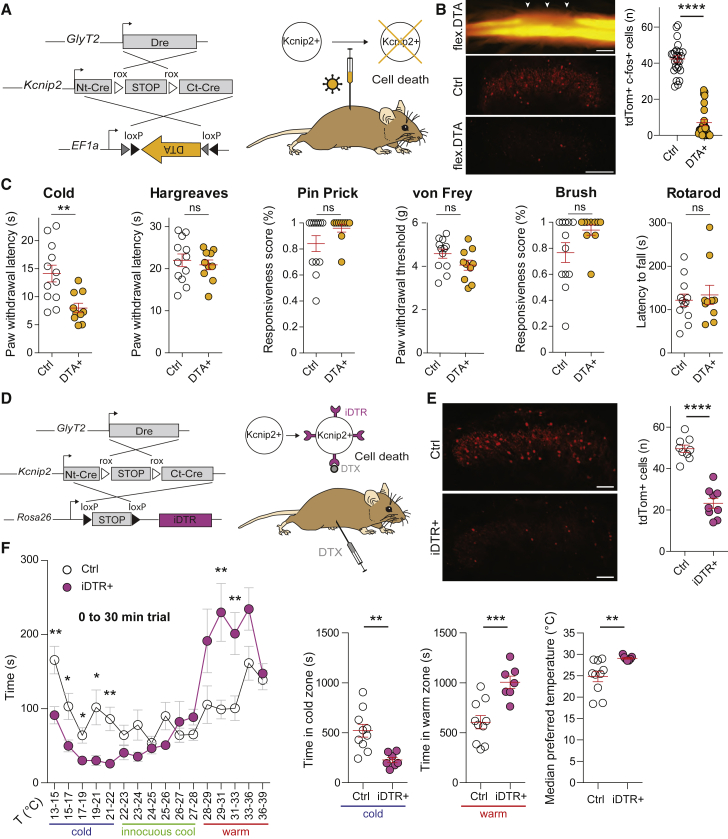
Figure 3.
DTX-mediated ablation of Kcnip2GlyT2 neurons
(A–C) Local ablation of Kcnip2GlyT2 neurons using AAV1.Ef1a.flex.DTA.
(A) Experimental strategy. Unilateral AAV injections were made into the lumbar dorsal horn of Kcnip2roxCre;GlyT2::Dre;ROSA26lsl-tdTom triple transgenic mice.
(B) (Left, top) Whole-mount spinal cord after Kcnip2GlyT2 ablation. White arrows indicate injection sites. Scale bar, 1 mm. (Middle and bottom) Loss of Kcnip2GlyT2 neurons (red) in the dorsal horn of AAV1.Ef1a.flex.DTA injected mice. Scale bar, 100 μm. (Right) Quantification of DTA mediated loss of Kcnip2GlyT2 neurons. n = 25 sections from five mice per group.
(C) Nociceptive sensitivity and motor performance of Kcnip2GlyT2 neuron-ablated mice, 3–4 weeks after AAV injection. n = 10 Kcnip2GlyT2 ablated mice, and n = 12 control mice. Scale bar, 1 mm.
(D–F) Spinal cord-wide ablation of Kcnip2GlyT2 neurons.
(D) Experimental strategy, i.p. injection of complete diphtheria toxin (DTX) in Kcnip2roxCre; GlyT2::Dre;ROSA26lsl-iDTR triple transgenic mice.
(E) Loss of tdTom (Kcnip2GlyT2) neurons in the dorsal horn of DTX treated mice. n = 9 sections from three mice per group; Scale bar, 100 μm.
(F) Thermal gradient test. (Left) Time spent by Kcnip2GlyT2 ablated and control mice in different temperature zones during a 30-min interval. (Right) Quantification of time spent in cold (13°C–22°C) and warm (28°C–39°C) zones. Median preferred temperature in Kcnip2GlyT2 ablated (n = 7) and control mice (n = 10);
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; unpaired t test. Mean ± SEM.