Abstract
Exposure to pneumolysin (8.37 and 41.75 ng/ml) caused a calcium-dependent increase in the generation of prostaglandin E2 and leukotriene B4 by both resting and chemoattractant-activated human neutrophils in vitro. These interactions of pneumolysin with neutrophils may result in dysregulation of inflammatory responses during pneumococcal infection.
Although pneumolysin has been reported to increase the activity of phospholipase A2 in both neutrophils (4) and pulmonary endothelial cells (9), the effects of the toxin on the production of arachidonic acid-derived mediators of inflammation have not been described. In the current study, we have investigated the effects of recombinant pneumolysin on the production of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) by human neutrophils in vitro.
Recombinant pneumolysin was expressed in Escherichia coli and purified from cell extracts as previously described (10). Protein homogeneity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The stock toxin protein concentration was 0.21 mg/ml, which corresponds to 1.3 × 106 hemolytic units/ml, and the stock was essentially free of contaminating bacterial endotoxin (<2 pg/ml). The toxin was diluted in endotoxin-free Hanks' balanced salt solution (HBSS [pH 7.4]; 1.25 mM CaCl2; Highveld Biological Pty. Ltd., Johannesburg, South Africa), which was also added to the pneumolysin-free control systems described below, and used at fixed final concentrations of 8.37 and 41.75 ng/ml. Under the experimental conditions used in the present study, pneumolysin at these concentrations possesses either no (8.37 ng/ml) or minimal (41.75 ng/ml) cytotoxicity (4).
Purified neutrophils were prepared from heparinized (5 U of preservative-free heparin/ml) venous blood of healthy adult volunteers as described previously (4) by centrifugation of blood on Histopaque-1077 (Sigma Diagnostics, St. Louis, Mo.) to separate neutrophils from mononuclear leukocytes, followed by removal of erythrocytes using gelatin (3%) sedimentation and selective lysis with 0.84% ammonium chloride. The neutrophils were routinely of high purity (>90%) and viability (>95%). These cells (2 × 106) in HBSS were subjected to preincubation at 37°C for 10 min, followed by addition of pneumolysin (8.37 and 41.75 ng/ml [final concentrations]) or an equal volume (100 μl) of HBSS (control systems). The final volume in each tube was 1 ml. For systems in which the cells were activated with the synthetic chemotactic tripeptide N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μM [final concentration]; Sigma Chemical Co.), a preincubation period of 5 min at 37°C was used, after which pneumolysin was added, followed 5 min later by FMLP. In a preliminary kinetic experiment using cells from a single donor and incubation times of 1, 3, 5, and 10 min after the addition of pneumolysin only or FMLP with or without the toxin, PGE2 and LTB4 were detectable in the supernatants of pneumolysin-treated neutrophils with or without FMLP at 1 min and reached a plateau at 3 to 5 min (data not shown). For this reason, a fixed incubation time of 5 min was used for a series of subsequent experiments designed to investigate the effects of pneumolysin on the production of PGE2 and LTB4 by FMLP-treated and untreated neutrophils from 10 different donors.
After incubation, the reactions were terminated by the addition of 1 ml of ice-cold HBSS to each tube and the tubes were transferred to an ice bath. The tubes were then centrifuged to pellet the cells, and PGE2 and LTB4 in the supernatants were assayed by competitive binding radioimmunoassay procedures (Du Pont NEN Research Products, Boston, Mass.). The results are expressed as nanograms per 107 cells.
In an additional series of experiments using cells from three different donors, the extracellular calcium-chelating agent EGTA (5 mM [final concentration]) was added to the cells 5 s prior to the pneumolysin.
The results of each series of experiments are expressed as the mean ± the standard error of the mean (SEM). Levels of statistical significance were calculated by the Mann-Whitney U test (two tailed), and statistical significance was assigned a P value of <0.05.
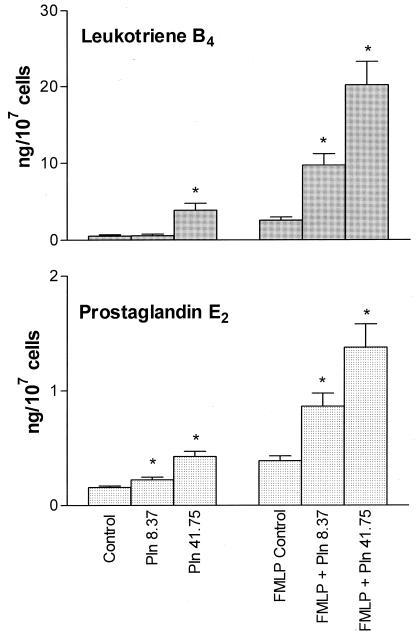
The effects of pneumolysin on the production of PGE2 and LTB4 are shown in Fig. 1. Exposure of neutrophils to pneumolysin in the absence of FMLP increased the production of both PGE2 and LTB4, which, in the case of PGE2, was statistically significant at both concentrations of the toxin, but only at the highest concentration for LTB4. FMLP per se significantly (P < 0.05) increased the production of both PGE2 and LTB4 by neutrophils, an effect which was strikingly and significantly (P < 0.05) potentiated by pretreatment of the cells with pneumolysin at both 8.37 and 41.75 ng/ml.
FIG. 1.
Effects of pneumolysin (Pln; 8.37 and 41.75 ng/ml) on the production of LTB4 and PGE2 by unstimulated and FMLP (1 μM)-activated neutrophils. The results of 9 (PGE2) or 10 (LTB4) experiments using cells from different donors are expressed as the mean ± the SEM. Asterisks indicate P < 0.05 for comparison with the appropriate pneumolysin-free control systems.
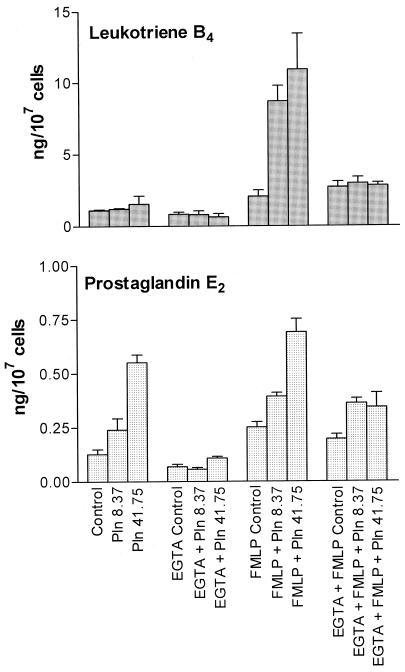
The effects of removal of extracellular Ca2+ by treatment of the cell-suspending medium with EGTA (5 mM) on the production of PGE2 and LTB4 by pneumolysin-treated neutrophils with or without FMLP are shown in Fig. 2. EGTA completely attenuated the pneumolysin-mediated enhancement of the production of PGE2 and LTB4 by FMLP-treated and untreated neutrophils but did not affect the production of these bioactive lipids in control systems with or without FMLP in the absence of pneumolysin. In the presence of EGTA, the pneumolysin-treated systems both with and without FMLP did not differ significantly from the corresponding pneumolysin-free control systems in PGE2 and LTB4 production.
FIG. 2.
Effects of EGTA (5 mM) on pneumolysin (Pln; 8.37 and 41.75 ng/ml)-mediated enhancement of production of LTB4 and PGE2 by unstimulated and FMLP (1 μM)-activated neutrophils. The results of two (PGE2) or three (LTB4) experiments using cells from different donors are expressed as the mean ± the SEM.
We have previously reported that pneumolysin at concentrations similar to those used in the present study, which had either no or minimal effects on cellular energy metabolism and viability, augmented the activity of phospholipase A2 in neutrophils (4). In the current study, most likely as a consequence of its enhancing effects on phospholipase A2, pneumolysin caused a dose-related increase in the production of both PGE2 and LTB4 by human neutrophils. Although this was evident with the toxin per se, it was most striking with the combination of pneumolysin and FMLP, which is most likely to mimic the situation at sites of inflammation.
Pneumolysin-mediated augmentation of the proinflammatory activities of human neutrophils is achieved by a pore-forming mechanism resulting in influx of extracellular Ca2+ (4). In the current study, inclusion of the Ca2+-chelating agent EGTA in the cell-suspending medium completely attenuated the pneumolysin-mediated enhancement of PGE2 and LTB4 production by neutrophils, probably by preventing Ca2+-dependent activation of phospholipase A2 (4, 9).
Receptor-mediated proadhesive, chemotactic, secretory, and pro-oxidative interactions of LTB4 with phagocytes, particularly neutrophils, have been well documented (3), and potentiation of these proinflammatory activities by pneumolysin may contribute to hyperacute inflammation during pneumococcal infection (7). Pneumolysin-mediated augmentation of the production of PGE2 by neutrophils may also contribute to dysregulated inflammatory responses by increasing vascular permeability, thereby facilitating influx of proinflammatory polypeptides, as well as by delaying neutrophil apoptosis (8).
In addition to augmentation of the production of proinflammatory cytokines, reactive oxidants, including nitric oxide, proteolytic enzymes, and adhesion molecules (2, 4, 6), data from the current study demonstrate that pneumolysin also increases the production of the proinflammatory lipids PGE2 and LTB4 by human neutrophils. Acting in concert these various proinflammatory agents may promote hyperacute inflammation during infection with Streptococcus pneumoniae. The relationship between the proinflammatory activities of pneumolysin and microbial persistence is more complex, with several possible scenarios. Exaggerated inflammatory responses may favor accelerated eradication of the microbial pathogen. Alternatively, hyperactivation of phagocytes may result in premature auto-oxidative inactivation of their protective functions (1), as well as inhibition of the proliferative responses of neighboring lymphocytes (5), favoring microbial persistence.
REFERENCES
- 1.Baehner R L, Boxer L A, Allen J M, Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977;50:327–335. [PubMed] [Google Scholar]
- 2.Braun J S, Novak R, Gao G, Murray P J, Shenep J L. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect Immun. 1999;67:3750–3756. doi: 10.1128/iai.67.8.3750-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleasson H E, Dahlén S-E. Asthma and leukotrienes: antileukotrienes as novel antiasthmatic drugs. J Int Med. 1999;245:205–227. doi: 10.1046/j.1365-2796.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 4.Cockeran R, Theron A J, Steel H C, et al. Proinflammatory interactions of pneumolysin with human neutrophils. J Infect Dis. 2001;183:604–611. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 5.El-Hag A, Lipsky P E, Bennett M, Clark R A. Immunomodulation by neutrophil myeloperoxidase and hydrogen peroxide: differential susceptibility of human lymphocyte functions. J Immunol. 1986;139:2406–2413. [PubMed] [Google Scholar]
- 6.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin 1 β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadioglu A, Gingles N A, Grattan K, Kerr A, Mitchell T J, Andrew P W. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68:492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi A G, Cousin J M, Dransfield I, Lason M F, Chilvers E R, Haslett C. Agents that elevate CAMP inhibit neutrophil apoptosis. Biochem Biophys Res Commnn. 1995;217:892–899. doi: 10.1006/bbrc.1995.2855. [DOI] [PubMed] [Google Scholar]
- 9.Rubins J B, Mitchell T J, Andrew P W, Niewoehner D E. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect Immun. 1994;62:3829–3836. doi: 10.1128/iai.62.9.3829-3836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders F K, Mitchell T W, Walker J A, Andrew P W, Boulnois G J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989;57:2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]