Abstract
Although cholera toxin (Ctx) and Escherichia coli heat-labile enterotoxin (Etx) are known to be potent mucosal adjuvants, it remains controversial whether the adjuvanticity of the holotoxins extends to their nontoxic, receptor-binding B subunits. Here, we have systematically evaluated the comparative adjuvant properties of highly purified recombinant EtxB and CtxB. EtxB was found to be a more potent adjuvant than CtxB, stimulating responses to hen egg lysozyme when the two were coadministered to mice intranasally, as assessed by enhanced serum and secretory antibody titers as well as by stimulation of lymphocyte proliferation in spleen and draining lymph nodes. These results indicate that, although structurally very similar, EtxB and CtxB have strikingly different immunostimulatory properties and should not be considered equivalent as prospective vaccine adjuvants.
Oral or nasal mucosal administration of protein antigen is thought to favor a state of immunological unresponsiveness by a process of peripheral tolerance, in order to avoid activation of detrimental immune responses to innocuous dietary and airborne environmental antigens (11, 23). Cholera toxin (Ctx) and heat-labile enterotoxin (Etx) from enterotoxinogenic strains of Escherichia coli, however, are strongly immunogenic by both parenteral and mucosal routes and elicit strong anti-toxin antibody responses when administered either orally or nasally (reviewed in reference 21). Ctx and Etx are also recognized as two of the most potent mucosal adjuvants yet identified, able to greatly enhance antibody responses to coadministered antigens (for recent reviews, see references 17 and 25). However, their toxicity is likely to preclude usage in human vaccines.
The toxins are hetero-oligomeric complexes each composed of an enzymatic A subunit and five identical B subunits (9). The A subunit is responsible for toxicity, catalyzing ADP ribosylation of Gsα, increasing cyclic AMP (cAMP) levels, and producing chloride efflux and fluid loss. The B subunits are arranged in a pentameric ring and contain five receptor-binding pockets for high-avidity association with cellular membranes containing GM1 ganglioside. The nontoxic B subunits, CtxB and EtxB, are also potent immunogens, avoiding tolerance induction when administered mucosally and generating strong secretory and systemic antibody responses (reviewed in references 17, 21, and 25).
While early studies suggested that the adjuvant activities of Ctx and Etx were completely dependent on the ability of the catalytic A subunit to raise intracellular cAMP levels and that nontoxic derivatives were devoid of adjuvanticity, it is now well established that this is not the case. Dickinson and Clements (2) constructed a single point mutation in EtxA which led to reduced ADP ribosylation activity, cAMP elevation, and cellular toxicity, though adjuvanticity was retained (5). Other completely nontoxic point mutants in both CtxA (8, 26–28) and EtxA (4, 6) are now being extensively characterized for their utility in promoting immune responses to mucosally administered antigens.
The possibility that GM1 binding itself may trigger altered responses to mucosally encountered antigens has been a recurrent subject of investigation. Recombinant B subunits have now been shown to possess distinct immunomodulatory activities. These activities include elevation of both Th1- and Th2-type cytokines (14), CD8+ and CD4+ T-cell apoptosis (24, 29), B-cell activation of major histocompatibility complex II, intracellular adhesion molecule 1 (ICAM-1), CD25, CD40, and B7 expression (15), and modulation of antigen presentation by B cells (10) and macrophages (12, 13). While often overshadowed by more potent holotoxin activity, recombinant B subunit has been found to stimulate antibody responses to coadministered protein antigens, and the GM1-binding function of the B subunit was found to be essential for both immunogenicity and adjuvant activity (1, 3, 7, 16). Because of the close structural similarity of CtxB and EtxB, which exhibit more than 80% sequence identity, most studies have not sought to carefully discriminate whether these molecules might exhibit differential activities.
In this paper we have directly compared the induction of mucosal and systemic antibody responses and examined antigen-specific lymphocyte proliferation, following intranasal (i.n.) administration of a model protein antigen together with purified recombinant CtxB or EtxB to identify their relative adjuvant and immunostimulatory activities. Contrary to what was expected, we observed striking differences in the immunomodulatory properties of these closely related molecules.
Preparation of toxin B subunits.
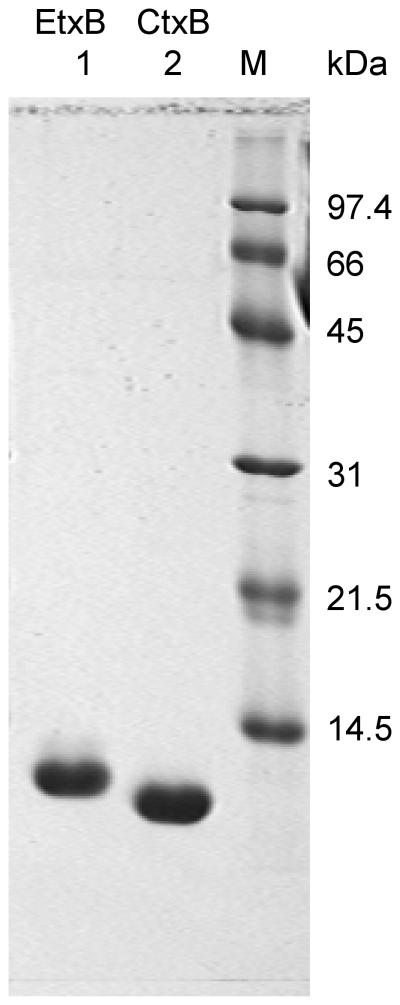
Recombinant EtxB and CtxB were purified from culture supernatants of Vibrio sp. 60 harboring plasmids pMMB68 or pATA13, respectively, by the method previously described (18). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the purified proteins revealed, for each, a single band at the expected molecular weight and demonstrated more than 98% purity (Fig. 1). The preparations of EtxB and CtxB contained equivalent, low levels of lipopolysaccharide (≤ 0.06 endotoxin unit (EU)/μg [data not shown]) as assessed by the Limulus amebocyte lysate endotoxin assay according to the manufacturer's protocol (E-Toxate; Sigma, Oakville, Ontario, Canada).
FIG. 1.
SDS-PAGE analysis of purified, recombinant ExtB and CtxB. The adjuvants (30 μg) were boiled in SDS sample buffer, separated by SDS–15% PAGE, and visualized using Coomassie Blue R-250 staining. Lane 1, EtxB; lane 2, CtxB; lane M, molecular size standards.
Stimulation of systemic and mucosal antibody responses by the B subunit.
Hen egg lysozyme (HEL) (three times crystallized, dialyzed, and lyophilized; catalog number L-6876; Sigma) was used as a model vaccine antigen with either EtxB or CtxB as an adjuvant. Proteins were aseptically prepared in sterile phosphate-buffered saline (PBS) at the appropriate concentration for i.n. administration in a total volume of 14 μl (7 μl per nare). To identify whether CtxB and EtxB had similar abilities to enhance systemic and mucosal antibody responses, groups of three to five female C3H/HeN (H-2k) mice (Charles River Laboratories, Montreal, Quebec, Canada) were immunized i.n. with HEL alone, HEL plus CtxB (HEL+CtxB), HEL plus EtxB (HEL+EtxB), or HEL plus Ctx (HEL+Ctx) (Fig. 2). Mice were given two immunizations, 7 days apart. Three weeks following immunization, mice were bled from the retro-orbital plexus, and the serum was collected by centrifugation after 1 h of clotting at room temperature. Sodium azide was added to a final concentration of 0.1%, and the serum samples were stored at −20°C until use. Fresh fecal samples were obtained from each mouse, and the individual samples within each experimental group were pooled and extracted overnight at 4°C in PBS containing 2 mM phenylmethylsulfonyl fluoride (PMSF), 200 μg of aprotinin per ml, 10 mM EDTA, and 0.1% NaN3. Insoluble fecal material was removed by centrifugation, and the supernatants were stored at −20°C until use. Antibody titers were determined by standard end-point enzyme-linked immunosorbent assay (ELISA). ELISA plates (Maxisorb; Nunc) were coated with 100 μg of HEL per ml overnight at 4°C, washed, and incubated with diluted serum, fecal, or nasal wash samples overnight at 4°C. Biotinylated goat anti-mouse immunoglobulin G1 (IgG1) or biotinylated goat anti-mouse IgA (Jackson Immunotech) was used to detect specific HEL-bound antibody isotypes, which were then detected with alkaline phosphatase-conjugated avidin (Extra-Avidin; Sigma), using p-nitrophenyl phosphate as the substrate in 10% ethanolamine–1 mM MgCl2 buffer [pH 9.8], and the intensity was measured spectrophotometrically at 405 nm. Titers were obtained from the highest dilution yielding an intensity twofold above that observed with samples taken from nonimmunized mice. Statistical significance was determined using Student's t test at the 95% confidence level.
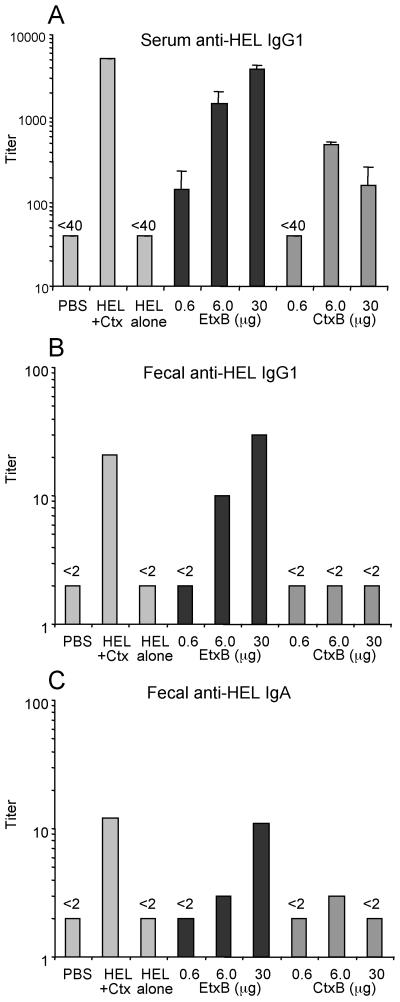
FIG. 2.
Serum and fecal antibody responses following i.n. immunization with HEL+EtxB or HEL+CtxB. C3H/HeN (H-2k) mice were immunized i.n. on two occasions, 7 days apart, with 1 μg of HEL either alone or mixed with different amounts of EtxB or CtxB. Controls received PBS alone or 1 μg of HEL plus 1 μg of Ctx holotoxin. Samples were collected 21 days following the first immunization and assayed for HEL-specific antibody by ELISA. Data bars, mean antibody titers (error bars, standard errors of the means [SEM]) (n = 3 mice per group). Fecal samples from all mice in each group were pooled to determine fecal antibody titers. (A) Serum anti-HEL IgG1; (B) fecal anti-HEL IgG1; (C) fecal anti-HEL IgA.
Antibody responses against HEL were undetectable in mice given saline or HEL alone (Fig. 2) but were significantly stimulated in mice immunized with various doses of HEL+EtxB (Fig. 2). IgG1 titers were 5- to 15-fold higher in feces and 4- to 100-fold higher in serum, and IgA titers were 2- to 5-fold higher than those observed after immunization with HEL alone, depending on the dose of EtxB. As little as 6 μg of EtxB stimulated anti-HEL IgG1 and IgA titers in feces, while even the lowest dose (0.6 μg) was able to elicit an anti-HEL IgG1 response in serum. CtxB admixed with HEL was unable to stimulate an anti-HEL IgG1 response in feces and elicited only a weak fecal IgA response. CtxB did stimulate a serum anti-HEL IgG1 response (Fig. 2A), though at titers consistently lower than those elicited by equivalent doses of EtxB. CtxB lost all stimulatory ability at the lowest dose used (0.6 μg). Serum anti-HEL IgA was undetectable following immunization with either B subunit (data not shown). Interestingly, increasing the dose of CtxB to 30 μg decreased the anti-HEL antibody responses (Fig. 2A and C), in contrast to the increased-dose response stimulated by EtxB. Antibody titers elicited by the highest dose of HEL+EtxB were similar to those produced by immunization with HEL plus 1 μg of Ctx holotoxin.
Next, the effect of admixed B subunit on the recall antibody responses of mice primed i.n. with HEL+EtxB or HEL+CtxB was examined following a secondary antigen challenge with Ctx holotoxin. Mice were primed with no antigen (PBS), HEL alone, HEL+EtxB, or HEL+CtxB and then challenged 3 weeks later with HEL+Ctx administered i.n., and anti-HEL antibody responses were measured (Fig. 3). Serum and fecal samples were obtained as described above, and the mice were sacrificed for nasal wash sampling as previously described (22). Briefly, the trachea was ligated with surgical thread and 60 G polyethylene tubing was inserted, aside the trachea, into the nasal cavity. PBS containing 2 mM PMSF, 200 μg of aprotinin per ml, 10 mM EDTA, and 0.1% NaN3 was injected into the nasal cavity via a syringe attached to the tubing and collected as it emerged from the nares into a 1.5-ml centrifuge tube placed below the nose. A total wash volume of 1 ml was injected and collected by this procedure. Nasal wash samples were clarified by centrifugation, and the supernatants were stored at −20°C until use.
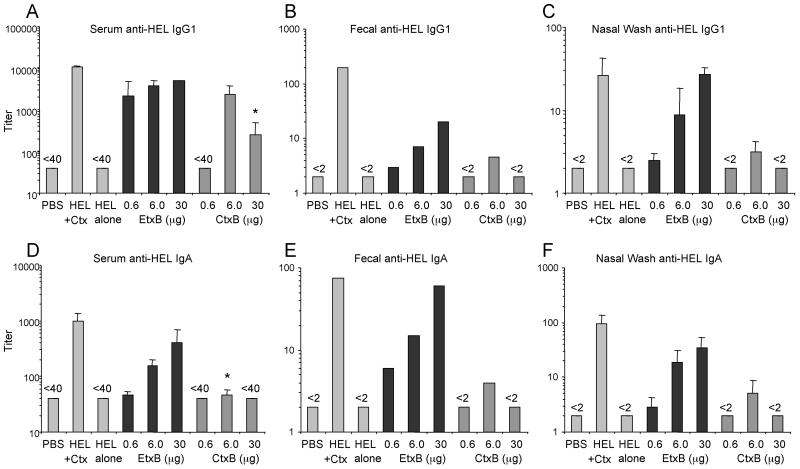
FIG. 3.
Recall antibody responses to i.n. priming with HEL+EtxB or HEL+CtxB. Mice were given two i.n. immunizations, 7 days apart, as described for Fig. 2. On day 22 after the first immunization, all mice were challenged i.n. with 1 μg of HEL plus 1 μg of Ctx. Ten days later, the mice were sacrificed and samples were obtained. Samples were assayed for HEL-specific antibody by ELISA. Data bars, mean antibody titers (error bars, SEM) (n = 3 mice per group). Fecal samples from all mice in each group were pooled to determine fecal antibody titers. ∗, P < 0.05 compared to equivalent dose of EtxB. (A) Serum anti-HEL IgG1; (B) fecal anti-HEL IgG1; (C) nasal anti-HEL IgG1; (D) serum anti-HEL IgA; (E) fecal anti-HEL IgA; (F) nasal anti-HEL IgA.
Priming i.n. with HEL alone yielded no antibody responses 10 days following a secondary challenge with HEL+Ctx (Fig. 3). By contrast, when EtxB was included with HEL during the initial priming (Fig. 3), high titers of serum and secretory anti-HEL-specific antibody were induced following the secondary challenge. Priming with HEL+CtxB, however, yielded much weaker antibody titers in feces and nasal secretions and low IgA responses in serum, following challenge. The titers of HEL-specific IgG1 and IgA in feces and nasal secretions, as well as those of anti-HEL IgG1 and IgA in serum, were significantly greater in mice primed with HEL+EtxB than in those given equivalent doses of HEL+CtxB. Only at an intermediate dose of B subunit (6 μg) were CtxB and EtxB able to induce similar, high titers of anti-HEL IgG1 in serum. A high dose of CtxB (30 μg) was less effective at stimulating anti-HEL antibody responses, even after Ctx challenge. Priming with HEL+Ctx holotoxin produced the highest titers of both systemic and secreted antibody observed (Fig. 3).
Parallel studies using an alternative model antigen, ovalbumin (OVA), were also undertaken. Mice immunized i.n. with OVA plus EtxB or OVA plus CtxB exhibited a similarly differential response, with EtxB triggering potent anti-OVA antibody responses, while CtxB elicited either meager or no anti-OVA response (data not shown).
Kinetics of antibody stimulation by recombinant B subunits.
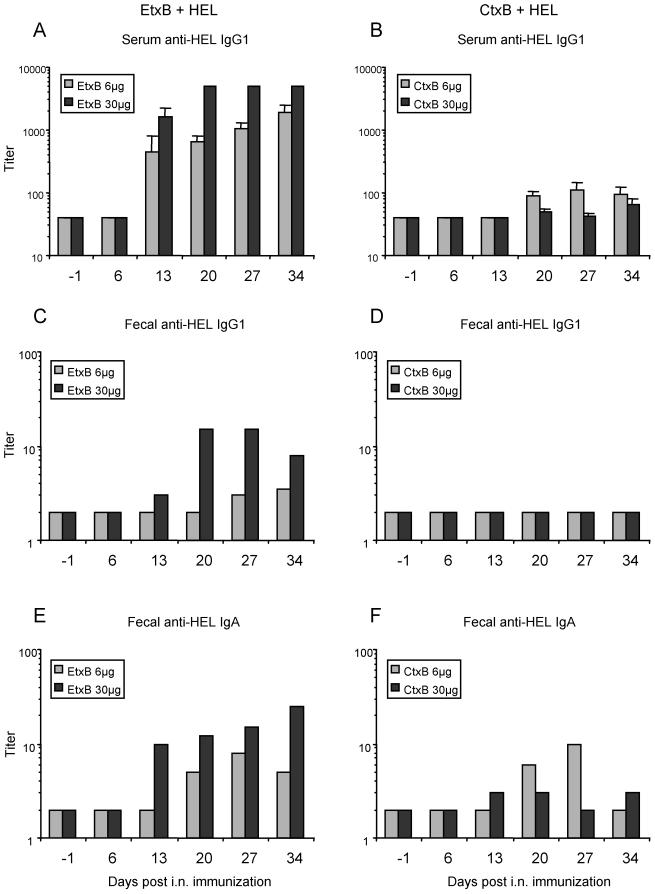
To determine whether CtxB was simply slower acting than EtxB and if its adjuvant effects might approach those of EtxB at later time points, the kinetics of anti-HEL antibody stimulation were examined over the course of 5 weeks using two doses of B subunit, 6 and 30 μg (Fig. 4). Following i.n. immunization with HEL+EtxB, elevated serum and fecal anti-HEL antibody titers were detectable 13 days after the primary administration (Fig. 4A, C, and E). Antibody titers in both serum and feces increased over the next 3 weeks. The serum anti-HEL IgG1 response when CtxB was used as the adjuvant (Fig. 4B, D, and F) lagged 1 week behind that observed for EtxB used as the adjuvant, appearing at 20 days postimmunization, and remained significantly lower over the next 3 weeks. Again, no detectable titers of fecal IgG1 were stimulated by CtxB. Fecal anti-HEL IgA induced by CtxB appeared between weeks 2 and 4 and decreased by week 5. Again, a high dose of CtxB yielded lower anti-HEL antibody titers in serum and feces than a low dose, in contrast to the dose response to EtxB.
FIG. 4.
Kinetics of antibody responses to i.n. immunization with HEL+EtxB or HEL+CtxB. Mice were immunized with 1 μg of HEL either alone or with the indicated amount of EtxB or CtxB exactly as described for Fig. 2. Samples were taken at the indicated time points before and after the first immunization and were then assayed for HEL-specific antibody by ELISA. Data bars, mean antibody titers in serum (error bars, SEM) (n = 3 mice per group). Fecal samples from all mice in each group were pooled to determine fecal antibody titers. (A and B) Serum anti-HEL IgG1; (C and D) fecal anti-HEL IgG1; (E and F) fecal anti-HEL IgA.
HEL-specific lymphocyte priming following B subunit immunization.
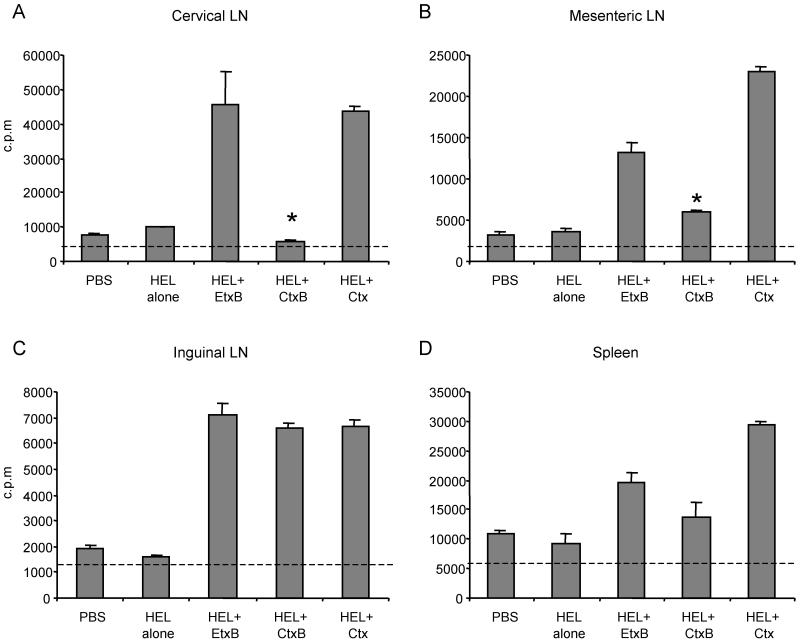
The presence of T cells primed to respond to HEL following i.n. immunization with HEL+CtxB or HEL+EtxB was determined by measuring the proliferation of lymphocytes restimulated in vitro with HEL (Fig. 5). The spleen, mesenteric lymph nodes (MLN), cervical lymph nodes (CLN), and inguinal lymph nodes (ILN) were removed from immunized mice and placed in sterile Hank's balanced salt solution at 4°C. Tissues were homogenized by grinding between frosted glass slides followed by gentle pipeting. A single cell suspension was obtained by passing the homogenate though 60 μm-opening nylon mesh, and the lymphocytes were recovered by centrifugation. Red blood cells were removed using osmotic lysis by resuspending the cells in 1 ml of cold erythrocyte lysis solution (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubating the suspension for 1 min before dilution with 10 volumes of Hank's balanced salt solution. Membrane debris and adipocytes were then removed by further centrifugation, the supernatant was discarded, and the lymphocyte pellet was resuspended in complete RPMI medium containing 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 100 μg of penicillin-streptomycin per ml, 100 μg of amphotericin B (Fungizone; GIBCO BRL) per ml. Total lymphocytes from each tissue were cultured at 5 × 105 cells in a 96-well flat-bottomed tissue culture plate (Nunc), in complete RPMI medium with or without 200 μg of HEL per ml. Cells were incubated for 72 h at 37°C in 5% CO2, and [3H]thymidine was added for the final 15 h to assess proliferation. Cells were harvested onto glass-fiber filter paper using a PHD (Cambridge, Mass.) harvester, and incorporated counts were measured by liquid scintillation counting in a Beckman beta counter. Optimum proliferation was observed during 72 h of incubation in culture medium containing 200 μg of HEL per ml (reference 20 and data not shown). Proliferative responses were detected 7 days following i.n. immunization with HEL+Ctx in all the tissues examined (Fig. 5). When EtxB was used as the adjuvant, similar cell proliferation was also seen in all locations examined (Fig. 5). However, when CtxB was used as adjuvant (Fig. 5), the cell proliferation level was not significantly above the background (nonimmune) level in either the CLN or spleen, and the response in MLN was significantly lower than that observed for HEL+EtxB or HEL+Ctx. Surprisingly, the proliferation of ILN lymphocytes following HEL+CtxB administration was equivalent to that stimulated by HEL+Ctx or HEL+EtxB. The restricted localization of HEL-primed lymphocytes to distal, peripheral lymph nodes, and their absence from spleen and local CLN, following immunization with HEL+CtxB correlated with the lack of systemic humoral responses and weak mucosal antibody responses. In contrast, HEL+EtxB immunization lead to primed lymphocytes in spleen, as well as in both local and distal lymph nodes, and stimulated both systemic and mucosally secreted antibody production.
FIG. 5.
HEL-specific lymphocyte proliferation following i.n. immunization with HEL+EtxB or HEL+CtxB. Mice were immunized once i.n. with either 1 μg of HEL alone, 1 μg of HEL plus 30 μg of EtxB, 1 μg of HEL plus 30 μg of CtxB, or 1 μg HEL plus 1 μg of Ctx, as indicated. Controls received PBS. Seven days later, cell suspensions were isolated from CLN (A), MLN (B), ILN (C), and spleen (D). The cells were cultured in the presence or absence of 200 μg of HEL per ml for 72 h. Proliferation was measured by incorporation of [3H]thymidine during the final 15 h of culture, and data are mean counts per minute ± SEM from triplicate cultures. Dashed line, background proliferation from cultures without HEL. ∗, P < 0.05 compared to HEL+EtxB.
Additionally, limiting dilution analysis revealed frequencies of HEL-specific lymphocytes in the peripheral lymph nodes (MLN and ILN) following immunization with HEL+CtxB that were lower than those observed following immunization with HEL+EtxB or HEL+Ctx (data not shown). This suggests that EtxB treatment produces a greater expansion of the antigen-specific T-cell population than CtxB, which may then lead to the enhanced immune responses observed.
Concluding remarks.
These findings demonstrate that although EtxB and CtxB are close homologs, their capacity to function as mucosal adjuvants is markedly different. Using recombinant EtxB and CtxB, we found EtxB to be more potent than CtxB at inducing high-titer antibody responses both systemically and at distant mucosal sites when coadministered i.n. with antigen. Moreover, it was recently reported that i.n. immunization with EtxB stimulated protective mucosal immunity to ocular herpesvirus infection, while CtxB did not (18). Why EtxB is such a potent adjuvant whereas CtxB exhibits such poor adjuvanticity remains to be fully explained. Since it is thought that EtxB is more promiscuous than CtxB in binding to a range of cell surface receptors in addition to GM1 ganglioside, it is possible that the enhanced adjuvanticity of EtxB stems from its ability to interact with non-GM1 receptors. The observation by Ruddock et al. (19) that the intrinsic stability of EtxB is much greater than that of CtxB, as measured by its resistance to pH-induced denaturation, thermostability, and susceptibility to detergents, may provide an alternative explanation for their differential adjuvanticity. It is conceivable that it is this differential ability to maintain a pentameric structure that permits EtxB to sustain its effects on the immune system while CtxB easily disassembles into a less active form. Interestingly, neither B subunit is a very effective adjuvant by the oral route (4), perhaps reflecting the ability of the harsh environment of the gut to denature both proteins. Furthermore, the presence of the A subunit, including nontoxic variants, may enable the stabilization of the CtxB pentamer structure and thereby account for the reported enhanced adjuvanticity of such molecules (3, 8, 26–28). However, because of concern about the safety of attenuated Ctx and Etx holotoxins, it is encouraging that EtxB (devoid of any A subunit) demonstrates such a potent adjuvant activity. In so doing, it should be suitably applicable as an immunoadjuvant for inclusion in mucosal vaccines.
Acknowledgments
D.G.M. gratefully acknowledges the support of the Medical Research Council of Canada in the form of a postdoctoral fellowship during the completion of this work.
We thank Abu Tholib Aman and Martin Kenny for help in preparing CtxB and EtxB, respectively, and Hong Liang for providing excellent technical assistance.
REFERENCES
- 1.de Haan L, Feil I K, Verweij W R, Holtrop M, Hol W G, Agsteribbe E, Wilschut J. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur J Immunol. 1998;28:1243–1250. doi: 10.1002/(SICI)1521-4141(199804)28:04<1243::AID-IMMU1243>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douce G, Giannelli V, Pizza M, Lewis D, Everest P, Rappuoli R, Dougan G. Genetically detoxified mutants of heat-labile toxin from Escherichia coli are able to act as oral adjuvants. Infect Immun. 1999;67:4400–4406. doi: 10.1128/iai.67.9.4400-4406.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannelli V, Fontana M R, Giuliani M M, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65:331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidry J J, Cardenas L, Cheng E, Clements J D. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infect Immun. 1997;65:4943–4950. doi: 10.1128/iai.65.12.4943-4950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagiwara Y, Komase K, Chen Z, Matsuo K, Suzuki Y, Aizawa C, Kurata T, Tamura S. Mutants of cholera toxin as an effective and safe adjuvant for nasal influenza vaccine. Vaccine. 1999;17:2918–2926. doi: 10.1016/s0264-410x(99)00135-8. [DOI] [PubMed] [Google Scholar]
- 9.Hirst T R. Biogenesis of cholera toxin and related oligomeric enterotoxins. In: Moss J, Vaughan M, Iglewski B, Tu A T, editors. Bacterial toxins and virulence factors in disease. Vol. 8. New York, N.Y: Marcel Dekker Inc.; 1995. pp. 123–184. [Google Scholar]
- 10.Li T K, Fox B S. Cholera toxin B subunit binding to an antigen-presenting cell directly co-stimulates cytokine production from a T cell clone. Int Immunol. 1996;8:1849–1856. doi: 10.1093/intimm/8.12.1849. [DOI] [PubMed] [Google Scholar]
- 11.Lowrey J L, Savage N D, Palliser D, Corsin-Jimenez M, Forsyth L M, Hall G, Lindey S, Stewart G A, Tan K A, Hoyne G F, Lamb J R. Induction of tolerance via the respiratory mucosa. Int Arch Allergy Immunol. 1998;116:93–102. doi: 10.1159/000023931. [DOI] [PubMed] [Google Scholar]
- 12.Matousek M P, Nedrud J G, Cieplak W, Jr, Harding C V. Inhibition of class II major histocompatibility complex antigen processing by Escherichia coli heat-labile enterotoxin requires an enzymatically active A subunit. Infect Immun. 1998;66:3480–3484. doi: 10.1128/iai.66.7.3480-3484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matousek M P, Nedrud J G, Harding C V. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J Immunol. 1996;156:4137–4145. [PubMed] [Google Scholar]
- 14.Nakagawa I, Takahashi I, Kiyono H, McGhee J R, Hamada S. Oral immunization with the B subunit of the heat-labile enterotoxin of Escherichia coli induces early Th1 and late Th2 cytokine expression in Peyer's patches. J Infect Dis. 1996;173:1428–1436. doi: 10.1093/infdis/173.6.1428. [DOI] [PubMed] [Google Scholar]
- 15.Nashar T O, Hirst T R, Williams N A. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–578. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999;20:493–500. doi: 10.1016/s0167-5699(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 18.Richards C M, Aman A T, Hirst T R, Hill T J, Williams N A. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J Virol. 2001;75:1664–1671. doi: 10.1128/JVI.75.4.1664-1671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruddock L W, Ruston S P, Kelly S M, Price N C, Freedman R B, Hirst T R. Kinetics of acid-mediated disassembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. Molecular basis of pH stability. J Biol Chem. 1995;270:29953–29958. doi: 10.1074/jbc.270.50.29953. [DOI] [PubMed] [Google Scholar]
- 20.Schaffeler M P, Brokenshire J S, Snider D P. Detection of precursor Th cells in mesenteric lymph nodes after oral immunization with protein antigen and cholera toxin. Int Immunol. 1997;9:1555–1562. doi: 10.1093/intimm/9.10.1555. [DOI] [PubMed] [Google Scholar]
- 21.Snider D P. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 22.Snider D P, Underdown B J, McDermott M R. Intranasal antigen targeting to MHC class II molecules primes local IgA and serum IgG antibody responses in mice. Immunology. 1997;90:323–329. doi: 10.1111/j.1365-2567.1997.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel S, Mowat A M. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 24.Truitt R L, Hanke C, Radke J, Mueller R, Barbieri J T. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infect Immun. 1998;66:1299–1308. doi: 10.1128/iai.66.4.1299-1308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams N A, Hirst T R, Nashar T O. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto S, Yamamoto M, Imaoka K, Yamamoto M, Fujihashi K, Kiyono H, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits mucosal immunity to vaccines given intranasally. J Allergy Clin Immunol. 1997;99:146. [Google Scholar]
- 29.Yankelevich B, Soldatenkov V A, Hodgson J, Polotsky A J, Creswell K, Mazumder A. Differential induction of programmed cell death in CD8+ and CD4+ T cells by the B subunit of cholera toxin. Cell Immunol. 1996;168:229–234. doi: 10.1006/cimm.1996.0070. [DOI] [PubMed] [Google Scholar]