Abstract
Background and Study Aims
Antiepileptic drugs are the first choice of treatment for patients with epilepsy. However, the withdrawal of antiepileptic drugs after seizure-free remains a significant focus for the majority of patients with epilepsy and their families. In this study, we evaluated the risk factors associated with relapse after drug withdrawal in patients with seizure free for 2 years. We aimed to guide patients in seizure-free to assess the risk of drug withdrawal.
Patients and Methods
Through screening, 452 patients with epilepsy were included in the study.Patients were followed up for at least 2 years or more. Analyzed their clinical data by applying the χ2-test, Kaplan-Meier survival analysis and multivariate Cox regression analysis.
Results
423 patients completed follow-up, of which 304 cases recurred (71.9%).Related recurrence factors include age of onset, type of seizure, number of AEDs, seizure-free time before withdrawal, and electroencephalogram (EEG) results before drug withdrawal (P<0.05). The results of correlation analysis showed that age of onset, seizure frequency, seizure type, number of AEDs, the period from AEDs treatment to a seizure-free status, EEG results before drug withdrawal, and pre-medication course, were all significantly related to the recurrence of seizures after drug reduction and withdrawal (P<0.05). We identified a range of independent risk factors, including onset age, seizure frequency, Multiple AEDs and the period from AEDs treatment to a seizure-free status.
Conclusion
The overall recurrence rate of epilepsy in our patient cohort was high, and the peak recurrence period was within one-year of drug withdrawal. Patients with partial seizures, a short seizure-free time before withdrawal, severe EEG abnormalities before drug reduction, and a long course of the disease, are prone to relapse. Patients with an older age at onset and a high frequency of attack, those taking multi-drug combination therapy, and those that take a long time to gain control, should be managed carefully to AEDs withdrawal.
Keywords: epilepsy, relapse, antiepileptic drugs, drug withdrawal
Introduction
Epilepsy is a chronic brain disease and one of the most common neurological diseases. The World Health Organization (WHO) estimates that 50 million people are affected by epilepsy worldwide and the incidence of this condition is known to be higher in developing countries.1 There are almost nine million people with epilepsy in China, with an incidence of 3–5 per thousand individuals.2 Epileptic seizures not only bring physical and mental harm to the patients themselves; they also create a significant medical burden to families and society.3 This factor needs to be considered by medical workers, the patients and their families, and society.
Antiepileptic drugs (AEDs) are the preferred treatment for patients with epilepsy; using these drugs, 70% of patients can be controlled and enter a seizure-free time before withdrawal after a reasonable time course of treatment.4 In order to eliminate these side effects and reduce the economic burden and of patients with epilepsy, most patients face the problem of whether to reduce or stop taking drugs after no seizure.5 Many studies have addressed the recurrence rate of epilepsy after drug withdrawal and the risk factors related to this recurrence.6–10 However, these studies have not reached a unified opinion and there is still a lack of guidance and suggestions for epilepsy patients and clinicians. In the present study, we investigated the relapse rate, resolved rate, relapse peak, and risk factors related to relapse in patients with epilepsy who entered a two-year seizure-free time before withdrawal. Our aim was to to guide patients in seizure-free to assess the risk of drug withdrawal.
Materials and Methods
Patient Recruitment
The study population consisted of patients with epilepsy from the Department of Neurology at the Second Affiliated Hospital of Xinxiang Medical University, between September 1990 and June 2016. All patients in the study were candidates for AED withdrawal. Patients who had not had seizures for 2 years or more and had begun to reduce their medication were followed up once every 2 months until drug withdrawal. Then, the patients were followed up 3 months, 6 months, 9 months, 12 months, 18 months and 24 months after drug withdrawal. Finally, the patients were followed-up at least once a year after more than 24 months until June 2016. The inclusion criteria are as follows: (1) epilepsy patients who met the diagnostic criteria of epilepsy according to the 2017 International League Against Epilepsy (ILAE); (2) EEG (electroencephalogram) or 24-hour video electroencephalogram is required before medication and drug reduction;(3) CT (computed tomography)/MRI (magnetic resonance imaging) examination was performed before medication or at the beginning of medication; (4) epilepsy patients with an onset age of more than 1 year and less than 60 years old; (5) seizure-free survival for at least 24 consecutive months during AED therapy; (6) follow-up time after drug withdrawal to relapse of 2 years or more after drug withdrawal; (7) female patients taking effective contraceptive measures during drug withdrawal and follow-up; (8) provided informed consent (patients or their families) and signed an informed consent form. Exclusion criteria: (1) subjects with epilepsy who had received unknown drug components in other hospitals and had already tried to reduce or stop AEDs, but relapsed; (2) special types of epilepsy (such as juvenile myoclonic epilepsy); (3) patients with severe brain trauma, cerebrovascular disease, lesions occupying the intracranial space and progressive diseases according to CT/MRI examination; (4) pregnant or lactating women; (5) psychogenic seizures or an uncertain diagnosis of epilepsy defined according to video-EEG and seizure attacks not explained by brain location and those that had many attacks full of variety; (6) patients with severe systemic diseases, such as malignant tumors, or liver cirrhosis; or (7) patients who could not be followed up regularly or for less than 2 years.
Classification of EEG Severity11
Mild abnormal EEG: (1) α rhythm of a is irregular, unstable, poorly regulated and amplitude modulated, the frequency slows down to 8Hz, the amplitude exceeds 100µv, and the physiological response is not obvious. (2) The amplitude difference of corresponding parts of both hemispheres exceeds 50%. (3)β activity increased significantly, and the amplitude was higher than 50µv. (4)θ activity increased significantly, mainly in the frontal area. (5)δ activity increased slightly.(5) Excessive ventilation shows moderate amplitude.Early appearance or delayed disappearance of slow wave activity in θ frequency band.Moderately abnormal EEG: (1) The basic rhythm is obviously slow, and the occipital region is slow at α rhythm of 7–8Hz, or α rhythm disappears completely, and is affected by θ rhythm of 4~7Hz replacement. (2) The left and right sides are obviously asymmetrical. (3) There are many medium amplitude waves scattered around 3Hz α wave α or activity. (4) Normal physiological sleep wave disappears on one or both sides, or normal sleep cycle disappears. (5) More widely scattered or less rhythmic epileptiform discharges.Severe abnormality EEG: (1) Background withδwaves are dominant, with a small amount θ activity, or few low amplitude fast wave of α or β frequency band, compounded on slow wave. (2) The background is dominated by θ rhythm, with a few scattered α,β, δ waves. (3) α generalization. (4) Irregular amplitude and frequency, completely out of rhythm. (5) Epileptic discharge with paroxysmal rhythm. (6) Periodic phenomenon. (7) Continuous low voltage or electrical quiescent state.
Methods
First, we collated a range of relevant information for the patients with epilepsy, including general demographic information of patients (name, gender, date-of-birth, age of onset, date of the first visit, the end date of follow-up, duration of follow-up, course of disease before medication, frequency of seizure before medication, type of seizure, and family history of epilepsy). We also collated a range of clinical information, including the date of the first treatment, name and dose, type and dose of AEDs before drug reduction, EEG description before drug reduction, seizure-free period before AED withdrawal, withdrawal time, failure date of drug reduction, the time elapsed after reducing drug relapse, relapse after drug withdrawal, the time elapsed after stopping drug relapse, and the specific situation of patients at the end of follow-up. This information was collected and recorded by epilepsy doctors who had received special training.
Previous studies have demonstrated that the recurrence rate of patients with epilepsy after drug withdrawal is the highest in the first 12 months, especially within the first 6 months.12 Generally, children and adults experience at least 2 years of complete remission without seizures prior to experiencing drug withdrawal of AEDs.13 The pre-medication course was defined as the time from the diagnosis of epilepsy to the start of formal medication. The period from AEDs treatment to seizure free was defined as the time from the beginning of antiepileptic drugs to the last attack before drug withdrawal. The withdrawal time was defined as the duration of AEDs from reduction to complete withdrawal (with no recurrence during drug reduction). The relapse of epilepsy was defined as the occurrence of seizures during drug reduction or after drug withdrawal. Epilepsy resolution was defined as no seizure within 10 years and the termination of AED treatment for at least 5 years.14
According to the follow-up results, the patients were divided into two groups: a no recurrence group and a recurrence group. At the end of the follow-up period, patients who had not had seizures after 2 years or more from drug withdrawal were included in the no recurrence group; patients who had relapsed during gradual drug withdrawal or after complete drug withdrawal were classified into the recurrence group.
Statistical Methods
SPSS 20.0 software was used for data analysis. The Chi-squared (R×C data table) was used to compare the classification variables between groups and P<0.05 was statistically significant. Kaplan-Meier survival analysis was used to analyze the recurrence of epilepsy. The survival time was defined as the time from the beginning of drug reduction to the end of epilepsy recurrence or observation. The Kaplan-Meier method, log rank test, and Breslow test were used for the univariate analysis of prognostic factors. Then, univariate Cox regression analysis was used to determine univariate statistical significance and identify variables that were clinically influential factors. P< 0.05 was considered as statistically significant.
Results
Follow Up
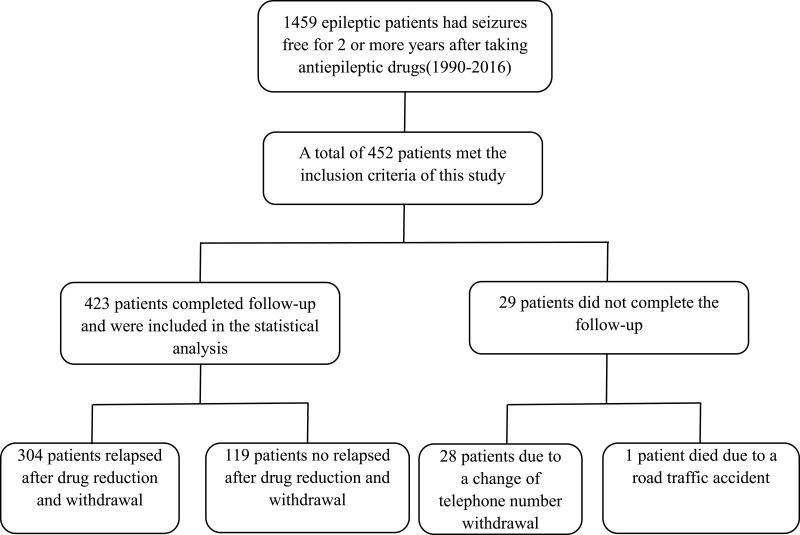
A total of 1459 patients achieved a seizure-free time before withdrawal of 2 years or more in the epilepsy database used in this study. 452 patients met the inclusion criteria of this study. Finally, 423 patients completed follow-up and were included in the statistical analysis. In total, 29 patients (6.4%) did not complete the follow-up; these were all patients after drug withdrawal. Of these, we lost contact with 28 patients due to a change of telephone number; one patient died due to a road traffic accident (Figure 1). The time span from the beginning of drug reduction to the end of follow-up was 0–318m; the median time was 13m.
Figure 1.
Flow chart for statistical analysis of study patients.
Recurrence Rate, Resolution Rate, and Recurrence Peak of Epilepsy After Drug Withdrawal
In this study, a total of 423 patients with epilepsy met the inclusion criteria and exclusion criteria. Table 1 shows the clinical characteristics of the general data related to these patients. The onset age of the patients was 1–58 years old, with a median of 11 years. The follow-up time ranged from 2–26 years, with a median of 8 years; Pre- medication course of epilepsy was 0–444 months, with a median of 8 months. The period from AEDs treatment to seizure free was 0–144 months, with a median of 0m (215 of the 423 patients did not have seizures after taking antiepileptic drugs). The seizure-free time before withdrawal was 2–23 years, with a median of 8 years. The median withdrawal time was 6 months (range, 2–192 months). Of the 423 patients, 304 (71.9%) were classified into the recurrence group and 119 (28.1%) were classified into the no recurrence group; and 74 (17.5%) patients were resolved.
Table 1.
Chi Square Test Results of Patients’ Basic Clinical Data and Relapse Related Risk Factors
| Related Factors | Total (n) | Recurrence Group (%) | No Recurrence Group (n) | χ2 | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 242 | 172 (71.7%) | 70 | 0.18 | 0.68 |
| Female | 181 | 132 (72.9%) | 49 | ||
| Age at seizure onset, years | |||||
| <18Y | 289 | 190 (65.7%) | 99 | 16.92 | 0.00 |
| ≥18Y | 134 | 114 (85.1%) | 20 | ||
| Pre-medication course, months | |||||
| ≤6M | 206 | 144 (69.9%) | 62 | 0.77 | 0.38 |
| >6M | 217 | 160 (73.7%) | 57 | ||
| Seizure frequency, time/months | |||||
| ≤4T/M | 349 | 245 (70.2%) | 104 | 2.74 | 0.09 |
| >4T/M | 74 | 59 (79.7%) | 15 | ||
| Seizure types | |||||
| Partial seizure | 210 | 165 (78.6%) | 45 | 9.82 | 0.00 |
| Generalized seizure | 186 | 123 (66.1%) | 63 | ||
| Others* | 27 | 16 (59.3%) | 11 | ||
| Family history | |||||
| No | 355 | 258 (72.7%) | 97 | 0.71 | 0.40 |
| Yes | 68 | 46 (67.6%) | 22 | ||
| Number of AEDs | |||||
| Monotherapy | 332 | 229 (69.0%) | 103 | 6.38 | 0.01 |
| Multi drug combination | 91 | 75 (82.4%) | 16 | ||
| The period from AEDs treatment to a seizure-free status, months | |||||
| ≤1M | 299 | 209 (69.9%) | 90 | 1.95 | 0.16 |
| >1M | 124 | 95 (76.6%) | 29 | ||
| Remission period, years | |||||
| 2Y≤R<3Y | 236 | 177 (75.0%) | 59 | 9.63 | 0.02 |
| 3Y≤R<4Y | 81 | 60 (74.1%) | 21 | ||
| 4Y≤R<5Y | 34 | 26 (76.5%) | 8 | ||
| R≥5Y | 72 | 41 (56.9%) | 31 | ||
| EEG before drug reduction | |||||
| Normal | 248 | 168 (67.7%) | 80 | 9.89 | 0.02 |
| Mild abnormality | 121 | 88 (72.7%) | 33 | ||
| Moderately abnormal | 29 | 26 (89.7%) | 3 | ||
| Severe abnormality | 25 | 22 (88.0%) | 3 | ||
| Duration of drug reduction | |||||
| ≤6M | 282 | 202 (71.6%) | 80 | 0.02 | 0.88 |
| >6M | 141 | 102 (72.3%) | 39 |
Note: Others* (Unclassified).
Abbreviations: AED, antiepileptic drug; EEG, electroencephalography.
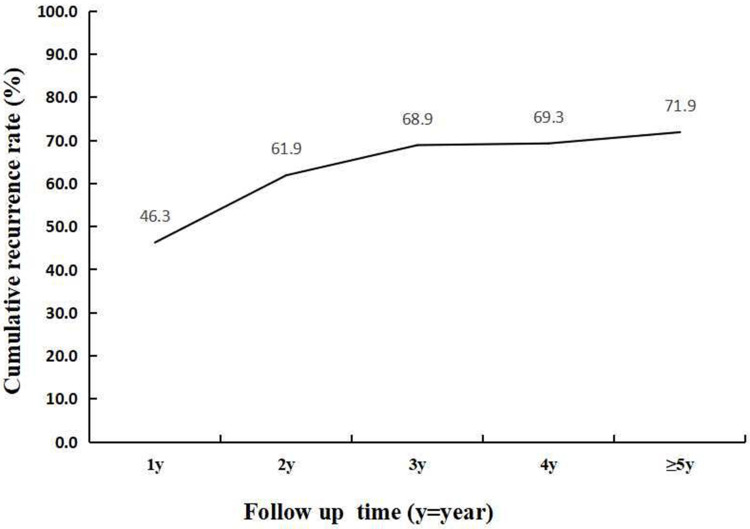
After drug withdrawal, the cumulative recurrence rate was 46.3% (196/423) in 1 year, 61.9% (262/423) in 2 years, 68.6% (290/423) in 3 years, 69.3% (293/423) in 4 years and 71.9% (304/423) in more than 5 years. Peak recurrence occurred within 1 year of drug withdrawal, as shown in Figure 2.
Figure 2.
The cumulative recurrence rate of patients after drug withdrawal.
Relationship Between Related Factors and Recurrence
The chi-squared analysis identified several factors that were associated with a significantly higher recurrence rate after drug reduction and withdrawal (P<0.05), including patients with an onset age over 18 years, partial seizures, multiple AEDs, a short seizure-free time before withdrawal, and an abnormal EEG before drug reduction. Other factors, such as gender, pre medication course before medication, seizure frequency, family history, the period from AEDs treatment to seizure free, seizure free time before drug withdrawal and the withdrawal time, were not significant (P> 0.05) (Table 1).
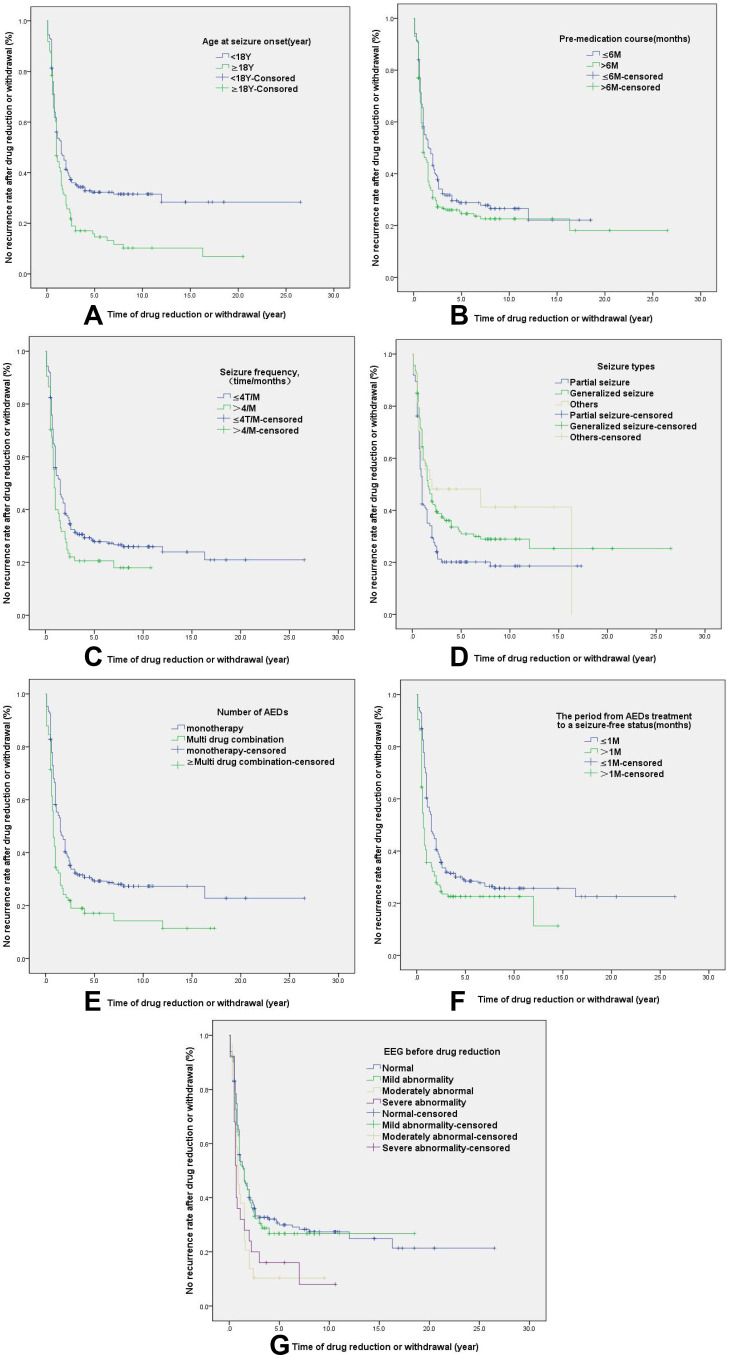
Kaplan-Meier survival analysis showed that an older onset age, a longer pre- medication course, higher seizure frequency, partial seizure type, multiple AEDs, the period from AEDs treatment to a seizure-free status and abnormal EEG results before drug reduction, were more likely to relapse after drug reduction and withdrawal (P<0.05), Therefore, these factors are directly related to drug reduction and recurrence after drug withdrawal (Table 2, Figure 3). Gender, family history, seizure-free time before withdrawal, and the withdrawal time had no direct correlation with relapse.
Table 2.
Kaplan-Meier Univariate Analysis of the Relationship Between Related Factors and Recurrence in Patients with Epilepsy
| Variable | Mean(M) | Median(M) | Log Rank P | Breslow P |
|---|---|---|---|---|
| Age at seizure onset, years | 0.00a | 0.01b | ||
| <18Y | 104.47 | 18.00 | ||
| ≥18Y | 39.23 | 12.00 | ||
| Pre-medication course, months | 0.07 | 0.03b | ||
| ≤6M | 68.39 | 19.00 | ||
| >6M | 77.20 | 12.00 | ||
| Seizure frequency, time/months | 0.03a | 0.01b | ||
| ≤4T/M | 87.32 | 18.00 | ||
| >4/M | 34.34 | 11.00 | ||
| Seizure types | 0.00a | 0.00b | ||
| Partial seizure | 49.93 | 12 | ||
| Generalized seizure | 98.32 | 19 | ||
| Others* | 91.22 | 24 | ||
| Number of AEDs | 0.00a | 0.00b | ||
| Monotherapy | 93.40 | 18.00 | ||
| Multi drug combination | 38.82 | 9.00 | ||
| The period from AEDs treatment to a seizure-free status, months | 0.00a | 0.00b | ||
| ≤1M | 91.18 | 18.00 | ||
| >1M | 43.32 | 8.000 | ||
| EEG before drug reduction | 0.00a | 0.00b | ||
| Normal | 90.38 | 18.00 | ||
| Mild abnormality | 69.52 | 18.00 | ||
| Moderately abnormal | 22.03 | 11.00 | ||
| Severe abnormality | 25.60 | 8.00 |
Notes: Others* (unclassified).aP<0.05=This index has influence on the long-term recurrence of epilepsy, bP<0.05, This index has influence on the recent recurrence of epilepsy.
Abbreviations: AED, antiepileptic drug; EEG, electroencephalography.
Figure 3.
Kaplan Meier univariate analysis of the relationship between related factors and recurrence: (A) the relationship between onset age and recurrence (B) The relationship between the pre-medication course and recurrence; (C). Relationship between seizure frequency and recurrence (D). Relationship between seizure type and recurrence (E). Relationship between the number of AEDs and recurrence; (F). The relationship between the period from AEDs treatment to a seizure-free status and recurrence; (G). The relationship between EEG results before reduction and recurrence.
Independent Risk Factors Affecting Epilepsy Recurrence After Drug Withdrawal
Analysis showed that age, frequency, AEDs, and time to achieve control, were independent risk factors for epilepsy recurrence after stopping drugs. To further eliminate the influence of interference factors, we added gender and the period from AEDs treatment to a seizure-free status as factors in multivariate Cox regression analysis. We found that onset age (HR:1.68, 95% CI: 1.32–2.14, P=0.00), seizure frequency (HR:1.53, 95% CI: 1.15–2.04, P =0.00), multiple AEDs (HR:1.62, 95% CI: 1.24–2.12, P=0.00) and the period from AEDs treatment to a seizure-free status (HR:1.57, 95% CI: 1.22–2.00, P=0.00) were independent risk factors that influenced the recurrence of epilepsy after drug withdrawal (Table 3).
Table 3.
Multivariate Analysis for the Relapse of Epilepsy After Drug Withdrawal
| Influence Factor | β | SE | Wald | p | HR | 95% CI |
|---|---|---|---|---|---|---|
| Age at seizure onset | 0.52 | 0.12 | 18.08 | 0.00 | 1.68 | 1.32–2.14 |
| Seizure frequency | 0.42 | 0.15 | 8.28 | 0.00 | 1.53 | 1.15–2.04 |
| Number of AEDs | 0.48 | 0.14 | 12.66 | 0.00 | 1.62 | 1.24–2.12 |
| The period from AEDs treatment to a seizure-free status | 0.45 | 0.13 | 12.48 | 0.00 | 1.57 | 1.22–2.00 |
Abbreviations: HR, risk index, 95%; CI, Confidence interval.
Discussion
In our analysis of 423 patients with epilepsy, we found that the overall recurrence rate was 71.9%; this was higher than the reported rates of 12% to 67% in other studies.15 This discrepancy may be due to the heterogeneity of the population, the criteria used for grouping and differences in follow-up periods.From the cumulative recurrence rate of different follow-up times, the recurrence rate of patients after drug withdrawal was 46.3% in 1 year, 61.9% in 2 years and 68.9% in 3 years. This finding was consistent with other studies.The recurrence rate of the patients decreased significantly after 3 years, thus indicating that there was no recurrence within 3 years after drug withdrawal, and the risk of recurrence in the future would be reduced. Some scholars also reported that 85% of epilepsy patients relapsed within 2 years after stopping AEDs.16 This suggests that any future randomized controlled trials for AED termination should include a follow-up period of at least 2 years after drug withdrawal to ensure that most recurrent cases are included.
In this study, we found that the peak of relapse occurred within 1 years after the start of drug withdrawal; this was similar to previous findings. For example, a previous study found that the relapse rate of epilepsy after drug withdrawal was highest in the first 3 months but then decreased steadily. There have some reported that the recurrence rate was highest within 12 months after the withdrawal of AED also.17–19 Furthermore, the recurrence rate decreased significantly after 3 years. As a consequence, these authors suggested that drug withdrawal should be commenced after 3 years of seizure-free.A Chinese study reported that most relapses of epilepsy patients after drug withdrawal occurred in the first 12 months and that the recurrence rate decreased significantly after 2 years; relapses beyond 5 years after withdrawal were rare,20 this is very consistent with our research results. These data remind us that no matter whether we consider the first three months or one or two years, the early recurrence rate is significantly higher than that during the later period; furthermore, the peak period is generally no more than three years. Therefore, in epilepsy patients who are stopping drug reduction, we should observe the patient for changes in condition and perform timely follow-up over the first three years, especially in the first three months, and fully inform patients of the peak of recurrence risk.
Our analysis identified a range of factors that can influence the recurrence of epilepsy patients after drug withdrawal, including the age at onset, the type of seizure, number of AEDs, and the degree of abnormality on EEG results prior to drug withdrawal. These findings are similar to those reported previously.6–9,21–23 Reported that the types of seizure, seizure frequency before treatment, intracranial lesions, and the time of initial treatment, had a greater impact on the recurrence of epilepsy.6 Lizana et al reported that compared with adults, children have a lower risk of recurrence after stopping antiepileptic drugs.7 In another study, reported that the severity of epilepsy, and the period of seizure-free time before withdrawal after treatment, are significant factors for predicting the recurrence of epilepsy.8 Other researchers suggested that female patients and patients with an abnormal EEG after drug reduction and withdrawal should be treated more cautiously due to the high risk of recurrence.10
As a special examination method related to epilepsy, EEG can provide significant reference data for the diagnosis and treatment of epilepsy, including the selection of AEDs, the adjustment of dosage, and the indication of drug withdrawal.9 However, there are still some controversy related to the prediction of epilepsy recurrence. A paper published in The Lancet previously reported that EEG may have a certain predictive value for epilepsy recurrence in children, and that such abnormalities on EEG may be related to some important clinical factors.24 However, a recent meta-analysis proposed that the recurrence rate of patients with abnormal EEGs during drug withdrawal was higher than that of patients with normal EEGs and that an abnormal EEG during drug withdrawal was a risk factor for recurrence.25 With technological development, the sensitivity and accuracy of EEG have been significantly improved. In this study, we divided EEG abnormalities into three levels: mild, moderate, and severe. The recurrence rate of these four groups of cases was statistically significant (P<0.05), thus suggesting that the larger the abnormality on EEG results before drug reduction, the greater the possibility of recurrence. These findings are consistent with the previous conclusion that abnormal dynamic EEG results are important indicators of epilepsy recurrence and have important value in predicting epilepsy recurrence after drug withdrawal.26 However, abnormal dynamic EEG results are not the only factor relevant for recurrence.15 In the present study, Cox multivariate analysis failed to prove that EEG abnormality before drug reduction was an independent factor of recurrence. This finding requires us to perform further research with large sample size and fully validate the direct relationship between EEG results and the recurrence of epilepsy after drug reduction.
Cox multivariate regression analysis showed that age of onset, seizure frequency, number of AEDs, and the period from AEDs treatment to a seizure-free status, were all independent risk factors for the recurrence of epilepsy after drug withdrawal. These findings provide us with some useful reference guidelines for patients who are ready to reduce or stop drugs, and can consider whether to start the reduction or stop the drug by taking into account the actual clinical situation. However, some researchers have emphasized that the only independent risk factor for epilepsy recurrence was an abnormal EEG epileptiform discharge before drug withdrawal.27 With regards to the outcome of patients with epilepsy after drug withdrawal, although many patients have unexpected recurrence after the withdrawal of AEDs for 2 years after seizure-free, in the long run, the withdrawal of AEDs does not appear to affect the long-term prognosis.
In addition to the factors described above, some researchers believed that visual fatigue (watching a video for a long time or using a computer), a lack of sleep, drinking alcohol, excessive fatigue, and other factors, cannot be ignored during the process of drug withdrawal. However, the precise causes of epilepsy recurrence are not yet clear. Buratti et al proposed that during the management of patients with epilepsy, it is possible that sleep disorders, emotional stress, drug use, and light stimulation, may lead to the risk of epilepsy recurrence.28 However, the present study did not consider the correlation between common inducing factors and drug withdrawal recurrence in patients with epilepsy; these factors will be considered in future studies.
Due to limitations associated with case selection, we were unable to perform a detailed evaluation for the degree of recurrence of epilepsy after drug withdrawal. Secondly, with regards to monitoring treatment with AEDs, we only collected AEDs and EEG data. In the future, more objective methods should be applied, such as monitoring the concentration of serum AEDs. In the present study, we excluded patients who had failed to reduce and stop AEDs again; however, other studies have shown that before such patients decide to reduce and stop AEDs again, they needed a longer period of seizure-free than the first time.29
It is a difficult task to stop AEDs. According to our present research, we suggest that people with epilepsy with high risk factors for epilepsy recurrence should continue to use AEDs cautiously.In this study, we investigated the recurrence rate, recurrence peak, and the risk factors leading to recurrence after drug withdrawal in patients with a 2-year seizure-free time before withdrawal, in addition, we have increased the number of enrolled patients as much as possible, extend the time of possible follow-up to ensure the reliability of the results, to provide a theoretical reference basis for patients entering remission.Our future research will investigate the patients at the peak of recurrence, and cooperate with other research centers as far as possible to obtain the follow-up results of patients in multiple centers, discuss the high-risk factors and characteristics of patients at the peak of recurrence, and determine the plan to reduce the recurrence rate of epilepsy after drug withdrawal.
Acknowledgments
The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.
Funding Statement
We greatly appreciate the enthusiastic support received from all study participants. This study was funded by the Henan Medical Science And Technology Research Project [grant number: LHGJ20200530], Xinxiang Science And Technology Research Plan Project [grant number: GG2020035], Capital Health Research And Development Project [grant numbers: 2016-1-2011 and 2020-1-2013].
Ethical Approval
The authors confirm the study complies with the Declaration of Helsinki.All the enrolled patients, children passed the informed consent of their immediate family members, while adults passed their own informed consent.
Informed Consent
The parents or guardians of all children enrolled in this study had provided written informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khan SA. Epilepsy awareness in Saudi Arabia. Neurosciences. 2015;20(3):205–206. doi: 10.17712/nsj.2015.3.20150338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang WZ, Wu JZ, Wang DS, et al. The prevalence and treatment gap in epilepsy in China: an ILAE/IBE/WHO study. Neurology. 2003;60(9):1544–1545. doi: 10.1212/01 [DOI] [PubMed] [Google Scholar]
- 3.Chang L. Progress in epidemiological investigation of epilepsy in China. J Int Neurology Neurosurg. 2012;39:161–164. [Google Scholar]
- 4.Beghi E, Giussani G, Grosso S. et al.Withdrawal of antiepileptic drugs: guidelinesof the Italian league against epilepsy. Epilepsia. 2013;54:2–12. doi: 10.1111/epi.12305 [DOI] [PubMed] [Google Scholar]
- 5.Tang X, Yu P, Ding D, et al. Risk factors for seizure reoccurrenc source after withdrawal from antiepileptic drugs in individuals who have been seizure-free for over 2 years. PLoS One. 2017;12(8):e0181710. doi: 10.1371/journal.pone.0181710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu F, Sun H. Openly study of high-risk factors of epilepsy relapse after single antiepileptic drug withdrawal.Chinese. J Neuromed. 2014;13:1053–1055. [Google Scholar]
- 7.Ramos-Lizana J, Aguirre-Rodríguez J, Aguilera-López P, Cassinello-García E. Recurrence risk after withdrawal of antiepileptic drugs in children with epilepsy: aprospective study. Eur J Paediatr Neurol. 2010;14(2):116–124. doi: 10.1016/j.ejpn.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Specchio LM, Tramacere L, La Neve A, Beghi E. Discontinuing antiepileptic drugs in patients who are seizure free on monotherapy. J Neurol Neurosurg Psychiatry. 2002;72(1):22–25. doi: 10.1136/jnnp.72.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olmez A, Arslan U, Turanli G, Aysun S. Risk of recurrence after drug withdrawal in childhood epilepsy. Seizure. 2009;18(4):251–256. doi: 10.1016/j.seizure.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 10.Benbadis SR, Beniczky S, Bertram E, MacIver S, Moshé SL. The role of EEG in patients with suspected epilepsy. Epileptic Disord. 2020;22(2):143–155. doi: 10.1684/epd.2020.1151 [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Wu W, Liu X, et al. Clinical EEG Training Course. People’s Health Publishing House; 2011. [Google Scholar]
- 12.Specchio LM, Beghi E. Should antiepileptic drugs be withdrawn in seizure-free patients? CNS Drugs. 2004;18(4):201–212. doi: 10.2165/00023210-200418040-00001 [DOI] [PubMed] [Google Scholar]
- 13.Strozzi I, Nolan SJ, Sperling MR, Wingerchuk DM, Sirven J. Early versus late antiepileptic drug withdrawal for people with epilepsy in remission. Cochrane Database Syst Rev. 2015;2015(2):CD001902. doi: 10.1002/14651858.CD001902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher RS, Acevedo C, Arzimanoglou A. Ilae official report: apractical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 15.Shih JJ, Ochoa JG. A systematic review of antiepileptic drug initiation and withdrawal. Neurologist. 2009;15(3):122–131. doi: 10.1097/NRL.0b013e3181901ad3 [DOI] [PubMed] [Google Scholar]
- 16.Rathore C, Panda S, Sarma PS, Radhakrishnan K. How safe is it to withdraw antiepileptic drugs following successful surgery for mesial temporal lobe epilepsy? Epilepsia. 2011;52(3):627–635. doi: 10.1111/j.1528-1167.2010.02890.x [DOI] [PubMed] [Google Scholar]
- 17.Aktekin B, Dogan EA, Oguz Y, Senol Y. Withdrawal of antiepileptic drugs in adult patients free of seizures for 4 years: a prospective study. Epilepsy Behav. 2006;8(3):616–619. doi: 10.1016/j.yebeh.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Gasparini S, Ferlazzo E, Giussani G. Rapid versus slow withdrawal of antiepileptic monotherapy in 2-year seizure-free adult patients with epilepsy (RASLOW) study: a pragmatic multicentre, prospective, randomized, controlled study. Neurol Sci. 2016;37(4):579–583. doi: 10.1007/s10072-016-2483-3 [DOI] [PubMed] [Google Scholar]
- 19.Karalok ZS, Guven A, Öztürk Z, Gurkas E. Risk factors for recurrence after drug withdrawal in childhood epilepsy. Brain Dev. 2020;42(1):35–40. doi: 10.1016/j.braindev.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 20.He R-Q, Zeng Q-Y, Zhu P, Bao Y-X, Zheng R-Y, Xu H-Q. Risk of seizure relapse after antiepileptic drug withdrawal in adult patients with focal epilepsy. Epilepsy Behav. 2016;64:233–238. doi: 10.1016/j.yebeh.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 21.Atalar AÇ, Şirin NG, Bebek N, Baykan B. Predictors of successful valproate withdrawal in women with epilepsy. Epilepsy Behav. 2021;119:107980. doi: 10.1016/j.yebeh.2021.107980 [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Amdur R, Pauldurai J, Koubeissi M. Seizure recurrence after prolonged seizure control: patterns and risk factors. Epilepsy Behav. 2021;124:108330. doi: 10.1016/j.yebeh.2021.108330 [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Zhang X, Long J, Wu Q, Han Y. Prediction of the recurrence risk in patients with epilepsy after the withdrawal of antiepileptic drugs. Epilepsy Behav. 2020;110:107156. doi: 10.1016/j.yebeh.2020.107156 [DOI] [PubMed] [Google Scholar]
- 24.Bessant P, Chadwick D, Eaton B, et al. Randomised study of antiepileptic in patients in remission. Lancet. 1991;337(8751):1175–1180. [PubMed] [Google Scholar]
- 25.Yao J, Wang H, Xiao Z. Correlation between EEG during AED withdrawal and epilepsy recurrence: a meta-analysis. Neurol Sci. 2019;40(8):1637–1644. doi: 10.1007/s10072-019-03855-x [DOI] [PubMed] [Google Scholar]
- 26.Yang L-H, Jiang L-Y, Lu RY. Correlation between the changes in ambulatory electroencephal-ography findings and epilepsy recurrence after medication withdrawal among the population in southern. China Neurol Med Chir. 2013;53(1):12–16. doi: 10.2176/nmc.53.12 [DOI] [PubMed] [Google Scholar]
- 27.Krumholz A, Wiebe S, Gronseth GS. Evidence-based guideline: management of an unprovoked first seizure in adults: report of the guideline development subcommittee of the American Academy of neurology and the American epilepsy society. Neurology Actions. 2015;84(16):1705–1713. doi: 10.1212/WNL.0000000000001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buratti L, Natanti A, Viticchi G. Impact of sleep disorders on the risk of seizure recurrence in juvenile myoclonic. Epilepsy Behav. 2018;80:21–24. doi: 10.1016/j.yebeh.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 29.McCarthy AL, Camfield CS, Camfield PR, Valencia I. What do epileptologists recommend about discontinuing antiepileptic drugs for a second time in children? Epilepsy Behav. 2015;47:120–126. doi: 10.1016/j.yebeh.2015.04.014 [DOI] [PubMed] [Google Scholar]