Abstract
Background
Glucocorticoids are the mainstay for the treatment of croup. The existing evidence demonstrates that glucocorticoids are effective in the treatment of croup in children. However, updating the evidence on their clinical relevance in croup is imperative. This is an update to a review first published in 1999, and updated in 2004, 2011, and 2018.
Objectives
To investigate the effects and safety of glucocorticoids in the treatment of croup in children aged 18 years and below.
Search methods
We searched the Cochrane Library, which includes the Cochrane Central Register of Controlled Trials (CENTRAL; 2022 Issue 9), Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 4 March 2022), Embase (Ovid) (1974 to 4 March 2022). We also searched the WHO ICTRP and ClinicalTrials.gov on 4 March 2022.
Selection criteria
We included randomised controlled trials (RCTs) in children (aged 18 years and below) with croup. We assessed the effect of glucocorticoids compared to the following: placebo, any other pharmacologic agents, any other glucocorticoids, any combination of other glucocorticoids, given by different modes of administration, or given in different doses. The included studies must have assessed at least one of our primary outcomes (defined as the change in croup score or return visits, (re)admissions to the hospital or both) or secondary outcomes (defined as the length of stay in hospital or emergency departments, patient improvement, use of additional treatments, or adverse events).
Data collection and analysis
Review authors independently extracted data, with another review author verified. We entered the data into Review Manager 5 for meta‐analysis. Two review authors independently assessed studies for risk of bias using the Cochrane risk of bias tool. Two review authors assessed the certainty of the evidence for the primary outcomes using the GRADE approach.
Main results
This updated review includes 45 RCTs with a total of 5888 children, an increase of two RCTs with 1323 children since the last update. We also identified one ongoing study and one study awaiting classification. We assessed most studies (98%) as at high or unclear risk of bias.
Any glucocorticoid compared to placebo
Compared to placebo, glucocorticoids may result in greater reductions in croup score after two hours (standardised mean difference (SMD) −0.65, 95% confidence interval (CI) −1.13 to −0.18; 7 RCTs, 426 children; low‐certainty evidence); six hours (SMD −0.76, 95% CI −1.12 to −0.40; 11 RCTs, 959 children; low‐certainty evidence); and 12 hours (SMD −1.03, 95% CI −1.53 to ‐0.53; 8 RCTs, 571 children; low‐certainty evidence). The evidence for change in croup score after 24 hours is very uncertain (SMD −0.86, 95% CI −1.40 to −0.31; 8 RCTs, 351 children; very low‐certainty evidence).
One glucocorticoid compared to another glucocorticoid
There was little to no difference between prednisolone and dexamethasone for reduction in croup score at two‐hour post‐baseline score (SMD 0.06, 95% CI −0.06 to 0.18; 1 RCT, 1231 children; high‐certainty evidence). There was likely little to no difference between prednisolone and dexamethasone for reduction in croup score at six‐hour post‐baseline score (SMD 0.21, 95% CI −0.21 to 0.62; 1 RCT, 99 children; moderate‐certainty evidence). However, dexamethasone probably reduced the return visits or (re)admissions for croup by almost half (risk ratio (RR) 0.55, 95% CI 0.28 to 1.11; 4 RCTs, 1537 children; moderate‐certainty evidence), and showed a 28% reduction in the use of supplemental glucocorticoids as an additional treatment (RR 0.72, 95% CI 0.53 to 0.97; 2 RCTs, 926 children).
Dexamethasone given in different doses
Compared to 0.15 mg/kg, 0.60 mg/kg dexamethasone probably reduced the severity of croup as assessed by the croup scoring scale at 24‐hour postbaseline score (SMD 0.63, 95% CI 0.16 to 1.10; 1 RCT, 72 children; moderate‐certainty evidence); however, this was not the case at two hours (SMD −0.27, 95% CI −0.76 to 0.22; 2 RCTs, 861 children; high‐certainty evidence). There was probably no reduction at six hours (SMD −0.45, 95% CI −1.26 to 0.35; 3 RCTs, 178 children; moderate‐certainty evidence), and the evidence at 12 hours is very uncertain (SMD −0.60, 95% CI −4.39 to 3.19; 2 RCTs, 113 children; very low‐certainty evidence). There was little to no difference between doses of dexamethasone in return visits or (re)admissions of children or both (RR 0.91, 95% CI 0.71 to 1.17; 3 RCTs, 949 children; high‐certainty evidence) or length of stay in the hospital or emergency department (mean difference 0.12, 95% CI −0.32 to 0.56; 2 RCTs, 892 children). The need for additional treatments, such as epinephrine (RR 0.78, 95% CI 0.34 to 1.75; 2 RCTs, 885 children); intubation (risk difference 0.00, 95% CI −0.00 to 0.00; 2 RCTs, 861 children); or use of supplemental glucocorticoids (RR 0.77, 95% CI 0.51 to 1.15; 2 RCTs, 617 children), also did not differ between doses of dexamethasone.
There were moderate to high levels of heterogeneity in the analyses for most comparisons. Adverse events were observed for some of the comparisons reported in the review.
Authors' conclusions
The evidence that glucocorticoids reduce symptoms of croup at two hours, shorten hospital stays, and reduce the rate of return visits or (re)admissions has not changed in this update. A smaller dose of 0.15 mg/kg of dexamethasone may be as effective as the standard dose of 0.60 mg/kg. More RCTs are needed to strengthen the evidence for effectiveness of low‐dose dexamethasone at 0.15 mg/kg to treat croup.
Keywords: Adolescent, Child, Humans, Croup, Croup/drug therapy, Dexamethasone, Dexamethasone/therapeutic use, Epinephrine, Epinephrine/therapeutic use, Glucocorticoids, Glucocorticoids/therapeutic use, Prednisolone, Prednisolone/therapeutic use, Randomized Controlled Trials as Topic, Respiratory Tract Infections
Plain language summary
Glucocorticoids for croup in children
Review question
What is the effectiveness and safety of glucocorticoids when treating children with croup?
Background
Respiratory viruses are the main cause of croup in children. Croup leads to a swelling of the throat and airway, which can make breathing difficult. Children also present with a special type of cough called a barking cough. Glucocorticoids are types of steroids that help reduce the swelling, thereby making it easier for children with croup to breathe.
This is an update of a review first published in 1999 and updated in 2004, 2011, and 2018.
Search date
The evidence is current to 4 March 2022.
Study characteristics
We included 2 new studies with 1323 children, for a total of 45 studies with 5888 children aged 0 to 18 years published between 1964 and 2021. The three types of glucocorticoids used in the new studies were budesonide, dexamethasone, and prednisolone. The most recent study compared the effectiveness of budesonide and dexamethasone. The other new study compared the effectiveness of dexamethasone and prednisolone, as well as a small dose of dexamethasone (0.15 mg/kg) versus 0.60 mg/kg dexamethasone. We added the data from the new study that compared the doses of dexamethasone to previously included studies looking at the same comparison.
Study funding sources
Funding sources included government (11%), academic or research institute (7%), industry (18%), or foundations (9%). More than half of the studies (55%) did not report funding sources.
Key results
Compared to prednisolone, dexamethasone showed no improvement in croup score at two and six hours after presenting to the hospital or emergency department, and probably reduced return visits or (re)admissions for croup by almost half. The addition of supplemental glucocorticoid favoured dexamethasone versus prednisolone. Compared to 0.15 mg/kg dexamethasone, the standard dose of 0.60 mg/kg probably reduced the severity of croup as assessed by the croup scoring scale at 24 hours after presenting to the hospital or emergency department. However, we did not find any important difference between groups in croup scoring scale at 2, 6, or 12 hours, return visits or (re)admissions of children, or length of stay in the hospital or emergency department. The need for additional treatments such as the use of other drugs like epinephrine, supplemental glucocorticoid, or the use of a tube to help breathing did not differ between 0.15 mg/kg and 0.60 mg/kg dexamethasone. No serious adverse events from the use of the glucocorticoids were reported in the newly included studies.
Conclusions
The evidence has not changed that glucocorticoids reduce symptoms of croup at two hours, shorten hospital stays, and reduce the rate of return visits or (re)admissions compared to placebo (dummy treatment). A small dose of dexamethasone at 0.15 mg/kg may be as effective as the standard dose of 0.60 mg/kg. More studies are needed to strengthen the evidence for the effectiveness of low‐dose dexamethasone at 0.15 mg/kg to treat croup. We conclude that glucocorticoids are effective in the treatment of croup in children.
Certainty of evidence
Most studies (98%) had problems related to their methods, reporting issues, or both. For any glucocorticoid compared to placebo, we downgraded the certainty of the evidence for change in croup score after 2, 6, 12, and 24 hours and return visits or (re)admissions due to study variability, imprecision and inconsistency of study results, and risk of bias. There is little evidence that reporting bias influenced our results for return visits or (re)admissions, or both. Similar threats to the certainty of the evidence were present in the other comparisons in this review, including concerns related to risk of bias and inconsistency and imprecision of study results.
Summary of findings
Summary of findings 1. Any glucocorticoid compared to placebo for croup.
| Any glucocorticoid compared to placebo for croup | ||||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: any glucocorticoid Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Placebo | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) |
The mean change in croup score was −1.50 to −0.81. | The mean change in croup score was 0.65 standard deviations in favour (1.13 more to 0.18 more). | ‐ | 426 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | A standard deviation of 0.65 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score was −3.23 to −0.65. | The mean change in croup score was 0.76 standard deviations in favour (1.12 more to 0.40 more). | ‐ | 959 (11 RCTs) | ⊕⊕⊝⊝ Lowc,d | A standard deviation of 0.76 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) |
The mean change in croup score was −7.62 to −1.00. | The mean change in croup score was 1.03 standard deviations in favour (1.53 more to 0.53 more). | ‐ | 571 (8 RCTs) | ⊕⊕⊝⊝ Lowe,f | A standard deviation of 1.03 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) |
The mean change in croup score was −2.56 to −1.05. | The mean change in croup score was 0.86 standard deviations in favour (1.40 more to 0.31 more). | ‐ | 351 (8 RCTs) | ⊕⊝⊝⊝ Very lowg,h | A standard deviation of 0.86 represents a large difference between groups. |
| Return visits or (re)admissions or both | 204 per 1000 | 106 per 1000 (74 to 153) | RR 0.52 (0.36 to 0.75) | 1679 (10 RCTs) | ⊕⊕⊝⊝ Lowi,j | |
| Adverse events | 13/26 (50%) studies reported collecting adverse events data, and 8/13 (62%) reported no serious adverse events. Bjornson 2004 reported 7 instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported 1 child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported 7 secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported 1 child with pneumonitis in the placebo group (1/13, 7.7%) and 2 children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported 1 instance of exacerbated symptoms, 5 children with emotional distress, 2 with vomiting, and 1 instance of eye irritation in the budesonide group (9/42, 21.4%), and 3 instances of exacerbated symptoms, 6 children with emotional distress, 3 with vomiting, 2 rashes, and 1 instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%). | 1399 (13 RCTs) |
⊕⊕⊝⊝ Lowk,l | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. bWe downgraded by one level for risk of bias. The contributing studies were at high (n = 3) and unclear (n = 4) risk of bias. cWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 83%), and variation in point estimates and in direction of effects for one study. dWe downgraded by one level for risk of bias. The contributing studies were at high (n = 3) and unclear (n = 8) risk of bias. eWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 86%), and variation in point estimates. fWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 6) risk of bias. gWe downgraded by two levels for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. The confidence intervals did not overlap for some studies. There was variation in the direction of effects. hWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 6) risk of bias. iWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 52%), and variation in point estimates. jWe downgraded by one level for risk of bias. The contributing studies were at high (n = 3) and unclear (n = 7) risk of bias. kWe downgraded by one level for imprecision. Narrative synthesis conducted, estimates are not precise. lWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 11) risk of bias.
Summary of findings 2. Any glucocorticoid compared to epinephrine for croup .
| Any glucocorticoid compared to epinephrine for croup | ||||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: any glucocorticoid Comparison: epinephrine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Epinephrine | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) |
The mean change in croup score was −4.24 to −3.74. | The mean change in croup score was 0.77 standard deviations not in favour (0.24 more to 1.77 less). | ‐ | 130 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | A standard deviation of 0.77 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score was −1.25 to −1.10. | The mean change in croup score was 0.10 standard deviations in favour (1.18 more to 0.97 less). | ‐ | 63 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | A standard deviation of 0.10 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) |
The mean change in croup score was −3.86 to −1.45. | The mean change in croup score was 0.07 standard deviations in favour (0.57 more to 0.43 less). | ‐ | 129 (3 RCTs) | ⊕⊕⊝⊝ Lowg,h | A standard deviation of 0.07 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) |
The mean change in croup score was −4.40 to −2.01. | The mean change in croup score was 0.17 standard deviations not in favour (0.18 more to 0.51 less). | ‐ | 129 (3 RCTs) | ⊕⊕⊝⊝ Lowg,i | A standard deviation of 0.17 represents a small difference between groups. |
| Return visits or (re)admissions or both | 0 per 1000 | 0 per 1000 (0 to 0) | RD 0.00 (−0.04 to 0.04) | 130 (2 RCTs) | ⊕⊕⊝⊝ Lowg,j | |
| Adverse events | 3/4 (75%) studies reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported 5 cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported 4 cases of tremor and tachycardia (4/25, 16%) in the epinephrine group. | 162 (3 RCTs) | ⊕⊕⊝⊝ Lowk,l, | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 87%), and variation in point estimates. There was minimal overlap of the confidence intervals. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and a clinically important benefit for epinephrine compared to glucocorticoids. cWe downgraded by one level for risk of bias. The contributing studies were at high risk of bias (n = 2). dWe downgraded by two levels for inconsistency. There was considerable heterogeneity (I² = 78%), and variation in point estimates and in the direction of effects. eWe downgraded by one level for imprecision. The sample size was small (did not meet optimal information size). The effect estimate included both the null effect and a clinically important effect for glucocorticoids compared to epinephrine. fWe downgraded by one level for risk of bias. The contributing studies were at unclear risk of bias (n = 2). gWe downgraded by one level for imprecision. The sample size was small (did not meet optimal information size). hWe downgraded by one level for risk of bias. The contributing studies were at high (n = 1) and unclear (n = 2) risk of bias. iWe downgraded by one level for risk of bias. The contributing studies were at high (n = 1) and unclear (n = 2) risk of bias. jWe downgraded by one level for risk of bias. The contributing studies were at high risk of bias (n = 2). kWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise. lWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 1) risk of bias.
Background
Description of the condition
Croup is a common childhood respiratory disease that often leads to frequent emergency department (ED) visits (Bjornson 2008). It is a spectrum of diseases including laryngotracheitis, laryngotracheobronchitis, and laryngotracheobronchopneumonitis (Sizar 2021). Patients may present with sudden onset of a seal‐like barking cough, often accompanied by stridor, voice hoarseness, and respiratory distress (Bjornson 2008). As with many other acute respiratory diseases, croup can be mild, moderate, or severe in presentation. In brief, the pathophysiology of croup involves upper‐airway obstruction due to generalised inflammation of the airways, triggered by viral infection (especially the parainfluenza virus, which accounts for over 75% of infections) (Bjornson 2013). Whilst croup is a self‐limiting viral infection, the burden of frequent hospitalisation contributes significantly to healthcare utilisation (Bjornson 2013; Denny 1983). Croup accounts for 7% and 3% of hospitalisation in children under five and children between six months and three years in North America (Johnson 2014; Weinberg 2009). Likewise, one European study found that 16% of children aged five to eight years old had suffered from croup at least once, and 5% had experienced recurrent croup (Van Bever 1999).
Description of the intervention
The clinical benefits of glucocorticoids in the management of croup are well documented in the literature (Griffin 2000; Kairys 1989). Unlike the controversies that existed in the 1970s concerning the treatment of croup (Cherry 1979), many clinical guidelines now support the use of glucocorticoids (Alberta Medical Association 2008). Glucocorticoids have also been shown to decrease the rate and length of hospitalisation, return visits, and admission to intensive care unit in children with croup (Brown 2002; Geelhoed 1996b; Kairys 1989). Studies have also continued to highlight the effectiveness of glucocorticoids in reducing the severity of croup (Brown 2002).
How the intervention might work
One of the cardinal features of inflammation is oedema or swelling. Whilst there are associated generalised swellings of the airway in croup, inflammation and oedema of the subglottic larynx (the narrowest part of the paediatric airway) and trachea, especially near the cricoid cartilage, are most clinically significant (Cherry 2008). Glucocorticoids have anti‐inflammatory properties through which they reduce croup‐related mucosal oedema and inflammation and as such reduce the associated difficulty in breathing (Cherry 2008).
Why it is important to do this review
Systematic reviews of randomised controlled trials (RCTs) on the use of glucocorticoid for the treatment of croup have contributed significantly to the evidence around the management of croup to date. The first Cochrane Review on this study question included 24 RCTs that examined the effectiveness of treating croup with glucocorticoids (Ausejo 2000). A few other reviews have been conducted since to update the existing evidence (Gates 2018; Russell 2004; Russell 2011). The current review is necessary to incorporate new evidence to help strengthen or refute the findings of previous reviews on this study question. As there is a growing debate about the lowest effective dose of glucocorticoid in the management of croup (Alshehr 2005; Chub‐Uppakarn 2007; Dobrovoljac 2009), this review aimed to address this, and to update the existing evidence on the effect of glucocorticoids on croup.
Objectives
To investigate the effects and safety of glucocorticoids in the treatment of croup in children aged 18 years and below.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs in child health research that met our inclusion criteria irrespective of language, publication status, trial conduct and reporting quality, or risk of bias. We excluded all other study designs.
Types of participants
We included RCTs on children aged 18 years and below diagnosed with croup, pseudo croup, or laryngotracheitis. We defined croup as a syndrome consisting of hoarseness, barking cough, and stridor, where an alternative diagnosis of acute stridor had been excluded. We included both inpatients and outpatients, and defined children admitted to the emergency department as outpatients.
Types of interventions
We included studies where the intervention was the use of one or more glucocorticoids via any route of drug administration. There were no restrictions on the type or dose of glucocorticoid administered. We defined the control as the use of a placebo or any other active pharmacologic agent. We considered the following scenarios: the use of any glucocorticoid compared to placebo, glucocorticoid compared to epinephrine, or one glucocorticoid compared to one or a combination of other glucocorticoids, or glucocorticoids given by different modes of administration, or glucocorticoids given in different doses. We excluded studies if none of the treatment groups received one or more glucocorticoids.
Types of outcome measures
We included RCTs that measured on one or more of our primary or secondary outcomes. We excluded studies that failed to meet all of our inclusion criteria.
Primary outcomes
Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours.
Return visits or (re)admissions to the hospital, or both.
Secondary outcomes
Length of stay in the hospital or emergency department.
Patient improvement at 2, 6, 12, and/or 24 hours (yes or no, as reported in the individual studies).
The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids.
Any adverse events.
Search methods for identification of studies
Electronic searches
We adopted the search strategy developed by a research librarian in the previous review (Gates 2018) on 4 March 2022 (Appendix 1). The update searches were conducted by the librarian Mê‐Linh Lê. We included subject headings and keywords for croup and glucocorticoids and restricted the search to RCTs. We searched the Cochrane Library, which includes the Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 9), Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 4 March 2022), and Embase (Ovid) (1974 to 4 March 2022).
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int/) and ClinicalTrials.gov (clinicaltrials.gov) on 4 March 2022 (Appendix 1). We scanned the reference lists of relevant systematic reviews identified during screening and the included studies to identify additional relevant primary studies.
Data collection and analysis
Selection of studies
We transferred the citations identified via the search to Rayyan software after de‐duplication (Ouzzani 2016). Three review authors (CT, AK, MR) independently screened the identified citations for eligibility using a two‐stage sifting approach to review the title, abstract, and full‐text article. Any disagreements were resolved by discussion or by involving another review author (AA) when necessary.
Data extraction and management
Three review authors (CT, AK, MR) independently extracted the data, which were all in the English language. We used Microsoft Excel to manage data extraction (Microsoft Excel). We leveraged the data extraction form used in our previous review (Gates 2018). The details of the data extracted based on participant characteristics, experimental and control interventions, and primary and secondary outcomes have all been previously published (Gates 2018). Any disagreements during data extraction were resolved by discussion or by involving another review author (AA) when necessary.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool to assess risk of bias of the included studies (Higgins 2011b). We judged the risk of bias for each study as low, high, or unclear for seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. We determined the overall risk of bias as follows: low when all domains were judged as low risk; unclear when one or more domains were judged as unclear risk; and high when one or more domains were judged as high risk. Two review authors (CT, MR) independently assessed risk of bias, resolving any disagreements by discussion or by involving another review author (AA) when necessary.
Measures of treatment effect
We added relevant data from the included studies into Review Manager 5 for analysis (Review Manager 2020). We computed the effect of treatment using the random‐effects model.
Croup scores were reported as the Westley score (Westley 1978), the telephone outpatient (TOP) score (Bjornson 2016), the Downes and Raphaelly score (Downes 1975), or various author‐created scales. We therefore used standardised mean differences (SMDs) to combine the outcome for any croup score. A treatment effect (difference between treatment means) divided by its measurement variation (e.g. a pooled standard deviation) gives the SMD. We did not find effect estimates to be significantly different between Westley and other croup scores, so we included studies that reported any croup score in the subgroup analyses. Of note, a decrease in Westley score of one point from baseline is thought to be a clinically important change.
We expressed length of stay as mean differences (MDs) and calculated an overall MD. We calculated risk ratios (RRs) for binary data (i.e. return visits or (re)admissions (or both), patient improvement, use of additional treatments). We calculated risk differences (RDs) where outcomes had zero events in both groups. For return visits or (re)admissions (or both), we calculated the number needed to treat for an additional beneficial outcome (NNTB) for significant results. Because there was substantial variation in control group event rates between studies, we reported the NNTB for the mean control group rate, as well as for the smallest and largest control group rate observed.
We reported data on adverse events narratively.
Unit of analysis issues
As reported in Gates 2018, we calculated the change from baseline croup score in 28 (62%) studies where the change from baseline measures was not reported directly (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
We pooled counts, means, and variances using standard formulae for seven (15%) studies that contained more than one experimental treatment group (Cetinkaya 2004; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Luria 2001; Parker 2019). One study by Geelhoed (Geelhoed 1995a; Geelhoed 1995b), and another by Skowron (Skowron 1966a; Skowron 1966a and b; Skowron 1966b), presented the results of two individual trials in one publication. We treated these as separate comparisons in the analyses and used pooled counts only when they were reported as such in the publications.
Dealing with missing data
When they were not directly reported, we estimated the variances for continuous data in accordance with the work of Abrams 2005 and Follmann 1992. Using standard formulae, we imputed standard deviations from standard errors in three (7%) studies (Alshehr 2005; Johnson 1998; Von Mühlendahl 1982), ranges in three (7%) studies (Alshehr 2005; Roorda 1998; Super 1989), 95% confidence intervals (CIs) in two (4%) studies (Fitzgerald 1996; Klassen 1998), and interquartile ranges (IQRs) in three (7%) studies (Johnson 1996; Klassen 1994; Klassen 1998). When the change in croup score from baseline was not directly reported (n = 14, 31%), we derived the variance of the change assuming a correlation of 0.5 between pre‐ and post‐treatment scores (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fitzgerald 1996; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
In 11 (26%) studies, data from which to impute variances for change in croup score or length of stay were inadequate; for these studies we substituted average variances from other studies in the main analysis (Cetinkaya 2004; Dobrovoljac 2012; Eboriadou 2010; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Kuusela 1988; Massicotte 1973; Roberts 1999; Skowron 1966a; Skowron 1966b). Furukawa and colleagues assert that when the number of studies with imputed data within a meta‐analysis is relatively small, variance data can be safely borrowed from other studies and still provide accurate results (Furukawa 2006). For certain outcomes only one study was included in the comparison, and that study did not report a variance estimate; in such a case we did not calculate a point estimate of effect (Cetinkaya 2004; Duman 2005; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Rittichier 2000).
We substituted medians for means in nine (20%) studies (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1998; Parker 2019; Super 1989; Von Mühlendahl 1982). When data for our prespecified time points (2, 6, 12, and 24 hours from baseline) were not reported, we used time points close to these if available. We substituted one hour for two hours in one study (Dobrovoljac 2012); four hours for six hours in 12 (28%) studies (Alshehr 2005; Amir 2006; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973); five hours or discharge for six hours in one study (Johnson 1998); and 14 hours for 12 hours in one study (Massicotte 1973).
Assessment of heterogeneity
In keeping with Gates 2018, we assessed heterogeneity quantitatively with the Chi² test for heterogeneity and the I² statistic (Higgins 2002). The I² statistic indicates the per cent variability due to between‐study (or interstudy) variability as opposed to within‐study (or intrastudy) variability. We considered an I² of less than 40% to be low (potentially unimportant), 30% to 60% to be moderate, 50% to 90% to be substantial, and 75% to 100% to be considerable (Higgins 2011a, Section 9.5.2).
Assessment of reporting biases
In addition to visually inspecting the funnel plots, we used the rank correlation test and weighted regression for the detection of publication bias (Begg 1994; Egger 1997; Light 1984). We used more than one method because the relative merits of the methods are not well established.
Data synthesis
We used random‐effects models to combine treatment effects regardless of quantified heterogeneity for the analyses of all outcomes.
Subgroup analysis and investigation of heterogeneity
We explored heterogeneity between studies using subgroup analyses for the primary outcomes of change in croup score from baseline to 2, 6, 12, and 24 hours, and return visits or (re)admissions or both, using the Chi² test for subgroup differences in meta‐analysis. We explored heterogeneity by croup score, by inpatient or outpatient status, and by glucocorticoid.
Sensitivity analysis
In some analyses, we imputed variance data for most of the included RCTs (e.g. any glucocorticoid compared to placebo, change in croup score after two hours). We undertook sensitivity analyses for these and all other analyses containing imputed variance data using the largest, smallest, and average variances from the other included RCTs. As per protocol, we did not undertake any additional sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables for our two main comparisons (any glucocorticoid compared to placebo and any glucocorticoid compared to epinephrine) for the primary outcomes: change in croup score at 2, 6, 12, and 24 hours from baseline, and return visits or (re)admissions or both. The findings for the two main comparisons have not changed since the previous version of the review, as no new data were identified in the current update (Gates 2018). As per protocol, we created summary of findings tables for the remaining comparisons; however, in order not to detract from the two main comparisons, these are included in the Additional tables section. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it relates to the studies that contributed data to the meta‐analyses (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding where necessary.
Results
Description of studies
Results of the search
We identified 100 records in the 2022 update search (Figure 1). We retrieved 83 citations from the database searches and 17 records from trial registers, from which we identified and removed 41 duplicates. We screened 59 records by title and abstract and excluded 49 citations. We screened 10 full‐text articles of which six were excluded, with reasons for their exclusion provided. A flow diagram illustrating the 2022 update selection process is shown in Figure 1. We added two new RCTs with 1323 children (Huang 2021; Parker 2019), one ongoing study (IRCT20190914044765N1), and one study awaiting classification (Chen 2018). This updated review includes 45 RCTs with a total of 5888 children.
1.
Flow diagram of study selection for this review.
Included studies
Participant and trial characteristics
We identified 42 studies (93%) published in English, and one each in French (Massicotte 1973), Spanish (Martinez Fernandez 1993), and Danish (Vad Pedersen 1998). Four studies (9%) included children with mild croup (Bjornson 2004; Geelhoed 1996a; Luria 2001, Parker 2019). Twenty‐three studies (51%) assessed outpatient children (n = 22 emergency department visits, n = 1 physician office visits) (Alshehr 2005; Amir 2006; Bjornson 2004; Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996a; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Parker 2019; Rittichier 2000; Soleimani 2013; Sparrow 2006). Twenty‐three studies (51%) assessed hospitalised children (Chub‐Uppakarn 2007; Eden 1964; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Parker 2019; Roberts 1999; Roorda 1998; Skowron 1966a; Skowron 1966b; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982).
Thirty‐two studies (71%) were two‐armed trials (Alshehr 2005; Amir 2006; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Eden 1964; Eden 1967; Fitzgerald 1996; Garbutt 2013; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Johnson 1996; Klassen 1994; Klassen 1996; Koren 1983; Leipzig 1979; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Soleimani 2013; Sparrow 2006; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982); eight studies (18%) were three‐armed trials (Duman 2005; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Klassen 1998; Luria 2001; Parker 2019); and three studies (7%) were four‐armed trials (Cetinkaya 2004; Kuusela 1988; Martinez Fernandez 1993). Two studies (4%) included two individual two‐armed trials each (Geelhoed 1995a; Geelhoed 1995b; Skowron 1966a; Skowron 1966b).
Characteristics of the comparisons
Twenty‐six studies (58%) investigated any glucocorticoid compared to placebo. Of these, 15 (58%) investigated dexamethasone (Bjornson 2004; Cruz 1995; Dobrovoljac 2012; Eden 1967; Geelhoed 1996a; James 1969; Johnson 1996; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Skowron 1966a and b; Super 1989; Von Mühlendahl 1982); four (15%) investigated budesonide (Godden 1997; Husby 1993; Klassen 1994; Roberts 1999); three (12%) investigated prednisolone (Eden 1964; Massicotte 1973; Tibballs 1992); one (4%) investigated fluticasone (Roorda 1998); and three (12%) investigated both dexamethasone and budesonide (Cetinkaya 2004; Geelhoed 1995c; Johnson 1998). Four studies (10%) investigated any glucocorticoid compared to epinephrine. Of these, one investigated budesonide (Fitzgerald 1996); two investigated dexamethasone (Kuusela 1988; Martinez Fernandez 1993); and one investigated both dexamethasone and beclomethasone (Eboriadou 2010).
Thirteen studies (29%) investigated one glucocorticoid compared to another glucocorticoid. Of these, one investigated budesonide compared to dexamethasone (Huang 2021); six investigated dexamethasone compared to budesonide (Cetinkaya 2004; Duman 2005; Geelhoed 1995c; Johnson 1998; Klassen 1998; Vad Pedersen 1998); one investigated dexamethasone compared to betamethasone (Amir 2006); one investigated dexamethasone compared to beclomethasone (Eboriadou 2010); and four investigated dexamethasone compared to prednisolone (Fifoot 2007; Garbutt 2013; Parker 2019; Sparrow 2006). Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. Of these, one investigated dexamethasone and budesonide compared to a combination of dexamethasone and budesonide (Klassen 1998), and two investigated dexamethasone compared to a combination of dexamethasone and budesonide (Geelhoed 2005; Klassen 1996).
Five studies (11%) investigated dexamethasone using different modes of administration. Of these, four investigated oral compared to intramuscular dexamethasone (Cetinkaya 2004; Donaldson 2003; Rittichier 2000; Soleimani 2013), and one investigated oral compared to nebulised dexamethasone (Luria 2001). Four studies investigated dexamethasone given in different doses. Of these, three investigated 0.60 mg/kg compared to 0.15 mg/kg dexamethasone (Alshehr 2005; Chub‐Uppakarn 2007; Fifoot 2007), and one investigated both 0.60 mg/kg compared to 0.30 mg/kg and 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Geelhoed 1995a; Geelhoed 1995b).
Reported outcomes: primary outcomes
Sixteen studies (35%) reported a two‐hour change in croup score (Amir 2006; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Parker 2019; Roberts 1999; Roorda 1998); 20 studies (44%) reported a six‐hour change in croup score (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Roorda 1998; Vad Pedersen 1998; Von Mühlendahl 1982); 12 studies (27%) reported a 12‐hour change in croup score (Alshehr 2005; Chub‐Uppakarn 2007; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982); and 11 studies (24%) reported a 24‐hour change in croup score (Alshehr 2005; Cetinkaya 2004; Fitzgerald 1996; Godden 1997; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989). Of the 30 studies (67%) that reported a change in croup score, 18 (60%) used a validated score (the Westley score or a modified Westley score) (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Fifoot 2007; Godden 1997; Husby 1993; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Parker 2019; Rittichier 2000; Roorda 1998; Super 1989); 11 (37%) used author‐created scales (Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 2005; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Vad Pedersen 1998; Von Mühlendahl 1982); and one used the score by Downes 1975 (Eboriadou 2010). The studies by Bjornson 2004 and Garbutt 2013 used another validated score, the telephone outpatient (TOP) score, to measure clinical improvement. The TOP score is a two‐item, three‐point score used to assess the presence of stridor and barky cough by asking parents about their child's symptoms in the previous 24 hours (Bjornson 2016). Twenty‐seven studies (60%) reported return visits or (re)admissions to the hospital or both (Alshehr 2005; Amir 2006; Bjornson 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Parker 2019; Rittichier 2000; Roberts 1999; Skowron 1966a; Skowron 1966a and b; Skowron 1966b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998).
Reported outcomes: secondary outcomes
A total of 13 studies (29%) reported length of stay in the hospital or emergency department (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Klassen 1998; Kuusela 1988; Leipzig 1979; Parker 2019; Roorda 1998; Skowron 1966a; Skowron 1966a and b; Skowron 1966b; Sparrow 2006; Super 1989). Twelve studies (27%) reported patient improvement; of these, one reported improvement after two hours (Roberts 1999); eight reported improvement after six hours (Eden 1964; Eden 1967; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973; Roberts 1999); six reported improvement after 12 hours (Eden 1964; Eden 1967; James 1969; Massicotte 1973; Roberts 1999; Super 1989); and seven reported improvement after 24 hours (Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; James 1969; Roberts 1999; Super 1989). About two‐thirds of the included studies (n = 30) reported the use of additional treatments; of these, 12 reported intubation/tracheotomies (Chub‐Uppakarn 2007; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; James 1969; Johnson 1996; Johnson 1998; Leipzig 1979; Parker 2019; Roorda 1998; Skowron 1966a; Skowron 1966a and b; Skowron 1966b); four reported the use of antibiotics (Husby 1993; James 1969; Koren 1983; Rittichier 2000); 14 reported the use of supplemental glucocorticoids (Dobrovoljac 2012; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Parker 2019; Rittichier 2000; Roorda 1998; Super 1989; Vad Pedersen 1998); 22 reported the use of epinephrine (Amir 2006; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Koren 1983; Parker 2019; Rittichier 2000; Roberts 1999; Sparrow 2006; Super 1989; Tibballs 1992); and five reported the use of a mist tent (Alshehr 2005; Johnson 1996; Klassen 1996; Rittichier 2000; Super 1989). Twenty‐four studies reported collecting adverse events data, of which eight reported serious adverse events following the administration of glucocorticoids (namely secondary bacterial infections, e.g. pneumonia, otitis media) (Alshehr 2005; Bjornson 2004; Johnson 1996; Klassen 1998; Kuusela 1988; Parker 2019; Roberts 1999; Super 1989), and 16 reported no serious adverse events (Chub‐Uppakarn 2007; Duman 2005; Eden 1967; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Huang 2021; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Sparrow 2006; Tibballs 1992; Vad Pedersen 1998).
Funding
The included studies received funding from government (11%), academic (7%), industry (18%), and foundations (9%) sources. However, more than half (55%) of the included studies did not report any funding sources.
Excluded studies
We excluded six studies following the searches in 2022 (Figure 1). Gursanscky 2019 and Tyler 2022 were not randomised trials; Lee 2019 and Meskina 2019 were randomised trials that did not investigate glucocorticoids; and Faraji‐Goodarzi 2018 was a randomised trial that did not report any relevant outcomes. See Characteristics of excluded studies table.
We edited the excluded studies list to remove legacy excluded studies that evidently did not meet the inclusion criteria (e.g. letters, commentaries, summaries, case studies). We made this change to comply with current Cochrane standards for methods and reporting. We excluded 38 studies in this 2022 updated review.
Ongoing studies
We identified one ongoing study, IRCT20190914044765N1, and one study awaiting classification, Chen 2018 (Figure 1). We will assess these studies for inclusion in a future update.
Risk of bias in included studies
We presented the risk of bias of all included studies as assessed using the Cochrane risk of bias tool in Figure 2 and Figure 3. We judged the overall risk of bias to be low in one study (Garbutt 2013), unclear in 32 studies (Alshehr 2005; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Johnson 1996; Johnson 1998; Klassen 1996; Klassen 1998; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Parker 2019; Roorda 1998; Skowron 1966a and b; Sparrow 2006; Super 1989; Tibballs 1992; Von Mühlendahl 1982), and high in 12 studies (Amir 2006; Cetinkaya 2004; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fitzgerald 1996; Geelhoed 1995c; Klassen 1994; Rittichier 2000; Roberts 1999; Soleimani 2013; Vad Pedersen 1998). Rationales for our risk of bias judgements are provided in the risk of bias tables in the Characteristics of included studies table.
2.

Risk of bias graph for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged risk of bias for random sequence generation to be low in 27 studies (60%) and unclear in 18 studies (40%). The 17 studies at unclear risk of bias were described as randomised; however, the method for generating the randomisation sequence was unclear or not reported (Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Godden 1997; Huang 2021; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Martinez Fernandez 1993; Roorda 1998; Skowron 1966a and b; Soleimani 2013; Von Mühlendahl 1982). Randomisation was adequately described in the remaining 27 studies. We judged risk of bias for allocation concealment to be low in 19 studies (42%) and unclear in 26 studies (58%); in the latter studies, there was insufficient information reported in the publication to determine whether or not the groups to which the children were allocated could have been foreseen (Amir 2006; Cetinkaya 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; Koren 1983; Leipzig 1979; Rittichier 2000; Roorda 1998; Skowron 1966a and b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998; Von Mühlendahl 1982). Allocation concealment was adequate in the remaining 19 studies.
Blinding
We judged risk of bias for blinding of participants and personnel to be low in 26 studies (58%), unclear in 11 studies (24%), and high in eight studies (18%). Of the eight studies at high risk of bias, four appeared to be open‐label (Amir 2006; Duman 2005; Rittichier 2000; Vad Pedersen 1998). Cetinkaya 2004 did not explicitly describe any measures taken to blind participants and personnel from treatment assignment, and any blinding could have been broken. Personnel were not blinded in Fitzgerald 1996. In Eboriadou 2010, the treatments were clearly distinguishable, and the method for blinding was not described even though the study was termed "double‐blind". In Soleimani 2013, only the outcome assessor was blinded. Of the 11 studies assessed as at unclear risk of bias, seven were described as double‐blind without any further details regarding who was blinded or how blinding was achieved (Eden 1964; Geelhoed 1996a; Huang 2021; Husby 1993; Leipzig 1979; Roorda 1998; Von Mühlendahl 1982). In Donaldson 2003, Geelhoed 1995a, Geelhoed 1995c, and Johnson 1998, blinding was attempted, but we judged that the blinding could have been broken; however, it was unclear how often this could have occurred. The remaining studies included satisfactory descriptions of how participants and personnel were blinded.
We judged risk of bias for blinding of outcome assessment to be low in 27 studies (60%), unclear in 13 studies (29%), and high in five studies (11%). For 22 studies (49%), there was no mention of a third‐party outcome assessor, so the judgement for outcome assessment was carried over from blinding of participants and personnel (Cetinkaya 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; Koren 1983; Kuusela 1988; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Sparrow 2006; Tibballs 1992). Of the remaining studies, we judged two as at high risk of bias because outcome assessors were not blinded (Amir 2006; Vad Pedersen 1998). We judged seven studies as at unclear risk of bias: in Donaldson 2003, Johnson 1998, and Rittichier 2000, blinding of the outcome assessors was attempted, but we judged that the blinding could have been broken, although it was unclear how often this could have occurred; the studies by Leipzig 1979, Roorda 1998, and Von Mühlendahl 1982 were described as double‐blind, but it was unclear if the outcome assessors were blinded; and in Soleimani 2013, the outcome assessor was described as blinded, but it was unclear how or if the blinding could have been broken. The remaining studies provided satisfactory descriptions of how outcome assessors were blinded.
Incomplete outcome data
We judged risk of bias for incomplete outcome data to be low in 27 studies (60%), unclear in 14 studies (31%), and high in four studies (9%). The four studies at high risk of bias reported large losses to follow‐up that were imbalanced between groups (Dobrovoljac 2012; Geelhoed 1995c; Klassen 1994; Roberts 1999). Dobrovoljac 2012 and Roberts 1999 used the last observation carried forward (LOCF) method to estimate endpoint outcome values. Regarding the studies at unclear risk of bias, in one study the number of children analysed was not reported (Amir 2006), and in seven studies it was either unclear to which group the children who were lost to follow‐up had been allocated, or whether or not the losses to follow‐up were balanced between groups (Cruz 1995; Eden 1964; Johnson 1996; Huang 2021; Kuusela 1988; Rittichier 2000; Soleimani 2013; Von Mühlendahl 1982). In four studies, losses to follow‐up ranged from 13% to 17% (Fifoot 2007; Luria 2001; Soleimani 2013; Super 1989). In Fitzgerald 1996, loss to follow‐up was 5%, and the LOCF method was used to estimate endpoint outcome values. In Parker 2019, 11% of participants were missing at one‐hour croup assessment with unexplained exclusion reasons. We judged risk of bias due to incomplete outcome data not a concern for the remaining studies.
Selective reporting
We judged risk of bias for selective reporting to be low in three studies (7%) and unclear in 42 studies (93%). In the three studies at low risk of bias, the outcomes in the trial registers matched those reported in the publications (Fifoot 2007; Garbutt 2013; Parker 2019). For the remaining 42 studies, no protocol or trial registry was cited in the publication or located via online searches. In all cases, the outcomes reported in the methods matched those reported in the results section of the publications.
Other potential sources of bias
We judged risk of bias from other sources to be low in 35 studies (78%), unclear in eight studies (18%), and high in two studies (4%). In the two studies at high risk of bias, there was a baseline imbalance in croup score (Amir 2006; Vad Pedersen 1998). For six studies at unclear risk of bias, there was the potential for bias in participant selection because some children were not enrolled due to manpower constraints, failure of the emergency department to contact the research team, or because the emergency department was busy (Geelhoed 1995a; Geelhoed 1995b; Godden 1997; Klassen 1994; Klassen 1996; Klassen 1998; Sparrow 2006). In one study at unclear risk of bias, baseline data were not presented, therefore it was not possible to estimate whether or not baseline imbalances existed between groups (Skowron 1966a and b). For the remaining study at unclear risk of bias, participants were enrolled more than once (Parker 2019).
Effects of interventions
Comparison 1: Any glucocorticoid compared to placebo
See Table 1.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to placebo, glucocorticoids may have resulted in greater reductions in croup score after two hours (standardised mean difference (SMD) −0.65, 95% confidence interval (CI) −1.13 to −0.18; P = 0.007, I² = 81%; 7 RCTs, 426 children; low‐certainty evidence; Analysis 1.1); six hours (SMD −0.76, 95% CI −1.12 to −0.40; P < 0.001, I² = 83%; 11 RCTs, 959 children; low‐certainty evidence; Analysis 1.2); and 12 hours (SMD −1.03, 95% CI −1.53 to −0.53; P < 0.001, I² = 86%; 8 RCTs, 571 children; low‐certainty evidence; Analysis 1.3). The evidence for change in croup score after 24 hours is very uncertain (SMD −0.86, 95% CI −1.40 to −0.31; P = 0.002, I² = 81%; 8 RCTs, 351 children; very low‐certainty evidence; Analysis 1.4).
1.1. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 1: Croup score (change baseline ‐ 2 hours) by score
1.2. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 2: Croup score (change baseline ‐ 6 hours) by score
1.3. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 3: Croup score (change baseline ‐ 12 hours) by score
1.4. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 4: Croup score (change baseline ‐ 24 hours) by score
There were no subgroup differences in reductions in croup score by score (Westley 1978 or otherwise) (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4) or by inpatient or outpatient status (Analysis 1.5; Analysis 1.6; Analysis 1.7) at any time point. At two hours, there was no subgroup difference in effect by glucocorticoid (Chi² = 5.65, P = 0.06, I² = 64.6%; Analysis 1.8). At six hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 11.46, P = 0.009, I² = 73.8%; Analysis 1.9), accounted for by the larger reduction in croup score for prednisolone (SMD −1.87, 95% CI −2.62 to −1.13; P < 0.001; 1 RCT, 42 children) compared to budesonide (SMD −0.81, 95% CI −1.04 to −0.58; P < 0.001, I² = 0%; 5 RCTs, 333 children) and dexamethasone (SMD −0.62, 95% CI −1.17 to −0.08; P = 0.03, I² = 85%; 6 RCTs, 567 children). Fluticasone did not show an effect (SMD 0.06, 95% CI −0.89 to 1.02; P = 0.90; 1 RCT, 17 children). At 12 hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 10.08, P = 0.006, I² = 80.2%; Analysis 1.10), accounted for by the larger reduction in croup score for prednisolone (SMD −2.40, 95% CI −3.26 to −1.55; P < 0.001; 1 RCT, 39 children) compared to budesonide (SMD −0.97, 95% CI −1.26 to −0.68; P < 0.001, I² = 0%; 3 RCTs, 209 children) and dexamethasone (SMD −0.85, 95% CI −1.55 to −0.15; P = 0.02, I² = 84%; 5 RCTs, 323 children). At 24 hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 9.02, P = 0.01, I² = 77.8%; Analysis 1.11). Although larger reductions in croup score were observed with budesonide (SMD −1.40, 95% CI −1.88 to −0.93; P < 0.001, I² = 0%; 2 RCTs, 89 children) and dexamethasone (SMD −0.89, 95% CI −1.55 to −0.22; P = 0.009, I² = 81%; 6 RCTs, 245 children) compared to placebo, fluticasone did not show an effect (SMD 0.21, 95% CI −0.75 to 1.17; P = 0.67; 1 RCT, 17 children).
1.5. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 5: Croup score (change baseline ‐ 2 hours) by inpatient/outpatient
1.6. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 6: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient
1.7. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 7: Croup score (change baseline ‐ 24 hours) by inpatient/outpatient
1.8. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 8: Croup score (change baseline ‐ 2 hours) by glucocorticoid
1.9. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 9: Croup score (change baseline ‐ 6 hours) by glucocorticoid
1.10. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 10: Croup score (change baseline ‐ 12 hours) by glucocorticoid
1.11. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 11: Croup score (change baseline ‐ 24 hours) by glucocorticoid
2. Return visits or (re)admissions to the hospital or both
Compared to placebo, glucocorticoids may have reduced the rate of return visits or (re)admissions to the hospital or both by almost half (risk ratio (RR) 0.52, 95% CI 0.36 to 0.75; P < 0.001, I² = 52%; 10 RCTs, 1679 children; low‐certainty evidence; Analysis 1.12). There were no subgroup differences in effect by glucocorticoid (budesonide or dexamethasone, Analysis 1.13); by inpatient or outpatient status (Analysis 1.12); or by croup severity (mild or moderate croup, Analysis 1.14).
1.12. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 12: Return visits or (re)admissions or both by inpatient/outpatient
1.13. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 13: Return visits or (re)admissions or both by glucocorticoid
1.14. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 14: Return visits or (re)admissions or both by croup severity
The number needed to treat for an additional beneficial outcome (NNTB) is presented in Table 3. The NNTB was 7 children (95% CI 5 to 12) for the mean placebo group rate (30.62%). The NNTB was 102 children (95% CI 78 to 179) for the smallest placebo group rate (2.06%). Lastly, the NNTB was 3 children (95% CI 2 to 5) for the largest placebo group rate (72.00%).
1. Number needed to treat for an additional beneficial outcome for return visits or (re)admissions or both for any glucocorticoid compared to placebo .
| Baseline rate (%) | NNTB (95% CI) |
| Mean baseline rate | |
| 30.62 | 7 (5 to 12) |
| Smallest baseline rate | |
| 2.06 | 102 (78 to 179) |
| Largest baseline rate | |
| 72.00 | 3 (2 to 5) |
NNTB: number needed to treat for an additional beneficial outcome
Secondary outcomes
1. Length of stay in the hospital or emergency department
Compared to those given a placebo, children treated with glucocorticoids spent fewer hours in the hospital (mean difference (MD) −14.90, 95% CI −23.58 to −6.22; P < 0.001, I² = 54%; 8 RCTs, 476 children; Analysis 1.15). All of the included studies investigated inpatients. There was no subgroup difference in effect by glucocorticoid (budesonide, dexamethasone, or fluticasone; Analysis 1.16).
1.15. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 15: Length of stay by inpatient
1.16. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 16: Length of stay by glucocorticoid
2. Patient improvement at 2, 6, 12, and/or 24 hours
Only one study investigated patient improvement two hours after the administration of glucocorticoids compared to placebo. Roberts 1999 studied 82 hospitalised children aged six months to eight years with moderate to severe croup who were given budesonide or placebo, and observed no difference in improvement after two hours (RR 1.81, 95% CI 0.96 to 3.40; P = 0.07; 1 RCT, 82 children; Analysis 1.17). Compared to placebo, glucocorticoids were associated with improvement in a greater proportion of children after six hours (RR 1.45, 95% CI 1.12 to 1.88; P = 0.005, I² = 34%; 6 RCTs, 332 children; Analysis 1.18); 12 hours (RR 1.33, 95% CI 1.09 to 1.62; P = 0.005, I² = 53%; 6 RCTs, 340 children; Analysis 1.19); and 24 hours (RR 1.28, 95% CI 1.01 to 1.61; P = 0.04, I² = 75%; 5 RCTs, 251 children; Analysis 1.20).
1.17. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 17: Improvement (at 2 hours) by inpatient
1.18. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 18: Improvement (at 6 hours) by inpatient/outpatient
1.19. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 19: Improvement (at 12 hours) by inpatient
1.20. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 20: Improvement (at 24 hours) by inpatient/outpatient
Only inpatients were included in the 12‐hour analysis (Analysis 1.19). There were no subgroup differences in estimates of effect by inpatient or outpatient status at six or 24 hours (Analysis 1.18; Analysis 1.20). There were no subgroup differences in effect by glucocorticoid at six hours (budesonide, dexamethasone, or prednisolone; Analysis 1.21), 12 hours (budesonide, dexamethasone, or prednisolone; Analysis 1.22), or 24 hours (dexamethasone or prednisolone; Analysis 1.23).
1.21. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 21: Improvement (at 6 hours) by glucocorticoid
1.22. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 22: Improvement (at 12 hours) by glucocorticoid
1.23. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 23: Improvement (at 24 hours) by glucocorticoid
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference between children treated with glucocorticoids and those given placebo in the use of antibiotics (risk difference (RD) 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 3 RCTs, 202 children; Analysis 1.24); the use of epinephrine (RD −0.03, 95% CI −0.08 to 0.01; P = 0.16, I² = 45%; 9 RCTs, 709 children; Analysis 1.25); the rate of intubation/tracheostomy (RD 0.00, 95% CI −0.01 to 0.01; P = 0.79, I² = 0%; 11 RCTs, 1090 children; Analysis 1.26); the use of a mist tent (RD −0.20, 95% CI −0.87 to 0.47; P = 0.55, I² = 95%; 2 RCTs, 84 children; Analysis 1.27); or the use of supplemental glucocorticoids (RR 0.61, 95% CI 0.36 to 1.03; P = 0.07, I² = 10%; 6 RCTs, 305 children; Analysis 1.28).
1.24. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 24: Additional treatments: antibiotics
1.25. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 25: Additional treatments: epinephrine
1.26. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 26: Additional treatments: intubation/tracheostomy
1.27. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 27: Additional treatments: mist tent
1.28. Analysis.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 28: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Of the 26 studies that investigated any glucocorticoid compared to placebo, 13 reported collecting adverse events data. Of these, eight reported no serious adverse events (Eden 1967; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Tibballs 1992). Bjornson 2004 reported seven instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported one child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported seven secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported one child with pneumonitis in the placebo group (1/13, 7.7%) and two children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported one instance of exacerbated symptoms, five children with emotional distress, two with vomiting, and one instance of eye irritation in the budesonide group (9/42, 21.4%), and three instances of exacerbated symptoms, six children with emotional distress, three with vomiting, two rashes, and one instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%).
Comparison 2: Any glucocorticoid compared to epinephrine
See Table 2.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to epinephrine, we do not know if there was no difference in the change in croup score following treatment with glucocorticoids after two hours (SMD 0.77, 95% CI −0.24 to 1.77; P = 0.13, I² = 87%; 2 RCTs, 130 children; very low‐certainty evidence; Analysis 2.1) and six hours (SMD −0.10, 95% CI −1.18 to 0.97; P = 0.85, I² = 78%; 2 RCTs, 63 children; very low‐certainty evidence; Analysis 2.2). There may be no difference between groups at 12 hours (SMD −0.07, 95% CI −0.57 to 0.43; P = 0.78, I² = 47%; 3 RCTs, 129 children; low‐certainty evidence; Analysis 2.3) or 24 hours (SMD 0.17, 95% CI −0.18 to 0.51; P = 0.35, I² = 0%; 3 RCTs, 129 children; low‐certainty evidence; Analysis 2.4).
2.1. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 1: Croup score (change baseline ‐ 2 hours) by inpatient/outpatient
2.2. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 2: Croup score (change baseline ‐ 6 hours) by inpatient
2.3. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 3: Croup score (change baseline ‐ 12 hours) by inpatient
2.4. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 4: Croup score (change baseline ‐ 24 hours) by inpatient
The analyses at six hours (Analysis 2.2), 12 hours (Analysis 2.3), and 24 hours (Analysis 2.4) included only inpatients. At two hours, there was a subgroup difference in effect by inpatient or outpatient status (Chi² = 7.44, P = 0.006, I² = 86.6%; Analysis 2.1). For outpatients, glucocorticoids were less effective at reducing croup score compared to epinephrine after two hours (SMD 1.29, 95% CI 0.73 to 1.84; P < 0.001; 1 RCT, 64 children). No difference was detected between the two treatments for inpatients (SMD 0.26, 95% CI −0.22 to 0.75; P = 0.29; 1 RCT, 66 children).
At two hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 7.37, P = 0.03, I² = 72.9%; Analysis 2.5). Epinephrine was more effective at reducing croup score compared to beclomethasone (SMD 1.41, 95% CI 0.62 to 2.19; P < 0.001; 1 RCT, 33 children) and dexamethasone (SMD 1.13, 95% CI 0.35 to 1.91; P = 0.005; 1 RCT, 31 children). At this time point, there was no difference in reduction in croup score between budesonide and epinephrine (SMD 0.26, 95% CI −0.22 to 0.75; P = 0.29; 1 RCT, 66 children). The 12‐ and 24‐hour analyses investigated budesonide and dexamethasone, and there were no subgroup differences in effect (Analysis 2.6; Analysis 2.7).
2.5. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 5: Croup score (change baseline ‐ 2 hours) by glucocorticoid
2.6. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 6: Croup score (change baseline ‐ 12 hours) by glucocorticoid
2.7. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 7: Croup score (change baseline ‐ 24 hours) by glucocorticoid
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 and Fitzgerald 1996 investigated return visits and (re)admissions, respectively, following the administration of glucocorticoids (dexamethasone and beclomethasone, and budesonide, respectively) compared to epinephrine. Both studies may not have reported any events (RD 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 2 RCTs, 130 children; low‐certainty evidence; Analysis 2.8).
2.8. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 8: Return visits or (re)admissions or both by inpatient/outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
Kuusela 1988 investigated length of stay for 32 children hospitalised with croup who were treated with dexamethasone, epinephrine, a combination of dexamethasone and epinephrine, or placebo. There was no difference in hours spent in the hospital between children treated with dexamethasone and those treated with epinephrine (MD −10.00, 95% CI −33.89 to 13.89; P = 0.41; 1 RCT, 32 children; Analysis 2.9).
2.9. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 9: Length of stay by inpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Fitzgerald 1996 investigated the use of additional treatments for children aged six months to six years admitted to the hospital with croup who were treated with budesonide or epinephrine. There was no difference in the proportion of children who required additional epinephrine between groups (RR 0.30, 95% CI 0.03 to 2.69; P = 0.28; 1 RCT, 66 children; Analysis 2.10). No child was intubated (RD 0.00, 95% CI −0.06 to 0.06; P = 1.00; 1 RCT, 66 children; Analysis 2.11). There was no difference between groups in the proportion of children who required supplemental glucocorticoids (RR 0.83, 95% CI 0.48 to 1.43; P = 0.49; 1 RCT, 66 children; Analysis 2.12).
2.10. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 10: Additional treatments: epinephrine
2.11. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 11: Additional treatments: intubation/tracheostomy
2.12. Analysis.

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 12: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Of the four studies that investigated glucocorticoids compared to epinephrine, three reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported five cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported four cases of tremor and tachycardia (4/25, 16%) in the epinephrine group.
Comparison 3: Dexamethasone compared to budesonide
See Table 4.
2. Dexamethasone compared to budesonide for croup.
| Dexamethasone compared to budesonide for croup | ||||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone Comparison: budesonide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Budesonide | Dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score was −2.93 to −2.00. | The mean change in croup score was 0.46 standard deviations in favour (0.79 more to 0.13 more). | ‐ | 326 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | A standard deviation of 0.46 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) |
The mean change in croup score was −3.07 to −2.33. | The mean change in croup score was 0.75 standard deviations in favour (1.19 more to 0.30 more). | ‐ | 84 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | A standard deviation of 0.75 represents a large difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.69 (0.40 to 1.22) | 374 (5 RCTs) | ⊕⊕⊕⊝ Moderatee | ||
| 122 per 1000 |
84 per 1000 (49 to 149) |
|||||
| Adverse events | 4/6 (67%) studies reported collecting adverse events data, and 3/4 (75%) studies reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and 1 case each of hives and violent behaviour in the dexamethasone group (2/69, 2.9%). | 335 (4 RCTs) |
⊕⊕⊝⊝ Lowf,g | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias. Allocation concealment was unclear in two studies; blinding was unclear in two studies; and one study was unblinded. There was a baseline imbalance in croup score in one study. bWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 51%), and variation in point estimates. cWe downgraded by one level for risk of bias. The contributing studies were at high risk of bias. Allocation concealment was unclear in both studies; blinding was unclear in one study, and the other study was unblinded. There was a baseline imbalance in croup score in one study. dWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). eWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included a null effect as well as considerable benefit for dexamethasone compared to budesonide. fWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise. gWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to budesonide, dexamethasone may have resulted in a greater reduction in croup score after six hours (SMD −0.46, 95% CI −0.79 to −0.13; P = 0.006, I² = 51%; 4 RCTs, 326 children; low‐certainty evidence; Analysis 3.1) and 12 hours (SMD −0.75, 95% CI −1.19 to −0.30; P = 0.001, I² = 0%; 2 RCTs, 84 children; low‐certainty evidence; Analysis 3.2). The analysis at 12 hours included only inpatients (Analysis 3.2). At six hours, there was no subgroup difference in effect by inpatient or outpatient status (Analysis 3.1).
3.1. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 1: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient
3.2. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 2: Croup score (change baseline ‐ 12 hours) by inpatient
2. Return visits or (re)admissions to the hospital or both
There was probably no difference in the rate of return visits or (re)admissions to the hospital (or both) between dexamethasone and budesonide groups (RR 0.69, 95% CI 0.40 to 1.22; P = 0.20, I² = 0%; 5 RCTs, 374 children; moderate‐certainty evidence; Analysis 3.3). There were no subgroup differences in effect by inpatient or outpatient status (Analysis 3.3).
3.3. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 3: Return visits or (re)admissions or both by inpatient/outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no difference in hours spent in the hospital or emergency department between children treated with dexamethasone and those treated with budesonide (MD −0.51, 95% CI −1.28 to 0.25; P = 0.15, I² = 51%; 2 RCTs, 184 children; Analysis 3.4). There was no subgroup difference in effect by inpatient or outpatient status (Analysis 3.4).
3.4. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 4: Length of stay by inpatient/outpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
Klassen 1998 investigated response to treatment, defined as a two‐point improvement in croup score, amongst 198 children aged three months to five years who were treated with budesonide, dexamethasone, or a combination of budesonide and dexamethasone in the emergency department for croup. There was no difference in response to treatment between those treated with dexamethasone and those treated with budesonide (RR 1.12, 95% CI 0.93 to 1.34; P = 0.22; 1 RCT, 134 children; Analysis 3.5).
3.5. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 5: Improvement (at 6 hours) by outpatient
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Compared to those treated with budesonide, children treated with dexamethasone were at a reduced risk of needing treatment with epinephrine (RR 0.45, 95% CI 0.21 to 0.96; P = 0.04, I² = 0%; 4 RCTs, 321 children; Analysis 3.6). Geelhoed 1995c and Johnson 1998 investigated the need for intubation/tracheostomy amongst children treated with dexamethasone or budesonide for croup. There were no events in either study (RD 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 2 RCTs, 145 children; Analysis 3.7). There was no difference in the need for additional glucocorticoids between children treated with dexamethasone and those treated with budesonide (RR 0.48, 95% CI 0.18 to 1.32; P = 0.15, I² = 0%; 3 RCTs, 240 children; Analysis 3.8).
3.6. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 6: Additional treatments: epinephrine
3.7. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 7: Additional treatments: intubation/tracheostomy
3.8. Analysis.

Comparison 3: Dexamethasone compared to budesonide, Outcome 8: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Of the six studies investigating dexamethasone compared to budesonide, three (50%) reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%) and one case each of hives and violent behaviour in the dexamethasone group (2/69, 2.9%).
Comparison 4: Dexamethasone compared to beclomethasone
See Table 5.
3. Dexamethasone compared to beclomethasone for croup .
| Dexamethasone compared to beclomethasone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone Comparison: beclomethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Beclomethasone | Dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RD 0.00 (−0.09 to 0.09) | 39 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| 0 per 1000 |
0 per 1000 (0 to 0) |
||||
| Adverse events (no events) | Eboriadou 2010 reported no adverse events related to the glucocorticoids. | 39 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise. cWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 investigated return visits to the emergency department amongst 39 children aged six months to five years treated with dexamethasone or beclomethasone for croup. There were probably no children that returned for additional care (RD 0.00, 95% CI −0.09 to 0.09; P = 1.00; 1 RCT, 39 children; moderate‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Dexamethasone compared to beclomethasone, Outcome 1: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies investigated the use of additional treatments for this comparison.
4. Any adverse events
Eboriadou 2010 investigated this comparison and reported no adverse events related to the glucocorticoids.
Comparison 5: Dexamethasone compared to betamethasone
See Table 6.
4. Dexamethasone compared to betamethasone for croup .
| Dexamethasone compared to betamethasone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone Comparison: betamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Betamethasone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) |
The mean change in croup score from 1 study was −1.68. | The mean change in croup score was 0.62 units in favour (1.17 more to 0.06 more). | ‐ | 52 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score from 1 study was −1.89. | The mean change in croup score was 0.67 units in favour (1.23 more to 0.11 more). | ‐ | 52 (1 RCT) | ⊕⊕⊝⊝ Lowb,c |
| Return visits or (re)admissions or both | Study population | RR 0.95 (0.67 to 1.34) | 52 (1 RCT) | ⊕⊕⊝⊝ Lowd | |
| 731 per 1000 |
694 per 1000 (490 to 979) |
||||
| Adverse events | Amir 2006 did not report collecting adverse events data. | 52 (1 RCT) | ⊕⊕⊝⊝ Lowa,e | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). cWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score. dWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit or harm for dexamethasone compared to betamethasone. eWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Amir 2006 investigated reduction in croup score for 52 children aged six months to six years were treated with dexamethasone or betamethasone in the emergency department for croup. Compared to betamethasone, dexamethasone may have resulted in a greater reduction in croup score after two hours (SMD −0.62, 95% CI −1.17 to −0.06; P = 0.03; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.1) and six hours (SMD −0.67, 95% CI −1.23 to −0.11; P = 0.02; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.2).
5.1. Analysis.

Comparison 5: Dexamethasone compared to betamethasone, Outcome 1: Croup score (change baseline ‐ 2 hours) by outpatient
5.2. Analysis.

Comparison 5: Dexamethasone compared to betamethasone, Outcome 2: Croup score (change baseline ‐ 6 hours) by outpatient
2. Return visits or (re)admissions to the hospital or both
Amir 2006 investigated re‐examinations by a primary care physician amongst 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. There may have been no difference in the rate of re‐examinations between dexamethasone and betamethasone groups (RR 0.95, 95% CI 0.67 to 1.34; P = 0.76; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.3).
5.3. Analysis.

Comparison 5: Dexamethasone compared to betamethasone, Outcome 3: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Amir 2006 investigated the need for epinephrine amongst 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. The risk for needing epinephrine was higher for those treated with dexamethasone compared to those treated with betamethasone (RR 2.11, 95% CI 1.18 to 3.76; P = 0.01; 1 RCT, 52 children; Analysis 5.4).
5.4. Analysis.

Comparison 5: Dexamethasone compared to betamethasone, Outcome 4: Additional treatments: epinephrine
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 6: Dexamethasone compared to prednisolone
See Table 7.
5. Dexamethasone compared to prednisolone for croup.
| Dexamethasone compared to prednisolone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone Comparison: prednisolone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Prednisolone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) |
The mean change in croup score from 1 study was −0.89. | The mean change in croup score was 0.06 units not in favour (0.06 more to 0.18 less). | ‐ | 1231 (1 RCT) | ⊕⊕⊕⊕ High |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score from 1 study was −2.35. | The mean change in croup score was 0.21 units not in favour (0.21 more to 0.62 less). | ‐ | 99 (1 RCT) | ⊕⊕⊕⊝ Moderatea |
| Return visits or (re)admissions or both | Study population | RR 0.55 (0.28 to 1.11) | 1537 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 212 per 1000 | 117 per 1000 (59 to 236) | ||||
| Adverse events | Fifoot 2007, Garbutt 2013, and Sparrow 2006 reported no serious adverse events related to the glucocorticoids. Parker 2019 reported 1 case of insomnia (1/411, 0.24%) and 13 cases of vomiting (13/411, 3.3%) in the prednisolone group, and 29 cases of vomiting (29/820, 3.5%), 1 case of 30‐second febrile convulsion (1/820, 0.1%), and 1 case of hyperactivity (1/820, 0.1%) in the dexamethasone group. | 1550 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 59%), and variation in point estimates. cWe downgraded by one level for imprecision. Narrative synthesis conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Two trials compared the effect of dexamethasone versus prednisolone. Parker 2019 found little to no difference at two‐hour postbaseline croup score assessment (SMD 0.06, 95% CI −0.06 to 0.18; P = 0.32; 1 RCT, 1231 children; high‐certainty evidence; Analysis 6.1). Fifoot 2007 found likely little to no difference between groups at six‐hour postbaseline croup score assessment (SMD 0.21, 95% CI −0.21 to 0.62; P = 0.33; 1 RCT, 99 children; moderate‐certainty evidence; Analysis 6.2).
6.1. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 1: Croup score (change baseline ‐ 2 hours) by outpatient
6.2. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 2: Croup score (change baseline ‐ 6 hours) by outpatient
2. Return visits or (re)admissions to the hospital or both
Dexamethasone probably reduced the rate of return visits or readmissions for croup by about half when compared to prednisolone (RR 0.55, 95% CI 0.28 to 1.11; P = 0.09, I² = 59%; 4 RCTs, 1537 children; moderate‐certainty evidence; Analysis 6.3).
6.3. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 3: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was little to no difference between groups in length of stay in the hospital or emergency department (MD −0.02, 95% CI −0.42 to 0.39; P = 0.94, I² = 12%; 2 RCTs, 1363 children; Analysis 6.4).
6.4. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 4: Length of stay by outpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
We found no difference in the addition of epinephrine to the treatment received by children treated with dexamethasone versus those treated with prednisolone (RR 0.90, 95% CI 0.50 to 1.64; P = 0.74, I² = 0%; 3 RCTs, 1463 children; high‐certainty evidence; Analysis 6.5). No child required intubation (RD 0.00, 95% CI −0.00 to 0.00; P = 1.00; 1 RCT, 1231 children; Analysis 6.6). However, there was a 28% reduction in the use of supplemental glucocorticoids as an additional treatment between children who received dexamethasone and those who received prednisolone (RR 0.72, 95% CI 0.53 to 0.97; P = 0.03, I² = 0%; 2 RCTs, 926 children; Analysis 6.7).
6.5. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 5: Additional treatments: epinephrine
6.6. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 6: Additional treatments: intubation/tracheotomy
6.7. Analysis.

Comparison 6: Dexamethasone compared to prednisolone, Outcome 7: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Although not specific to this comparison, Parker 2019 reported a few adverse events in four participants: one participant in the dexamethasone group had a febrile convulsion, and one participant in the prednisolone group had insomnia. Unlike Parker 2019, three trials did not report serious adverse events (Fifoot 2007; Garbutt 2013; Sparrow 2006).
Comparison 7: Budesonide compared to dexamethasone
See Table 8.
6. Budesonide compared to dexamethasone for croup.
| Budesonide compared to dexamethasone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: budesonide Comparison: dexamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone | Budesonide | ||||
| Adverse events |
Huang 2021 reported no adverse events. |
92 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies reported on change in clinical croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
No studies reported on return visits or (re)admission to the hospital (or both) for this comparison.
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies reported on length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies reported on the use of additional treatments for this comparison.
4. Any adverse events
Huang 2021 investigated the effect of inhaled budesonide versus dexamethasone in children with acute infectious laryngitis and found no adverse condition following treatment. The study authors did not report on any other outcomes relevant to this review.
Comparison 8: Budesonide and dexamethasone compared to dexamethasone
See Table 9.
7. Budesonide and dexamethasone compared to dexamethasone.
| Budesonide and dexamethasone compared to dexamethasone for croup | ||||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: budesonide and dexamethasone Comparison: dexamethasone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Dexamethasone | Budesonide and dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score was −3.24 to −1.80. | The mean change in croup score was 0.05 standard deviations not in favour (0.19 more to 0.30 less). | ‐ | 255 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | A standard deviation of 0.05 represents a minimal difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.91 (0.45 to 1.83) | 254 (3 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 100 per 1000 | 91 per 1000 (45 to 183) | |||||
| Adverse events | 1/3 (33%) studies reported collecting adverse events data. Klassen 1998 reported no adverse events in either the dexamethasone group or the dexamethasone and budesonide group. | 133 (1 RCTs) |
⊕⊕⊕⊝ Moderatec | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and a significant benefit or harm for dexamethasone and budesonide compared to dexamethasone alone. cWe downgraded by one level for imprecision. Narrative sythesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
There was probably no difference in reduction in croup score after six hours between children treated with combined dexamethasone and budesonide versus those treated with dexamethasone alone (SMD 0.05, 95% CI −0.19 to 0.30; P = 0.67, I² = 0%; 3 RCTs, 255 children; moderate‐certainty evidence; Analysis 7.1). There was no difference in effect by inpatient or outpatient status (Analysis 7.1).
7.1. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 1: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient
2. Return visits or (re)admissions to the hospital or both
There may have been no difference in the rate of admissions or return visits between children treated with combined dexamethasone and budesonide versus those treated with dexamethasone alone (RR 0.91, 95% CI 0.45 to 1.83; P = 0.79, I² = 0%; 3 RCTs, 254 children; low‐certainty evidence; Analysis 7.2). There was no subgroup difference in effect by inpatient or outpatient status (Analysis 7.2).
7.2. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 2: Return visits or (re)admissions or both by inpatient/outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no difference in hours spent in the hospital or emergency department amongst children treated with dexamethasone and budesonide versus those treated with dexamethasone alone (MD 0.44, 95% CI −0.05 to 0.92; P = 0.08, I² = 0%; 2 RCTs, 204 children; Analysis 7.3). There were no subgroup differences in effect by inpatient or outpatient status (Analysis 7.3).
7.3. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 3: Length of stay by inpatient/outpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
After six hours, there was no difference in the clinical improvement of children treated with dexamethasone and budesonide versus those treated with dexamethasone alone (RR 1.11, 95% CI 0.65 to 1.90; P = 0.70; 2 RCTs, 183 children; Analysis 7.4). This analysis only included outpatients (Analysis 7.4).
7.4. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 4: Improvement (at 6 hours) by outpatient
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference in the need for epinephrine (RR 1.42, 95% CI 0.27 to 7.39; P = 0.67, I² = 0%; 2 RCTs, 183 children; Analysis 7.5); a mist tent (RR 1.07, 95% CI 0.69 to 1.65; P = 0.77; 1 RCT, 50 children; Analysis 7.6); or supplemental glucocorticoids (RR 1.10, 95% CI 0.07 to 16.66; P = 0.95, I² = 66%; 2 RCTs, 182 children; Analysis 7.7) amongst children treated with dexamethasone and budesonide versus those treated with dexamethasone alone.
7.5. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 5: Additional treatments: epinephrine
7.6. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 6: Additional treatments: mist tent
7.7. Analysis.

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 7: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Klassen 1998 reported no adverse events in either the dexamethasone group or the dexamethasone and budesonide group.
Comparison 9: Budesonide and dexamethasone compared to budesonide
See Table 10.
8. Budesonide and dexamethasone compared to budesonide.
| Budesonide and dexamethasone compared to budesonide for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: budesonide and dexamethasone Comparison: budesonide | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Budesonide | Budesonide and dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score from 1 study was −2.30. | The mean change in croup score was 0.18 units in favour (0.52 more to 0.17 less). | ‐ | 129 (1 RCT) | ⊕⊕⊕⊝ Moderatea |
| Return visits or (re)admissions or both | Study population | RD 0.00 (−0.03 to 0.03) | 129 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Adverse events | Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group. | 129 (1 RCT) | ⊕⊕⊕⊝ Moderateb | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Klassen 1998 investigated children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was probably no difference in reduction in croup score after six hours amongst children treated with combined dexamethasone and budesonide versus those treated with budesonide alone (SMD −0.18, 95% CI −0.52 to 0.17; P = 0.32; 1 RCT, 129 children; moderate‐certainty evidence; Analysis 8.1).
8.1. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 1: Croup score (change baseline ‐ 6 hours) by outpatient
2. Return visits or (re)admissions to the hospital or both
Klassen 1998 investigated return visits to the emergency department amongst children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There were probably no events in either treatment group (RD 0.00, 95% CI −0.03 to 0.03; P = 1.00; 1 RCT, 129 children; moderate‐certainty evidence; Analysis 8.2).
8.2. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 2: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
Klassen 1998 investigated hours spent in the emergency department amongst children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There was no difference in length of stay amongst children treated with dexamethasone and budesonide versus those treated with budesonide alone (MD 0.25, 95% CI −0.36 to 0.86; P = 0.42; 1 RCT, 129 children; Analysis 8.3).
8.3. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 3: Length of stay by outpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
Klassen 1998 investigated response to treatment, defined as a two‐point reduction in croup score, amongst children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no difference in response to treatment amongst children treated with dexamethasone and budesonide versus those treated with budesonide alone (RR 0.97, 95% CI 0.79 to 1.20; P = 0.80; 1 RCT, 129 children; Analysis 8.4).
8.4. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 4: Improvement (at 6 hours) by outpatient
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Klassen 1998 investigated the need for additional treatments amongst children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no difference between groups in the need for epinephrine (RR 1.02, 95% CI 0.15 to 6.99; P = 0.99; 1 RCT, 129 children; Analysis 8.5) or supplemental glucocorticoids (RR 1.31, 95% CI 0.52 to 3.29; P = 0.57; 1 RCT, 129 children; Analysis 8.6).
8.5. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 5: Additional treatments: epinephrine
8.6. Analysis.

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 6: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group.
Comparison 10: Oral compared to intramuscular dexamethasone
See Table 11.
9. Oral dexamethasone compared to intramuscular dexamethasone for croup .
| Oral dexamethasone compared to intramuscular dexamethasone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: oral dexamethasone Comparison: intramuscular dexamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Intramuscular dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.81 (0.58 to 1.12) | 440 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 259 per 1000 | 210 per 1000 (150 to 290) | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The effect estimate included both a null effect and substantial benefit for oral compared to intramuscular dexamethasone.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
There was probably no difference in the rate of return visits or admissions following treatment with oral dexamethasone compared to intramuscular dexamethasone (RR 0.81, 95% CI 0.58 to 1.12; P = 0.21, I² = 0%; 3 RCTs, 440 children; moderate‐certainty evidence; Analysis 9.1). The analysis only included outpatients (Analysis 9.1).
9.1. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 1: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
Donaldson 2003 investigated clinical improvement, defined as parents' assessment that their child's condition had improved at least somewhat after 24 hours, amongst children aged three to 84 months treated in the emergency department with oral or intramuscular dexamethasone for croup. There was no difference between groups in rate of clinical improvement (RR 1.07, 95% CI 0.95 to 1.19; P = 0.27; 1 RCT, 95 children; Analysis 9.2).
9.2. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 2: Improvement (at 24 hours) by outpatient
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference in the need for antibiotics (RR 0.14, 95% CI 0.02 to 1.15; P = 0.07; 1 RCT, 277 children; Analysis 9.3); epinephrine (RR 0.94, 95% CI 0.71 to 1.24; P = 0.64, I² = 0%; 2 RCTs, 372 children; Analysis 9.4); a mist tent (RR 1.34, 95% CI 0.31 to 5.89; P = 0.70; 1 RCT, 277 children; Analysis 9.5); or supplemental glucocorticoids (RR 1.10, 95% CI 0.50 to 2.41; P = 0.81; 1 RCT, 277 children; Analysis 9.6) amongst children treated with oral dexamethasone versus those treated with intramuscular dexamethasone.
9.3. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 3: Additional treatments: antibiotics
9.4. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 4: Additional treatments: epinephrine
9.5. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 5: Additional treatments: mist tent
9.6. Analysis.

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 6: Additional treatments: supplemental glucocorticoids
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 11: Oral compared to nebulised dexamethasone
See Table 12.
10. Oral dexamethasone compared to nebulised dexamethasone for croup.
| Oral dexamethasone compared to nebulised dexamethasone for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: oral dexamethasone Comparison: nebulised dexamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Nebulised dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.39 (0.17 to 0.89) | 176 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| 209 per 1000 | 81 per 1000 (35 to 186) | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Luria 2001 investigated returns to medical care of children aged six months to six years following treatment with oral or nebulised dexamethasone in the emergency department for croup. There were probably fewer return visits to medical care amongst those treated with oral dexamethasone versus those treated with nebulised dexamethasone (RR 0.39, 95% CI 0.17 to 0.89; P = 0.03; 1 RCT, 176 children; moderate‐certainty evidence; Analysis 10.1).
10.1. Analysis.

Comparison 10: Oral compared to nebulised dexamethasone, Outcome 1: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies investigated the use of additional treatments for this comparison.
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 12: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg
See Table 13.
11. Dexamethasone 0.30 mg/kg compared to dexamethasone 0.15 mg/kg for croup.
| Dexamethasone 0.30 mg/kg compared to dexamethasone 0.15 mg/kg for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone 0.30 mg/kg Comparison: dexamethasone 0.15 mg/kg | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.30 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 0.94 (0.06 to 14.27) | 60 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| 34 per 1000 | 32 per 1000 (2 to 492) | ||||
| Adverse events | Geelhoed 1995b did not report collecting adverse events data. | 60 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential harm for 0.30 mg/kg compared to 0.15 mg/kg dexamethasone. bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995b investigated re‐presentations to medical care for croup amongst children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone. There may have been no difference in the rate of re‐presentations to medical care between groups (RR 0.94, 95% CI 0.06 to 14.27; P = 0.96; 1 RCT, 60 children; low‐certainty evidence; Analysis 11.1).
11.1. Analysis.

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 1: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Geelhoed 1995b investigated the need for additional treatments amongst children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.43, 95% CI 0.19 to 0.98; P = 0.05; 1 RCT, 60 children; Analysis 11.2). No child required supplemental glucocorticoids (RD 0.00, 95% CI −0.06 to 0.06; P = 1.00; 1 RCT, 60 children; Analysis 11.3).
11.2. Analysis.

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 2: Additional treatments: epinephrine
11.3. Analysis.

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 3: Additional treatments: supplemental glucocorticoids
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg
See Table 14.
12. Dexamethasone 0.60 mg/kg compared to dexamethasone 0.30 mg/kg for croup.
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.30 mg/kg for croup | |||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone 0.60 mg/kg Comparison: dexamethasone 0.30 mg/kg | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone 0.30 mg/kg | Dexamethasone 0.60 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 1.40 (0.25 to 7.81) | 60 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| 69 per 1000 | 97 per 1000 (17 to 539) | ||||
| Adverse events | Geelhoed 1995a did not report collecting adverse events data. | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential for harm for 0.60 mg/kg compared to 0.30 mg/kg dexamethasone. bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995a investigated re‐presentations to medical care for croup amongst children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone. There may have been no difference in the rate of re‐presentations to medical care amongst children treated with 0.60 mg/kg versus 0.30 mg/kg dexamethasone (RR 1.40, 95% CI 0.25 to 7.81; P = 0.70; 1 RCT, 60 children; low‐certainty evidence; Analysis 12.1).
12.1. Analysis.

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 1: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Geelhoed 1995a investigated the need for additional treatments amongst children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.78, 95% CI 0.27 to 2.28; P = 0.65; 1 RCT, 60 children; Analysis 12.2) or supplemental glucocorticoids (RR 2.81, 95% CI 0.12 to 66.40; P = 0.52; 1 RCT, 60 children; Analysis 12.3).
12.2. Analysis.

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 2: Additional treatments: epinephrine
12.3. Analysis.

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 3: Additional treatments: supplemental glucocorticoids
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 14: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg
See Table 15.
13. Dexamethasone 0.60 mg/kg compared to dexamethasone 0.15 mg/kg for croup.
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.15 mg/kg for croup | ||||||
| Patient or population: children with croup Setting: emergency department, inpatients and outpatients Intervention: dexamethasone 0.60 mg/kg Comparison: dexamethasone 0.15 mg/kg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.60 mg/kg | |||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) |
The mean change in croup score was −1.05 to −0.75. | The mean change in croup score was 0.27 standard deviations in favour (0.76 more to 0.22 less). | ‐ | 861 (2 RCTs) | ⊕⊕⊕⊕ High | A standard deviation of 0.14 represents a small difference between groups. |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) |
The mean change in croup score was −3.10 to −2.09. | The mean change in croup score was 0.45 units in favour (1.26 more to 0.35 less). | ‐ | 178 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) |
The mean change in croup score was −3.50 to −2.95. | The mean change in croup score was 0.60 units in favour (4.39 more to 3.19 less). | ‐ | 113 (2 RCTs) | ⊕⊝⊝⊝ Very Lowb,c | |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) |
The mean change in croup score from 1 study was −4.00. | The mean change in croup score was 0.63 units not in favour (0.16 less to 1.10 less). | ‐ | 72 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Return visits or (re)admissions or both | Study population | RR 0.91 (0.71 to 1.17) | 949 (3 RCTs) | ⊕⊕⊕⊕ High | ||
| 208 per 1000 | 189 per 1000 (148 to 243) | |||||
| Adverse events | Parker 2019 reported 16 cases of vomiting (16/410, 4.0%) and 1 case of 30 seconds of febrile convulsion (1/410, 0.2%) in the 0.60 mg/kg dexamethasone group, and 13 cases of vomiting (13/410 (3.3%), 1 case of stridor (1/410, 0.2%), and 1 case of hyperactivity (1/410, 0.2%) in the 0.15 mg/kg dexamethasone group. Alshehr 2005 reported 1 case of bacterial tracheitis and 2 cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%) and no adverse events in the 0.15 mg/kg dexamethasone group. Chub‐Uppakarn 2007 and Fifoot 2007 reported no adverse events in either treatment group. | 170 (3 RCTs) | ⊕⊕⊕⊝ Moderated | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by two levels level for inconsistency. There was considerable heterogeneity (I² = 99%), and variation in point estimates. The 95% confidence intervals did not overlap. cWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit and harm for 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. dWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
There was no reduction at two hours (SMD −0.27, 95% CI −0.76 to 0.22; P = 0.28, I² = 62%; 2 RCTs, 861 children; high‐certainty evidence; Analysis 13.1). There was probably no difference at six hours (SMD −0.45, 95% CI −1.26 to 0.35; P = 0.27, I² = 85%; 3 RCTs, 178 children; moderate‐certainty evidence; Analysis 13.2). We do not know if there was no difference at 12 hours (SMD −0.60, 95% CI −4.39 to 3.19; P = 0.76, I² = 98%; 2 RCTs, 113 children; very low‐certainty evidence; Analysis 13.3). We found that treating children with 0.60 mg/kg versus 0.15 mg/kg dose of dexamethasone probably reduced the severity of croup scores at 24‐hour postbaseline score (SMD 0.63, 95% CI 0.16 to 1.10; P = 0.009; 1 RCT, 72 children; moderate‐certainty evidence; Analysis 13.4).
13.1. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 1: Croup score (Westley) (change baseline ‐ 2 hours) by inpatient/outpatient
13.2. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 2: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient
13.3. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 3: Croup score (change baseline ‐ 12 hours) by inpatient/outpatient
13.4. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 4: Croup score (change baseline ‐ 24 hours) by outpatient
2. Return visits or (re)admissions to the hospital or both
There was little to no difference in return visits or (re)admissions or both between children treated with dexamethasone 0.60 mg/kg versus 0.15 mg/kg (RR 0.91, 95% CI 0.71 to 1.17; P = 0.48, I² = 0%; 3 RCTs, 949 children; high‐certainty evidence; Analysis 13.5).
13.5. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 5: Return visits or (re)admissions or both by outpatient
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was little to no difference in hours spent at the outpatient clinic between children treated with dexamethasone 0.60 mg/kg versus 0.15 mg/kg (MD 0.12, 95% CI −0.32 to 0.56; P = 0.59, I² = 0%; 2 RCTs, 892 children; Analysis 13.6).
13.6. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 6: Length of stay by outpatient
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
We found no difference in the need of additional treatments between treating children with croup with dexamethasone at 0.60 mg/kg versus 0.15 mg/kg in cases of epinephrine use (RR 0.78, 95% CI 0.34 to 1.75; P = 0.54, I² = 0%; 2 RCTs, 885 children; Analysis 13.7); intubation (RD 0.00, 95% CI −0.00 to 0.00; P = 1.00, I² = 0%; 2 RCTs, 861 children; Analysis 13.8); or use of supplemental glucocorticoids (RR 0.77, 95% CI 0.51 to 1.15; P = 0.19, I² = 0%; 2 RCTs, 617 children; Analysis 13.9).
13.7. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 7: Additional treatments: epinephrine
13.8. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 8: Additional treatments: intubation/tracheotomy
13.9. Analysis.

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 9: Additional treatments: supplemental glucocorticoids
4. Any adverse events
Of the four studies investigating dexamethasone at 0.60 mg/kg versus dexamethasone at 0.15 mg/kg, two (50%) reported no adverse events in either treatment group (Chub‐Uppakarn 2007; Fifoot 2007). Parker 2019 reported 16 cases of vomiting (16/410, 4.0%) and one case of 30 seconds of febrile convulsion (1/410, 0.2%) in the 0.60 mg/kg dexamethasone group, and 13 cases of vomiting (13/410, 3.3%), one case of stridor (1/410, 0.2%), and one case of hyperactivity 30 minutes after the dose (1/410, 0.2%) in the 0.15 mg/kg dexamethasone group. Alshehr 2005 reported one case of bacterial tracheitis and two cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%), and no adverse events in the 0.15 mg/kg dexamethasone group.
Publication bias
The publication bias for change in croup score (at six hours), and return visits or (re)admissions to the hospital (or both), for glucocorticoids compared to placebo remains the same as in the previous version of this review (Gates 2018). Insufficient numbers of included studies precluded testing for publication bias for any of the other comparisons or outcomes.
Discussion
This updated review includes 45 RCTs with a total of 5888 children. This is an increase of two RCTs with 1323 children from the last update (Huang 2021; Parker 2019). Parker 2019 reported relevant data to update the existing evidence on the effects of glucocorticoid compared to prednisolone, and the optimal dosage of glucocorticoid for the treatment of croup. Huang 2021 reported only on adverse events related to the use of budesonide compared to dexamethasone for croup.
Summary of main results
Any glucocorticoid compared to placebo
Twenty‐six studies investigated glucocorticoids compared to placebo. Glucocorticoids may have reduced the symptoms of croup within two hours of treatment, with the effect lasting at least 24 hours. The effect was dependent on the glucocorticoid administered. Budesonide and dexamethasone reduced the symptoms of croup within two hours of treatment, with the effect lasting at least 24 hours. One trial showed that prednisolone reduced the symptoms of croup within six hours, with the effect lasting at least 12 hours. One trial showed that fluticasone did not reduce the symptoms of croup after two, six, or 24 hours compared to placebo. The certainty of the evidence for the effect of glucocorticoids compared to placebo for reducing the symptoms of croup from two to 12 hours was low, as there was considerable between‐study heterogeneity in effect estimates (Table 1). The certainty of the evidence for the effect of glucocorticoids compared to placebo for reducing the symptoms of croup after 24 hours was very low, as there was considerable between‐study heterogeneity in the magnitude and direction of the effect (Table 1).
Compared to placebo, both budesonide and dexamethasone may have reduced the rate of return visits and/or (re)admissions to the hospital or emergency department. The certainty of the evidence for the effect of glucocorticoids in reducing the rate of return visits or (re)admissions or both was low, as there was considerable between‐study heterogeneity in the effect estimates (Table 1).
Compared to placebo, glucocorticoids reduced length of stay in the hospital by approximately 15 hours and resulted in clinical improvement in a greater proportion of children after six hours. The effect lasted at least 24 hours. There was little to no difference in the need for additional treatments between children treated with glucocorticoids and those treated with placebo. Treatment with glucocorticoids was infrequently associated with serious adverse events.
Any glucocorticoid compared to epinephrine
Four studies investigated glucocorticoids compared to epinephrine. We do not know if there was no difference in the reduction in symptoms of croup for children treated with epinephrine compared to those treated with glucocorticoids at two and six hours. There may not have been a reduction in croup symptoms at 12 or 24 hours following administration of glucocorticoids or epinephrine. After two hours, the effect was dependent on the glucocorticoid administered. Epinephrine resulted in greater reductions in symptoms of croup compared to beclomethasone and dexamethasone. There was little no difference in reduction in croup symptoms between epinephrine and budesonide two hours after treatment. The certainty of the evidence for the effect of glucocorticoids compared to epinephrine for reducing the symptoms of croup was very low to low. The sample sizes for the six‐, 12‐, and 24‐hour analyses were small, and there was considerable between‐study heterogeneity in effect estimates for the six‐hour analysis. There was considerable between‐study heterogeneity in the magnitude and direction of the effect estimates for the two‐hour analysis; the sample size for the comparison was small; and the pooled effect estimate was imprecise (Table 2).
There may have been no difference in the rate of return visits or (re)admissions or both following treatment with glucocorticoids compared with epinephrine. The certainty of the evidence for the effect of glucocorticoids compared to epinephrine for reducing the rate of return visits or (re)admissions or both was low, as the sample size did not meet the optimal information size, and the contributing studies reported no events (Table 2).
There was little to no difference in length of stay in the hospital for children treated with glucocorticoids compared to those treated with epinephrine, nor were there any differences between groups in the need for additional treatments. One study reported a 31.3% rate of secondary bacterial infections amongst children treated with dexamethasone. Another study reported a 16% rate of tremor and tachycardia amongst children treated with epinephrine.
One glucocorticoid compared to another glucocorticoid
Thirteen studies investigated one glucocorticoid compared to another glucocorticoid. Compared to budesonide, dexamethasone may have resulted in greater reductions in symptoms of croup after six and 12 hours. The certainty of the evidence for the effect of dexamethasone compared to budesonide for reducing the symptoms of croup was low, as the contributing studies were all at high or unclear risk of bias; there was substantial between‐study heterogeneity in effect estimates for the six‐hour analysis; and the sample size did not meet the optimal information size for the 12‐hour analysis (Table 4). Compared to betamethasone, dexamethasone may have resulted in greater reductions in symptoms of croup after two and six hours. The certainty of the evidence for the effect of dexamethasone compared to betamethasone for reducing the symptoms of croup was low, as the only study contributing to the analysis was at high risk of bias and had a small sample size (Table 6). There was no difference in the reduction in symptoms of croup two hours following treatment with dexamethasone or prednisolone, and likely no difference at six hours. The certainty of the evidence for the effect of dexamethasone compared to prednisolone for reducing the symptoms of croup was moderate, as the only study that contributed to the analysis had a small sample size (Table 7).
There was probably no difference between dexamethasone and budesonide in the rate of return visits or (re)admissions or both. The certainty of the evidence for the effect of dexamethasone compared to budesonide for reducing the rate of return visits or (re)admissions or both was moderate, as few events were reported, and the effect estimate included the null effect as well as considerable benefit for dexamethasone compared to budesonide (Table 4). There was probably no difference between dexamethasone and beclomethasone, and there may have been no difference between dexamethasone and betamethasone in the rate of return visits or (re)admissions or both. The certainty of the evidence for the effect of dexamethasone compared to beclomethasone for reducing the rate of return visits or (re)admissions or both was moderate, as the only study that contributed to the analysis had a small sample size and reported no events (Table 5). The certainty of the evidence for the effect of dexamethasone compared to betamethasone for reducing the rate of return visits or (re)admissions or both was low, as only one small study contributed to the analysis, and the effect estimate included the null effect as well as appreciable benefit and harm (Table 6). Compared to prednisolone, dexamethasone probably reduced the rate of return visits or (re)admissions or both by about 50%. The certainty of the evidence for the effect of dexamethasone compared to prednisolone for reducing the rate of return visits or (re)admissions or both was moderate, as the sample size did not reach the optimal information size (Table 7). The addition of data from Parker 2019 attenuated the magnitude of the difference (61%) previously reported in this comparison category.
There was no difference in length of stay in the hospital or emergency department between children treated with dexamethasone compared to budesonide, or dexamethasone compared to prednisolone. One study showed no difference in clinical improvement between children treated with dexamethasone and those treated with budesonide. Compared to those treated with budesonide, children treated with dexamethasone were at a reduced risk for needing epinephrine. There was no difference between children treated with dexamethasone and those treated with budesonide in need for intubation or supplemental glucocorticoids. Compared to those treated with betamethasone, children treated with dexamethasone were at a increased risk for needing epinephrine. There was no difference between children treated with dexamethasone and those treated with prednisolone in the need for epinephrine. However, there was a 28% reduction in the use of supplemental glucocorticoids as an additional treatment. Adverse events were infrequently reported.
Huang 2021 was the only study that investigated the effect of inhaled budesonide versus dexamethasone. The study authors found no adverse condition following the treatment of infectious laryngitis with inhaled budesonide or dexamethasone. They did not report on change in clinical croup scores between baseline and 2, 6, 12, and/or 24 hours, or any other outcomes relevant to this review.
One glucocorticoid compared to a combination of glucocorticoids
Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. There was probably no difference in reduction in symptoms of croup for children treated with dexamethasone compared to combined dexamethasone and budesonide, and probably no difference for children treated with budesonide compared to combined budesonide and dexamethasone. The certainty of the evidence for the effect of dexamethasone compared to dexamethasone and budesonide for reducing the symptoms of croup was moderate, as the sample size for the analysis did not meet the optimal information size (Table 10). The certainty of the evidence for the effect of budesonide compared to budesonide and dexamethasone for reducing the symptoms of croup was moderate (Table 10), as only one small study contributed to the analysis.
There may have been no difference in rate of return visits or (re)admissions to the hospital or both following treatment with dexamethasone compared to combined dexamethasone and budesonide, and there was probably no difference for this outcome following treatment with budesonide compared to combined budesonide and dexamethasone. The certainty of the evidence for the effect of dexamethasone compared to dexamethasone and budesonide for reducing the rate of return visits or (re)admissions or both was low (Table 9), as the sample size for the analysis did not meet the optimal information size; there were few events; and the estimate was imprecise. The certainty of the evidence for the effect of budesonide compared to budesonide and dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate (Table 10), as only one small study contributed to the analysis.
There was no difference in hours spent in the hospital or emergency department, clinical improvement, or the need for additional treatments for children treated with dexamethasone compared to those treated with combined dexamethasone and budesonide, nor for children treated with budesonide compared to combined budesonide and dexamethasone. Only one study collected adverse events data, which included one case (1.5%) of oral thrush in the budesonide group and no events in the budesonide and dexamethasone group (Klassen 1998).
Glucocorticoids given by different modes of administration
Five studies investigated dexamethasone given by different modes of administration. There was probably no difference in the rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to those treated with intramuscular dexamethasone. There was probably a reduced rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to those treated with nebulised dexamethasone. The certainty of the evidence for the effect of oral compared to intramuscular dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate, as the contributing studies reported few events, and the estimate was imprecise (Table 11). The certainty of the evidence for the effect of oral compared to nebulised dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate, as only one study contributed to the analysis, and the sample size did not meet the optimal information size (Table 12).
There was no difference in clinical improvement or in the need for additional treatments between children treated with oral dexamethasone and those treated with intramuscular dexamethasone. None of the studies comparing dexamethasone given by different modes of administration reported collecting adverse events data.
Dexamethasone given in different doses
Five studies investigated dexamethasone given in different doses. There was no reduction in croup score after two hours for inpatients treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone. There was probably no reduction in croup score after six hours for children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone. After 12 hours, we do not know if there was no difference in the change in croup score amongst children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. The effect differed by inpatient and outpatient status. One study showed that there was probably a reduction in croup score with 0.60 mg/kg after 24 hours (Alshehr 2005).
In inpatients, the 0.60 mg/kg dose resulted in a greater reduction in croup score after 12 hours, whereas in outpatients, the 0.15 mg/kg dose was more effective. One study investigated change in croup score after 24 hours for inpatients treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone (Alshehr 2005). Children treated with 0.15 mg/kg probably experienced greater reductions in croup score after 24 hours compared to those treated with 0.60 mg/kg dexamethasone. The certainty of the evidence for the effect of 0.60 mg/kg dexamethasone compared to 0.15 mg/kg dexamethasone for reducing croup score was very low to high (Table 15). The six‐hour analysis included three studies, but the sample size did not meet the optimal information size. In the 12‐hour analysis, there was considerable between‐study heterogeneity in effect estimates, and the sample sizes did not meet the optimal information size. In the 12‐hour analysis, the pooled effect estimate included the null effect as well as appreciable benefit and harm. The 24‐hour analysis included only one study with a small sample size.
There may have been no difference in the rate of return visits or (re)admissions or both for children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone and 0.60 mg/kg compared to 0.30 mg/kg dexamethasone. There was no difference in the rate of return visits or (re)admissions for children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. The certainty of the evidence was low for the effect of 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Table 13), and 0.60 mg/kg compared to 0.30 mg/kg dexamethasone (Table 14), for reducing the rate of return visits or (re)admissions or both for croup, as the analysis included only one small study that reported few events, and the effect estimate included benefit, the null effect, and potential for harm. The certainty of the evidence for the effect of 0.60 mg/kg compared to 0.15 mg/kg dexamethasone on return visits or (re)admissions or both was high (Table 15).
Likewise, we found no difference in length of stay in the hospital or emergency department for children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. There was no difference in the need for additional treatments between children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone; 0.60 mg/kg compared to 0.30 mg/kg dexamethasone; or 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. Adverse events were infrequently reported for the 0.15 mg/kg and 0.60 mg/kg doses of dexamethasone.
Overall completeness and applicability of evidence
We searched for RCTs that compared glucocorticoids to placebo, or any other active pharmacologic treatment for croup. However, in this update we found only two new studies that investigated one glucocorticoid compared to another glucocorticoid (Huang 2021; Parker 2019), and one study that investigated glucocorticoids given in different doses (Parker 2019). Overall, the number of included studies was large (n = 45), of which 26 (58%) investigated glucocorticoids compared to placebo. Only four studies investigated glucocorticoids compared to epinephrine; 13 investigated one glucocorticoid compared to another glucocorticoid; three investigated one glucocorticoid compared to a combination of glucocorticoids; five investigated glucocorticoids given by different modes of administration; and five investigated glucocorticoids given in different doses. Most studies (67%) reported a change in croup score for at least one time point, and 58% used the Westley croup score (Westley 1978), which has been shown to be a valid and reliable measure of croup severity. Most studies (51%) investigated outpatients presenting to emergency departments or outpatient clinics, generally with mild to moderate croup. In a study conducted by Rosychuk 2010 that described the epidemiology of croup presentation to emergency departments within the Alberta, Canada emergency databases, less than 6% of children presenting to the emergency department with croup symptoms required hospitalisation. We have therefore presented subgroup analyses by inpatient or outpatient setting as a form of sensitivity analysis because of the possible overrepresentation of studies with inpatient cases of croup. However, the findings from these subgroup analyses should be interpreted with caution.
We found no evidence of publication bias for our two primary outcomes: change in croup score (at six hours), and return visits or (re)admissions to the hospital or both for glucocorticoids compared to placebo.
Certainty of the evidence
This systematic review included 45 RCTs of 5888 children. Most studies were at unclear or high overall risk of bias (98%). We assessed risk of bias for random sequence generation as low in 60% of studies. The allocation sequence was adequately concealed between randomisation and assignment to treatment groups in 42% of studies. We were unable to ascertain whether the conduct of these studies was methodologically flawed. However, based on the information provided in the publications, we cannot exclude the possibility of selection bias. Empirically, selection bias has been associated with exaggerated estimates of treatment effects (Jüni 2001; Wood 2008). Inadequate allocation concealment is more likely to result in biased estimates of treatment effects when the outcomes of a study are subjective (Wood 2008). Croup score, one of our primary outcomes, is typically assessed by the healthcare provider, and interobserver variability has been reported to be fair to moderate (Chan 2001). Hartling 2014 demonstrated that the association between selection bias and the estimate of treatment effects may not hold true for RCTs in child health. We are therefore uncertain as to how selection bias may have impacted our results.
More than half (58%) of the included studies were at low risk of bias for blinding of participants and personnel, and 60% were at low risk for blinding of outcome assessors. Many of the studies judged as at unclear risk of bias for the blinding domains were described as "blind" or "double‐blind". However, details about who was blinded or how (or both) were omitted from the publications. Whilst it is possible that these studies were well conducted but inadequately reported, we cannot confidently exclude the potential for performance and detection bias. In eight (18%) studies, participants and personnel were not blinded. All of these studies but one investigated glucocorticoids given via different modes of administration (e.g. orally, intramuscularly, nebulised), therefore blinding participants and personnel to the treatment assignment would not have been feasible. Studies that are not blinded or that are inadequately blinded can result in exaggerated estimates of treatment effects (Wood 2008). This association may not be true for RCTs in child health (Hartling 2014), therefore we are uncertain as to how the inclusion of unblinded or inadequately blinded trials may have impacted our results.
Most studies (60%) were at low risk of bias for incomplete outcome data. Although most studies (93%) were at unclear risk of bias for selective reporting, the outcomes reported in the results matched those reported in the methods sections of the publications in most cases.
For the comparison any glucocorticoid versus placebo, we detected between‐study heterogeneity in point estimates of effect as well as heterogeneity in the pooled estimates of effect by glucocorticoid for change in croup score. For this reason, we downgraded the certainty of the evidence for change in croup score after 2, 6, 12, and 24 hours. With respect to the estimates for individual glucocorticoids, after two hours the between‐study estimates for budesonide were heterogeneous. Two studies showed a clear benefit for dexamethasone, whilst Johnson 1996 showed the potential for no difference in effect between dexamethasone and placebo. Between‐study estimates for the effectiveness of budesonide compared to placebo after 6, 12, and 24 hours showed a consistent beneficial effect. For dexamethasone, between‐study estimates were highly heterogeneous at all time points and included the potential for benefit, no effect, or harm compared to placebo. In future updates of this review, we may use meta‐regression analyses to explore factors that could explain at least some of the observed heterogeneity (e.g. the 'effective' dosage of the active comparator). If such an analysis is deemed important to clinicians and researchers, it should be planned and documented a priori before future updates of this review. Only one very small study (N = 17) investigated croup score for fluticasone compared to placebo 2, 6, and 24 hours after treatment (Roorda 1998). Another single study (N = 42) investigated croup score for prednisolone compared to placebo 6 and 12 hours after treatment (Massicotte 1973). We caution against drawing any conclusions based on the evidence from these small, single studies.
Accounting for the pooled estimates of effect by glucocorticoid, the test for subgroup differences between the effects of budesonide, dexamethasone, and fluticasone two hours following their administration indicated marginal differences in croup scores (P = 0.06). Whilst fluticasone (based on one study) compared to placebo showed no reduction in croup scores (P = 0.36), the pooled effect estimate for budesonide indicated a reduction in croup scores (P = 0.005), and a marginal reduction for dexamethasone (P = 0.06). There was a subgroup difference in effect between budesonide, dexamethasone, fluticasone, and prednisolone six hours following their administration (P = 0.009). This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone, and fluticasone (based on one study) had no effect (P = 0.90). There was a subgroup difference in effect between budesonide, dexamethasone, and prednisolone 12 hours following their administration (P = 0.006). This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone. There was a subgroup difference in effect between budesonide, dexamethasone, and fluticasone 24 hours following their administration (P = 0.01). This was accounted for by the fact that the effect estimate for fluticasone (based on one study) indicated no effect (P = 0.36), whilst the pooled estimates for budesonide and dexamethasone both showed beneficial effects.
For the comparison any glucocorticoid versus placebo, we also downgraded the certainty of the evidence for return visits or (re)admissions or both due to inconsistency. There was little evidence that publication bias influenced our results for return visits or (re)admissions or both.
Similar threats to the certainty of the evidence were present in the other 12 comparisons in this review, including concerns regarding risk of bias, inconsistency, and imprecision. Aside from the comparison of glucocorticoids versus placebo, for which seven to 11 RCTs made up the analyses for the primary outcomes, all of the other comparisons included between one and five studies. Combined with the fact that the studies mostly included small samples of children (median n = 72, interquartile range 54 to 99), many analyses had to be downgraded due to imprecision, as the optimal information size criteria were not met. Since many of the analyses contained only one or two small RCTs, we caution against drawing any firm conclusions from the results of these few small studies. There exist very few within‐study comparisons of one glucocorticoid compared to another, of glucocorticoids given by different modes of administration, or of different doses of the same glucocorticoid.
Potential biases in the review process
We know that the risk of bias of studies used in a meta‐analysis is crucial to the certainty of evidence produced. This may affect the translation and uptake of research evidence to practice. We used the Cochrane risk of bias tool to assess the risk of bias of all included studies, and judged most of the studies as at unclear or high risk of bias. We were unable to update some of the other comparisons due to a lack of new data. These comparison categories are highlighted in the Results section. The lack of new data in these areas may signal areas where more RCTs on glucocorticoids and croup are needed. We also know that some comparisons may not warrant new RCTs at this time because of the considerable high‐certainty evidence that is available. To the best of our knowledge, 26 RCTs have compared any glucocorticoids versus placebo, and the findings have been consistently in support of the use of glucocorticoids in the treatment of croup (Gates 2018). That said, other areas that may benefit from more RCTs are comparisons around the lowest optimal glucocorticoids dose, and the most effective mode of administration.
Agreements and disagreements with other studies or reviews
This update strengthens evidence from previous review publications that supported the use of a lower dose of glucocorticoids (Chub‐Uppakarn 2007; Geelhoed 1995). It would be preferable to treat croup with the lowest effective dose of glucocorticoids to limit steroid exposure in these children. Data from Parker 2019 and Chub‐Uppakarn 2007 suggest that 0.15 mg/kg is as effective in the treatment of croup as 0.60 mg/kg. Their findings are also supported by Geelhoed 1995, which found no difference in return visits or (re)admissions amongst children treated for croup using 0.30 mg/kg compared to 0.15 mg/kg. Likewise, there was no difference between the two treatment groups in the need for additional treatment with epinephrine or supplemental glucocorticoid.
Authors' conclusions
Implications for practice.
The evidence has not changed that glucocorticoids reduce symptoms of croup at two hours, which may last up until 24 hours; shorten hospital stays; and reduce the rate of return visits or (re)admission.
Apart from dexamethasone and prednisolone, we found insufficient data to draw conclusions about the role of other glucocorticoids (e.g. fluticasone, beclomethasone) for reducing the symptoms of croup. Adverse events were reported from the use of glucocorticoids in some of the included studies.
Implications for research.
This update further strengthens the evidence base for the effectiveness of glucocorticoids in the treatment of croup. Dexamethasone reduces return visits or (re)admission of croup by about half. A small dose of dexamethasone at 0.15 mg/kg may be as effective as the current standard dose of 0.60 mg/kg. More randomised controlled trials are needed to strengthen the evidence for the effectiveness of low‐dose dexamethasone at 0.15 mg/kg to treat croup.
The findings of this update review are in keeping with previous versions of the review which asserted that additional trials assessing the effectiveness of dexamethasone and budesonide compared to placebo are not warranted. The cumulative meta‐graph by year for change in croup score six hours after treatment shows that the standardised mean difference for the effect of glucocorticoids compared to placebo has been stable (Figure 4). Accordingly, we also found no new studies published since 1999 that reported on this outcome for this comparison. For return visits or (re)admissions or both, the cumulative meta‐graph by year indicates that the pooled risk ratio has also been relatively constant (Figure 5). No new trials reporting on this outcome for this comparison have been published since 2004.
4.
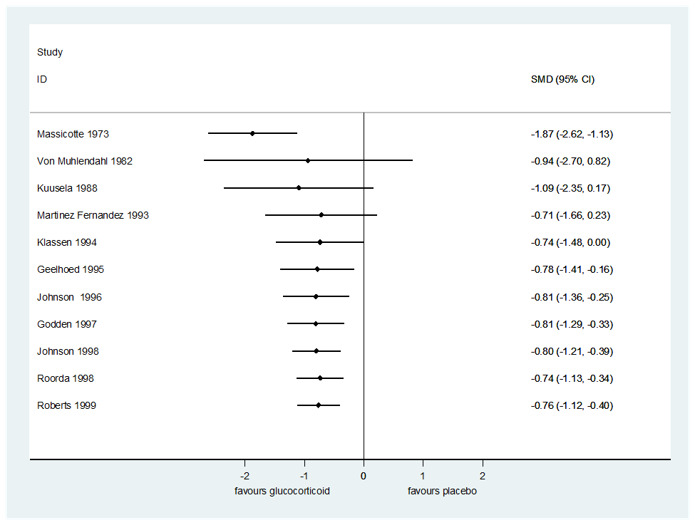
Cumulative meta‐graph by year for change in croup score six hours after treatment for any glucocorticoid compared to placebo.
5.
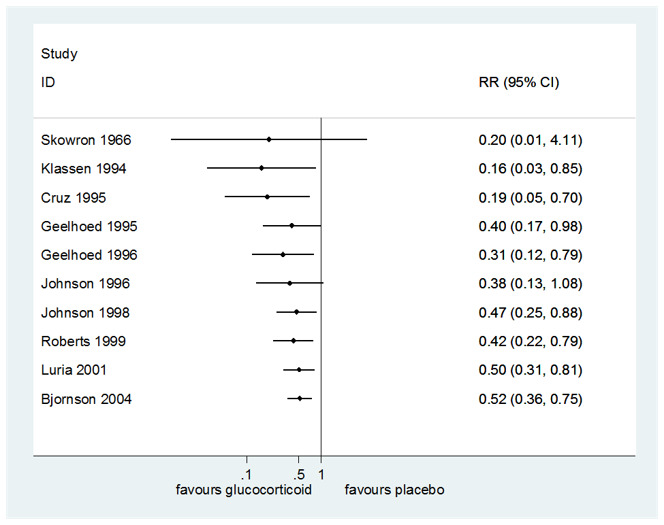
Cumulative meta‐graph by year for return visits or (re)admissions or both for any glucocorticoid compared to placebo.
Feedback
Taste of oral steroids may be a problem,
Summary
A recent letter in the Lancet has questioned the results of a study on oral prednisolone for wheeze in young children on the basis that (amongst other things) oral prednisolone tastes very bitter and may not have been taken well by the children in the study.(1)
Whilst the authors have replied that they overcame the problem by asking parents to mix the powder with the child's favorite juice, I have had comments from parents in the past that their children did not like the taste of soluble prednisolone tablets, and I gather that dexamethasone solution is also very bitter.
For this reason I have abandoned the use of prednisolone and dexamethasone in children with croup or acute asthma, and use soluble betamethasone tablets instead. Betamethasone and dexamethasone are equal in potency and both are more potent than oral prednisolone; the British National Formulary states that the equivalent dose is that 5 mg of prednisolone is equivalent to 750 µg betamethasone (which equates to one and a half 500 µg tablets). It should also be noted that dexamethasone oral solution costs about 10 times as much as betamethasone tablets!
My extrapolation of the results of this review to the use of betamethasone in primary care is based on two assumptions. Firstly that betamethasone is equivalent to dexamethasone, and secondly that the outpatient trials in secondary care contain patients that are similar to those presenting in primary care. I wonder if the authors agree that this is reasonable?
Reference
1. Weinberger M, Ahrens R. Oral prednisolone for viral wheeze in young children. Lancet 2004;363(9405):330
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
In response to Dr. Cates' comment regarding the use of betamethasone for the treatment of croup, we are unable to conclude that betamethasone is efficacious for the treatment of croup.
Among the included studies, only Klassen et al (1998) reported the results of the blinding methodology. Children were randomized to identically tasting and appearing budesonide, dexamethasone or both treatments. Research assistants and parents were asked to identify which study medication the child received. The responses were similar and this indicates that blinding was successful. In addition, Klassen has conducted RCTs using intravenous dexamethasone with a 70% sucrose solution. This has been very well‐tolerated with a very low incidence of vomiting. Paediatric croup and asthma trials have shown that when compared to prednisone/prednisolone, oral dexamethasone combined with flavoured syrup is both well‐tolerated and an inexpensive treatment.
To date, we are not aware of any RCTs in children with croup that compared betamethasone to placebo or an active treatment, such as dexamethasone. Although betamethasone is theoretically as potent as dexamethasone, there is no actual empirical data to prove this. Therefore, we cannot judge the equivalency, or the tolerability, of betamethasone versus dexamethasone. Perhaps a randomized controlled trial should be conducted that directly compares betamethasone to dexamethasone so the palatability and equivalency can be assessed.
In response to the second stated assumption, there are guidelines for generalising results of trials to clinical practice and physicians need to carefully consider the comparability of participants in any one study to their own patients.1
1Guyatt G, Haynes B, Jaeschke R, Cook D, Greenhalgh T, Meade M, Green L, Naylor C, Wilson M, McAlister F, Richardson M. Introduction: the philosophy of evidence‐based medicine. In: Guyatt G, Rennie D, editors. Users' guides to the medical literature: a manual for evidence‐based clinical practice. Chicago: AMA Press; 2002. pp. 3‐12.
Kelly Russell Terry Klassen David Johnson
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Contributors
Chris Cates
What's new
| Date | Event | Description |
|---|---|---|
| 4 March 2022 | New search has been performed | The following new authors joined the review group for this 2022 update: Alex Aregbesola, Clara Tam, Asha Kothari, Mê‐Linh Lê, and Mirna Ragheb. As in the previous review, we added an age range for children in our inclusion criteria (0 to 18 years). We did not extract data for some outcomes prespecified in the protocol because the new included studies did not report these outcomes. We used the Cochrane risk of bias tool to assess risk of bias for all studies included in the meta‐analysis. We used the GRADE approach to assess the certainty of the evidence (GRADEpro GDT). We added summary of findings tables for two comparisons. |
| 4 March 2022 | New citation required and conclusions have changed | We included two new trials (Huang 2021; Parker 2019), and identified one ongoing trial (IRCT20190914044765N1), and one trial awaiting classification (Chen 2018). We excluded six new trials (Faraji‐Goodarzi 2018; Gursanscky 2019; Kotaniemi‐Syrjanen 2018; Lee 2019; Meskina 2019; Tyler 2022). The previous version of this review concluded that glucocorticoids reduced symptoms of croup at two hours, shortened hospital stays, and reduced the rate of return visits to care. We concluded that dexamethasone probably reduces revisits or readmission of croup by about half. A smaller dose of 0.15 mg/kg of dexamethasone may be as effective as the standard dose of 0.60 mg/kg. More randomised controlled trials are needed to strengthen the evidence for effectiveness of low‐dose dexamethasone at 0.15 mg/kg to treat croup. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 3 April 2018 | New search has been performed | New authors joined the team to update the review. We updated the searches and included five new trials. Three were newly identified trials (Dobrovoljac 2012; Garbutt 2013; Soleimani 2013); one was a previously excluded trial (Chub‐Uppakarn 2007); and one trial was previously awaiting classification (Eboriadou 2010). We excluded six new trials, Eghbali 2016; Faghihinia 2007; Faraji‐Goodarzi 2018; Gill 2017; Mohammadzadeh 2014; Roked 2015, and one ongoing trial (NCT01748162). We included one new ongoing trial (ACTRN12609000290291). We assessed risk of bias of the included studies and the certainty of the evidence. We added two new primary outcomes: change in croup score after two hours and patient improvement after two hours. We added adverse events as a secondary outcome and summary of findings tables. We included two new comparisons: oral compared to nebulised dexamethasone, and dexamethasone compared to beclomethasone. |
| 3 April 2018 | New citation required and conclusions have changed | Our conclusions have changed. The previous version of this review concluded that when compared to placebo, glucocorticoids reduce croup symptoms within six hours and that the effect lasts 12 hours. In this update we concluded that when compared to placebo, glucocorticoids reduce croup symptoms within two hours and that the effect lasts at least 24 hours. |
| 16 September 2014 | New search has been performed | Searches updated. We included one new trial, Dobrovoljac 2012, and excluded one new trial (Faghihinia 2007). We added a two‐hour croup score and a two‐hour improvement outcome. |
| 16 September 2014 | New citation required but conclusions have not changed | Review updated; conclusions remain unchanged. |
| 1 December 2011 | Amended | Grammatical correction made to the Plain language summary. |
| 18 July 2011 | Amended | Analysis 5.2 contained an error, as the negative signs for the change in croup scores at six hours were not included. The mean difference remains non‐significant. |
| 23 July 2010 | New search has been performed | Searches conducted. We added seven new trials since the 2004 publication (Alshehr 2005; Amir 2006; Cetinkaya 2004; Duman 2005; Fifoot 2007; Geelhoed 2005; Sparrow 2006). We excluded three new trials (Chub‐Uppakarn 2007; Custer 2005; Schooff 2005). |
| 20 May 2010 | New citation required but conclusions have not changed | New authors joined the team to update the review. The conclusions remain unchanged. |
| 16 August 2008 | Amended | Converted to new review format |
| 3 February 2004 | Feedback has been incorporated | Feedback incorporated. |
| 7 April 2003 | New search has been performed | Searches conducted. |
| 17 August 1997 | New search has been performed | Searches conducted. |
Acknowledgements
We thank the administrative staff of the Children's Hospital Research Institute of Manitoba. We acknowledge the contributions of Allison Gates, Michelle Gates, Ben Vandermeer, Cydney Johnson, Lisa Hartling, and David W Johnson, authors of the 2018 review update.
For this 2022 update, we acknowledge the contribution of Veronica Lai, who participated in the update.
The following people conducted the editorial process for this update.
Sign‐off Editors (final editorial decision): Mark Jones (Bond University, Australia); Mieke van Driel (The University of Queensland, Australia).
Managing Editors (provided editorial guidance to authors, edited the review, selected peer reviewers, and collated peer‐reviewer comments): Liz Dooley (Bond University, Australia); Fiona Russell (Bond University, Australia).
Contact Editor (assessed peer‐review comments and recommended an editorial decision): Lubna Al‐Ansary (Department of Family and Community Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia).
Statistical Editor (provided comments): Robert S Ware (Menzies Health Institute Queensland, Griffith University, Australia).
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support.
Peer reviewers (provided comments and recommended an editorial decision):
Clinical/content review: Dr Gina Neto (University of Ottawa, Canada).
Consumer review: A Fraiz (Registered Nurse and Consumer).
Methods review: Rachel Richardson (Associate Editor, Cochrane).
Search review: Justin Clark (Institute for Evidence‐Based Healthcare, Bond University, Australia); Liz Doney (Cochrane Skin, University of Nottingham, UK).
Appendices
Appendix 1. Search strategies for the 2022 update
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations and Daily 1946 to 4 March 2022
| Search Strategy: | ||
| # | Searches | Results |
| 1 | Adrenal Cortex Hormones/ | 68009 |
| 2 | Beclomethasone/ | 3062 |
| 3 | exp Betamethasone/ | 7720 |
| 4 | Budesonide/ | 4755 |
| 5 | Cortisone/ | 19687 |
| 6 | Corticosterone/ | 26436 |
| 7 | Cortodoxone/ | 893 |
| 8 | Dexamethasone/ | 53872 |
| 9 | exp Glucocorticoids/ | 201487 |
| 10 | Hydrocortisone/ | 74655 |
| 11 | Hydroxycorticosteroids/ | 375 |
| 12 | exp Methylprednisolone/ | 20351 |
| 13 | Prednisolone/ | 33795 |
| 14 | Prednisone/ | 40638 |
| 15 | Pregnenolone/ | 4436 |
| 16 | Pregnenediones/ | 2273 |
| 17 | Tetrahydrocortisol/ | 269 |
| 18 | Triamcinolone/ | 3962 |
| 19 | adrenal cortex hormone*.tw,kf,nm. | 68324 |
| 20 | becl?met*.tw,kf,nm. | 4015 |
| 21 | betamet?asone*.tw,kf,nm. | 8204 |
| 22 | budesonide*.tw,kf,nm. | 6808 |
| 23 | clobetasol*.tw,kf,nm. | 1961 |
| 24 | corticoid*.tw,kf,nm. | 6443 |
| 25 | corticosteroid*.tw,kf,nm. | 114283 |
| 26 | corticosterone*.tw,kf,nm. | 35897 |
| 27 | cortisone*.tw,kf,nm. | 23717 |
| 28 | cortodoxone*.tw,kf,nm. | 893 |
| 29 | dexamet?asone*.tw,kf,nm. | 76779 |
| 30 | glucocortico*.tw,kf,nm. | 122905 |
| 31 | hydrocortisone*.tw,kf,nm. | 80392 |
| 32 | hydroxycorticosteroid*.tw,kf,nm. | 6735 |
| 33 | hydroxypregnenolone*.tw,kf,nm. | 1008 |
| 34 | methylprednisolone*.tw,kf,nm. | 28760 |
| 35 | prednisolone*.tw,kf,nm. | 48416 |
| 36 | prednisone*.tw,kf,nm. | 55854 |
| 37 | pregnenedione*.tw,kf,nm. | 2279 |
| 38 | pregnenolone*.tw,kf,nm. | 7389 |
| 39 | tetrahydrocortisol*.tw,kf,nm. | 508 |
| 40 | triamcinolone*.tw,kf,nm. | 12442 |
| 41 | or/1‐40 [Glucocorticoids Concept] | 513682 |
| 42 | exp Laryngitis/ | 4066 |
| 43 | (croup* or pseudocroup*).tw,kf. | 1883 |
| 44 | (laryngo tracheo bronch* or laryngotracheobronch*).tw,kf. | 558 |
| 45 | (laryngo tracheit* or laryngotracheit*).tw,kf. | 951 |
| 46 | laryngit*.tw,kf. | 2167 |
| 47 | or/42‐46 [Croup Concept] | 6844 |
| 48 | and/41,47 [Glucocorticoids and Croup Concepts] | 632 |
| 49 | randomized controlled trial.pt. | 560028 |
| 50 | controlled clinical trial.pt. | 94719 |
| 51 | randomized.ab. | 552306 |
| 52 | placebo.ab. | 225982 |
| 53 | drug therapy.fs. | 2450860 |
| 54 | randomly.ab. | 377029 |
| 55 | trial.ab. | 589312 |
| 56 | groups.ab. | 2317503 |
| 57 | or/49‐56 [RCT Filter] | 5275938 |
| 58 | exp animals/ not humans.sh. | 4966780 |
| 59 | 57 not 58 [Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook] | 4590783 |
| 60 | and/48,59 [RCT filter applied to glucocorticoid & croup results] | 396 |
| 61 | (201804* or 2019* or 2020*).ed. [Update Limit] | 2108579 |
| 62 | and/60‐61 [Update Limit Applied] | 17 |
| 63 | remove duplicates from 62 | 17 |
| 64 | (202009* or 2021* or 2022*).ed. [Update Limit] | 1811972 |
| 65 | 60 and 64 | 11 |
| 66 | remove duplicates from 65 | 11 |
Embase 1974 to 2022 Week 8
| Search Strategy: | ||
| # | Searches | Results |
| 1 | beclomethasone/ | 7662 |
| 2 | betamethasone/ | 18188 |
| 3 | budesonide/ | 22808 |
| 4 | corticosteroid/ | 257015 |
| 5 | corticosterone/ | 32159 |
| 6 | cortisone/ | 13015 |
| 7 | cortodoxone/ | 2123 |
| 8 | dexamethasone/ | 167648 |
| 9 | exp glucocorticoid/ | 778646 |
| 10 | hydrocortisone/ | 133565 |
| 11 | hydroxycorticosteroid/ | 304 |
| 12 | methylprednisolone/ | 107667 |
| 13 | prednisolone/ | 135821 |
| 14 | prednisone/ | 184211 |
| 15 | pregnane derivative/ | 2049 |
| 16 | pregnenolone/ | 4757 |
| 17 | steroid hormone/ | 12245 |
| 18 | tetrahydrocortisol/ | 539 |
| 19 | adrenal cortex hormone*.tw,kw. | 438 |
| 20 | becl?met*.tw,kw. | 4671 |
| 21 | betamet?asone*.tw,kw. | 7424 |
| 22 | budesonide*.tw,kw. | 9804 |
| 23 | clobetasol*.tw,kw. | 2089 |
| 24 | corticoid*.tw,kw. | 7327 |
| 25 | corticosteroid*.tw,kw. | 172123 |
| 26 | corticosterone*.tw,kw. | 32198 |
| 27 | cortisone*.tw,kw. | 8488 |
| 28 | cortodoxone*.tw,kw. | 8 |
| 29 | dexamet?asone*.tw,kw. | 86461 |
| 30 | glucocortico*.tw,kw. | 105249 |
| 31 | hydrocortisone*.tw,kw. | 20385 |
| 32 | hydroxycorticosteroid*.tw,kw. | 758 |
| 33 | hydroxypregnenolone*.tw,kw. | 733 |
| 34 | methylprednisolone*.tw,kw. | 29433 |
| 35 | prednisolone*.tw,kw. | 42598 |
| 36 | prednisone*.tw,kw. | 53777 |
| 37 | pregnenedione*.tw,kw. | 6 |
| 38 | pregnenolone*.tw,kw. | 5931 |
| 39 | tetrahydrocortisol*.tw,kw. | 461 |
| 40 | triamcinolone*.tw,kw. | 10669 |
| 41 | or/1‐40 [Glucocorticoids Concept] | 1062940 |
| 42 | croup/ | 1994 |
| 43 | laryngitis/ | 3965 |
| 44 | laryngotracheobronchitis/ | 702 |
| 45 | pseudocroup/ | 252 |
| 46 | (croup* or pseudocroup*).tw,kw. | 2154 |
| 47 | laryngit*.tw,kw. | 2246 |
| 48 | (laryngo tracheit* or laryngotracheit*).tw,kw. | 854 |
| 49 | (laryngo tracheo bronch* or laryngotracheobronch*).tw,kw. | 544 |
| 50 | or/42‐49 [Croup Concept] | 8520 |
| 51 | and/41,50 [Combined Glucocorticoids and Croup Concepts] | 1494 |
| 52 | crossover procedure/ | 69541 |
| 53 | double blind procedure/ | 192593 |
| 54 | randomized controlled trial/ | 696988 |
| 55 | single blind procedure/ | 45282 |
| 56 | allocat*.tw,kw. | 178563 |
| 57 | assign*.tw,kw. | 443634 |
| 58 | (cross over* or crossover*).tw,kw. | 116961 |
| 59 | (doubl* adj blind*).tw,kw. | 227957 |
| 60 | factorial*.tw,kw. | 43563 |
| 61 | placebo*.tw,kw. | 339624 |
| 62 | random*.tw,kw. | 1763875 |
| 63 | (singl* adj blind*).tw,kw. | 28391 |
| 64 | volunteer*.tw,kw. | 277008 |
| 65 | or/52‐64 [Recommended terms to limit to trials in Embase] | 2632950 |
| 66 | exp animals/ not exp humans/ | 4907469 |
| 67 | 65 not 66 | 2387867 |
| 68 | and/51,67 [RCT Filter and main search concept] | 202 |
| 69 | ("2018" or "2019" or "2020").yr. | 4984140 |
| 70 | and/68‐69 [Update Date Applied] | 26 |
| 71 | remove duplicates from 70 | 26 |
| 72 | ("2020" or "2021" or "2022").yr. | 3880081 |
| 73 | and/68,72 | 14 |
Cochrane Library (via Wiley) 4 March 2022
| Search Strategy: | ||
| ID | Search | Hits |
| #1 | [mh ^"Adrenal Cortex Hormones"] | 2502 |
| #2 | [mh ^Beclomethasone] | 1141 |
| #3 | [mh Betamethasone] | 1519 |
| #4 | [mh ^Budesonide] | 1861 |
| #5 | [mh ^Cortisone] | 160 |
| #6 | [mh ^Corticosterone] | 41 |
| #7 | [mh ^Cortodoxone] | 32 |
| #8 | [mh ^Dexamethasone] | 4938 |
| #9 | [mh Glucocorticoids] | 4764 |
| #10 | [mh ^Hydrocortisone] | 6154 |
| #11 | [mh ^Hydroxycorticosteroids] | 10 |
| #12 | [mh Methylprednisolone] | 2851 |
| #13 | [mh ^Prednisolone] | 3157 |
| #14 | [mh ^Prednisone] | 4135 |
| #15 | [mh ^Pregnenolone] | 22 |
| #16 | [mh ^Pregnenediones] | 566 |
| #17 | [mh ^Tetrahydrocortisol] | 12 |
| #18 | [mh ^Triamcinolone] | 684 |
| #19 | adrenal cortex hormone*:ti,ab,kw | 0 |
| #20 | becl?met*:ti,ab,kw | 2650 |
| #21 | (betametasone* or betamethasone*):ti,ab,kw | 2617 |
| #22 | budesonide*:ti,ab,kw | 5030 |
| #23 | clobetasol*:ti,ab,kw | 746 |
| #24 | corticoid*:ti,ab,kw | 739 |
| #25 | corticosteroid*:ti,ab,kw | 23957 |
| #26 | corticosterone*:ti,ab,kw | 154 |
| #27 | cortisone*:ti,ab,kw | 585 |
| #28 | cortodoxone*:ti,ab,kw | 40 |
| #29 | (dexametasone* or dexamethasone*):ti,ab,kw | 13356 |
| #30 | glucocortico*:ti,ab,kw | 9736 |
| #31 | hydrocortisone*:ti,ab,kw | 9741 |
| #32 | hydroxycorticosteroid*:ti,ab,kw | 107 |
| #33 | hydroxypregnenolone*:ti,ab,kw | 8 |
| #34 | methylprednisolone*:ti,ab,kw | 5679 |
| #35 | prednisolone*:ti,ab,kw | 7576 |
| #36 | prednisone*:ti,ab,kw | 10186 |
| #37 | pregnenedione*:ti,ab,kw | 566 |
| #38 | pregnenolone*:ti,ab,kw | 113 |
| #39 | tetrahydrocortisol*:ti,ab,kw | 40 |
| #40 | triamcinolone*:ti,ab,kw | 3418 |
| #41 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 | 71548 |
| #42 | [mh Laryngitis] | 128 |
| #43 | (croup* or pseudocroup*):ti,ab,kw | 237 |
| #44 | ("laryngo tracheo bronch*" or laryngotracheobronch*):ti,ab,kw | 28 |
| #45 | ("laryngo tracheit*" or laryngotracheit*):ti,ab,kw | 15 |
| #46 | laryngit*:ti,ab,kw | 250 |
| #47 | #43 or #43 or #44 or #45 or #46 | 478 |
| #48 | #41 and #47 | 159 |
| #49 | #41 and #47 with Publication Year from 2020 to 2022, in Trials | 6 |
Trial registry: ClinicalTrials.gov (https://clinicaltrials.gov/)
Search strategy
Advanced search >
Search Terms: croup OR laryngitis OR laryngotracheobronchitis OR laryngotracheitis
Age: Child
Intervention: Anti‐Inflammatory Agents
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int.uml.idm.oclc.org/trialsearch/)
Search strategy
Advanced search >
Title: croup OR laryngitis OR laryngotracheobronchitis OR laryngotracheitis
Search for clinical trials in children
Recruitment Status is: ALL
Date Selection: 2018‐2022
Data and analyses
Comparison 1. Any glucocorticoid compared to placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Croup score (change baseline ‐ 2 hours) by score | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 1.1.1 Westley score | 5 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.44, 0.01] |
| 1.1.2 Non‐Westley score | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.93, ‐0.10] |
| 1.2 Croup score (change baseline ‐ 6 hours) by score | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 1.2.1 Westley score | 5 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.02, ‐0.56] |
| 1.2.2 Non‐Westley score | 6 | 623 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.43, ‐0.18] |
| 1.3 Croup score (change baseline ‐ 12 hours) by score | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.53, ‐0.53] |
| 1.3.1 Westley score | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.56, ‐0.53] |
| 1.3.2 Non‐Westley score | 6 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.45, ‐0.30] |
| 1.4 Croup score (change baseline ‐ 24 hours) by score | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 1.4.1 Westley score | 4 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.72, ‐0.37] |
| 1.4.2 Non‐Westley score | 4 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.56, 0.16] |
| 1.5 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 1.5.1 Inpatient | 5 | 301 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.44, ‐0.16] |
| 1.5.2 Outpatient | 2 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.93, 0.29] |
| 1.6 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 1.6.1 Inpatient | 8 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.22, ‐0.23] |
| 1.6.2 Outpatient | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.11, ‐0.56] |
| 1.7 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 1.7.1 Inpatient | 7 | 291 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.46, ‐0.19] |
| 1.7.2 Outpatient | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.71, ‐0.48] |
| 1.8 Croup score (change baseline ‐ 2 hours) by glucocorticoid | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.10, ‐0.22] |
| 1.8.1 Budesonide | 4 | 246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.71, ‐0.30] |
| 1.8.2 Dexamethasone | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.00, 0.03] |
| 1.8.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.52, 1.42] |
| 1.9 Croup score (change baseline ‐ 6 hours) by glucocorticoid | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.07, ‐0.41] |
| 1.9.1 Budesonide | 5 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.04, ‐0.58] |
| 1.9.2 Dexamethasone | 6 | 567 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.08] |
| 1.9.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.89, 1.02] |
| 1.9.4 Prednisolone | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.62, ‐1.13] |
| 1.10 Croup score (change baseline ‐ 12 hours) by glucocorticoid | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.51, ‐0.56] |
| 1.10.1 Budesonide | 3 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.26, ‐0.68] |
| 1.10.2 Dexamethasone | 5 | 323 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.55, ‐0.15] |
| 1.10.3 Prednisolone | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐3.26, ‐1.55] |
| 1.11 Croup score (change baseline ‐ 24 hours) by glucocorticoid | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.41, ‐0.37] |
| 1.11.1 Budesonide | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.88, ‐0.93] |
| 1.11.2 Dexamethasone | 6 | 245 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.55, ‐0.22] |
| 1.11.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.75, 1.17] |
| 1.12 Return visits or (re)admissions or both by inpatient/outpatient | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.75] |
| 1.12.1 Inpatient | 3 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.30] |
| 1.12.2 Outpatient | 7 | 1356 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 1.13 Return visits or (re)admissions or both by glucocorticoid | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| 1.13.1 Budesonide | 4 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.90] |
| 1.13.2 Dexamethasone | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.81] |
| 1.14 Return visits or (re)admissions or both by croup severity | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.76] |
| 1.14.1 Mild croup | 3 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.95] |
| 1.14.2 Moderate croup | 7 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.86] |
| 1.15 Length of stay by inpatient | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 1.15.1 Inpatient | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 1.16 Length of stay by glucocorticoid | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.55 [‐22.70, ‐6.41] |
| 1.16.1 Budesonide | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐15.29 [‐26.89, ‐3.69] |
| 1.16.2 Dexamethasone | 6 | 328 | Mean Difference (IV, Random, 95% CI) | ‐18.25 [‐27.87, ‐8.62] |
| 1.16.3 Fluticasone | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐12.34, 21.94] |
| 1.17 Improvement (at 2 hours) by inpatient | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 1.17.1 Inpatient | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 1.18 Improvement (at 6 hours) by inpatient/outpatient | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 1.18.1 Inpatient | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.96, 1.90] |
| 1.18.2 Outpatient | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.16, 2.74] |
| 1.19 Improvement (at 12 hours) by inpatient | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.19.1 Inpatient | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.20 Improvement (at 24 hours) by inpatient/outpatient | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 1.20.1 Inpatient | 4 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.43] |
| 1.20.2 Outpatient | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.14, 3.51] |
| 1.21 Improvement (at 6 hours) by glucocorticoid | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 1.21.1 Budesonide | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.19, 2.32] |
| 1.21.2 Dexamethasone | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.76, 2.72] |
| 1.21.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.69, 2.62] |
| 1.22 Improvement (at 12 hours) by glucocorticoid | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.22.1 Budesonide | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.08, 1.84] |
| 1.22.2 Dexamethasone | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.06, 2.18] |
| 1.22.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.55] |
| 1.23 Improvement (at 24 hours) by glucocorticoid | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 1.23.1 Dexamethasone | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.05, 1.84] |
| 1.23.2 Prednisolone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| 1.24 Additional treatments: antibiotics | 3 | 202 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 1.25 Additional treatments: epinephrine | 9 | 709 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 1.26 Additional treatments: intubation/tracheostomy | 11 | 1090 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 1.27 Additional treatments: mist tent | 2 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.87, 0.47] |
| 1.28 Additional treatments: supplemental glucocorticoids | 6 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
Comparison 2. Any glucocorticoid compared to epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.24, 1.77] |
| 2.1.1 Inpatient | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 2.1.2 Outpatient | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.73, 1.84] |
| 2.2 Croup score (change baseline ‐ 6 hours) by inpatient | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 2.2.1 Inpatient | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 2.3 Croup score (change baseline ‐ 12 hours) by inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.3.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.4 Croup score (change baseline ‐ 24 hours) by inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.4.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.5 Croup score (change baseline ‐ 2 hours) by glucocorticoid | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.13, 1.63] |
| 2.5.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 2.5.2 Dexamethasone | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.35, 1.91] |
| 2.5.3 Beclomethasone | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 1.41 [0.62, 2.19] |
| 2.6 Croup score (change baseline ‐ 12 hours) by glucocorticoid | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.6.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.47, 0.50] |
| 2.6.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.09, 0.82] |
| 2.7 Croup score (change baseline ‐ 24 hours) by glucocorticoid | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.7.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.27, 0.70] |
| 2.7.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.38, 0.61] |
| 2.8 Return visits or (re)admissions or both by inpatient/outpatient | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 2.8.1 Inpatient | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.8.2 Outpatient | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.9 Length of stay by inpatient | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐33.89, 13.89] |
| 2.9.1 Inpatient | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐33.89, 13.89] |
| 2.10 Additional treatments: epinephrine | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 2.69] |
| 2.11 Additional treatments: intubation/tracheostomy | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.12 Additional treatments: supplemental glucocorticoids | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
Comparison 3. Dexamethasone compared to budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 4 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.13] |
| 3.1.1 Inpatient | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.04, ‐0.22] |
| 3.1.2 Outpatient | 2 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.90, 0.18] |
| 3.2 Croup score (change baseline ‐ 12 hours) by inpatient | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 3.2.1 Inpatient | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 3.3 Return visits or (re)admissions or both by inpatient/outpatient | 5 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.22] |
| 3.3.1 Inpatient | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.14, 2.79] |
| 3.3.2 Outpatient | 3 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.30] |
| 3.4 Length of stay by inpatient/outpatient | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.28, 0.25] |
| 3.4.1 Inpatient | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.93, ‐0.07] |
| 3.4.2 Outpatient | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 3.5 Improvement (at 6 hours) by outpatient | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 3.5.1 Outpatient | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 3.6 Additional treatments: epinephrine | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.96] |
| 3.7 Additional treatments: intubation/tracheostomy | 2 | 145 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 3.8 Additional treatments: supplemental glucocorticoids | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.32] |
Comparison 4. Dexamethasone compared to beclomethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Return visits or (re)admissions or both by outpatient | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| 4.1.1 Outpatient | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
Comparison 5. Dexamethasone compared to betamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Croup score (change baseline ‐ 2 hours) by outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.06] |
| 5.1.1 Outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.06] |
| 5.2 Croup score (change baseline ‐ 6 hours) by outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.23, ‐0.11] |
| 5.2.1 Outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.23, ‐0.11] |
| 5.3 Return visits or (re)admissions or both by outpatient | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 5.3.1 Outpatient | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 5.4 Additional treatments: epinephrine | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.18, 3.76] |
Comparison 6. Dexamethasone compared to prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Croup score (change baseline ‐ 2 hours) by outpatient | 1 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.1.1 Outpatient | 1 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.2 Croup score (change baseline ‐ 6 hours) by outpatient | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.21, 0.62] |
| 6.2.1 Outpatient | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.21, 0.62] |
| 6.3 Return visits or (re)admissions or both by outpatient | 4 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.11] |
| 6.3.1 Outpatient | 4 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.11] |
| 6.4 Length of stay by outpatient | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.39] |
| 6.4.1 Outpatients | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.39] |
| 6.5 Additional treatments: epinephrine | 3 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.64] |
| 6.6 Additional treatments: intubation/tracheotomy | 1 | 1231 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 6.7 Additional treatments: supplemental glucocorticoids | 2 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.97] |
Comparison 7. Budesonide and dexamethasone compared to dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 3 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.19, 0.30] |
| 7.1.1 Inpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.30, 0.63] |
| 7.1.2 Outpatient | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.39] |
| 7.2 Return visits or (re)admissions or both by inpatient/outpatient | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.83] |
| 7.2.1 Inpatient | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.46, 2.29] |
| 7.2.2 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.13, 2.60] |
| 7.3 Length of stay by inpatient/outpatient | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.05, 0.92] |
| 7.3.1 Inpatient | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.75, 4.15] |
| 7.3.2 Outpatient | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.94] |
| 7.4 Improvement (at 6 hours) by outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 7.4.1 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 7.5 Additional treatments: epinephrine | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.39] |
| 7.6 Additional treatments: mist tent | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.65] |
| 7.7 Additional treatments: supplemental glucocorticoids | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 16.66] |
Comparison 8. Budesonide and dexamethasone compared to budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Croup score (change baseline ‐ 6 hours) by outpatient | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.52, 0.17] |
| 8.1.1 Outpatient | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.52, 0.17] |
| 8.2 Return visits or (re)admissions or both by outpatient | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 8.2.1 Outpatient | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 8.3 Length of stay by outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 8.3.1 Outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 8.4 Improvement (at 6 hours) by outpatient | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 8.4.1 Outpatient | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 8.5 Additional treatments: epinephrine | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.15, 6.99] |
| 8.6 Additional treatments: supplemental glucocorticoids | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.52, 3.29] |
Comparison 9. Oral compared to intramuscular dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Return visits or (re)admissions or both by outpatient | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 9.1.1 Outpatient | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 9.2 Improvement (at 24 hours) by outpatient | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 9.2.1 Outpatient | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 9.3 Additional treatments: antibiotics | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 9.4 Additional treatments: epinephrine | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 9.5 Additional treatments: mist tent | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.31, 5.89] |
| 9.6 Additional treatments: supplemental glucocorticoids | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.41] |
Comparison 10. Oral compared to nebulised dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Return visits or (re)admissions or both by outpatient | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
| 10.1.1 Outpatient | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
Comparison 11. Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Return visits or (re)admissions or both by outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 11.1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 11.2 Additional treatments: epinephrine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.98] |
| 11.3 Additional treatments: supplemental glucocorticoids | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
Comparison 12. Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Return visits or (re)admissions or both by outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 12.1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 12.2 Additional treatments: epinephrine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.28] |
| 12.3 Additional treatments: supplemental glucocorticoids | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 66.40] |
Comparison 13. Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Croup score (Westley) (change baseline ‐ 2 hours) by inpatient/outpatient | 2 | 861 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.76, 0.22] |
| 13.1.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.25, 0.00] |
| 13.1.2 Outpatient | 1 | 820 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.23, 0.04] |
| 13.2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.26, 0.35] |
| 13.2.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.13, ‐0.74] |
| 13.2.2 Outpatient | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.35, 0.32] |
| 13.3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐4.39, 3.19] |
| 13.3.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.55 [‐3.39, ‐1.71] |
| 13.3.2 Outpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 1.32 [0.81, 1.83] |
| 13.4 Croup score (change baseline ‐ 24 hours) by outpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.16, 1.10] |
| 13.4.1 Outpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.16, 1.10] |
| 13.5 Return visits or (re)admissions or both by outpatient | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 13.5.1 Outpatient | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 13.6 Length of stay by outpatient | 2 | 892 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.32, 0.56] |
| 13.6.1 Outpatient | 2 | 892 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.32, 0.56] |
| 13.7 Additional treatments: epinephrine | 2 | 885 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.34, 1.75] |
| 13.8 Additional treatments: intubation/tracheotomy | 2 | 861 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 13.9 Additional treatments: supplemental glucocorticoids | 2 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alshehr 2005.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants |
Study period: September 1998 to December 2002 Setting: emergency rooms and outpatient clinics in 3 medical institutes, Abha City, Saudi Arabia Inclusion criteria: children aged 3 months to 9 years who had been given a diagnosis of croup and had persistent, moderately severe respiratory distress (Westley croup score > 3) Exclusion criteria: symptoms or signs suggesting another cause of stridor; history of chronic pulmonary disease; severe systemic disease; immune dysfunction; stridor or intubation for more than 1 month; glucocorticoid therapy in the last 4 weeks before study entry Baseline characteristics (N = 72):
|
|
| Interventions | Treatment (N = 36): single dose 0.15 mg/kg oral dexamethasone Comparator (N = 36): single dose 0.6 mg/kg oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 4, 12, and 24 hours; hospitalisation; length of stay in hospital; use of mist tent | |
| Notes | All children received mist therapy throughout the observation period. Funding source: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked randomization code was produced by random‐number generating software" |
| Allocation concealment (selection bias) | Low risk | Quote: "To make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution." "A blocked randomization code was produced ... and the code was not broken until after the study ended and all decisions regarding data analysis were finalized" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" "To make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind"; "to make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution"; "the code was not broken until after the study ended and all the decisions regarding data analysis were finalized" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 14% (N = 12) of children recruited were excluded prior to randomisation. All randomised children were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Amir 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: November 2002 to March 2003 Setting: emergency department of Schneider Children’s Medical Center, Israel Inclusion criteria: children aged 6 months to 6 years with a clinical picture of mild to moderate acute laryngotracheitis presenting to the emergency department. Mild to moderate croup defined as a Westley croup score of 1 to 11 Exclusion criteria: spasmodic croup; acute epiglottitis; bacterial tracheitis; pneumonia; foreign body aspiration; chronic lung disease; congenital or acquired anatomical airway anomalies; immunosuppressed or immunocompromised; treated before arrival at the emergency department with inhaled bronchodilators or corticosteroids in any form; exposed to varicella in the previous 28 days; contradictions to corticosteroid treatment Baseline demographics (N = 52):
|
|
| Interventions | Treatment (N = 26): single 0.60 mg/kg (maximum 10 mg) dose of intramuscular dexamethasone Comparator (N = 26): single 0.40 mg/kg dose of oral betamethasone Treatments were of equivalent potency; supplemental oxygen was provided to children in whom air saturation was < 95%. |
|
| Outcomes | Change in Westley croup score from baseline to 2 and 4 hours; return visits to the emergency department; use of epinephrine | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study participants were assigned by a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding described. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: used a third‐party outcome assessor. No blinding. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the number of children analysed is not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | High risk | Comment: baseline imbalance in croup score |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Bjornson 2004.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: September 2001 to April 2002 and September 2002 to February 2003 Setting: 4 paediatric emergency departments in Canada (Alberta Children’s Hospital in Calgary, Stollery Children’s Health Centre in Edmonton, Winnipeg Children’s Hospital in Winnipeg, or Children’s Hospital of Eastern Ontario in Ottawa) Inclusion criteria: children aged 3 months to 9 years with mild croup based on an initial medical evaluation. Mild croup was defined as onset within the past 72 hours of a seal‐like, barking cough and a Westley croup score of ≤ 2. Exclusion criteria: symptoms or signs of another cause of stridor; history of congenital or acquired stridor, asthma, exposure to varicella within the previous 21 days, chronic pulmonary disease, severe systemic disease, or known immune dysfunction; treatment with corticosteroids within the past 2 weeks; treatment of respiratory distress with epinephrine prior to enrolment; those enrolled in another clinical trial in the past 4 weeks; parents unable to speak either English or French; lack of a telephone at home; a prior visit to the emergency department because of croup during this episode of the disease Baseline demographics (N = 720):
|
|
| Interventions | Treatment (N = 359): single 0.60 mg/kg (maximum of 20 mg) dose of oral dexamethasone Control (N = 361): single dose of oral placebo (10 mL distilled water) Both treatment and placebo included 50 mL of wild cherry‐flavoured syrup. |
|
| Outcomes | Return visits to any healthcare provider within 7 days | |
| Notes | Funding source: Canadian Institutes of Health Research; Alberta Children's Hospital Foundation; Children's Hospital of Eastern Ontario Research Institute; Stollery Children's Hospital Foundation; Cumberland Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme, stratified by centre, used random permuted blocks of 6‐20 children to ensure the comparable assignment of eligible patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "Codes were secured at each center's pharmacy until enrolment and all decisions regarding data analysis had been finalized." The preparations were "not distinguishable" and "packaged in sequentially numbered, sealed bags". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "Parents were unable to determine which preparation their child had received"; "Preparations were not distinguishable by appearance, volume, weight, taste, or smell." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind"; "Preparations were not distinguishable by appearance, volume, weight, taste, or smell and were packaged in identical syringes in sequentially numbered, sealed bags"; "biostatistician who was not otherwise involved in the study performed the data analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing data imputed using intention‐to‐treat analysis. 5% (N = 37) protocol deviations equally distributed between groups. 2% (N = 13) had incomplete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Cetinkaya 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: not reported Setting: paediatric emergency department at the Şişlu Etfal Education and Research Hospital in Istanbul, Turkey Inclusion criteria: children aged 6 to 36 months who were admitted to the paediatric emergency clinic with a diagnosis of croup, defined as the acute (< 48 h) onset of stridor, chest wall retraction, barking cough, and hoarse voice Exclusion criteria: epiglottitis; reactive airway exacerbation; foreign body aspiration; acute bacterial pneumonia, acquired or congenital upper airway anomalies; intubated in the previous month; received steroids within the preceding 2 weeks Baseline characteristics (N = 60):
|
|
| Interventions | All children received 5 to 6 L/min of moisturised oxygen for 20 minutes upon arriving at the hospital, along with a single dose of 0.16 mg/kg of salbutamol. Treatment 1 (N = 15): 500 µg nebulised budesonide, a single dose of oral placebo (multivitamin syrup), and 2 mL intramuscular placebo (saline) Treatment 2 (N = 15): 2 mL nebulised placebo (saline), a single dose oral placebo (multivitamin syrup), and 0.60 mg/kg (maximum 8 mg) intramuscular dexamethasone Treatment 3 (N = 15): 2 mL nebulised placebo (saline), a single dose of 0.60 mg/kg (maximum 8 mg) oral dexamethasone, and 2 mL intramuscular placebo (saline) Control (N = 15): 2 mL intramuscular placebo (saline), 2 mL nebulised salbutamol solution with saline, and oral placebo (multivitamin syrup) |
|
| Outcomes | Change in Westley croup score from baseline to 24 hours | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding not explicitly described; it appears there was an attempt to blind parents and children by using similar‐appearing oral, nebulised, and intramuscular placebos. It is unclear if personnel were blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of blinding or third‐party outcome assessors. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Chub‐Uppakarn 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: March 2001 to October 2003 Setting: paediatric ward of Hatyai Hospital in the southern part of Thailand Inclusion criteria: children aged 6 months to 5 years who were admitted to the paediatric ward with moderate to severe croup. Westley croup score 4 to 7 Exclusion criteria: history of contact with chicken pox within the preceding 3 weeks; history of congenital or acquired stridor; chronic pulmonary disease; asthma; severe systemic disease or known immune dysfunction; treatment with corticosteroids within the preceding 2 weeks; treatment with epinephrine for respiratory distress before enrolment Baseline demographics (N = 41):
|
|
| Interventions | Treatment 1 (N = 21): single 0.15 mg/kg dose (maximum 3 mg) of intramuscular dexamethasone Treatment 2 (N = 20): single 0.60 mg/kg dose (maximum 12 mg) of intramuscular dexamethasone |
|
| Outcomes | Change in Westley croup score at 2, 6, and 12 hours; intubations; adverse events | |
| Notes | All children were treated with a single nebulisation of epinephrine (1:1000) 1 mL in 0.9% saline 3 mL at baseline. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme used random permuted blocks of four children" |
| Allocation concealment (selection bias) | Low risk | Quote: "codes were secured at the hospital pharmacy until enrolment and all decisions regarding data analysis had been finalized" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the preparations of dexamethasone suspension consisted of 10 mL of dexamethasone phosphate injection in concentrations of 1.2 and 0.3 mg/mL. The preparations were packaged in identical containers by a hospital pharmacist and were not distinguishable in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Cruz 1995.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: November 1992 to December 1993 Setting: emergency department at the Children's Hospital of Michigan, USA Inclusion criteria: children aged 6 months to 5 years reporting to the emergency department with a clinical diagnosis of acute laryngotracheitis or viral croup. Westley croup score of at least 2 and able to be managed as outpatients Exclusion criteria: history of prior intubation; structural airway anomalies; those requiring more than 1 racaemic epinephrine treatment; hospitalisation; β‐agonist therapy; received steroids in the past 24 hours Baseline demographics (N = 45):
|
|
| Interventions | Treatment (N = 19): single 0.60 mg/kg (maximum 10 mg) dose of intramuscular dexamethasone Control (N = 19): equal volume of placebo (normal saline) |
|
| Outcomes | Return visits to the emergency department; patient improvement 24 hours after discharge | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind"; "Both the patients and the investigators were blinded to the content of the syringe"; "the drug code was broken only after the last patient had completed the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% (N = 7) were excluded or lost to follow‐up; it is unclear if losses were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Dobrovoljac 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of the Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children over 6 months of age presenting to the emergency department with mild to moderate croup (harsh cough with or without stridor, Westley croup score 1 to 6) Exclusion criteria: Westley croup score < 1; severe croup (Westley croup score > 6) requiring epinephrine upon arrival; received steroids within the last week; other significant co‐existing illnesses Baseline demographics (N = 70):
|
|
| Interventions | Treatment (N = 35): single 0.15 mg/kg (1 mg/mL solution) dose of oral dexamethasone Control (N = 35): same volume of placebo solution All children also received 0.15 mg/kg oral dexamethasone at 60 minutes (after study completion). |
|
| Outcomes | Change in Westley croup score from baseline to 2 hours; use of epinephrine and supplemental glucocorticoids | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the pharmacy department and the randomization list was kept concealed from emergency physicians, nurses and parents until the end of the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the PMH pharmacy ensured that the two preparations could not be differentiated" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 6% loss to follow‐up due to worsening condition, all in the placebo group (N = 4, 11%). LOCF method used, which could have biased results in favour of the treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Donaldson 2003.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants |
Study period: January 1999 to December 1999 Setting: emergency department of William Beaumont Hospital, USA Inclusion criteria: children aged 3 to 84 months with history of inspiratory stridor or a barky cough and a Westley croup score of ≥ 2 after 10 to 15 minutes of cool mist therapy in the emergency department Exclusion criteria: Westley croup score < 2; signs suggesting another cause for stridor such as epiglottitis, bacterial tracheitis, foreign body, chronic lung disease; severe comorbidities; inability of parents to give informed consent; glucocorticoid therapy within 4 weeks of presenting Baseline demographics (N = 96):
|
|
| Interventions | Treatment 1 (N = 49): 0.60 mg/kg intramuscular dexamethasone and oral placebo (syrup) Treatment 2 (N = 46): 0.60 mg/kg oral dexamethasone and intramuscular placebo (direct pressure with hub of syringe on thigh) |
|
| Outcomes | Unscheduled revisits; parent‐reported symptom relief after 24 hours; use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomization method from a random number generator performed by the department of Pharmacy" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In both groups, neither the parents nor the treating physicians were present in the treatment room during the administration of medications"; "The emergency medicine faculty... were blinded to the route of administration of the drug"; "If the child vomited while in the ED, the treatment given was unblinded" Comment: blinding was attempted but could be broken if the child vomited whilst in the emergency department. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: third‐party outcome assessor described as blinded. Because blinding of children and parents could have been broken, the assessors could have become unblinded during conversation with parents. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 1% (N = 1) loss to follow‐up, unclear from which group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Duman 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: September 2002 to September 2003 Setting: paediatric emergency department of Dokuz Eylül University Faculty of Medicine, Izmir, Turkey Inclusion criteria: children aged more than 6 months presenting to the emergency department with a history of inspiratory stridor, barking cough, hoarseness and signs of inspiratory distress, and a Westley croup score of ≥ 2 Exclusion criteria: Westley croup score < 2, and with other suggested causes for stridor (epiglottitis, bacterial tracheitis, foreign body aspiration); past history of laryngoscopy, tracheal intubation, chronic lung disease, or severe comorbidities; immediate intubation or transfer to intensive care; corticosteroid therapy within 4 weeks of presentation; history of tuberculosis personally or in the family; chickenpox within the preceding 21 days; known immunodeficiency Baseline demographics (N = 76):
|
|
| Interventions | Treatment 1 (N = 31): 2.5 mL (0 to 20 kg) or 5.0 mL (20 to 40 kg) nebulised epinephrine with same volume normal saline and 0.60 mg/kg intramuscular dexamethasone Treatment 2 (N = 19): 2.5 mL (0 to 20 kg) or 5.0 mL (20 to 40 kg) nebulised epinephrine with same volume normal saline and 2 mg nebulised budesonide Comparator (N = 26): cool mist therapy and 0.60 mg/kg intramuscular dexamethasone In all groups, the drug was administered for a period of 20 minutes using a nebuliser with oxygen at a rate of 6 to 7 L/min through a face mask. |
|
| Outcomes | Change in Westley croup score from baseline to 2 hours; admissions from the emergency department; use of epinephrine | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to treatment according to a randomisation list produced at the beginning of the study. Patients were randomised in blocks of three." Comment: assumed that the blocked randomisation was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding. No description of a third‐party outcome assessor. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Eboriadou 2010.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: January 2000 to December 2001 Setting: emergency department at a hospital in Greece Inclusion criteria: children aged 6 months to 5 years presenting to the emergency department with a clinical diagnosis of viral croup by a history of a short period of viral upper respiratory symptoms followed by hoarseness or barking cough and clinical evidence of hoarseness, barking cough, or stridor in the emergency department; modified Downes and Raphaelly croup score ≥ 2; could be managed as outpatients Exclusion criteria: known structural airways anomalies; acute epiglottitis; bacterial tracheitis; pneumonia; foreign body aspiration or past history of laryngoscopy; history of prior intubation; chronic airway obstruction; received steroids in the past 24 hours; required more than 1 treatment with nebulised L‐epinephrine or hospitalisation Baseline characteristics (N = 64):
|
|
| Interventions | Treatment 1 (N = 25): single 5 mL (1:1000 mg/mL) dose of nebulised L‐epinephrine via nebuliser with oxygen at a rate of 5 L/minute Treatment 2 (N = 19): single 0.60 mg/kg (maximum 8 mg) dose of intramuscular dexamethasone Treatment 3 (N = 20): single 200 µg dose of inhaled beclomethasone dipropionate delivered via AeroChamber device Supplemental oxygen was used for children with oxygen saturation values < 95%. |
|
| Outcomes | Change in modified Downes and Raphaelly croup score from baseline to 2 hours; return visits to the emergency department | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned by a random numbers table to receive..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: described as "double‐blind", though the interventions were clearly distinguishable, and the mechanism of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Eden 1964.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: hospital in the USA Inclusion criteria: children hospitalised for treatment of acute croup, including all children with acute respiratory infections characterised by hoarseness, inspiratory stridor, and a barking cough Exclusion criteria: not reported Baseline characteristics (N = 50):
|
|
| Interventions | All children received as routine therapy oxygen, increased humidity, and tetracycline. Treatment (N = 25): 1 mg/kg intramuscular methyl prednisolone every 6 hours for 24 hours Control (N = 25): 1 mg/kg placebo preparation every 6 hours for 24 hours |
|
| Outcomes | Patient improvement at 6, 12, 24 hours | |
| Notes | Funding source: Upjohn Company (supplied drugs for the trial) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were divided into two groups according to a table of random sampling" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The composition of each preparation was unknown to the investigators until the end of the study" Comment: described as double‐blind. Investigators blinded, but it is unclear if participants or personnel (or both) were blinded because who administered the treatments is not stated. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor, unclear who performed the measurements. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 6% (N = 3) lost to follow‐up due to inadequate evaluation. All losses were in 1 group, but it is unclear which group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Eden 1967.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: hospital in the USA Inclusion criteria: children hospitalised with acute croup, including those presenting with acute respiratory infections characterised by barking cough, hoarseness, sternal retractions, and respiratory stridor Exclusion criteria: not reported Baseline demographics (N = 50):
|
|
| Interventions | Treatment (N = 25): 0.1 mL/kg per dose schedule (0.1 mg/kg) intramuscular dexamethasone every 6 hours for 48 hours (total daily dose of 0.40 mg/kg) Control (N = 25): volume of 0.1 mL/kg per dose schedule of placebo preparation intramuscularly every 6 hours for 48 hours |
|
| Outcomes | Patient improvement at 6, 12, 24 hours; tracheostomy | |
| Notes | Funding source: dexamethasone (Decadron) by Merck Sharp & Dohme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were divided into two groups according to a table of random sampling" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "The composition of each preparation was unknown to the investigators until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Fifoot 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants |
Study period: July 2004 to October 2005 Setting: emergency department of Mater Childrens' Hospital, Brisbane, Australia Inclusion criteria: children aged 6 months to 6 years presenting to the emergency department with croup (Westley croup score ≥ 2) with parents available for telephone follow‐up 1 week following enrolment Exclusion criteria: chronic respiratory disease (excluding asthma); severe croup (Westley croup score > 7); known allergy or relative contraindication to steroids (varicella or exposure to varicella within the past 3 weeks, history of tuberculosis, diabetes, or hypertension, known immunodeficiency); treatment with steroids in the preceding week or with nebulised adrenaline en route or immediately on arrival in the emergency department Baseline demographics (N = 99):
|
|
| Interventions | Treatment 1 (N = 34): single 1 mg/kg dose of oral prednisolone Treatment 2 (N = 34): single 0.15 mg/kg dose of oral dexamethasone Treatment 3 (N = 31): single 0.60 mg/kg dose of oral dexamethasone Children who did not tolerate oral therapy after 2 attempts received nebulised budesonide. |
|
| Outcomes | Change in Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; use of epinephrine and use of supplemental glucocorticoids | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized (using a computer‐generated sequence)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Recruiting staff and study investigators were blinded to treatment assignments. MCH pharmacists prepared each steroid agent as a solution, such that each child would receive an identical volume of preparation... flavoured to standardize taste and palatability" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recruiting staff and study investigators were blinded to treatment assignments. MCH pharmacists prepared each steroid agent as a solution, such that each child would receive an identical volume of preparation... flavoured to standardize taste and palatability" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 13% (N = 13) loss to follow‐up. Losses balanced between groups. Did not use intention‐to‐treat analysis for the telephone follow‐up outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: no deviations from protocol detected |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Fitzgerald 1996.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency departments of 3 paediatric tertiary referral hospitals, Sydney, Australia Inclusion criteria: children aged 6 months to 6 years admitted to the emergency department based on a decision by the medical staff regarding croup severity. To be included in study children were required to have acute (viral) or spasmodic croup and a minimum Westley croup score of 6. Exclusion criteria: significant past or present systemic disease; pre‐existing known airway abnormalities; confirmed hypersensitivity to budesonide or L‐adrenaline; suspected epiglottitis; foreign body aspiration; bronchiolitis or asthma; need for immediate intubation or transfer to intensive care; treated with glucocorticoids in the 4 weeks prior to the study Baseline demographics (N = 67):
|
|
| Interventions | Treatment 1 (N = 35): single 2 mg/4 mL dose of nebulised budesonide Treatment 2 (N = 31): single 4 mg/4 mL dose of nebulised L‐epinephrine |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 12, and 24 hours; readmission to the hospital; intubation, use of epinephrine, and use of additional steroids | |
| Notes | Funding source: Astra Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: described as double‐blind, but nursing staff (personnel) were unblinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study medication was administered by nursing staff and the investigator was not present when the medication was placed in the opaque nebulizer bowl" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 5% (N = 3) loss to follow‐up. All‐patients‐treated analysis excluded N = 1 child (1.5%). 13 children who received medications appropriately did not remain for the entire 24 hours. Last value extended for those who recovered before the 24‐hour period; however, no children returned or were readmitted; it is unclear how this may have affected the findings. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Garbutt 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: 26 October 2009 to 16 April 2010, and 6 September 2010 to 29 April 2011 Setting: 10 offices of primary care practitioners in St Louis, MO, USA Inclusion criteria: children aged 1 to 8 years with croup symptoms for ≤ 48 hours and a clinical diagnosis of mild or moderate croup at an office visit, based on symptoms in the past 24 to 36 hours Exclusion criteria: diagnosis of severe croup or impending respiratory failure; prior treatment with epinephrine or oral corticosteroids for this croup episode; symptoms or signs suggesting other cause of stridor; chronic respiratory disease including asthma; known contraindication to steroid use; parent not in the same household as the child in the subsequent 4 days, could not participate in telephone interviews, or was not English speaking Baseline demographics (N = 87):
|
|
| Interventions | Treatment (N = 41): single 2 mg/kg (maximum 60 mg/day) dose of oral prednisolone once per day for 3 days Comparator (N = 46): single 0.60 mg/kg (maximum 18 mg) dose of oral dexamethasone, followed by 2 days of placebo comparable in appearance, smell, and taste |
|
| Outcomes | Additional health care for croup within 11 days of randomisation | |
| Notes | Funding source: National Center for Research Resources (National Institutes of Health); National Institutes of Health Roadmap for Medical Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized blocks were used to assign subjects to treatment groups, with randomization stratified by site. Computer generated random numbers determined how the two treatments were allocated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drug packages were prepared offsite by the pharmacist"; "The pharmacist... packaged the bottles in a sealed opaque envelope"; "For allocation concealment, the drug formulation ensured the volume of the weight‐based dose was equivalent for each medication." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pharmacist... packaged the bottles in a sealed opaque envelope"; "comparable in appearance, smell and taste"; "patients, parents, PCPs, and study team members were blinded to treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The pharmacist... packaged the bottles in a sealed opaque envelope"; "comparable in appearance, smell and taste"; "patients, parents, PCPs, and study team members were blinded to treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. Losses to follow‐up on days 1, 2, 3, 4, and 11 were 7%, 9%, 9%, 3%, and 2% (non‐cumulative). Participation in follow‐up interviews was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no deviations from protocol detected |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Low risk | Comment: all domains judged as low risk |
Geelhoed 1995a.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial (trial A; see Geelhoed 1995b for trial B) | |
| Participants |
Study period: July 1994 to August 1994 Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months admitted to the hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, and hoarse voice) and a minimum modified Westley croup score of 3 Exclusion criteria: other acute or chronic medical problems; modified Westley croup score < 3 (mild croup); families without a telephone or with limited English language abilities; any kind of steroid therapy in the past week; pre‐existing upper airway condition; history of prolonged stridor; those presenting with a clinical picture suggesting a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 60):
|
|
| Interventions | Treatment (N = 29): single 0.30 mg/kg (maximum 6 mg) dose of oral dexamethasone Comparator (N = 31): single 0.60 mg/kg (maximum 12 mg) of oral dexamethasone |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; length of hospital stay; use of epinephrine and use of additional glucocorticoids | |
| Notes | Study reports on 2 comparisons. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the child was withdrawn from the study, their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken, but it is unclear how frequently this occurred. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 2% (N = 1) in trial A withdrew, 5% (N = 3) in trial A lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: some children were not enrolled when the emergency department was busy, potential to bias participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Geelhoed 1995b.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial (trial B; see Geelhoed 1995a for trial A) | |
| Participants |
Study period: August 1994 to December 1994 Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months admitted to the hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, and hoarse voice) and a minimum modified Westley croup score of 3 Exclusion criteria: other acute or chronic medical problems; modified Westley croup score < 3 (mild croup); families without a telephone or with limited English language abilities; any kind of steroid therapy in the past week; pre‐existing upper airway condition; history of prolonged stridor; those presenting with a clinical picture suggesting a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 60):
|
|
| Interventions | Treatment (N = 29): single 0.15 mg/kg (maximum 3 mg) dose of oral dexamethasone Comparator (N = 31): single 0.30 mg/kg (maximum 6 mg) dose of oral dexamethasone |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; length of hospital stay; use of epinephrine and use of additional glucocorticoids | |
| Notes | Study reports on 2 comparisons. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the child was withdrawn from the study, their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken, but it is unclear how frequently this occurred. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 8% (N = 5) in trial B were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: some children were not enrolled when the emergency department was busy, potential to bias participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Geelhoed 1995c.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children aged 3 months and older admitted to hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, hoarse voice) and a minimum croup score of 3 Exclusion criteria: croup score < 3 (mild croup); caregivers did not consent; family did not have a telephone; caregivers had limited English language abilities; received steroid therapy in the past week; pre‐existing conditions of the upper airway or prolonged stridor; clinical examination suggested a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 80):
|
|
| Interventions | Treatment 1 (N = 23): single dose (0.60 mg/kg) oral dexamethasone and 4 mL nebulised saline Treatment 2 (N = 27): single 2 mg (4 mL) dose nebulised budesonide and placebo Control (N = 30): single dose oral placebo and 4 mL of nebulised saline |
|
| Outcomes | Change in croup score from baseline to 2, 4, and 12 hours; re‐presentations with croup; length of hospital stay; use of epinephrine, use of supplemental glucocorticoids, and intubations | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the attending doctors considered patients to be severely ill or failing to improve, they could be withdrawn at any time to receive steroids, at which time their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: did not use intention‐to‐treat analysis for the telephone follow‐up. 17% (N = 9) withdrew, more in placebo than in treatment group (23% compared to 9%). An additional 10% (N = 8) were lost to follow‐up, unclear from which group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Geelhoed 1996a.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months presenting to the emergency department with a diagnosis of croup (acute onset of inspiratory stridor, barking cough, hoarseness, and chest wall retractions) not severe enough to warrant admission Exclusion criteria: other acute or chronic medical problems; families that did not have a telephone or had limited English language abilities; received any type of steroids in the preceding week; pre‐existing upper airway condition; history of prolonged stridor; clinical picture that suggested a diagnosis other than croup Baseline demographics (N = 100):
|
|
| Interventions | Treatment (N = 50): single 0.15 mg/kg dose of oral dexamethasone Control (N = 50): single dose of oral placebo |
|
| Outcomes | Reattendance at the emergency department with croup | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Treatments were given double blind" Comment: no further explanation given. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4% (N = 4) lost to follow‐up. Equal between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Geelhoed 2005.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months who presented to the emergency department with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, hoarse voice) and no other acute or chronic medical problems requiring admission. Croup was defined as the acute onset of inspiratory stridor, barking cough, hoarse voice, and chest wall retractions. Exclusion criteria: other acute or chronic medical problems; families that did not have a telephone or had limited English language abilities; received any type of steroids in the preceding week; pre‐existing upper airway condition; history of prolonged stridor; clinical picture that suggested a diagnosis other than croup Baseline demographics (N = 72):
|
|
| Interventions | Treatment (N = 36): single 0.15 mg/kg dose of oral dexamethasone and 2 mg of nebulised budesonide Control (N = 36): single 0.15 mg/kg dose of oral dexamethasone and a dose of equivalent volume of nebulised placebo (saline) |
|
| Outcomes | Change in croup score from baseline to 2 and 4 hours; readmissions for croup; length of stay in hospital | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized to 1 of 2 groups based on hospital pharmacy computer‐generated numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a nurse who had no further part in the management of the child administered treatment. Other staff and subjects were blinded to the nebulized treatments given." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1% (N = 1) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Godden 1997.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: November 1993 to April 1995 Setting: paediatric wards of Poole NHS Trust Hospital, Dorset, England Inclusion criteria: children admitted to hospital with a clinical diagnosis of croup based on the modified Westley croup score Exclusion criteria: receiving bronchodilators or received systemic steroids within the previous month Baseline demographics (N = 89):
|
|
| Interventions | Treatment (N = 47): initial 2 mg (4 mL) dose of nebulised budesonide, followed by a repeating dose of 1 mg every 12 hours Control (N = 42): initial 4 mL dose of nebulised placebo (normal saline), followed by a repeating dose of 2 mL placebo (normal saline) every 12 hours Both treatment and placebo were delivered via an opaque nebuliser chamber, driven by wall oxygen at a rate of 8 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 4, 12, and 24 hours; length of stay in hospital; use of epinephrine and intubation | |
| Notes | Baseline croup score not presented for 2 children due to prior treatment with nebulised L‐epinephrine. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "trial solution was supplied in an opaque respule within a sealed silver foil packet." "The patient initially received 4 mL of a solution containing either normal saline vehicle or 4mg (4mL) of budesonide, via an opaque nebuliser chamber." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8% (N = 7) withdrew, equal between groups (9% in study group, 7% in control group) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: some potentially eligible children were not enrolled due to manpower constraints, which could have biased participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Huang 2021.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: January 2019 to January 2020 Setting: Wuhan Children’s Hospital, Wuhan, China Inclusion criteria: children meeting the diagnostic criteria of acute infectious laryngitis in Zhufutang practical paediatrics confirmed by laryngoscope examination and diagnosed for the first time; had complete clinical data; written informed consent obtained from all guardians of patients Exclusion criteria: children with systemic diseases, congenital larynx and other systemic serious diseases, allergic to drugs used in this study, and presence of bronchial foreign bodies Baseline demographics (N = 92):
|
|
| Interventions | Treatment 1 (N = 46): 1.00 mg/kg of dexamethasone injection twice a day Treatment 2 (N = 46): budesonide inhalation therapy and 2.00 mL of budesonide added 3.00 mL normal saline twice a day |
|
| Outcomes | Time for clinical symptoms to disappear after 3 days of treatment; therapeutic effects; adverse events | |
| Notes | No sources of funding reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to either the study and control group with 46 cases in each group" Comment: stated only as randomly allocated. No details provided on the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description provided on the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no description provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "46 cases in each group; control group involved 18 males and 14 females" Comment: no participant flow diagram provided. Unclear if any participants were lost to follow‐up. Baseline characteristics of proportion of males and females do not add up in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified |
| Other bias | Low risk | Quote: "general data of the two groups were comparable (P > 0.05)" Comment: baseline characteristics between groups were comparable with no significant differences |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Husby 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1990 to December 1991 Setting: Department of Paediatrics, Kolding Hospital, Denmark Inclusion criteria: children admitted to hospital with croup (inspiratory stridor, cough, and respiratory distress) with a modified Westley croup score > 5 and informed parental consent Exclusion criteria: clinical condition consistent with epiglottitis, foreign body aspiration, bronchiolitis, or asthma; received local or systemic steroid treatment or epinephrine Baseline demographics (N = 36) (1 child excluded before placebo was administered):
|
|
| Interventions | Treatment (N = 20): 2, 1000 µg (2 mL 500 µg/mL) doses of nebulised budesonide, 30 minutes apart Control (N = 16): 2, 2 mL doses of placebo (0.9% saline), 30 minutes apart Both treatment and placebo were given with a dynamic flow rate of 8 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 hours; use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind, no further explanation. Insufficient information provided to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 child omitted as did not receive treatment due to technical problems. No other missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
James 1969.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: September 1965 to November 1966 (excluding 2 periods during May and August 1966, total of 6 weeks) Setting: children's division of the Los Angeles County‐University of Southern California Medical Center, USA Inclusion criteria: children admitted to hospital with a diagnosis of croup or laryngotracheobronchitis (dyspnoea with inspiratory stridor, subcostal, suprasternal, or sternal retractions, and a barking, seal‐like cough) Exclusion criteria: very mild stridor at admission; history of persistent or congenital stridor; suspected diagnosis of acute epiglottitis; clinical or roentgenographic evidence of an associated pneumonitis Baseline demographics (N = 88):
|
|
| Interventions | Treatment (N = 45): single 4 mg/mL dose of intramuscular dexamethasone sodium phosphate Control (N = 43): single 4 mg/mL dose of placebo solution identical in appearance Both treatment and placebo were administered 0 to 3 hours after admission. |
|
| Outcomes | Patient improvement at 12 and 24 hours; use of antibiotics and tracheostomy | |
| Notes | Funding source: Merck Sharp & Dohme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "dexamethasone... and a placebo solution of identical appearance were provided in randomly numbered vials. As each patient was admitted to the study, he received the predetermined dose of medication from the next bottle in the series, after which the bottle was discarded." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...solution of identical appearance were provided in randomly numbered unlabeled vials" "All of the evaluations were completed before the drug code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...solution of identical appearance were provided in randomly numbered unlabeled vials" "All of the evaluations were completed before the drug code was broken" Comment: outcome measures assessed by staff and in some cases also by the investigator. Both staff and investigator appear to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Johnson 1996.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1989 to November 1991 Setting: emergency department of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children presenting to the emergency department between 8:00 a.m. and midnight with signs and symptoms consistent with acute laryngotracheitis, including seal‐like barking cough and inspiratory stridor that had developed 2 to 72 hours before they were seen; persistent moderate respiratory distress (modified Westley croup score 2.5 to 5) after having been treated for at least 30 minutes with humidified oxygen provided by plastic tubing aimed toward the nose and mouth; written parental informed consent Exclusion criteria: history of congenital stridor or endotracheal intubation longer than 1 month; signs or symptoms suggesting another cause of stridor (e.g. bacterial tracheitis, epiglottitis, supraglottic foreign body, spasmodic croup); presence of marked expiratory wheeze that responded to treatment with bronchodilators; history of chronic respiratory problems such as asthma requiring routine daily treatment with bronchodilators; presence of a severe systemic disease that would affect the decision to admit or discharge; history of receiving corticosteroids in the last 2 weeks or racaemic epinephrine hydrochloride in the last 4 hours Baseline demographics (N = 55):
|
|
| Interventions | Treatment (N = 28): single 10 mg (< 8 kg body weight), 15 mg (8 to 12 kg body weight), or 20 mg (> 12 kg body weight) dose of nebulised parenteral dexamethasone sodium phosphate solution (10 mg/mL) mixed with normal saline to make 4 mL provided with 100% oxygen at a flow rate of 6 to 7 L/min Control (N = 27): placebo (normal saline) provided in the same fashion All children were also treated with humidified oxygen supplied by plastic tubing aimed towards the nose and mouth throughout the study period. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; hospitalisation rate; improvement at 4 hours; use of epinephrine, use of mist tent, intubation, and use of additional glucocorticoids | |
| Notes | Funding source: Pediatric Consultants, The Hospital for Sick Children, Toronto, Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized in blocks of 10" Comment: block randomisation assumed to be computer‐generated |
| Allocation concealment (selection bias) | Low risk | Quote: "the randomized code was generated and held by the hospital pharmacy until after enrolment of all patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pharmacy dispensed the appropriate medication in a vial specially prepared for this study, based on a randomization schedule" "To ensure blinding of investigators, staff, and parents, nebulizer containers were covered during and after nebulization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The pharmacy dispensed the appropriate medication in a vial specially prepared for this study, based on a randomization schedule" "To ensure blinding of investigators, staff, and parents, nebulizer containers were covered during and after nebulization" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% (N = 9) protocol deviations not included in the analysis. More losses in the intervention group, but unclear if this was significant |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Johnson 1998.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: September 1993 to May 1996 Setting: emergency departments of The Hospital for Sick Children in Toronto and Alberta Children's Hospital in Calgary, Canada Inclusion criteria: children aged from 3 months to 9 years presenting to the emergency department between 3:00 p.m. and 6:00 a.m. who were given a diagnosis of croup (acute onset of inspiratory stridor associated with a seal‐like barking cough) with persistent moderately severe respiratory distress (modified Westley croup score of 3 to 6) after being treated with humidified oxygen for 30 minutes Exclusion criteria: signs and symptoms suggesting another cause of stridor (e.g. epiglottitis, bacterial tracheitis, supraglottic foreign body); parents who were unable to speak English well enough to give informed consent; history of chronic pulmonary disease, severe systemic disease, immune dysfunction, stridor, intubation for more than 1 month; glucocorticoid therapy in the 4 weeks prior to entering the study Baseline demographics (N = 144):
|
|
| Interventions | Treatment 1 (N = 48): single 4 mg dose of nebulised budesonide Treatment 2 (N = 47): single 0.6 mg/kg dose of intramuscular dexamethasone Control (N = 49): single dose of nebulised placebo suspension All children received 0.5 mL of 2.25% racepinephrine and normal saline combined with either treatment or placebo (total volume 8 mL) via nebuliser with oxygen from a wall outlet at a rate of 6 to 7 L/min through a face mask held tightly to the child's face over a period of 20 minutes. |
|
| Outcomes | Change in modified Westley croup score from baseline to 5 hours or discharge; rate of hospitalisation; use of epinephrine, use of supplemental glucocorticoids, intubations | |
| Notes | Funding source: Astra Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked randomization code was produced by random‐number generating software." |
| Allocation concealment (selection bias) | Low risk | Quote: "A blocked randomization code... provided only to the pharmacy at the hospital" "The pharmacies prepared sequential patient packets containing study drugs that were sealed and were identical in appearance and weight." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "To make the nebulized study drugs indistinguishable from each other, they were packaged in opaque containers and discharged directly into a colored nebulizer" "to maintain masking, the study nurse temporarily took the parents away from their child while an emergency staff nurse not otherwise involved in the care of the child injected the dexamethasone into the child's thigh, placed a bandage over the injection site (all children received a bandage whether or not they received dexamethasone), and initiated nebulization." Comment: blinding was attempted, but it could have been broken. Unmasking occurred in 3 cases. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessor described as blinded, but blinding could have been broken. Unmasking occurred in 3 cases. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used, including 17% (N = 25) with protocol deviations. 2% (N = 1) from the treatment group were lost to follow‐up because parents could not be reached. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Klassen 1994.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1992 to October 1993 Setting: emergency department at the Children's Hospital of Eastern Ontario, Canada Inclusion criteria: children aged from 3 months to 5 years presenting to the emergency department between 9:00 a.m. and midnight (except holidays) with mild to moderate croup consisting of hoarseness, inspiratory stridor, and barking cough, and a Westley croup score ≥ 2 after breathing humidified oxygen for at least 15 minutes Exclusion criteria: diagnosis of epiglottitis or chronic upper or lower airway disease (not including asthma); corticosteroids administered within the past 2 weeks; severe croup (defined as a Westley croup score of 8 or higher or requiring treatment with racaemic epinephrine immediately on arrival) Baseline demographics (N = 54):
|
|
| Interventions | Treatment (N = 27): single 2 mg (4 mL) dose of nebulised budesonide Control (N = 27): single 4 mL dose of nebulised placebo (0.9% saline solution) Both treatment and placebo administered by an updraft nebuliser with a continuous flow of oxygen at 5 to 6 L/min. |
|
| Outcomes | Change in Westley croup score from baseline to 4 hours; admissions to the hospital; 2‐point improvement in croup score at 4 hours; use of epinephrine, use of supplemental glucocorticoids | |
| Notes | Funding source: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 10 by the pharmacy department, with a random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization list was kept concealed from the research assistants, parents and emergency physicians and from the child's regular physician until the end of the trial." "the pharmacy provided both budesonide and normal saline in opaque brown syringes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the pharmacy provided both budesonide and normal saline in opaque brown syringes to ensure blinding. The research assistants then placed the study drug directly into an opaque nebulizer reservoir." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the pharmacy provided both budesonide and normal saline in opaque brown syringes to ensure blinding. The research assistants then placed the study drug directly into an opaque nebulizer reservoir." Comment: research assistants blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not use intention‐to‐treat analysis. 7% (N = 4) lost, all from the placebo group (15%) due to worsening condition or lack of satisfaction with treatment. Unbalanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: 24 children were not enrolled because the emergency department failed to contact the study team; this could potentially have biased participant selection |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Klassen 1996.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1993 to April 1994 Setting: emergency department of the Children's Hospital of Eastern Ontario, Canada Inclusion criteria: children aged 3 months to 5 years presenting to the emergency department with mild to moderate croup (hoarseness, inspiratory stridor, barking cough) and a modified Westley croup score ≥ 3 after at least 15 minutes of mist therapy Exclusion criteria: diagnosis of epiglottitis, chronic upper or lower airway disease (excluding asthma), severe croup (modified Westley croup score ≥ 8); received glucocorticoids within the previous 2 weeks; needed immediate racaemic epinephrine on arrival Baseline demographics (N = 50):
|
|
| Interventions | All children received a single 0.60 mg/kg dose of oral dexamethasone upon entry into the study. Treatment (N = 25): single 2 mg (4 mL) dose of nebulised budesonide Control (N = 25): single 4 mL dose of placebo (0.9% saline solution) Both treatment and control delivered by an updraft nebuliser with a continuous flow of oxygen at a rate of 5 to 6 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 4 hours; admissions to the hospital; 2‐point improvement in croup score at 4 hours; use of epinephrine, use of supplemental glucocorticoids, and use of mist tent | |
| Notes | Funding source: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 10 by the pharmacy department, using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed... by the pharmacy department"; "the pharmacy provided both budesonide and normal saline in opaque, brown syringes."; "the randomization code was revealed only after all patients had completed the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Budesonide is slightly opaque; therefore, to conceal its identity, the pharmacy provided both budesonide and normal saline in opaque, brown syringes. The research assistants placed the drugs directly into an opaque nebulizer reservoir. Once nebulized, the drugs were indistinguishable by sight and smell." "Both the research assistants and the physicians caring for the patients in the emergency department were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Budesonide is slightly opaque; therefore, to conceal its identity, the pharmacy provided both budesonide and normal saline in opaque, brown syringes. The research assistants placed the drugs directly into an opaque nebulizer reservoir. Once nebulized, the drugs were indistinguishable by sight and smell." "Both the research assistants and the physicians caring for the patients in the emergency department were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2% (N = 1) in the placebo group required racaemic epinephrine and was excluded; 2% (N = 1) lost to follow‐up in the treatment group because parent could not be contacted for follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: 33 children were not enrolled because the study team was not contacted; this could potentially have biased participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Klassen 1998.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1995 to April 1996 and October 1996 to January 1997 Setting: emergency departments of the Children's Hospital of Eastern Ontario, Ottawa, or the Winnipeg Children's Hospital, Winnipeg, Canada Inclusion criteria: children aged 3 months to 5 years who presented to the emergency department with croup (hoarseness, inspiratory stridor, and barking cough) and Westley croup score of ≥ 2 following at least 15 minutes of mist therapy; parents available for telephone follow‐up a week after enrolling in the study Exclusion criteria: epiglottitis; chronic respiratory disease (except asthma); severe croup (Westley croup score ≥ 8); racaemic epinephrine treatment upon arriving at the emergency department; glucocorticoids in the last 2 weeks; history of tuberculosis in child or household; chickenpox or exposure to it within the past 21 days; known immunodeficiency Baseline demographics (N = 198):
|
|
| Interventions | Treatment 1 (N = 65): single 2 mg (4 mL) dose of nebulised budesonide plus the appropriate volume of oral placebo (clear syrup solution) Treatment 2 (N = 69): single 4 mL dose of nebulised placebo (saline solution) plus 0.6 mg/kg oral dexamethasone Treatment 3 (N = 64): single 4 mL dose of nebulised budesonide plus 0.6 mg/kg of oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 4 hours (or until discharge); admissions to hospital; length of stay in the emergency department; 2‐point improvement in croup score at 4 hours; use of epinephrine and use of additional glucocorticoids | |
| Notes | Funding source: Ontario Ministry of Health; Emergency Health Services, Toronto, Ontario; Manitoba Medical Services Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central pharmacy... randomized individuals to the 3 groups, using computer‐generated random numbers in random blocks of 6 or 9" |
| Allocation concealment (selection bias) | Low risk | Quote: "the list was kept at a central pharmacy until the end of the study to ensure allocation concealment. Because the drugs were packaged identically and identified only by a sequential study number, the research assistant who administered the intervention remained unaware of the next group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dexamethasone syrup and placebo dexamethasone syrup were identical in taste and appearance... All solutions were packaged in brown syringes and the research assistant instilled either solution directly into an opaque nebulizer reservoir." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Dexamethasone syrup and placebo dexamethasone syrup were identical in taste and appearance... All solutions were packaged in brown syringes and the research assistant instilled either solution directly into an opaque nebulizer reservoir." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 1% (N = 1) loss to follow‐up in the treatment group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: 10 children were not enrolled because the study team was not contacted; this could potentially have biased participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Koren 1983.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: January 1979 to January 1980 Setting: paediatric division of the Chaim Sheba Medical Center, Tel Hashomer, Israel Inclusion criteria: children aged 8 months to 8 years hospitalised with croup (all with inspiratory stridor, dyspnoea, subcostal and/or suprasternal retraction, and a barking cough); informed consent given by the parents Exclusion criteria: evidence of associated bronchitis, bronchopneumonia, and acute epiglottitis Baseline demographics (N = 78):
|
|
| Interventions | Children in all groups were sedated via rectal administration of 75 mg/kg of body weight of chloral hydrate. Treatment (N = 40): single 0.60 mg/kg dose of intramuscular dexamethasone sodium phosphate (4 mg/mL) Control (N = 38): single dose of intramuscular placebo solution of identical appearance |
|
| Outcomes | Use of epinephrine and use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dexamethasone sodium phosphate and a placebo solution of identical appearance were administered" "All evaluations were completed before the study code was opened" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Kuusela 1988.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1984 to March 1985 and January 1986 to April 1986 Setting: Department of Paediatrics, Tampere University Central Hospital, Finland Inclusion criteria: children diagnosed and admitted for acute laryngitis (croup) Exclusion criteria: not reported Baseline demographics (N = 72):
|
|
| Interventions | All children received initial treatment in the emergency department, then were transferred to a ward where they were placed in a humid room and given oral fluids at a minimum of 20 mL/kg body weight over the next 4 hours. Treatment 1 (N = 19): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular dexamethasone plus at least 1, 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised L‐epinephrine (2.25% epinephrine base with 0.5% chlorobutanol as preservative). Additional doses of nebulised L‐epinephrine every 2 hours as needed Treatment 2 (N = 16): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular dexamethasone plus at least 1, 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised placebo solution. Additional doses of nebulised placebo every 2 hours as needed Treatment 3 (N = 16): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular placebo plus at least 1, 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised L‐epinephrine (2.25% epinephrine base with 0.5% chlorobutanol as preservative). Additional doses of nebulised L‐epinephrine every 2 hours as needed Control (N = 21): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular placebo plus at least 1, 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised placebo solution. Additional doses of nebulised placebo every 2 hours as needed |
|
| Outcomes | Change in clinical score based on dyspnoea and cough scale from baseline to 6, 12, and 24 hours; length of stay in hospital | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "the ampules were unlabelled, numbered, and randomized; the code was not available for investigators until the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo preparation consisted of the corresponding diluent supplied in similar ampules."; "the code was not available for the investigators until the end of the study"; "The active solution and the placebo preparation were identically packed in individual randomized vials containing 10 ml" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: did not use intention‐to‐treat analysis. 8% (N = 6) excluded due to protocol violations, unclear what group they were in |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Leipzig 1979.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: November 1976 to March 1978 Setting: Pediatric Service of the State University Hospital or the Crouse‐Irving Memorial Hospital, Syracuse, NY, USA Inclusion criteria: all children admitted to hospital with a diagnosis of croup with disease of sufficient severity on a predetermined scoring system; consent of the child's physician and parents Exclusion criteria: not reported Baseline demographics (N = 30):
|
|
| Interventions | Treatment (N = 16): 2, 0.30 mg/kg doses of intramuscular dexamethasone (4 mg/mL) Control (N = 14): 2 doses of intramuscular placebo (sterile saline) (1 dose initially and another 2 hours later) |
|
| Outcomes | Change in croup score from baseline to 24 hours; length of stay at hospital; intubation | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned from a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Vials had been previously prepared containing either dexamethasone (4 mg/L) or sterile saline. They were marked only with a number, assigned from a table of random numbers" Comment: described as double‐blind. Unclear who was blinded and who prepared the vials. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Vials had been previously prepared containing either dexamethasone (4 mg/L) or sterile saline. They were marked only with a number, assigned from a table of random numbers" Comment: described as double‐blind. Unclear who was blinded and who prepared the vials. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Luria 2001.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: September 1995 to December 1997 Setting: emergency departments at either Children's Hospital Medical Center in Cincinnati or Children's Hospital in Columbus, OH, USA Inclusion criteria: children aged 6 months to 6 years presenting to the emergency department with mild croup (barky cough, stridor and/or hoarseness for < 48 hours) and having a viral prodrome consisting of fever, cough, or rhinorrhoea (in an attempt to exclude children with spasmodic croup) Exclusion criteria: treated with corticosteroids 14 days prior to enrolling in the study; a clinical picture consistent with spasmodic croup; history of prolonged endotracheal intubation; history of chronic respiratory illness (i.e. asthma or cystic fibrosis); a condition associated with airway abnormalities; those without a working telephone or with a severe disease (i.e. received nebulised racaemic epinephrine or corticosteroids at the order of the emergency department physician or had < 94% oxygen saturation) Baseline demographics (N = 264):
|
|
| Interventions | Treatment group 1 (N = 85): single 0.60 mg/kg (maximum 10 mg) dose of oral dexamethasone (1 mg/mL) plus nebulised placebo solution Treatment group 2 (N = 91): single dose of oral placebo plus a 160 μg dose of nebulised dexamethasone sodium phosphate Control (N = 88): single dose of oral placebo plus nebulised placebo solution Nebulised study preparations were delivered with a nebuliser that had a fill volume of 3 mL and the oxygen flow set at 5 to 6 L/min. |
|
| Outcomes | Return visits to the emergency department | |
| Notes | Funding source: the Bremer Foundation at the Ohio State University, Columbus, OH, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed in blocks of 15 by the study pharmacist at each enrolling site with the use of a random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study pharmacist then assembled numbered 'croup kits' containing study preparations that reflected the results of the randomization." "The study physician retrieved the lowest numbered kit when enrolling a new subject to maintain the randomization order. Only pharmacists knew the results of the randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The kits were sealed to prevent any tampering and were kept in the EDs... Only the study pharmacists knew the results of the randomization... All oral study preparations were mixed 1:1 with a commercially available grape flavouring to minimize taste bias." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: intention‐to‐treat analysis used, including N = 9 protocol deviations. 16% (N = 43) loss on day 7 for the telephone follow‐up (14% in the oral treatment group, 15% in the nebulised treatment group, 19% in the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Martinez Fernandez 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1989 to September 1990 Setting: children's hospital in Spain Inclusion criteria: children hospitalised with symptoms suggestive of croup (acute laryngitis, laryngotracheobronchitis, spasmodic croup) Exclusion criteria: child's croup judged by the physician to be too severe Baseline characteristics (N = 66):
|
|
| Interventions | Treatment 1 (N = 15): single dose of intramuscular placebo, plus 0.14% nebulised L‐epinephrine initially and every 4 hours as needed Treatment 2 (N = 16): single 0.5 mg/kg dose of intramuscular dexamethasone, plus nebulised placebo (saline) initially and every 4 hours as needed Treatment 3 (N = 18): single 0.5 mg/kg dose of intramuscular dexamethasone, plus 0.14% nebulised L‐epinephrine initially and every 4 hours as needed Control (N = 17): single dose of intramuscular placebo, plus nebulised placebo (saline) initially and every 4 hours as needed All children received humidified oxygen and fluid therapy. |
|
| Outcomes | Change in croup score from baseline to 6, 12, and 24 hours | |
| Notes | Written in Spanish Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Low risk | Comment: treatments shipped in pre‐numbered ampoules, unlabelled and randomly ordered by the pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: described as double‐blind. Treatments shipped in pre‐numbered ampoules, unlabelled and randomly ordered by the pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Massicotte 1973.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: December 1971 to March 1972 Setting: L'Hôpital Sainte‐Justine, Montreal, Quebec, Canada Inclusion criteria: children admitted to hospital with severe croup (croup score ≥ 9) Exclusion criteria: mild or moderate croup (croup score < 9); require tracheotomy on admission (altered consciousness, peribuccal cyanosis); those with epiglottitis, foreign body aspiration, diphtheria, pharyngeal abscess, acute or chronic medical conditions; corticosteroids in the past 48 hours, allergy to penicillin or ampicillin Baseline characteristics (N = 42):
|
|
| Interventions | All children were placed in a humidified room and received intravenous saline, and additionally received 100 mg ampicillin/24 hours in 4 doses (1 dose every 6 hours) over the course of 10 days. Treatment (N = 25): single 4 mg/kg dose of intravenous methyl‐prednisolone initially (40 mg for 6 to 8 kg; 60 mg for 9 to 12 kg; 80 mg for 13 to 16 kg; 120 mg for 17 to 20 kg), followed by repeated doses at 4 and 8 hours, if needed Control (N = 17): single 4 mg/kg dose of intravenous placebo (lactose) initially, followed by repeated doses at 4 and 8 hours, if needed |
|
| Outcomes | Change in croup score from baseline to 4 and 14 hours; patient improvement at 4 and 14 hours | |
| Notes | Written in French Funding source: Upjohn Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random numbers table used |
| Allocation concealment (selection bias) | Low risk | Comment: the treatments were identical in appearance, containing either methyl‐prednisolone or placebo. Administration was double‐blind, and the code was not broken until the last child had completed the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the treatments were identical in appearance, containing either methyl‐prednisolone or placebo. Administration was double‐blind, and the code was not broken until the last child had completed the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Parker 2019.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: March 2009 to July 2012 Setting: paediatric emergency department of Princess Margaret Hospital for Children and emergency department of Joondalup Health Campus, Perth, Western Australia Inclusion criteria: children aged > 6 months, contactable by telephone, and English‐speaking caregivers. A maximum weight of 20 kg was imposed to limit the maximum possible dexamethasone dose to 12 mg (adult dose). Exclusion criteria: known prednisolone or dexamethasone allergy, immunosuppressive disease or treatment, steroid therapy or enrolment in the same study within the previous 14 days, and a high clinical suspicion of an alternative diagnosis, with specific prompts to include bacterial tracheitis, inhaled foreign body, retropharyngeal abscess, epiglottitis, angio‐oedema, vascular ring, and subglottic stenosis Baseline demographics (N = 1231):
|
|
| Interventions | Treatment 1 (N = 410): single 0.60 mg/kg dose of oral dexamethasone Treatment 2 (N = 410): single 0.15 mg/kg dose of oral dexamethasone Treatment 3 (N = 411): single 1.00 mg/kg dose of prednisolone |
|
| Outcomes | Change in Westley croup score from baseline to 2 hours; reattendance with croup; length of stay; use of epinephrine; intubation; use of supplemental glucocorticoids; any adverse events | |
| Notes | Funding source: Princess Margaret Hospital Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization list was computer‐generated at www.randomization.com by using randomly permuted block sizes in the ratio of 4 patients from each group, with block randomization by center" |
| Allocation concealment (selection bias) | Low risk | Quote: "All medications were prepared, randomly assigned, and labeled by a clinical trials pharmacist at Princess Margaret Hospital." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both staff (administering and assessing treatments) and patients were therefore blinded to the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both staff (administering and assessing treatments) and patients were therefore blinded to the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The analysis was conducted per protocol via intention to treat" "The distribution of repeat enrollments and patients meeting an exclusion criterion was relatively balanced across treatment groups (dexamethasone/low‐dose dexamethasone/prednisolone distributed at 1:1.48:1.14 and 1:1.29: 1.71, respectively)" Comment: 141/1231 (11%) participants were missing at hour 1 of croup assessment with reasons for their exclusion unexplained |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified study outcomes of interest have been reported in the results |
| Other bias | Unclear risk | Comment: several children were enrolled in this study more than once |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain was judged as unclear risk |
Rittichier 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: October 1996 to June 1999 Setting: emergency department at children's hospital in Denver, CO, USA Inclusion criteria: children aged 3 months to 12 years presenting to the emergency department with moderate croup (hoarseness and barky cough associated with either a history/presence of stridor at rest, and/or retractions) of < 48 hours onset of illness (defined as onset of barky cough) Exclusion criteria: children with epiglottitis; foreign body aspiration; reactive airway exacerbation; acute bacterial pneumonia; acquired or congenital upper airway anomalies such a tracheomalacia; immunocompromised; history of steroid exposure in the previous 2 weeks; children with mild croup (history or presence of a barky cough without the presence/history of the associated stridor or retractions); children with severe croup who had altered mental status, severe retractions, cyanosis associated with their croup; admitted to the hospital during the initial emergency department visit, either before consideration or after being enrolled in the study Baseline demographics (N = 277):
|
|
| Interventions | All children were given a cool mist therapy per emergency department protocol. Treatment 1 (N = 139): single 0.60 mg/kg (maximum 8 mg) dose of intramuscular dexamethasone Treatment 2 (N = 138): single 0.60 mg/kg (maximum 8 mg) dose of oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 24 hours; unscheduled return visits to the emergency department; use of epinephrine, use of additional glucocorticoids, use of mist tent, and use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random allocation chart based on a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization code was held by the nursing staff in the ED" Comment: randomisation code was held by nurses in the ED, and enrolment was performed by physicians, fellows, and residents. Unclear whether they could have determined the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Nurses administered the dexamethasone either orally or intramuscularly per hospital protocol." Comment: no blinding of participants and personnel. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To help with blinding of the physician... Band‐Aids were placed on all patients whether they received PO or IM medicine." "Caretakers were contacted... by a caller who was blinded to the route of administration... Caretakers were instructed to not disclose the route of administration of the medicine to the caller." Comment: blinding was attempted but could have been broken. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% lost to follow‐up (N = 13 protocol deviations; N = 27 could not be reached, of which data for N = 10 were lost in a hospital move). Unclear if losses were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Roberts 1999.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: April 1994 to April 1996 Setting: infectious diseases ward of the Women's and Children's Hospital in North Adelaide, Australia Inclusion criteria: children aged 6 months to 8 years admitted with croup (inspiratory stridor, barking cough, hoarse voice, and respiratory distress) and a croup score of ≥ 4; stable croup (2 scores taken 15 minutes apart within 1 point of each other); written informed consent from the parent Exclusion criteria: suspected epiglottitis, foreign body aspiration, bronchiolitis, pneumonia, or active asthma; intubation due to airways disease in the previous 12 months; acute wheezing; treatment with corticosteroids in the previous 4 weeks; treatment with adrenaline in the previous week; significant past or present pulmonary, cardiovascular, renal, hepatic, gastrointestinal, neurological, or endocrine disease that could interfere with the study; children unable to inhale the nebuliser mist for at least 1.5 minutes Baseline demographics (N = 82):
|
|
| Interventions | Treatment (N = 42): 2 mg/4 mL dose of nebulised budesonide every 12 hours for a maximum of 4 doses Control (N = 40): dose of nebulised placebo (same formulation but without budesonide) every 12 hours for a maximum of 4 doses Both treatment and placebo driven by an air or oxygen flow of 6 L/min. |
|
| Outcomes | Change in croup score from baseline to 2, 6, 12, 24 hours; revisits to the hospital for follow‐up; 2‐point improvement in croup score at 2, 6, and 12 hours; use of epinephrine | |
| Notes | Used a croup score similar to Leipzig 1979 with alterations to stridor assessment, oxygen saturation, and temperature Funding source: Astra Draco |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization in blocks of six were performed by Astra Draco" |
| Allocation concealment (selection bias) | Low risk | Quote: "All study medication was packaged identically, identified only by a study number... the randomisation code was kept at Astra Draco and broken only after study completion, hence all treatment decisions were made without awareness of study allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All respules and all ventstreams used in the study were made of an opaque plastic to conceal any differences between the active and placebo doses" "The randomization code was kept at Astra Draco and only broken after study completion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All respules and all ventstreams used in the study were made of an opaque plastic to conceal any differences between the active and placebo doses" "The randomization code was kept at Astra Draco and only broken after study completion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 20% (N = 10) lost due to withdrawals, protocol deviations, inability to contact parents. Used the last value extended principle for analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Roorda 1998.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1995 to March 1997 Setting: hospital in the Netherlands Inclusion criteria: children aged 4 to 52 months hospitalised with moderate croup Exclusion criteria: received systemic steroids, any bronchodilators, or antibiotics in the previous 48 hours Baseline demographics (N = 17):
|
|
| Interventions | Treatment (N = 9): 2 doses of 1000 µg of fluticasone propionate administered by metred dose inhaler (4 puffs of 250 µg), 30 minutes apart Control (N = 8): placebo administered in a similar fashion |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 6, and 24 hours; length of stay in hospital; use of additional glucocorticoids and intubation | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Skowron 1966a.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: December 1964 to March 1965 Setting: tracheitis ward of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children hospitalised with croup Exclusion criteria: not reported Baseline demographics (N = 200 in total, N = 94 for 1.0 mL dexamethasone compared to placebo, 6 excluded):
|
|
| Interventions | 1.0 mL (4 mg) dexamethasone compared to placebo (see Skowron 1966b for 1.5 mL (6 mg) dexamethasone compared to placebo) All children were placed in a croupette with moist air, given twice‐daily intramuscular crystalline sodium penicillin (825,000 IU/day) and streptomycin sulphate (0.5 g), as well as secobarbital, 3/4 grain per rectum on admission for children over 6 months. Treatment (N = 41): 1.0 mL (4 mg, based on approximately 0.4 mg/kg) subcutaneous dexamethasone every 6 hours for a total of 4 doses Control (N = 53): 1.0 mL placebo every 6 hours for a total of 4 doses |
|
| Outcomes | Readmissions to the hospital; length of stay in the hospital; tracheotomy | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators. As each child was admitted to the series, he received subcutaneously either material A or material B, according to a random selection code" Comment: bottles were not sequentially numbered, but instead labelled A or B. Unclear where the random selection code was held |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 6) lost due to protocol deviations |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: no baseline data presented, impossible to judge if baseline imbalances existed |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Skowron 1966a and b.
| Study characteristics | ||
| Methods | See Skowron 1966a and Skowron 1966b | |
| Participants | See Skowron 1966a and Skowron 1966b | |
| Interventions | See Skowron 1966a and Skowron 1966b | |
| Outcomes | See Skowron 1966a and Skowron 1966b | |
| Notes | See Skowron 1966a and Skowron 1966b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Allocation concealment (selection bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Selective reporting (reporting bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Other bias | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Overall risk of bias All outcomes | Unclear risk | See Skowron 1966a and Skowron 1966b |
Skowron 1966b.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: December 1964 to March 1965 Setting: tracheitis ward of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children hospitalised with croup Exclusion criteria: not reported Baseline demographics (N = 200 in total, N = 100 for 1.5 mL dexamethasone compared to placebo):
|
|
| Interventions | 1.5 mL (6 mg) dexamethasone compared to placebo (see Skowron 1966a for 1.0 mL dexamethasone compared to placebo) All children were placed in a croupette with moist air, given twice‐daily intramuscular crystalline sodium penicillin (825,000 IU/day) and streptomycin sulphate (0.5 g), as well as secobarbital, 3/4 grain per rectum on admission for children over 6 months. Treatment (N = 56): 1.5 mL (6 mg, based on approximately 0.5 mg/kg) subcutaneous dexamethasone every 6 hours for a total of 4 doses Control (N = 44): 1.5 mL placebo every 6 hours for a total of 4 doses |
|
| Outcomes | Readmissions to the hospital; length of stay in the hospital; tracheotomy | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators. As each child was admitted to the series, he received subcutaneously either material A or material B, according to a random selection code" Comment: bottles were not sequentially numbered, but instead labelled A or B. Unclear where the random selection code was held |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 6) lost due to protocol deviations |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: no baseline data presented, impossible to judge if baseline imbalances existed |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Soleimani 2013.
| Study characteristics | ||
| Methods | Randomised, single‐blind controlled trial | |
| Participants |
Study period: January 2009 to March 2010 Setting: emergency department at Ali‐Ebne Abitaleb Hospital, Iran Inclusion criteria: children aged 6 months to 6 years admitted to the emergency department with barking cough, stridor, hoarseness, and respiratory distress Exclusion criteria: chronic pulmonary disease; severe croup (croup score > 7); recurrent croup; allergy to corticosteroids; contraindication of corticosteroid (history of tuberous sclerosis, history of varicella infection during the past 3 weeks); history of corticosteroid administration during the last 4 weeks; foreign body; epiglottitis; bacterial tracheitis; immune deficiency Baseline demographics (N = 68):
|
|
| Interventions | Treatment 1 (N = 36): single dose 0.60 mg/kg intramuscular dexamethasone Treatment 2 (N = 32): single dose 0.60 mg/kg oral dexamethasone |
|
| Outcomes | Return visits or (re)admissions or both | |
| Notes | Not indexed in the databases searched (located via a search of Google.ca) Funding source: Zahedan University of Medical Sciences, Zahedan, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as "randomly divided"; unclear how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel were not blinded. Treatments were clearly distinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessor described as blinded. Unclear how they were blinded and if the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 15% loss to follow‐up; unclear from which group children were lost. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Sparrow 2006.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months who presented to the emergency department with mild to moderate croup defined by clinical symptoms (acute onset of inspiratory stridor and a hoarse voice accompanied by a barking cough) and a modified Taussig croup score of < 5 Exclusion criteria: children whose families did not have a telephone; limited English language proficiency; received steroids Baseline demographics (N = 133):
|
|
| Interventions | Treatment (N = 65): single 1 mg/kg dose of oral prednisolone Comparator (N = 68): single 0.15 mg/kg dose of oral dexamethasone |
|
| Outcomes | Unscheduled re‐presentations to medical care; time spent in the emergency department; use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated orders randomised into blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The PMH pharmacy ensured that the two steroid preparations could not be differentiated, the code being held by the pharmacy. Bottles were simply labelled solution A or solution B" Comment: bottles were not sequentially numbered, but instead labelled A or B |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Bottles were simply labelled solution A or solution B, and following randomization... given by a nurse who took no further part in the child's care."; "the code was not broken until all data were collected and all follow up completed"; "similar taste and appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Unclear risk | Comment: many children were not approached because the emergency department was busy in the winter; this could potentially have biased participant selection |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Super 1989.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: October 1983 to April 1985 Setting: Cleveland Metropolitan General Hospital or Rainbow Babies and Children's Hospital, OH, USA Inclusion criteria: children admitted to hospital with a diagnosis of croup (barking cough, inspiratory stridor, hoarseness) with a viral prodrome consisting of rhinorrhoea, cough, or fever and a modified Westley croup score ≥ 3 after 30 minutes of mist therapy Exclusion criteria: clinical picture consistent with acute epiglottitis, spasmodic croup, or pneumonia; history of a chronic illness except asthma; history of tracheal intubation or laryngoscopy Baseline demographics (N = 29):
|
|
| Interventions | All children received mist therapy for at least 30 minutes. Treatment (N = 16): single 0.6 mg/kg dose of parenteral dexamethasone Control (N = 13): single dose of parenteral placebo (saline) |
|
| Outcomes | Change in modified Westley croup score from baseline to 12 and 24 hours; length of stay in hospital; 2‐unit improvement in croup score at 12 and 24 hours; use of supplemental glucocorticoids and use of mist tent | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned, by a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "either parenterally administered dexamethasone or saline solution of the same color, volume and consistency as the dexamethasone. Randomization and drug preparation were done in the pharmacy" "The drug code was broken only after the last patient completed the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patient nor the investigators knew whether the patient received dexamethasone or placebo. The drug code was broken only after the last patient completed the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patient nor the investigators knew whether the patient received dexamethasone or placebo. The drug code was broken only after the last patient completed the study." "All decisions regarding the data analysis were made before the drug code was broken. Whenever possible these decisions were made in favour of the null hypothesis" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 17% (N = 5) lost to follow‐up (19% in treatment group due to early discharge and protocol deviation, 15% in placebo group due to early discharge or missed observations) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Tibballs 1992.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: Royal Children's Hospital, Melbourne, Australia Inclusion criteria: hospitalised children aged 6 months or older who required endotracheal intubation for upper airway obstruction caused by croup (defined as coryzal symptoms, fever, barking cough, hoarse voice, retraction, inspiratory stridor, or cyanosis developing over several days) Exclusion criteria: children younger than 6 months old; congenital airway anomalies; previous intubations; spasmodic croup (sudden onset without preceding fever or symptoms of upper respiratory tract infection) Baseline demographics (N = 70, 3 excluded):
|
|
| Interventions | All children received endotracheal intubation under inhalational anaesthesia with halothane, first with an oral endotracheal tube, in order to secure the airway rapidly and assess the diameter, and second substituted with a nasal tube. Humidification was provided with heat and moisture exchangers, with oxygen added as required. The tube was aspirated routinely every 1 to 2 hours to remove secretions. Treatment (N = 38): 1 mg/kg nasogastric prednisolone within 24 hours of intubation and then every 12 hours until 24 hours after extubation Control (N = 32, 3 excluded): 1 mg/kg of placebo within 24 hours of intubation and then every 12 hours until 24 hours after extubation |
|
| Outcomes | Use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "order determined by a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Unidentified placebo and prednisolone were supplied by the pharmacy in an order determined by a table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "Unidentified placebo and prednisolone were supplied by the pharmacy" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4% (N = 3) lost because of exclusion due to bacterial infection or protocol deviations, all in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
Vad Pedersen 1998.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Study period: October 1989 to September 1990 Setting: paediatric department of Esbjerg Centralsygehus (Central Hospital), Denmark Inclusion criteria: children hospitalised with croup based on a modified Westley croup score ≥ 3 (including chest wall retractions, barking cough, respiratory frequency, and stridor) Exclusion criteria: required immediate intensive care; clinical suspicion of epiglottitis; cyanosis; croup recurrence; had received local or systemic steroid treatment; being treated with carbamazepine, phenobarbital, or rifampicin Baseline demographics (N = 59, 2 excluded):
|
|
| Interventions | Treatment 1 (N = 27): 2, 1000 μg doses of inhaled budesonide at 30‐minute intervals Treatment 2 (N = 29): single 0.6 mg/kg (0.15 mL/kg) dose of intramuscular dexamethasone |
|
| Outcomes | Change in croup score from baseline to 6 and 12 hours; return visits to the hospital; use of supplemental glucocorticoids | |
| Notes | Written in Danish Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: block randomisation with varying block size |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unblinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: unblinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 2) who were randomised were excluded due to protocol deviations |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | High risk | Comment: baseline imbalance in croup score |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk |
Von Mühlendahl 1982.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial | |
| Participants |
Study period: January 1979 to April 1980 Setting: 3 paediatric clinics in West Berlin, Germany Inclusion criteria: children admitted to hospital with a diagnosis of pseudo‐croup to 1 of 3 paediatric clinics in West Berlin Exclusion criteria: children who were already somnolent or cyanotic at admission (stage III or IV or pseudo‐croup) Baseline demographics (N = 406; 349 included in the evaluation):
|
|
| Interventions | Treatment (N = 176): single dose 6 mg oral dexamethasone Control (N = 173): single dose 6 mg oral placebo |
|
| Outcomes | Change in croup score from baseline to 6 and 12 hours | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of 406 children, 57 (14%) failed to complete the study. 24 (7%) were eliminated due to protocol violation; 19 (5%) received further doses of steroids; and 4 (1%) developed measles. Unclear what group the lost children were in. Did not use intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes in the methods appeared in the results. |
| Other bias | Low risk | Comment: no other sources of bias identified |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk |
CI: confidence interval ED: emergency department IU: international units LOCF: last observation carried forward NHS: National Health Service SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anene 1996 | Randomised controlled trial; children were not diagnosed with croup |
| Bollobas 1965 | Not a randomised controlled trial; children were not diagnosed with croup |
| Cichy 1983 | Not a randomised controlled trial |
| Connolly 1969 | Randomised controlled trial; children were not diagnosed with croup |
| Couser 1992 | Randomised controlled trial; children were not diagnosed with croup |
| Eghbali 2016 | Randomised controlled trial; the intervention was L‐epinephrine |
| Faghihinia 2007 | Randomised controlled trial; no usable results were presented (unclear how many children were in each group) |
| Faraji‐Goodarzi 2018 | Randomised controlled trial; no relevant outcomes |
| Flisberg 1973 | Not a randomised controlled trial |
| Freezer 1990 | Not a randomised controlled trial; study was a retrospective chart review |
| Gill 2017 | Not a randomised controlled trial |
| Goddard 1967 | Randomised controlled trial; children were not diagnosed with croup |
| Gursanscky 2019 | Not a randomised controlled trial; review article |
| Haque 1981 | Not a randomised controlled trial; children in the control group received no treatment |
| Havaldar 1997 | Not a randomised controlled trial; children were not diagnosed with croup |
| Kelley 1992 | Not a randomised controlled trial; study was a retrospective chart review |
| Kotaniemi‐Syrjanen 2018 | Abstract only |
| Kunkel 1996 | Not a randomised controlled trial; intervention was epinephrine |
| Ledwith 1995 | Not a randomised controlled trial; intervention was epinephrine |
| Lee 2019 | Wrong intervention; intervention was epinephrine |
| Martensson 1960 | Not a randomised controlled trial |
| McDonogh 1994 | Not a randomised controlled trial; study was a retrospective chart review |
| Meskina 2019 | Wrong intervention; intervention was homeopathic drug |
| Mohammadzadeh 2014 | Randomised controlled trial; intervention was epinephrine |
| NCT01748162 | Randomised controlled trial; did not report any relevant outcomes |
| Novik 1960 | Not a randomised controlled trial |
| Osváth 1994 | Not a randomised controlled trial |
| Prendergast 1994 | Not a randomised controlled trial; intervention was epinephrine |
| Rizos 1998 | Not a randomised controlled trial |
| Roked 2015 | Not a randomised controlled trial; study was a retrospective chart review |
| Ross 1969 | Not a randomised controlled trial; study was a retrospective chart review |
| Serra 1997 | Not a randomised controlled trial |
| Sumboonnanonda 1997 | Randomised controlled trial; children in the control group received no treatment |
| Sussman 1964 | Randomised controlled trial; children were not diagnosed with croup |
| Tal 1983 | Randomised controlled trial; children were not diagnosed with croup |
| Tellez 1991 | Randomised controlled trial; children were not diagnosed with croup |
| Tyler 2022 | Not a randomised controlled trial; nested case‐control study |
| Wilhelmi 1976 | Not a randomised controlled trial |
Characteristics of studies awaiting classification [ordered by study ID]
Chen 2018.
| Methods | Randomised controlled trial |
| Participants |
Study period: November 2016 to April 2017 Participants: children with acute laryngitis |
| Interventions | Treatment (N = 40): 1.0 mg budesonide inhalation, and single injection of 0.3 to 0.5 mg/kg dexamethasone Comparator (N = 38): single injection of 0.3 to 0.5 mg/kg dexamethasone |
| Outcomes | Change in croup score from baseline to 12 and 24 hours |
| Notes | Full text could not be found via library. |
Characteristics of ongoing studies [ordered by study ID]
IRCT20190914044765N1.
| Study name | Comparison the effect of oral and intravenous dexamethasone effect on the mild and moderate croup treatment in children |
| Methods | Randomised controlled trial (parallel) |
| Participants |
Inclusion criteria: age 6 months until 72 months; temperature < 38 °C; no lung disease; no asthma; no recurrent use of bronchodilator in last week; not Westley croup score 2 until 7; no steroid use in last week Exclusion criteria: lung disease; severe croup; recurrent croup; allergy to steroid; prohibition to steroid use; steroid in last week; epiglottitis; tracheitis; temperature > 38 °C |
| Interventions | Treatment 1: oral dexamethasone Treatment 2: intravenous dexamethasone |
| Outcomes | Westley croup score; respiratory rate; heart rate; oxygen saturation; follow‐up 3‐day relapse |
| Starting date | 27 August 2019 |
| Contact information | Dr Ehsan Khoshnejad Afkham Kashan University of Medical Sciences, No. 65, Rajaii Ave, Somayeh Ave, Qom, Ghoum, 3715815548, Iran email: dr.ehkhaf@yahoo.com |
| Notes | Trial registration at the Iranian Registry of Clinical Trials (IRCT) (IRCT20190914044765N1) Funding source: no external funding specified |
Differences between protocol and review
The following new authors joined the review group for the 2022 update: Alex Aregbesola, Clara Tam, Asha Kothari, Mê‐Linh Lê, and Mirna Ragheb. As in the previous version of this review, we added an age range for children in our inclusion criteria (0 to 18 years). We did not extract data for some of the outcomes in the protocol because the newly included studies did not report these outcomes. We used GRADEpro GDT software to assess the certainty of the body of evidence (GRADEpro GDT). We updated the summary of findings tables for two comparisons and added a summary of findings table for one new comparison in the Additional tables section.
Contributions of authors
Alex Aregbesola (AA): study selection, data extraction and verification, risk of bias assessment, GRADE assessment, statistical analyses, manuscript preparation. Clara Tam (CT): data extraction and verification, risk of bias assessment, GRADE assessment, statistical analyses, manuscript preparation. Asha Kothari (AK): data extraction and verification, risk of bias assessment, GRADE assessment, manuscript preparation. Mê‐Linh Lê (ML): study selection, contribution to the manuscript. Mirna Ragheb (MR): data extraction and verification, risk of bias assessment, contribution to the manuscript. Terry P Klassen (TPK): clinical adviser, contribution to the manuscript.
Sources of support
Internal sources
-
The Children’s Hospital Foundation of Manitoba, Canada
Funding to support this update review
-
Alberta Research Centre for Health Evidence (ARCHE), University of Alberta, Canada
Funding for the completion of previous update and for previous versions of this review
-
TRanslating Emergency Knowledge for Kids (TREKK), Manitoba, Canada
Funding for the completion of this update
-
Canadian Coordinating Office for Health Technology Assessment (CCOHTA), Ottawa, Canada
Funding for previous versions of this review
-
Children's Hospital of Eastern Ontario Research Institute (CHEO RI), Ottawa, Canada
Funding for previous versions of this review
-
Thomas C. Chalmers Centre for Systematic Reviews, Ottawa, Canada
Funding for previous versions of this review
-
Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Madrid, Spain
Funding for previous versions of this review
-
Instituto Nacional de la Salud (INSALUD), Madrid, Spain
Funding for previous versions of this review
External sources
-
Alberta Heritage Foundation for Medical Research, Canada
Funding for previous versions of this review
Declarations of interest
Alex Aregbesola: declared that they have no conflict of interest. Clara Tam: declared that they have no conflict of interest. Asha Kothari: declared that they have no conflict of interest. Mê‐Linh Lê: declared that they have no conflict of interest. Mirna Ragheb: declared that they have no conflict of interest. Terry P Klassen: is an author of four of the included studies (Bjornson 2004; Klassen 1994; Klassen 1996; Klassen 1998).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alshehr 2005 {published data only}
- Alshehr M, Almegamsi T, Hammdi A. Efficacy of a small dose of oral dexamethasone in croup. Biomedical Research 2005;65(1):65-72. [Google Scholar]
Amir 2006 {published data only}
- Amir L, Hubermann H, Halevi A, Mor M, Mimouni M, Waisman Y. Oral betamethasone versus intramuscular dexamethasone for the treatment of mild to moderate viral croup. Pediatric Emergency Care 2006;22(8):541-4. [DOI] [PubMed] [Google Scholar]
Bjornson 2004 {published and unpublished data}
- Bjornson C, Klassen TP, Williamson J, Brant R, Plint A, Bulloch B, et al. A randomized trial of single dose of oral dexamethasone for mild croup. New England Journal of Medicine 2004;351(13):1306-13. [DOI] [PubMed] [Google Scholar]
Cetinkaya 2004 {published data only}
- Cetinkaya F, Tufekci BS, Kutluk G. A comparison of nebulized budesonide, and intramuscular, and oral dexamethasone for treatment of croup. International Journal of Pediatric Otorhinolaryngology 2004;68(4):453-6. [DOI] [PubMed] [Google Scholar]
Chub‐Uppakarn 2007 {published data only}
- Chub-Uppakarn S, Sangsupawanich P. A randomized comparison of dexamethasone 0.15 mg/kg versus 0.6 mg/kg for the treatment of moderate to severe croup. International Journal of Pediatric Otorhinolaryngology 2007;71(3):473-7. [DOI] [PubMed] [Google Scholar]
Cruz 1995 {published data only}
- Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics 1995;96(2 Pt 1):220-3. [PubMed] [Google Scholar]
Dobrovoljac 2012 {published data only}
- Dobrovoljac M, Geelhoed G. How fast does oral dexamethasone work in mild to moderately severe croup? A randomized double-blinded clinical trial. Emergency Medicine Australasia 2012;24(1):79-85. [DOI] [PubMed] [Google Scholar]
Donaldson 2003 {published data only}
- Donaldson D, Poleski D, Knipple E, Filips K, Reetz L, Pascula RG, et al. Intramuscular versus oral dexamethasone for the treatment of moderate-to-severe croup: a randomized double-blind trial. Academy of Emergency Medicine 2003;10(1):16-21. [DOI] [PubMed] [Google Scholar]
Duman 2005 {published data only}
- Duman M, Ozdemir D, Atasever S. Nebulized L-epinephrine and steroid combination in the treatment of moderate to severe croup. Clinical Drug Investigation 2005;25(3):183-9. [DOI] [PubMed] [Google Scholar]
Eboriadou 2010 {published data only}
- Eboriadou M, Chryssanthopoulou D, Stamoulis P, Damianidou L, Haidopoulou K. The effectiveness of local corticosteroids therapy in the management of mild to moderate viral croup. Minerva Pediatrica 2010;62(1):23-8. [PubMed] [Google Scholar]
Eden 1964 {published data only}
- Eden A, Larkin VP. Corticosteroid treatment of croup. Pediatrics 1964;33:768-9. [PubMed] [Google Scholar]
Eden 1967 {published data only}
- Eden AN, Kaufman A, Yu R. Corticosteroids and croup. Controlled double-blind study. JAMA 1967;200(5):403-4. [PubMed] [Google Scholar]
Fifoot 2007 {published data only}
- Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup: a randomized, double-blinded clinical trial. Emergency Medicine Australasia 2007;19(1):51-8. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1996 {published data only}
- Fitzgerald D, Mellis C, Johnson M, Allen H, Cooper P, Van Asperen P. Nebulized budesonide is as effective as nebulized adrenaline in moderately severe croup. Pediatrics 1996;97(5):722-5. [PubMed] [Google Scholar]
Garbutt 2013 {published data only}
- Garbutt JM, Conion B, Sterkel R, Baty J, Schechtman KB, Mandrell K, et al. The comparative effectiveness of prednisolone and dexamethasone for children with croup: a community-based randomized trial. Clinical Pediatrics 2013;52(11):1014-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geelhoed 1995a {published data only}
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. [DOI] [PubMed] [Google Scholar]
Geelhoed 1995b {published data only}
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. [DOI] [PubMed] [Google Scholar]
Geelhoed 1995c {published data only}
- Geelhoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo‐controlled trial. Pediatric Pulmonology 1995;20(6):355-61. [DOI] [PubMed] [Google Scholar]
Geelhoed 1996a {published data only}
- Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ 1996;313(7050):140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geelhoed 2005 {published data only}
- Geelhoed GC. Budesonide offers no advantage when added to oral dexamethasone in the treatment of croup. Pediatric Emergency Care 2005;21(6):359-62. [DOI] [PubMed] [Google Scholar]
Godden 1997 {published data only}
- Godden CW, Campbell MJ, Hussey M, Cogswell JJ. Double blind placebo controlled trial of nebulised budesonide for croup. Archives of Disease in Childhood 1997;76(2):155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2021 {published data only}
- Huang T, Xia Z, Li W. Efficacy of inhaled budesonide on serum inflammatory factors and quality of life among children with acute infectious laryngitis. American Journal of Otolaryngology - Head and Neck Medicine and Surgery 2021;42(1):102820. [DOI] [PubMed] [Google Scholar]
Husby 1993 {published data only}
- Husby S, Agertoft L, Mortensen S, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Archives of Disease in Childhood 1993;68(3):352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen S, Agertoft L, Husby S, Pedersen S. Pseudocroup treated with inhaled steroid (budesonide). A double-blind placebo-controlled trial. Ugeskrift for Laeger 1994;156(45):6661-3. [PubMed] [Google Scholar]
James 1969 {published data only}
- James JA. Dexamethasone in croup. A controlled study. American Journal of Diseases of Children 1969;117(5):511-6. [DOI] [PubMed] [Google Scholar]
Johnson 1996 {published data only}
- Johnson DW, Schuh S, Koren G, Jaffee DM. Outpatient treatment of croup with nebulized dexamethasone. Archives of Pediatrics & Adolescent Medicine 1996;150(4):349-55. [DOI] [PubMed] [Google Scholar]
Johnson 1998 {published data only}
- Johnson DW, Jacobson S, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. New England Journal of Medicine 1998;339(8):498-503. [DOI] [PubMed] [Google Scholar]
Klassen 1994 {published data only}
- Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild-to-moderate croup. New England Journal of Medicine 1994;331(5):285-9. [DOI] [PubMed] [Google Scholar]
Klassen 1996 {published data only}
- Klassen TP, Watters LK, Feldman ME, Sutcliffe T, Rowe PC. The efficacy of nebulized budesonide in dexamethasone‐treated outpatients with croup. Pediatrics 1996;97(4):463-6. [PubMed] [Google Scholar]
Klassen 1998 {published data only}
- Klassen TP, Craig WR, Moher D, Osmond MH, Pasterkamp H, Sutcliffe T, et al. Nebulized budesonide and oral dexamethasone for treatment of croup: a randomized controlled trial. JAMA 1998;279(20):1629-32. [DOI] [PubMed] [Google Scholar]
Koren 1983 {published data only}
- Koren GF. Corticosteroid treatment of laryngotracheitis v spasmodic croup in children. American Journal of Diseases of Children 1983;137(10):941-4. [DOI] [PubMed] [Google Scholar]
Kuusela 1988 {published data only}
- Kuusela AL, Vesikari T. A randomized double-blind, placebo-controlled trial of dexamethasone and racemic epinephrine in the treatment of croup. Acta Paediatrica Scandinavica 1988;77(1):99-104. [DOI] [PubMed] [Google Scholar]
Leipzig 1979 {published data only}
- Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in treatment of croup. Journal of Pediatrics 1979;94(2):194-6. [DOI] [PubMed] [Google Scholar]
Luria 2001 {published data only}
- Luria JW, Gonzalez-del-Rey JA, DiGuilio GA, McAneney CM, Olsen JJ, Ruddy RM. Effectiveness of oral or nebulized dexamethasone for children with mild croup. Archives of Pediatrics & Adolescent Medicine 2001;155(12):1340-5. [DOI] [PubMed] [Google Scholar]
Martinez Fernandez 1993 {published data only}
- Martinez Fernandez A, Sanchez GE, Rica EI, Echaniz UI, Alonso DM, Vilella CM, et al. Randomized double-blind study of treatment of croup with adrenaline and/or dexamethasone in children. Anales Españoles de Pediatria 1993;38:29-32. [PubMed] [Google Scholar]
Massicotte 1973 {published data only}
- Massicotte P, Tetreault L. Evaluation of methyl‐prednisolone in the treatment of acute laryngitis in children. Unión Médicale du Canada 1973;102(10):2064-72. [PubMed] [Google Scholar]
Parker 2019 {published data only}
- Parker CM, Cooper MN. Prednisolone versus dexamethasone for croup: a randomized controlled trial. Pediatrics 2019;144(3):e20183772. [DOI] [PubMed] [Google Scholar]
Rittichier 2000 {published data only}
- Rittichier KK, Ledwith CA. Outpatient treatment of moderate croup with dexamethasone: intramuscular versus oral dosing. Pediatrics 2000;106(6):1344-8. [DOI] [PubMed] [Google Scholar]
Roberts 1999 {published data only}
- Roberts GW, Master VV, Staugas RE, Raftos JV, Parsons DW, Coulthard KP, et al. Repeated dose inhaled budesonide versus placebo in the treatment of croup. Journal of Paediatrics and Child Health 1999;35(2):170-4. [DOI] [PubMed] [Google Scholar]
Roorda 1998 {published data only}
- Roorda RJ, Walhof CM. Effects of inhaled fluticasone propionate administered with metered dose inhaler and spacer in mild to moderate croup: a negative preliminary report. Pediatric Pulmonology 1998;25(2):114-7. [DOI] [PubMed] [Google Scholar]
Skowron 1966a {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528-31. [PMC free article] [PubMed] [Google Scholar]
Skowron 1966a and b {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528-31. [PMC free article] [PubMed] [Google Scholar]
Skowron 1966b {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528-31. [PMC free article] [PubMed] [Google Scholar]
Soleimani 2013 {published data only}
- Soleimani G, Daryadel A, Moghadam AA, Sharif MR. The comparison of oral and IM dexamethasone efficacy in croup treatment. Journal of Comprehensive Pediatrics 2013;4(4):175-8. [Google Scholar]
Sparrow 2006 {published data only}
- Sparrow A, Geelhoed G. Prednisolone versus dexamethasone in croup: a randomised equivalence trial. Archives of Disease in Childhood 2006;91(7):580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Super 1989 {published data only}
- Super DM, Cartelli NA, Brooks LJ, Lembo RM, Kumar ML. A prospective randomized double‐blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. Journal of Pediatrics 1989;115(2):323-9. [DOI] [PubMed] [Google Scholar]
Tibballs 1992 {published data only}
- Tibballs J, Shann FA, Landau LI. Placebo-controlled trial of prednisolone in children intubated for croup. Lancet 1992;340(8822):745-8. [DOI] [PubMed] [Google Scholar]
Vad Pedersen 1998 {published data only}
- Vad Pedersen L, Dahl M, Falk-Petersen HE, Larsen SE. Inhaled budesonide versus intramuscular dexamethasone in the treatment of pseudocroup [Inhaleret budesonid versus dexamethasone i.m. til behandling at pseudocroup]. Ugeskrift for Laeger 1998;160(15):2253-6. [PubMed] [Google Scholar]
Von Mühlendahl 1982 {published data only}
- Von Mühlendahl KE, Kahn D, Spohr HL, Dressler F. Steroid treatment of pseudo‐croup. Helvetica Paediatrica Acta 1982;37(5):431-6. [PubMed] [Google Scholar]
References to studies excluded from this review
Anene 1996 {published data only}
- Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double‐blind, placebo‐controlled trial. Critical Care Medicine 1996;24(10):1666-9. [DOI] [PubMed] [Google Scholar]
Bollobas 1965 {published data only}
- Bollobas B. On local adrenocortical hormone treatment of rhinolaryngologic diseases. Zeitschrift für Laryngologie, Rhinologie, Otologie und Ihre Grenzgebiete 1965;44(7):476-81. [PubMed] [Google Scholar]
Cichy 1983 {published data only}
- Cichy M, Pawlik J. Treatment of subglottic laryngitis in children. Otolaryngologia Polska 1983;37(1):11-3. [PubMed] [Google Scholar]
Connolly 1969 {published data only}
- Connolly JH, Field C, Glasgow J. A double blind trial of prednisolone in epidemic bronchiolitis due to respiratory syncytial virus. Acta Pediatrica Scandinavica 1969;58(2):116-20. [DOI] [PubMed] [Google Scholar]
Couser 1992 {published data only}
- Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE. Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. Journal of Pediatrics 1992;121(4):591-6. [DOI] [PubMed] [Google Scholar]
Eghbali 2016 {published data only}
- Eghbali A, Sabbagh A, Bagheri B, Taherahmade H, Kahbazi M. Efficacy of nebulized L-epinephrine for treatment of croup: a randomized, double-blind study. Fundamental & Clinical Pharmacology 2016;30(2016):70-5. [DOI: 10.1111/fcp.12158] [DOI] [PubMed] [Google Scholar]
Faghihinia 2007 {published data only}
- Faghihinia J. A comparison between intramuscular dexamethasone and fluticasone propionate inhaler in treatment of croup. World Allergy Organization Journal 2007;S104:327. [Google Scholar]
Faraji‐Goodarzi 2018 {published data only}
- Faraji-Goodarzi M, Taee N, Mohammadi-Kamalvand M. Comparison of the effect of cold drink and dexamethasone, and their combined effect on children with croup. Drug Research 2018;68(4):185-8. [DOI] [PubMed] [Google Scholar]
Flisberg 1973 {published data only}
- Flisberg K, Olsholt R. Pseudocroup with stridor. Acta Oto-Laryngologica 1973;76(4):295-9. [DOI] [PubMed] [Google Scholar]
Freezer 1990 {published data only}
- Freezer N, Butt W, Phelan P. Steroids in croup: do they increase the incidence of successful extubation? Anaesthesia and Intensive Care 1990;18(2):224-8. [DOI] [PubMed] [Google Scholar]
Gill 2017 {published data only}
Goddard 1967 {published data only}
- Goddard JE, Phillips OC, Marcy JH. Betamethasone for prophylaxis of postintubation inflammation: a double‐blind study. Anesthesia and Analgesia 1967;46(3):348-53. [PubMed] [Google Scholar]
Gursanscky 2019 {published data only}
- Gursanscky L. Prednisolone versus dexamethasone for croup. Journal of Paediatrics and Child Health 2019;55(12):1511. [Google Scholar]
Haque 1981 {published data only}
- Haque KN. Efficacy of dexamethasone in acute laryngotracheobronchitis (croup). Saudi Medical Journal 1981;2(3):143-5. [Google Scholar]
Havaldar 1997 {published data only}
- Havaldar PV. Dexamethasone in laryngeal diphtheritic croup. Annals of Tropical Paediatrics 1997;17(1):21-3. [DOI] [PubMed] [Google Scholar]
Kelley 1992 {published data only}
- Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. American Journal of Emergency Medicine 1992;10(3):181-3. [DOI] [PubMed] [Google Scholar]
Kotaniemi‐Syrjanen 2018 {published data only}
- Kotaniemi-Syrjanen A, Klemola T, Koponen P, Aito H, Malmstrom K, Malmberg P, et al. Intermittent tiotropium in early childhood wheezing? Preliminary safety results of a pilot study. European Respiratory Journal 2018;52(Suppl 62):PA1042. [DOI: 10.1183/13993003.congress-2018.PA1042] [DOI] [Google Scholar]
Kunkel 1996 {published data only}
- Kunkel NC, Baker MD. Use of racemic epinephrine, dexamethasone, and mist in the outpatient management of croup. Pediatric Emergency Care 1996;12(3):156-9. [DOI] [PubMed] [Google Scholar]
Ledwith 1995 {published data only}
- Ledwith CA, Shea LM, Mauro RD. Safety and efficacy of nebulized racemic epinephrine in conjunction with oral dexamethasone and mist in the outpatient treatment of croup. Annals of Emergency Medicine 1995;25(3):331-7. [DOI] [PubMed] [Google Scholar]
Lee 2019 {published data only}
- Lee JH, Jung JY, Lee HJ, Kim DK, Kwak YH, Chang I, et al. Efficacy of low-dose nebulized epinephrine as treatment for croup: a randomized, placebo-controlled, double-blind trial. American Journal of Emergency Medicine 2019;31(12):2171-6. [DOI] [PubMed] [Google Scholar]
Martensson 1960 {published data only}
- Martensson B, Nilsson G, Torbjar J. The effect of corticosteroids in the treatment of pseudo‐croup. Acta Oto-Laryngologica 1960;158(Suppl):62-71. [DOI] [PubMed] [Google Scholar]
McDonogh 1994 {published data only}
- McDonogh AJ. The use of steroids and nebulised adrenaline in the treatment of viral croup over a seven year period at a district hospital. Anaesthesia and Intensive Care 1994;22(2):175-8. [DOI] [PubMed] [Google Scholar]
Meskina 2019 {published data only}
- Meskina ER, Khadisova MK, Tselipanova EE. Efficacy of a homeopathic drug in combination treatment of acute obstructive laryngitis in children. Voprosy Prakticheskoi Pediatrii 2019;14(4):36-43. [Google Scholar]
Mohammadzadeh 2014 {published data only}
- Mohammadzadeh I, Noorouzi AR, Nakhjavani N, Barari-Savadkoohi R, Mohammadpor-Mir A, Alizadeh-Navaei R. The effect of dexamethasone and nebulised L-epinephrine in treatment of croup. Journal of Babol University of Medical Sciences 2014;16(2):12-6. [Google Scholar]
NCT01748162 {published data only}
- NCT01748162. Management of recurrent croup. clinicaltrials.gov/ct2/show/NCT01748162 (first received 12 December 2012).
Novik 1960 {published data only}
- Novik A. Corticosteroid treatment of non-diphtheric croup. Acta Oto-Laryngologica 1960;158(Suppl):20-2. [PubMed] [Google Scholar]
Osváth 1994 {published data only}
- Osváth P, Kelenhegyi K, Szánthó A. Management of childhood pseudocroup with budesonide inhalation. Orvosi Hetilap 1994;135(46):2535-7. [PubMed] [Google Scholar]
Prendergast 1994 {published data only}
- Prendergast M, Jones JS, Hartman D. Racemic epinephrine in the treatment of laryngotracheitis: can we identify children for outpatient therapy? American Journal of Emergency Medicine 1994;12(6):613-6. [DOI] [PubMed] [Google Scholar]
Rizos 1998 {published data only}
- Rizos J, DiGravio B, Sehl M, Tallon J. The disposition of children with croup treated with racemic epinephrine and dexamethasone in the emergency department. Journal of Emergency Medicine 1998;16(4):535-9. [DOI] [PubMed] [Google Scholar]
Roked 2015 {published data only}
- Roked F, Atkinson M, Hartshorn S. Best practice: one or two doses of dexamethasone for the treatment of croup? Archives of Disease in Childhood 2015;100(Suppl 3):A40-1. [Google Scholar]
Ross 1969 {published data only}
- Ross JA. Special problems in acute laryngotracheobronchitis. Laryngoscope 1969;79(7):1218-26. [DOI] [PubMed] [Google Scholar]
Serra 1997 {published data only}
- Serra A, Bonarrigo A, Cupido GF, Manciagli M, Pantalena V, Raso D, et al. Experience with flunisolide in inflammatory diseases in otorhinolaryngology. Multicentric trial [Impiego di flunisolide nel trattamento di laringiti e sinusiti]. Otorinolaringologia 1997;47(3):137-44. [Google Scholar]
Sumboonnanonda 1997 {published data only}
- Sumboonnanonda A, Suwanjutha S, Sirinavin S. Randomized controlled trial of dexamethasone in infectious croup. Journal of the Medical Association of Thailand 1997;80(4):262-5. [PubMed] [Google Scholar]
Sussman 1964 {published data only}
- Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16 alpha‐methyl, 9 alpha fluoroprednisolone) in obstructive respiratory tract infections in children. Pediatrics 1964;34:851-5. [PubMed] [Google Scholar]
Tal 1983 {published data only}
- Tal A, Bavilski C, Yohai D. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71(1):13-8. [PubMed] [Google Scholar]
Tellez 1991 {published data only}
- Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. Journal of Pediatrics 1991;118(2):289-94. [DOI] [PubMed] [Google Scholar]
Tyler 2022 {published data only}
- Tyler A, Bryan MA, Zhou C, Mangione-Smith R, Williams D, Johnson DP, et al. Variation in dexamethasone dosing and use outcomes for inpatient croup. Hospital Pediatrics 2022;12(1):22-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelmi 1976 {published data only}
- Wilhelmi J. High dosage rectal prednisone therapy (Rectodelt 100) in viral croup of the small child. Medizinische Monatsschrift 1976;30(10):467-9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2018 {published data only}
- Chen QP, Zhou RF, Zhang YM, Yang L. Efficacy of systemic glucocorticoids combined with inhaled steroid on children with acute laryngitis. Zhonghua er bi yan hou tou jing wai ke za zhi [Chinese Journal of Otorhinolaryngology Head and Neck Surgery] 2018;53(1):53-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT20190914044765N1 {published data only}
- IRCT20190914044765N1. Comparison the effect of oral and intravenous dexamethasone effect on the mild and moderate croup treatment in children. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190914044765N1 (first received 24 September 2019).
Additional references
Abrams 2005
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823-44. [DOI] [PubMed] [Google Scholar]
Alberta Medical Association 2008
- Alberta Medical Association Toward Optimized Practice Working Group for Croup. Guideline for the diagnosis and management of croup. actt.albertadoctors.org/CPGs/Lists/CPGDocumentList/croup-guideline.pdf (accessed prior to 22 December 2022).
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grade quality of evidence and strength of recommendations. BMJ 2004;328(7457):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. [PubMed] [Google Scholar]
Bjornson 2008
- Bjornson C, Johnson DW. Croup. Lancet 2008;371(9609):329-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornson 2013
- Bjornson CL, Johnson DW. Croup in children. CMAJ: Journal de l'Association Medicale Canadienne 2013;185(15):1317-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornson 2016
- Bjornson C, Williamson J, Johnson D. Telephone Out Patient Score: the derivation and validation of a telephone follow-up assessment tool for use in clinical research in children with croup. Pediatric Emergency Care 2016;32(5):290-7. [DOI] [PubMed] [Google Scholar]
Brown 2002
- Brown JC. The management of croup. British Medical Bulletin 2002;61:189-202. [DOI] [PubMed] [Google Scholar]
Chan 2001
- Chan AK, Langley JM, LeBlanc JC. Interobserver variability of croup scoring in clinical practice. Paediatrics & Child Health 2001;6(6):347-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherry 1979
- Cherry JD. The treatment of croup: continued controversy due to failure of recognition of historic, ecologic, etiologic and clinical perspectives. Journal of Pediatrics 1979;94(2):352-4. [DOI] [PubMed] [Google Scholar]
Cherry 2008
- Cherry JD. Clinical practice. Croup. New England Journal of Medicine 2008;358(4):384-91. [DOI] [PubMed] [Google Scholar]
Denny 1983
- Denny FW, Murphy TF, Clyde WA Jr, Collier AM, Henderson FW. Croup: an 11-year study in a pediatric practice. Pediatrics 1983;71(6):871-6. [PubMed] [Google Scholar]
Dobrovoljac 2009
- Dobrovoljac M, Geelhoed GC. 27 years of croup: an update highlighting the effectiveness of 0.15 mg/kg of dexamethasone. Emergency Medicine Australasia 2009;21(4):309-14. [DOI] [PubMed] [Google Scholar]
Downes 1975
- Downes JJ, Raphaelly RC. Pediatric intensive care. Anesthesiology 1975;43:238-50. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh l, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(2):769-73. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI: 10.1016/j.jclinepi.2005.06.006] [DOI] [PubMed] [Google Scholar]
Geelhoed 1995
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. [DOI] [PubMed] [Google Scholar]
Geelhoed 1996b
- Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ (Clinical Research Ed.) 1996;313(7050):140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 February 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griffin 2000
- Griffin S, Ellis S, Fitzgerald-Barron A, Rose J, Egger M. Nebulized steroid in the treatment of croup: a systematic review of randomised controlled trials. British Journal of General Practice 2000;50(451):135-41. [PMC free article] [PubMed] [Google Scholar]
Hartling 2014
- Hartling L, Hamm MP, Fernandes RM, Dryden D, Vandermeer B. Quantifying bias in randomized controlled trials in child health: a meta‐epidemiological study. PLOS ONE 2014;9(2):e88008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI: 10.1136/bmj.d5928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2014
- Johnson DW. Croup. BMJ Clinical Evidence 2014;2014:0321. [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kairys 1989
- Kairys SW, Olmstead EM, O'Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from randomized trials. Pediatrics 1989;83(5):683-93. [PubMed] [Google Scholar]
Light 1984
- Light RS, Pillemar DB. Summing Up: The Science of Reviewing Research. Cambridge: Harvard University Press, 1984. [Google Scholar]
Microsoft Excel [Computer program]
- Microsoft Excel. Redmond (WA): Microsoft Corporation, 2016.
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI: 10.1186/s13643-016-0384-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rosychuk 2010
- Rosychuk RJ, Klassen TP, Metes D, Voaklander DC, Senthilselvan A, Rowe BH. Croup presentations to emergency departments in Alberta, Canada: a large population-based study. Pediatric Pulmonology 2010;45(1):83-91. [DOI: 10.1002/ppul.21162] [DOI] [PubMed] [Google Scholar]
Sizar 2021
- Sizar O, Carr B. Croup. www.ncbi.nlm.nih.gov/books/NBK431070/?report=classic (accessed prior to 23 November 2022).
Van Bever 1999
- Van Bever HP, Wieringa MH, Weyler JJ, Nelen VJ, Fortuin M, Vermeire PA. Croup and recurrent croup: their association with asthma and allergy. An epidemiological study on 5-8-year-old children. European Journal of Pediatrics 1999;158(3):253-7. [DOI] [PubMed] [Google Scholar]
Weinberg 2009
- Weinberg GA, Hall CB, Iwane MK, Poehling KA, Edwards KM, Griffin MR, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. Journal of Pediatrics 2009;154(5):694-9. [DOI] [PubMed] [Google Scholar]
Westley 1978
- Westley CR, Cotton EK, Brooks JG. Nebulized racemicepinephrine by IPPB for the treatment of croup. American Journal of Diseases in Children 1978;132(5):484-7. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Lotte Gluud L, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: a meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ausejo 1999
- Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. The effectiveness of glucocorticoids in treating croup: meta-analysis. BMJ 1999;319(7210):595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ausejo 2000
- Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD001955. [DOI: 10.1002/14651858.CD001955.pub2] [DOI] [PubMed] [Google Scholar]
Gates 2018
- Gates A, Gates M, Vandermeer B, Johnson C, Hartling L, Johnson DW, et al. Glucocorticoids for croup in children. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD001955. [DOI: 10.1002/14651858.CD001955.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2019
- Gates A, Johnson DW, Klassen TP. Glucocorticoids for croup in children. JAMA Pediatrics 2019;173(6):595-6. [DOI: 10.1001/jamapediatrics.2019.0834] [DOI] [PubMed] [Google Scholar]
Russell 2004
- Russell K, Wiebe N, Saenz A, Ausejo Segura M, Johnson D, Hartling L, et al. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD001955. [DOI: 10.1002/14651858.CD001955.pub2] [DOI] [PubMed] [Google Scholar]
Russell 2011
- Russell KF, Liang Y, O'Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD001955. [DOI: 10.1002/14651858.CD001955.pub3] [DOI] [PubMed] [Google Scholar]


