Abstract
Background/purpose
Ethylenediaminetetraacetic acid (EDTA) is used as an irrigant in regenerative endodontic treatment. The present study aimed to investigate the effects of EDTA on stem cells from apical papilla (SCAPs) in vitro.
Materials and methods
Human SCAPs were isolated and characterised. The cells were treated with media supplemented with EDTA at concentrations ranging from 1.25% to 17%. Cell proliferation and apoptosis were examined using MTT assay and annexin V/propidium iodide staining. Cell migration was determined by a scratch assay. Gene expression was evaluated using a real-time polymerase chain reaction. Mineral deposition, a hallmark of osteogenesis in vitro, was determined using alizarin red s staining.
Results
Overall, SCAPs exhibited mesenchymal stem cell characteristics. EDTA treatment at 2.50% and 1.25% did not significantly exhibit cytotoxicity and alter cell morphology. However, EDTA attenuated cell proliferation and reduced MKI67 mRNA expression in SCAPs. Further, EDTA significantly induced early cell apoptosis at 48 h. Cell migration was delayed with EDTA treatment. After maintaining SCAPs in an osteogenic induction medium, EDTA diminished mineral deposition by SCAPs on day 14.
Conclusion
EDTA treatment exhibits adverse effects on SCAPs in vitro. Hence, EDTA exposure to periapical tissues should be avoided to minimise the negative impacts on SCAPs cells in regenerative processes.
Keywords: EDTA, Stem cells from the apical papilla, Cell proliferation, Cell migration, Cell apoptosis, Osteogenic differentiation
Introduction
Regenerative endodontics is currently a valid treatment option for necrotic immature teeth.1 Numerous publications indicate the benefit and success of regenerative endodontic procedures as determined by the continued root formation and increase in dentin thickness.1 Various root canal irrigation regimens and final irrigants have been utilised.2 EDTA at the concentration of 17% is recommended as the canal irrigant between each root canal treatment appointment and before creating a blood clot inside the canal space.3 The use of EDTA results in the release of bioactive molecules from dentin, influencing stem cell migration and differentiation in the root canal in vitro.4 EDTA irrigation removes the smear layer and conditions dentin surfaces.5,6 The exposed collagen after EDTA treatment could further modulate cell functions, including odonto/osteogenic differentiation.6
Stem cells from apical papilla (SCAPs) reside in apical papilla tissues. These cells can differentiate into odontoblast-like cells and form dentin/pulp complex in vivo.7 SCAPs can survive root canal infection even though total pulp degeneration usually occurs.8,9 These cells have been speculated to be the primarily responsible cells in dentin/pulp regeneration following the regenerative endodontic procedure.9 The proliferation, migration, and differentiation abilities of SCAPs play a crucial role in dental pulp tissue regeneration following regenerative endodontics. The effects of several irrigants, including 17% EDTA in SCAPs, are reported. 17% EDTA exposure promotes more cell survival than other irrigants.5,10 Hence, the present study aimed to investigate further the direct effect of different EDTA concentrations on cell proliferation, apoptosis, migration, and differentiation of SCAPs in vitro.
Materials and methods
Cell isolation and culture
All experimental protocols were approved by the Human Research Ethical Committee, Faculty of Dentistry, Chulalongkorn University (approval No. 031/2020). All methods were carried out in accordance with the Declaration of Helsinki guidelines. Informed consent was obtained from all participants and/or legal guardian(s).
Apical papilla tissues from the immature impacted third molars scheduled for surgical removal according to the patient's treatment plan were collected for cell isolation using the cell explantation technique.11 The isolated cells were maintained in Dulbecco modified Eagle medium (DMEM cat. No. 11960, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (cat. No.10270, Gibco), 2 mM l-glutamine (GlutaMAX™−1, cat. No. 35050, Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B (Antibiotic-Antimycotic, cat. No. 15240, Gibco) at 37 °C in a humidified atmosphere of 5% CO2. According to previous protocols, stem cell characteristics were analysed by cell surface marker expression and differentiation ability toward osteogenic and adipogenic lineages.11,12 For EDTA treatment, based on previous publications, cells were exposed to EDTA at different concentrations (17%, 12%, 10%, 5%, 2.5% and 1.25% v/v)13,14 for 30 and 60 s and subsequently rinsed with SCAPs growth medium.
Cell viability assays
Cells (5 × 104 cells) were seeded in 24 well-plates and maintained in a growth medium. At designated time points, cells were incubated with MTT solution for 15 min, and the formed formazan crystals were dissolved using DMSO/glycine buffer. The solubilised dye was measured at the absorbance of 570 nm using a microplate reader.
Cell morphology assay
After EDTA exposure, cells were permeabilised with 0.1% Triton X-100 and stained with rhodamine-phalloidin (Invitrogen, Carlsbad, CA, USA) in 10% horse serum at 1:100 dilution. Nuclei were counterstained with DAPI at 1:1000 dilution. F-actin and nucleus orientation were examined using a fluorescence microscope.
Cell apoptosis assay
Cells (1 × 105 cells/well) were seeded in 6 well-plates and maintained in a growth medium. According to the manufacturer's protocol, cells were stained with Propidium Iodide/Annexin V staining (Roche Diagnostics, Mannheim, Germany) and further analysed using a FACSCalibur flow cytometer (BD Biosciences Pharmingen, San Diego, CA, USA).
Cell migration assay
Cells (1 × 105 cells/well) were seeded in 6 well-plates and maintained in a growth medium. A scratch assay was created by scratching the monolayer of cells. Subsequently, cells were treated with EDTA. Images of the wound area were captured at 24 and 48 h. The percentage of wound area was calculated using ImageJ software.
Gene expression assay
Total RNA was extracted with RiboEx™ solution (cat. No. 301-001, GeneAll®, Seoul, South Korea) and converted to cDNA using reverse transcriptase Improm-II ™ RT system kit (ImProm-II™, cat. No. A3800, Promega, Madison, WI, USA). A real-time polymerase chain reaction was performed in a real-time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) with SYBR® green detection system (FastStart™ Essential DNA Green Master; Roche Diagnostic). The primer sequences were MKI67 forward: 5′- CGTTTGTTTCCCCAGTGTCT-3'; reverse: 5′- CTCCCTGCCCCTTTCTATTC-3'; GAPDH forward: 5′-TCATGGGTGTGAACCATGAGAA-3’; reverse: 5′-GGCATGGACTGTGGTCATGAG-3’.
Mineral deposition assay
Cells were fixed with cold methanol for 10 min and further incubated with the alizarin red s solution (2% w/v) for 3 min. The deposited dye was solubilised with a 10% cetylpyridium chloride solution. The solubilised solution was measured at the absorbance of 570 nm using the same microplate ready as above.
Statistical analysis
Data were presented as mean ± standard error of the mean. Statistical analysis was performed using Prism version 8 software (GraphPad Software, San Diego, CA, USA). Mann Whitney U test was employed for two-group comparisons. Kruskal Wallis test followed by Dunnett post hoc test was utilised for three or more group comparisons. All experiments were repeated with cells from at least four donors. A statistically significant difference was considered when the P-value < 0.05.
Results
Stem cells characterisation
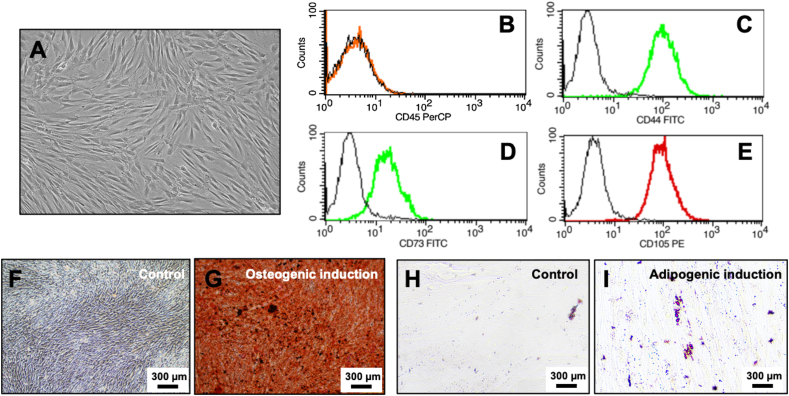
Isolated human cells exhibited spindle shape morphology and expressed CD44, CD73, and CD105 but lacked CD45 expression (Fig. 1A-E). After maintaining these cells in an induction medium, cells could differentiate into osteoblasts and adipocytes, as confirmed by the increase in mineral deposition (Fig. 1F and G) and intracellular lipid accumulation (Fig. 1H and I).
Figure 1.
Characterization of stem cells from apical papilla. (A) Morphology of the isolated cells. (B–E) Immunostaining of protein surface markers CD45, CD44, CD73, and CD105. (F–G) Mineralisation assay after osteogenic induction for 14 days. (H–I) Lipid accumulation assay after adipogenic induction for 16 days.
EDTA attenuated cell proliferation and promoted early apoptosis
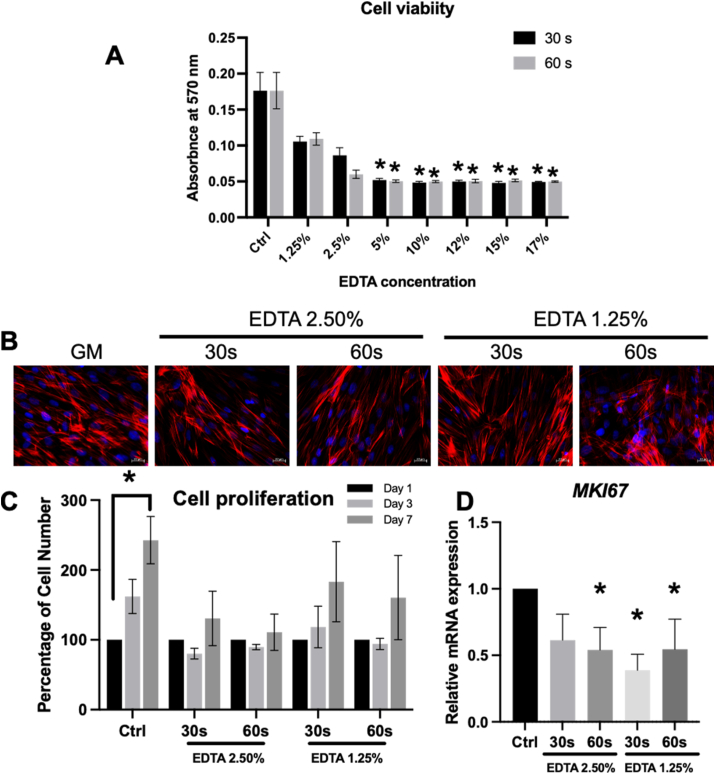
Cells were treated with EDTA at concentrations ranging from 1.25% to 17% for the 30s or 60s and subsequently maintained in a growth medium for 24 h. Cells treated with EDTA exhibited a significant decrease in cell viability compared with the control. However, there was no statistically significant difference when cells were treated with 1.25% and 2.5% EDTA (Fig. 2A). Cells treated with 1.25% and 2.5% EDTA appeared to have similar morphology to those of the control group while maintaining intact actin filaments at the cytoskeletal level (Fig. 2B). Hence, 1.25% and 2.5% EDTA were employed in further experiments from this point onwards.
Figure 2.
SCAPs were exposed to 2.5% and 1.25% EDTA for the 30 s and 60 s. (A) Cell viability assay at 24 h (B) Cell morphology evaluated by phalloidin staining at 24 h. (C) Cell proliferation assay on day 1, 3, and 7. (D) MKI67 mRNA expression was assessed by real-time polymerase chain reaction at 24 h. Asterisks indicated a statistically significant difference compared with the control (P < 0.05).
The proliferation ability was attenuated when cells were treated with 1.25% or 2.5% EDTA for the 30s and 60s (Fig. 2C). In the control condition, significantly increased cell numbers were observed from baseline to day 7. However, in EDTA-treated cells, a slight increase of SCAP numbers on day seven was noted but not markedly different from baseline on day 1. Congruently, SCAPs treated with 1.25% or 2.5% EDTA also showed a decrease in MKI67 mRNA expression compared to the control group (Fig. 2D).
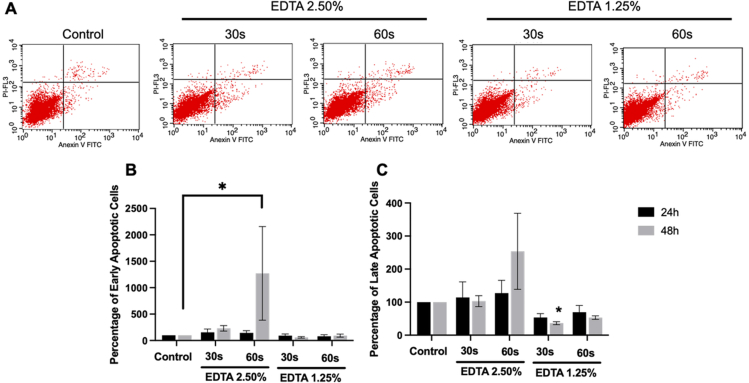
Next, SCAPs were treated with 1.25% or 2.5% EDTA for the 30s and 60s and subsequently maintained in a growth medium for apoptosis assessment. Cells treated with 2.5% EDTA for the 60s had a higher percentage of early and late apoptotic cells than control cells. However, only a significant difference was observed in the percentage of early apoptotic cells at 48 h (Fig. 3A-C).
Figure 3.
SCAPs were exposed to 2.5% and 1.25% EDTA for the 30 s and 60 s. Apoptosis assay was performed using Annexin V/PI staining at 24 h and 48 h. (A) Representative cytograms. (B–C) The graphs demonstrated the percentage of early and late apoptotic cells. Asterisks indicated a statistically significant difference compared with the control (P < 0.05).
EDTA attenuated cell migration and osteogenic differentiation
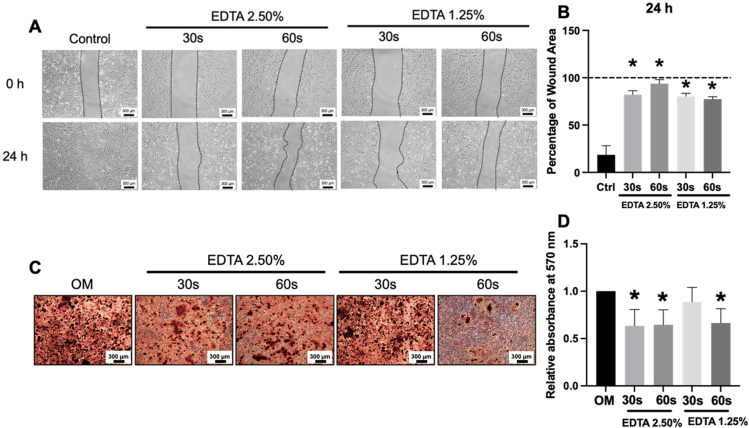
Cell migration into the “scratched wound” area was observed in the control conditions at 24 h. On the contrary, in all EDTA-treated SCAPs, delayed cell migration was noted (Fig. 4A). Thus, the percentage of wound area was significantly higher with EDTA treatments compared with the control (Fig. 4B).
Figure 4.
SCAPs were exposed to 2.5% and 1.25% EDTA for the 30 s and 60 s. Cell migration was examined using the scratch assay. (A) Representative images of cell migration at 0 h and 24 h. (B) The graphs demonstrated the percentage of the wound area. (C) Representative images of mineral deposition determined by alizarin red s staining. (D) The graphs showed the quantitative analysis of solubilised dye. Asterisks indicated a statistically significant difference compared with the control (P < 0.05). OM: osteogenic inductive medium condition; Ctrl: normal growth medium condition.
After maintaining SCAPs in an osteogenic induction medium for 14 days of culture, the mineral deposition was examined. The results demonstrated that EDTA treatment suppressed the mineralisation deposition process in SCAPs (Fig. 4C and D).
Discussion
Current clinical guidelines in regenerative endodontics are trending towards including cell-homing approaches. The laceration of periapical tissue lets progenitor cells from the periapical tissue migrate into the root canal system. SCAPs, which reside in the apical papilla, would migrate upward into the root canal and differentiate into dental pulp- and odontoblast-like cells. These cells play a crucial role in dentin/pulp regeneration. The present study described the adverse effects of EDTA on SCAPs. In this regard, EDTA attenuated cell proliferation and induced early apoptosis. Further, cell migration and osteogenic differentiation were compromised with EDTA regardless of the concentration. These in vitro effects of EDTA on SCAPs should be considered for clinical implications.
17% EDTA root canal irrigation alone or combined with other irrigants enhanced SCAPs in vitro survival compared to other groups.5,10 Dentin conditioning with 17% EDTA allowed human dental pulp stem cell proliferation similar to those treated with distilled water.15 However, the present study described the attenuative effects of EDTA on cell viability and proliferation of SCAPs. These distinctions might be ascribable to variations in methodology upon EDTA exposure to cells. For example, in previous studies, the root dentin was irrigated with EDTA and then allowed to dry before cell exposure.10 In Martin et al., cells were seeded in hyaluronic acid-based constructs before EDTA treatment.10 However, in our study, cells were directly exposed to EDTA in a monolayer culture system.
The present study showed that cell proliferation and MKI67 mRNA expression were attenuated in EDTA-treated conditions. MKI67 is detectable through the G1, S, G2, and M phases of the cell cycle.16 The expression levels were low in G1 and S phases and peaked in the M phase.16 Hence, the reduction of MKI67 expression indicates that the cell proliferation rate is attenuated. EDTA not only negatively affects cell proliferation but also influences cell apoptosis. The present study revealed that the percentage of early cell apoptosis was significantly increased in 2.5% EDTA treatment for the 60 s at 48 h time point. Our study suggests that EDTA treatment attenuated SCAPs cell proliferation and promoted apoptosis in vitro.
SCAPs cell migration was also decreased when cells were exposed to EDTA. However, another study in human dental pulp cells demonstrated that 12% EDTA treatment significantly enhanced stromal cell-derived factor-induced cell migration via transforming growth factor β and chemokine receptor 4.17 It should be noted that there were major differences between these two studies regarding cell types and migration assay techniques used, and the use of growth factor-induced migration.
EDTA-treated dentin discs promoted mineralisation by human dental pulp stem cells in vitro. The same irrigant drove bone/cartilage formation by murine-induced pluripotent stem cells adjacent to treated dentin in vivo.18 Direct 12% EDTA treatment increased osteogenic differentiation of human dental pulp cells in vitro and promoted dentin bridge formation in direct pulp capping in vivo canine models.13 However, this present study indicates a negative impact of EDTA on osteogenic differentiation in SCAPs after direct exposure, as shown by the decrease of mineral deposition ability. Despite the different cell types used in previous studies, comprehensive mechanisms related to cell signalling should be investigated in future in vitro research using EDTA treatment procedures.
When compared to clinical settings, it is essential to note the main differences in our findings, such as the absence of dentin and its components as well as the dimension of the cells exposed to root canal irrigants. The lack of dentinal components means that the effect of them being irrigated by EDTA generally attained from removing the smear layer exposes dentinal tubules leading to the release of growth factors such as transforming growth factor β (TGF-β), which were not involved in the present study in term of cell responses. Another notable distinction was that SCAPs in our study were treated in a monolayer culture system. In the clinical setting, SCAPs reside in the apical papilla tissues which have other components including the extracellular matrix. Hence, the way that SCAPs are actually exposed to the irrigant is much different from this in vitro study. Moreover, in standard clinical practice in endodontics, the root canal is usually treated with EDTA, dried with paper points and followed by blood clot creation in the root canal. Thus, this process could reduce the remaining EDTA in the canal after irrigation and also dilute the final EDTA concentration at the apex.
From a clinical point of view, the present study supported the current clinical consideration for the regenerative procedure,3 which encouraged the use of irrigation systems that minimise the chance of irrigant extrusion, for example, the use of the side-vents with a closed-ended needle, the proper position of irrigation needle, the use of gently irrigation force, the use of negative pressure irrigation system, and the appropriate use of irrigation activation with ultrasonic or nickel-titanium finishing rotary files. The root canal should be dried entirely after EDTA is used as a final rinse to avoid adverse effects on incoming SCAPs.
Despite the beneficial use of EDTA in clinical regenerative endodontic treatment procedures, EDTA herein exhibited a direct negative effect on cell proliferation, cell migration, and osteogenic differentiation in SCAPs in vitro. Hence, the clinical use of EDTA should be carried forward with caution. The flowing of EDTA liquid remnants into periapical tissue must be carefully avoided. The use of EDTA should be limited. Future ex vivo or in vivo effects of EDTA on SCAPs should be further clarified. Integrating a biological rationale into a clinical approach might accomplish the goals of regenerative endodontics.3
Declaration of competing interest
The authors have no competing interests relevant to this article.
Acknowledgement
This study was supported by the National Research Council of Thailand (N41A640135) and the Faculty Research Fund, Faculty of Dentistry, Chulalongkorn University. The author thanks Assistant Professor Joao N Ferreira for language editing.
Contributor Information
Nunthawan Nowwarote, Email: nunthawan.nowwarote@u-paris.fr.
Thanaphum Osathanon, Email: thanaphum.o@chula.ac.th.
References
- 1.Ong T.K., Lim G.S., Singh M., Fial A.V. Quantitative assessment of root development after regenerative endodontic therapy: a systematic review and meta-analysis. J Endod. 2020;46:1856–18566 e2. doi: 10.1016/j.joen.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Kontakiotis E.G., Filippatos C.G., Tzanetakis G.N., Agrafioti A. Regenerative endodontic therapy: a data analysis of clinical protocols. J Endod. 2015;41:146–154. doi: 10.1016/j.joen.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3.AAE clinical considerations for a regenerative procedure (Revised 5/18/2021) 2021. https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wp-content/uploads/REC062921.pdf Available at:
- 4.Hancerliogullari D., Erdemir A., Kisa U. The effect of different irrigation solutions and activation techniques on the expression of growth factors from dentine of extracted premolar teeth. Int Endod J. 2021;54:1915–1924. doi: 10.1111/iej.13589. [DOI] [PubMed] [Google Scholar]
- 5.Trevino E.G., Patwardhan A.N., Henry M.A., et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–1115. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K., Kawashima N., Ichinose S., et al. EDTA treatment for sodium hypochlorite-treated dentin recovers disturbed attachment and induces differentiation of mouse dental papilla cells. J Endod. 2018;44:256–262. doi: 10.1016/j.joen.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Na S., Zhang H., Huang F., et al. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J Tissue Eng Regen Med. 2016;10:261–270. doi: 10.1002/term.1686. [DOI] [PubMed] [Google Scholar]
- 8.Tobias Duarte P.C., Gomes-Filho J.E., Ervolino E., et al. Histopathological condition of the remaining tissues after endodontic infection of rat immature teeth. J Endod. 2014;40:538–542. doi: 10.1016/j.joen.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Diogenes A., Hargreaves K.M. Microbial modulation of stem cells and future directions in regenerative endodontics. J Endod. 2017;43:S95–S101. doi: 10.1016/j.joen.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Martin D.E., De Almeida J.F., Henry M.A., et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–55. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Damrongsri D., Nowwarote N., Sonpoung O., Photichailert S., Osathanon T. Differential expression of notch related genes in dental pulp stem cells and stem cells isolated from apical papilla. J Oral Biol Craniofac Res. 2021;11:379–385. doi: 10.1016/j.jobcr.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowwarote N., Osathanon T., Fournier B.P.J., et al. PTEN regulates proliferation and osteogenesis of dental pulp cells and adipogenesis of human adipose-derived stem cells. Oral Dis. 2021;24 doi: 10.1111/odi.14030. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Leng S., Tang L., et al. EDTA promotes the mineralization of dental pulp in vitro and in vivo. J Endod. 2021;47:458–465. doi: 10.1016/j.joen.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Galler K.M., Widbiller M., Buchalla W., et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J. 2016;49:581–590. doi: 10.1111/iej.12492. [DOI] [PubMed] [Google Scholar]
- 15.Deniz Sungur D., Aksel H., Ozturk S., Yilmaz Z., Ulubayram K. Effect of dentine conditioning with phytic acid or etidronic acid on growth factor release, dental pulp stem cell migration and viability. Int Endod J. 2019;52:838–846. doi: 10.1111/iej.13066. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J.P., Wang L.W., Qu A.P., et al. Quantum dots-based quantitative and in situ multiple imaging on ki67 and cytokeratin to improve ki67 assessment in breast cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Leng S., Yue J., et al. EDTA enhances stromal cell-derived factor 1 alpha-induced migration of dental pulp cells by up-regulating chemokine receptor 4 expression. J Endod. 2019;45:599–605 e1. doi: 10.1016/j.joen.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Ser-Od T., Al-Wahabi A., Inoue K., et al. Effect of EDTA-treated dentin on the differentiation of mouse iPS cells into osteogenic/odontogenic lineages in vitro and in vivo. Dent Mater J. 2019;38:830–838. doi: 10.4012/dmj.2018-161. [DOI] [PubMed] [Google Scholar]