Abstract
Significant progress in fabricating new multifunctional soft materials and the advances of additive manufacturing technologies have given birth to a new generation of soft robots with complex capabilities, such as crawling, swimming, jumping, gripping, and releasing. Within this vast array of responsive soft materials, hydrogels receive considerable attention due to their fascinating properties, including biodegradable, self-healing, stimuli-responsive, and large volume transformation. Konjac glucomannan (KGM) is an edible polysaccharide that forms a pH-responsive, self-healing hydrogel when crosslinked with borax, and it is the focus of this study. A novel KGM-Borax ink for three-dimensional (3D) printing of free-form structures and soft robots at room temperature is presented. A complete process from ink preparation to the fabrication of a completely cross-linked part is demonstrated. Print setting parameters, rheological properties of the ink and crosslinked gels were characterized. Print quality was found to be consistent across a wide range of print settings. The minimum line width achieved is 650 μm. Tensile testing was carried out to validate the self-healing capability of the KGM-Borax gel. Results show that KGM-Borax has a high self-healing efficiency of 98%. Self-healing underwater was also demonstrated, a rarity for crosslinked gels. The means to form complex structures via 3D printing, reacting to environmental stimuli and the resilience against damage, make this new KGM-Borax gel a promising candidate for the fabrication of the next generation of soft robots.
Keywords: 3D printing, polysaccharide gel, self-healing, konjac glucomannan
Introduction
Soft materials are inspiring the next generation of intelligent and versatile devices.1 These soft robots are able to adapt and react to challenges in their operating environments; for instance, exploration in confined spaces or locomotion on uneven terrain. The choice of material is an important factor when designing soft robots for various applications.2 Silicone elastomers, urethanes, and foams are typically used in the construction of soft robots. However, these materials are generally not responsive to environmental stimuli or environmentally friendly. As such, hydrogels have been proposed as promising alternative materials3 due to their attractive properties. They are capable of maintaining their structure while holding or expelling large quantities of water, many times the mass of the substance itself.4 They are capable of physically and chemically reacting to external stimuli, such as heat, electric fields, magnetic fields, pH, ionic concentration and light intensity, and, in some cases, combinations of what have been cited earlier.5 Depending on the composition, hydrogels are also biocompatible and can degrade benignly at the end of their life. There is also the possibility of fabricating edible robots.6,7
Polysaccharides are one class of hydrogels with great potential as a material for soft robots. These are commonly derived from biological sources—from animals (chitin, chitosan), from plants (pectin, galactomannan, glucomannan), from algae (alginate, carrageenan, agarose), or from microbes (xanthan, gellan).8 Consequently, they are typically biodegradable, and they are considered to be nontoxic and biocompatible.9 Gels of this type have been studied for a wide variety of applications: as biomaterials in tissue engineering,10,11 for the transport and controlled release of drugs,12,13 as rheology modifiers in cosmetics, and as food additives.
Konjac glucomannan (KGM), a polysaccharide, is derived from the corms of Amorphophallus konjac and it consists of β-1-4 linked d-glucose and d-mannose in a molar ratio of 1:1.6.14,15 Like many polysaccharides, konjac has a history of use as a foodstuff, in particular in the Levant (using an analogue derived from the salep orchard16) or as konnyaku noodles or jelly in East Asian cuisine. The customary method of preparation of konjac gel is to induce gelation through heating an aqueous KGM solution in the presence of an alkali. Glucomannan chains contain randomly scattered acetyl groups that are removed on addition of the alkali. Heating causes aggregation of the chains due to hydrogen bonding and hydrophobic interactions.17–19 Unlike other gels held together by hydrogen bonding (such as gelatine), these chains will not disentangle on reheating, and so the gellation of KGM prepared in this manner is thermo-irreversible. This makes the freeform fabrication and three-dimensional (3D) printing of alkali-based KGM challenging, since the gel cannot be shaped once it is set.
An alternative means of gellation of konjac is through the addition of the crosslinking agent sodium tetraborate (borax).20 This is the basis for the ink presented in this article (Fig. 1 demonstrates printing with this ink). Borax, in aqueous solution, exists as equal parts of boric acid and tetrahydroxyborate ion [Eq. (1)].
FIG. 1.
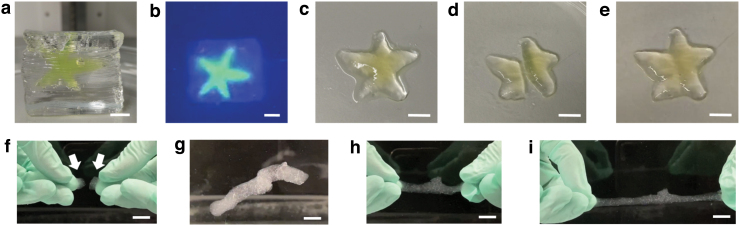
Demonstration of printing and self-healing with KGM-Borax ink. (a) A 3D printed upright star shape with KGM-Borax ink and Ultrez support. The ink has been mixed with a fluorescent dye for visualization. (b) Washing the printed structure in salt water. The star is clearly visible in the presence of UV light. (c) The star after the support structure has been washed away. (d) A cut is made to split the star into two parts. (e) When the cut surfaces are brought together, the material heals and the star shape is regained. (f–i) Demonstration of healing under water. (f) Two separated KGM-Borax samples, indicated by arrows, submerged under water before healing. (g) The healed sample after 5 min of contact. (h) Healed sample at rest, before stretching. (i) Healed sample as it is being stretched. Scale bar = 1 cm. KGM, konjac glucomannan. Color images are available online.
| (1) |
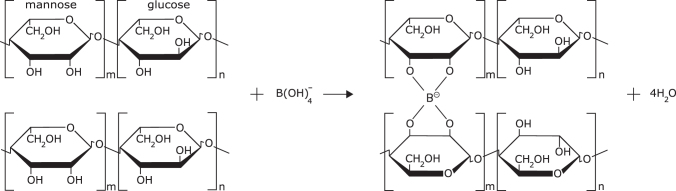
The tetrahedral borate ion interacts with the cis-hydroxy groups on mannose to form a crosslink (Fig. 2). This manner of crosslinking gives the KGM-Borax gel unique properties. First, the equilibrium represented in Equation (1) can be altered by the addition of an acid or an alkali.21 Consequently, the crosslinked KGM-Borax gel is responsive to changes in pH, with alkaline conditions producing a mechanically stronger gel.
FIG. 2.
(Left) A pair of KGM chains, each with d-mannose and d-glucose linked through a β-1-4 glycosidic bond. (Right) KGM chains cross-linked through the borate ion.
Second, in the presence of free borate ions, the KGM-Borax gel also exhibits self-healing, while remaining stiff enough to maintain its form.21 Several approaches have been proposed to realize polymers that repair themselves, among which the popular ones are dynamic covalent bonds22,23 and reversible chemical reactions.24,25 The latter has been employed in the context of soft robotics. The advantage of the KGM-Borax gel is that the healing is autonomous, requiring no external stimulus, and the participating ions are intrinsic to the gel. Sensitivity to pH and the ability to self-heal make this a particularly important material for future gel-based robots.
A common method to fabricate soft robots is by casting a soft material into a carefully designed mold. This manner of fabrication is low cost, well characterized, and offers a low barrier to entry. As such, many biocompatible, high tensile strength hydrogels reported in literature use casting as a main fabrication method to form shapes.26–29 However, molding can be challenging when it comes to manufacturing complex internal structures as parts can be easily damaged during the removal process. Although this could be mitigated through the self-healing process, it often requires external stimulus and could impact the mechanical properties of the materials. In addition, it would add a layer of complexity into the fabrication process. In contrast, 3D free-form fabrication of soft robots has the potential to unlock many new opportunities.30 Using additive manufacturing, it is possible to fabricate complex geometries that incorporate multiple functions such as sensing and actuation into the mechanical structure.31 Unfortunately, most of the high tensile strength hydrogels mentioned earlier are not compatible with 3D printing due to their high viscosity and low shear thinning properties.29
Hydrogels are commonly used as inks in printing scaffolds for cell culture.32 However, printing large 3D structures with these gels is challenging due to their inability to support their own weight. To overcome this, a support gel can be used.33 Yet, other challenges, such as precise control of temperature in the case of gelatine-based gels, remain unaddressed. In this context, the KGM-Borax gel presented in this article is an attractive choice for 3D printing because the entire fabrication process takes place at room temperature. Aqueous KGM solutions have recently been deposited by bio-printing,34 whereas borax crosslinked KGM has only been used in casting. The ability to print with the gel allows the fabrication of intricate geometries that are not realizable with other forms of fabrication. Further, since the KGM-Borax gel is responsive to an external stimulus, the geometry and function of a printed part can be altered to suit a desired function after it has been fabricated, enabling 4D printing.35
In this article, we show a novel method of printing with borax-crosslinked KGM gels. Our work is the first demonstration of printing and curing using a KGM-Borax gel. We also provide a rheological characterization of the printable ink and the resultant crosslinked gel. We also determine the most appropriate printing parameters. In addition, we investigated the self-healing property of KGM-Borax gels, which occurs without requiring an external stimulus. We present the results of tensile testing to demonstrate the robustness of the gel after it has been damaged and repaired. We present the entire process of preparing a printable ink, fabricating free form structures by using additive manufacturing, curing the printed structure, washing the support material to extract the part, and self-healing of the structure on infliction of damage. We also demonstrate a novel feature of the KGM-Borax gel, which is the self-healing of the gel when it is completely submerged in water. This behavior is demonstrated, for the first time, in this article.
Materials and Methods
Ink preparation
KGM was purchased as konjac gum powder from Special Ingredients Ltd., Chesterfield, United Kingdom. Borax (sodium tetraborate decahydrate American Chemical Society reagent >99.5%, S9640) was purchased from Sigma Aldrich. Five hundred milligrams of KGM powder was gradually dissolved in 50 mL of deionized water at room temperature. The mixture was continuously agitated by using an overhead stirrer (50006-03; Cole Parmer) at 1000 rpm for 30 min. The mixture was then allowed to rest for 16 h to ensure thorough wetting of solute particles. Borax was dissolved in deionized water to make 11.8 mM solutions. Before printing, 3 mL of 11.8 mM borax solution was added to the prepared KGM solution. The addition of borax partially crosslinked the KGM. It was then mixed in a dual-axis planetary mixer (ARE250; Thinky) for 5 min at 2000 rpm. This partially crosslinked gel was used as the ink for 3D printing as soon as it was prepared.
Support gel preparation
To print complex 3D shapes with overhangs, a support structure is required. This was realized by using a support gel made of high-molecular-weight polyacrylic acid. Carbopol Ultrez 10 NF was obtained in powder form from Lubrizol. Sodium hydroxide was purchased from Fisher Scientific (15678110). Four grams of Ultrez was dispersed in 400 mL deionized water by stirring to form a solution. Eight molar sodium hydroxide was added to neutralize the pH, which immediately resulted in the formation of a gel. This gel was prepared in bulk and stored in air-tight containers at room temperature. Just before printing, the gel was transferred to a smaller container of 30 mL volume, and it was spun in a planetary mixer at 2000 rpm for 10 s to remove air bubbles.
Printing protocol
A bioprinter (Bio-X; Cellink) was used to print the ink. Partially crosslinked KGM-Borax ink, prepared as described in the Ink Preparation section, was loaded into 3 mL syringes. A regulated positive pressure was used to drive the ink through a nozzle. A secondary syringe was loaded in a similar manner with Ultrez (prepared as in the Support Gel Preparation section) and used to deposit the support gel.
Printing was carried out at ambient temperature (20°C) with the syringes and the print-bed maintained at this temperature. Glass was used as a substrate for printing. After printing, a two-step process was followed to produce a crosslinked structure free of support material:
-
1.
The print (including support material) was removed from the substrate and immersed in a container of 200 mL 94 mM borax solution. The excess of borate ions ensured thorough crosslinking of the KGM chains and cured the ink.
-
2.
The support structure was removed by gently agitating the print in a 3 wt% aqueous solution of sodium chloride (obtained as a powder from Honeywell Fluka; 71382). Ultrez disassociated in the presence of the salt and was easily washed off, leaving the finished KGM print.
Print parameter characterization
The controllable parameters for a KGM-Borax gel print are the pressure applied, the speed at which the print-head moves, and the diameter of the nozzle. With the aim of achieving a detailed print, the minimum workable nozzle diameter was first established. A syringe nozzle of diameter 0.41 mm (22 gauge) and length 32 mm was the smallest size through which the ink could consistently flow. To determine the optimum printer parameters for this nozzle, a series of tests was performed by printing zig-zag lines on a glass substrate with varying values of pressure and speed. In each case, the width of the printed line was measured before curing at five positions under a microscope (Hirox KH-7700, Japan). The distance between the tip of the nozzle and the print-bed, that is, the layer height, was maintained at 0.4 mm.
Rheological characterization
Rheological characterization of our KGM-Borax printable gel material was carried out by using a rotational rheometer (TA Instruments Discovery HR-30). Two materials were studied: the ink used for printing and the crosslinked gel.
For the ink, the mixture was prepared as described in the Ink Preparation section. As the ink was a medium viscosity liquid, a cone-plate geometry was used, with a cone angle of 4° and a plate diameter of 40 mm. Ink was loaded onto the plate by using a syringe, the cone was lowered to its truncation gap, and excess ink was removed.
For the crosslinked gel, samples were printed without support as 40 mm diameter, 1mm-thick disks by using the prepared ink with a nozzle of 0.4 mm diameter and a rectilinear infill of 100% density. The disks were cured after printing with a 94 mM borax solution. They were used within 24 h of their preparation. As the crosslinked gel is a soft solid, samples were tested by using a 40 mm diameter Peltier plate with parallel geometry. The samples were carefully placed onto the surface of the lower plate, and the upper flat plate was lowered until a force was detected.
For both materials, the linear viscoelastic (LVE) region was first determined by using an amplitude sweep of the rotational strain at a frequency of 63 rad/s with deformation ranging from 0.5% to 500%. Frequency sweeps were then performed on fresh samples within the LVE with a fixed oscillation strain of 1% and frequency ranging between 0.4 and 100 rad/s.
In all cases, the temperature was controlled at 20°C. Before testing, samples were left for 1 min to reach mechanical and thermal equilibrium. Each study was repeated with a new material sample three times.
Tensile test
Tensile testing was performed to characterize the self-healing ability of the fully cured gel. Cast test specimens for measuring the peak load and elongation at break were prepared in a manner similar to that followed in making the ink for printing (the Ink Preparation section) up to the addition of borax. A higher-concentration solution of 94 mM borax was then used to crosslink the gel. Three milliliters of borax solution was used for every 50 mL of ink. On mixing of the borax, the gel was immediately cast into cylindrical containers (radius 5 mm, length 65 mm) and left to set for 24 h to ensure complete crosslinking had occurred.
Samples for investigating the mechanical anisotropy in printed specimens were prepared as in the Printing Protocol section. To produce a grain in the piece, the cylindrical specimens (radius 5 mm, length 65 mm) were sliced with an aligned rectilinear infill at 100% density with the direction of extrusion for each layer orthogonal to the axis of the cylinder. These samples were left for 24 h between printing and testing.
Tensile tests were performed by using a universal testing machine (Instron 3343 with 1 kN load cell). A gauge length of 35 mm and a test speed of 100 mm/min were used. The samples had an initial diameter of 5 mm. The samples were loaded by hand between parallel grips, the faces of which were tightened until the gel was held firm.
Samples for self-healing experiments were prepared identically to the cast samples above. They were cut in half and the exposed surfaces were placed next to each other with no external load. Points were marked on either side of the join to show its position. Samples were left to heal for 5, 30, or 60 min, respectively. During this resting time, no external force was applied to the ends. After resting, these samples were tested under tension as described earlier. The tensile test results were post-processed by using MATLAB 2018a and the moving median technique to filter out undesirable noise.
Results
Rheological characterization
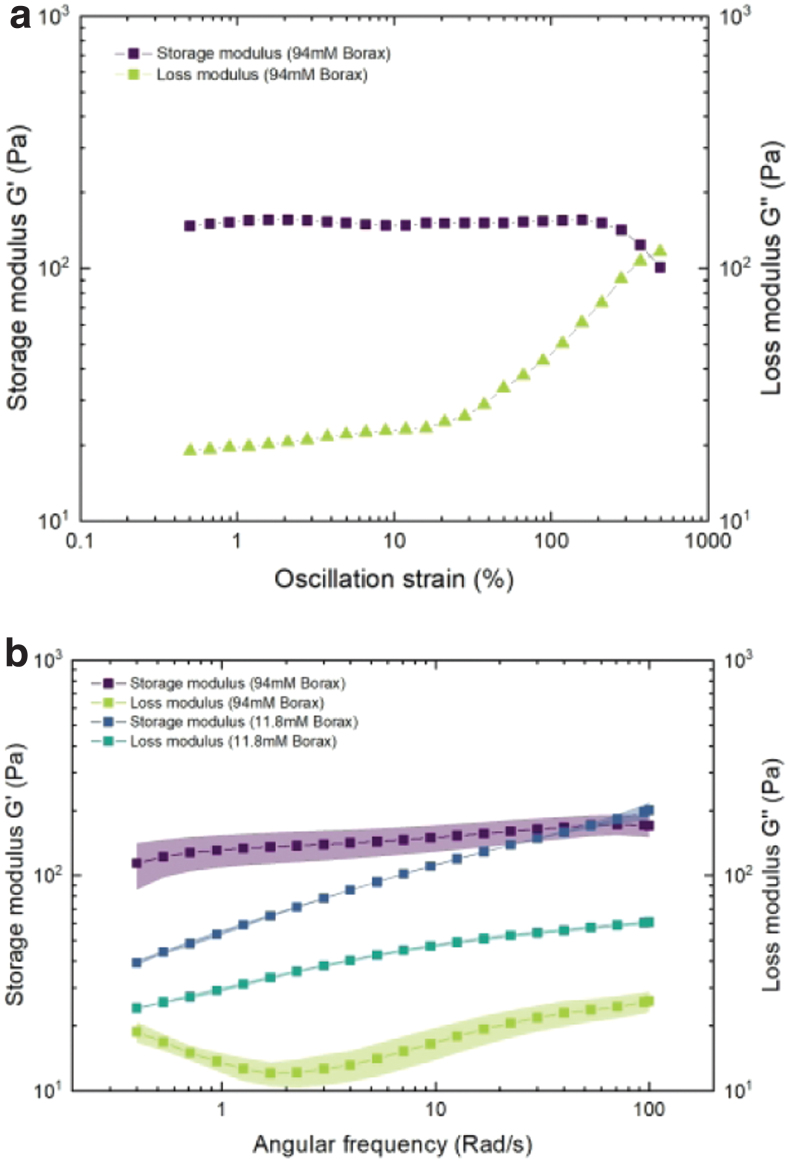
The result of the rheological examination can be seen in Figure 3. It can be seen from Figure 3b that with increasing frequency, the storage modulus (G′) and the loss modulus (G′′) gradually increase. Since G′ is higher than G′′ across the full frequency range, the elastic behavior of the materials predominates over its viscous behavior. In addition, with increasing frequency, that is, low relaxation time, sample flexibility is diminished and they become increasingly rigid. The error of the test method was less than 8%.
FIG. 3.
(a) Storage and loss moduli of the KGM-Borax ink as a function of amplitude of the rotational strain at a frequency of 63 rad/s. (b) Storage and loss moduli of the KGM-Borax ink (11 mM Borax) and fully cured KGM gel (94 mM Borax) as a function of frequency, with a fixed oscillation strain of 1% and within the LVE region. LVE, linear viscoelastic. Color images are available online.
Printing
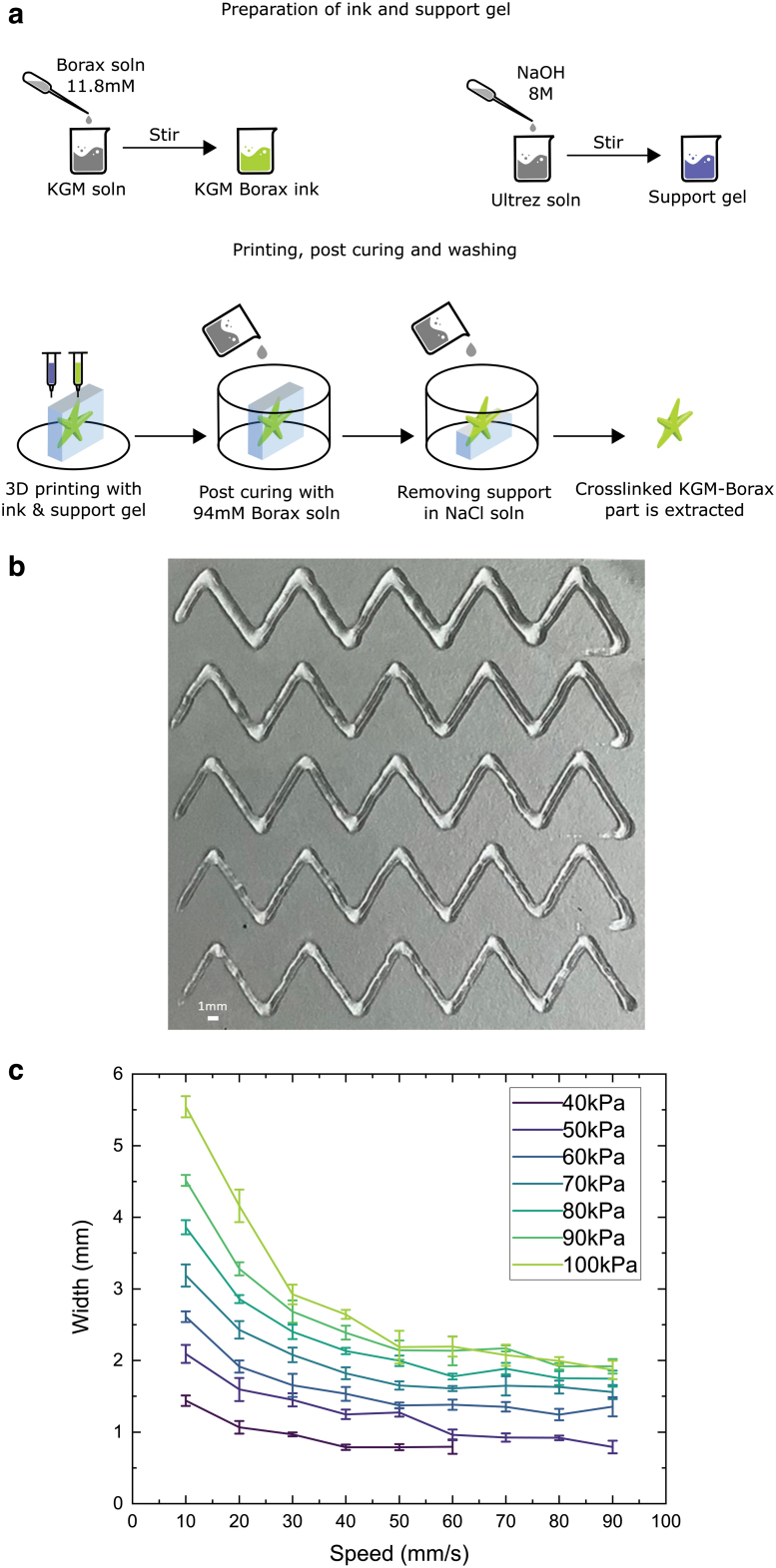
The results of experiments to determine print quality for various printing parameters are shown in Figure 4. The width of the printed track varies as a function of print speed and extrusion pressure for a given nozzle size. For instance, the minimum line width of 650 μm was achieved at 40 kPa and 60 mm/s and the maximum line width of 5700 μm was measured at 100 kPa and 10 mm/s. At constant pressure, a decrease in width was observed on increasing print speed. At a set print speed, higher pressures resulted in wider print tracks. The mean standard deviation in width across all pressures was measured to be 4%, 5%, and 6.5% of the average track width at 40, 50, and 60 mm/s speeds, respectively. This provides important information for 3D fabrication.
FIG. 4.
(a) A schematic showing the preparation of the KGM-Borax ink, printing with it, post-curing and washing away the support. (b) Optimization of printing parameters by printing zig-zag lines with various parameters. One of the samples at 40 kPa pressure and speeds of 10–50 mm/s. Scale bar = 1 mm. (c) Mean and standard deviation of print width measured at five locations for various printing parameters. Color images are available online.
Tensile characterization
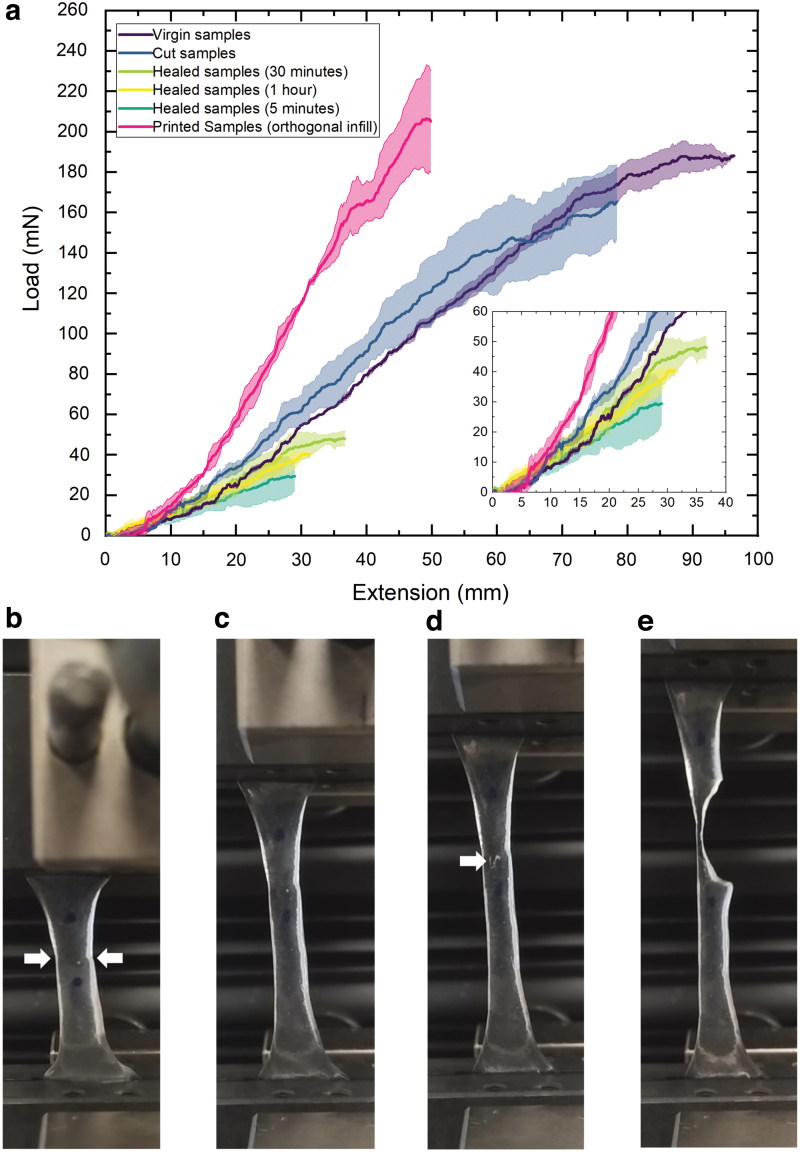
Results from the tensile testing can be seen in Figure 5. To characterize the self-healing capability of the gel, an approach similar to that of López-Díaz et al.36 was followed. Two parameters are considered: maximum load before failure (LF) of the gel and the load at 50% extension (). The healing efficiency () for each of these parameters is the ratio of the value measured for the healed gel versus that of the virgin gel. Therefore, for the load at failure, healing efficiency,
FIG. 5.
(a) Load-extension profiles of crosslinked KGM-borax samples as prepared and after healing. (b–e) A self-healed sample being stretched to break. (b) Sample before test, at a rest length of 35 mm. Arrows indicate the position of the seam where the sample was cut and healed. (c) Sample at an intermediate extension. (d) A crack appears in the material at the location indicated by the arrow. (e) Snapshot of the sample before breaking. Color images are available online.
| (2) |
The mean value of was 39.5 mN and the mean value of was 188.2 mN, giving = 21%. This can be seen in Figure 5a. The load at failure of the virgin specimens is roughly five times that of the healed specimens.
In a similar manner, for the load at 50% extension, healing efficiency,
| (3) |
The mean value of was 20.6 mN and the mean value of was 21 mN, giving = 98%, a high healing efficiency (see Fig. 5 inset).
Discussion
Evaluation of print quality showed that the KGM-Borax ink remains printable at pressures as low as 40 kPa and speeds from 10 up to 90 mm/s. At higher speeds and lower pressures, the print fails. This was due to insufficient flow of the material and poor adhesion of the extrudate to the print bed. At higher pressures and lower speeds, the width of the track was more than 13 times larger than the diameter of the nozzle. The smallest average printed gel track width was 0.78 mm, and it was attained at a speed of 40 mm/s and pressure of 40 kPa. Extrapolating from the optimization study presented for alginate-gelatine gels in Ref.,37 the KGM-Borax ink prints at a finer resolution (0.78 mm vs. 1.13 mm) even with a larger nozzle (0.41 mm vs. 0.26 mm) while requiring less extrusion pressure (40 kPa vs. 100 kPa).
The KGM-Borax was designed to be of a low enough viscosity to extrude through small nozzles. This was achieved by partial crosslinking. Printed structures, therefore, required further curing with a solution of higher borax concentration to stabilize the structure. The abundance of borate ions in the curing solution facilitates high crosslinking of the polysaccharide chains, resulting in more rigid structures. The high concentration of the borax solution and the long (24 h) curing time ensure that the low-volume (<10 mL) samples cure fully, and therefore the degree and rate of curing were not considered for this investigation. For larger samples, it is expected that accounting for these will be necessary, as KGM at the core of the sample will remain uncured until the borax diffuses to it, and there will be a stiffness gradient corresponding to the degree of curing. The temperature of the sample while curing may also have an effect. It is anticipated that temperatures above room temperature will reduce curing time, but at the cost of lowering the quality of the print due to the decreased viscosity of both the support gel and KGM ink.
The gel presented here is soft and can autonomously self-heal. Other biocompatible gels in the literature offer higher tensile strengths26 but do not self-heal autonomously. Previous work38 suggests that modification of the gel by the addition of microfibrillated cellulose can improve gel strength while retaining the other properties. Alternative nanofillers, in particular nanoclay, have also been shown to improve the tensile strength of KGM gels, although these were not crosslinked with borax.39
There is also potential for improving the characteristics of the gel through blending with other polysaccharides. Intermixing with xanthan has been shown to significantly increase the strength of thermally gelled KGM,40 and experimentation with KGM-Guar-Borax blends within our lab has shown initial results of qualitatively stronger gels that retain the autonomous self-healing and pH responsivity of KGM-Borax.
A range of 3D KGM-borax structures were printed. A polyacrylic acid-based support ink was used to maintain the 3D structure of the KGM-Borax ink while printing. This support material showed no noticeable change in properties in the presence of the borax curing agent and is easily removed by NaCl solution. The support material was extruded from a second nozzle at the same time as the ink. Figure 1a–e shows a 3D star as printed in its upright position with support material. The support gel holds the structure intact until it is subsequently cured in a concentrated borax solution. When cut and placed next to each other without additional force, the samples were able to repair the damage (Fig. 1e). The 3D printed parts retain the ability to mechanically heal and regain mechanical strength.
The tensile tests demonstrated an increase in stiffness but a retention of maximum load at failure of printed samples over cast samples where the grain was perpendicular to the axis of loading (Fig. 5a). This is similar to the behaviour seen in other filament-extruded materials. Variations to the fabrication protocol—such as the addition of a relaxation period between printing and full crosslinking—may allow tuning of this effect.
An alternative approach to achieving complex 3D structures is to print the ink directly into a container filled with polyacrylic acid support material.33,41 However, this method requires long preparation time as air bubbles need to be removed from the gel to ensure high-quality printing. Another problem is that the support gel inhibits the permeation of borax solution at the post-curing stage. This leads to the printed parts being incompletely crosslinked and easily damaged during the extraction process. The last drawback is that the maximum height of the printed structures is limited to the length of the nozzle. In contrast, printing support gel simultaneously with the KGM-Borax ink requires significantly less preparation time and quantity of support material. We also observed that this method allowed the borax solution to permeate through the support material during post-curing. This ensures the printed parts are fully cross-linked. Another advantage is that it allows the printing of taller structures while using nozzles of a shorter length.
The Carbopol Ultrez support as currently used is neutral in pH and has no effect on the surface of the KGM-Borax print. However, it retains the gelation and shear-thinning properties, which make it an effective support material even when excess sodium hydroxide is added. There is, therefore, potential for stiffening of the KGM-Borax gel due to an increased pH if an alkali support was used.
The KGM-Borax gel presented here is made with readily available materials and does not require a carefully controlled temperature profile to manufacture. Since it only requires mixing of the reagents at ambient temperatures, it is easier to manufacture, in contrast to other polysaccharide gels, which, for example, require thermal gelling with an alkali.
The KGM-Borax gel is a pH-responsive material. This is due to the shifting of the borate ion equilibrium described in Equation (1). A decrease in pH favors the dissociation of ions from the polysaccharide chains and results in a weakening of the gel. An increase in pH has the opposite effect, stabilizing the tetrahedral structure of the borate ions and strengthening the gel. Song et al.21 consider the rheological properties of a similar KGM-Borax gel at both pH 2 and pH 10. They demonstrate an increase in shear storage modulus within the LVE region on the order of 15 × when transitioning from acidic to alkali conditions. This property can be exploited to design programmable, task-specific, and responsive soft robots that react to environmental changes.
Self-healing polymers have been used in the context of soft robotics.24,25 Typically, polymer networks are crosslinked with a thermoreversible Diels-Alder reaction. A recent study investigated 3D printing with these gels.42 An external stimulus, in the form of heat, is required to trigger the process of healing. In contrast, the printable KGM-Borax gel presented here does not require an external stimulus to initiate healing. The borate ion, which contributes to the healing, is intrinsic to the gel. Another intrinsic and autonomous self-healing material has been presented where polyborosiloxane was used to repair damage.43 Dynamic bonds between boron and oxygen in the polyborosiloxane networks contribute toward healing. Polyborosiloxane is not prone to deterioration such as evaporation of the solvent, which is common in the case of hydrogels. However, natural degradation of the gel may be a desirable property in applications, including environmental monitoring and remediation, that employ large numbers of micro- or nano-robots. A timed, benign decay of the gel would eliminate postoperative cleanup and additional pollution of the environment. Further study is required to quantify the biodegradability of the KGM-Borax gel.
A further feature of the printable KGM gel is its ability to heal under water (Fig. 1j–m). Cylindrical samples, made in a manner similar to those used in the tensile tests, were cut in half and brought together when completely submerged in water. The samples healed within 5 min. Under tension, they were able to withstand stretching in excess of twice the initial length (Fig. 1m). This is the first demonstration of underwater healing in KGM-Borax gels. This ability is highly desirable in robots operating in aquatic settings. The healing efficiency under water will be further studied in future work.
The 3D printable polysaccharide gels are a potential route to edible, functional structures and soft robots. The edibility of KGM gels depends on the crosslinker used. Although KGM itself is edible, the presence of borax makes it unsafe for ingestion in large quantities. Though not toxic in minute quantities, any potentially detrimental effects of borax on humans and the environment can be avoided by using alternative crosslinkers or incorporating KGM into other gels such as Xanthan gum and Carrageenan.44
Conclusion
We have presented a new borax-crosslinked KGM-Borax gel ink for additive manufacturing. Results show a consistent print quality at a wide range of print speeds and pressures, with a mean standard deviation of 70 μm. The end-to-end process of preparing the ink, printing with it, curing it, and washing away support material was demonstrated (Fig. 4a). The ink is of a suitable viscosity to print complex 3D structures, while being supported by a polyacrylic acid-based support gel. Successful curing with 94 mM borax solution and extraction of a cleaned part from a sodium chloride solution was demonstrated (Fig. 1b, c). Subsequent recovery from damage was also shown (Fig. 1d, e). Characterization of the rheology of the cured part confirmed that it is a crosslinked gel.
We demonstrated the self-healing of KGM-Borax printed gels. We characterized the ability to recover mechanical strength after being damaged and healed by conducting tension tests. Our experiments revealed that at an extension of 50%, the mechanical strength of a damaged-and-healed sample is minimally different from that of an undamaged one (98% healing efficiency). This ability to repair is retained in the printed parts, as is shown in the healing of the star shape (Fig. 1d, e). We also demonstrated, for the first time, healing of the KGM-Borax gel under water. The tested sample was able to heal within 5 min and stretch in excess of twice its body length (Figs. 1l, m).
In conclusion, the new 3D printable KGM-Borax gel shows promise as a gel for fabricating soft robots due to its preparation with readily available materials, the ease of printing and curing, the responsiveness to stimuli, and the ability to autonomously repair after mechanical damage. Future work will investigate edibility, toxicity, and biodegradability of the printed konjac gels, as well as the viability of scaling the printing process to accommodate larger prints. A further avenue of investigation is how the characteristics of KGM-Borax can be tuned by blending the KGM with other polysaccharides.
Authors' Contributions
All the authors were involved in the conception, design of experiments, analysis of data, and drafting the article. K.M.D., D.G., and N.H.L. performed the experiments. All authors gave final approval for publication.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the EPSRC Centre for Doctoral Training in Future Autonomous and Robotic Systems (FARSCOPE, grant EP/L015293/1) at the Bristol Robotics Laboratory where D.G. is a PhD student. K.M.D. was supported by the University of Bristol. N.H.L. was supported by the EPSRC Doctoral Training Partnership. J.R. was supported through EPSRC research grants EP/S026096/1, EP/R02961X/1, and EP/M020460/1, and by the Royal Academy of Engineering as a Chair in Emerging Technologies.
Data Accessibility Statement
Data presented in this study are available from the University of Bristol's research database and can be accessed using the doi: 10.5523/bris.flotdeagj80y2tnhs08i0acim.
References
- 1. Kim S, Laschi C, Trimmer B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol 2013;31:287–294. [DOI] [PubMed] [Google Scholar]
- 2. Coyle S, Majidi C, LeDuc P, et al. . Bio-inspired soft robotics: Material selection, actuation, and design. Extreme Mech Lett 2018;22:51–59. [Google Scholar]
- 3. Lee Y, Song W, Sun J-Y. Hydrogel soft robotics. Mater Today Phys 2020;15:100258. [Google Scholar]
- 4. Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res 2015;6:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn S, Kasi RM, Kim S-C, et al. . Stimuli-responsive polymer gels. Soft Matter 2008;4:1151–1157. [DOI] [PubMed] [Google Scholar]
- 6. Chambers LD, Winfield J, Ieropoulos I, et al. Biodegradable and Edible Gelatine Actuators for Use as Artificial Muscles. EAPAD. March 2014. San Diego, CA. Bellingham, WA: SPIE, 2014. [Google Scholar]
- 7. Shintake J, Sonar H, Piskarev E, et al. Soft pneumatic gelatin actuator for edible robotics. 2017. IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS)Vancouver, BC. New York, NY: IEEE, 2017; pp. 6221–6226. [Google Scholar]
- 8. Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int 2008;57:397–430. [Google Scholar]
- 9. Dumitriu S. Polysaccharides as biomaterials. In: Dumitriu S (ed). Polymeric Biomaterials. New York, NY: Marcel Dekker; 2001; pp. 1–11. [Google Scholar]
- 10. Jia J, Richards DJ, Pollard S, et al. . Engineering alginate as bioink for bioprinting. Acta Biomater 2014;10:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Ao Q, Tian X, et al. . Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017;9:401–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 2010;62:83–99. [DOI] [PubMed] [Google Scholar]
- 13. Ahmadi F, Oveisi Z, Samani S M, et al. . Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res Pharm Sci 2015;10:1–16. [PMC free article] [PubMed] [Google Scholar]
- 14. Maeda M, Shimahara H, Sugiyama N. Detailed examination of the branched structure of konjac glucomannan. Agr Biol Chem 1980;44:245–252. [Google Scholar]
- 15. Kato K, Matsuda K. Studies on the chemical structure of konjac mannan: Part I. Isolation and characterization of oligosaccharides from the partial acid hydrolyzate of the mannan. Agr Biol Chem 1969;33:1446–1453. [Google Scholar]
- 16. Kurt A, Kahyaoglu T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr Polym 2014;104:50–58. [DOI] [PubMed] [Google Scholar]
- 17. Maekaji K. The mechanism of gelation of konjac mannan. Agr Biol Chem 1974;38:315–321. [Google Scholar]
- 18. Williams MA, Foster TJ, Martin DR, et al. . A molecular description of the gelation mechanism of konjac mannan. Biomacromolecules 2000;1:440–450. [DOI] [PubMed] [Google Scholar]
- 19. Du X, Li J, Chen J, et al. . Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res Int 2012;46:270–278. [Google Scholar]
- 20. Gao S, Guo J, Nishinari K. Thermoreversible konjac glucomannan gel crosslinked by borax. Carbohydr Polym 2008;72:315–325. [Google Scholar]
- 21. Song C, Lv Y, Qian K, et al. . Preparation of konjac glucomannan–borax hydrogels with good self-healing property and pH-responsive behavior. J Polym Res 2019;26:52. [Google Scholar]
- 22. Yu K, Xin A, Wang Q. Mechanics of self-healing polymer networks crosslinked by dynamic bonds. J Mech Phys Solids 2018;121:409–431. [Google Scholar]
- 23. Dahlke J, Zechel S, Hager MD, et al. . How to design a self-healing polymer: General concepts of dynamic covalent bonds and their application for intrinsic healable materials. Adv Mater Interfaces 2018;5:1800051. [Google Scholar]
- 24. Terryn S, Brancart J, Lefeber D, et al. . Self-healing soft pneumatic robots. Sci Robot 2017;2. [DOI] [PubMed] [Google Scholar]
- 25. Roels E, Terryn S, Brancart J, et al. . Additive manufacturing for self-healing soft robots. Soft Robot 2020;76 [Epub ahead of print]; DOI: 10.1089/soro.2019.0081. [DOI] [PubMed] [Google Scholar]
- 26. Balitaan JN, Hsiao CD, Yeh JM, et al. . Innovation inspired by nature: Biocompatible self-healing injectable hydrogels based on modified-β-chitin for wound healing. Int J Biol Macromol 2020;162:723–736. [DOI] [PubMed] [Google Scholar]
- 27. Yuan T, Cui X, Liu X, et al. . Highly tough, stretchable, self-healing, and recyclable hydrogels reinforced by in situ-formed polyelectrolyte complex nanoparticles. Macromolecules 2019;52:3141–3149. [Google Scholar]
- 28. Li Z, Su Y, Xie B, et al. . A novel biocompatible double network hydrogel consisting of konjac glucomannan with high mechanical strength and ability to be freely shaped. J Mater Chem B 2015;3:1769–1778. [DOI] [PubMed] [Google Scholar]
- 29. Xu C, Dai G, Hong Y. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater 2019;95:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipson H. Challenges and opportunities for design, simulation, and fabrication of soft robots. Soft Robot 2014;1:21–27. [Google Scholar]
- 31. Wallin T, Pikul J, Shepherd R. 3D printing of soft robotic systems. Nat Rev Mater 2018;3:84–100. [Google Scholar]
- 32. Gungor-Ozkerim PS, Inci I, Zhang YS, et al. . Bioinks for 3D bioprinting: An overview. Biomater Sci 2018;6:915–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin Y, Compaan A, Bhattacharjee T, et al. . Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures. Biofabrication 2016;8:025016. [DOI] [PubMed] [Google Scholar]
- 34. Yulong W, Lu H, Jia Y, et al. . 3D Bioprintability of konjac glucomannan hydrogel. Mater Sci 2020;26:109–113. [Google Scholar]
- 35. Tibbits S. 4D printing: Multi-material shape change. Archit Design 2014;84:116–121. [Google Scholar]
- 36. López-Díaz A, Martín-Pacheco A, Naranjo A, et al. Autonomous self-healing pneumatic McKibben muscle based on a new hydrogel material. 3rd IEEE International Conference on Soft Robotics. May 2020. New Haven, CT. New York: IEEE, 2020; pp. 13–18. [Google Scholar]
- 37. Webb B, Doyle BJ. Parameter optimization for 3D bioprinting of hydrogels. Bioprinting 2017;8:8–12. [Google Scholar]
- 38. Lu B, Lin F, Jiang X, et al. . One-pot assembly of microfibrillated cellulose reinforced PVA–borax hydrogels with self-healing and pH-responsive properties. ACS Sustain Chem Eng 2017;5:948–956. [Google Scholar]
- 39. Resano-Goizueta I, Ashokan BK, Trezza TA, et al. . Effect of nano-fillers on tensile properties of biopolymer films. J Polym Environ 2018;26:3817–3823. [Google Scholar]
- 40. Tako M. Synergistic interaction between xanthan and konjac glucomannan in aqueous media. Biosci Biotech Bioch 1992;56:1188–1192. [Google Scholar]
- 41. Hinton TJ, Hudson A, Pusch K, et al. . 3D printing PDMS elastomer in a hydrophilic support bath via freeform reversible embedding. ACS Biomater Sci Eng 2016;2:1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou Q, Gardea F, Sang Z, et al. . A tailorable family of elastomeric-to-rigid, 3D printable, interbonding polymer networks. Adv Funct Mater 2020;30:2002374. [Google Scholar]
- 43. Narumi K, Qin F, Liu S, et al. Self-healing UI: Mechanically and Electrically Self-healing Materials for Sensing and Actuation Interfaces. 32nd Annual ACM Symposium on User Interface Software and Technology. October 2019. New Orleans, LA. New York, NY: ACM, 2019; pp. 293–306. [Google Scholar]
- 44. Dave V, McCarthy SP. Review of konjac glucomannan. J Environ Polym Degrad 1997;5:237–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available from the University of Bristol's research database and can be accessed using the doi: 10.5523/bris.flotdeagj80y2tnhs08i0acim.