Abstract
Objective:
Characterizing the pathophysiology of irritability symptoms from a dimensional perspective above and beyond diagnostic boundaries is key to developing mechanism-based interventions that can be applied broadly. Face emotion processing deficits are present in youths with elevated levels of irritability. The present study aimed to identify the neural mechanisms of face emotion processing, in a sample enriched for irritability by including youth with high functioning autism spectrum disorder (HF-ASD).
Method:
Youths (N=120, age=8.3–19.2 years) completed an implicit face emotion task during fMRI acquisition. We evaluated how irritability, measured dimensionally, above and beyond diagnostic group, relates to whole-brain neural activation and amygdala connectivity in response to face emotions.
Results:
Both neural activation and amygdala connectivity differed as a function of irritability level and face emotion in the prefrontal cortex. Youths with higher irritability levels had decreased activation in response to both fearful and happy faces in the left middle frontal gyrus and to happy faces in the left inferior frontal gyrus. Furthermore, increased irritability levels were associated with altered right amygdala connectivity to the left superior frontal gyrus when viewing fearful and sad faces.
Conclusion:
The neural mechanisms of face emotion processing differ in youths with higher irritability compared to their less irritable peers. The findings suggest that these irritability mechanisms may be common to both typically developing and HF-ASD youths. Understanding the neural mechanisms of pediatric irritability symptoms that cut across diagnostic boundaries may be leveraged for future intervention development.
Keywords: irritability, face emotion, fMRI, children, autism spectrum disorder
Social media promotion text:
Facebook:
Study finds that irritable children (including irritable children with autism spectrum disorder) show altered brain activity in regions responsible for regulating their own emotions when they see other people’s emotional faces. #irritability #autism #ASD #emotion #brain #SDSU
Twitter:
New @SDSU @JAACAP study finds that irritable children (including children with autism) show altered brain activity in regions responsible for regulating their own emotions when they see other people’s emotional face expressions. #irritability #autism #ASD #emotion #brain
Lay Summary:
Irritability in youths can lead to worse mental health, including suicidality, and lower financial outcomes in adulthood, but little is known about how perceiving others’ emotions may lead to irritability. This study examined brain responses in children with varying levels of irritability, including children with autism spectrum disorder who often have high levels of irritability, when viewing emotional faces. More irritable children showed different brain responses in areas responsible for controlling their emotions. Knowing that irritable children show altered emotion regulation-related brain activity in response to other people’s emotional faces may be helpful for designing treatments for irritability.
Introduction
Irritability symptoms, conceptualized as a relatively lower threshold for anger,1 are among the most common self- and parent-reported mood symptoms in youth,2 and are prevalent across multiple diagnostic categories, including depressive, anxiety, and disruptive behavior disorders,3 as well as autism spectrum disorder (ASD)4 and typical development.5 Elevated irritability levels are associated with severe, pervasive impairment in childhood6 and moreover predict poorer mental health7–9 and socioeconomic10 outcomes in adulthood. Thus, addressing pediatric irritability is crucial,1 not only to improve the child’s wellbeing, but also to prevent poor outcomes in adulthood. However, treatment options, both pharmacological and non-pharmacological, are lacking for irritability symptoms. Indeed, the only FDA-approved medication for irritability is in the context of ASD.11 Because irritability symptoms occur across multiple diagnostic contexts,12 studying the irritability dimension’s neural basis may point to common pathophysiology and potential treatment targets that could be applied across diagnostic boundaries.
In line with this, Research Domain Criteria (RDoC) efforts have promoted a dimensional perspective to evaluate the neural underpinnings of symptom dimensions, such as irritability, across diagnostic boundaries.13 The present work aims to answer RDoC-based calls for research14 to investigate the neural correlates of irritability symptoms from a dimensional perspective in a sample enriched for irritability by including youth with high-functioning ASD (HF-ASD), a diagnostic category with high prevalence of irritability.4 Irritability symptoms are common among youths without a psychiatric diagnosis15 as well as youths with HF-ASD,4 yet potential shared pathophysiology of the irritability dimension is largely unexplored.
Face emotion processing deficits have been suggested as one of the mechanisms underlying irritability symptoms1,16 and have been documented in irritable youths,17–22 as well as in HF-ASD.16,21,22 Irritability-related face emotion processing deficits manifest in difficulties accurately labeling emotions16,20 and in neural differences both when explicitly labeling face emotions18,19 as well as during implicit processing (e.g., gender identification of emotional faces).17,21,22 For example, irritable youths may interpret ambiguous faces as more hostile23 or may need more intense emotional information to accurately label an emotion.16 Difficulties understanding the facial expressions of others can negatively impact social interactions leading to confusion, frustration, and ultimately, irritability symptoms. The extant irritability literature has focused on threatening, happy, and neutral faces,19,24,25 yet other emotional expressions, such as sadness and fear, may also be perceived as ambiguous in youth with emotion processing difficulties.18,21,26 The associated neural mechanisms may be informative for capturing the pathophysiology of irritability.
Few studies have examined the neural mechanisms of face processing across the irritability dimension in youths, but findings primarily examining highly-irritable diagnostic groups point to aberrant neural patterns in the amygdala18,19,25 and prefrontal cortex (PFC),24 as well as amygdala connectivity with the PFC17 during both explicit (i.e., labeling face emotions)18,19 and implicit17,24,25 face emotion fMRI paradigms. Therefore, aberrant amygdala and prefrontal engagement may be related to aberrant face emotion processing among irritable youth. Neural activation has thus far been the predominant means of identifying the neural mechanisms of emotion processing in irritability,19,25 but identifying how brain areas interact (i.e., functional connectivity), may provide a more comprehensive picture of aberrant brain function in irritability.
Despite the recent focus on the importance of identifying transdiagnostic neural mechanisms of symptom dimensions,27 few studies have included multiple diagnostic groups to examine neural mechanisms of irritability levels. To our knowledge, there are only three such studies,17,18,24 yet those studies were not focused on the irritability dimension itself but rather on disentangling irritability from anxiety17,24 or bipolar disorder from disruptive mood dysregulation disorder.18 Furthermore, pediatric irritability has not yet been evaluated in a sample that includes youths with ASD, despite the high prevalence of irritability in this population.4 Examining irritability beyond diagnostic boundaries using a dimensional approach is important, because it is more likely to identify underlying mechanisms that are common across diagnoses.14 Moreover, an enriched sample increases the variance of irritability in the sample, leading to more robust statistical analyses.17, 24
The current study examines the neural mechanisms of face emotion processing in a sample enriched for irritability by including youth with HF-ASD, addressing the aforementioned gaps in the literature. Youths completed an implicit face emotion task during fMRI acquisition, probing bottom-up processing of face emotions, which has been suggested to contribute to irritability.17,25 During the task, participants labeled the gender of actors displaying fearful, sad, happy, or neutral facial expressions. We evaluated how irritability, measured dimensionally, above and beyond diagnostic group and core autism symptoms, relates to whole-brain neural activation and amygdala connectivity, and interacts with differing face emotions. Based on previous research of the neural mechanisms of implicit emotion processing in irritable youth,17,19,24,25 we hypothesized that irritability would be associated with aberrant activation in the amygdala and PFC as well as amygdala-prefrontal connectivity in response to viewing differing face emotions.
Method
Participants
Data from 120 youths, aged 8.3–19.2 years (mean age [SD]=14.22[2.7]), were included. Additional participants were excluded due to missing key measures (n=11), movement-related fMRI data quality (n=7), or poor accuracy (<80%) on the gender identification task (n=6). The age range in the present sample (i.e., late childhood through adolescence) encompasses important developmental periods during which irritability is a primary concern for parents seeking psychiatric care for their children.8,28 Irritability during this age range is predictive of serious mental health, academic, social, and financial problems across the lifespan.5,8 Therefore, understanding the mechanisms of irritability across this developmental range is important groundwork for preventing short and long-term effects of irritability.
Irritability is common among typically developing youths,2 but variability in the higher ranges is sparse. To examine irritability dimensionally, beyond diagnostic boundaries, youths with HF-ASD were included because irritability is prevalent in this population.4 Mean between-group irritability levels differed, as expected, but covered an overlapping range (see Table 1). The final sample included 73 youth recruited from the community and 47 youth with ASD recruited through the University of Michigan Autism and Communication Disorders Center (UMACC), who had been diagnosed based on the Autism Diagnostic Observation Schedule (ADOS)29 and the Autism Diagnostic Interview-Revised (ADI-R).30 Exclusion criteria included cognitive functioning scores <85, co-occurring neurological disorders or MRI contraindications. The Institutional Review Board of the University of Michigan approved all procedures and all participants provided consent/assent. Parental permission was obtained for participants <18 years. Data from a subset of participants were included in previous studies,21,22,26,31 including three on diagnostic group differences of neural face emotion processing.21,22,26
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Full Sample (N=120) |
HF-ASD (n=47) |
Non-ASD (n=73) |
Independent-samples t-test |
|---|---|---|---|---|
|
| ||||
| Sex (%female) | 23.30% | 17.00% | 27.40% | χ2(1)=1.72, p>.05 |
| Age (years) | 14.22(2.70) | 13.94(2.35) | 14.40(2.9) | t(111.88)=.96, p>.05 |
| Verbal cognitive functioning | 114.17(15.94) | 112.83(19.23) | 115.03(13.49) | t(74.94)= 68, p>.05 |
| Non-verbal cognitive functioning | 103.22(16.26) | 106(20.36) | 101.39(12.69) | t(67.96)=−1.37, p>.05 |
| Gender ID accuracy (%) | 95.70%(3.94) | 94.56%(3.81) | 96.44%(3.89) | t(118)=2.6, p=.01 |
| Irritability | 1.17(1.49) | 2.02(1.73) | .62(.98) | t(65.32)=−5.08, p<.001 |
| Range | 0–6 | 0–6 | 0–5 | |
| Social communication impairment | 47.78(40.92) | 90.53(28.57) | 19.88(15.95) | t(64.89)=−15.46, p<.001 |
| Range | 0–163 | 21–163 | 0–82 | |
| Anxiety | 18.91(11.78) | 24.89(14.02) | 15(8) | t(65.71)=−4.39, p<.001 |
| Range | 1–61 | 3–61 | 1–41 | |
| Depression | 6.28(5.54) | 8.36(6.08) | 4.93(4.74) | t(81.20)=−3.28, p<.01 |
| Range | 0–28 | 0–24 | 0–28 | |
Note: ASD=autism spectrum disorder. Irritability=sum of Child Behavior Checklist Items 86, 87, and 95. Social communication impairment=score on Social Responsiveness Scale. Anxiety=score on Spence Children’s Anxiety Scale. Depression=score on Children’s Depression Inventory. Statistically significant group differences based on independent samples t-test are bolded. Values indicate mean(SD) unless otherwise noted.
Irritability
Three items previously identified and validated as comprising an irritability factor32,33 from the Child Behavior Checklist (CBCL; version for 6–18 year olds)34 were used to assess level of irritability symptoms (“temper tantrums or hot temper,” “stubborn, sullen or irritable,” and “sudden changes in mood or feelings”), rated on a scale of 0–2. Items were summed and then used as a continuous variable as in prior studies.32,33 This subscale has acceptable psychometric properties (alpha = .73),32,33 and is sensitive to variation along the irritability spectrum.18,33
This subscale was selected for two reasons. First, because it can be used across diagnostic categories, unlike the Aberrant Behavior Checklist (ABC) which is specifically geared toward ASD. Second, the CBCL irritability scale is particularly useful in the dimensional measurement of irritability in typical youths because it provides more variability in the lower ranges compared to other irritability measures.18,33 This is an important consideration given that the majority of youths included in the present study were recruited from the community.
See Supplement 1 for details on additional symptom measures, participant screening, and fMRI acquisition.
Gender Identification Task
During functional magnetic resonance imaging (fMRI) acquisition, participants completed an event-related gender identification task with emotional (happy, sad, fearful, or neutral) faces.21,22 Threatening faces were excluded from the paradigm to limit the number of contrasts. Faces came from the NimStim set35 and included an equal number of male and female faces. One trial consisted of a fixation cross (500ms), a face (250ms), and a black screen (1500ms) during which participants indicated the gender of the face by pressing the thumb button of a button box for male, and the index finger button for female. Participants thus viewed the faces without making explicit judgments on the face emotion (and thus either “tamping down” on the emotion and/or probing a particular labeling process related to language). All participants used the right hand to respond. Unlike emotional face task paradigms in other studies17 that present faces for multiple seconds and cannot control for attention, our implicit face processing task minimized cross-subject differences in attention by having a very quick (250ms) presentation of the face (i.e., enough for one saccade but not enough for multiple saccades around the screen) and a task (identifying gender) that required attention. Only trials in which gender was correctly identified were included in the analyses. Inter-trial intervals were jittered, ranging from 0 to 6000ms at intervals of 2000ms. Each of the two runs consisted of 60 trials, 15 of each emotion, and randomized for each participant.
Analytic Plan
Participant characteristics.
Since our sample consisted of individuals with and without the HF-ASD diagnosis, we conducted independent samples t-tests and chi square tests to assess whether the diagnostic groups differed on variables that could be relevant to irritability and potentially confound our results, i.e., gender, age, verbal and non-verbal cognitive functioning, core ASD symptoms (social communication impairment), anxiety, and depression. In the absence of statistical significance, potentially clinically meaningful differences were also considered.
Behavioral data analysis.
To examine potential differences in accuracy of labeling gender in relation to irritability, we conducted a univariate ANOVA with percent accuracy as the dependent variable and irritability level as the independent variable. We also examined potential group (HF-ASD vs. non-ASD) differences in task accuracy.
fMRI data analysis.
fMRI preprocessing.
Preprocessing of fMRI data was done in AFNI. See Supplement 1 for additional details.
First-level models.
Three models were used for individual level analyses: one for neural activation, and one each for right and left amygdala functional connectivity. Face emotion regressors (i.e., happy, fearful, sad, and neutral) were included as described below. All models also included incorrect trials as a nuisance regressor as well as motion parameters (estimated in the x, y, z, roll, pitch, and yaw directions), and fourth-degree polynomials modeling low-frequency drift in the baseline model.
Neural activation.
In this analysis, the four emotion conditions were modeled as regressors convolved with AFNI’s GAM basis function over 250ms of face presentation for each trial. The resulting beta coefficient maps represent the magnitude of average neural activation for each emotion compared to implicit baseline.
Amygdala functional connectivity.
Given the extensive literature on the role of the amygdala in emotion processing (e.g.,36), we completed generalized psychophysiological interaction (gPPI) analyses using right and left amygdala seeds. The amygdala masks (right amygdala=972mm3; left amygdala=756mm3) were created using the Talairach atlas in AFNI. gPPI analyses calculated the change in correlations between the amygdala seeds and each voxel in the brain, for each condition compared to implicit baseline. The analyses result in a set of voxel-wise images that represent connectivity between the seed region and the rest of the brain for each face condition.
Addressing head motion.
Excessive head motion was addressed with multiple methods: realigning functional images, scrubbing TR pairs with framewise displacement >1mm, and including nuisance covariates for motion parameters in the individual level models.
Second-level models.
Addressing the potential confound of diagnostic group.
The aim of the present study was to evaluate the neural mechanisms of irritability from an RDoC-based dimensional perspective, and the present sample of youths was enriched for irritability by including youths with HF-ASD, a diagnostic category with characteristic social communication impairment.37 We controlled for potential effects of autism as follows: a) across all second-level models, diagnostic group (HF-ASD vs. non-ASD) was included as a binary covariate; b) additionally, we examined the impact of social communication impairment (Social Responsiveness Scale scores; see Supplement 1) on our results in post-hoc analyses. Between group effects of irritability are also of interest, yet they are outside the scope of the present study. Nevertheless, for the interested reader, we completed these additional analyses (i.e., Irritability × Diagnosis × Face Emotion), in Supplement 1. However, the results should be interpreted with caution as the variability of irritability is limited among the non-ASD sample.
Whole-brain analyses.
AFNI’s 3dMVM was used to conduct second-level, repeated- measures ANOVAs with the individual-level beta coefficients as the dependent variable, face emotion (neutral, happy, sad, and fearful) as a within-subject factor, irritability as the between-subject quantitative variable. Diagnosis (HF-ASD vs non-ASD) was included as a covariate to examine neural mechanisms of irritability above and beyond potential diagnosis effects. Three whole-brain ANOVA models were conducted, one each for activation, and right and left amygdala to whole-brain functional connectivity. These models generated the contrast of primary interest, Irritability × Emotion, which examines neural correlates of face emotion processing depending on irritability level, in addition to lower-order terms (e.g., Emotion, Irritability, Diagnosis main effects).
A whole-brain cluster corrected threshold of α=.05 was used, calculated via AFNI’s 3dClustsim program using a mixed-model spatial autocorrelation function (-acf) and the NN1 2-sided option, per recent recommendations.38 The cluster extent threshold resulting from the 3dClustsim analysis was k≥28 voxels for all second-level analyses. Additionally, a height threshold of p<.005 was used.
Amygdala Region-of-Interest (ROI) analyses.
Given the previously documented role that the amygdala may play in irritability (e.g.,36), we additionally utilized ROI analyses to specifically probe potential neural mechanisms of irritability related to amygdala activation. We extracted average activation for each condition in each individual based on the right and left amygdala masks from the Talairach daemon atlas in AFNI. ANCOVA in SPSS v. 24 (IBM; Armonk, NY) was used to assess the Irritability × Emotion interaction covarying for Diagnostic Group.
Post-hoc analyses.
To evaluate significant omnibus findings for the Irritability × Emotion interaction, values from resulting clusters in each analysis were extracted and partial correlations conducted in SPSS: irritability was correlated with activation (or connectivity) for each face emotion condition separately, controlling for diagnosis. False discovery rate (FDR) was used to correct for multiple comparisons.
Additional analyses evaluating potential confounds.
Extracted values were also used to conduct post-hoc ANCOVAs using SPSS to assess the potential influence of confounding variables, including age, gender, anxiety and depression symptoms, core ASD symptoms (social communication impairment), task accuracy, and use of psychotropic medications. Additionally, whole-brain models were repeated with gender as an additional covariate.
Results
Participant characteristics for the overall sample as well as each participant pool separately are listed in Table 1. There were no significant diagnostic group differences in gender, age, verbal and non-verbal cognitive functioning (Table 1). Overall accuracy to label gender (mean=95.70%, SD=3.9%) was well above chance (50%). Irritability was not significantly associated with task accuracy (r=.10, p=.26), and did not significantly predict overall task accuracy controlling for diagnostic group (F1,117=0.003, p=.95). There was a significant between group difference in gender identification accuracy (t118=2.6, p=.01); thus, we subsequently evaluated whether our results remain after controlling for accuracy (see Additional Analyses).
Associations of Irritability with Neural Activation
We found that the Irritability × Emotion interaction was associated with activation in the left middle frontal gyrus (MFG, F3,351=10.23, xyz=−35, 32, 33, k=98, p<0.05 corrected, Figure 1A), and left inferior frontal gyrus (IFG, F3,351=8.26, xyz=−44, 8, 9, k=38, p<0.05 corrected, Figure 1B). Post-hoc analyses indicated that in the left MFG, there was a significant negative association between irritability and activation in response to fearful (r=−0.26, p<0.05, corrected) and happy faces (r=−0.25, p<0.05, corrected), and in the left IFG there was a significant negative association between irritability and activation to happy faces (r=−0.39, p<0.001, corrected). In other words, greater levels of irritability are associated with less activation to fearful and happy faces in the left MFG, and to happy faces in the left IFG. Post-hoc partial correlations were not significant for any other face emotion conditions. Clusters that emerged for other contrasts are listed in Table 2.
Figure 1. Irritability × Emotion Interaction is Associated with Prefrontal Cortex Activation.
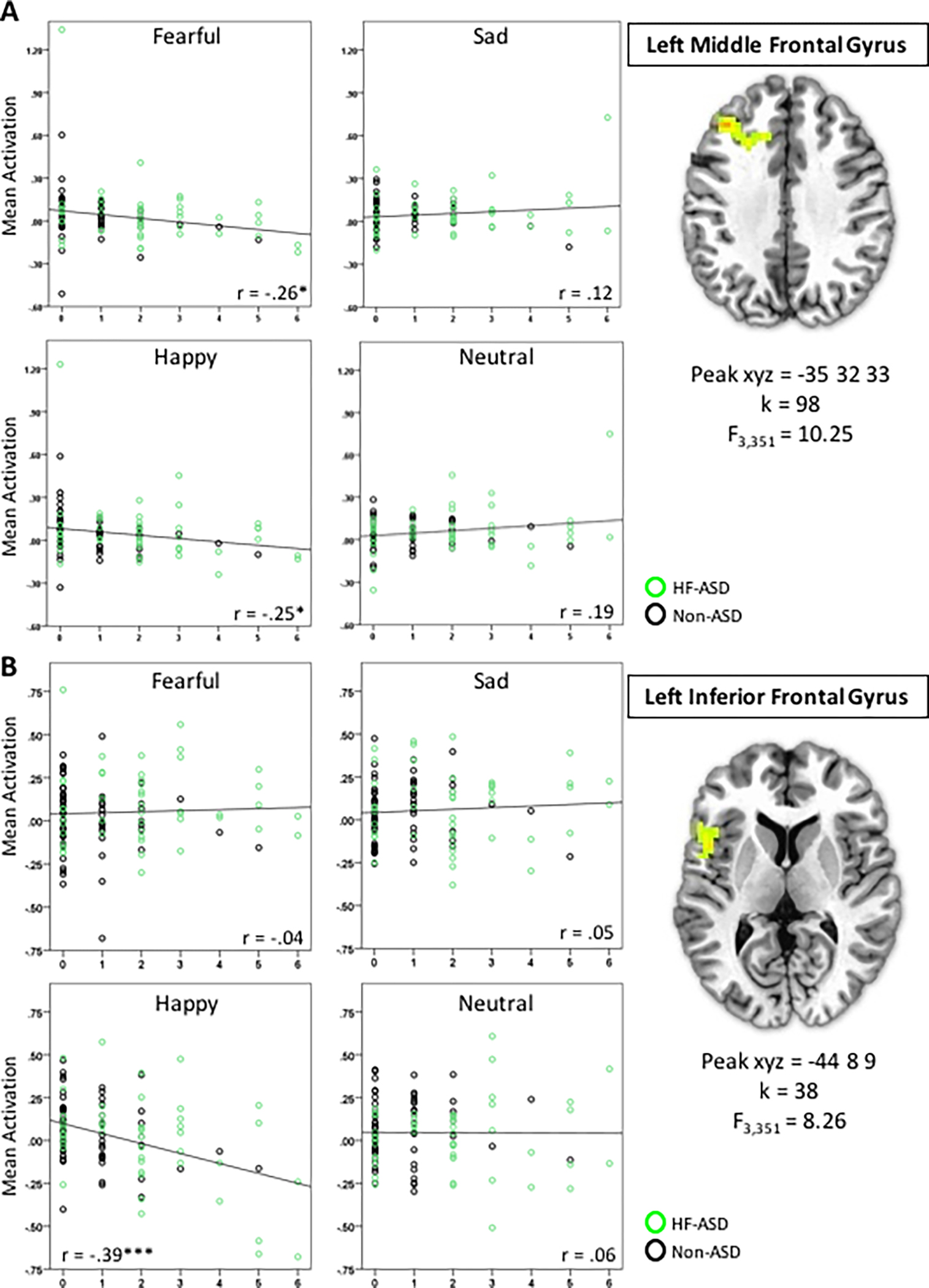
In A) Left Middle Frontal Gyrus and B) Left Inferior Frontal Gyrus. Partial correlation coefficients are shown. * p<0.05, corrected, ***p<0.001, corrected. For this and all figures, brain images represent axial sections (left=left) with threshold set at whole-brain-corrected p<.05.
Table 2.
Significant Clusters Resulting from Whole Brain Analyses
| Activation | ||||||
| a Irritability × Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| 98 | 10.25 | −35 | 32 | 33 | 9,32 | Left Middle Frontal Gyrus |
| 38 | 8.26 | −44 | 8 | 9 | 44,45 | Left Inferior Frontal Gyrus |
| Irritability | ||||||
| k | F 1,117 | x | y | z | BA | Region |
| 36 | 17.61 | −8 | −62 | 39 | 7 | Left Precuneus |
| Diagnostic Group | ||||||
| k | F 1,117 | x | y | z | BA | Region |
| 46 | 18.91 | −41 | −80 | 9 | 19 | Left Middle Occipital Gyrus |
| 28 | 19.04 | 38 | −80 | 6 | 19 | Right Middle Occipital Gyrus |
| Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| 35 | 7.56 | −44 | −56 | 9 | 39,22 | Left Middle Temporal Gyrus, Left Superior Temporal Gyrus |
| 31 | 9.60 | 60 | −47 | 9 | 22,21 | Right Superior Temporal Gyrus, Right Middle Temporal Gyrus |
|
| ||||||
| Right Amygdala Connectivity | ||||||
| a Irritability × Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| b127 | 11.60 | 20 | −23 | 33 | 31 | Bilateral Cingulate Gyrus |
| 56 | 8.27 | −20 | 14 | 48 | 8 | Left Superior Frontal Gyrus |
| Diagnostic Group × Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| 37 | 7.90 | 8 | 59 | 18 | 10 | Right Medial Frontal Gyrus, Left Medial Frontal Gyrus |
|
| ||||||
| Left Amygdala Connectivity | ||||||
| a Irritability × Emotion | ||||||
| k | F 1,117 | x | y | z | BA | Region |
| 1294 | 11.30 | −5 | 38 | 24 | 32 | Bilateral Anterior Cingulate Cortex and Medial Frontal Gyrus |
| 30 | 9.15 | 62 | 14 | 21 | 44 | Right Inferior Frontal Gyrus |
| 29 | 8.41 | 50 | −2 | 40 | 6 | Right Precentral Gyrus |
| Diagnostic Group × Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| 37 | 7.46 | 38 | 14 | 42 | 6,9 | Right Middle Frontal Gyrus, Right Precentral Gyrus |
| Emotion | ||||||
| k | F 3,351 | x | y | z | BA | Region |
| 49 | 10.71 | −11 | −68 | 12 | 30,18,31 | Left Cuneus, Left Posterior Cingulate, Left Lingual Gyrus |
| 36 | 9.25 | −5 | −53 | −23 | - | Bilateral Nodule |
| 33 | 9.50 | 29 | −83 | 21 | 19 | Right Middle Occipital Gyrus |
| 29 | 6.85 | −8 | −8 | 60 | 6 | Bilateral Medial Frontal Gyrus, Left Middle Frontal Gyrus |
Note: BA=Brodmann Area.
contrast of interest in this study; extracted values for bolded clusters are presented in Figures 1–3; for any contrasts not listed no significant clusters emerged in the analyses.
85% of this cluster’s voxels were situated in the hemispheric white matter and most likely represent noise. We therefore focused our post-hoc analyses on the left SFG cluster for the contrast of interest among right amygdala functional connectivity results.
Amygdala ROI analysis.
Irritability × Emotion was not significantly associated with right or left amygdala activation.
Associations of Irritability with Right Amygdala Functional Connectivity
We detected that the Irritability × Emotion interaction was associated with right amygdala connectivity with the left superior frontal gyrus (SFG; F3,351=8.27, xyz=−20,14,48, k=56, Figure 2). Post-hoc analyses indicated a significant negative association between irritability and right amygdala connectivity in response to fearful faces (r=−0.33, p<0.001, corrected) and a significant positive association between irritability and right amygdala connectivity with sad faces (r=−0.25, p<0.05, corrected). Post-hoc partial correlations were not significant for other face emotion conditions. Clusters that emerged for other contrasts are listed in Table 2.
Figure 2. Irritability × Emotions Interaction is Associated with Right Amygdala Connectivity with Left Superior Frontal Gyrus.
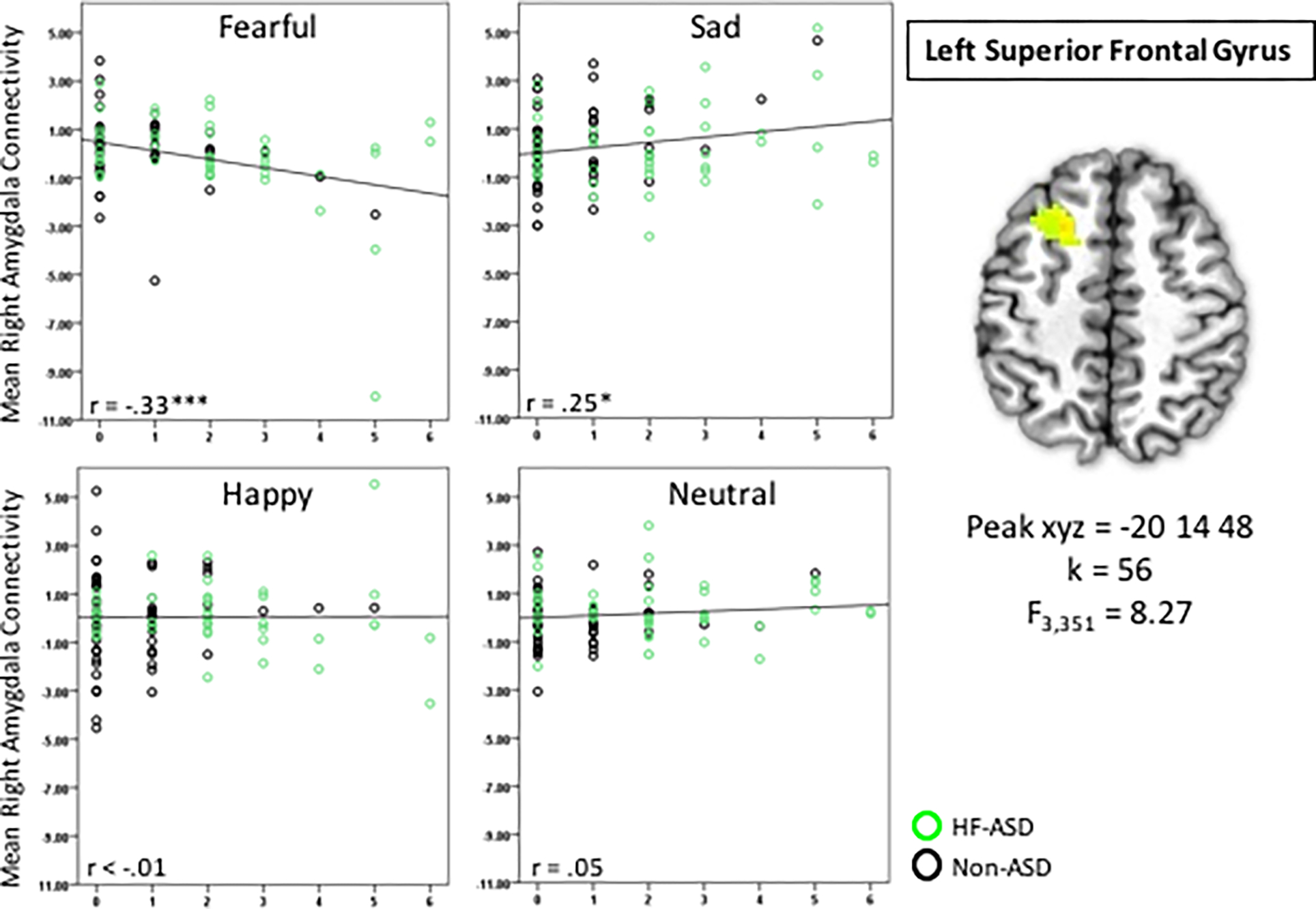
Partial correlation coefficients are shown. * p<0.01, corrected, ***p<0.001, corrected
Associations of Irritability with Left Amygdala Functional Connectivity
The Irritability × Emotion interaction was associated with left amygdala connectivity with the bilateral anterior cingulate cortex (ACC)/MFG (F3,351=11.30, xyz=−5,38,24, k=1294), the right IFG (F3,351=9.15, xyz=62,14,21, k=30, Supplemental Figure S1), and the right precentral gyrus (F3,351=8.14, xyz=50,−2,40, k=29) (Table 2). Inspection of the extracted mean connectivity values for these clusters revealed that the results were outlier driven (i.e., driven by connectivity values >3 standard deviations from the mean; see Supplement 1 for outlier analyses). Additionally, follow-up analyses (see section below) revealed that that the bilateral ACC/MFG and right precentral gyrus clusters are no longer significant when controlling for potential confounding variables (see Supplement 1 for details). Overall, left amygdala connectivity results are therefore less robust and are not further discussed further in the main manuscript. Clusters that emerged for other contrasts are listed in Table 2.
Additional Analyses
After taking the potential impact of age, gender, anxiety, depression, core ASD symptoms (social communication impairment), task accuracy, and psychotropic medication usage into account, main results for neural activation and right amygdala connectivity remain significant (see Supplement 1 for additional details).
Discussion
The present study focused on examining the neural mechanisms of implicit emotion processing in youth across the irritability spectrum by adopting an RDoC-based dimensional perspective on irritability in a sample of youths enriched for irritability by including youths with HF-ASD. As hypothesized, we found that both neural activation and amygdala connectivity differed as a function of irritability level and face emotion in the prefrontal areas, suggesting that the underlying neural mechanisms of face emotion processing vary with level of irritability. These findings are largely consistent with the literature,24,25 as outlined below, and are significant above and beyond diagnostic category as well as core ASD symptoms (i.e., social communication impairment), indicating that these irritability mechanisms may be common to both typically developing and HF-ASD youths.
Our whole-brain activation analyses revealed that youths with higher irritability levels have decreased activation in response to both fearful and happy faces in the left middle frontal gyrus and to happy faces in the left inferior frontal gyrus. These findings are consistent with previous studies investigating the neural mechanisms of face emotion processing in irritable youths. For example, deactivation of prefrontal areas in response to implicit processing of fearful faces was demonstrated in youths with severe mood dysregulation compared to non-irritable youths.25 Another study evaluating how neural mechanisms of threat bias differ based on severity of irritability symptoms found that higher irritability was associated with aberrant activation in the prefrontal cortex when youths were to attend away from a threatening face.24 Aberrant activation in frontal areas in response to emotional faces may therefore represent deficits in face emotion interpretation, which may underlie irritability.
Our amygdala connectivity findings implicated amygdala-prefrontal cortex connectivity, not amygdala-ventral visual area connectivity as in a prior study.17 Moreover, we did not find amygdala activation differences with varying levels of irritability, unlike other findings in irritable youths.19,25,39 This may be due to differences in task stimuli/design. Unlike our gender identification face paradigm, tasks in studies that found amygdala activation effects varied intensity of emotional faces19,25 or had participants judge nose width, which did not require attention to the entire face.39
Although more research is needed to consolidate differential findings, the fact that similar brain regions (i.e., prefrontal cortex, prefrontal-amygdala connectivity) emerged in our two orthogonal whole-brain analyses (activation and amygdala connectivity) is corroborating evidence that these regions are involved in irritability. That is, altered activation in the prefrontal cortex to fearful and happy faces as well as altered amygdala-prefrontal connectivity to fearful and sad faces, all in relation to irritability, suggest that the amygdala and the prefrontal cortex play an important role in face emotion mechanisms of irritability. Moreover, it is interesting to note that across both orthogonal analyses, prefrontal circuitry was implicated in response to fearful faces (i.e., decreased activation and amygdala connectivity with greater levels of irritability). Fearful faces, like angry faces, can signal a threat in the environment40 and may thus elicit high-arousal, hostile responses in those prone to irritability.
Overall, the regions that emerged as significant in the present study have been linked to brain circuits associated with executive functions41 and emotion regulation, such as reappraisal of negative emotions.42 Irritability symptoms have likewise been linked to deficits in executive functioning in childhood.43 In the present study, youths with higher irritability levels demonstrated decreased recruitment of these regions when viewing happy and fearful faces, which could be a marker for weaker engagement of the self-regulation circuit in response to the faces. Amygdala-prefrontal connectivity has been shown to be related to successful emotion regulation, with increased connectivity between the amygdala and the inferior frontal gyrus related to down-regulation of emotions.44 The dysregulation of amygdala connectivity with the inferior frontal gyrus among youths with higher irritability symptoms may represent deficits in emotion regulation in response to fearful and sad faces. Recent evidence that mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity45 suggests that this may not only be a mechanism of irritability but also a potential intervention route for irritable youths. In addition, our findings that prefrontal recruitment relates to irritability symptoms suggests that youths with increased irritability may have difficulty recruiting prefrontal areas to appropriately interpret social stimuli. Indeed, the inferior frontal gyrus and medial prefrontal cortex have also been implicated in social cognition46 and are likely related to interpretation of social information. As youths with irritability have difficulty labeling emotional faces,20 this may lead to difficulty and frustration in interpreting social cues, and thus lead to irritability.47
In our study, the inclusion of an HF-ASD group not only enriched the sample for irritability but also aligns with Research Domain Criteria efforts to redefine categorical diagnostic categories based on dimensionally measured symptom manifestations by evaluating the neural underpinnings of the irritability dimension across diagnostic categories.13 Of note, such dimensional conceptualizations may be more sensitive than traditional diagnostic group-based comparisons. For example, other studies comparing high-irritable to non-irritable diagnostic groups generally failed to detect neural alterations in response to happy faces during implicit face tasks;25 by contrast, we found differences in relation to happy faces when irritability is captured dimensionally in the model. This dimensional approach of conceptualizing psychopathology may be promising as characterization of mechanism can reach beyond what are often arbitrary diagnostic boundaries and thus be more accurate. Moreover, understanding the dimensional features of irritability that expand across diagnostic boundaries may aid in intervention and prevention efforts that can be applied across a wide range of diagnoses.
We note several limitations of this study. First, the non-ASD participants in this study did not undergo full diagnostic screening and it is therefore possible that some subjects met diagnostic criteria for psychopathology. However, the primary focus of this study was to capture dimensionally-measured irritability levels, rather than diagnostic categorization. Additionally, to control for potential effects of affective symptomatology, our results were adjusted for depression and anxiety, also measured dimensionally, across all subjects. Second, a more rigorous approach to controlling for confounding variables would have been to add them to the second-level fMRI analyses; however, to retain power and avoid collinearity, we chose a post-hoc method to control for confounding variables. Third, although our measure of irritability, like all Likert-style measures, is an ordinal scale, we modeled it continuously to be consistent with prior studies. A true “continuous” measure (i.e., where each step increase reflects exactly the same increase in irritability rather than “0=Not True”, “1=Somewhat or Sometimes True”, “2=Very True or Often True” for each item) would better capture the dimension, yet such a scale does not yet exist for irritability. Future psychometric work improving measures must go hand in hand with neuroscientific advances to best move the field forward. Fourth, despite controlling for differences between youths with and without ASD diagnosis and adjusting for social communication impairment symptom severity, residual confounding factors may have remained. There may be pathophysiological mechanisms of irritability that are unique to youths with ASD. The focus of the current study was to evaluate neural mechanisms of irritability above and beyond ASD symptoms, and we did not additionally focus on comparing HF-ASD and non-ASD youths on neural mechanisms of the irritability dimension due to inherent differences in variance of irritability in the two diagnostic groups. However, we included these analyses, which have limited interpretability, in Supplement 1. Furthermore, despite enriching our sample by including youth with HF-ASD to increase the variability in irritability, lower levels of irritability were still more prominently represented compared to higher levels which may have introduced effects of heteroscedasticity in our analyses. Fifth, because our paradigm did not include angry faces, our study is less directly comparable to the many studies that investigated irritability in response to angry faces. However, our inclusion of other emotional faces (i.e., fearful, happy, and sad), which all showed involvement in irritability symptom mechanisms, contributes to the literature as many studies had looked at responses to angry faces exclusively. Finally, we did not use eye-tracking during fMRI acquisition and cannot rule out that eye fixation patterns differed between samples and influenced our results.48 However, these differences were likely minimized due to the very brief stimulus presentation (250ms), which limited opportunities for multiple saccades, and having participants identify the gender of the face, which required them to look at the entire face.
Our findings suggest that alterations in neural processing of face emotion may contribute to irritability symptoms. Understanding the neural mechanisms of pediatric irritability may be leveraged for future intervention development. Our findings and the research program for which they pave the way are important because interventions targeting irritability during adolescence may not only help us improve the wellbeing of children, but also help us prevent long term sequelae in adulthood. Prevention-focused interventions for irritable adolescents that target implicit emotion processing may be effective. Computer-based behavioral interventions,23 mindfulness approaches,45 and emerging treatments such as neurofeedback49 may be promising approaches.
The present study is one of the first to evaluate the neural mechanisms of irritability from an RDoC-based dimensional perspective; nevertheless, to document the full dimension of irritability and to assess generalizability across other diagnoses on the irritability spectrum, future work with other diagnoses will be necessary. As youth with ASD are included in the future transdiagnostic studies of irritability symptoms, it will be important to examine the overlap of symptoms, such as rigidity, with irritability. Furthermore, the present study explored emotional face processing related to varying levels of irritability, yet because there are documented changes in irritability levels across development50 as well as maturation of the emotional networks of the brain,51 future longitudinal studies may wish to follow youths with varying levels of irritability to assess for changes in neural patterns as the youths develop.
Supplementary Material
Acknowledgments:
We thank the families for participating. We also gratefully acknowledge Richard Reynolds, M.S. of the Scientific and Statistical Computing Core, National Institute of Mental Health, National Institutes of Health, and Jill Weisberg, Ph.D. of San Diego State University, for their assistance in setting up the data processing pipeline. We thank Catherine Lord, Ph.D. of the University of California, Los Angeles, for her support in the recruitment of youths with HF-ASD, and Nader Amir, Ph.D. and Lisa Eyler, Ph.D. of the San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology for helpful comments on an earlier version of this manuscript. We also thank Yonca Cam, B.A. of San Diego State University, for her assistance with preprocessing.
Funding:
This work was supported by an Autism Speaks Predoctoral Fellowship (4773 to J.L.W.) and grant (2573 to C.S.M.).
Contributor Information
Maria Kryza-Lacombe, San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology.
Natalia Iturri, Department of Psychology, San Diego State University..
Christopher S. Monk, Department of Psychology, University of Michigan.
Jillian Lee Wiggins, San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology and the Department of Psychology, San Diego State University.
References
- 1.Brotman MA, Kircanski K, Stringaris A, Pine DS, Leibenluft E. Irritability in Youths: A Translational Model. Am J Psychiatry. Jun 01 2017;174(6):520–532. [DOI] [PubMed] [Google Scholar]
- 2.Collishaw S, Maughan B, Natarajan L, Pickles A. Trends in adolescent emotional problems in England: a comparison of two national cohorts twenty years apart. J Child Psychol Psychiatry. Aug 2010;51(8):885–894. [DOI] [PubMed] [Google Scholar]
- 3.Stringaris A Irritability in children and adolescents: a challenge for DSM-5. Eur Child Adolesc Psychiatry. Feb 2011;20(2):61–66. [DOI] [PubMed] [Google Scholar]
- 4.McGuire K, Fung LK, Hagopian L, et al. Irritability and Problem Behavior in Autism Spectrum Disorder: A Practice Pathway for Pediatric Primary Care. Pediatrics. Feb 2016;137 Suppl 2:S136–148. [DOI] [PubMed] [Google Scholar]
- 5.Hameed U, Dellasega CA. Irritability in Pediatric Patients: Normal or Not? Prim Care Companion CNS Disord. 2016;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibenluft E, Stoddard J. The developmental psychopathology of irritability. Dev Psychopathol. Nov 2013;25(4 Pt 2):1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fichter MM, Kohlboeck G, Quadflieg N, Wyschkon A, Esser G. From childhood to adult age: 18-year longitudinal results and prediction of the course of mental disorders in the community. Soc Psychiatry Psychiatr Epidemiol. Sep 2009;44(9):792–803. [DOI] [PubMed] [Google Scholar]
- 8.Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. Sep 2009;166(9):1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickles A, Aglan A, Collishaw S, Messer J, Rutter M, Maughan B. Predictors of suicidality across the life span: the Isle of Wight study. Psychol Med. Sep 2010;40(9):1453–1466. [DOI] [PubMed] [Google Scholar]
- 10.Copeland WE, Shanahan L, Egger H, Angold A, Costello EJ. Adult diagnostic and functional outcomes of DSM-5 disruptive mood dysregulation disorder. Am J Psychiatry. Jun 2014;171(6):668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibenluft E Irritability in children: what we know and what we need to learn. World Psychiatry. Feb 2017;16(1):100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brotman MA, Kircanski K, Leibenluft E. Irritability in Children and Adolescents. Annu Rev Clin Psychol. May 8 2017;13:317–341. [DOI] [PubMed] [Google Scholar]
- 13.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. Mar 2012;14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. May 14 2013;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayes SD, Calhoun SL, Murray MJ, Ahuja M, Smith LA. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Res Autism Spect Dis. Jan-Mar 2011;5(1):474–485. [Google Scholar]
- 16.Griffiths S, Jarrold C, Penton-Voak IS, Woods AT, Skinner AL, Munafo MR. Impaired Recognition of Basic Emotions from Facial Expressions in Young People with Autism Spectrum Disorder: Assessing the Importance of Expression Intensity. J Autism Dev Disord. Mar 31 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoddard J, Tseng WL, Kim P, et al. Association of Irritability and Anxiety With the Neural Mechanisms of Implicit Face Emotion Processing in Youths With Psychopathology. JAMA Psychiatry. Jan 01 2017;74(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins JL, Brotman MA, Adleman NE, et al. Neural Correlates of Irritability in Disruptive Mood Dysregulation and Bipolar Disorders. Am J Psychiatry. Jul 01 2016;173(7):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas LA, Brotman MA, Muhrer EJ, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Arch Gen Psychiatry. Dec 2012;69(12):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. Sep 2007;48(9):863–871. [DOI] [PubMed] [Google Scholar]
- 21.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. Jan 2013;52(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng SJ, Carrasco M, Swartz JR, et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. Mar 2011;52(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoddard J, Sharif-Askary B, Harkins EA, et al. An Open Pilot Study of Training Hostile Interpretation Bias to Treat Disruptive Mood Dysregulation Disorder. J Child Adolesc Psychopharmacol. Feb 2016;26(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kircanski K, White LK, Tseng WL, et al. A Latent Variable Approach to Differentiating Neural Mechanisms of Irritability and Anxiety in Youth. JAMA Psychiatry. Apr 6 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas LA, Kim P, Bones BL, et al. Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. Neuroimage Clin. Jan 1 2013;2:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Soc Cogn Affect Neurosci. Jun 2014;9(6):832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. Apr 2014;171(4):395–397. [DOI] [PubMed] [Google Scholar]
- 28.Peterson BS, Zhang H, Santa Lucia R, King RA, Lewis M. Risk factors for presenting problems in child psychiatric emergencies. J Am Acad Child Adolesc Psychiatry. Sep 1996;35(9):1162–1173. [DOI] [PubMed] [Google Scholar]
- 29.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. Jun 2000;30(3):205–223. [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. Oct 1994;24(5):659–685. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins JL, Bedoyan JK, Carrasco M, Swartz JR, Martin DM, Monk CS. Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Hum Brain Mapp. Feb 2014;35(2):646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringaris A, Zavos H, Leibenluft E, Maughan B, Eley TC. Adolescent irritability: phenotypic associations and genetic links with depressed mood. Am J Psychiatry. Jan 2012;169(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiggins JL, Mitchell C, Stringaris A, Leibenluft E. Developmental trajectories of irritability and bidirectional associations with maternal depression. J Am Acad Child Adolesc Psychiatry. Nov 2014;53(11):1191–1205, 1205 e1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms and profiles. Vol 30: Burlington, VT: University of Vermont, Research center for children, youth, & families; 2000. [Google Scholar]
- 35.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. Aug 15 2009;168(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dannlowski U, Ohrmann P, Bauer J, et al. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. Jan 15 2007;154(1):13–20. [DOI] [PubMed] [Google Scholar]
- 37.American Psychological Association. Diagnostic and statistical manual of mental disorders. 5 ed. Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- 38.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. Apr 2017;7(3):152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. Jan 2010;167(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z, Gendron M, Liu Q, Zhao G, Li H. Trait Anxiety Impacts the Perceived Gaze Direction of Fearful But Not Angry Faces. Front Psychol. 2017;8:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sylvester CY, Wager TD, Lacey SC, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41(3):357–370. [DOI] [PubMed] [Google Scholar]
- 42.Vanderhasselt MA, Kuhn S, De Raedt R. ‘Put on your poker face’: neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Soc Cogn Affect Neurosci. Dec 2013;8(8):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granvald V, Marciszko C. Relations between key executive functions and aggression in childhood. Child Neuropsychol. 2016;22(5):537–555. [DOI] [PubMed] [Google Scholar]
- 44.Morawetz C, Bode S, Baudewig J, Heekeren HR. Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci. Apr 1 2017;12(4):569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doll A, Holzel BK, Mulej Bratec S, et al. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage. Jul 1 2016;134:305–313. [DOI] [PubMed] [Google Scholar]
- 46.Adolfi F, Couto B, Richter F, et al. Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex. Mar 2017;88:124–142. [DOI] [PubMed] [Google Scholar]
- 47.Deveney CM, Connolly ME, Haring CT, et al. Neural mechanisms of frustration in chronically irritable children. Am J Psychiatry. Oct 2013;170(10):1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klin A Three things to remember if you are a functional magnetic resonance imaging researcher of face processing in autism spectrum disorders. Biol Psychiatry. Oct 1 2008;64(7):549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramot M, Kimmich S, Gonzalez-Castillo J, et al. Direct modulation of aberrant brain network connectivity through real-time NeuroFeedback. Elife. Sep 16 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Copeland WE, Brotman MA, Costello EJ. Normative Irritability in Youth: Developmental Findings From the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. Aug 2015;54(8):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casey BJ, Heller AS, Gee DG, Cohen AO. Development of the emotional brain. Neurosci Lett. Dec 1 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


