Abstract
Electronic cigarette liquids (e-liquids) with sweetener additives such as sucralose, a synthetic chlorinated disaccharide, are popular among some e-cigarette consumers; sucralose can be added either by the manufacturer or by the consumer. The prevalence of sucralose in commercial e-liquids is not known, nor is the typical concentration of sucralose when present; labels are not required to disclose ingredient information. Here, we report the effects of sucralose on e-liquid degradation upon e-cigarette vaping as studied using 1H NMR spectroscopy, ion chromatography, and gas chromatography coupled with detection by mass spectrometry or flame ionization detector. Sucralose was found to be subject to degradation when included in propylene glycol + glycerol based e-liquids and vaped; the presence of sucralose in the e-liquids also resulted in altered and enhanced solvent degradation. In particular, production of aldehydes (carbonyls) and hemiacetals (which have implications for health) was enhanced, as demonstrated by 1H NMR. The presence of sucralose at 0.03 mol % (0.14 wt %) in an e-liquid also resulted in production of potentially harmful organochlorine compounds and catalyzed the cyclization of aldehydes with solvents to acetals upon vaping; the presence of chloride in e-liquid aerosols was confirmed by ion chromatography. Quantities of sucralose as low as 0.05 mol % (0.24 wt %) in e-liquids lead to significant production of solvent degradation products.
Graphical Abstract
1. INTRODUCTION
Sweeteners are common additives to conventional smoked and smokeless tobacco products, presumably to improve palatabilitiy.1,2 For electronic cigarettes (e-cigarettes), sweet and fruit-flavored e-cigarette liquids (e-liquids) are preferred over tobacco or menthol flavors by some former and current adult smokers,1 and fruit-flavored e-liquids in particular (which tend to be sweet) have been reported to be preferred by adolescents (less-so by adults).3 Chemicals added to enhance e-liquid sweetness include but are not limited to very low volatility compounds (e.g., steviol glycosides, mogrosides, sucrose, glucose, fructose, and sucralose), semivolatile compounds (e.g., maltol, ethyl maltol, and erythritol), and volatile flavor constituents (e.g., some esters, lactones, and aldehydes, which have often been described as “fruity”).4-8 Degradation of glucose and some other sugars have been shown to generate 5-hydroxymethylfurfural and furfural9-11 which are regarded as respiratory irritants12,13 and are volatile components in caramel/tobacco flavor profiles.14 For low volatility sweeteners such as glucose, volatile degradants may be more important for flavor profile enhancement than the parent compounds.
Sucralose, a synthetic sugar substitute commonly used in reduced-calorie foods, is a component of some e-liquids. Also, concentrated sucralose mixtures are also available from e-liquid companies so that consumers can add as much sucralose as desired to their e-liquids, which can be via a 5–15% sucralose mixture in propylene glycol (PG).15 Similar in structure to sucrose, sucralose differs in that it contains three chlorines in place of three hydroxyls, and one Cl has an inverted stereochemistry relative to the hydroxyl it replaces in sucrose. These structural differences combine to make sucralose 400–700-fold sweeter by weight than sucrose.16 In e-cigarettes, sucralose has been reported to result in a small amount of sweetness enhancement, but this effect has been reported to be device-dependent,17 perhaps because volatile flavor compounds may have a greater influence on perceived sweetness than sucralose. Although sucralose is regarded as safe for gastrointestinal consumption,18 neither sucralose itself nor its thermal degradation products have been shown to be safe upon inhalation.
Although there is little published regarding sucralose stability during vaping, this molecule has been examined for its inherent thermal stability and its stability in food products. Degradation of pure sucralose has been found to occur at temperatures as low as ~98 °C, as evidenced by the production of polychlorinated aromatic hydrocarbons; visible degradation was reported at 125 °C.19 Degradation pathways for sucralose under stronger pyrolysis conditions at 250 °C and in the presence of glycerol (GL) (which is used in e-liquids) have been explored.20 Sucralose degradation (in glycerol) was proposed to occur via dehydration and dehydrochlorination reactions and was reported to generate hydrochloric acid, water, and chloropropanols.20 E-cigarettes are likely to achieve temperatures capable of degrading sucralose because the boiling points of the main solvents (PG and GL) and their mixtures range from ~189 to 292 °C.21
Here we used various techniques to study the chemical reactions occurring in sucralose-containing e-liquids upon vaping from a commercial tank-style e-cigarette. 1H NMR excels at direct nondestructive analyses, especially for known compounds.22-25 In particular, the effect of sucralose on aldehyde (carbonyl) production, as indicated by levels of propanal, acetaldehyde, glycolaldehyde, and acrolein, as well as PG and/or GL formaldehyde hemiacetals can be monitored as formed when formaldehyde reacts with either PG or GL in a reversible reaction (i.e., formaldehyde can be released) and thus contribute to the level of total formaldehyde produced by an e-cigarette. Mass spectrometry (MS) methods were also used because low-concentration degradation products can be difficult to quantify by NMR, particularly when the resonances are overlapping or very near the resonances of high concentration-compounds, in this case the e-liquid solvents PG and GL. Gas chromatography/mass spectrometry (GC/MS) allows for separation of known analyte compounds from the abundant PG and GL in the captured aerosol as well as identification of unknown compounds by way of the MS data. Thus, GC/MS allows quantification of cyclic acetals that could be undetected or underdetected by other techniques;25 recent research indicates that cyclic acetals of common e-cigarette flavorants and PG exhibit different toxicological properties.26 Gas chromatography flame-ionization detection (GC/FID) is a complement to GC/MS by exhibiting a nearly proportional response with respect to the number of C atoms in each compound.27 Ion chromatography (IC) allows the direct determination of released chloride from sucralose during the vaping process.
2. MATERIALS AND METHODS
2.1. Chemicals and E-cigarette Devices.
For All Experiments.
United States Pharmacopeia (USP) grade glycerol was obtained from Sigma-Aldrich (St. Louis, MO). Coils rated at 1.2 Ω OCC (suggested by the manufacturer for use at 10–26 W) were used for all experiments (KangerTech US, LLC, Shenzhen, China).
For 1H NMR Experiments.
USP grade propylene glycol was purchased from Sigma-Aldrich. (S)-(−)-nicotine (99%) was obtained from Alfa Aesar (Haverhill, MA). Sucralose (>98%) and methanol (ACS grade) were obtained from TCI Co., Ltd. (Portland, OR). Details about methods used during sample collection for determination of free-base levels and degradation products have been reported previously.21,28
For GC/MS, GC/FID, and IC Experiments.
USP grade propylene glycol was purchased from TCI. A commercially available “sweetener” (sucralose in PG) was purchased from EcigExpress (Bellingham, WA) in October of 2016. Aliquots of this commercial mixture were tested in triplicate via 1H NMR and found to contain 3 mol % (corresponding to 12 wt % or 8 vol %) sucralose relative to PG as determined by integration analysis.
Sodium chloride (99.2%) and HPLC-grade isopropanol (IPA) were purchased from Fisher Scientific (Pittsburgh, PA). 4-Methyl-1,3-dioxolane (>98%) (PG formaldehyde acetal), a mixture of 4-hydroxymethy-1,3-dioxolane/5-hydroxy-1,3-dioxane (>98%) (GL formaldehyde acetals), 3-chloropropan-1,2-diol (>98%), and 1,3-dichloropropan-2-ol (>98%) were purchased from TCI. 1,2,3-Trichlorobenzene (99%) was purchased from Sigma-Aldrich.
A KangerTech Subtank Nano was obtained from KangerTech. A Model NE-1010 (New Era Pump Systems Inc., Farmingdale, NY) syringe pump outfitted with a custom 300 mL syringe was used for sample puff generation.
2.2. E-liquid Preparation with the Compositions Confirmed by 1H NMR.
Simplified e-liquids were prepared to contain 1:1 (by mol) PG and GL. Concentrations of sucralose ranged from 0.05 to 0.12 mol % (equivalent to 0.24 to 0.57 wt %) in the final liquid, as was found in commercial e-liquids tested by 1H NMR. To evaluate coil and sample variability, the following e-liquids were prepared: sucralose-free PG+GL, 0.05 mol % sucralose in PG+GL, and 0.10 mol % sucralose in PG+GL. To test the effect of increasing amounts of sucralose on degradation using a single coil, the same 0.05 and 0.10 mol % sucralose in PG+GL e-liquids were used but a 0.075 mol % sucralose in PG+GL was also prepared. To assess the vaping impact of sucralose on the fraction of the nicotine in the free-base (unprotonated) state versus the monoprotonated state (αfb), a sample was prepared to contain 24 mg/mL nicotine (equivalent to 1.1 mol % or 2.1 wt %) in PG+GL; aliquots of this mixture were then combined with sucralose to obtain 0.12 mol % sucralose. E-liquid compositions were verified by 1H NMR peak integration.
2.3. Sample Collection Protocol and 1H NMR.
Vaporized e-liquid samples were collected following a protocol outlined previously,21 using the modified CORESTA puffing method (where the power button was activated one second prior to each 3 s, 55 mL puff, with 27 s between puffs).29 This puff protocol was selected to be consistent with other researchers. The sample collection protocol for the determination of free-base nicotine content and subsequent 1H NMR methods has been described.28
All coils (1.2 Ω) were conditioned with 10 puffs at 26 W prior to first-time sample collection, similar to prior studies.30-32 All other puffs (for wicking and sample collection) were generated at 20 W. Either 10 or 20 “wicking puffs” were generated and discarded prior to aerosol sample collection with each new e-liquid.
Each degradation sample contained three puffs. Three different 1.2 Ω coils were tested with three different sucralose concentrations (0, 0.05, and 0.10 mol %) to determine coil variability. Between e-liquid conditions, each tank was emptied of fluid and wiped down with lint-free tissues to minimize the residual e-liquid from the previous condition. Obvious excess e-liquid on the coil was removed, but the wicking material was not cleaned with solvent. The tank was then filled with the new e-liquid and 20 “wicking puffs” were generated to ensure that the previous e-liquid had been removed from the wicking material and had been replaced by the new e-liquid. For the coil and sample variability experiment, 3 samples were collected for each of 3 coils and each of the 3 e-liquid compositions, for a total of 27 samples. A second experiment was conducted using a single coil and 4 different sucralose concentrations (0, 0.05, 0.075, and 0.10 mol % sucralose in PG+GL) with 3 samples per condition to examine degradation trends for a total of 12 samples.
Each sample for determination of fraction free-base nicotine was derived from 15 puffs, following methods previously described.28 The same 1.2 Ω coil was used to test the 24 mg/mL nicotine samples to eliminate variability between coils. Because of the cleaning of the coil between conditions, only 10 wicking puffs were generated prior to sample collection between e-liquids (without and then with 0.12 mol % sucralose).
Degradation samples were tested by 1H NMR at 25 °C and free-base nicotine samples were tested at 40 °C per previous methods.21,28 Spectra for degradation samples generated using sucralose-containing e-liquids frequently exhibited acid-induced broadening, likely because of HCl (a strong acid) production by degradation of sucralose, which made the hemiacetal degradation peaks minimally visible. To neutralize the acid, small quantities of DMSO-d6 saturated with sodium bicarbonate were added to each sample until the hemiacetal peaks could be resolved. Spectra were normalized relative to the PG resonance at ~1.05 ppm.
2.4. Preparation of E-liquids, E-cigarette, and Calibration Standards for GC/MS, GC/FID, and IC Experiments.
An e-liquid containing 0.03 mol % (0.14 wt %) sucralose was prepared by combining a 1:1 (by mol) PG/GL mixture with commercial sucralose “sweetener” (sucralose in PG). A KangerTech Subtank Nano was used with a KangerTech 1.2 Ω OCC atomizer. The atomizer was “primed” per the manufacturer’s instructions by saturating the inner wicking material with e-liquid. The tank was then filled to 80% capacity and left to wick for ~30 min.
A mixture of standards was prepared in IPA containing 4-methyl-1,3-dioxolane, a mixture of 4-hydroxymethyl-1,3-dioxolane/5-hydroxy-1,3-dioxane, 3-chloropropan-1,2-diol, and 1,3-dichloropropan-2-ol. This mixture was used to prepare calibration standards at approximate concentrations of 200, 100, 50, 10, and 2 ng/μL. Samples and calibration standards were spiked with 1,2,3-trichlor-obenzene as a GC internal standard.
A chloride stock solution was prepared using deionized water (MilliporeSigma; Burlington, MA) and sodium chloride (Fisher Scientific). IC calibration standards were made from the sodium chloride stock in 98% IPA/2% H2O at approximate concentrations of 60, 12, 6, 3, and 1 ppm (mg/L) chloride.
2.5. Sampling Methods for GC/MS, GC/FID, and IC.
The prepared Subtank Nano was installed on an Efusion DNA200 power supply (Lost Vape Ltd.; London, England), the resistance was confirmed to be 1.2 ± 0.1 ohms, and the power level was set to 20 W. Puffs were generated using the 300 mL syringe pump. Each 5 s puff was 50 mL in volume and had an interpuff interval of 35 s.
Aerosol generated by the e-cigarette was drawn through an ~4.5 cm section of silicone tubing connected to an 18-gauge inlet needle which was inserted into a capped 2 mL autosampler vial. The orifice of the needle was positioned to impact aerosol particles against the vial wall. Another 18-gauge exit needle was positioned above the inlet needle and attached to an ~8 cm length of tubing, connected to a solenoid valve, and then the syringe pump. An ~7 cm length section of tubing connected the valve to the custom syringe.
Each aerosol sample consisted of three puffs. A total of 30 consecutive samples were collected for a total of 90 puffs. Samples were collected, then dispersed into 980 μL of IPA and 20 μL of GC internal standard solution (1,2,3-trichlorobenzene in IPA) ~1 h after collection, giving a total volume of ~ 1030–1050 μL, depending on the quantity of captured aerosol for each sample. A Teflon-lined screw cap was installed and each sample was mixed. An unvaped blank e-liquid sample was prepared by diluting 50 μL of the e-liquid in 930 μL IPA with 20 μL of internal standard solution. The unvaped blank was tested with experimental samples by GC/MS, GC/FID, and IC.
2.6. Analytical Methods for GC/MS, GC/FID, and IC.
After sample collection, dilution, and the addition of an internal standard, sample vials along with calibration standards and blanks were tested by GC/MS and GC/FID, using the same sample order for both. After analysis by GC/FID, the contents of each vial were transferred to a 1.5 mL polypropylene IC vial and analyzed by IC. Additional solvent blanks for IPA and H2O were also tested using IC.
The limit of quantitation (LOQ) for free chloride IC samples was calculated using the standard deviation of free chloride detected in an unvaped e-liquid blank (0.004 ppm) multiplied by a factor of 10 resulting in a limit of 0.04 ppm. For compounds with mass concentrations estimated using total ion chromatogram (TIC) peak areas, which were normalized relative to internal standard TIC peak areas, the LOQ was estimated by the internal standard response factor and a minimum TIC peak area of 1000, resulting in the LOQ of 0.016 ng/μL. The LOQ for compounds quantitated using GC/FID (multipoint calibration standard) was conservatively estimated to be one-tenth of the lowest concentration standard resulting in the following LOQs: 4-methyl-1,3-dioxolane (0.16 ng/μL), 4-hydroxymethyl-1,3-dioxolane (0.04 ng/μL), 5-hydroxy-1,3-dioxane (0.2 ng/μL), and 3-chloro-1,2-propandiol (0.24 ng/μL).
2.6.1. GC/MS.
Sample analyses were conducted using an Agilent 7890A GC equipped with a Restek 5Sil-MS column (30 m × 0.25 mm ID × 0.25 μm df), which was coupled to an Agilent 5975C MSD. The autosampler injected 1 μL of sample at a 10:1 split under 12 mL min−1 constant injector He flow (99.9999% pure, AirGas; Radnor, PA). The injection port temperature was 200 °C; after injection, the oven temperature was held at 40 °C for 2 min, then increased at 10 °C per minute until it reached 300 °C. The MS was operated in electron impact ionization mode using an ionization energy of 70 eV; detection was configured for positive ions scanning a range of 34–400 amu. The electron multiplier voltage was set to 1730 V. Other conditions were interface temperature, 230 °C; source temperature, 226 °C; and quadrupole temperature, 150 °C.
2.6.2. GC/FID.
An Agilent 7890B GC with a Restek 5Sil-MS column (30 m × 0.25 mm ID × 0.25 μm df) was used for flame ionization detection (FID). The same GC oven temperature program was used as described earlier in Section 2.6.1. Other conditions were injection volume, 1 μL; split ratio, 10:1 (He) at 16 mL min−1; injection port temperature, 200 °C; detector temperature, 280 °C; FID hydrogen flow, 30 mL min−1; FID air flow, 300 mL min−1; FID makeup gas (N2) flow, 25 mL min−1.
2.6.3. Ion Chromatography.
All IC equipment, columns, and software used in this study were obtained from Dionex (Sunnyvale, CA). Anion analyses of samples were conducted using an ICS-5000 IC system outfitted with a conductivity detector cell and electrolytically regenerated suppressor (AERS 500, 4 mm). Aliquots of samples (25 μL) were injected into the system for each test. Separation was carried out using an IonPac-AS15 column with an IonPac-AG15 guard column and a flow of 1.20 mL min−1. An eluent concentration of 38 mM of potassium hydroxide was maintained for the entire 20 min run.
3. RESULTS AND DISCUSSION
3.1. 1H NMR of E-liquid Aerosol: Sample Variability, Device Differences, and Degradation Products.
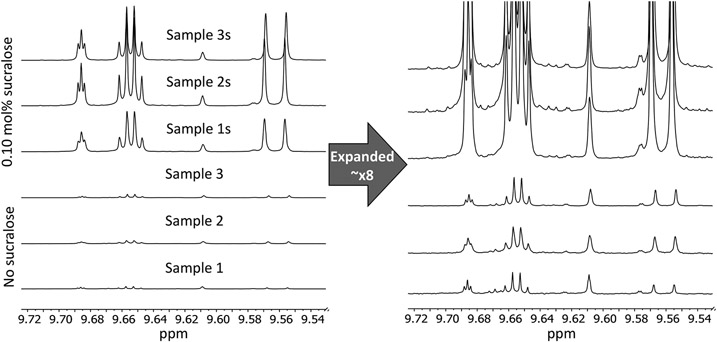
The extent of PG and GL degradation, as shown by the most abundant nonsolvent species detected (aldehydes) relative to the PG methyl resonance, was found to be consistent when using the same coil and e-liquid, both at zero and high tested sucralose concentrations (Figure 1). Different coils were shown to produce different concentrations of degradation products relative to PG (Figure 2). Replicates using a single coil produced highly consistent results, indicating that individual samples were representative of each e-liquid condition. For the three coils, the average percent of the aerosol trapped in the sample vial for each condition ranged from 35 to 54%, 45–57%, and 15–36% for the 0, 0.05, and 0.10 mol % sucralose samples, respectively; similar to our previous results.23 As the sucralose concentration increased, the percent of the aerosol trapped decreased. The increase in degradation production observed with increased sucralose concentration reported herein is relative to the molar quantity of PG, rather than an absolute quantity of each degradation product. Because of this, it is possible that degradation production is underestimated by this method.
Figure 1.
Sample degradant variability using the same coil with and without sucralose (0.10 mol %) by1H NMR. (Left) Variability between samples collected using 0.10 mol % sucralose e-liquid was found to be low. (Right) The spectra were expanded to allow comparison of the variability between samples collected without sucralose and again found to be minimal. Samples were vaporized using the CORESTA puff method at 20 W using a conditioned 1.2 Ω coil. The intensities were relative to the PG methyl resonance.
Figure 2.
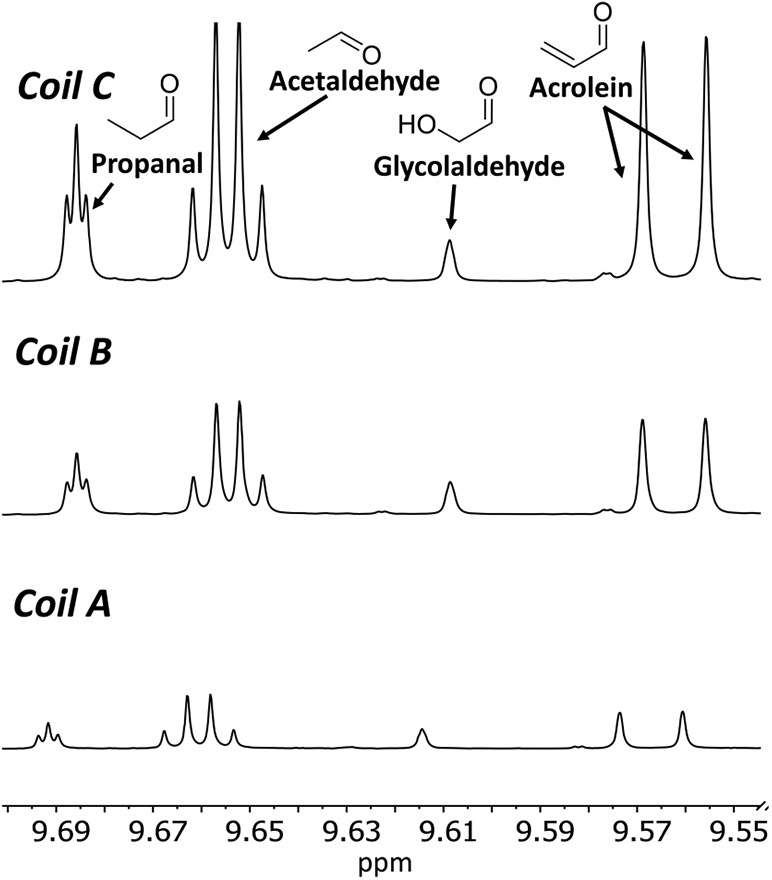
Degradant variability for three coils (A, B, and C), all at 0.10 mol % sucralose by 1H NMR. All spectra are normalized relative to the propylene glycol doublet at ~1.05 ppm. Samples were vaporized using the CORESTA puff method at 20 W using a conditioned 1.2 Ω coil.
The effect of sucralose concentration (0, 0.05, 0.075, and 0.10 mol % sucralose in PG+GL) on degradation produced by a single coil was compared by 1H NMR. The concentrations of aldehydes (propanal, acetaldehyde, glycolaldehyde, and acrolein, Figure 3) and hemiacetals (Figure 4) all increased as sucralose concentration increased. Hemiacetals of formaldehyde with PG or GL are of concern because the formation reactions (Figure 5) are reversible: formaldehyde can be released by these hemiacetals and contribute to the total formaldehyde level delivered by an e-cigarette.23 Sucralose levels as low as 0.05 mol % in PG+GL increased both aldehyde and hemiacetal output. Acid-induced broadening of OH resonances, including from the hemiacetals, was observed (likely due to the degradation of sucralose, which is known to produce hydrochloric acid19,20 as well as increase the production of other acids such as acetic acid), so sodium bicarbonate was added to sucralose-containing NMR samples to reduce the broadening until the hemiacetal peaks were visible (Figure 4). The average (±SD) percent of the aerosol trapped in the sample vial for each condition (0, 0.05, 0.075, and 0.10 mol % sucralose) was 48 ± 4, 36 ± 4, 16 ± 5, 18 ± 4%, respectively, all collected using the same coil. Again, increased sucralose concentration was found to result in a lower percent of the aerosol captured.
Figure 3.
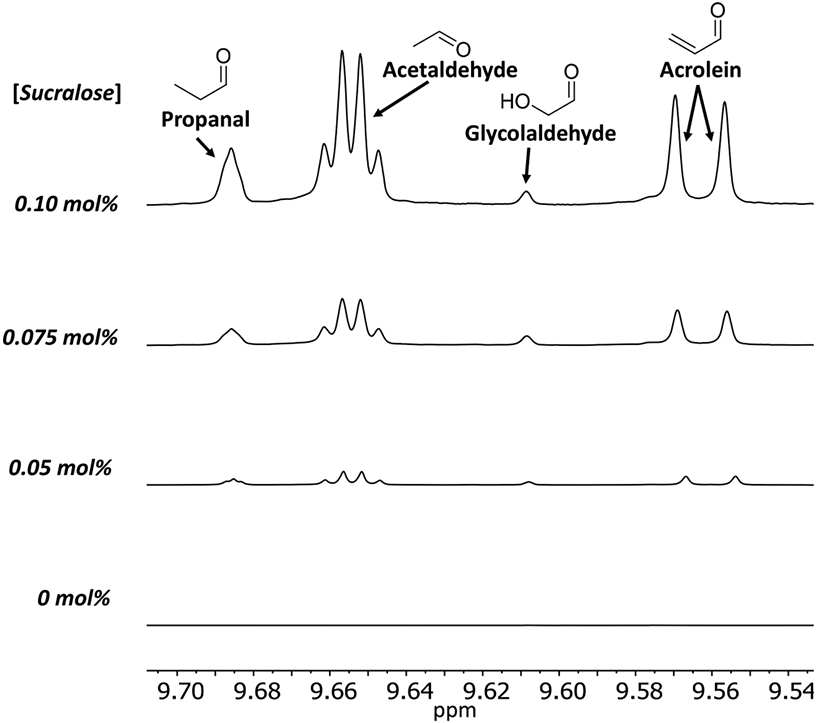
Increased sucralose concentrations generated a greater concentration of aldehydes using the same device, coil, and vaping conditions by 1H NMR. Samples (three puffs each) were generated at 20 W using a conditioned 1.2 Ω coil. The intensities were relative to the PG methyl resonance.
Figure 4.
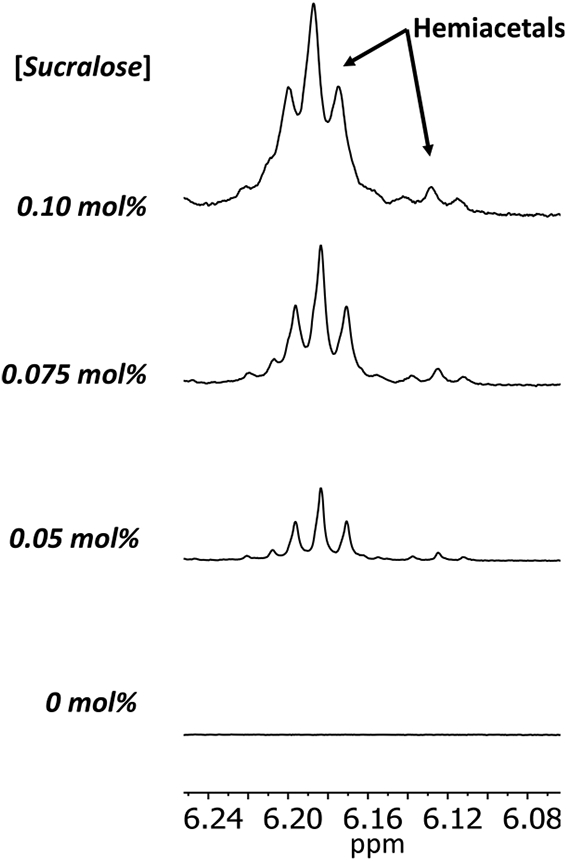
Increased sucralose concentrations generated a greater concentration of hemiacetals using the same device and coil by1H NMR. For these spectra, sodium bicarbonate (not present in the samples depicted in Figure 3) was added to buffer the mixture in order to slow the hydrogen exchange of the hemiacetal OH groups. Samples were vaporized using the CORESTA puff method at 20 W using a conditioned 1.2 Ω coil. The intensities were relative to the PG methyl resonance.
Figure 5.
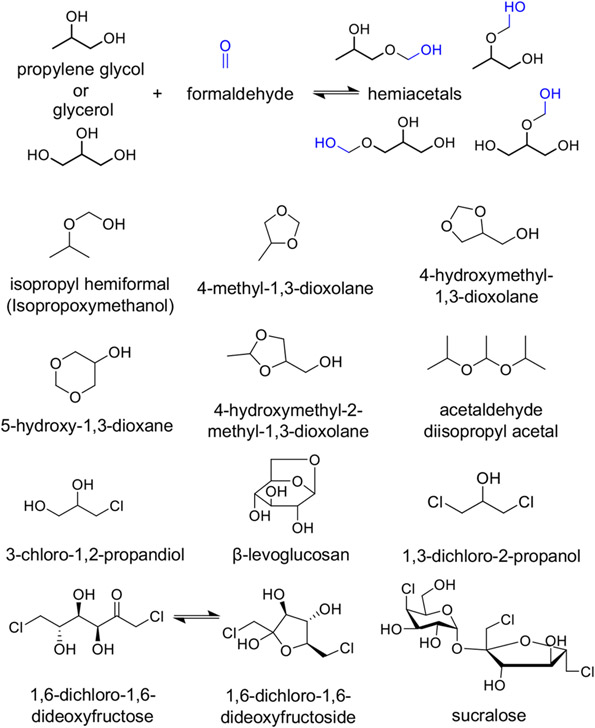
Propylene glycol- and glycerol-based hemiacetal production,23 other degradation products identified in this study, and related structures.
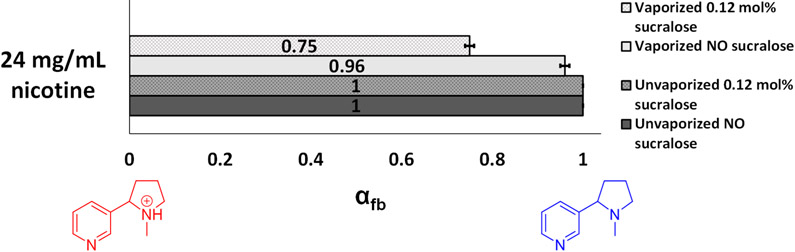
The protonation state of nicotine was evaluated before and after vaping, in order to assess the possible production of acid due to sucralose degradation.20 Nicotine can exist in nonprotonated (freebase) and protonated forms. Neglecting insignificant quantities of the diprotonated state, the fraction of nicotine in the free-base relative to the monoprotonated state (αfb) can be determined by 1H NMR.28 The αfb value for the unvaped e-liquids used in this study (Figure 6) was 1.00 ± ≤ 0.01. Vaping the 24 mg/mL nicotine-containing PG+GL mixture (no sucralose) produced aerosol characterized by αfb = 0.96 ± 0.01. Vaping the same e-liquid, but with 0.12 mol % sucralose added produced aerosol characterized by αfb = 0.75 ± 0.01. The change in degree of protonation for nicotine was then used to calculate the approximate number of protons produced due to the presence of sucralose during vaporization, possibly as HCl.20 The αfb for the vaporized 24 mg/mL nicotine samples decreased by 0.21 (0.96 to 0.75) when the sucralose was added; if this decrease in αfb is entirely attributed to sucralose, an average of ~2.2 protons would be released from every vaped sucralose molecule. This is consistent with the pyrolysis mechanism proposed by Rahn and Yaylayan, which suggested that each sucralose molecule should release 2 equiv of hydrochloric acid.20 The extra 0.2 protons taken up by nicotine may be from other acids (such as acetic acid) that may be produced during degradation. Possible evidence for this includes the enhanced solvent degradation after the addition of sucralose (Figures 1, 3, and 4), that more acetate was observed in the high sucralose concentration samples (not shown), and that the samples depicted in Figure 4 required addition of a sodium bicarbonate in order to slow the acid-catalyzed exchange of OH groups for NMR detection. The αfb results show that more acid is being produced with the addition of 0.12 mol % sucralose than without. Sucralose thus appears to increase acid production from PG and GL degradation, including hydrochloric acid, directly during the breakdown. One outcome of sucralose degradation producing acid(s) could be to reduce αfb for nicotine, making the e-liquid less harsh.28 This warrants further study.
Figure 6.
Fraction of nicotine in the free-base state (αfb) in e-liquids (PG, GL, and nicotine) before and after being vaporized with and without sucralose (0.12 mol %) as determined using differences in 1H NMR chemical shifts. The same 1.2 Ω coil was used to vaporize all e-liquids shown. Samples were vaporized using the CORESTA puff method at 20 W using a conditioned coil and collected directly into a micro-NMR tube.
3.2. Aerosol Collection and Target Analytes for GC/MS, GC/FID, and IC Analyses.
The average (±SD) mass of aerosol condensate collected in each vial (three puffs per vial), considering all samples, was 37 ± 12 mg. The range was 21–52 mg, indicating that there was variability in the mass captured. Overall, for all the puffs 1.56 g of the starting e-liquid material was vaporized and 1.12 g of aerosol condensate was collected (30 vials, 90 puffs total) resulting in an overall capture efficiency of 72%. Total capture efficiency was calculated using tank mass after 90 puffs and tank starting mass versus total mass collected in all vials.
Results for target analytes (structures in Figure 5) are given in Table 1. Observed products included direct sucralose degradation products (1,6-dideoxy-1,6-dichlorofructose and free chloride) as previously reported,20,33 formaldehyde (4-methyl-1,3-dioxolane; 4-hydroxymethyl-1,3-dioxolane; 5-hydroxy-1,3-dioxane), and acetaldehyde acetals (4-hydroxymethyl-2-methyl-1,3-dioxolane) (structures in Figure 5) which are promoted to form under acidic conditions, reaction products between hydrochloric acid and GL reaction products (3-chloropropan-1,2-diol) as well as a marker of cellulose degradation (levoglucosan). An acetal of acetaldehyde and isopropanol (acetaldehyde diisopropyl acetal) and the hemiacetal of formaldehyde and isopropanol (IPA hemiformal) were also observed.
Table 1.
Compounds Detected (μg per mg Aerosol Condensate)a
| compound nameb | low (μg mg−1) | high (μg mg−1) | average ± SD (μg mg−1) |
|---|---|---|---|
| IPA formaldehyde hemiacetald,h | 2820 | 3780 | 3360 ± 340 |
| 4-methyl-1,3-dioxolanec | 121 | 163 | 141 ± 17 |
| 4-hydroxymethyl-1,3-dioxolanec | 84 | 127 | 104 ± 18 |
| 5-hydroxy-1,3-dioxanec | 292 | 413 | 373 ± 42 |
| 4-hydroxymethyl-2-methyl-1,3-dioxolaned | 156 | 183 | 168 ± 9 |
| acetaldehyde diisopropyl acetald | 336 | 621 | 469 ± 99 |
| free chloridef | 44 | 68 | 57 ± 10 |
| 3-chloro-1,2-propandiolc | 7.6 | 11.8 | 9.5 ± 1.4 |
| 1,6-dichloro-1,6-dideoxyfructosed | 3.5 | 5.4 | 4.3 ± 0.8 |
| unidentified chlorinated compounde,g | 1.4 | 2.0 | 1.7 ± 0.2 |
| β-levoglucosand | 29 | 34 | 32 ± 2 |
Detection range of target compounds represented as μg per mg aerosol condensate collected, as determined by GC/MS, GC/FID, or IC (samples 25–30, generated using a 1.2 Ω coil at 20 W comprised of 1:1 molar propylene glycol and glycerol). Free chloride was the only target analyte detected in an unvaped starting material blank (Table 2).
Structures are depicted in Figure 5.
Multipoint calibration prepared from authentic chemical standards, GC/FID peak area normalized to internal standard peak area and mass concentration calculated.
Analyte mass concentration estimated from GC/MS total ion chromatogram (TIC) peak area normalized to internal standard TIC peak area.
Multipoint calibration standard used to establish response factor from 1,3-dichloropropan-2-ol, target ion peak area extracted from GC/MS data, and normalized to internal standard extracted target ion peak area to estimate approximate analyte mass concentration.
IC used with multipoint calibration standard prepared from sodium chloride.
Spectral match identified 1,3-dichloropropanol with high certainty but retention time did not match that of an authentic chemical standard. Spectra suggest this unidentified compound is at least monochlorinated.
See Supporting Information for details on identification.
Formaldehyde acetals and acetaldehyde acetals could be formed with e-liquid solvents PG and GL during aerosol generation and/or sample condensation.22,23 Aldehydes are known to react with alcohols to form acetals through an acid catalyzed mechanism.26,34 The presence of acetals supports the assertion that acids are formed during the vaporization process. As formaldehyde and acetaldehyde are highly volatile and expected to favor partitioning into the aerosol gas phase35 it is more likely that these carbonyls were present in the particle phase of the collected aerosol as less volatile hemiacetals with PG or GL.22-25 Acetaldehyde diisopropyl acetal and IPA-formaldehyde hemiacetal are therefore more likely to have formed after addition of IPA where exchange could occur with existing acetaldehyde/formaldehyde hemiacetals of PG and/or GL (see Supporting Information for additional information on the identification of IPA-formaldehyde hemiacetal).
The IC results showed the presence of free chloride in the vaporized samples, ostensibly as hydrochloric acid.19,20 Considering samples 25–30, only a small amount (0.005 μmol g−1) of free chloride was detected in the starting material before vaporization compared to a total of 0.397 μmol g−1 detected in the aerosol condensate samples, an ~80-fold increase. The presence of free chloride in e-cigarette aerosol indicates that sucralose is unstable in the e-cigarette environment tested, which was within bounds of the settings recommended by the manufacturer for this coil, device, and PG to GL ratio. This is likely because the boiling points for PG +GL mixtures (must meet or exceed these for vaporization) range from 188.6 to 292 °C, with 50:50 (by mol) PG and GL boiling at 210 °C,21 which well exceeds the temperature at which pure sucralose has been shown to degrade, 125 °C.19
IC results (Table 1) for sample numbers 25–30 (at the end of the experiment) show that during vaporization, each sucralose produces an average of ~0.9 free chlorides. Differences between IC findings and NMR results are like due to IC measuring the chloride concentrations as well as the formation of organochlorine compounds detected by GC/MS (Table 1), whereas NMR was used to determine the presence of protons accepted by nicotine. Another difference involves the presence of nicotine, which was only used in the NMR experiments. Other differences include the concentration of sucralose used, as well as sample collection protocol. In general, both the IC and NMR results indicate that sucralose degrades, leading likely production of HCl.
Samples of the unvaped e-liquid starting material contained no detectable levels of 1,6-dideoxy-1,6-dichlorofructose (a known sucralose hydrolysis product) providing evidence that its formation must have occurred during the vaporization process rather than in the heated zones of the GC or MS.
As discussed previously, chloropropanols have been demonstrated to form when sucralose is heated in the presence of glycerol under pyrolysis conditions, which is especially relevant to e-cigarettes where glycerol is a ubiquitous solvent.20 While the total amount of 3-chloropropan-1,2-diol detected in samples 25–30 (~10 μg g−1 of e-liquid vaporized) was below a threshold of concern (European Commission tolerable daily intake of 2 μg/kg body weight),36 it should be noted that there is no literature to date on the effects of 3-chloropropan-1,2-diol inhalation though it is considered a Group 2B possible human carcinogen.
Vaporization of e-liquids containing sucralose may degrade the atomizer wicking material, which is comprised of cellulose (advertised as “organic cotton”) for the atomizers used in this study. For samples 25–30, ~32 μmols mg−1 β-levoglucosan were captured, far exceeding ~3 × 10−3 μmols mg−1 sucralose in the starting material. This suggests that sucralose was not a major source of β-levoglucosan captured in samples. One of the main thermal degradation products of cellulose (a β(1 → 4) linked polymer of d-glucose) is levoglucosan which is generated from hydrolyzed d-glucose units through dehydration.37 In addition, production of levoglucosan from cellulose can be catalyzed by acids.38 1H NMR, GC, and IC results support the generation of acids. Therefore, it is conceivable that the majority of levoglucosan detected arose from acid catalyzed degradation of the cellulose wicking material in the atomizer. This is likely a key factor in the “coil killer” properties of sucralose. d-glucose (which is nonvolatile) is unlikely to be carried away by vaporization and instead left to further degrade into compounds such as hydroxymethylfurfural (HMF) and furfural, which are volatile.11 Production of HMF and furfural may not be an unpleasant experience for the consumer as they are components in caramel/tobacco flavor profiles.14
Some proponents of e-cigarettes have claimed that consumers may discontinue use of a particular coil/e-liquid if exposed to significant degradants and have dismissed degradation findings as having been produced under unrealistic conditions.39 However, it is possible that consumers build a tolerance to irritating substances, may even seek a level of irritation,32,33 and in many cases continue consumption due to nicotine addiction despite harm.40,41 E-liquid components (nicotine, cinnamaldehyde, and menthol) and degradation products (formaldehyde and acrolein) are known agonists of TRPA1 ion channels, which respond to irritants.42-46 There have been reports that chronic exposure to irritants can desensitize the response, indicating that e-liquids containing nicotine, menthol, and/or cinnamaldehyde could potentially lower a consumer’s sensitivity to toxic e-liquid degradants.45,47 Some level of PG and/or GL degradation, especially the formation of acetaldehyde, may even be desirable for some consumers. Acetaldehyde has been demonstrated to react with biogenic amines to form monoamine oxidase inhibiting compounds which act synergistically with nicotine.48
3.3. Forms of Chlorine Released from Sucralose.
Approximately 1% of the total possible chloride produced by sucralose was accounted for as organic compounds determined by GC methods (Table 2). Because sucralose is a very low volatility compound, it is likely that much of the sucralose in the e-liquid was simply not vaporized along with the PG and GL and perhaps concentrated in the wicking material. Unidentified organochlorine compounds could be a source of unaccounted for chlorine such as the unidentified chlorine compound noted in Table 1. When pure sucralose is heated, it has been demonstrated to generate organochlorine compounds volatile enough to be collected from the headspace gas phase.19 These compounds include a chlorinated furan derivative, a chlorinated tetrahydropyran, and a polychlorinated aromatic hydrocarbon.19 Of the compounds identified by de Oliveira et al.,19 only the chlorinated furan (originating from the fructose moiety of sucralose) was identified by GC/MS in the present study (as 1,6-dichloro-1,6-dideoxyfructose, before dehydration to a furan derivative20) with certainty. Using infrared spectroscopy, de Oliveira et al. identified chloroacetaldehyde generated during the heating of pure sucralose.19 Chloroacetaldehyde, which readily forms acetals in the presence of alcohols, was not identified in the present study though it may be related to the unidentified chlorinated compound in Table 1. Some reactions/pathways for sucralose degradation in the electronic cigarette setting are undoubtedly different than those in the pyrolysis of pure sucralose due to the addition of PG and GL in an e-cigarette as well as the temperature/environmental differences.
Table 2.
Total Chlorine Found from Samples Containing Sucralosea
|
μ mol chlorine equivalentsb |
|||
|---|---|---|---|
| compound | unvaped | captured | % of total |
| sucralose | 1.39 | NDc | NDc |
| free chloride | 0.005 | 0.397 | 28.4 |
| 3-chloropropan-1,2-diol | NDc | 0.012 | 0.9 |
| 1,6-dichloro-1,6-dideoxyfructose | NDc | 0.005 | 0.4 |
The μmol chlorine equivalents b of chlorinated target compounds detected in captured aerosol condensate compared to the amount in unvaped starting material as determined by GC/MS, GC/FID, or IC (samples 25–30, generated using a 1.2 Ω coil at 20 W containing comprised of 1:1 molar propylene glycol and glycerol).
Each μmol of free chloride and 3-chloropropan-1,2-diol each contribute one chlorine equivalent, whereas 1,6-dichloro-1,6-dideoxyfructose contributes two chlorines, while sucralose contributes three.
ND: not detected.
4. CONCLUSIONS
This study provides evidence that sucralose is unstable in the e-cigarette environment tested, as evaluated within the bounds of the settings recommended by the manufacturer of the contemporary device that was used. The vaporization of a sucralose-containing e-liquid (0.05 mol %, equivalent to 0.24 wt %, or greater) was found by NMR to increase the production of aldehydes (such as propanal, acetaldehyde, glycolaldehyde, and acrolein) as well as formaldehyde hemiacetals (which can release formaldehyde). Analysis by GC/MS and GC/FID showed that chloropropanols (3-chloropropan-1,2-diol) were formed during vaporization for sucralose-containing e-liquids (0.03 mol %, equivalent to 0.14 wt %, sucralose). The use of IC confirmed that while a small portion of the total possible chlorines on sucralose was liberated during e-liquid vaporization, chloropropanols were still formed and free chloride was detected. The presence of free chloride indicates that sucralose is unstable in e-liquids when vaporized,19,20 and the presence of acid was confirmed using NMR by determining the protonation state of nicotine before and after vaporization. Production of acid from sucralose degradation likely enhances aldehyde and hemiacetal formation from PG and/or GL during vaporization due to the acid catalyzed nature of these degradation pathways. By NMR it was determined that ~2.2 protons were absorbed by nicotine after vaping a sucralose-containing e-liquid (0.12 mol % sucralose and 24 mg/mL nicotine which is equivalent to 1.1 mol %). IC analysis of samples 25–30 indicated that an average of ~0.9 free chlorides were released per sucralose molecule when vaping a sucralose-containing e-liquid (0.03 mol % sucralose). This apparent difference may be attributed not only to the different concentrations of sucralose (the NMR-based data were for a higher concentration of sucralose, which induces a greater overall level of total degradation) but also that the NMR experiments examined the uptake of protons by nicotine and IC allows detection of free chloride, rather than protons. Because of the increase in e-liquid degradation and the production of chloropropanols, the use of sucralose in e-liquids should be avoided; the presence of sucralose as an ingredient in commercial e-liquids should be disclosed by means of appropriate labeling.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mr. Ari J. Bluffstone for experimental assistance and Professor James F. Pankow for his critical reading of and suggestions for this manuscript.
Funding
This work was supported by the U.S. National Institutes of Health, Grant R01ES025257; the research reported was supported by the NIEHS and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
ABBREVIATIONS LIST
- α fb
fractional free-base nicotine content
- e-cigarette
electronic cigarette
- e-liquid
electronic cigarette liquid
- FID
flame ionization detector
- GC/MS
gas chromatography/mass spectrometry
- GC/FID
gas chromatography with flame ionization detector
- IC
ion chromatography (IC)
- GC
gas chromatography
- GL
glycerol
- HMF
hydroxymethylfurfural
- IPA
isopropanol
- MS
mass spectrometry
- NMR spectroscopy
NMR spectroscopy
- PG
propylene glycol
- IPA
isopropyl alcohol
- HPLC-MS/MS
high performance liquid chromatography tandem mass spectrometry
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.9b00047.
Details for the MS analyses of samples (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, and Vouthis V (2013) Impact of Flavour Variability on Electronic Cigarette Use Experience: An Internet Survey. Int. J. Environ. Res. Public Health 10 (12), 7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Felton RE (1972) What flavoring can do to improve sales of tobacco products. World Tobacco 38, 147–148. [Google Scholar]
- (3).Morean ME, Butler ER, Bold KW, Kong G, Camenga DR, Cavallo DA, Simon P, O’Malley SS, and Krishnan-Sarin S (2018) Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS One 13 (1), No. e0189015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Behar RZ, Luo WT, McWhirter KJ, Pankow JF, and Talbot P (2018) Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fagan P, Pokhrel P, Herzog TA, Moolchan ET, Cassel KD, Franke AA, Li X, Pagano I, Trinidad DR, Sakuma KK, Sterling K, Jorgensen D, Lynch T, Kawamoto C, Guy MC, Lagua I, Hanes S, Alexander LA, Clanton MS, Graham-Tutt C, Eissenberg T, and Addictive Carcinogens W (2018) Sugar and Aldehyde Content in Flavored Electronic Cigarette Liquids. Nicotine Tob. Res 20 (8), 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kubica P, Wasik A, Kot-Wasik A, and Namiesnik J (2014) An evaluation of sucrose as a possible contaminant in e-liquids for electronic cigarettes by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 406 (13), 3013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Miao S, Beach ES, Sommer TJ, Zimmerman JB, and Jordt SE (2016) High-Intensity Sweeteners in Alternative Tobacco Products. Nicotine Tob. Res 18 (11), 2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tierney PA, Karpinski CD, Brown JE, Luo W, and Pankow JF (2016) Flavour chemicals in electronic cigarette fluids. Tob Control 25 (e1), No. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Capuano E, and Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. Lwt-Food Sci. Technol 44 (4), 793–810. [Google Scholar]
- (10).Jing Q, and Lu XY (2008) Kinetics of Non-catalyzed Decomposition of Glucose in High-temperature Liquid Water. Chin. J. Chem. Eng 16 (6), 890–894. [Google Scholar]
- (11).Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, and Saliba NA (2016) Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control 25, No. ii88. [DOI] [PubMed] [Google Scholar]
- (12).Briganti M, Delnevo CD, Brown L, Hastings SE, and Steinberg MB (2019) Bibliometric Analysis of Electronic Cigarette Publications: 2003(–)2018. Int. J. Environ. Res. Public Health 16 (3), 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Krishnan-Sarin S, Jackson A, Morean M, Kong G, Bold KW, Camenga DR, Cavallo DA, Simon P, and Wu R (2019) E-cigarette devices used by high-school youth. Drug Alcohol Depend. 194, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Burdock GA, and Fenaroli G Fenaroli’s handbook of flavor ingredients, 6th ed.; CRC Press/Taylor & Francis Group: Boca Raton, 2010. [Google Scholar]
- (15).https://e-liquid-recipes.com, E-Liquid Recipes, accessed 10 June 2019.
- (16).Wiet SG, and Beyts PK (1992) Sensory Characteristics of Sucralose and Other High-Intensity Sweeteners. J. Food Sci 57 (4), 1014–1019. [Google Scholar]
- (17).Rosbrook K, Erythropel HC, DeWinter TM, Falinski M, O’Malley S, Krishnan-Sarin S, Anastas PT, Zimmerman JB, and Green BG (2017) The effect of sucralose on flavor sweetness in electronic cigarettes varies between delivery devices. PLoS One 12 (10), e0185334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Grotz VL, and Munro IC (2009) An overview of the safety of sucralose. Regul. Toxicol. Pharmacol 55 (1), 1–5. [DOI] [PubMed] [Google Scholar]
- (19).de Oliveira DN, de Menezes M, and Catharino RR (2015) Thermal degradation of sucralose: a combination of analytical methods to determine stability and chlorinated byproducts. Sci. Rep 5, 9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rahn A, and Yaylayan VA (2010) Thermal degradation of sucralose and its potential in generating chloropropanols in the presence of glycerol. Food Chem. 118 (1), 56–61. [Google Scholar]
- (21).Duell AK, Pankow JF, Gillette SM, and Peyton DH (2018) Boiling points of the propylene glycol + glycerol system at 1 atm pressure: 188.6–292°C without and with added water or nicotine. Chem. Eng. Commun 205, 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jensen RP, Luo W, Pankow JF, Strongin RM, and Peyton DH (2015) Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med 372 (4), 392–4. [DOI] [PubMed] [Google Scholar]
- (23).Jensen RP, Strongin RM, and Peyton DH (2017) Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Salamanca JC, Meehan-Atrash J, Vreeke S, Escobedo JO, Peyton DH, and Strongin RM (2018) E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci. Rep 8 (1), 7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Salamanca JC, Munhenzva I, Escobedo JO, Jensen RP, Shaw A, Campbell R, Luo W, Peyton DH, and Strongin RM (2017) Formaldehyde Hemiacetal Sampling, Recovery, and Quantification from Electronic Cigarette Aerosols. Sci. Rep 7 (1), 11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, and Zimmerman JB, Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res 2018. DOI: 10.1093/ntr/nty192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).de Saint Laumer JY, Cicchetti E, Merle P, Egger J, and Chaintreau A (2010) Quantification in gas chromatography: prediction of flame ionization detector response factors from combustion enthalpies and molecular structures. Anal. Chem 82 (15), 6457–62. [PubMed] [Google Scholar]
- (28).Duell AK, Pankow JF, and Peyton DH (2018) Free-Base Nicotine Determination in Electronic Cigarette Liquids by 1H NMR Spectroscopy. Chem. Res. Toxicol 31 (6), 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).CORESTA Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection - Definitions and Standard Conditions. https://www.coresta.org/routine-analytical-machine-e-cigarette-aerosol-generation-and-collection-definitions-and-standard (accessed 03/22/2018). [Google Scholar]
- (30).Beauval N, Antherieu S, Soyez M, Gengler N, Grova N, Howsam M, Hardy EM, Fischer M, Appenzeller BMR, Goossens JF, Allorge D, Garcon G, Lo-Guidice JM, and Garat A (2017) Chemical Evaluation of Electronic Cigarettes: Multi-component Analysis of Liquid Refills and their Corresponding Aerosols. J. Anal. Toxicol 41 (8), 670–678. [DOI] [PubMed] [Google Scholar]
- (31).Beauval N, Verriele M, Garat A, Fronval I, Dusautoir R, Antherieu S, Garcon G, Lo-Guidice JM, Allorge D, and Locoge N (2019) Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int. J. Hyg. Environ. Health 222, 136. [DOI] [PubMed] [Google Scholar]
- (32).Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, and Peyton DH (2017) Benzene formation in electronic cigarettes. PLoS One 12 (3), No. e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Grice HC, and Goldsmith LA (2000) Sucralose–an overview of the toxicity data. Food Chem. Toxicol 38, S1–6. [DOI] [PubMed] [Google Scholar]
- (34).Jang M, Czoschke NM, Lee S, and Kamens RM (2002) Heterogeneous atmospheric aerosol production by acid-catalyzed particle-phase reactions. Science 298 (5594), 814–7. [DOI] [PubMed] [Google Scholar]
- (35).Pankow JF (2017) Calculating compound dependent gasdroplet distributions in aerosols of propylene glycol and glycerol from electronic cigarettes. J. Aerosol Sci 107, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).European Commission, S. C. o. F. Opinion of the Scientific Committee on Food on 3-monochloro-propande-1,2-diol (3-MCPD); Rue de la Loi 200, B-1049 Bruxelles/Wetstraat 200, B-1049 Brussel - Belgium, 2001. [Google Scholar]
- (37).Lin YC, Cho J, Tompsett GA, Westmoreland PR, and Huber GW (2009) Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 113 (46), 20097–20107. [Google Scholar]
- (38).Meng X, Zhang HY, Liu C, and Xiao R (2016) Comparison of Acids and Sulfates for Producing Levoglucosan and Levoglucosenone by Selective Catalytic Fast Pyrolysis of Cellulose Using Py-GC/MS. Energy Fuels 30 (10), 8369–8376. [Google Scholar]
- (39).Farsalinos KE, Voudris V, and Poulas K (2015) E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 110 (8), 1352–6. [DOI] [PubMed] [Google Scholar]
- (40).Benowitz NL (2010) Nicotine addiction. N. Engl. J. Med 362 (24), 2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Koob GF, and Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24 (2), 97–129. [DOI] [PubMed] [Google Scholar]
- (42).Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, and Patacchini R (2008) Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest 118 (7), 2574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, and Morice AH (2009) TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med 180 (11), 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, and Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U. S. A 104 (33), 13525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, and Voets T (2009) Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci 12 (10), 1293–9. [DOI] [PubMed] [Google Scholar]
- (46).Willis DN, Liu B, Ha MA, Jordt SE, and Morris JB (2011) Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 25 (12), 4434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Carstens E, Kuenzler N, and Handwerker HO (1998) Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol 80 (2), 465–92. [DOI] [PubMed] [Google Scholar]
- (48).Talhout R, Opperhuizen A, and van Amsterdam JG (2007) Role of acetaldehyde in tobacco smoke addiction. Eur. Neuropsychopharmacol 17 (10), 627–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.