Abstract
The epidemic and pandemic potential of Vibrio cholerae O139 is such that a vaccine against this newly emerged serogroup of V. cholerae is required. A conjugate made of the polysaccharide moiety (O-specific polysaccharide plus core) of the lipopolysaccharide (LPS) of V. cholerae O139 (pmLPS) was prepared by derivatization of the pmLPS with adipic acid dihydrazide and coupling to tetanus toxoid (TT) by carbodiimide-mediated condensation. The immunologic properties of the conjugate were tested using BALB/c mice injected subcutaneously three times at 2 weeks interval and then a fourth time 4 weeks later. Mice were bled 7 days after each injection and then once each month for the following 6 months. LPS and TT antibody levels were determined by enzyme-linked immunosorbent assay using immunoplates coated with either O139 LPS or TT. Both pmLPS and pmLPS-TT conjugate elicited low levels of immunoglobulin M (IgM), peaking 5 weeks after the first immunization. The conjugate elicited high levels of IgG antibodies, peaking 3 months after the first immunization and declining slowly during the following 5 months. TT alone, or as a component of conjugate, induced mostly IgG antibodies. Antibodies elicited by the conjugate recognized both capsular polysaccharide and LPS from V. cholerae O139 and were vibriocidal. They were also protective in the neonatal mouse model of cholera infection. The conjugation of the O139 pmLPS, therefore, enhanced its immunogenicity and conferred T-dependent properties to this polysaccharide.
Since the appearance of Vibrio cholerae O139 in the suburb of Madras, India, in October 1992, epidemic cholera caused by this strain has spread rapidly throughout the Indian subcontinent (1). Clinical illness associated with V. cholerae O139 infection appears to be virtually identical to that due to V. cholerae O1 E1 Tor infections. However, in contrast to infection with V. cholerae O1, V. cholerae O139 infection has largely affected the adult population in areas of V. cholerae O1 endemicity, indicating a lack of protective immunity against this newly evolved strain (1). Presumably, there are differences between the immune responses against O1 and O139 strains, which may be of considerable importance in terms of protection (33). A quiescent period followed the appearance of V. cholerae O139, and it was thought that it was a one-time event. However, there was an upsurge of cases in Calcutta, India, in 1996, and the O139 serogroup again became the dominant serogroup causing cholera in India by September 1996 (32). The O139 serogroup has remained present in India and Bangladesh since this last outbreak (15) and requires careful monitoring.
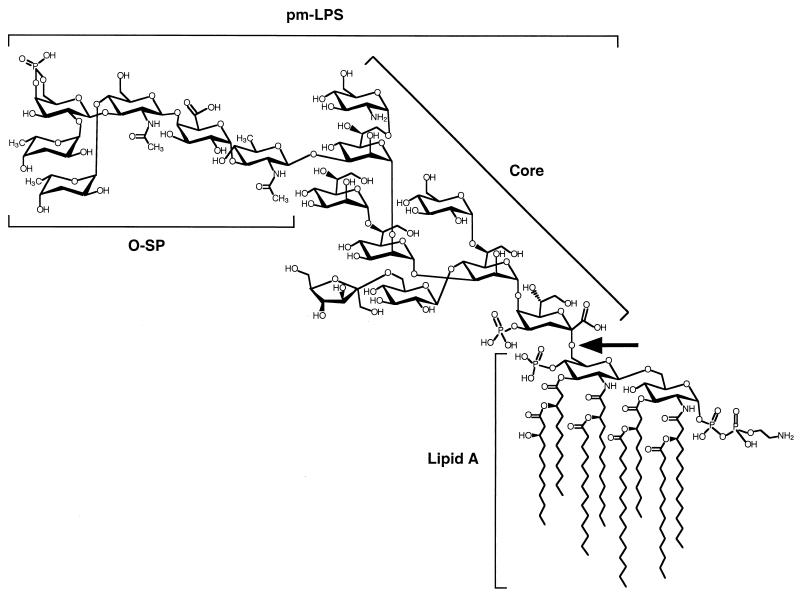
It has been suggested that the emergence of V. cholerae O139 is the result of a complex chromosomal rearrangement involving the horizontal transfer of genes encoding enzymes involved in O-specific polysaccharide (O-SP) biosynthesis (3, 8, 14, 43). Indeed, the major differences between V. cholerae O1 and V. cholerae O139 reside in their cell surface components. V. cholerae O139, unlike V. cholerae O1, expresses capsular polysaccharide (CP) (43, 46). Both the structure of the CP and that of the lipopolysaccharide (LPS) from V. cholerae O139 have been characterized (Fig. 1) (11, 12, 28, 36). Although, O139 LPS and CP share the same repeat unit, only the CP is polymerized (12). Nevertheless, CP and LPS share common epitopes (43).
FIG. 1.
Overall structure of the LPS of V. cholerae O139. The O-SP and the core structure are taken from Cox et al. (11, 12) and the lipid A structure is arranged according to Kabir (26) and Wilkinson (48). The arrow indicates the lipid A-core bond hydrolyzed by acetic acid treatment: this treatment releases the polysaccharide moiety (O-SP plus core) of the LPS (pmLPS; molecular weight, 2,701).
Several oral cholera vaccines, either inactivated or live attenuated, have been developed to elicit protection against this new serogroup of V. cholerae (10, 23, 40, 44). Various subcellular fractions of V. cholerae O139 administered subcutaneously have been evaluated in the rabbit ileal loop model of experimental cholera, and the immune response directed against the O139 serogroup antigen appeared to be determinant for protective immunity (4). It has been proposed that serum immunoglobulin G (IgG) antibodies (Abs) confer protection against enteric diseases by inactivating the inoculum on the mucosal surfaces (38). Systemic administration of IgG Abs specific for the O-SP of V. cholerae O1 was found to protect neonatal mice against loss of weight and death following intragastral challenge with V. cholerae O1 (5). A V. cholerae O139 CP-tetanus toxoid (TT) conjugate vaccine induced protection in the rabbit ileal loop model of experimental cholera (24). More recently, V. cholerae O139 CP conjugated with a recombinant mutant diphtheria toxin was shown to elicit high levels of serum anti-CP IgG in mice with vibriocidal activity (30). These results encourage the development of vaccines based on polysaccharide-protein conjugate to prevent cholera (16, 17).
In this study, we synthesized a conjugate prepared with the polysaccharide moiety (O-SP plus core) of the LPS (pmLPS) from V. cholerae O139 bound to TT. The synthesis, characterization, and immunologic properties in mice of this conjugate were assessed.
Preparation and characterization of LPS, pmLPS, and CP.
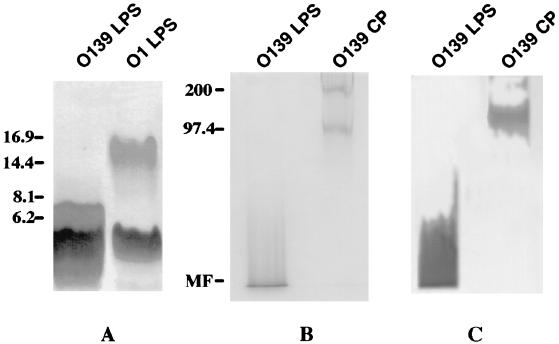
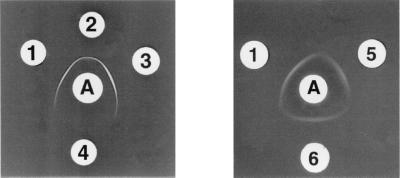
V. cholerae O139 (strain MO45, kindly provided by Y. Takeda, Kyoto University, Kyoto, Japan) was grown on tryptic soy agar (Difco) at 37°C for 18 h. LPS was obtained by hot phenol-water extraction (47), followed by enzymatic treatment (DNase, RNase, and protease) and ultracentrifugation. The pellet containing the LPS had 0.5% (wt/vol) protein and less than 0.2% (wt/vol) nucleic acid. LPS was treated with acetic acid to hydrolyze the lipid A-core linkage (Fig. 1) (19). The resulting product is referred to as pmLPS. For the preparation of CP, LPS was removed from the ultracentrifugation supernatant by passage through a Sephacryl S-200 column in a buffer containing deoxycholic acid (37). Void volume fractions containing CP, detected by refractive index and sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) in gels treated with Alcian blue (a cationic dye that binds acidic polysaccharides) prior to silver staining (9), were dialyzed extensively against 10% (vol/vol) ethanol to remove deoxycholic acid (37). The LPS had 2 × 104 endotoxin units/μg, and the pmLPS had 10 endotoxin units/μg as assessed by the Limulus amebocyte lysate assay (21). This reduction by a factor of 2,000 is consistent with previous data (16, 42). LPS from V. cholerae O139 gave two dense silver-stained bands (41) in Tricine–SDS–16.5% PAGE (31) with Mrs of approximately 4,000 and 6,200 (Fig. 2A). LPS from V. cholerae O1 gave two bands with Mrs of 4,000 and 15,000 (Fig. 2A). This is consistent with the observation that O139 O-SP has 1 hexasaccharide unit (12) whereas O1 O-SP has 12 to 18 repeating monosaccharide units (27). In SDS–10% PAGE (Fig. 2B), in gels treated with Alcian blue prior to silver staining, O139 LPS gave one band with a smear at the bottom of the gel and O139 CP gave two bands with Mrs of 100,000 and 200,000, consistent with the polymerized structure of this polysaccharide (28, 36). Both V. cholerae O139 LPS and CP were recognized by an anti-O139 hyperimmune mouse serum in immunoblotting experiments (Fig. 2C). This is consistent with the observation that O139 O-SP shares an epitope with O139 CP (43). This hyperimmune mouse serum did not react with V. cholerae O1 LPS under the same conditions (data not shown). Monoclonal Abs (MAbs), prepared as previously described (6), were screened by enzyme-linked immunosorbent assay (ELISA) against purified O139 LPS and checked for specificity by immunoblot analysis against O139 and O1 LPS, and by agglutination with V. cholerae O139 and O1 bacterial cells. Clone B-16-5, IgM class, was selected for its high avidity to O139 pmLPS and O139 CP, as determined by ELISA inhibition. Double-immunodiffusion assay showed a single band of precipitate between LPS, pmLPS, CP, and the B-16-5 MAb (Fig. 3). That pmLPS yielded a line of identity with LPS suggests that the O139-specific antigenic determinant was preserved during the purification of the pmLPS. No cross-reactivity was observed with LPS from V. cholerae O1 serotype Inaba. The 1H and 31P nuclear magnetic resonance (NMR) spectra of the pmLPS, recorded on a Bruker AC 300P spectrometer, were identical to those previously reported (not shown) (11). The 1H NMR spectrum confirmed the absence of small organic molecules.
FIG. 2.
Analysis of polysaccharide preparations of V. cholerae O139. (A) Tricine–SDS–16.5% PAGE. The gel was stained with silver. (B) SDS–10% PAGE. The gel was pretreated with Alcian blue, a cationic dye that binds acidic polysaccharides, prior to silver staining. (C) Immunoblot analysis with hyperimmune O139 mouse antiserum as the probe. Mrs are shown on the left. MF, migration front.
FIG. 3.
Double immunodiffusions. A, MAb anti-LPS O139. 1, pmLPS O139; 2, LPS O139; 3, CP O139; 4, LPS O1; 5, derivatized pmLPS O139; 6, pmLPS-TT.
Preparation and characterization of conjugate.
pmLPS was derivatized with adipic acid dihydrazide (ADH) (7, 22, 29). The derivatized pmLPS (pmLPS-AH) was bound to TT (Pasteur-Mérieux, Marcy-1'Etoile, France) with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC). The reaction mixture was separated by gel permeation chromatography through CL-6B Sepharose. TT was detected by measuring optical density at 280 nm, and polysaccharide was measured by determining the refractive index. The conjugate eluting in the void volume fractions was collected and pooled. The hydrazide content of pmLPS-AH was measured by the trinitrobenzene sulfonic acid assay, using ADH as a standard (18). Hexose concentrations were measured by a colorimetric method based on the anthrone reaction, using pmLPS as a standard (20). The extent of derivatization of the activated pmLPS was calculated as the ADH/polysaccharide ratio and was 5.2% (mol/mol). For the conjugate, the pmLPS/protein (wt/wt) ratio was 1.90%, corresponding to a 0.99 mol/mol ratio. The yield was 9.6%, as calculated by the ratio of the amount of the saccharide in the conjugate to the initial amount of derivatized polysaccharide. In double-immunodiffusion assays, MAb B-16-5 gave a line of identity with pmLPS, derivatized pmLPS and TT-pmLPS, suggesting that the O139 antigenic determinant common to O-SP and CP was preserved during the conjugation of the pmLPS (Fig. 3).
Anti-O139 and anti-TT Abs response of mice.
Six-week-old female BALB/c mice were injected subcutaneously with 2.5 μg of pmLPS O139 alone, or as a conjugate, as described in the footnote of Table 1. A group of mice was immunized similarly with 2.5 μg of TT. LPS and TT Ab levels were determined by ELISA. Plates were coated with either LPS or TT. Serial twofold dilutions of mouse sera (1/100 to 1/6,400) were analyzed. The secondary Abs used were either peroxidase-conjugated anti-mouse IgG (γ chain specific) or IgM (μ chain specific). The results were calculated for each immunoglobulin class, as a percent of a high-titered reference serum arbitrarily assigned a value of 100 ELISA units by parallel line analysis with a program from the Centers for Disease Control and Prevention and expressed as the geometric mean (35). Following the same method, anti-TT Ab level was expressed with respect to a hyperimmune mouse pooled standard serum prepared in the laboratory by repeated immunizations of mice with TT. Serum anti-O139 Ab titers are shown in Table 1. Preimmune sera and phosphate-buffered saline control sera contained no detectable levels of Abs. After the second immunization, pmLPS elicited a moderate IgM response and a very weak IgG response, consistent with the response induced by a T-independent antigen. After the third immunization with pmLPS-TT, IgM titers were equivalent to those elicited in response to pmLPS. After the fourth immunization, pmLPS-TT elicited an IgG response very much higher than that of pmLPS (P = 0.0011), lasting at least 231 days (P = 0.0046). This IgG response demonstrates a booster effect and an immunoglobulin isotype switch. This strongly suggests that the pmLPS was functionally converted, due to the protein carrier effect, into a T-dependent antigen. In inhibition ELISA (42), the binding of anti-pmLPS-TT antibodies to O139 LPS was inhibited by either O139 LPS or O139 CP (concentration of antigen yielding 50% inhibition, 8 or 1 μg/ml, respectively). Serum anti-TT Ab titers are shown in Table 1. Preimmune sera contained no detectable levels of anti-TT Abs. After the third immunization, pmLPS-TT elicited a significant increase in anti-TT IgG levels (P < 0.01), similar to that in mice immunized with TT alone (data not shown).
TABLE 1.
ELISA titers of serum anti-LPS and anti-TT Abs elicited in mice following immunization with pmLPS alone or as a conjugatea
| Dayb | Geometric mean ELISA titer (25th–75th percentiles of:
|
Pc | ||||
|---|---|---|---|---|---|---|
| Anti-TT IgG with pmLPS-TT | Anti-LPS IgM with:
|
Anti-LPS IgG with:
|
||||
| pmLPS | pmLPS-TT | pmLPS | pmLPS-TT | |||
| 0* | <1 | <1 | <1 | <1 | <1 | NSd |
| 7 | <1 | 2.8 (2.3–3.5) | 1.8 (1.5–2.2) | 1.4 (1.3–1.5) | 1.2 (0.9–1.6) | NS |
| 14* | ||||||
| 21 | 9.3 (5.3–12.2) | 7.9 (4.8–14.2) | 6.2 (5.4–8.8) | <1 | 1 (0.8–1) | NS |
| 28* | ||||||
| 35 | 61.9 (52.8–109.5) | 17.5 (7.5–41.8) | 17.7 (12.3–24.3) | <1 | 1.8 (0.9–4.6) | NS |
| 56* | ||||||
| 63 | 193.5 (155.9–219.2) | 6.5 (3.6–11.4) | 8.1 (6.2–9) | 2 (1.2–4.9) | 11.7 (8.1–15.3) | 0.0011 |
| 91 | 280.5 (204.1–454.8) | 4.2 (3.1–6.8) | 7.5 (5.7–8.8) | <1 | 36.3 (11.7–67.3) | 0.0357 |
| 119 | 191.3 (156.4–308.8) | 4.4 (2.5–6.9) | 2.8 (2–3.5) | 1.2 (1.1–3.3) | 21.4 (7.3–45.8) | 0.0249 |
| 152 | 209.5 (181.1–295.3) | 4.2 (2.9–6.4) | 12.7 (10.1–15.3) | 2.5 (2.1–3.1) | 29.9 (14.8–78.3) | 0.0131 |
| 190 | 192.2 (147.9–277.9) | 4.6 (3.5–6.6) | 7.2 (6–9.5) | 1.3 (1.1–2.2) | 30.6 (15.7–72.3) | 0.0055 |
| 231 | 183e (144.4–249.5) | 4.5 (3.1–6.5) | 6.3e (4.5–9.3) | <1 | 23.8e (12.3–60.6) | 0.0046 |
Ten mice were injected subcutaneously with saline solutions containing 2.5 μg of the antigen three times at 2-weeks interval and were then given a fourth injection 4 weeks later. The mice were bled 7 days after each injection and then again each month for 6 months after the fourth injection.
Days of immunization are marked with an asterisk.
Comparison of titers of anti-LPS IgG elicited by pmLPS-TT versus pmLPS (P values are calculated by Student's t test).
NS, not significant.
Nine mice were tested.
Vibriocidal Abs response.
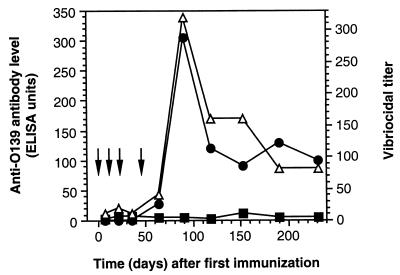
The vibriocidal tests were performed as previously described (5) with twofold dilutions (beginning with an initial 1:10 dilution) using V. cholerae O139 strain MO10-T4, a spontaneous nonencapsulated variant of MO10 (43), kindly provided by A. Weintraub (Karolinska Institute, Huddinge, Sweden), as the target strain and guinea pig serum as the source of complement. The vibriocidal titer was defined as the reciprocal of the highest dilution of serum causing 100% bacterial lysis. Controls for each assay included, in addition to the usual cell control and complement control, a positive hyperimmune control serum with a titer of 1/2,560. Consecutive sera of one mouse immunized with pmLPS-TT were tested for vibriocidal activity (Fig. 4). There was a correlation between the kinetics of the vibriocidal Ab titer and the anti-O139 IgG level (correlation coeficient = 0.89). Findings for sera from other mice immunized with pmLPS-TT supported this correlation.
FIG. 4.
Time course of amounts of IgM (■) and IgG (●) anti-O139 Abs and O139 vibriocidal Ab titer (Δ) in the serum of a single mouse immunized four times (arrows) with pmLPS-TT.
Protective activity of anti-pmLPS-TT Abs.
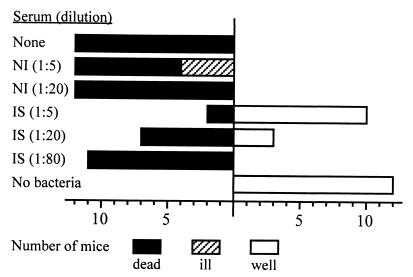
Suckling Swiss mice 5 days old and weighing 3.3 to 4.4 g were used for oral challenge experiments with V. cholerae O139. A V. cholerae O139 strain, isolated in 1992 from a patient in India and selected for its capacity to produce high levels of cholera toxin (5 μg/ml), was used for oral challenge in mice. After removing secreted cholera toxin, a dose of 3.5 × 108 V. cholerae cells (10 times the 50% lethal dose), preincubated for 30 min at 37°C with immune serum at various dilutions in 0.1 ml, was delivered into the stomach with a blunt-tip feeding needle. Groups of mice that received vibrio suspension alone, phosphate-buffered saline alone, or vibrio suspension with nonimmunized-mouse serum served as controls. Mice were maintained at 30°C for 48 h or until death, and all surviving mice were scored as well or ill at 48 h. Mice were considered ill if they met all of the following criteria: diarrhea, markedly reduced skin turgor, and poor response to stimuli. Mice that received pooled immune sera, collected on days 152 and 231 from mice immunized with pmLPS-TT, diluted 1:5, were significantly protected (Fig. 5). The level of protection decreased as the dilution of the pooled immune sera increased: protection was therefore dependent on dose. No protection was observed in mice that received pooled nonimmune control sera.
FIG. 5.
Protective activity of anti-pmLPS-TT Abs against challenge with 10 times the 50% lethal dose of V. cholerae O139 in the suckling-mouse model. NI, pooled non immune sera; IS, pooled immune sera obtained on days 152 and 231 from mice immunized with pmLPS-TT. Health status was scored 48 h after challenge.
Discussion.
The emergence in 1992 of a new V. cholerae strain assigned to the serogroup O139 was an unprecedented change in the history of cholera. The epidemic and pandemic potential of V. cholerae O139 poses a serious threat to developing countries, and a vaccine against this novel strain is therefore required. The absence of cross-protection between V. cholerae O1 and V. cholerae O139 serogroups, documented in rabbits either immunized with live bacteria (2) or passively protected with sera of convalescent cholera patients (33), suggested that protection against cholera is LPS specific. This is supported by the correlation observed between the protective effect of rabbit O139 antisera and anti-LPS Ab titers (25).
Accordingly, we designed a conjugate vaccine to elicit anti-O139 Abs in mice and studied the immunologic properties of these Abs. As observed in many LPSs from various gram-negative bacteria, the V. cholerae pmLPS is attached to the lipid A portion of the molecule through 3-deoxy-d-manno-octulosonic acid (Kdo) (12, 48). This bond is cleaved by mild acid hydrolysis (Fig. 1) to release a polysaccharide bearing a Kdo residue at its reducing end (22). The use of the carboxylic group of the Kdo moiety for polysaccharide-protein coupling results in a saccharide with a single terminal active site for conjugation. This single-end activated pmLPS has a high potential for use as a vaccine: (i) the O139 specific antigenic determinant(s) are conserved; (ii) it is the simplest conjugate configuration in which polysaccharide chains radiate from the protein carriers; (iii) the coupling procedure is the easiest to control, producing well-defined non-cross-linked, water-soluble conjugate molecules of known configuration (22).
It has been shown that phenol-water extraction of capsulated bacteria yields a mixture of LPS and CP in the aqueous phase (11, 28, 37, 46). To confirm the effective separation of LPS from CP after further purification steps, these two types of cell surface polysaccharide were identified by Tricine–SDS-PAGE using differential staining. Only the rapidly migrating material, corresponding to LPS, was silver stained, but the slowly migrating forms of the O139 antigen were not. This result is consistent with the previous observation that O139 CP is not stained with silver (34). It is thought that the silver staining of polysaccharides depends on the presence of periodate-sensitive cis-hydroxyl groups in the monosaccharide residues (9). Thus, as the O139 CP repeating unit, unlike the LPS core, lacks cis-hydroxyls (11, 12, 28, 36) it is not silver stained. However, this CP, which is acidic, is stained by the cationic dye Alcian blue.
The derivatization ratio of pmLPS, an essential step in our coupling procedure, was lower than usual with other polysaccharides (13, 16). Nevertheless, the polysaccharide/protein ratio (0.99 mol/mol) obtained herein was sufficient for a strong IgG response in immunized mice. The unconjugated pmLPS elicited mostly IgM Abs, whereas only low levels of IgG anti-LPS Abs were detected. This response was similar to those previously reported for polysaccharides tested in mice (45). In contrast, the pmLPS-TT conjugate elicited mostly IgG anti-LPS Abs, which were boosted following reimmunization. Moreover, after the fourth immunization, a high level of these IgG Abs was maintained for 5 months. We found that pmLPS-TT had typical T-dependent properties. Similar results have been obtained with O-SP from several other enteric bacterial pathogens (7, 29).
Interestingly, Abs obtained in mice immunized with pmLPS conjugated to TT recognized both O-SP and CP purified from V. cholerae O139. This result is entirely consistent with the observation that CP and LPS share a common epitope(s) expressed by a common hexasaccharide unit (12, 43). This cross-reactivity between O139 pmLPS and CP accounts for our finding that pmLPS-TT Abs reacted with both encapsulated and nonencapsulated V. cholerae O139 strains and is consistent with observations that protection against V. cholerae O139 can be mediated by Abs directed against either the LPS or CP of this novel cholera vibrio (24, 25, 33, 34, 39). Our results demonstrate the efficiency of a conjugated pmLPS in eliciting an IgG response in mice and justify clinical evaluation of this V. cholerae O139 conjugate.
Acknowledgments
We thank S. Ughetto-Monfrin, Unité de Chimie Organique, Institut Pasteur, for performing the NMR experiments and S. Dartevelle and A. Guénolé for expert technical assistance. We are grateful to L. Mulard and I. Kansau Silva for critical reading of the manuscript.
REFERENCES
- 1.Albert M J. Vibrio cholerae O139 Bengal. J Clin Microbiol. 1994;32:2345–2349. doi: 10.1128/jcm.32.10.2345-2349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M J, Alam K, Rahman A S M H, Huda S, Sack R B. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J Infect Dis. 1994;169:709–710. doi: 10.1093/infdis/169.3.709. [DOI] [PubMed] [Google Scholar]
- 3.Berche P, Poyart C, Abachin E, Lelievre H, Vandepitte J, Dodin A, Fournier J M. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis. 1994;170:701–704. doi: 10.1093/infdis/170.3.701. [DOI] [PubMed] [Google Scholar]
- 4.Bondre V P, Sinha V B, Srivastava B S. Evaluation of different subcellular fractions of Vibrio cholerae O139 in protection to challenge in experimental cholera. FEMS Immunol Med Microbiol. 1998;19:323–329. doi: 10.1111/j.1574-695X.1997.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 5.Bougoudogo F, Vely F, Nato F, Boutonnier A, Gounon P, Mazié J C, Fournier J M. Protective activities of serum immunoglobulin G on the mucosal surface to Vibrio cholerae O1. Bull Inst Pasteur. 1995;93:273–283. [Google Scholar]
- 6.Boutonnier A, Nato F, Bouvet A, Lebrun L, Audurier A, Mazié J C, Fournier J M. Direct testing of blood cultures for detection of the serotype 5 and 8 capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1989;27:989–993. doi: 10.1128/jcm.27.5.989-993.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu C Y, Liu B K, Watson D, Szu S S, Bryla D, Shiloach J, Schneerson R, Robbins J B. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock L E, Johnson J A, Michalski J M, Morris J G, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 9.Corzo J, Pérez-Galdona R, Léon-Barrios M, Gutiérrez-Navarro A M. Alcian Blue fixation allows silver staining of the isolated polysaccharide component of bacterial lipopolysaccharides in polyacrylamide gels. Electrophoresis. 1991;12:439–441. doi: 10.1002/elps.1150120611. [DOI] [PubMed] [Google Scholar]
- 10.Coster T S, Killeen K P, Waldor M K, Beattie D T, Spriggs D R, Kenner J R, Trofa A, Sadoff J C, Mekalanos J J, Taylor D N. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;345:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 11.Cox A D, Brisson J R, Varma V, Perry M B. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:43–58. doi: 10.1016/0008-6215(96)00135-8. [DOI] [PubMed] [Google Scholar]
- 12.Cox A D, Perry M B. Structural analysis of the O-antigen-core region of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:59–65. doi: 10.1016/0008-6215(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 13.Devi S J N, Hayat U, Frasch C E, Kreger A S, Morris J G. Capsular polysaccharide-protein conjugate vaccines of carbotype 1 Vibrio vulnificus: construction, immunogenicity, and protective efficacy in a murine model. Infect Immun. 1995;63:2906–2911. doi: 10.1128/iai.63.8.2906-2911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumontier S, Berche P. Vibrio cholerae O22 might be a putative source of exogenous DNA resulting in the emergence of the new strain of Vibrio cholerae O139. FEMS Microbiol Lett. 1998;164:91–98. doi: 10.1111/j.1574-6968.1998.tb13072.x. [DOI] [PubMed] [Google Scholar]
- 15.Faruque S M, Siddique A K, Saha M N, Asadulghani, Rahman M M, Zaman K, Albert M J, Sack D A, Sack R B. Molecular characterization of a new ribotype of Vibrio cholerae O139 Bengal associated with an outbreak of cholera in Bangladesh. J Clin Microbiol. 1999;37:1313–1318. doi: 10.1128/jcm.37.5.1313-1318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R K, Szu S C, Finkelstein R A, Robbins J B. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect Immun. 1992;60:3201–3208. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R K, Taylor D N, Bryla D A, Robbins J B, Szu S S C. Phase 1 evaluation of Vibrio cholerae O1, serotype Inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect Immun. 1998;66:3095–3099. doi: 10.1128/iai.66.7.3095-3099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habeeb A F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 19.Hancock I C, Poxton I R. Appendix 1. General methods. In: Hancock I C, Poxton I R, editors. Bacterial cell surface techniques. Chichester, United Kingdom: John Wiley & Sons; 1988. pp. 269–286. [Google Scholar]
- 20.Herbert D, Phipps P J, Strange R E. Chemical analysis of microbial cells. Methods Microbiol. 1971;5B:209–344. [Google Scholar]
- 21.Hochstein H D. Role of the FDA in regulating the Limulus amoebocyte lysate test. In: Prior R B, editor. Clinical applications of the Limulus amoebocyte lysate test. Boca Raton, Fla: CRC Press; 1990. pp. 38–49. [Google Scholar]
- 22.Jennings H J, Sood R K. Synthetic glycoconjugates as human vaccines. In: Lee Y C, Lee R T, editors. Neoglycoconjugates: preparation and applications. San Diego, Calif: Academic Press; 1994. pp. 325–371. [Google Scholar]
- 23.Jertborn M, Svennerholm A M, Holmgren J. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine. 1996;14:1459–1465. doi: 10.1016/s0264-410x(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J A, Joseph A, Morris J G. Capsular polysaccharide-protein conjugate vaccines against Vibrio cholerae O139 Bengal. Bull Inst Pasteur. 1995;93:285–290. [Google Scholar]
- 25.Jonson G, Osek J, Svennerholm A M, Holmgren J. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun. 1996;64:3778–3785. doi: 10.1128/iai.64.9.3778-3785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabir S. Characterization of the lipopolysaccharides from Vibrio cholerae 395 (Ogawa) Infect Immun. 1982;38:1263–1272. doi: 10.1128/iai.38.3.1263-1272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenne L, Lindberg B, Unger P, Gustasfson B, Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr Res. 1982;100:341–349. doi: 10.1016/s0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- 28.Knirel Y A, Paredes L, Jansson P E, Weintraub A, Widmalm G, Albert M J. Structure of the capsular polysaccharide of Vibrio cholerae O139 synonym Bengal containing D-galactose 4,6-cyclophosphate. Eur J Biochem. 1995;232:391–396. doi: 10.1111/j.1432-1033.1995.391zz.x. [DOI] [PubMed] [Google Scholar]
- 29.Konadu E, Robbins J B, Shiloach J, Bryla D A, Szu S C. Preparation, characterization, and immunological properties in mice of Escherichia coli O157 O-specific polysaccharide-protein conjugate vaccines. Infect Immun. 1994;62:5048–5054. doi: 10.1128/iai.62.11.5048-5054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kossaczka Z, Shiloach J, Johnson V, Taylor D N, Finkelstein R A, Robbins J B, Szu S C. Vibrio cholerae O139 conjugate vaccines: synthesis and immunogenicity of V. cholerae O139 capsular polysaccharide conjugates with recombinant diphtheria toxin mutant in mice. Infect Immun. 2000;68:5037–5043. doi: 10.1128/iai.68.9.5037-5043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay A K, Basu A, Garg P, Bag P K, Ghosh A, Bhattacharya S K, Takeda Y, Nair G B. Molecular epidemiology of reemergent Vibrio cholerae O139 Bengal in India. J Clin Microbiol. 1998;36:2149–2152. doi: 10.1128/jcm.36.7.2149-2152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandy R K, Albert M J, Ghose A C. Serum antibacterial and antitoxin responses in clinical cholera caused by Vibrio cholerae O139 Bengal and evaluation of their importance in protection. Vaccine. 1996;14:1137–1142. doi: 10.1016/0264-410x(96)00035-7. [DOI] [PubMed] [Google Scholar]
- 34.Nandy R K, Mukhopadhyay S, Ghosh A N, Ghose A C. Antibodies to the truncated (short) form of ‘O’ polysaccharides (TFOP) of Vibrio cholerae O139 lipopolysaccharides protect mice against experimental cholera induced by encapsulated O139 strains and such protection is mediated by inhibition of intestinal colonization of vibrios. Vaccine. 1999;17:2844–2852. doi: 10.1016/s0264-410x(99)00097-3. [DOI] [PubMed] [Google Scholar]
- 35.Plikaytis B D, Holder P F, Carlone G M. Program ELISA for Windows user's manual, version 1.00. Atlanta, Ga: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 36.Preston L M, Xu Q W, Johnson J A, Joseph A, Maneval D R, Husain K, Reddy G P, Bush C A, Morris J G. Preliminary structure determination of the capsular polysaccharide of Vibrio cholerae O139 Bengal Al1837. J Bacteriol. 1995;177:835–838. doi: 10.1128/jb.177.3.835-838.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins J B, Schneerson R, Szu S C. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta D K, Boesman-Finkelstein M, Finkelstein R A. Antibody against the capsule of Vibrio cholerae O139 protects against experimental challenge. Infect Immun. 1996;64:343–345. doi: 10.1128/iai.64.1.343-345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 41.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 42.Villeneuve S, Boutonnier A, Mulard L A, Fournier J M. Immunochemical characterization of an Ogawa-Inaba common antigenic determinant of Vibrio cholerae O1. Microbiology. 1999;145:2477–2484. doi: 10.1099/00221287-145-9-2477. [DOI] [PubMed] [Google Scholar]
- 43.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Kabat E. Carbohydrate antigens (polysaccharides) In: Van Regenmortel M H V, editor. Structure of antigens. Vol. 3. New York, N.Y: CRC Press; 1996. pp. 247–276. [Google Scholar]
- 46.Weintraub A, Widmalm G, Jansson P E, Jansson M, Hultenby K, Albert M J. Vibrio cholerae O139 Bengal possesses a capsular polysaccharide which may confer increased virulence. Microb Pathog. 1994;16:235–241. doi: 10.1006/mpat.1994.1024. [DOI] [PubMed] [Google Scholar]
- 47.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol-water and further applications of procedure. In: Wistler R L, editor. Methods in carbohydrates chemistry. Vol. 5. New York, N.Y: Academic Press Inc.; 1965. pp. 83–91. [Google Scholar]
- 48.Wilkinson S G. Bacterial lipopolysaccharides: themes and variations. Prog Lipid Res. 1996;35:283–343. doi: 10.1016/s0163-7827(96)00004-5. [DOI] [PubMed] [Google Scholar]