1α2 chains) from conditioned culture media of human cell lines by Western blot. Representative Western blot of three independently experiments was shown.
(D) The expression levels of Col1a1 and Col1a2 in primary pancreatic cancer cell lines from indicated mouse models, as compared to mouse fibroblasts (n = 3).
(E) Genome-wide DNA methylation analysis of human pancreatic cancer cell lines and HPNE cell line (n = 3). DNA methylation at COL1A2 gene promoter regions was shown. Methylation percentages of the individual CpGs examined are depicted as bars (with Y axis ranging from 0 to 100) at their genome.
(F) The reverse correlation between COL1A2 expression level and COL1A2 methylation fraction in human pancreatic cancer cells and fibroblasts based on the RNA-seq and DNA methylation data from the CCLE database. Pearson’s correlation test was used.
(G) Methylated DNA immunoprecipitation (MeDIP) assay of COL1A1 and COL1A2 genes in various human cancer cell lines, as compared with BJ fibroblasts (n = 3).
(H) The expression of COL1A1 and COL1A2 in human cancer cells and fibroblasts treated with 5-azacytidine (5-AZA; 10 μg/mL) for 14 days (n = 3).
Data are represented as mean ± SEM. **p < 0.01, ****p < 0.0001; NS: not significant, Student’s t test was used unless otherwise indicated. All experiments were conducted in triplicate.
See also Figure S1.
Consistent with the observations made with human PDAC cell lines, cancer cell lines from different genetically engineered mouse models (GEMMs) of PDAC exhibited only Col1α1 chain (encoded by Col1a1) expression but not Col1α2 chain (encoded by Col1a2) (Figure 1D). In contrast, mouse fibroblast lines displayed expression of both Col1α1 and Col1α2 chains (Col1a1 and Col1a2) (Figure 1D). We generated KrasG12D/+;Pdx1-Cre;LSL-YFP (KC;YFP) mice and LSL-KrasG12D/+;Trp53loxP/loxP;Pdx1-Cre;LSL-YFP (KPPC;YFP) mice. We confirmed the expression Col1a1 but not Col1a2 in YFP-sorted KC;YFP early lesion cells and KPPC;YFP cancer cells (Figure S1A). Collectively, the production of Col1 homotrimer consisting of only the Col1α1 chain is likely an overarching feature of both human and mouse pancreatic cancer cells.
Pancreatic cancer cells exhibit hypermethylation of the COL1A2 promoter and epigenetic silencing of the COL1A2 gene
We next analyzed the CCLE database for potential genetic events in cancer cells that could explain the loss of Col1α2 chain. Copy number alterations or deletion of COL1A2 were not identified in the cancer cell lines examined (Figure S1B). Next, our unbiased genome-wide DNA methylation analysis revealed prominent hypermethylation in the promoter regions of COL1A2 gene in multiple human PDAC cell lines, but not in normal human pancreatic epithelial HPNE cell line (Figure 1E). Consistently, the COL1A2 hypermethylation status inversely correlated with the expression level of COL1A2 in pancreatic cancer cells and fibroblasts (Figure 1F). Next, methylated DNA immunoprecipitation (MeDIP) assay was conducted employing DNA isolated from cancer cells or fibroblasts. DNA hypermethylation in the promoter of the COL1A2 gene but not the COL1A1 gene was detected in all human and mouse PDAC cell lines, while COL1A2 hypermethylation was not detected in fibroblasts (Figures 1G and S1C). Treatment with the demethylating agent 5-azacitidine recovered the expression of COL1A2 in human and mouse pancreatic cancer cells (Figures 1H and S1D).
Col1 deletion in pancreatic cancer cells delays PDAC progression and prolongs overall survival of mice
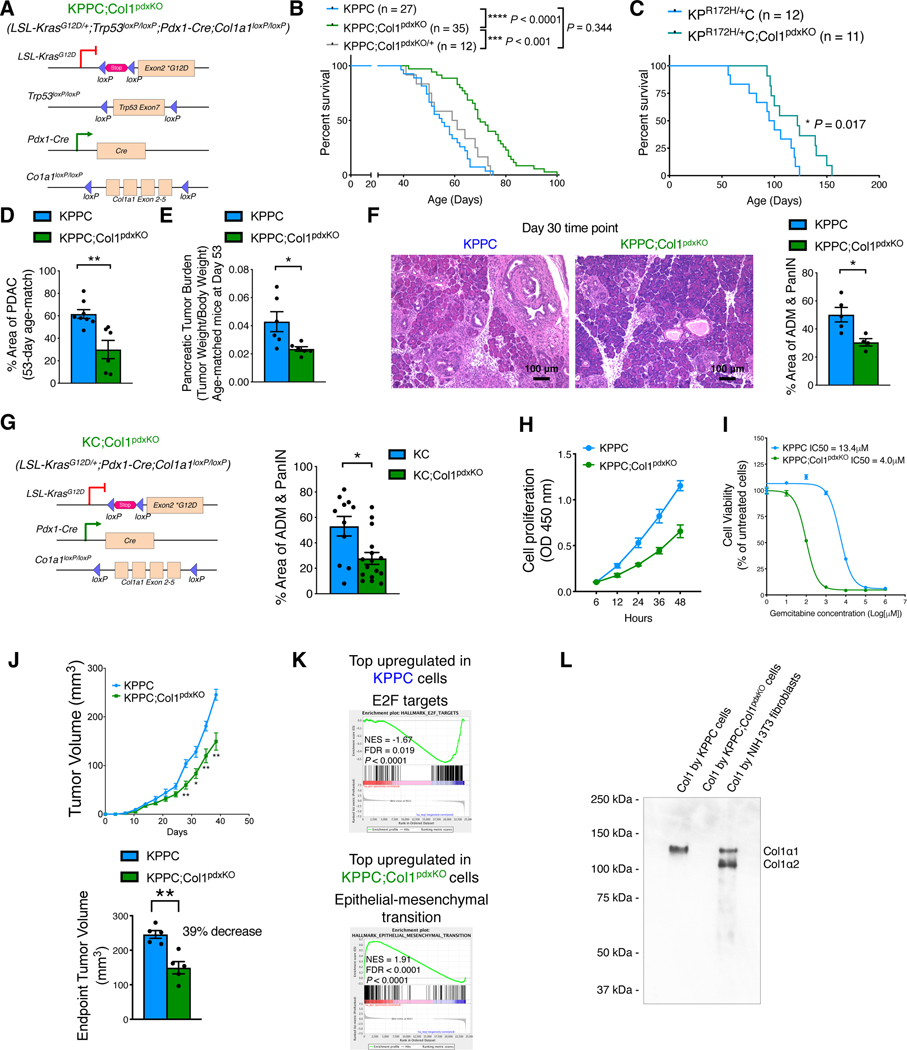
To test the functional role of Col1 homotrimer produced by cancer cells, we generated LSL-KrasG12D/+;Trp53loxP/loxP;Pdx1-Cre;Col1a1loxP/loxP (KPPC;Col1pdxKO) mice, with genetic deletion of Col1 in cancer cells (Figure 2A). Col1 deletion in cancer cells significantly impeded PDAC progression and prolonged the overall survival of KPPC;Col1pdxKO mice as compared to KPPC control mice (Figure 2B). KPPC;Col1pdxKO/+ mice with heterozygous Col1a1 deletion in cancer cells revealed similar overall survival when compared to KPPC mice (Figure 2B). We also confirmed this phenotype using LSL-KrasG12D/+;Trp53R172H/+;Pdx1-Cre;Col1a1loxP/loxP (KPC;Col1pdxKO) mice (Figure 2C).
Figure 2. Deletion of Col1 as the α1/α1/α1 homotrimer format in pancreatic cancer cells delays PDAC progression.
(A) Genetic strategy to delete Col1α1 (Col1a1) in pancreatic cancer cells using the KPPC;Col1pdxKO mice, as compared to background-matched KPPC mice.
(B) Survival of KPPC (n = 27), KPPC;Col1pdxKO (n = 35), and KPPC;Col1pdxKO/+ (heterozygous Col1a1 deletion, n = 12) mice. Log-rank (Mantel-Cox) test was used.
(C) Survival of KPC;Col1pdxKO (n = 11) mice, as compared to background-matched KPC (n = 12) mice. Log-rank (Mantel-Cox) test was used.
(D) Histology evaluation of tumors from KPPC (n = 8) and KPPC;Col1pdxKO (n = 6) mice at the same age of 53 days.
(E) Pancreatic tumor burden (tumor weight/body weight) of KPPC (n = 6) and KPPC;Col1pdxKO (n = 6) mice at the same 53 days of age.
(F) Percentage of ADM/PanIN lesion areas of KPPC (n = 5) and KPPC;Col1pdxKO (n = 4) mice at the same 30 days of age. Scale bar: 100 μm.
(G) Genetic strategy to delete Col1a1 in cancer cell lineage in the context of pancreatic cancer using the KC;Col1pdxKO mice, as compared to background-matched KC mice. Percentage of ADM/PanIN lesion areas was quantified based on H&E sections from KC (n = 11) and KC;Col1pdxKO (n = 16) mice.
(H) Cell proliferation of KPPC and KPPC;Col1pdxKO cell lines over time (n = 3 biological replicates).
(I) Cell viability of KPPC and KPPC;Col1pdxKO cell lines (% normalized to untreated group of each cell line) treated with gemcitabine (n = 3).
(J) Xenograft tumor formation of KPPC and KPPC;Col1pdxKO cancer cell lines subcutaneously in nude mice (n = 5/group).
(K) Gene set enrichment analysis (GSEA) of RNA-seq data on KPPC and KPPC;Col1pdxKO cancer cells. Top upregulated pathways were shown. NOM. p. val: nominal P value. NES, normalized enrichment score. FDR, false discovery rate (q value).
(L) Detection of purified Col1 (mouse Col1α1 and/or Col1α2 chains) from conditioned culture media of mouse cancer cells or fibroblasts. Representative Western blot of three independently experiments was shown.
Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Student’s t test was used unless otherwise indicated.
See also Figures S1–S3; Table S1.
At 53 days of age, KPPC;Col1pdxKO revealed significantly less PDAC lesions, lower pancreatic tumor burden, and elevated stromal Col1 deposition predominantly produced by fibroblasts (Figures 2D, 2E, and S1E), when compared with age-matched KPPC control mice. Tumor desmoplasia at endpoint was not significantly different between KPPC;Col1pdxKO mice and KPPC mice (Figure S1F). The protein levels of other ECM components including type IV collagen, thrombospondin-1, and laminin were also compared (Figures S1G and S1H).
Col1 deletion in pancreatic cancer cells delays ADM and PanIN development
In order to evaluate the role of Col1 in early-stage disease, we examined the 30-day-old KPPC;Col1pdxKO mice, which revealed significantly less ADM and PanIN lesions compared to KPPC control mice (Figure 2F). Furthermore, we generated the KC;Col1pdxKO mice (Figures 2G and S2A), which revealed significantly delayed ADM and PanINs when compared to KC control mice at 6 months of age. The total level of Col1 deposition (Figure S2B) was not significantly different between the two groups, likely due to the dominant Col1 deposition by fibroblasts (Chen et al., 2021). Nevertheless, a more targeted evaluation revealed that KC;Col1pdxKO mice exhibit decreased Col1 around early stage lesions of neoplasia, before the significant emergence of fibroblasts and fibroblast-derived Col1 (Figure S2C). These observations suggest that Col1 homotrimers by cancer cells support the initiation and early progression of pancreatic cancer.
Col1 homotrimers produced by cancer cells increase capacity for proliferation, tumor growth, and resistance to gemcitabine
We established multiple cancer cell lines from pancreatic tumors of KPPC;Col1pdxKO and KPPC mice (3 independent cell lines per group) (Figure S2D). Primary KPPC;Col1pdxKO cancer cells revealed diminished cell-cell adhesion and prominent spindle-shaped cell morphology in culture, in contrast to primary KPPC cancer cells that were cobblestone-shaped and grew as colonies of cell clusters with prominent cell-cell adhesion. KPPC;Col1pdxKO cancer cells exhibited significantly lower proliferation rate, increased gemcitabine sensitivity, and diminished rate of subcutaneous tumor formation (Figure 2H–J). Bulk RNA-sequencing (RNA-seq) revealed significant cancer cell-intrinsic changes in gene expression profile upon the deletion of Col1a1 in KPPC;Col1pdxKO cancer cell lines (Figures 2K and S2E). Gene set enrichment analysis (GSEA) identified epithelial-mesenchymal-transition and ECM remodeling as the most upregulated transcriptional signature in KPPC;Col1pdxKO cells, in contrast to the proliferation-related E2F target pathway as the most upregulated in KPPC cells (Figure 2K; Table S1). These consistent results demonstrated the mesenchymal features and decreased proliferation of KPPC;Col1pdxKO cells (Figures 2H, S2D, S2E and S3A).
The KPPC cancer cells revealed a single band of α1 chain indicating the synthesis of unique Col1 (αl/αl/αl) homotrimers, in contrast to NIH-3T3 fibroblasts which revealed two bands representing αl and α2 chains of Col1 forming normal Col1 (αl/α2/αl) heterotrimers; the KPPC;Col1pdxKO cancer cells did not show any expression of Col1 α-chains as expected, confirming the genetic deletion of Col1a1 (Figure 2L). At the transcript level, KPPC cancer cells revealed detectable expression of Col1a1, but not Col1a2; KPPC;Col1pdxKO cancer cells exhibited efficient knockdown of Col1a1 and elevation in Col5a2 expression but no other fibrillar collagen genes (Figure S3B).
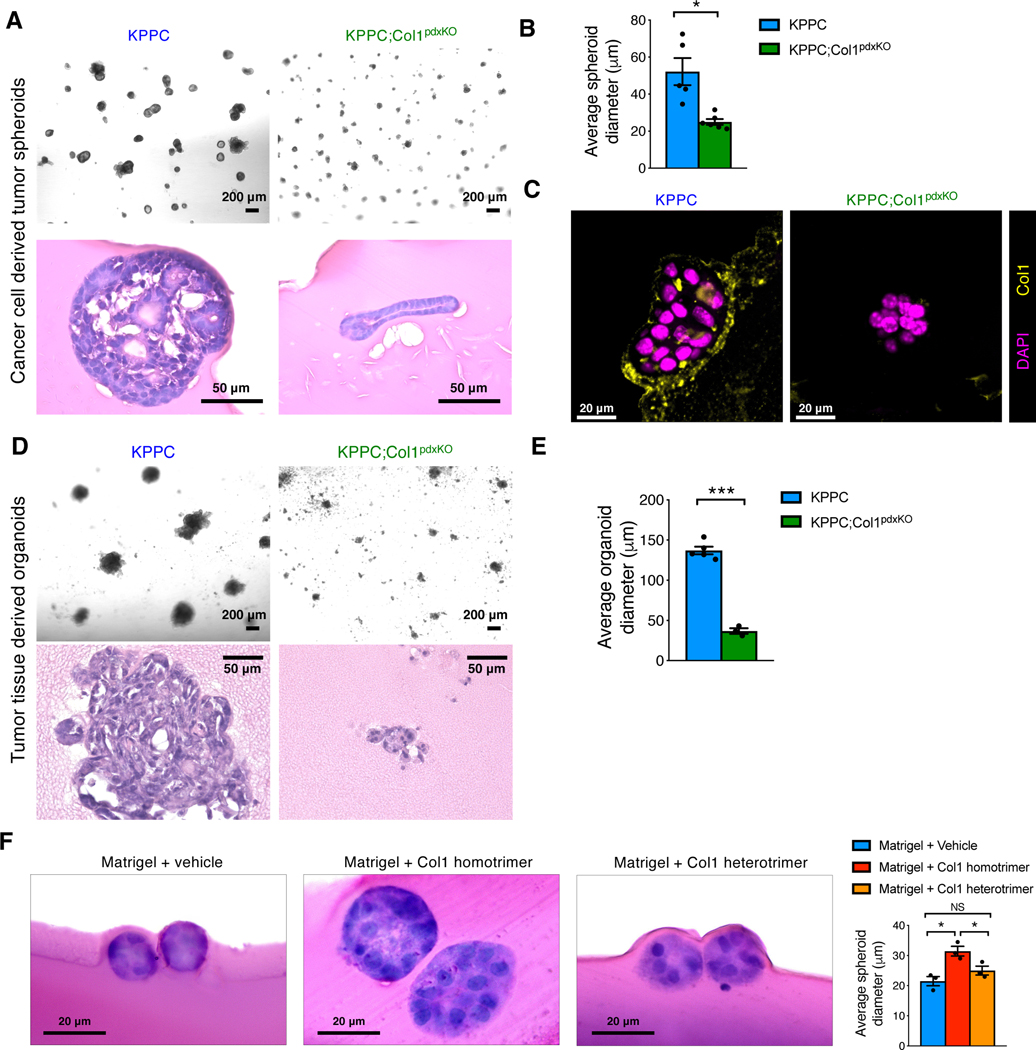
KPPC;Col1pdxKO cells had diminished ability to form tumor spheroids within 3D Matrigel when compared to KPPC cells (Figures 3A and 3B). While KPPC cell-derived tumor spheroids contained a surrounding layer of Col1, KPPC;Col1pdxKO cell-derived tumor spheroids did not (Figure 3C). Given that Matrigel does not contain detectable Col1 (Figure S3C), we surmised that the deposited Col1 was generated by cancer cells. Organoids established from KPPC;Col1pdxKO tumors and cultured in 3D Matrigel revealed stunted growth expansion when compared to KPPC tumor organoids (Figures 3D and 3E).
Figure 3. Deletion of oncogenic Col1 homotrimers impedes the proliferation and tumorigenesis of pancreatic cancer cells.
(A-C) 3D tumor spheroids established from KPPC and KPPC;Col1pdxKO cancer cell lines (n ≥ 5 biological replicates). Tumor spheroids were subjected to H&E (A and B) and Col1 staining (C). Average diameter of spheroids was quantified in (B). Scale bar for tumor spheroids: 200 μm. Scale bar for H&E staining: 50 μm. Scale bar for immunofluorescence staining: 20 μm.
(D and E) Organoids were established from fresh tumor mixtures of KPPC (n = 5) and KPPC;Col1pdxKO (n = 3) mice (D), and quantified for average diameter (E). Scale bar: 200 μm in 3D culture images; 50 μm in H&E images.
(F) Tumor spheroids established from KPPC;Col1pdxKO cells grown in 3D Matrigel culture system supplemented with Col1 homotrimers or Col1 heterotrimers (n = 3/group). Tumor spheroids were subjected to H&E staining. Scale bar for H&E staining: 20 μm.
Data are represented as mean ± SEM. *p < 0.05, ***p < 0.001; NS: not significant, Student’s t test was used.
See also Figure S3.
Col1 homotrimers are resistant to MMP degradation and favor enhanced survival of cancer cells
We next assessed whether Col1 homotrimers isolated from the conditioned medium of KPPC cells and Col1 heterotrimers isolated from the conditioned medium of NIH-3T3 mouse fibroblasts exhibit different susceptibility to degradation by matrix metalloproteinases (MMPs) 1 and 2 (Han et al., 2010; Han et al., 2008; Makareeva et al., 2010). Col1 homotrimers exhibited resistance to MMP degradation when compared to Col1 heterotrimers, which revealed degradation fragments (Figure S3D).
In search for a more robust source of Col1 homotrimers to conduct cell biology experiments and phenotypic rescue studies, we employed well-established biochemical techniques to isolate Col1 homotrimers from the skin of Col1a2oim/oim mice. The Col1a2oim/oim mice with homozygous mutated Col1a2 gene cannot generate normal Col1 heterotrimers and only produce Col1 homotrimers in their body (Chipman et al., 1993; Kuznetsova et al., 2001; Kuznetsova et al., 2004; Kuznetsova et al., 2003; Miles et al., 2002). Col1 heterotrimers were isolated from the skin of age-matched wild-type littermate mice. The Col1 homotrimer or heterotrimer compositions were confirmed by Western blot and Coomassie staining of SDS-PAGE gels (Figure S3E). The purified Col1 homotrimers, but not Col1 heterotrimers, significantly enhanced cancer cell proliferation and rescued the suppressed spheroid formation of KPPC;Col1pdxKO cells due to the genetic deletion of Col1 homotrimers (Figure 3F).
Col1 homotrimers induce persistent proliferation signals via DDR1 and integrin α3β1
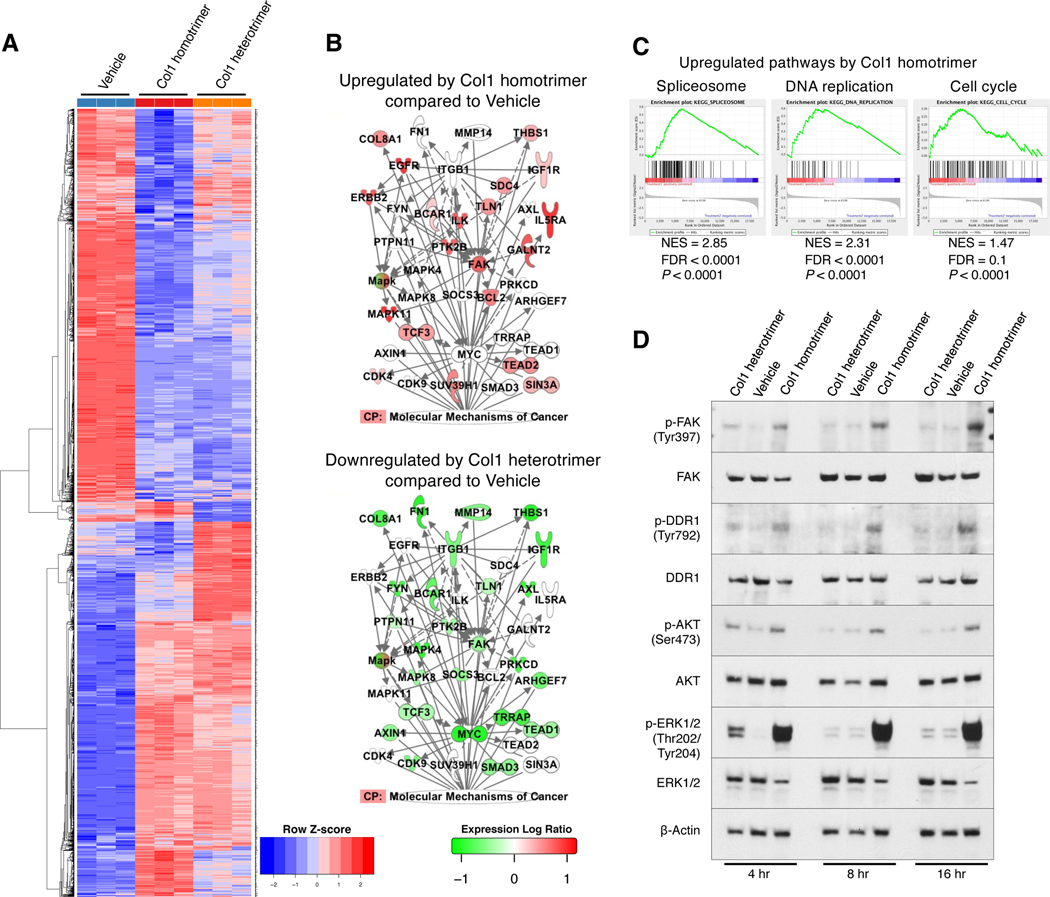
Next, RNA-seq was conducted on KPPC;Col1pdxKO cancer cells grown on pre-coated Col1 homotrimers, Col1 heterotrimers, or vehicle (Figure 4A). Col1 homotrimers, when compared with Col1 heterotrimers, significantly upregulated the signaling network involving FAK/EGFR/MAPK/MYC at the transcriptional level (Figure 4B). In contrast, this signaling axis was downregulated by Col1 heterotrimer treatment (Figure 4B). Col1 homotrimers upregulated a variety of gene clusters related to nucleotide editing/replication/repair and cell cycle pathways, as compared with either Col1 heterotrimer or vehicle treatment (Figures 4C and S3F; Table S2). Additionally, when compared to vehicle, Col1 homotrimer significantly upregulated glutathione metabolism, DNA replication, RNA splicing, and cell cycle pathways (Figure S3G), associated with promoted cancer cell proliferation.
Figure 4. Oncogenic Col1 homotrimers produced by pancreatic cancer cells induce persistent proliferation signals.
(A-C) RNA sequencing (RNA-seq) analysis on KPPC;Col1pdxKO cancer cells cultured on pre-coated vehicle, Col1 homotrimer, or Col1 heterotrimer (n = 3/group). Heatmap of differentially expressed genes was shown in (A). IPA was performed to visualize the most differentially regulated signaling network (B). CP: canonical pathway. GSEA revealed upregulated pathways by Col1 homotrimers as compared to Col1 heterotrimer (C).
(D) KPPC;Col1pdxKO cancer cells were treated with Col1 homotrimers or heterotrimers for 4–16 hours and examined for intracellular signaling by Western blotting. Representative Western blot of three independently experiments was shown.
See also Figures S3 and S4; Table S2.
We further identified that Col1 homotrimers induced more persistent and robust activation of discoidin domain receptor 1 (DDR1), FAK, AKT, and ERK signaling when compared to Col1 heterotrimers (Figure 4D) in both KPPC;Col1pdxKO and KPPC cancer cells (Figure S4A). These results support the proliferation-promoting effect of Col1 homotrimers and demonstrate that the impact of Col1 deletion on cancer cell proliferation in KPPC;Col1pdxKO cells can be rescued by replenishment of Col1 homotrimers, in either soluble or pre-coated condition (Figure S4B).
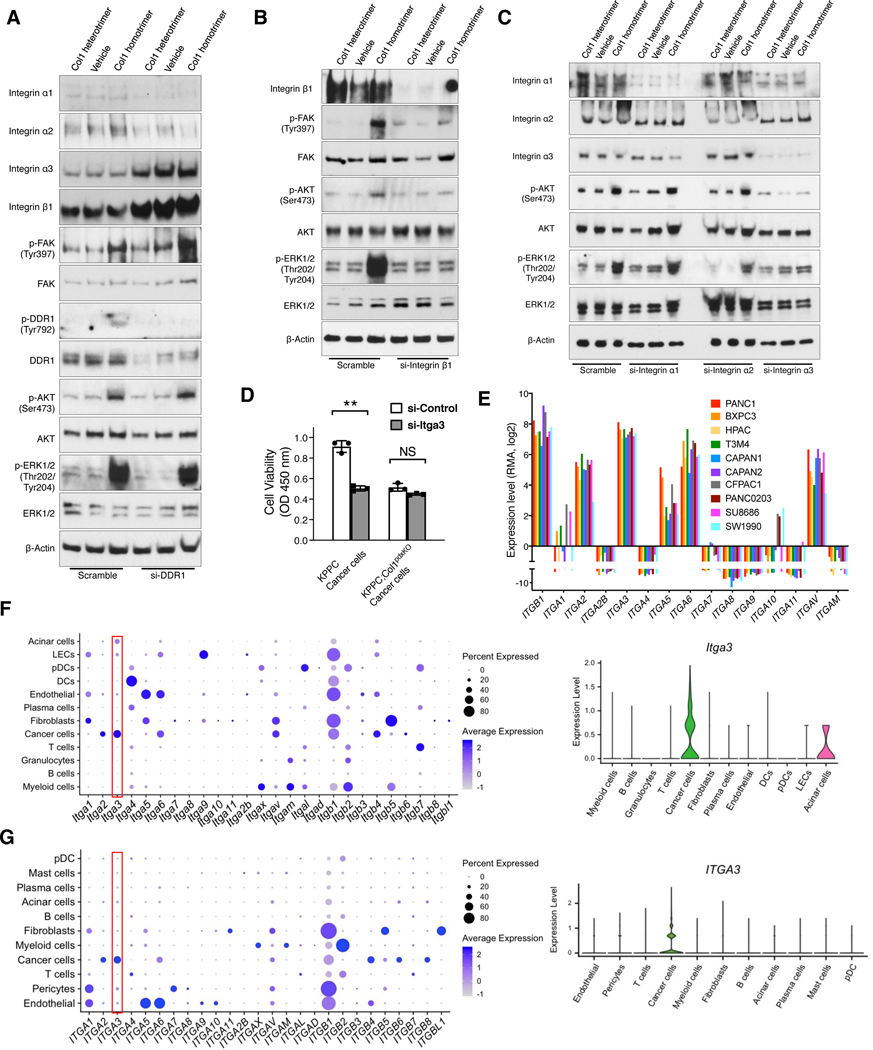
We next examined the involvement of DDR1 and integrins on Col1 homotrimer-induced signaling, given their potential roles as collagen receptors on cancer cells (Egeblad et al., 2010; Leitinger, 2011; Yeh et al., 2012). DDR1 knockdown by siRNAs did not inhibit the signaling activation by Col1 homotrimers (Figures 5A and S4C). In contrast, integrin β1 knockdown by siRNAs significantly diminished the Col1 homotrimer-induced signaling (Figures 5B and S4D). Knockdown experiments of integrin subunits α1, α2, and α3 revealed that integrin α3 knockdown (Figures 5C and S4E) significantly inhibited Col1 homotrimer-induced downstream signaling, whereas integrin α2 knockdown moderately inhibited Col1 homotrimer-induced signaling. In contrast, integrin α1 knockdown did not alter Col1 homotrimer-induced signaling. Blocking antibody of integrin α3 (P1B5) inhibited Col1 homotrimer induced cancer cell adhesion via integrin α3(β1) (Figure S4F). Integrin α3 knockdown inhibited the proliferation of KPPC cancer cells, but not Col1-depleted KPPC;Col1pdxKO cells (Figure 5D).
Figure 5. Col1 homotrimer-induced signals involve integrin α3(β1) that is specifically expressed by pancreatic cancer cells.
(A-C) KPPC;Col1pdxKO cancer cells were transfected with siRNAs of DDR1 (A), integrin β1 (B), integrin α1, integrin α2, or integrin α3 (C). Cells were treated with Col1 homotrimers or heterotrimers for 16 hours. Cells were examined by Western blotting. Representative Western blot of three independently experiments was shown.
(D) KPPC and KPPC;Col1pdxKO cancer cells were transfected with siRNA of integrin α3. Cells were then cultured for 48 hours and examined by cell viability assay (n = 3). Data are represented as mean ± SEM. **p < 0.01; NS: not significant, Student’s t test was used.
(E) Expression levels of selected integrin subunits in human PDAC cell lines based on RNA-seq data from the CCLE database. RMA normalization was used and data were presented as the log2 gene expression.
(F and G) Expression profile of Itga3 (F) and ITGA3 (G) among various cell clusters from the single-cell RNA-sequencing datasets of mouse (GEO: GSE166298) and human (GSA: CRA001160) pancreatic tumors, as shown in dot plot and violin plot.
See also Figure S4.
Integrin α3(β1) is associated with poor prognosis and T cell suppression in pancreatic cancer patients
RNA-seq data from human (Figure 5E, based on CCLE database) and mouse (Figure S4G) pancreatic cancer cell lines confirmed that integrin α3 is the most abundantly expressed α subunit of integrin by pancreatic cancer cells. Integrin α10 and α11, which are also known collagen-binding integrins, revealed minimal expression in pancreatic cancer cells (Figures 5E and S4G). The extensive and specific expression of integrin α3 by cancer cells was further validated by recently published single-cell RNA-sequencing (scRNA-seq) datasets of mouse (Chen et al., 2021) and human (Peng et al., 2019) pancreatic tumors (Figures 5F and 5G), and by IHC staining of mouse and human pancreatic tumor tissue sections (Figure S4H).
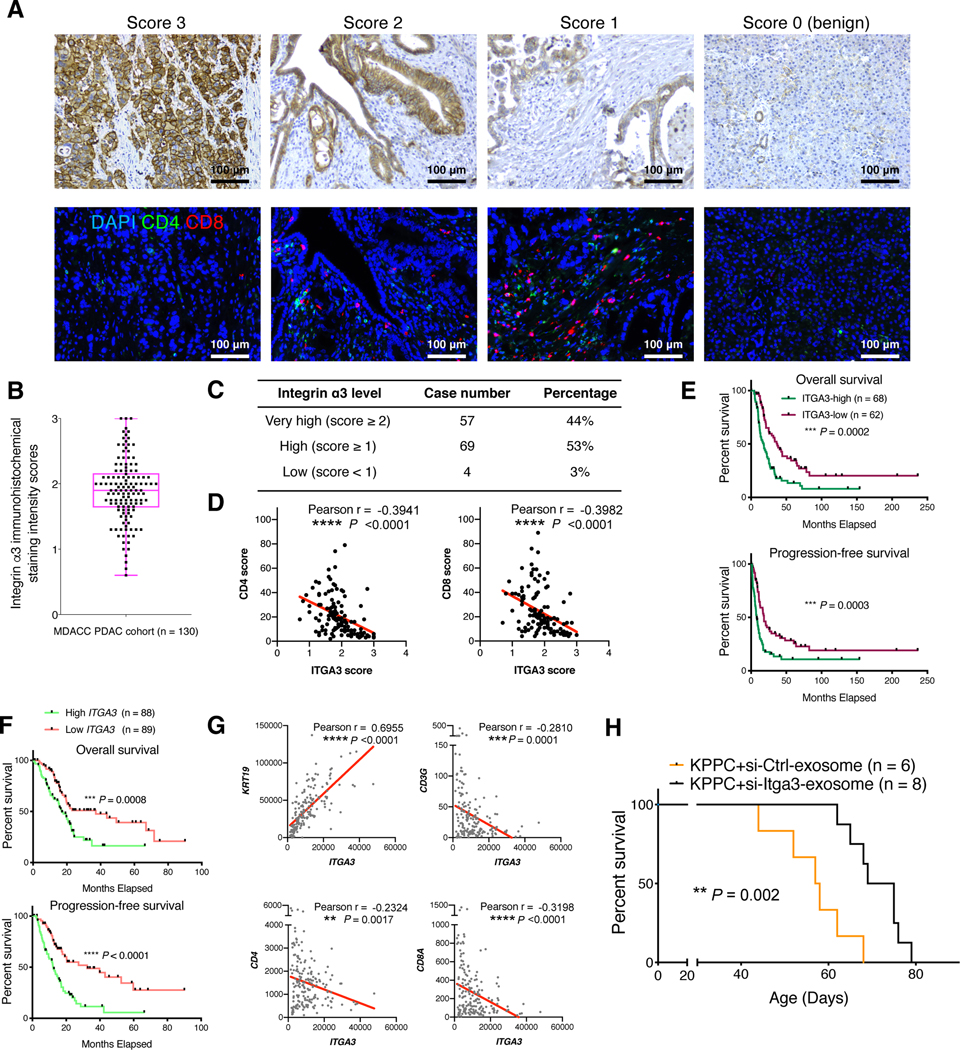
Human PDAC sections of an MDACC cohort (n = 130) were examined for integrin α3 expression, showing an average IHC score of 1.86 (Figures 6A and 6B). The majority (97%) of human PDAC sections revealed prominent integrin α3 expression with IHC scores greater than 1.0 (Figure 6C). High integrin α3 expression level negatively correlated with T cell presence (Figures 6A and 6D), associated with poor survival of patients (Figure 6E). Consistently, we validated that higher ITGA3 transcript level correlates with significantly worse survival of PDAC patients in the TCGA cohort (Figure 6F). High ITGA3 transcript level also correlates with high expression of KRT19 (cytokeratin-19) and low expression of T cell-associated genes in the TCGA cohort (Figure 6G). We next utilized the previously described iExosome therapeutic system (Kamerkar et al., 2017; Mendt et al., 2018) containing siRNAs targeting integrin α3, which significantly suppressed integrin α3 expression, increased T cell infiltration, and prolonged KPPC mouse survival (Figures 6H and S5A). These results suggest the important role and therapeutic potential of Col1 homotrimer-integrin α3 pathway in PDAC progression and T cell inhibition.
Figure 6. Integrin α3 correlates with poor prognosis and T cell suppression in pancreatic cancer patients.
(A-E) Representative images showing the immunohistochemical (IHC) scores of integrin α3 (A) on a scale of 0–3 in human PDAC tissue microarray sections. CD4 and CD8 staining was also conducted on the serial sections of the same samples (A). The average score of integrin α3 expression was 1.86 for the entire cohort (B). The top and bottom lines of the boxplot represent the interquartile range (IQR); center line represents median; and the whiskers represent minimum and maximum values. Case number and percentage of PDAC samples with indicated integrin α3 expression scores was counted (C). Scatterplot illustrating the reverse correlation between integrin α3 IHC score and CD4/CD8 score was shown in (D). Pearson’s correlation test was used. Kaplan-Meier survival curves showed the correlation between integrin α3 expression level and survival (E). Log-rank (Mantel-Cox) test was used. Scale bar: 100 μm.
(F and G) Survival (F) of pancreatic adenocarcinoma patients from TCGA dataset correlated with ITGA3 expression level. Log-rank (Mantel-Cox) test was used. Patients with available survival data and RNA-seq data (n = 177) were stratified into two groups based on the median ITGA3 expression level. Scatterplot illustrating the correlation between the gene expression levels of ITGA3 and KRT19 (or T cell related genes) in PDAC patients was shown in (G). Pearson’s correlation test was used.
(H) Survival of KPPC mice treated with mesenchymal stem cell-derived exosomes electroporated with either scrambled siRNA-control or siRNA-Itga3. Log-rank (Mantel-Cox) test was used.
**p < 0.01, ***p < 0.001, ****p < 0.0001; NS: not significant.
See also Figure S5.
Deletion of Col1 homotrimer in pancreatic cancer cells alters intratumoral microbiome and the immune landscape
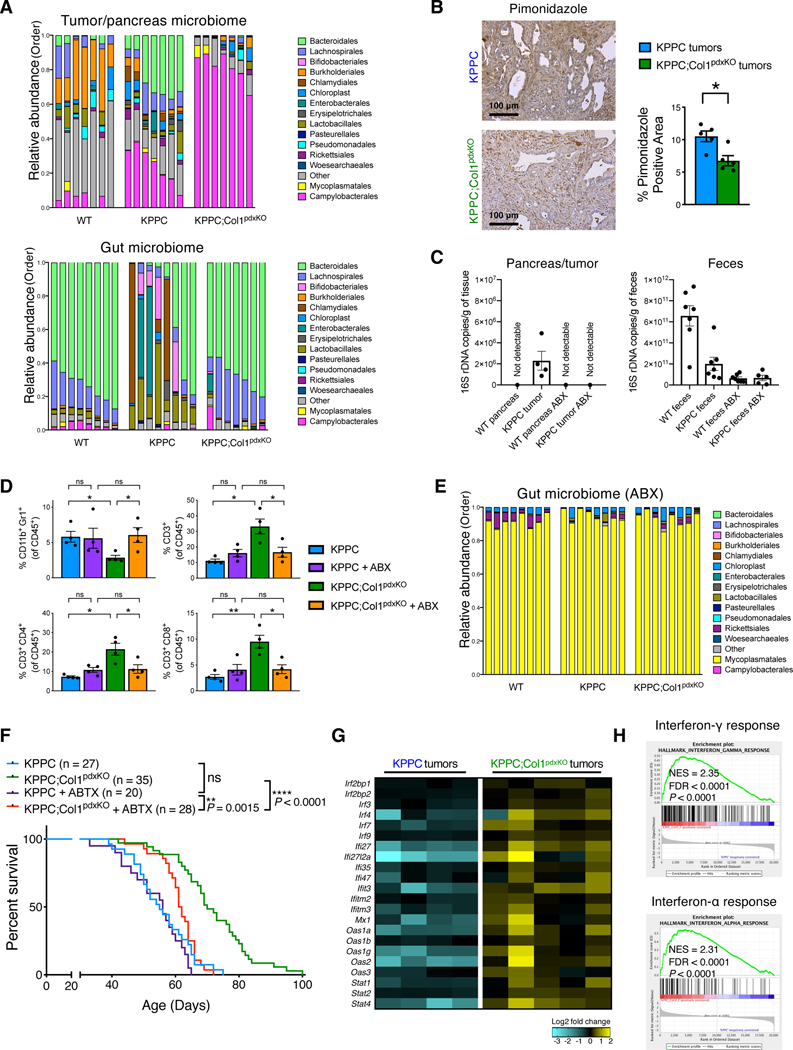
Recent studies support the notion that gut and tumor microbiome can regulate tumor immunity (Gopalakrishnan et al., 2018; Ma et al., 2018; Mager et al., 2020; Pushalkar et al., 2018; Riquelme et al., 2019; Sivan et al., 2015; Vetizou et al., 2015; Wang et al., 2018). Therefore, we examined the impact of Col1 deletion on tumor/gut microbiome and tumor immunity. 16S rRNA gene sequencing analysis of the tumors of KPPC;Col1pdxKO mice revealed significantly altered intratumoral microbiome profile with decreased Bacteroidales and increased Campylobacterales, as compared with tumors from KPPC control mice or normal pancreas from co-housed wild-type littermate mice (Figures 7A, S5B, and S5C). These examined KPPC tumors and KPPC;Col1pdxKO tumors are stage-matched samples with comparable tumor burden (Figure S5D). Col1 deletion preserved gut microbiome homeostasis of KPPC;Col1pdxKO mice with a taxonomic pattern similar to that of tumor-free (WT) littermate mice. The KPPC control mice exhibited aberrant gut microbiome (compared to WT mice or KPPC;Col1KO mice) composition usually associated with more advanced tumor progression (Pushalkar et al., 2018) (Figures 7A, S6A, and S6B). The decreased Bacteroidales (anaerobic) and increased Campylobacterales (microaerophilic) in the tumors were associated with the alleviated intratumoral hypoxia in KPPC;Col1pdxKO tumors, when compared to KPPC tumors (Figure 7B). Bulk RNA-seq revealed elevated gene expression levels of oxidative phosphorylation (OXPHOS) and angiogenesis pathways in KPPC;Col1pdxKO tumors (Figure S6C). Normal pancreas from healthy littermate mice exhibited very low levels of total bacterial DNA content while pancreatic tumors from KPPC mice exhibited detectable bacterial DNA content by quantitative PCR (Figure 7C). The altered microbiome in KPPC;Col1pdxKO mice was associated with changes in intratumoral immune cell profile including decreased myeloid-derived suppressor cells (MDSCs) and increased T cells with putative benefit in restraining PDAC (Figure 7D).
Figure 7. Col1 homotrimer deletion in cancer cells confers beneficial tumor microbiome and immune landscape.
(A) Bacterial 16S rRNA gene sequencing analysis of intratumoral microbiome and gut (fecal) microbiome from co-housed KPPC mice and KPPC;Col1pdxKO mice with stage-matched end-point tumors (n = 7–8/group). Pancreatic microbiome and gut microbiome from co-housed wild-type (tumor-free) littermate control mice were also shown. Sequences were classified by taxonomy at the Order level.
(B) Representative images of hypoxyprobe/pimonidazole staining and positivity quantification of KPPC and KPPC;Col1pdxKO tumors (n = 5/group). Scale bar: 100 μm.
(C) The evaluation of total bacterial DNA content by 16S rRNA gene qRT-PCR. Pancreatic tissue (as pancreatic tumor or normal pancreas) samples (n = 4/group) and fecal samples (n = 5–7/group) were collected from KPPC mice and healthy (tumor-free) littermate mice, treated with either broad-spectrum antibiotics (ABX) or vehicle.
(D) Immune profiling (flow cytometry) assay of CD11b+Gr1+ myeloid cells, CD3+ T cells, CD4+/CD3+ T cells, and CD8+/CD3+ T cells in the tumors from KPPC mice and KPPC;Col1pdxKO mice with or without ABX treatment (n = 4/group).
(E) Bacterial 16S rRNA gene sequencing analysis of gut microbiome (fecal samples) from mice with ABX treatment (n = 8/group). Sequences were classified by taxonomy at the Order level.
(F) The overall survival of KPPC mice (n = 20) and KPPC;Col1pdxKO mice (n = 28) with ABX treatment, compared with untreated KPPC and KPPC;Col1pdxKO mice (originally shown in Figure 2B). Log-rank (Mantel-Cox) test was used.
(G and H) Significantly upregulated expression of interferon response pathway genes (G) in KPPC;Col1pdxKO tumors (n = 5) compared to KPPC tumors (n = 4), based on bulk RNA-seq data. GSEA revealing significantly upregulated interferon pathways was shown in (H).
Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001; NS: not significant, Student’s t test was used unless otherwise indicated.
See also Figures S5, S6, and S7; Table S3.
To determine the impact of microbiome on the tumor immunity, we used a combination of broad-spectrum antibiotics (ABX) treatment (Pushalkar et al., 2018). This combination of ABX ablated the bacteria in the tumors to undetected levels analogous to normal pancreas (Figure 7C). Similarly, and as expected, ABX treatment also reduced the gut microbiome content but did not completely eliminate it (Figure 7C and 7E), likely due to the dramatically abundant microbial content in feces (Toure et al., 2019; Ubeda et al., 2010). Nevertheless, 16S rRNA gene sequencing analysis of the remaining gut microbiome after ABX treatment revealed complete loss of the microbial taxonomy difference between KPPC;Col1pdxKO mice and KPPC mice (Figures 7E and S6D).
Elimination of the tumor and gut microbiome by ABX treatment resulted in altered immune profile in KPPC;Col1pdxKO tumors with increased MDSCs and decreased T cells (Figure 7D), which led to significantly shortened survival of KPPC;Col1pdxKO mice (Figure 7F). Such impact of ABX treatment was not observed in KPPC mice. Col1 homotrimer deletion from cancer cells leads to the recruitment of protective microbiome associated with beneficial immune cell profile, consistent with previous studies showing the correlation between the immunosuppressive MDSCs and the presence of intratumoral Bacteroidales (Gopalakrishnan et al., 2018; Pushalkar et al., 2018; Riquelme et al., 2019). Bulk RNA-seq of KPPC;Col1pdxKO tumors further revealed the increased expression profiles of genes related to type I interferon (IFN) pathway (Figure 7G and 7H), which is associated with microbiome-regulated antitumor immune responses (Fessler et al., 2019; Vetizou et al., 2015).
Cancer cell intrinsic impact on tumor immunity is reversed by deletion of Col1
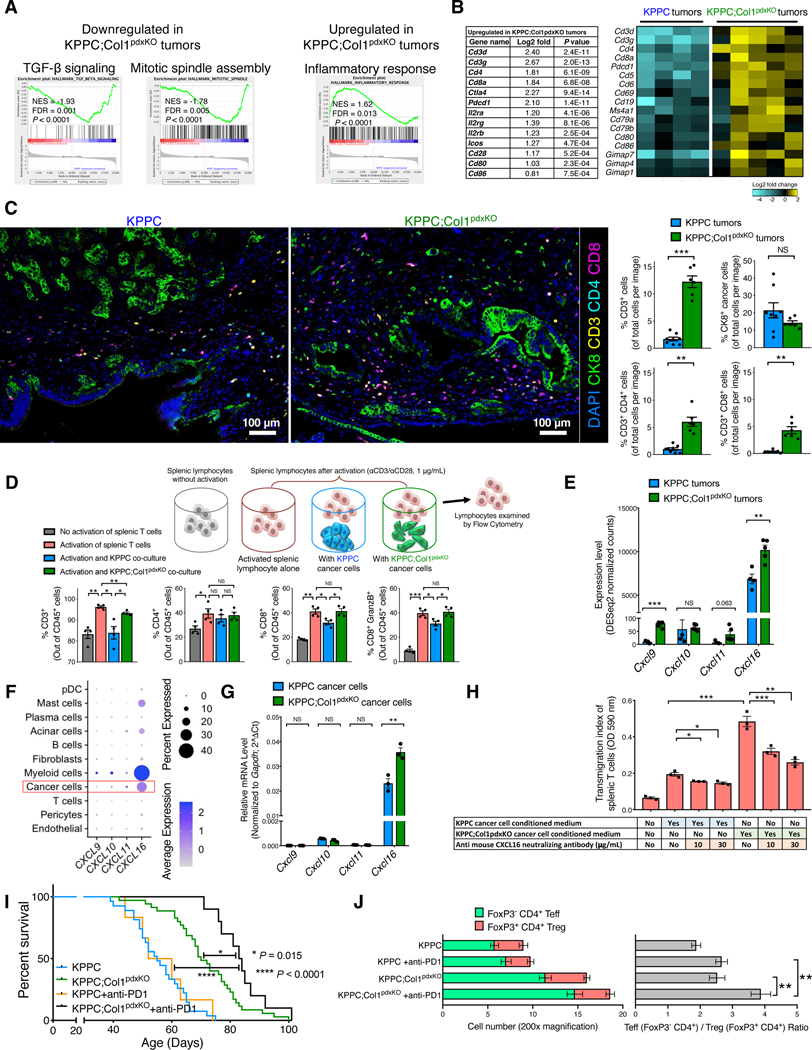
Despite significant reversal of phenotype by antibiotic ablation of the tumor-restraining microbiome, KPPC;Col1pdxKO mice still exhibited better survival than KPPC mice in the context of antibiotics treatment (Figure 7F). Therefore, we probed whether microbiome-independent, cancer cell-intrinsic effects might further contribute to the altered tumor immunity. Bulk RNA-seq data revealed upregulated immune response pathways in KPPC;Col1pdxKO tumors, in contrast to upregulated TGF-β and mitotic spindle pathways in KPPC tumors associated with more advanced tumor progression (Figures 8A, S7A, and S7B; Table S3). In particular, T cell signature genes and a variety of immune related genes were significantly upregulated in KPPC;Col1pdxKO tumors (Figure 8B). The multispectral imaging validated the increased CD3, CD4 and CD8 T cell infiltration in KPPC;Col1pdxKO tumors (Figure 8C), consistent with our previous results (Figure 7D).
Figure 8. Deletion of Col1 homotrimers from cancer cells increases T cell accumulation and enables the efficacy of anti-PD-1 therapy.
(A and B) GSEA revealing differentially regulated pathways in KPPC;Col1pdxKO tumors (n = 5), as compared to KPPC tumors (n = 4) (A), based on bulk RNA-seq data (partly shown in Figures 7G and 7H). Upregulated T cell signature genes in KPPC;Col1pdxKO tumors were listed with log2-fold change and P value (Wald Test) in (B). Heatmap of representative genes encoding immune regulatory molecules was also shown.
(C) T cell quantification from multispectral imaging of multiplex stained sections of stage-matched endpoint KPPC tumors (n = 8) and KPPC;Col1pdxKO tumors (n = 6). Scale bar: 100 μm.
(D) Splenic lymphocytes (n = 4 mice) were cultured in the presence or absence of: anti-CD3/anti-CD28 activation, KPPC cancer cell co-culture, or KPPC;Col1pdxKO cancer cell co-culture. Lymphocytes were then examined by flow cytometry.
(E) Expression profile of genes encoding chemokines related to T cell recruitment based on bulk RNA-seq of KPPC (n = 4) and KPPC;Col1pdxKO (n = 5) tumors.
(F) Expression profile of indicated genes among various cell clusters from the single-cell RNA-sequencing dataset of human pancreatic tumors (GSA: CRA001160), as shown in dot plot.
(G) Expression profile of indicated genes in KPPC and KPPC;Col1pdxKO cancer cells (n = 3) as examined by qRT-PCR.
(H) Transwell migration assay of mouse splenic T cells (in upper chambers, treated with vehicle or CXCL16 neutralizing antibody) towards conditioned medium from KPPC or KPPC;Col1pdxKO cancer cells (n = 3).
(I,J) Survival of KPPC;Col1pdxKO (n = 10) and KPPC mice (n = 6) treated with anti-PD-1 therapy (I), compared to untreated KPPC (n = 27) and KPPC;Col1pdxKO (n = 35) mice originally from Figure 2B. Log-rank (Mantel-Cox) test was used. Indicated cell number and ratio were examined by immunofluorescence (J).
Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; NS: not significant, Student’s t test was used unless otherwise indicated.
See also Figures S7 and S8; Table S3.
In addition, Col1 homotrimer deletion in cancer cells increased the level of stromal Col1 heterotrimer in the tumors, associated with enhanced stromal response and inhibited tumor progression (Figure S7C). The elevated stromal Col1 and Col3 (type III collagen) are predominantly produced by cancer-associated fibroblasts (Figure S7D). These results are in concordance with our recent findings that decreased stromal Col1 is associated with accelerated pancreatic tumor progression (Chen et al., 2021).
To evaluate potential interactions between cancer cells and T cells, we conducted the co-culture assays. The KPPC cancer cells significantly suppressed the activation and expansion of co-cultured mouse splenic CD3+ T cells, especially CD8+ T cells (Figure 8D). In contrast, KPPC;Col1pdxKO cancer cells, deleted for Col1 homotrimer, lost such immunosuppressive impact on co-cultured mouse splenic T cells (Figure 8D). Bulk tumor RNA-seq analysis revealed the upregulation of genes encoding T cell chemoattractants, especially CXCL16 in KPPC;Col1pdxKO tumors (Figure 8E). Human PDAC scRNA-seq analysis further identified CXCL16 as the most abundantly expressed T cell chemoattractant in cancer cells (Figure 8F), consistent with recent observations on ovarian cancer (Hornburg et al., 2021). Significant upregulation of CXCL16 expression was also confirmed by qRT-PCR in isolated KPPC;Col1pdxKO cancer cells (Figure 8G). Transwell migration assays of activated mouse splenic T cells demonstrated that the conditioned medium of KPPC;Col1pdxKO cancer cells (as compared to that of KPPC cancer cells) elicited more T cell migration, which could be dose-dependently inhibited by CXCL16 neutralizing antibody (Figure 8H). These results suggest that Col1 homotrimers generated by cancer cells in KPPC tumors decreased T cell activation and infiltration, possibly via downregulation of cancer cell produced CXCL16.
We also identified that KPPC;Col1pdxKO cancer cells exhibited significantly downregulated expression of CXCL5 (Figure S8A), which is a potent chemoattractant for MDSCs (Bezzi et al., 2018; Chen et al., 2021; Wang et al., 2016). Conditioned medium from KPPC cancer cells significantly induced the transwell migration of mouse RAW264.7 macrophages, and such migration could be dose-dependently inhibited by CXCR2 inhibitor and CXCL5 neutralizing antibody. In contrast, conditioned medium from KPPC;Col1pdxKO cancer cells revealed significantly lower capacity for inducing macrophage migration, which was minimally impacted by CXCR2 inhibitor or CXCL5 antibody (Figure S8B).
Collectively, these results suggest that the survival benefit of KPPC;Col1pdxKO mice is a combined result from altered tumor microbiome and immune profile, as well as the cancer-intrinsic changes upon the deletion of cancer-specific Col1 homotrimers.
Col1 deletion in cancer cells enables therapeutic efficacy of anti-PD1 immune checkpoint blockade in PDAC
Consistent with our bulk RNA-seq and immunostaining data (Figure 8A–D), flow cytometry analyses confirmed that KPPC;Col1pdxKO tumors have elevated T cell infiltration and associated T cell activation markers (Figures S8C and S8D), when compared to KPPC tumors. Specifically, a significant increase in CD4+PD-1+ and CD8+PD-1+ cells was observed in KPPC;Col1pdxKO tumors. In order to determine whether such increase in PD-1+ T cells of KPPC;Col1pdxKO tumors has any functional significance (Sharpe and Pauken, 2018), KPPC mice and KPPC;Col1pdxKO mice with advanced PDAC were treated with PD-1 antibodies. Similar to previous reports (Feig et al., 2013; Winograd et al., 2015), KPPC mice were recalcitrant to anti-PD-1 treatment (Figure 8I). In contrast, KPPC;Col1pdxKO mice responded positively to anti-PD-1 treatment and exhibited prolonged overall survival (Figure 8I). Anti-PD-1 treatment resulted in a significantly increased ratio between effector T cells (Teff; CD4+FoxP3-) and regulatory T cells (Treg; CD4+FoxP3+) in KPPC;Col1pdxKO tumors with increased total number of CD4+ T cells (Figure 8J).
These results reveal that deletion of oncogenic Col1 homotrimer in cancer cells alters the immunosuppressive microenvironment, promotes T cell infiltration into the tumors, and renders PDAC responsive to anti-PD-1 checkpoint blockade therapy.
Discussion
For several decades, type I collagen (Col1) has been reported by many investigators as a significant component of the deposited extracellular matrix (ECM) in PDAC stroma (Imamura et al., 1995; Mollenhauer et al., 1987; Tian et al., 2019). However, direct assessment of the specific function of Col1 produced by cancer cells in PDAC remained undetermined.
Our recent study showed that loss of Col1 in αSMA+ myofibroblasts leads to acceleration of PDAC and aggravated immunosuppression (Chen et al., 2021). In contrast, this present study identifies that deletion of Col1 in cancer cells leads to the suppression of PDAC, alleviates immunosuppression, and induces beneficial microbiome associated with enhanced CD4+ Teff:Treg ratio and CD8+ T cell infiltration. Cancer cell derived oncogenic Col1 consists of unique α1 chain homotrimers (α1/α1/α1), instead of the (α1/α2/α1) heterotrimer produced by fibroblasts or other normal cells. Our mechanistic studies demonstrate that cancer cells produce their own small amounts of a specific oncogenic Col1 homotrimer variant that facilitates cancer cell proliferation and survival. Deletion of cancer cell derived Col1 does not impact the total Col1 content of the PDAC stroma of established tumors but still has significant biological effect on PDAC progression. There is no current evidence supporting the presence of Col1 homotrimers in normal tissue and therefore its specific production by cancer cells constitutes a unique oncogenic variant with cancer-promoting properties, similar to oncogenic proteins generated by mutated oncogenes in cancer cells.
We demonstrate that PDAC cancer cells produce Col1 homotrimer of α1 chains due to the suppression of Col1a2 gene via DNA hypermethylation of the promoter (DeClerck et al., 1987; Misawa et al., 2011; Sengupta et al., 2003). Although it remains unknown what precise signals prompt such epigenetic downregulation of Col1a2 gene, some studies suggest that Ras can downregulate α2 chain expression (Du et al., 1999; Noda, 1993; Travers et al., 1996). We demonstrate that Col1 homotrimers robustly induce phosphorylation of DDR1, FAK, AKT, and ERK in cancer cells when compared to heterotrimers. Suppression of DDR1 leads to continued signaling activation, suggesting that Col1 homotrimers can activate other receptors in parallel or as a compensatory mechanism. Our results also underscore the role of cancer cell associated α3β1 integrin in mediating Col1 homotrimer induced signaling transduction.
Cancer cell derived oncogenic Col1 homotrimers also influence the tumor immune microenvironment to facilitate immunosuppression. Our studies suggest that tumor-promoting Col1 homotrimers likely repel T cells and recruit MDSCs through Col1 homotrimer-regulated cancer cell secretome. Such immune modulation was associated with unique intratumoral microbiome. Our results provide insights into the connection between cancer cell produced collagen homotrimer and its impact on tumor-promoting microbiome. Nevertheless, functional studies utilizing fecal microbiome transplantation, strain-specific transplantation, metabolomics, and germ-free mouse model system are essential to further uncover the precise roles of relevant microbial species in PDAC progression and immune response.
In summary, our results establish a function for Col1 in PDAC progression with the identification of an oncogenic Col1 homotrimer variant that is specifically produced by cancer cells that impacts tumor immunity and microbiome, with implications for development of new therapeutic strategies.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources, reagents and samples should be directed to and will be fulfilled by the Lead Contact, Raghu Kalluri (rkalluri@mdanderson.org).
Materials Availability
Materials and reagents used in this study are listed in the Key Resources Table. Reagents generated in our laboratory in this study or previous studies are available upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat Collagen I | SouthernBiotech | Cat# 1310-01, RRID:AB_2753206 |
| Rabbit α3 integrin | Sigma-Aldrich | Cat# HPA008572, RRID:AB_2668094 |
| Mouse Pimonidazole | HPI | Cat# HP1-100, RRID:AB_2811309 |
| Goat Collagen IV | Abcam | Cat# ab6585, RRID:AB_305583 |
| Rabbit Thrombospondin-1 | Abcam | Cat# ab85762, RRID:AB_10674322 |
| Rabbit Laminin | Sigma-Aldrich | Cat# L9393, RRID:AB_477163 |
| Rabbit FSP1 | DAKO | Cat# A5114, RRID:AB_2335679 |
| Rabbit CD3 | Abcam | Cat# ab16669, RRID:AB_443425 |
| Rabbit CD4 | Cell Signaling | Cat# 25229, RRID:AB_2798898 |
| Rabbit CD8a | Cell Signaling | Cat# 98941, RRID:AB_2756376 |
| Rat Cytokeratin 8 | DSHB | Cat# TROMA-I, RRID:AB_531826 |
| Rat FoxP3 | eBioscience | Cat# 14-4771-80, RRID:AB_529583 |
| Rabbit phospho-ERK1/2 | Cell Signaling | Cat# 4376, RRID:AB_331772 |
| Rabbit ERK | Cell Signaling | Cat# 9102, RRID:AB_330744 |
| Rabbit phospho-AKT | Cell Signaling | Cat# 4060, RRID:AB_2315049 |
| Rabbit AKT | Cell Signaling | Cat# 4691, RRID:AB_915783 |
| Rabbit β-actin | Cell Signaling | Cat# 4970, RRID:AB_2223172 |
| Rabbit phospho-DDR1 | Cell Signaling | Cat#11994, RRID:AB_2797793 |
| Rabbit DDR1 | Cell Signaling | Cat# 5583, RRID:AB_10694842 |
| Rabbit phospho-FAK | Abcam | Cat# ab39967, RRID:AB_955850 |
| Rabbit FAK | Cell Signaling | Cat# 3285, RRID:AB_2269034 |
| Rabbit α1 integrin | Abcam | Cat# ab78479, RRID:AB_2280644 |
| Rabbit α2 integrin | Abcam | Cat# ab133557, RRID:AB_2833020 |
| Mouse α3 integrin | BD Biosciences | Cat# 611044, RRID:AB_2129599 |
| Mouse α3 integrin | Santa Cruz | Cat# sc-13545, RRID:AB_668044 |
| Rabbit β1 integrin | Santa Cruz | Cat# sc-8978, RRID:AB_2130101 |
| Rat PDGFRα(CD140a)-PE | BioLegend | Cat# 135905, RRID:AB_1953268 |
| Rat EpCAM(CD326)-AF488 | BioLegend | Cat# 118210, RRID:AB_1134099 |
| Rat CD45-Pacific Blue | BioLegend | Cat# 103126, RRID:AB_493535 |
| Rat CD3-AF700 | eBioscience | Cat# 56-0032-82, RRID:AB_529507 |
| Rat CD11b-BV711 | BD | Cat# 563168, RRID:AB_2716860 |
| Rat CD45RB-FITC | eBioscience | Cat# 11-0455-82, RRID:AB_465064 |
| Hamster CD3e PE-Cy7 | eBioscience | Cat# 25-0031-82, RRID:AB_469572 |
| Rat CD4-BV605 | Biolegend | Cat# 100548, RRID:AB_2563054 |
| Rat CD8a-BV650 | Biolegend | Cat# 100742, RRID:AB_2563056 |
| Mouse Granzyme B APC | Invitrogen | Cat# GRB05, RRID:AB_2536539 |
| Rat CD279 (PD-1) PerCP-Cy5.5 | Biolegend | Cat# 135208, RRID:AB_2159184 |
| Mouse CD25 APC-R700 | BD Biosciences | Cat# 659126, RRID:AB_2870475 |
| Hamster CD69 PE-CF594 | BD Biosciences | Cat# 562455, RRID:AB_11154217 |
| Hamster CD3e | BD Biosciences | Cat# 553057, RRID:AB_394590 |
| Hamster CD28 | BD Biosciences | Cat# 553294, RRID:AB_394763 |
| Rat CXCL16 | R&D Systems | Cat# MAB503, RRID:AB_2276752 |
| Rat CXCL5 | Novus Biological | Cat# NBP2-22026 |
| Rat PD-1 | Bio X Cell | Cat# BE0146, RRID:AB_10949053 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Tissue microarray sections of human PDAC | MDACC | IRB LAB05-0854 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Phosphatase inhibitor | Roche | Cat# 04906845001 |
| Protease inhibitor | Roche | Cat# 4693116001 |
| SB 225002 | Tocris | Cat# 2725/10 |
| Collagenase IV | Gibco | Cat# 17104019 |
| Dispase II | Gibco | Cat# 17105041 |
| Liberase | Roche | Cat# 5401020001 |
| DNase I | Roche | Cat# 10104159001 |
| Type I collagen solution from rat tail | Sigma-Aldrich | Cat# C3867 |
| Critical Commercial Assays | ||
| Direct-zol RNA Kit | Zymo Research | Cat# R2050 |
| Reverse Transcription Kit | Applied Biosystems | Cat# 4368814 |
| SYBR Green Master Mix | Applied Biosystems | Cat# 4367659 |
| Cell Counting Kit-8 | Abcam | Cat# ab228554 |
| Live-Dead-eFluor780 | eBioscience | Cat# 65-0865-14 |
| Foxp3/Transcription Factor Staining Buffer Set | eBioscience | Cat# 00-5523-00 |
| Gomori’s Trichrome Stain Kit | Leica Biosystems | Cat# 38016SS2 |
| Chromium Single Cell 3’ Reagent Kits (v2) | 10x Genomics | Cat# PN-120237 |
| ABC-Kit | Vector | Cat# PK-6100 |
| Stable DAB | Invitrogen | Cat# 750118 |
| Direct Red 80 | Sigma-Aldrich | Cat# 365548 |
| Pierce BCA protein assay kit | Thermo Fisher | Cat# 23208 |
| Sircol collagen assay kit | Biocolor | Cat# S1000 |
| TruSeq Stranded mRNA Sample Prep Kit | Illumina | Cat# 20020594 |
| Deposited Data | ||
| KPPC and KPPC;Col1pdxKO tumor RNA-sequencing | This paper | GEO: GSE131357 |
| KPPC and KPPC;Col1pdxKO cell RNA-sequencing | This paper | GEO: GSE131064 |
| Col1 treated cancer cells RNA-sequencing | This paper | GEO: GSE131666 |
| Single-cell RNA-sequencing of mouse PDAC tumors | Chen et al., 2021 | GEO: GSE166298 |
| Single-cell RNA-sequencing of human PDAC tumors | Peng et al., 2019 | GSA: CRA001160 |
| TCGA pancreatic adenocarcinoma cohort survival and gene expression data (GDAC Firehose PAAD) | Broad Institute | http://gdac.broadinstitute.org/runs/stddata__2016_01_28/data/PAAD/20160128/ |
| Experimental Models: Cell Lines | ||
| Primary mouse KPPC cancer cell lines | This paper | N/A |
| Primary mouse KPPC;Col1pdxKO cancer cell lines | This paper | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: LSL-KrasG12D/+;Pdx1-Cre | Hingorani et al., 2005 | N/A |
| Mouse: Trp53loxP/+ | Chen et al., 2005 | N/A |
| Mouse: Col1a1loxP/loxP | Chen et al., 2021 | N/A |
| Oligonucleotides | ||
| See Table S5 | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Flowjo v10.7.1 | Flowjo, L.L.C. | RRID: SCR_008520 |
| Prism v8.0.0 | GraphPad Software Inc. | RRID: SCR_002798 |
| Fiji v2.0.0 | ImageJ | RRID: SCR_002285 |
| cBioportal v2.2.0 | MSK Center for Mol Onc | https://www.cbioportal.org/ |
| Seurat R package (3.5.3) | Satija et al., 2015 | https://satijalab.org/seurat/ |
| DESeq2 | Anders and Huber, 2010 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| STAR aligner | Dobin et al., 2013 | https://github.com/alexdobin/STAR |
| Gene Set Enrichment Analysis (GSEA) | Broad Institute | http://software.broadinstitute.org/gsea/index.jsp |
| Other | ||
Data and Code Availability
The accession numbers for the data reported in this paper are GEO: GSE131357, GSE131064, and GSE131666. The single-cell RNA sequencing analyses in this study were based on recently published datasets that are available as GEO: GSE166298 and GSA: CRA001160. The survival and gene expression data of TCGA pancreatic adenocarcinoma cohort were based on the GDAC Firehose PAAD dataset (previously known as the TCGA Provisional dataset).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
FSF-KrasG12D/+ (Schonhuber et al., 2014), Pdx1-Flp (Schonhuber et al., 2014), Trp53frt/+ (Lee et al., 2012), LSL-KrasG12D/+ (Hingorani et al., 2005), Trp53loxP/+ (Chen et al., 2005), and Pdx1-Cre (Hingorani et al., 2005) mouse strains were previously documented. Col1a1loxP/loxP mouse strain (with loxP-flanked exons 2–5) was established in the Genetically Engineered Mouse Facility at MD Anderson Cancer Center (MDACC) using the Col1a1tm1a(EUCOMM)Wtsi embryonic stem cells that were obtained from the European Mouse Mutant Cell Repository (EuMMCR) (Chen et al., 2021). We crossed the LSL-KrasG12D;Pdx1-Cre (referred to as KC), LSL-KrasG12D/+;Trp53R172H/+;Pdx1-Cre;Col1a1loxP/loxP (referred to as KPC), or LSL-KrasG12D;Trp53loxP/loxP;Pdx1-Cre (referred to as KPPC) mice with the Col1a1loxP/loxP mouse strain, resulting in the generation of the KC;Col1a1loxP/loxP (referred to as KC;Col1pdxKO), KPC;Col1a1loxP/loxP (referred to as KPC;Col1pdxKO), and KPPC;Col1a1loxP/loxP (referred to as KPPC;Col1pdxKO). Specifically, the original KPPC strain was crossed with Col1a1loxP/loxP strain for at least 6 generations, leading to the generation of KPPC;Col1pdxKO mice and background-matched KPPC littermate control mice. Such breeding strategy minimized the potential genetic background difference between the original mouse strains, which was applied to the generation of background-matched KC and KC;Col1pdxKO mice, or background-matched KPC and KPC;Col1pdxKO mice. Characterization of genotyping and disease phenotypes for the FSF-KrasG12D/+;Pdx1-Flp (referred to as KF), FSF-KrasG12D/+;Trp53frt/+;Pdx1-Flp (referred to as KPF), and FSF-KrasG12D/+;Trp53frt/frt;Pdx1-Flp (referred to as KPPF) mice were previously described (Chen et al., 2021; Chen et al., 2018; Schonhuber et al., 2014). Osteogenesis imperfecta murine (OIM) strain harboring Col1a2 mutation was purchased from Jackson Laboratory (001815; B6C3Fe a/a-Col1a2oim/J). Anti-PD-1 RMP1–14 monoclonal antibody (BE0146; Bio X Cell) was intraperitoneally administered at 200 μg per mouse, twice per week. The anti-PD-1 treatment was started upon the detection of palpable solid pancreatic tumors for KPPC mice (around 34–39 days of age) and KPPC;Col1pdxKO mice (around 44–50 days of age). The aforementioned experimental mice with desired genotypes were monitored and analyzed with no randomization or blinding. Both female and male mice with desired genotype(s) for PDAC were used for experimental mice. For xenograft tumor formation experiment, 8-week-old female nude mice (n = 5 per group) were subcutaneously injected with KPPC and KPPC;Col1pdxKO cancer cell lines (5 × 105 cells per injection). All mice were housed under standard housing conditions at MDACC animal facilities, and all animal procedures were reviewed and approved by the MDACC Institutional Animal Care and Use Committee.
Cell culture
Isolation of primary PDAC cells from mouse pancreatic tumors was performed as previously described with minor modifications (Chen et al., 2018; Zheng et al., 2015). Fresh tumor tissues were minced with sterilized lancets, digested with collagenase IV (17104019, Gibco, 4 mg/mL)/dispase II (17105041, Gibco, 4 mg/mL)/RPMI at 37 °C for 0.5 hour, filtered by 70 μm cell strainers to generate single cell suspension and resuspended in RPMI/2%FBS. The subsequent single-cell suspension was stained with CD140a(PDGFRα)-PE (135905; BioLegend) for fibroblast sorting, and CD326(EpCAM)-AlexaFluor-488 (118210; BioLegend) for cancer cell sorting, as previously described (Chen et al., 2021). Samples were filtered through a 40 μm mesh and then sorted with Aria II sorter (BD Biosciences) at the South Campus Flow Cytometry Core Laboratory of MDACC. Cells were cultured in RPMI medium containing 20% FBS and 1% penicillin-streptomycin-amphotericin B (PSA) antibiotic mixture. Human cell lines, such as Panc1, BxPC3, PSN1, CAPAN1, T3M4, HPNE (human pancreatic epithelial cells), and BJ (fibroblasts), were cultured in RPMI with 10% FBS and 1% penicillin–streptomycin (PS). All cell lines were from American Type Culture Collection (ATCC) except for T3M4 (Cell Bank, RIKEN BioResource Center). Cells were routinely tested to be negative for mycoplasma.
Patient cohort for PDAC tissue microarray
All human PDAC sections were fixed on tissue microarray slides that contain three representative 1 mm cores from each patient (two representative cores of tumor and one core of matched benign pancreatic tissue). The tissue microarrays were constructed from FFPE blocks of archived PDAC specimens using a methodology as previously described (Wang et al., 2002). This study was approved by the Institutional Review Board of MDACC (IRB LAB05–0854). Informed consent was obtained from all patients. The patients received no neoadjuvant therapy. Cases and clinical information were retrieved from the surgical pathology files of the Department of Pathology, MDACC (Table S4).
METHOD DETAILS
Histology and immunohistochemistry.
For formalin-fixed samples, mouse tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm thickness. Sections were processed for hematoxylin and eosin (H&E) staining. Masson’s trichrome staining (MTS) was conducted using Gomori’s Trichrome Stain Kit (38016SS2, Leica Biosystems). Picrosirius red staining for collagen was conducted using 0.1% Picrosirius Red (Direct Red80; Sigma-Aldrich) and counterstained with Weigert’s haematoxylin. Images were captured with a Leica DM 1000 LED microscope and an MC120 HD Microscope Camera with LAS V4.4 Software (Leica). Formalin-fixed, paraffin-embedded sections were processed for immunohistochemical staining as previously documented (Chen et al., 2018; Zheng et al., 2015). Sections were incubated with primary antibodies: type I collagen (Col1; ab34710, Abcam, 1:200) and integrin α3 (HPA008572, Sigma-Aldrich, 1:300), followed by biotinylated secondary antibodies, and streptavidin HRP (Biocare Medical). For all immunolabeling experiments, sections were developed by DAB and counterstained with hematoxylin. Quantification of staining techniques was conducted as recently described (Chen et al., 2021). For intratumoral hypoxia detection, Hypoxyprobe-1 (HP1, HPI Inc., 60 mg/kg intraperitoneally) was injected into KPPC and KPPC;Col1pdxKO mice 30 min before euthanasia. Staining for pimonidazole adduct (PAB2627, HPI Inc., 1:100) on formalin-fixed paraffin-embedded KPPC and KPPC;Col1pdxKO tumor tissue sections was performed using the same immunohistochemistry method as described above. The staining intensity of integrin α3 (HPA008572, Sigma-Aldrich, 1:300) was quantified by visual scoring of staining on a scale of 0–3 (3-very high, 2-high, 1-low, and 0-negative). The IHC scores of integrin α3 for all samples are graded by combined score of the intensity of staining and the percentage of positive tumor cells. The formula for staining score was used: S = p1 × 1 + p2 × 2 + p3 × 3, in which p1, p2 and p3 represent fractions of tumor cells representing each staining categories of 1, 2 and 3 respectively. The average score of integrin α3 expression was 1.86 for the entire cohort. The expression of integrin α3 was categorized as ITGA3-high (n = 68) and ITGA3-low (n = 62) using the average combined score 1.86 as a cutoff. All source data for the quantification of aforementioned staining and other experiments in this study are provided in Data S1.
Immunofluorescence
Immunofluorescence staining of formalin-fixed, paraffin-embedded sections were conducted using primary antibodies of CD4 (25229s, Cell Signaling Technology, 1:100) and FoxP3 (14–4771-80, eBioscience, 1:100), followed by TRITC- and FITC-labeled secondary antibodies (Jackson ImmunoResearch). Slides were then mounted with DAPI-containing Vectashield Mounting Medium (Vector Laboratories), visualized under the LSM800 confocal laser scanning microscope, and analyzed with ZEN software (Zeiss).
qRT-PCR
Total RNA was extracted from cells using Direct-zol RNA Kit (Zymo Research), processed for cDNA synthesis using the Reverse Transcription Kit (Applied Biosystems), and subjected to the qRT-PCR using SYBR Green Master Mix (Applied Biosystems). The expression level of indicated genes was normalized to the expression of mouse Gapdh or human GAPDH as housekeeping gene. The qRT-PCR primers are listed in Table S5.
Total mRNA sequencing
For mRNA sequencing (RNA-seq) analysis of tumor tissues, freshly dissected tumor samples were frozen in RNase-free tubes with liquid nitrogen and preserved at −80°C. Tumor samples were collected from KPPC;Col1pdxKO mice (n = 5) and age-matched (53 days) KPPC mice (n = 4). Samples were homogenized using bead tubes with ceramic beads on Fisherbrand Bead Mill 24 homogenizer (Fisher Scientific). For RNA-seq of cell lines, KPPC and/or KPPC;Col1pdxKO cancer cells were cultured in 6-well plates (Corning) pre-coated with vehicle (PBS with 0.5 M Glycerol), homotrimer Col1 (50 μg/mL), or heterotrimer Col1 (50 μg/mL). Cells were harvested after 48 hours of culture. For both tissues and cells, total RNA was extracted using Direct-zol RNA Kit (Zymo Research). Quality control analysis was conducted using RNA 6000 Nano Kit on Bioanalyzer 2100 (Agilent). Total mRNA sequencing was performed using Illumina TrueSeq stranded mRNAseq Library and High-Output sequencing PE 75×75 nt on NextSeq 500 (Illumina) by MDACC Sequencing and ncRNA Program core facility. Raw sequencing data from the Illumina platform were converted into Fastq files and aligned to the reference genome mm10 using the Spliced Transcripts Alignment to a Reference (STAR) algorithm. HTSeq-count was then utilized to generate the raw counts for each gene. Raw counts were then analyzed by DESeq2 for data processing, normalization, and differential expression analysis according to standard procedures. Functional categorization and pathway reconstitution from the RNA-seq data were conducted using gene set enrichment analysis (GSEA; Broad Institute) and Ingenuity Pathway Analysis (IPA) software (Qiagen). Unsupervised hierarchical clustering heatmap reveals the differentially expressed genes that are defined by DESeq2 P-value < 0.05 and fold change > 1.5, comparing Col1 homotrimers with Col1 heterotrimers, comparing Col1 homotrimers with vehicle, or comparing KPPC tumors with KPPC;Col1pdxKO tumors. Color scheme of the heatmap represents row Z-score distribution. All analyses were implemented in R.
Genome-wide DNA methylation assay
DNA was isolated from validated human cell lines (Panc1, BxPC3, T3M4, CAPAN1, PSN1, HPNE) using the Qiagen DNeasy Blood & Tissue Kit (69506) and examined for concentration and quality using Nanodrop (Thermo Scientific). The DNA was then subjected to bisulfite conversion and library preparation at the MDACC Epigenome Profiling Core Facility, after which reduced representation bisulfite sequencing was performed using the Illumina HiSeq4000 for each cell line in biological triplicate at the MDACC Science Park Next Generation Sequencing Core-Smithville. A custom pipeline analysis was generated in collaboration with Dr. Kunal Rai (Department of Genomic Medicine at MDACC), enabling site-specific comparison of methylation status across all samples as well as alignment and base read quality assessment. The DNA methylation results were visualized with the Broad Institute Integrative Genomics Viewer (IGV, v2.4.14)
Single-cell RNA-sequencing (scRNA-seq)
Mouse KPPF (FSF-KrasG12D/+;Trp53frt/frt;Pdx1-Flp) pancreatic tumor scRNA-seq analyses were conducted based on our recently reported dataset (deposited to GEO under the accession number GSE166298) using the same methodology as our recent study (Chen et al., 2021). Human pancreatic tumor scRNA-seq analyses were conducted based on a recently reported dataset deposited to GSA under the accession number CRA001160 (Peng et al., 2019). Library Seurat (version 3.5.3), dplyr and cowplot were loaded into R to explore QC metrics, filter cells, normalize data, cluster cells, and identify cluster biomarkers. To filter out low-quality cells, a threshold with a minimum of 200 and a maximum of 7000 genes per cell was used. Cells with more than 10% of the mitochondrial genome were also removed for further analysis. “RunUMAP” function was used for clustering the cells. Based on the “JackStrawPlot” and “ElbowPlot” functions, the first 27 principal components were used for UMAP projection and clustering analysis. “FindAllMarkers” function was used to identify the specific markers for each cell cluster. “DoHeatmap” function was used to show the top 10 genes in each cluster. “VlnPlot” and “DotPlot” functions were used to show expression probability distributions across cell clusters of the genes we selected to assign the cell type identity, and the genes that we were interested in.
3D culture of PDAC organoids and tumor spheroids
Organoids were established from primary tumors of KPPC and KPPC;Col1pdxKO mice at the same age of 53 days. Fresh tumor tissues were minced with sterilized lancets, digested with collagenase IV (17104019, Gibco, 4 mg/mL)/dispase II (17105041, Gibco, 4 mg/mL)/RPMI at 37 °C for 0.5 hour, filtered by 70 μm cell strainers, resuspended in RPMI/20%FBS, and counted for cell density. The same number (105) of total cells per tumor sample were seeded into cell culture chambers (177402, Nunc/Thermo Fisher) coated with growth-factor-reduced Matrigel (354230, Corning). Tumor spheroids were established using primary cancer cell lines from KPPC and KPPC;Col1pdxKO tumors. The same number (105) of cancer cells per tumor sample were seeded into cell culture chambers coated with growth-factor-reduced Matrigel. The 3D culture condition of organoids and tumor spheroids was based on previously published system (Huang et al., 2015). In brief, organoids and tumor spheroids were cultured in RPMI medium containing 5% Matrigel, 20% FBS, and 1% penicillin-streptomycin-amphotericin B (PSA) antibiotic mixture. Average diameter of organoids and tumor spheroids from KPPC and KPPC;Col1pdxKO tumors was quantified using the NIH ImageJ software from 10x microscopic images (BZ-X710 fluorescence microscope, Keyence). Matrigel layer containing established organoids or tumor spheroids after 3D culture and/or treatment was fixed with paraformaldehyde, carefully dissected from the cell culture chamber, subjected to manual dehydration/paraffinization, and prepared as paraffin-embedded sections. Sections were then processed for H&E and/or immunofluorescence staining.
Flow cytometry
For the characterization of immune infiltration, fresh tumor tissues (KPPC and KPPC;Col1pdxKO mice, either without treatment or with broad-spectrum antibiotics treatment) were weighed, minced, and digested in 2 mL solution containing 0.1 mg/mL Liberase TL (Roche, 05401020001) and 0.2 mg/mL DNase I in RPMI media (Roche, 10104159001) at 37°C for 30 min with gentle mixing, followed by further dissociation with gentleMACS Dissociator (Miltenyi Biotec). The digestion was stopped with RPMI with 10% FBS and 10 mM EDTA. Cells were filtered by 70 μm cell strainers, washed, resuspended, and subjected to a gradient centrifugation using Histopaque-1119 (Sigma-Aldrich, 11191). Alternatively, co-culture cell mixtures of mouse (KPPC or KPPC;Col1pdxKO) cancer cells and mouse splenic cells were collected and filtered before immunostaining. Cells were stained in the dark on ice for 30 min with 100 μL antibody cocktail for membrane markers, diluted in FACS buffer containing 20% Coomassie Brilliant Stain Buffer (BD Bioscience, 566349) and 50 μg/mL anti-mouse CD16/CD32 (2.4G2) FC block (BD Biosciences, 553142). Cells were washed twice with PBS/2%FBS, fixed-permeabilized with Foxp3/Transcription Factor Staining Buffer Set (eBioscience, 00–5523-00), washed twice with 1x Permeabilization buffer, and stained in the dark on ice for 30 min with antibody cocktail (for intracellular markers) diluted in 1x Permeabilization buffer. Cells were washed twice with 1x Permeabilization buffer and washed with PBS/2%FBS. The Fixable Viability Dye eFluor 780 (eBioscience) and antibodies used for single-cell suspension were specified in Key Resources Table. Samples were filtered through a 40 μm mesh and examined using a BD LSR Fortessa X20. The percentage positive cells were analyzed by FlowJo Version 10.1 and gated on CD45 positivity. Unstained, viability stain only, and single-stained beads (eBioscience) were used as compensation controls. Gating strategy was shown in Figure S8D.
Multispectral imaging of multiplex stained tissue sections
The tyramide signal amplification (TSA) multiplex staining procedures, spectral unmixing and cell segmentation using the Nuance and inForm imaging softwares were described previously (Carstens et al., 2017; Chen et al., 2021). Antibody concentrations used for the multiplex staining can be found in Key Resources Table. Multiplex stained slides were scanned with the Vectra Multispectral Imaging System version 2, using Vectra software version 3.0.3 (Perkin Elmer). Each tissue section was scanned in its entirety using a 4x objective. Up to 80 regions (at 20x) per section (sample) were selected for multispectral imaging using the Phenochart software (Perkin Elmer). Each multiplex field was scanned at 10 nm interval of the emission light spectrum across the range of each emission filter cube. Filter cubes used for multispectral imaging were DAPI (440–600 nm), FITC (520 nm-680 nm), Cy3 (570–690 nm), Texas Red (580–700 nm) and Cy5 (680–720 nm). Multispectral images of single marker stained slides with the corresponding fluorophores were used to generate a spectral library using the Nuance Image Analysis software (Perkin Elmer). The library contained the emitting spectral peaks of all fluorophores and was used to unmix each multispectral image (spectral unmixing) to its individual 6 components by using the inForm 2.2 image analysis software. Thresholds of detection for the indicated markers were adjusted across different cohorts in order to ensure consistent capture of positive signal across all controls. All images of various samples and groups were processed using the same thresholds of staining positivity. After spectral unmixing and cell segmentation, the images were then subjected to a proprietary inForm active learning phenotyping algorithm. This algorithm enables the individual identification of each DAPI-stained cell based on the fluorescence staining signal and nuclear/cell morphological features. Cells were classified into the following classes: cytokeratin-8+ (CK8+) cancer cells, total T cells (CD3+), CD8+ T cells (CD3+CD8+), CD4+ T cells (CD3+CD4+), and other (CK8−CD3−). All the procedures for staining, imaging, image processing, and quantifications were performed blinded to the sample identity.
Methylated DNA immunoprecipitation (MeDIP)
Methylated DNA was isolated from 1.0 μg of sonicated DNA using Methylamp Methylated DNA Capture (MeDIP) Kit. DNA was added to each antibody coated well and incubated for 120 min at room temperature on an orbital shaker. After releasing with proteinase K for 60 min at 65 °C, DNA was eluted from the column and adjusted to a final volume of 100 μL with nuclease-free water. For each sample, an input vial (1:20) was performed using total sonicated DNA for further normalization. For DNA amplification, a total of 5 μL of eluted DNA was added to the reaction mixture containing the primer pair (200 nM each) and diluted with 2x Fast SYBR Green Master Mix (Applied Biosystems) in a final volume of 20 μL for each PCR reaction. The real-time PCR reactions were performed in a 96-well reaction plate using the StepOne Real-Time System (Applied Biosystems) and were performed in triplicate. PCR reaction was stopped when the fluorescent signal increased over the threshold and electrophoresis of PCR products was done on a Bioanalyzer 2100 (Agilent Technologies) according to the manufacturer’s protocol. Electrophoresis results are visualized as virtual gel images as previously described (Tampe et al., 2017; Tampe et al., 2014). For quantification, the Ct values of the input samples were subtracted from the Ct values of the MeDIP samples after enrichment (1:20) to generate the dCt values used in the equation 2−dCt. Oligonucleotide sequences are shown in Table S5.
Type I collagen purification
Type I collagen (Col1) extraction from either mouse tissues or cell culture medium was conducted according to previous description with minor modifications (Han et al., 2010; Kuznetsova et al., 2001; Kuznetsova et al., 2004; Kuznetsova et al., 2003; Makareeva et al., 2010; Miles et al., 2002). Mouse Col1 homotrimers and heterotrimers were extracted from 2-month-old homozygous osteogenesis imperfecta murine (OIM; Col1a2oim/oim) and wild-type (WT) littermate mice, respectively. Sliced skins and tail tendons of mice were washed in protease-inhibiting buffer (3.5 M NaCl, 10 mM Tris, 20 mM EDTA, 2 mM N-ethylmaleimide, 1 mM phenylmethylsulfonyl fluoride, pH 7.5) for three days at 4 °C. Col1 was solubilized with 0.5 M of acetic acid (pH 2.8) and digested by pepsin (Calbiochem) at 4 °C for two days. After removing the insoluble materials by centrifugation at 3,000g for 10 min, solubilized collagen was precipitated by 2 M NaCl at 4 °C overnight. Alternatively, mouse Col1 homotrimers and heterotrimers were also extracted from cell culture media of mouse primary pancreatic cancer cell lines and mouse fibroblast (NIH-3T3) line, respectively. Human Col1 homotrimers and heterotrimers were extracted from cell culture medium of Panc1 human pancreatic cancer cell line and BJ human fibroblast line, respectively. Cells were cultured in T225 flasks with RPMI/10%FBS, and then switched to serum-free RPMI medium containing 2 mM glutamine and 50 μg/mL ascorbate (Sigma-Aldrich). After 24 h of incubation, serum-free medium was subjected to sequential centrifugation steps at 800g for 5 min, and 2,000g for 10 min to remove cells and debris. Supernatant was collected, cooled on ice, and buffered with 100 mM Tris-HCl, pH 7.4 containing Complete EDTA-free protease inhibitor cocktail (Roche). Collagen was precipitated by gradual addition of ammonium sulfate to a final concentration of 176 mg/mL and incubation at 4 °C overnight. Precipitated Col1 protein was collected after centrifugation at 12,000 g for 2 h and resuspended in 0.2 M sodium phosphate, 0.5 M glycerol, pH 7.4. The resuspended Col1 extraction solutions were further dialyzed against 0.2 M sodium phosphate, 0.5 M glycerol, pH 7.4 (referred as PBS/Glycerol) using Pur-A-Lyzer Maxi-50000 Dialysis Kit (Sigma-Aldrich) so as to remove remaining components (and proteins under 50 kDa) from previous Col1 precipitation steps. Total protein concentration of aforementioned Col1 extraction was determined by Pierce BCA assay kit (Thermo Fisher Scientific). Collagen concentration was determined by Sircol assay (Biocolor).
Col1 solution treatment in multi-well plates
Purified Col1 (homotrimers or heterotrimers) or purchased Col1 (heterotrimers from rat tails) solutions were diluted in sterilized PBS/Glycerol (0.2 M sodium phosphate, 0.5 M glycerol, pH 7.4) and used for cell culture experiments in multi-well plates. The labeled concentration of Col1 solution referred to the total Col1 protein amount per well divided by the cell culture medium volume per well. Specifically, 50 μg/mL Col1 solution per well of a 6-well plate indicated that 100 μg Col1 protein (dissolved in PBS/Glycerol) was directly added to the cell culture system with 2 mL medium (as for 96-well plates, 10 μg Col1 protein into 0.2 mL culture medium per well).
Cell viability assay
KPPC or KPPC;Col1pdxKO cells (3 × 103 cells per well in 100 μL RPMI with 1% FBS) were seeded into 96-well plates and then treated with indicated conditions such as indicated siRNAs (Sigma-Aldrich MISSION Predesigned siRNAs and Universal Negative Control siRNAs, listed in Table S5) for 48 h. Cell viability/number in each well of 6-or 96-well plates was determined using the Cell Counting Kit-8 (CCK8; Abcam ab228554), examined at OD 450 nm on a microplate reader following the manufacturer’s instructions.
MMP degradation assay
MMP degradation assay of extracted type I collagen (Col1) homotrimers or heterotrimers was conducted according to previous description with minor modifications (Han et al., 2010; Makareeva et al., 2010). As described above, mouse Col1 heterotrimers and homotrimers were extracted from cell culture media of mouse primary pancreatic cancer cell lines and mouse fibroblast line, respectively. MMP-1 (901-MP, R&D Systems) and MMP-2 (Biolegend) were activated before the assay using p-aminophenylmercuric acetate (APMA, A9563, Sigma-Aldrich) according to manufacturer instructions. Col1 samples were prepared in TCNB assay buffer (pH 7.5, 50 mM Tris-HCl, 0.15 M NaCl, 10 mM CaCl2, 0.05% Brij 35 and incubated with activated MMP-1 (2 nM) or MMP-2 (50 nM) at 37 °C for 20 min. The degradation reaction was terminated by adding 6x reducing SDS Laemmli Sample Buffer (Bio-world) and denatured at 60 °C for 20 min. The degradation of Col1 were examined using Western blotting method showing the cleavage fragments of Col1α1 and Col1α2 chains.
Western blotting
Extracted type I collagen (Col1) solution and solubilized Matrigel (growth-factor-reduced, 354230, Corning) were prepared in 6x reducing SDS Laemmli Sample Buffer (Bio-world) and denatured at 95 °C for 20 min. Samples were then subjected to electrophoresis using Mini-PROTEAN TGX precast polyacrylamide gels (Bio-Rad) and transblotted onto polyvinylidene fluoride (PVDF) membranes using Trans-Blot Turbo Transfer System (Bio-Rad). Col1 was blotted with goat anti-Col1 antibody (1310, SouthernBiotech, 1:1000) and HRP-conjugated donkey anti-goat secondary antibody. Type IV collagen (Col4) was blotted with rabbit anti-Col4 antibody (ab52235, Abcam, 1:300) and HRP-conjugated secondary goat anti-rabbit antibody. The information for all antibodies can be found in Key Resources Table. Uncropped Western blot images are provided in Data S2.
Cell signaling detection
KPPC;Col1pdxKO cancer cells grown in 6-well plates were transfected with indicated siRNAs (Sigma-Aldrich MISSION Predesigned siRNAs and Universal Negative Control siRNAs, listed in Table S5) using Lipofectamine reagent (11668019, Invitrogen) following the manufacturer’s instructions. Cells were cultured with regular medium (RPMI with 10% FBS) to reach 70% confluence. Cells were then incubated with RPMI medium with 1% FBS for 6 hours before the treatment of Col1 homotrimers or heterotrimers (50 μg/mL) for indicated time periods (4–16 hours). Cells were then harvested and lysed in RIPA lysis buffer (Gibco) supplied with phosphatase inhibitor (04906845001, Roche) and protease inhibitor (4693116001, Roche). Cell lysates were mixed with 6x reducing SDS Laemmli Sample Buffer (Bio-world), denatured at 95 °C for 20 min, and then examined by Western blotting. Samples were subjected to electrophoresis and transblotted as described above. The samples were then blotted with the following primary antibodies: actin (4970, Cell Signaling, 1:2000), AKT (4691, Cell Signaling, 1:1000), phospho-AKT Ser473 (4060, Cell Signaling, 1:500), DDR1 (5583, Cell Signaling, 1:500), phospho-DDR1 Tyr792 (11994, Cell Signaling, 1:500), ERK1/2 (9102, Cell Signaling, 1:500), phospho-ERK1/2 Thr202/Tyr204 (4376, Cell Signaling, 1:500), FAK (3285, Cell Signaling, 1:500), phospho-FAK Tyr397 (ab39967, Abcam, 1:500), integrin α1 (ab78479, Abcam, 1:300), integrin α2 (ab133557, Abcam, 1:2000), integrin α3 (611044, BD Biosciences, 1:1000), integrin β1 (sc-8978, Santa Cruz, 1:200). Each of Western blot experiments was independently repeated for at least three times with consistent results.
Col1 coating for multi-well plates
Purified Col1 (homotrimers or heterotrimers) solutions were used for pre-coating the multi-well plates at indicated concentrations. Pre-coated Col1 homotrimers or heterotrimers at 20 μg/mL indicated that 40 μg Col1 protein was diluted in 200 μL PBS/Glycerol to coat one well of a 6-well plate overnight at room temperature, and then used for the cell culture system with 2 mL culture medium (as for 96-well plates, 4 μg Col1 protein in 20 μL PBS/Glycerol coated for 0.2 mL culture medium per well). For the signaling detection in cells attached to coated Col1, KPPC;Col1pdxKO cancer cells were starved for 6 hours in RPMI medium with 1% FBS for 6 hours and then seeded into 6-well plates pre-coated with Col1 homotrimers, Col1 heterotrimers, or vehicle. After cell culture for indicated time periods, cells were harvested and examined as described above.
Exosome preparation and electroporation with siRNAs
Exosomes were produced from human mesenchymal stem cells using previously established system (Kamerkar et al., 2017; Mendt et al., 2018). 4 × 109 number of total exosomes (measured by Nanosight™ analysis) and 4 μg of siRNA (scrambled siRNA-control or siRNA-Itga3) were mixed in 400 μL of plasma-lyte (BHL2B2533Q; Baxter Healthcare). These exosomes were electroporated using a single 4 mm cuvette using a Gene Pulser Xcell Electroporation System (BioRad, 165–2081), as previously described. The mice were injected with 109 exosomes containing 1 μg of siRNAs per intraperitoneal injection in 100 μL volume. This dosage represents approximately 0.8 to 1.2 μg of exosome protein load per injection in mice every 48 hours. The treatment starts at 5-week-old age for the KPPC mice.
In vitro migration of splenic T lymphocytes
Spleen from 8 healthy wild-type mice of 2.5-month age was minced, filtered through a 40 μm mesh, washed with ice-cold PBS, and then suspended in 5 mL of red blood cell lysis solution (sc-296258, Santa Cruz) on ice for 5 min and then washed with PBS. Splenic T lymphocytes were cultured in RPMI medium containing 10% FBS and activated using anti-CD3 (553057, BD Biosciences) and anti-CD28 (553294, BD Biosciences) antibodies (1 μg/mL) for 24 h. Activated splenic T lymphocytes were counted and seeded (5 × 105 cells/well) into the of transwell upper chambers (pore size 5 μm; Millipore, MCMP24H48), which were placed in 24-well plates containing 600 μL conditioned medium from KPPC cancer cells or KPPC;Col1pdxKO cancer cells, or serum-free RPMI medium. T cells in the upper chambers were treated with CXCL16 neutralizing antibody (MAB503, R&D Systems, Clone #142417). After 24 hours of incubation, migrated splenic T lymphocytes, that migrated to the bottom chambers of 24-well plates, were centrifuged at 800g for 5 min, fixed with PFA, and stained with crystal violet. After PBS washes (30 min, thrice), crystal violet was dissolved by DMSO and measured at OD 590 nm using a plate reader.
Co-culture of mouse splenic lymphocytes and PDAC cancer cells
KPPC and KPPC;Col1pdxKO cancer cells (2 × 104 cells/well) were seeded into 96-well plates. Spleen from 4 healthy mice of 2.5-month age (with identical genetic background to KPPC and KPPC;Col1pdxKO mice) was minced, filtered through a 40 μm mesh, washed with ice-cold PBS, and then resuspended in 5 mL of red blood cell lysis solution (sc-296258, Santa Cruz) on ice for 5 min and then washed with PBS. Splenic lymphocytes were counted and seeded into round-bottom 96-well plates (2 × 105 cells/well) with or without activation for 24 h before being transferred into the 96-well plates containing KPPC or KPPC;Col1pdxKO cancer cells (or only culture medium without cancer cells). The co-culture system was based on a lymphocyte : cancer cell ratio of 10:1. After 24 h of co-culture, splenic lymphocytes were harvested, stained using the above method of flow cytometry, and examined for lymphocyte activation. Anti-CD3 (553057, BD Biosciences) and anti-CD28 (553294, BD Biosciences) antibodies (1 μg/mL) were used for the in vitro activation of T cells.
In vitro macrophage migration
Mouse RAW264.7 macrophages (2 × 104 cells, suspended in 100 μL serum-free medium) were seeded into the of transwell upper chambers (pore size 5 μm; Millipore, MCMP24H48), which were placed in 24-well plates containing 600 μL conditioned medium from KPPC cancer cells or KPPC;Col1pdxKO cancer cells, or serum-free medium. Macrophages in the upper chambers were treated with CXCR2 inhibitor SB225002 (ab120895, Abcam) or CXCL5 neutralizing antibody (NBP2–22026, Novus Biological, clone 32B3). After incubation of 12 hours, macrophages were fixed by PFA and stained with 0.05% crystal violet. The migrated macrophages (that attached to the lower surface of transwell membrane) were counted under the microscope after the removal of remaining macrophages on the upper surface of transwell membrane. For the generation of conditioned medium from cancer cells, KPPC cancer cells or KPPC;Col1pdxKO cancer cells in T225 flasks were washed with PBS twice and incubated with serum-free RPMI medium for 24 hours. Conditioned medium collection was conducted by removing cellular components using centrifugation at 800g for 5 min and then 2000g for 10 min.
Microbial DNA 16S rRNA gene sequencing
Fresh tumor samples and fecal samples were collected from co-housed KPPC mice, KPPC;Col1pdxKO mice, or wild-type littermate control mice with or without antibiotics treatment. Stage-matched end-point tumors were examined and compared among indicated groups. Broad-spectrum antibiotics (ABX) treatment via oral gavage, as previously described (Pushalkar et al., 2018), contained vancomycin (50 mg/mL; Sigma-Aldrich), neomycin (10 mg/mL; Sigma-Aldrich), metronidazole (100 mg/mL; Alfa Aesar), and amphotericin (1 mg/mL; X-GEN Pharmaceuticals). During the experiments, mouse drinking water was supplied with ampicillin (1 mg/mL; Sigma-Aldrich), vancomycin (0.5 mg/mL; Sigma-Aldrich), neomycin (0.5 mg/mL; Sigma-Aldrich), metronidazole (1 mg/mL; Alfa Aesar), and amphotericin (0.5 μg/mL; X-GEN Pharmaceuticals). For mouse stool samples, genomic DNA was isolated with the QIAamp fast DNA stool kit (Qiagen) following the manufacturer’s instructions, with an additional step of intensive bead-beating lysis. For mouse tissue samples, genomic DNA was extracted from samples using the Qiagen Powersoil kit (Qiagen) as per the manufacturer’s instructions. The V4 region of 16S rRNA gene was amplified by PCR using the 515 forward and 806 reverse primer pairs from 100 ng of purified genomic DNA sample (Caporaso et al., 2012). The DNA libraries were purified using the QIAquick gel extraction kit (Qiagen) and sequenced with the Illumina Miseq sequencer system using 2×250 bp paired-end protocol. The paired-end reads were de-multiplexed by QIIME, followed by being merged and de-replicated for chimeras using VSEARCH. UNOISE 3 command algorithm was used as the denoising method of reads (Edgar, 2016). Operational taxonomic units (OUTs) were classified with the Silva database version 138 using the Mothur method. The detailed computational pipeline for data analysis was based on previous studies (Wang et al., 2018). Total bacterial DNA content in tumor and fecal samples was examined by qRT-PCR of 16S rRNA. Quantitative PCR (qPCR) assays were conducted using a QuantStudio 6 Flex Real-Time PCR system (Thermo Fisher). All qPCR mixtures contained 10 μL of 2× Kapa fast SYBR master mix, 0.8 μL of universal bacterial 926F primer (5’-AAACTCAAAKGAATTGACGG-3’) and 1062R primer (5’-CTCACRRCACGAGCTGAC-3’) at final concentration 0.4 μM, and 9.2 μL of the DNA template. Samples were heated to 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Melting curves were performed after amplification.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses of flow cytometry and immunostaining quantifications were performed with unpaired, two-tailed t test, one-way ANOVA with Tukey’s multiple comparison test, or Fisher’s exact test using GraphPad Prism (GraphPad Software, San Diego, CA, USA). The expression data of indicated genes among TCGA PDAC cohort samples were based on the RNA Seq V2 RSEM data. TCGA data were downloaded from cBioPortal (Cerami et al., 2012; Gao et al., 2013). For the survival analysis based on ITGA3 expression, patients with available OS data and RNA-seq data (n = 177) were stratified into ITGA3-high (n = 88) and ITGA3-low (n = 89) groups based on the median IL18 expression level. Kaplan-Meier plots were drawn for survival analysis and the log rank Mantel-Cox test was used to evaluate statistical differences. Data met the assumptions of each statistical test, where variance was not equal (determined by an F-test) Welch’s correction for unequal variances was applied. A P value < 0.05 was considered statistically significant. Error bars represented standard error of the mean (S.E.M.) when multiple visual fields were averaged to produce a single value for each animal, which was then averaged again to represent the mean bar for the group in each graph.
Supplementary Material
Data S2 Uncropped Western blot images for Figures 1, 2, 4, and 5. Related to STAR Methods.
Table S1 List of the differentially expressed genes between Col1 homotrimer deleted (KPPC;Col1pdxKO) cancer cells and control (KPPC) cancer cells by the RNA-sequencing analysis data. Related to Figure 2.
Table S2 List of the differentially expressed genes in KPPC;Col1pdxKO cancer cells grown on pre-coated Col1 homotrimers, Col1 heterotrimers, or vehicle by the RNA-sequencing analysis data. Related to Figure 4.
Table S3 List of the differentially expressed genes between Col1 homotrimer deleted (KPPC;Col1pdxKO) tumors and control (KPPC) tumors by the RNA-sequencing analysis data. Related to Figures 7 and 8.
Table S4 Clinical data of the patient cohort for PDAC tissue microarray. Related to STAR Methods.
Table S5 List of the sequences of primers and siRNAs. Related to STAR Methods.
Highlights.
Col1 produced by pancreatic cancer cells is the abnormal oncogenic homotrimer variant
Col1 homotrimer deletion in cancer cells inhibits pancreatic tumor progression
Col1 homotrimer deletion enriches T cells and beneficial tumor microbiome
Col1 homotrimer deletion enables the efficacy of anti-PD-1 immunotherapy
Acknowledgements
We thank D. Saur for providing the KF/KPPF mouse strain, Dr. Valerie S. LeBleu for helpful discussions, C.G. Liu at the MDACC Sequencing and ncRNA Program for RNA-seq analysis, K.M. Ramirez, K. Clise-Dwyer, and R. Jewell at the South Campus Flow Cytometry Core Laboratory of MDACC for flow cytometry cell sorting (in part supported by NCI P30CA16672), E.J. Thompson, D.P. Pollock and Y. Chen for their assistance for the single-cell RNA-sequencing analysis, the Genetically Engineered Mouse Facility team of MDACC for the generation of Col1a1loxP/loxP mouse strain. J. Zhang, X. Song, and X. Mao for RNA-seq data processing, K. Rai and M. Tang for the genome-wide DNA methylation analysis. This work was supported by NCI P01CA117969. R. Kalluri is a Distinguished University Chair supported by Sid W. Richardson Foundation. This work used the CCSG Microbiome Core Facility at MDACC supported in part by the NCI P30CA016672.
Footnotes
DECLARATOIN OF INTERESTS
The authors declare no competing interests.
References
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607. 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi M, Seitzer N, Ishikawa T, Reschke M, Chen M, Wang G, Mitchell C, Ng C, Katon J, Lunardi A, et al. (2018). Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nature medicine 24, 165–175. 10.1038/nm.4463. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, Affo S, Filliol A, Chin L, Savage TM, Yin D, et al. (2021). Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. The Journal of clinical investigation 131. 10.1172/JCI146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6, 1621–1624. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, and Kalluri R. (2017). Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nature communications 8, 15095. 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404. 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kim J, Yang S, Wang H, Wu CJ, Sugimoto H, LeBleu VS, and Kalluri R. (2021). Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer cell 39, 548–565 e546. 10.1016/j.ccell.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, LeBleu VS, Carstens JL, Sugimoto H, Zheng X, Malasi S, Saur D, and Kalluri R. (2018). Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO molecular medicine 10. 10.15252/emmm.201809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. (2005). Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730. 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman SD, Sweet HO, McBride DJ Jr., Davisson MT, Marks SC Jr., Shuldiner AR, Wenstrup RJ, Rowe DW, and Shapiro JR (1993). Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proceedings of the National Academy of Sciences of the United States of America 90, 1701–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClerck YA, Bomann ET, Spengler BA, and Biedler JL (1987). Differential collagen biosynthesis by human neuroblastoma cell variants. Cancer research 47, 6505–6510. [PubMed] [Google Scholar]
- Du W, Lebowitz PF, and Prendergast GC (1999). Elevation of alpha2(I) collagen, a suppressor of Ras transformation, is required for stable phenotypic reversion by farnesyltransferase inhibitors. Cancer research 59, 2059–2063. [PubMed] [Google Scholar]
- Edgar RC (2016). UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv, 081257. 10.1101/081257. [DOI] [Google Scholar]
- Egeblad M, Rasch MG, and Weaver VM (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 22, 697–706. 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America 110, 20212–20217. 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J, Matson V, and Gajewski TF (2019). Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer 7, 108. 10.1186/s40425-019-0574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. (2013). Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M, Huang FW, Jane-Valbuena J, Kryukov GV, Lo CC, McDonald ER 3rd, Barretina J, Gelfand ET, Bielski CM, Li H, et al. (2019). Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569, 503–508. 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103. 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Makareeva E, Kuznetsova NV, DeRidder AM, Sutter MB, Losert W, Phillips CL, Visse R, Nagase H, and Leikin S. (2010). Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. The Journal of biological chemistry 285, 22276–22281. 10.1074/jbc.M110.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, McBride DJ, Losert W, and Leikin S. (2008). Segregation of type I collagen homo- and heterotrimers in fibrils. J Mol Biol 383, 122–132. 10.1016/j.jmb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, and Tuveson DA (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell 7, 469–483. 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hornburg M, Desbois M, Lu S, Guan Y, Lo AA, Kaufman S, Elrod A, Lotstein A, DesRochers TM, Munoz-Rodriguez JL, et al. (2021). Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer cell 39, 928–944 e926. 10.1016/j.ccell.2021.04.004. [DOI] [PubMed] [Google Scholar]
- Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et al. (2015). Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature medicine 21, 1364–1371. 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, Suwa H, Ishigami S, and Imamura M. (1995). Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas 11, 357–364. [DOI] [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, and Kalluri R. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503. 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova N, McBride DJ Jr., and Leikin S. (2001). Osteogenesis imperfecta murine: interaction between type I collagen homotrimers. J Mol Biol 309, 807–815. 10.1006/jmbi.2001.4682. [DOI] [PubMed] [Google Scholar]
- Kuznetsova NV, Forlino A, Cabral WA, Marini JC, and Leikin S. (2004). Structure, stability and interactions of type I collagen with GLY349-CYS substitution in alpha 1(I) chain in a murine Osteogenesis Imperfecta model. Matrix biology : journal of the International Society for Matrix Biology 23, 101–112. 10.1016/j.matbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Kuznetsova NV, McBride DJ, and Leikin S. (2003). Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. J Mol Biol 331, 191–200. [DOI] [PubMed] [Google Scholar]
- Lee CL, Moding EJ, Huang X, Li Y, Woodlief LZ, Rodrigues RC, Ma Y, and Kirsch DG (2012). Generation of primary tumors with Flp recombinase in FRT-flanked p53 mice. Disease models & mechanisms 5, 397–402. 10.1242/dmm.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B. (2011). Transmembrane collagen receptors. Annu Rev Cell Dev Biol 27, 265–290. 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. (2018). Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360. 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, et al. (2020). Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489. 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, and Leikin S. (2010). Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer research 70, 4366–4374. 10.1158/0008-5472.CAN-09-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight 3. 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CA, Sims TJ, Camacho NP, and Bailey AJ (2002). The role of the alpha2 chain in the stabilization of the collagen type I heterotrimer: a study of the type I homotrimer in oim mouse tissues. J Mol Biol 321, 797–805. [DOI] [PubMed] [Google Scholar]
- Minafra S, Luparello C, Rallo F, and Pucci-Minafra I. (1988). Collagen biosynthesis by a breast carcinoma cell strain and biopsy fragments of the primary tumour. Cell Biol Int Rep 12, 895–905. [DOI] [PubMed] [Google Scholar]
- Misawa K, Kanazawa T, Misawa Y, Imai A, Endo S, Hakamada K, and Mineta H. (2011). Hypermethylation of collagen alpha2 (I) gene (COL1A2) is an independent predictor of survival in head and neck cancer. Cancer Biomark 10, 135–144. 10.3233/CBM-2012-0242. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J, Roether I, and Kern HF (1987). Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas 2, 14–24. [DOI] [PubMed] [Google Scholar]
- Moro L, and Smith BD (1977). Identification of collagen alpha1(I) trimer and normal type I collagen in a polyoma virus-induced mouse tumor. Arch Biochem Biophys 182, 33–41. [DOI] [PubMed] [Google Scholar]
- Noda M. (1993). Mechanisms of reversion. FASEB J 7, 834–840. [DOI] [PubMed] [Google Scholar]
- Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, Liu L, Huang D, Jiang J, Cui GS, et al. (2019). Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res 29, 725–738. 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. (2018). The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 8, 403–416. 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. (2019). Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 178, 795–806 e712. 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupard JH, Dimari SJ, Damjanov I, and Haralson MA (1988). Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. The American journal of pathology 133, 316–326. [PMC free article] [PubMed] [Google Scholar]
- Schonhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, Eser S, Feyerabend TB, Paul MC, Eser P, et al. (2014). A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nature medicine 20, 1340–1347. 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta PK, Smith EM, Kim K, Murnane MJ, and Smith BD (2003). DNA hypermethylation near the transcription start site of collagen alpha2(I) gene occurs in both cancer cell lines and primary colorectal cancers. Cancer research 63, 1789–1797. [PubMed] [Google Scholar]
- Sharpe AH, and Pauken KE (2018). The diverse functions of the PD1 inhibitory pathway. Nature reviews. Immunology 18, 153–167. 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089. 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampe B, Steinle U, Tampe D, Carstens JL, Korsten P, Zeisberg EM, Muller GA, Kalluri R, and Zeisberg M. (2017). Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney international 91, 157–176. 10.1016/j.kint.2016.07.042. [DOI] [PubMed] [Google Scholar]
- Tampe B, Tampe D, Muller CA, Sugimoto H, LeBleu V, Xu X, Muller GA, Zeisberg EM, Kalluri R, and Zeisberg M. (2014). Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. Journal of the American Society of Nephrology : JASN 25, 905–912. 10.1681/ASN.2013070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Clauser KR, Ohlund D, Rickelt S, Huang Y, Gupta M, Mani DR, Carr SA, Tuveson DA, and Hynes RO (2019). Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1908626116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure AM, Landry M, Souchkova O, Kembel SW, and Pilon N. (2019). Gut microbiota-mediated Gene-Environment interaction in the TashT mouse model of Hirschsprung disease. Sci Rep 9, 492. 10.1038/s41598-018-36967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers H, French NS, and Norton JD (1996). Suppression of tumorigenicity in Ras-transformed fibroblasts by alpha 2(I) collagen. Cell Growth Differ 7, 1353–1360. [PubMed] [Google Scholar]
- Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, and Pamer EG (2010). Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation 120, 4332–4341. 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084. 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, et al. (2016). Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov 6, 80–95. 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang H, Zhang W, and Fuller GN (2002). Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol 12, 95–107. 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. (2018). Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nature medicine 24, 1804–1808. 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, and Vonderheide RH (2015). Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res 3, 399–411. 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YC, Lin HH, and Tang MJ (2012). A tale of two collagen receptors, integrin beta1 and discoidin domain receptor 1, in epithelial cell differentiation. Am J Physiol Cell Physiol 303, C1207–1217. 10.1152/ajpcell.00253.2012. [DOI] [PubMed] [Google Scholar]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, and Kalluri R. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530. 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S2 Uncropped Western blot images for Figures 1, 2, 4, and 5. Related to STAR Methods.
Table S1 List of the differentially expressed genes between Col1 homotrimer deleted (KPPC;Col1pdxKO) cancer cells and control (KPPC) cancer cells by the RNA-sequencing analysis data. Related to Figure 2.
Table S2 List of the differentially expressed genes in KPPC;Col1pdxKO cancer cells grown on pre-coated Col1 homotrimers, Col1 heterotrimers, or vehicle by the RNA-sequencing analysis data. Related to Figure 4.
Table S3 List of the differentially expressed genes between Col1 homotrimer deleted (KPPC;Col1pdxKO) tumors and control (KPPC) tumors by the RNA-sequencing analysis data. Related to Figures 7 and 8.
Table S4 Clinical data of the patient cohort for PDAC tissue microarray. Related to STAR Methods.
Table S5 List of the sequences of primers and siRNAs. Related to STAR Methods.
Data Availability Statement
The accession numbers for the data reported in this paper are GEO: GSE131357, GSE131064, and GSE131666. The single-cell RNA sequencing analyses in this study were based on recently published datasets that are available as GEO: GSE166298 and GSA: CRA001160. The survival and gene expression data of TCGA pancreatic adenocarcinoma cohort were based on the GDAC Firehose PAAD dataset (previously known as the TCGA Provisional dataset).