Abstract
Fibronectin binding proteins (FnBP) on the surface of Staphylococcus aureus have previously been shown to mediate adherence of the organism to resting endothelial cells in static adhesion assays. However, in this study using well-defined flow assays, we demonstrate that physiologic levels of shear stress prevent FnBP-mediated adhesion of S. aureus 8325-4 to resting endothelial cells. This result suggests that mechanical forces present in vivo may influence the ability of staphylococci to bind endothelial cell surfaces.
With the increase in antibiotic resistance of Staphylococcus aureus, there is a critical need to develop innovative therapeutics to combat infections, such as infective endocarditis, osteomyelitis, and other S. aureus infections of organs. S. aureus adhesion to endothelial cells followed by invasion has been implicated in the pathogenesis of these infections (reviewed extensively in references 11, 12, and 15).
The hematogenous spread of S. aureus from local sites of infection to deeper tissues (reviewed in references 7 and 15) may be an important aspect of the infection process. Adhesion of S. aureus to endothelial cells may be important in this bacterial metastasis when there is no damage to the blood vessel wall. For example, infective endocarditis is sometimes seen in individuals with previously undamaged heart valves, suggestive of initial bacterial adhesion to the intact endothelium on the cardiac valve surface (11).
The endothelial cell receptors and bacterial adhesins involved in this adhesion have not been clearly identified. Recently, Peacock and colleagues (15) demonstrated the significant role played by the S. aureus adhesins fibronectin (Fn) binding protein A (FnBP A) and FnBP B (5, 8) by comparing adherence of mutant strains defective in expression of FnBPs with the adherence of isogenic parent strain 8325-4 to resting human endothelial cells under static conditions. FnBPs were also implicated in the subsequent internalization of S. aureus by endothelial cells. However, the significance of FnBPs has been questioned by an in vivo study of the pathogenesis of endocarditis (4). Fn and fibrinogen present on mammalian cells may act as bridging molecules between mammalian cells and the bacteria (1, 2, 18, 19). However, Peacock and colleagues found no change in S. aureus adhesion to resting human endothelial cells when the endothelial cells were precoated with Fn (15).
In the vasculature, fluid shear is an important physiological parameter that has been shown to modulate the interactions between blood cells and endothelial cells (6, 9, 10). Fluid shear stress is the hydrodynamic force generated by the flow of blood on the vessel wall. This force is proportional to the rate of deformation of the fluid at a surface, which is known as the shear rate (expressed as units per second). Physiological shear rates can range between 40 s−1 and 2,000 s−1 for stable laminar flow, and even higher shear rates are prevalent in turbulent flow and at vessel entrances and bifurcations (17). Therefore, as was stated in the study by Peacock et al. (15), it is important to incorporate well-defined flow conditions in the assay of bacterial adhesion to endothelial cells to further elucidate the relative importance of various molecular adhesion pathways. Parallel plate flow chamber assays have been used extensively to study this dynamic adhesion of cells to surface bound proteins and cells (6, 9, 10, 14). The aim of this study was to examine the role of FnBPs in mediating the adhesion of S. aureus 8325-4 to resting endothelial cells under physiological flow conditions.
S. aureus strain 8325-4 and its isogenic mutant lacking FnBPs, DU5883 (5), were grown for approximately 20 h (primary culture) in 50 ml of tryptic soy broth (TSB) under constant rotation at 37°C followed by a secondary culture (transfer of 1 ml of bacterial suspension to 49 ml of TSB under constant rotation at 37°C). After approximately 2 h of growth to early growth phase (peak expression of FnBPs [16]) in secondary culture, bacteria were harvested into cold phosphate-buffered saline (PBS) with a final concentration of 0.02% sodium azide and 0.1% bovine serum albumin (BSA). The bacterial cells were labeled with fluorescein isothiocyanate (FITC; Molecular Probes Inc, Eugene, Oreg.) and washed repeatedly to remove excess FITC. Sodium azide was used to preserve the number of FnBP adhesins on the bacterial surface during labeling. Preliminary studies demonstrated that short-term bacterial exposure to sodium azide did not affect FnBP-mediated binding to immobilized Fn under dynamic conditions (data not shown). Just before use in any assay, the labeled bacteria were removed from the PBS with 0.02% sodium azide and diluted into assay buffer without sodium azide (PBS with 0.1% BSA) to a concentration of 108 cells/ml to avoid any adverse effect of azide on the endothelial cells.
Bovine aortic endothelial cells (BAECs; Clonetics, San Diego, Calif.) were maintained in Dulbecco's Modified Eagle Medium (Clonetics) supplemented with 10% fetal bovine serum and penicillin-streptomycin according to standard tissue culture techniques. BAECs (second to sixth passages) were seeded onto gelatin-coated glass slides or 35-mm tissue culture dishes and grown to 100% confluence. For experiments requiring precoating of BAECs with Fn, the growth medium was replaced with serum-free medium containing 200 μg of Fn/ml and incubated for 30 min in a humidified incubator before use in an adhesion assay.
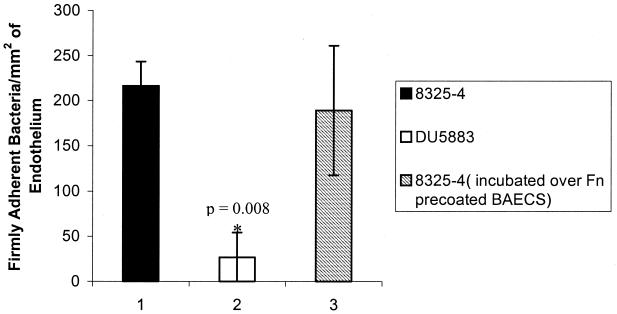
In order to verify our experimental system, we first performed a static adhesion assay to reproduce previously published results (15). BAECs or Fn-precoated BAECs in 35-mm tissue culture dishes were incubated with FITC-labeled S. aureus (108 cells/ml in assay buffer with no azide) for 20 min at 37°C. The bacterial suspension was aspirated, and the BAEC monolayer was washed gently with PBS to rinse away nonadherent bacteria. The adherent bacteria were visually counted using an inverted-stage microscope equipped with standard FITC filters, epifluorescent illumination, and a charged-coupled device camera. The results of this static adhesion assay are shown in Fig. 1. We examined the relative adhesion (adhesion of DU5883 to endothelial cells as a percentage of 8325-4 adhesion to endothelial cells) in order to compare our data to a previous study (15). Our results show 12.5% relative adhesion of DU5883 (versus that of 8325-4) to BAECs (Fig. 1) compared to the previously published result of approximately 19% relative adhesion of DU5883 versus 8325-4 to human umbilical vein endothelial cells (absolute numbers are given in reference 15). This result substantiates the role of FnBPs in mediating adhesion to resting endothelial cells under static conditions. Precoating of the BAECs with Fn did not affect FnBP-mediated S. aureus adhesion to endothelial cells under static conditions, in agreement with the previous study (15). These results demonstrate that we have reproduced previously published findings despite differences in bacterial labeling technique and endothelial cell source (15).
FIG. 1.
Static adhesion of S. aureus 8325-4 and DU5883 to BAECs and Fn-precoated BAECs. Data are plotted as the means ± standard errors of the means. The asterisk denotes statistically significant compared to the 8325-4 control (P < 0.05 by analysis of variance).
In order to reproduce the static assay in the parallel plate flow chamber (13), a FITC-labeled bacterial cell suspension (108 cells/ml in assay buffer with no azide) was perfused over the endothelial cell monolayer at a shear rate of 100 s−1 for 5 min before the flow was stopped, and the bacteria were allowed to settle onto the endothelial surface for 30 min. The nonadherent bacteria were rinsed away by switching the inlet tube to wash buffer (PBS with 0.1% BSA) and restarting flow at 25 s−1 for 5 min. This experiment is termed a “stopped flow” assay. The number of firmly adherent bacteria per square millimeter as visualized on an inverted epifluorescent microscope using a computer-controlled stage and image-capturing system is reported (eight frames of view in the center of the flow field for each experiment; each experiment was repeated at least three times; image capturing was set up as described in reference 13). The results from the stopped flow assay are shown in Fig. 2. Our results show 15.7% relative adhesion of DU5883 (versus 8325-4) to BAECs in the stopped flow assay, in agreement with our findings for the static adhesion assay (Fig. 1). Precoating of BAECs with Fn did not significantly alter the stopped flow adhesion results. These results demonstrate that FnBP-mediated S. aureus adhesion to endothelial cells under static conditions has been reproduced in the parallel plate flow chamber in the stopped flow assay.
FIG. 2.
Adhesion of S. aureus 8325-4 and DU5883 to BAECs under stopped flow and dynamic conditions. Shear rate is the rate of deformation of the fluid at the surface and is expressed as units per second. This parameter is directly proportional to the force generated on the surface by the flowing fluid. Data are plotted as the means ± standard errors of the means. The asterisk denotes statistically significant compared to the 8325-4 control (P < 0.05 by analysis of variance).
Dynamic adhesion of S. aureus to endothelial cells was examined in a parallel plate flow chamber under well-defined flow conditions as previously described (13). The shear rates examined were in the range of 10 to 200 s−1 corresponding to flow in veins and venules (17). A suspension of FITC-labeled bacteria (108 cells/ml in assay buffer with no azide) was perfused through the flow chamber over the endothelial cells (both BAECs and Fn-precoated BAECs) at a particular wall shear rate for 10 min. Immediately, the inlet tube was switched to wash buffer, which was perfused through the flow chamber to rinse away nonadherent cells for 5 min (6). With the buffer flowing, the number of firmly adherent cells was determined (13) and reported as firmly adherent cells per square millimeter of endothelium (the experiment was repeated three times). No bacteria adhered firmly to either BAECs or Fn-precoated BAECs during the dynamic flow assay at any of the shear rates examined (Fig. 2). Invasion of BAECs by S. aureus under shear conditions was not examined since there was no adhesion in the flow assay.
This result in the dynamic flow assay was unexpected because preliminary experiments in our laboratory demonstrated 8325-4 and DU5883 adhesion to immobilized Fn at shear rates as high as 300 s−1, suggesting that FnBPs can mediate binding under shear conditions (data not shown). To explain these results, we considered that the flow over BAECs could strip away any cellular Fn already present on the endothelial cell surface or any Fn precoated on the BAECs. This possibility was addressed by incorporating Fn at a concentration of 100 μg/ml in the flow assay buffer at two of the lower shear rates (10 s−1 and 25 s−1). No firm adhesion of bacteria was observed (data not shown). Therefore, the removal of Fn from the endothelial surface is not the cause for the surprising results of the flow assay.
The results of the dynamic study stand in sharp contrast to the assay results performed under static conditions. There are several possible explanations for the contradictory results. Shear at the surface of the endothelial cell could prevent formation of sufficient bonds by reducing the contact time between either the FnBPs and the Fn complexed to endothelial surface receptors or between the Fn and the endothelial cell receptors. The dramatic increase in bacterial adhesion when flow was stopped for 30 min and then restarted suggests that an increase in contact time could lead to formation of sufficient bonds. However, the adhesion with increased contact time could be a result of an increased number of receptors being expressed on the endothelial cell surface. For example, S. aureus resting on the surface of the endothelial cells during the static assay could be stimulating the cells during the 20- to 30-min period, resulting in receptor trafficking to the endothelial cell surface. Alternately, the increased contact time could bring into play stabilization mechanisms such as cytoskeleton linkage or a change in receptor confirmation. Finally, the number of receptors on the surface of resting endothelial cells may simply be insufficient to support firm adherence of the bacterium during flow.
This study is an initial step in the incorporation of hydrodynamic forces in in vitro assays involving bacterial adhesion to endothelial cells. In the future, we plan to examine the effects of cytokine stimulation (3) on the adhesion of S. aureus to human umbilical vein endothelial cells under dynamic flow conditions. In addition, other adhesion pathways, such as clumping factor A (ClfA) ClfB, and fibrinogen, will be investigated. Well-established static adhesion assays have identified various molecular mechanisms of S. aureus adhesion. We submit that the static assay needs to be complemented with dynamic adhesion assays to further elucidate the relative importance of each adhesion mechanism.
Acknowledgments
Bacterial strains in this study were kindly provided by Magnus Höök (Center for Extracellular Matrix Biology, Texas A & M University, Houston, Tex.) and Timothy J. Foster (Microbiology Department, Moyne Institute of Preventive Medicine, Trinity College, Dublin, Ireland).
This work was supported by a grant from the Whitaker Foundation.
REFERENCES
- 1.Becker R C, DiBello P M, Lucas F V. Bacterial tissue tropism: an in vitro model for infective endocarditis. Cardiovasc Res. 1987;21:813–820. doi: 10.1093/cvr/21.11.813. [DOI] [PubMed] [Google Scholar]
- 2.Cheung A L, Krishnan M, Jaffe E A, Fischetti V A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Investig. 1991;87:2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung A L, Koomey J M, Lee S, Jaffe E A, Fischetti V A. Recombinant human tumor necrosis factor alpha promotes adherence of Staphylococcus aureus to cultured human endothelial cells. Infect Immun. 1991;59:3827–3831. doi: 10.1128/iai.59.10.3827-3831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flock J L, Hienz S A, Heimdahl A, Schennings T. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect Immun. 1996;64:1876–1878. doi: 10.1128/iai.64.5.1876-1878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales R S, Wick T M. Hemodynamic modulation of monocytic cell adherence to vascular endothelium. Ann Biomed Eng. 1996;24:382–393. doi: 10.1007/BF02660887. [DOI] [PubMed] [Google Scholar]
- 7.Ing M B, Baddour L M, Bayer S A. Bacteremia and infective endocarditis: pathogenesis, diagnosis and complications. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. 1st ed. New York, N.Y: Churchill Livingstone; 1997. pp. 331–354. [Google Scholar]
- 8.Joh D, Wann E R, Kreikemeyer B, Speziale P, Höök M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 9.Jones D A, Abbassi O, McIntire L V, McEver R P, Smith C W. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993;65:1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence M B, McIntire L V, Eskin S G. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 11.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 12.Lowy F D. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000;8:341–343. doi: 10.1016/s0966-842x(00)01803-5. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed N, Teeters M A, Patti J M, Höök M, Ross J M. Inhibition of Staphylococcus aureus adherence to collagen under dynamic conditions. Infect Immun. 1999;67:589–594. doi: 10.1128/iai.67.2.589-594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed N, Rainier T R, Ross J M. Novel experimental study of receptor-mediated bacterial adhesion under the influence of fluid shear. Biotechnol Bioeng. 2000;68:628–636. [PubMed] [Google Scholar]
- 15.Peacock S J, Foster T J, Cameron B J, Berendt A R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 16.Saravia-Otten P, Muller H P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turrito V T, Goldsmith H L. Rheological aspects of thrombosis and hemostasis: basic principles and applications. Thromb Haemost. 1986;55:415–435. [PubMed] [Google Scholar]
- 18.Vann J M, Hamill R J, Albrecht R M, Mosher D F, Proctor R A. Immunoelectron microscopic localization of fibronectin in adherence of Staphylococcus aureus to cultured bovine endothelial cells. J Infect Dis. 1989;160:538–542. doi: 10.1093/infdis/160.3.538. [DOI] [PubMed] [Google Scholar]
- 19.Vercellotti G M, Lussenhop D, Peterson P K, Furcht L T, McCarthy J B, Jacob H S, Moldow C F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984;103:34–43. [PubMed] [Google Scholar]