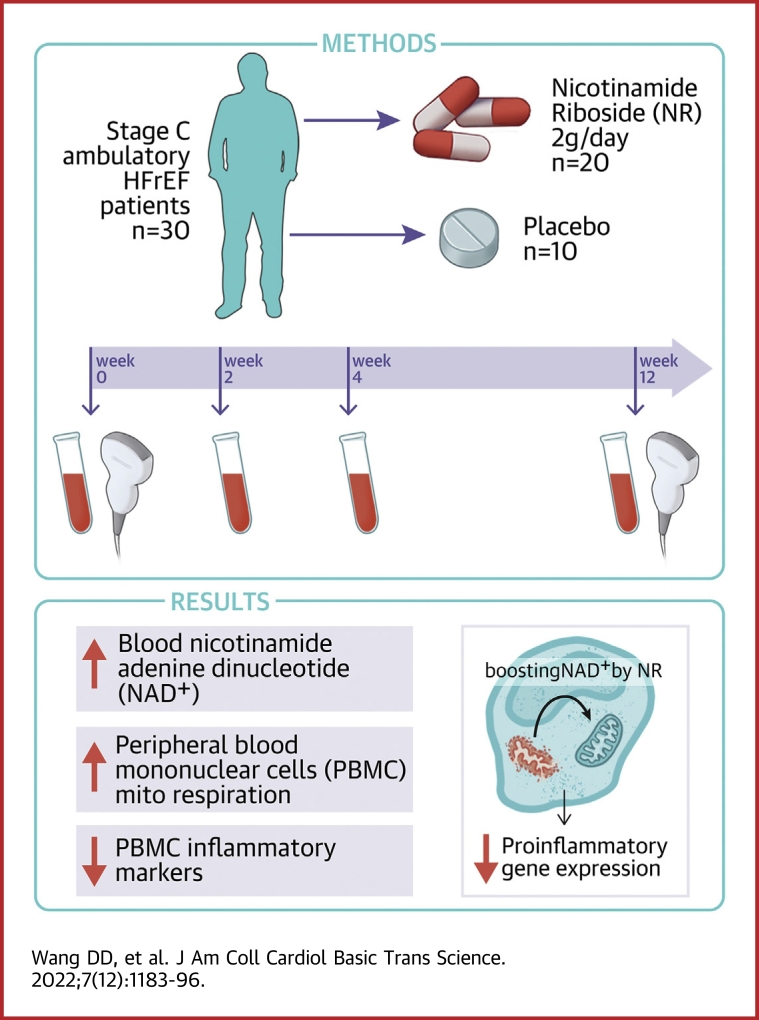
Visual Abstract
Key Words: nicotinamide riboside, NR, nicotinamide adenine dinucleotide, NAD+, heart failure with reduced ejection fraction, HFrEF, sterile inflammation, mitochondrial dysfunction
Abbreviations and Acronyms: AE, adverse event; E/e′, ratio of the early transmitral flow velocity to the early diastolic tissue velocity; GLS, global longitudinal strain; HF, heart failure; HFrEF, heart failure with reduced rejection fraction; IL, interleukin; LV, left ventricular; NAD+, nicotinamide adenine dinucleotide; NLRP3, NOD-like receptor family pyrin domain containing 3; NR, nicotinamide riboside; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor
Highlights
-
•
Boosting NAD+ by NR has been shown to blunt worsening of cardiac function in murine cardiomyopathy models and suggested to have anti-inflammatory effects a small, nonrandomized clinical trial.
-
•
In in this study, NR at 2 g/d over 12 weeks was safe, well tolerated, and significantly increased whole blood NAD+ levels in ambulatory stage C HFrEF patients.
-
•
The whole blood NAD+ response to NR correlated with increased respiration and decreased proinflammatory cytokine expression in PBMCs, providing evidence that boosting NAD+ acts on peripheral immune cells to reduce systemic inflammation.
Summary
The mitochondrial dysfunction characteristic of heart failure (HF) is associated with changes in intracellular nicotinamide adenine dinucleotide (NAD+) and NADH levels. Raising NAD+ levels with the NAD+ precursor, nicotinamide riboside (NR), may represent a novel HF treatment. In this 30-participant trial of patients with clinically stable HF with reduced ejection fraction, NR, at a dose of 1,000 mg twice daily, appeared to be safe and well tolerated, and approximately doubled whole blood NAD+ levels. Intraindividual NAD+ increases in response to NR correlated with increases in peripheral blood mononuclear cell basal (R2 = 0.413, P = 0.003) and maximal (R2 = 0.434, P = 0.002) respiration, and with decreased NLRP3 expression (R2 = 0.330, P = 0.020). (Nicotinamide Riboside in Systolic Heart Failure; NCT03423342)
Despite the use of guideline-directed medical therapies,1,2 heart failure (HF) remains a major cause of morbidity and mortality in the United States, affecting more than 6,000,000 individuals at an estimated annual cost of over $30 billion.3 Because current medical treatments for HF primarily target neurohormonal activation, novel therapeutic approaches are needed.4
One potential target is suggested by the observation that myocardium is characterized by decreases in intracellular nicotinamide adenine dinucleotide (NAD+) levels as well as the ratio of NAD+ to its reduced form, NADH.5, 6, 7 In vitro and preclinical model studies suggest that these changes adversely affect redox balance, cell death, and inflammation, as well as posttranslational protein modifications important to cellular metabolic processes and energy transduction, reviewed in Zhou and Tian,8 O’Brien and Tian,9 and Tian et al.10
Nicotinamide riboside (NR), an orally bioavailable NAD+ precursor, recently has been shown to be both well tolerated and effective in increasing peripheral blood NAD+ levels in small, phase 1 clinical trials of healthy volunteers.11, 12, 13 Additional human studies have found NR to be tolerated in individuals with obesity,14,15 advanced age16,17 and acute kidney injury.18 Chronic HFrEF patients represent a vulnerable population with high morbidity and mortality,3 and safety and tolerability data for NR presently are lacking in this population.
Thus, to explore the therapeutic potential of oral NR supplementation in HF, we undertook a safety and tolerability trial of NR in 30 participants with clinically stable, American College of Cardiology/American Heart Association stage C HFrEF. Secondary endpoints included the incidence of on-trial abnormal laboratory values and adverse events (AEs), as well as the effects of NR on whole blood NAD+ levels. Additional, exploratory endpoints assessed included changes in functional capacity by 6-minute walk test, quality of life by the Minnesota Living with Heart Failure Questionnaire, and transthoracic echocardiogram parameters including left ventricular (LV) systolic function (2-dimensional LV ejection fraction and LV global longitudinal strain), left ventricular filling pressure as assessed by the ratio of the early transmitral flow velocity to the early diastolic tissue velocity (E/e′), and LV end-diastolic and LV end-systolic volumes. NR effects on participant peripheral blood mononuclear cell (PBMC) mitochondrial function and inflammation also were assessed.19,20
Methods
Participants
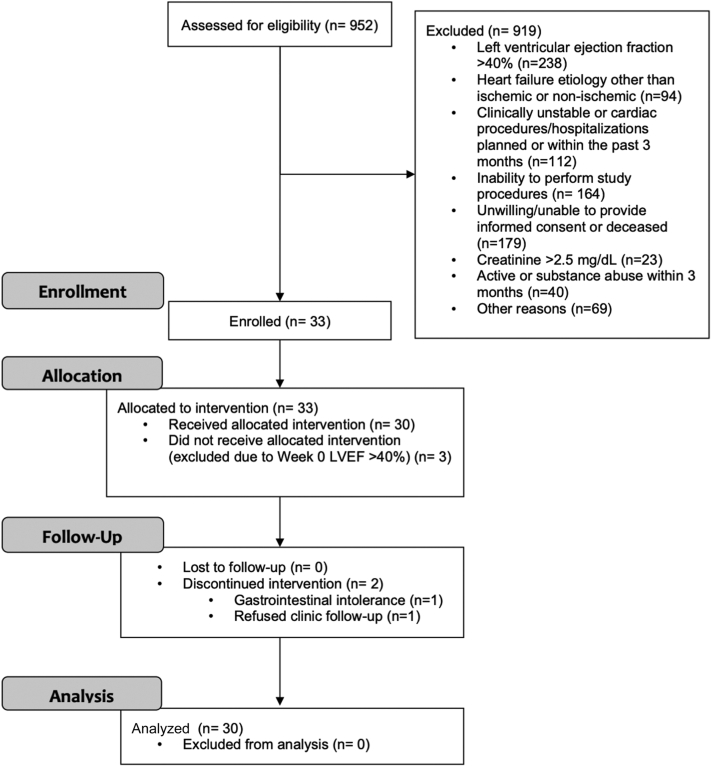
From 952 University of Washington (UW) HF patients screened, 33 participants were recruited. Of these, 3 were found at their randomization visit (week 0) to have an LV ejection fraction that had improved to >40%, and so were withdrawn from the trial prior to receiving randomized therapy. Therefore, final trial enrollment was 30 randomized participants. The first participant was enrolled on May 19, 2016, and the final participant completed follow-up on March 11, 2019. Primary reasons for exclusion were: 1) LV ejection fraction >40% (25.9%); 2) unwillingness or inability to provide informed consent, or deceased status (19.5%); 3) inability to undergo trial procedures (17.8%); 4) being clinically unstable, or cardiac procedures or hospitalizations within the past 3 months (12.2%); and 5) HF owing to inflammatory or toxin-induced cardiomyopathies (10.2%). Flow of participants through the trial is shown in Figure 1.
Figure 1.
Trial Participant Flow (The CONSORT Diagram)
Shown is the flow of participants from screening (assessment) through analysis phases, with reasons for inclusion/exclusion. LVEF = left ventricular ejection fraction.
To compare baseline PBMC mitochondrial function in American College of Cardiology/American Heart Association stage C HFrEF (ie, trial participants) versus healthy control subjects, blood samples also were collected from 9 healthy adult participants under a separate, University of Washington Institutional Review Board–approved protocol.
The 30 randomized participants met all prespecified inclusion criteria of: 1) HF with reduced ejection fraction (LV ejection fraction ≤40%, as determined by transthoracic echocardiogram) of nonischemic or ischemic etiologies; 2) clinical stability (no cardiac procedures or hospitalizations for cardiac causes, including HF, ischemia, or arrhythmia) within the prior 3 months; 3) ability to perform trial procedures (including trial visits, blood draws, and 6-minute walk tests); and 4) willingness or ability to provide informed consent. None had any trial exclusion criteria, which comprised: 1) HF with preserved ejection fraction (LV ejection fraction >40%); 2) HF etiologies other than nonischemic or ischemic (ie, primary valvular heart disease, hypertrophic cardiomyopathy, or infiltrative or inflammatory cardiomyopathies); 3) cardiac procedures within the previous 3 months; 4) hospitalizations for cardiovascular causes, including HF, chest pain, stroke or transient ischemic attack, or arrhythmias within the prior 3 months; 5) inability to perform trial visits or procedures; 6) unwillingness or inability to provide informed consent; 7) alanine aminotransferase >3× the upper limit of normal, hepatic insufficiency, or active liver disease; 8) recent history of acute gout; 9) chronic renal insufficiency with serum creatinine ≥2.5mg/dL; 10) pregnant or likely to become pregnant; 11) significant comorbidity likely to cause death within 6 months; 12) significant history of cannabis, methamphetamine, or opioid abuse within the previous 5 years; 13) current participation in a clinical trial of a drug or intervention; or 14) prior intolerance to NAD+ precursors, including niacin or nicotinamide.
Follow-up was completed for all 30 participants. Trial medication was discontinued in 1 participant due to gastrointestinal intolerance, but he completed all follow-up visits and procedures. The final participant refused to complete any trial visits or procedures after the randomization visit, but his vital status was confirmed at the end of the trial. No participants were excluded from analysis, which followed the principal of intention to treat. The standardized method for characterizing AEs was the Common Terminology Criteria for Adverse Events v3.0.
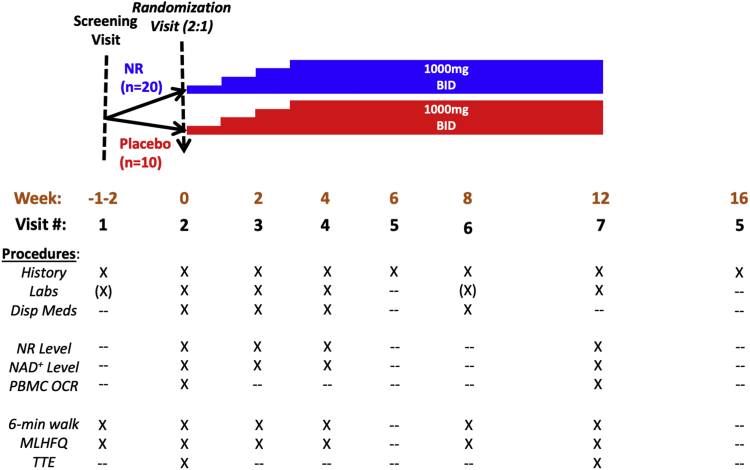
Trial design
The overall trial design is depicted graphically in Figure 2. Participant clinic visits, medication dispensation, and data collection were performed at the UW Translational Research Unit. At the screening visit, participants provided medical history and blood for baseline lab tests as well as for PBMC isolation and mitochondrial respiration assay (Seahorse XF, Agilent). Participants also performed a 6-minute walk test. History included assessment with the Minnesota Living with Heart Failure Questionnaire under license from the University of Minnesota (Minneapolis, Minnesota, USA).
Figure 2.
Schedule of Trial Procedures
Shown are participant allocation, schedule for uptitration of NR vs. matching placebo, and procedures performed at each study visit. BID = twice daily; MLHFQ = Minnesota Living with Heart Failure Questionnaire; NR = nicotinamide riboside; NAD = nicotinamide adenine dinucleotide; OCR = oxygen consumption rate; PBMC = peripheral blood mononuclear cell; TTE = transthoracic echocardiogram.
In this double-blind study, participants and study investigators or personnel were blinded to treatment assignment, as well as to blood NR and NAD+ levels. Using a randomization table provided by the trial biostatistician, the University of Washington Investigational Drug Service assigned trial IDs and dispensed trial medications. Participants were randomized to NR or matching placebo at a 2:1 allocation ratio.
At the week 0 (randomization) visit, participants underwent a medical history, a second 6-minute walk test and a baseline transthoracic echocardiogram (Figure 2). NR or matching placebo were initiated at a dose of 250 mg orally twice daily, then uptitrated by 250 mg twice daily each week to a final dose of 1,000 mg twice daily at week 3. Participants were continued on the 1,000 mg twice daily dose until the final in-person clinic visit at week 12. Blood draws for trial labs, including NR and NAD+ levels, were obtained at weeks 0, 2, 4, and 12. At week 4, a second blood draw for NR and NAD+ levels was done 4 hours after the morning NR dose. Medications were dispensed at weeks 0, 2, 4, and 8, with pill counts performed at weeks 2, 4, 8, and 12. A telephone visit was performed at week 6. Final transthoracic echocardiograms and PBMC mitochondrial respiration assays were performed at week 12. A follow-up telephone visit was performed at week 16 to confirm clinical status. Trial labs were performed by the University of Washington Research Testing Service, except that NR and NAD+ assays were performed by the University of Washington School of Pharmacy Pharmacokinetics Laboratory. Procedures for blood collection and processing, as well as for NR and NAD+ assay procedures, have been described previously.12
Ethics
The trial protocol and the separate, healthy volunteer blood collection followed UW ethical standards in accordance with the 2013 Helsinki Declaration. Trial participant and healthy volunteer recruitment were conducted according to UW Institutional Review Board policies as well as Health Insurance Portability and Accountability Act policies. All participants provided written, informed consent. The trial was registered on ClinicalTrials.gov (NCT03423342).
In addition, blood samples were obtained for PBMC isolation and mitochondrial function from 9 healthy, age-matched control subjects under a separate, University of Washington Institutional Review Board–approved protocol.
A trial Data and Safety Monitoring Committee reviewed unblinded safety, tolerability, laboratory results and endpoints following trial completion by 10, 20, and 30 participants. All trial personnel remained blinded to randomized treatment assignment until after trial completion and the final review by the Data and Safety Monitoring Committee.
NR: Source and authentication
NR and matching placebo were supplied by the manufacturer as 250-mg capsules (Niagen, ChromaDex). NR was manufactured in a GMP-compliant facility according to ISO/IEC 18025:2005 standards. Two Certificates of Analysis provided by the manufacturer and performed on separate lots reported ∼99% purity of the NR preparation.
PBMC studies
PBMCs were isolated from freshly collected whole blood by centrifugation in Sepmate-50 PBMC isolation tubes (STEMCELL Technologies, Cat. 85450).
PBMC mitochondrial function
Freshly isolated PBMCs were resuspended in Seahorse XF medium (Cat. 102353-100) and then plated (106 cells per well) onto Seahorse 24-well plates. PBMC mitochondrial respiratory function was assessed by measuring the oxygen consumption rate at basal and maximal stimulated conditions as described previously.20
PBMC inflammatory gene expression
For 23 participants (NR: n = 16, placebo: n = 7), total RNA for analysis was successfully isolated from residual, frozen PBMC pellet pairs obtained at the baseline (week 0) and week 12 visits. Following complementary DNA synthesis, real-time quantitative polymerase chain reaction was performed as described previously.20 Gene expression was calculated using the ΔΔCt method. Polymerase chain reaction primer pairs for NOD-like receptor family pyrin domain containing 3 (NLRP3), interleukin (IL)-1B, IL-6, tumor necrosis factor (TNF)-α, IL-18, and 18S ribosomal RNA have been described previously.20
Exploratory endpoints
The following endpoints were considered exploratory, as the trial was underpowered to detect differences.
6-minute walk test
Any potential training effect21 for 6-minute results was mitigated by performing a training test at the screening visit, while the second test, performed at the randomization visit, was considered the baseline test.
Echocardiography
Serial echocardiograms were performed using a standardized protocol. Two-dimensional LV ejection fraction, LV end-diastolic volume, and LV end-systolic volume were quantified using the biplane method of discs on for 29 participants (NR = 19, placebo = 10) with 2 evaluable studies. LV early diastolic function was assessed by E/e′ on 24 trial participants (NR = 17, placebo = 7) with 2 evaluable studies. All measurements were made using IntelliSpace Cardiovascular (Philips). peak systolic LV global longitudinal strain (GLS) was quantified using TOMTEC AutoSTRAIN for 29 trial participants (NR, n = 19; placebo, n = 10) with 2 evaluable studies. Each echocardiographic measurement was made by a single observer (C.M.R. for 2-dimensional volumes, LVEF, and GLS; J.N.K. for E/e′) blinded to participant clinical data, randomized treatment assignment, and echocardiogram temporal sequence.
Minnesota Living With Heart Failure Questionnaire
The Minnesota Living with Heart Failure Questionnaire was administered to trial participants at the screening (week 2) and randomization (week 0) visits, as well as at trial visits at weeks 2, 4, 8, and 12.
Statistical analyses
Descriptive statistics are presented as the mean, standard deviation, standard error of the mean, or range for continuous variables and as count and percentage for categorical variables. Statistical significance of differences in patient characteristics and outcomes between NR and placebo patients was evaluated using the 2-sample t test with unequal variances, the chi-square test, or Fisher’s exact test, as appropriate. For values with nonparametric distributions, the Wilcoxon signed rank test was used to compare within-group changes from baseline and the Mann-Whitney test was used to compare between-group changes. Normal quantile-quantile plots were used to visually assess departures from normality. Highly skewed variables were analyzed as log values. Simple linear regression analysis was used to determine the square of the correlation coefficient (R2) between observed continuous outcome and predictor variables that passed formal tests of normality following log2-transformation. Statistical analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing) or GraphPad Prism version 9.2.0 (GraphPad Software). A value of P < 0.050 was used to denote statistical significance.
Results
Participant characteristics
Trial participants had a mean age of 59 ± 8 years, and 23.0% were female with the following self-reported ethnic distribution: 83.0% non-Hispanic White, 7.0% Hispanic, 7.0% Asian, and 3.0% African American. HF etiology was nonischemic in 63% and mean LV ejection fraction at baseline was 28%. Overall, 29 (97.0%) of 30 participants were on beta-blockers and 29 (97.0%) of 30 were on an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or sacubitril-valsartan. In addition, 22 (73.0%) of 30 were on a mineralocorticoid receptor antagonist and 18 (60.0%) of 30 were on diuretics. Trial participant characteristics in the 2 randomized groups were well matched at baseline for demographics, HF medications, physical exam, social and medical history (Table 1), and baseline laboratory values (Table 2).
Table 1.
Baseline Demographics, Physical Exam Data, and Medical History by Randomized Group
| NR |
Placebo |
|||
|---|---|---|---|---|
| n | Mean (Range) or n (%) | n | Mean (Range) or n (%) | |
| Demographics | ||||
| Age, y | 20 | 58 (37-81) | 10 | 60 (39-76) |
| Female | 20 | 5 (25.0) | 10 | 2 (20.0) |
| White | 20 | 17 (85.0) | 10 | 10 (100) |
| Hispanic | 20 | 1 (5.0) | 10 | 1 (10.0) |
| Baseline HF medications | ||||
| Beta-blocker | 20 | 19 (95.0) | 10 | 10 (100) |
| ACE inhibitor/ARB | 20 | 14 (70.0) | 10 | 7 (70.0) |
| Sacubitril-valsartan | 20 | 5 (25.0) | 10 | 3 (30.0) |
| Mineralocorticoid receptor antagonist | 20 | 15 (75.0) | 10 | 7 (70.0) |
| Diuretics | 20 | 12 (60.0) | 10 | 6 (60.0) |
| Physical exam | ||||
| Sitting systolic blood pressure, mm Hg | 20 | 106 (85-140) | 10 | 109 (93-133) |
| Sitting diastolic blood pressure, mm Hg | 20 | 63 (49-76) | 10 | 67 (52-75) |
| Heart rate, beats/min | 20 | 64 (48-83) | 10 | 68 (52-80) |
| Weight, kg | 19 | 94 (63-142) | 10 | 96 (57-116) |
| Height, cm | 20 | 170 (104-196) | 10 | 176 (161-188) |
| Medical and social history | ||||
| Smoking history | 20 | 10 | ||
| Never smoker | 8 (40.0) | 4 (40.0) | ||
| Former smoker | 10 (50.0) | 6 (60.0) | ||
| Current smoker | 2 (10.0) | 0 (0) | ||
| Alcohol: never | 20 | 3 (15.0) | 10 | 3 (30.0) |
| Nonischemic HF | 19 | 13 (68.4) | 10 | 6 (60.0) |
| Left ventricular ejection fraction (most recent prior value), % | 20 | 28 (11-40) | 10 | 28 (14-39) |
| Previous MI | 20 | 7 (35.0) | 10 | 4 (40.0) |
| Previous CABG | 20 | 2 (10.0) | 10 | 4 (40.0) |
| Previous PCI | 20 | 4 (20.0) | 10 | 1 (10.0) |
| History of HTN | 19 | 7 (36.8) | 10 | 2 (20.0) |
| History of dyslipidemia | 20 | 11 (55.0) | 10 | 6 (60.0) |
| History of atherosclerotic disease (ie, CAD by angiogram, PAD, carotid stenosis) | 20 | 8 (40.0) | 10 | 4 (40.0) |
| History of atrial fibrillation | 20 | 6 (30.0) | 10 | 7 (70.0) |
| History of atrial flutter | 20 | 0 (0) | 10 | 3 (30.0) |
| History of diabetes | 20 | 8 (40.0) | 10 | 1 (10.0) |
| Liver disease | 20 | 1 (5.0) | 10 | 0 (0) |
| Emphysema | 20 | 1 (5.0) | 10 | 0 (0) |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CABG = coronary artery bypass grafting; CAD = coronary artery disease; HF = heart failure; HTN = hypertension; MI = myocardial infarction; NR = nicotinamide riboside; PAD = peripheral artery disease; PCI = percutaneous coronary intervention.
Table 2.
Baseline Demographic and Laboratory Values by Randomized Group
| NR |
Placebo |
|||
|---|---|---|---|---|
| n | Mean (Range) | n | Mean (Range) | |
| Potassium, mEq/L | 20 | 4.2 (3.4-4.8) | 10 | 4.3 (3.9-4.7) |
| Glucose, mg/dL | 20 | 131.7 (73.0-364.0) | 10 | 114.1 (72.0-185.0) |
| Uric acid, mg/dL | 20 | 6.6 (3.8-9.5) | 10 | 6.8 (4.2-8.3) |
| Creatinine, mg/dL | 20 | 1.1 (0.6-2.3) | 10 | 1.2 (0.6-1.7) |
| Alanine aminotransferase, U/L | 20 | 19.9 (10.0-39.0) | 10 | 27.3 (16.0-40.0) |
| Sitting systolic blood pressure, mm Hg | 20 | 106 (65-139) | 10 | 106 (96-122) |
| Sitting diastolic blood pressure, mm Hg | 20 | 67 (45-104) | 10 | 64 (54-68) |
| Body temperature, °C | 20 | 37 (36-37) | 10 | 36 (36-37) |
| Weight, kg | 20 | 95 (62-141) | 10 | 96 (56-115) |
| White blood count, ×103/μL | 20 | 6.8 (3.9-10.6) | 10 | 6.4 (4.1-8.5) |
| Hematocrit, % | 20 | 41 (32-46) | 10 | 42 (34-46) |
| Hemoglobin, g/dL | 20 | 13.5 (10.4-15.9) | 10 | 13.8 (11.5-15.9) |
| Platelet count, ×103/μL | 20 | 194 (113-327) | 10 | 194 (144-263) |
| Aspartate aminotransferase, U/L | 20 | 19.2 (13.0-34.0) | 10 | 24.9 (18.0-42.0) |
| HOMA-IRa | 20 | 7.0 (0.6-41.3) | 10 | 4.4 (0.7-13.6) |
| eGFR, mL/min/1.73 m2,b | 20 | 71 (31-123) | 10 | 67 (30-109) |
| BNP, pg/mL | 20 | 276 (24-911) | 10 | 378 (34-1,405) |
BNP = B-type natriuretic peptide; eGFR = estimated glomerular filtration rate; HOMA-IR = Homeostatic Model Assessment of Insulin Resistance; NR = nicotinamide riboside.
Basal.
By the Modification of Diet in Renal Disease, 4-component model.
NR safety and tolerability
In this relatively small trial, no between-group differences were observed in treatment-emergent AEs. On trial, a total of 97 AEs were reported, with 63 in the 20-participant NR group and 34 in the 10-participant placebo group. Table 3 summarizes total AEs and per-participant AEs by randomized group. There was 1 serious AE, a hospitalization for HF exacerbation and pancreatitis, in a participant randomized to placebo.
Table 3.
AEs (Total and Per Participant) by Randomized Group
| Total AEs |
Per-Participant AEs |
|||
|---|---|---|---|---|
| NR (n = 20) | Placebo (n = 10) | NR | Placebo | |
| Nervous system disorders | 12 | 5 | 6 (30.0) | 5 (50.0) |
| Infections and infestations | 12 | 5 | 6 (30.0) | 5 (50.0) |
| Renal and urinary disorders | 1 | 1 | 1 (5.0) | 1 (10.0) |
| Musculoskeletal and connective tissue disorders | 7 | 1 | 6 (30.0) | 1 (10.0) |
| General disorders and administration site conditions | 12 | 5 | 9 (45.0) | 4 (40.0) |
| Gastrointestinal disorders | 4 | 3 | 3 (15.0) | 2 (20.0) |
| Vascular disorders | 1 | 0 | 1 (5.0) | 0 (0) |
| Respiratory, thoracic, and mediastinal disorders | 4 | 9 | 3 (15.0) | 3 (30.0) |
| Skin and subcutaneous tissue disorders | 1 | 1 | 1 (5.0) | 1 (10.0) |
| Metabolism and nutrition disorders | 2 | 1 | 2 (10.0) | 1 (10.0) |
| Cardiac disorders | 3 | 2 | 3 (15.0) | 1 (10.0) |
| Injury, poisoning, and procedural complications | 1 | 0 | 1 (5.0) | 0 (0) |
| Psychiatric disorders | 1 | 0 | 1 (5.0) | 0 (0) |
| Investigations (“out-of-range” laboratory values) | 2 | 1 | 1 (5.0) | 1 (10.0) |
Values are n or n (%).
AE = adverse event; NR = nicotinamide riboside.
Out-of-range lab values (classified as investigations in Table 3) were rare, with creatinine >1.5× but <3× baseline on 2 occasions in 1 participant randomized to NR and alanine aminotransferase >3× but <5× baseline in 1 participant randomized to placebo.
NR tolerability was high, as assessed by on-trial compliance with randomized treatments. Based on returned pill counts, percentages of dispensed capsules taken were similar for NR group compliance (97% [range: 91%-100%] of dispensed capsules) and placebo group compliance (95% [range 89%-100%] of dispensed capsules) (P = 0.15).
There were no significant, on-trial changes (week 12 vs week 0) in prespecified, laboratory, or clinical variables (Table 4).
Table 4.
Changes (Week 12 vs Baseline) in Laboratory and Clinical Variables by Randomized Group
| NR |
Placebo |
|||||
|---|---|---|---|---|---|---|
| n | Week 12 to Week 0 Change | 95% CI | n | Week 12 to Week 0 Change | 95% CI | |
| Potassium, mEq/L | 19 | 0.01 ± 0.07 | -0.15 to 0.16 | 10 | 0.11 ± 0.12 | -0.17 to 0.39 |
| Glucose, mg/dL | 19 | 2.1 ± 6.6 | -11.8 to 16.0 | 10 | 2.0 ± 2.6 | -3.9 to 7.9 |
| Log10 glucose | 19 | 0.020 ± 0.017 | -0.016 to 0.055 | 10 | 0.009 ± 0.011 | -0.017 to 0.035 |
| Uric acid, mg/dL | 19 | -0.01 ± 0.17 | -0.37 to 0.36 | 10 | 0.22 ± 0.40 | -0.68 to 1.10 |
| Creatinine, mg/dL | 19 | 0.021 ± 0.050 | -0.083 to 0.130 | 10 | -0.004 ± 0.035 | -0.083 to 0.075 |
| Log10 creatinine | 19 | 0.006 ± 0.019 | -0.033 to 0.045 | 10 | -0.006 ± 0.012 | -0.034 to 0.022 |
| Alanine aminotransferase, U/L | 19 | 0.7 ± 1.5 | -2.5 to 3.9 | 10 | -3.1 ± 2.9 | -9.6 to 3.4 |
| Sitting systolic blood pressure, mm Hg | 19 | 2.80 ± 3.90 | -5.30 to 11.00 | 10 | -0.10 ± 3.60 | -8.17 to 8.00 |
| Sitting diastolic blood pressure, mm Hg | 19 | 0.1 ± 3.8 | -7.9 to 8.1 | 10 | 1.3 ± 2.6 | -4.5 to 7.1 |
| Body temperature, °C | 19 | -0.06 ± 0.08 | -0.24 to 0.11 | 10 | -0.10 ± 0.14 | -0.42 to 0.22 |
| Weight, kg | 19 | -0.96 ± 0.58 | -2.20 to 0.26 | 10 | 0.13 ± 0.48 | -0.96 to 1.20 |
| White blood count, ×103/μL | 19 | 0.14 ± 0.21 | -0.30 to 0.58 | 10 | -0.20 ± 0.29 | -0.86 to 0.46 |
| Hematocrit, % | 19 | -0.8 ± 0.6 | -2.1 to 0.5 | 10 | -0.4 ± 0.8 | -2.3 to 1.5 |
| Hemoglobin, g/dL | 19 | -0.43 ± 0.26 | -0.97 to 0.10 | 10 | -0.10 ± 0.24 | -0.65 to 0.45 |
| Platelet count, ×103/μL | 19 | -2.0 ± 8.0 | -19.7 to 15.5 | 10 | -3.0 ± 5.0 | -15.6 to 9.0 |
| Aspartate aminotransferase, U/L | 19 | -0.3 ± 0.9 | -2.2 to 1.7 | 10 | -1.5 ± 1.7 | -5.4 to 2.4 |
| HOMA-IRa | 19 | 0.20 ± 0.70 | -1.20 to 1.60 | 10 | -0.50 ± 0.60 | -1.80 to 0.78 |
| eGFR, mL/min/1.73 m2,b | 19 | -1.0 ± 4.0 | -8.8 to 6.7 | 10 | 2.0 ± 2.0 | -2.8 to 6.9 |
| BNP, pg/mL | 19 | 67.0 ± 32.0 | -1.3 to 135.0 | 10 | -75.0 ± 53.0 | -195.0 to 45.0 |
Abbreviations as in Table 2.
Basal.
By the Modification of Diet in Renal Disease, 4-component model.
On-trial NR and NAD+ levels
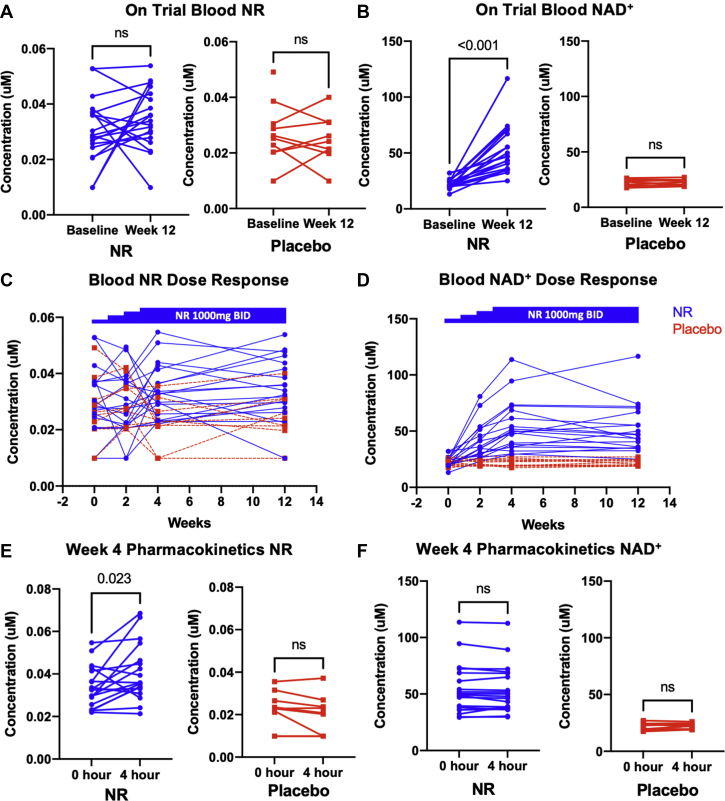
On-trial change (week 12 vs week 0) in morning trough whole blood NR levels did not differ between the NR and placebo groups (mean changes: NR 0.002 ± 0.009 μM vs placebo 0.001 ± 0.007 μM; P = 0.295). Notably, there was a wide interindividual variability in the change in NR levels (Figure 3A). However, as expected, on-trial changes in whole blood NAD+ levels were significantly higher in those randomized to NR versus placebo (mean changes: NR group 30.0 ± 20.0 μM vs placebo group -0.3 ± 2.0 μM; P < 0.001) (Figure 3B).
Figure 3.
On-Trial Blood NR and NAD+ Levels in Individual Participants
Morning trough blood (A) NR and (B) NAD+ at week 0 and week 12 are shown for participants randomized to NR (blue) (n = 19) and placebo (red) (n = 10). For the NR group, NR level changes did not reach statistical significance, but NAD+ levels were significantly higher at the end of randomized treatment. Blood (C) NR and (D) NAD+ levels in individual participants during dose titration over the first 3 weeks and stable dosing at 1,000 mg over weeks 4 through 12. Week 4 pharmacokinetics of (E) NR and (F) NAD+ as revealed by blood levels just prior to (trough) and at 4 hours following the morning dose of 1,000 mg NR. Patients had been on the maximum dose of 1,000 mg twice daily for 1 week prior to this visit. While NR levels rose significantly in the NR group over the 4-hour postdose period, NAD+ remained relatively unchanged over 4 hours. (A, B, D, F) Data analyzed by Wilcoxon signed rank test. Abbreviations as in Figure 2.
Figures 3C and 3D show the blood NR and NAD+ levels in individual participants during dose titration of NR or placebo over the first 3 weeks and during regular administration of the final dose from weeks 4 through 12. During the titration period, trough NR levels were generally higher in participants randomized to NR than those randomized to placebo; however, levels in the NR participants were quite variable over time and did not show an increasing trend corresponding to the escalation in dose (Figure 3C). In contrast, dose-dependent increases in blood NAD+ levels were observed in those participants randomized to NR (Figure 3D); NAD+ levels increased from week 0 to week 2 (after 1 week on NR 500 mg twice daily) and further increased by week 4 (after 1 week on NR 1,000 mg twice daily). From thereon, NAD+ levels remained relatively stable on the 1,000 mg twice daily dose through week 12 (Figure 3D) confirming that steady-state blood NAD+ was achieved within 1 week of a dose change. Thus, as reported previously,12 while interindividual variation in NAD+ level rise was wide among participants randomized to NR, intraindividual NAD+ levels were quite stable on the 1,000-mg twice-daily dose.
At the week 4 visit, blood NR and NAD+ levels were obtained just prior to and 4 hours after their morning 1,000-mg NR dose. As expected, NR levels tended to increase by 4 hours postdose, reflecting absorption following NR dosing (Figure 3E). However, NAD+ levels did not change over this time period (Figure 3F). These findings are similar to those reported previously in healthy volunteers, and suggest that blood NAD+ has reached the maximum, achievable steady-state level at the 1,000-mg twice-daily dose.12
Studies with PBMCs
PBMC mitochondrial respiration in stage C HFrEF (trial participants) versus healthy, age-matched control subjects
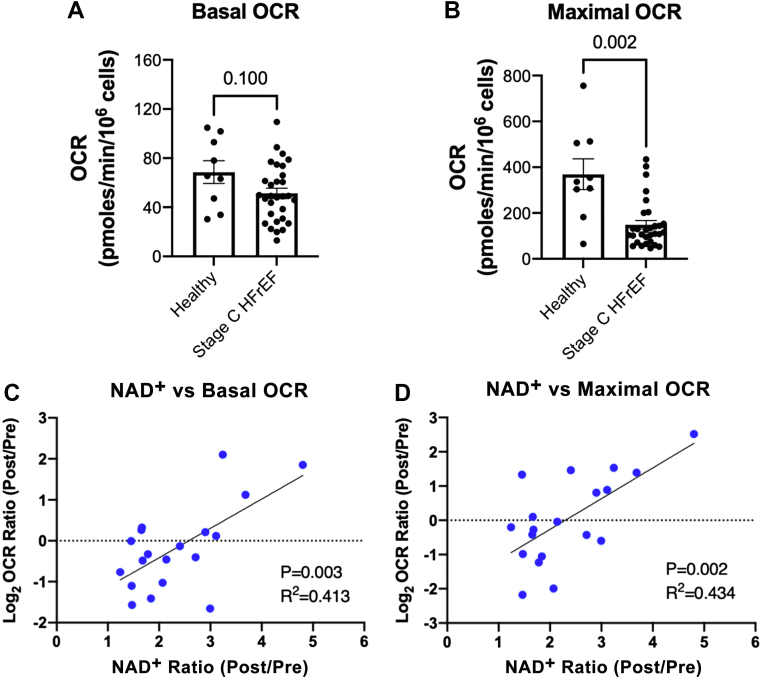
PBMC mitochondrial respiration at baseline in 30 trial participants was compared with that of a group of 9 healthy volunteers matched for age (stage C HFrEF participants mean age 60.0 ± 13.1 years vs healthy control subjects mean age 54.1 ± 8.9 years; P = 0.218). While PBMC basal respiration did not differ significantly between groups (Figure 4A), PBMC maximal respiration was significantly impaired in stage C HFrEF participants as compared with healthy control subjects (Figure 4B). There were no on-trial changes in the proportions or absolute numbers of white blood cell categories (Supplemental Figure 1), suggesting that observed PBMC oxygen consumption rate differences were not due to changes in white blood cell absolute numbers or proportions.
Figure 4.
Correlations of Whole Blood NAD+ Response to NR With Increased PBMC Mitochondrial Respiration
PBMC mitochondrial respiration (OCR) is depressed in American College of Cardiology/American Heart Association stage C heart failure with reduced ejection fraction (HFrEF) but increases in proportion to blood NAD+ level increase. (A) Differences in basal mitochondrial respiration between age-matched, healthy control subjects and stage C HFrEF patients did not reach statistical significance (mean + SEM and individual values shown, P = 0.0996). (B) Stage C HFrEF was associated with significantly lower maximal mitochondrial respiration (mean + SEM and individual values shown, P = 0.0016). In stage C HFrEF patients randomized to NR, change in blood NAD+ levels correlated positively with log-transformed changes in both (C) basal and (D) maximal mitochondrial respiration. (A, B) Analyzed by 2-tailed nonparametric t test (Mann-Whitney test). (C, D) Analyzed by simple linear regression. Abbreviations as in Figure 2.
Relationships of change in NAD+ levels with changes in PBMC respiration and inflammatory marker expression in the NR group
For PBMC respiration, individual participant changes in whole blood NAD+ levels were compared with changes in basal and maximal mitochondrial respiration. Within the NR group, as shown in Figures 4C and 4D, strong correlations were found between relative increases in NAD+ levels and increases in both basal and maximal mitochondrial respiration (basal: R2 = 0.413, P = 0.003; maximal: R2 = 0.434, P = 0.002).
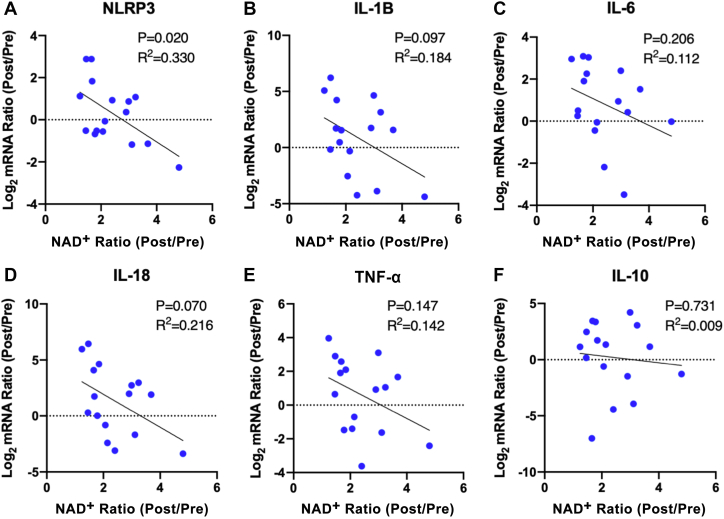
For PBMC inflammatory marker expression, potential correlations between changes in NAD+ and in PBMC expression of 5 proinflammatory genes were evaluated. The selected markers included NLRP3, IL-1β, IL-6, IL-18, and TNF-α. As a negative control, messenger RNA expression of IL-10, a marker of the anti-inflammatory macrophage phenotype, also was assessed. As shown in Figure 5, there was a significant correlation of change in NLRP3 with change in NAD+ (R2 = 0.330, P = 0.020) (Figure 5A), while there were directionally similar, though nonsignificant, changes in expression of the other 4 proinflammatory cytokines (Figures 5B to 5E). In contrast, there was no suggestion of a relationship between increased NAD+ levels and change in IL-10 expression (Figure 5F).
Figure 5.
Correlations of Whole Blood NAD+ Response to NR With Decreased PBMC Inflammatory Cytokine Expression
PBMC inflammatory marker expression is decreased in proportion to blood NAD+ level increase. (A) Within the NR-treated group, changes in blood NAD+ levels were negatively correlated with changes in PBMC expression of NOD-like receptor family pyrin domain containing 3 (NLRP3). Potential negative correlations of changes in NAD+ with changes in PBMC expression of other inflammatory markers, including (B) interleukin (IL)-1B, (C) IL-6, (D) IL-18, and (E) and tumor necrosis factor (TNF)-α, did not reach statistical significance. (F) Changes in NAD+ level were not correlated with changes in expression of the profibrotic cytokine, IL-10. Data analyzed by simple linear regression. mRNA = messenger RNA; other abbreviations as in Figure 2.
Post hoc analyses of the NR group raised the possibility of a threshold effect for whole blood NAD+ response (expressed as the ratio of week 12 to week 0 NAD+ levels) for improvement in PBMC mitochondrial respiration (expressed as the ratio of week 12 to week 0 maximal mitochondrial respiration). For example, as shown in Supplemental Figure 2, comparison of NR group participants with whole blood NAD+ changes above versus below the group median NAD+ change of 2.1 were consistent with a threshold for improvement in PBMC mitochondrial respiration. A similar, threshold response was suggested for whole blood NAD+ change and decreased PBMC expression of proinflammatory markers (Supplemental Figure 3).
Other exploratory endpoints
To investigate a potential range of NR effects on prespecified surrogate endpoints, serial measurements were obtained for: 1) functional capacity as assessed by 6-minute walk test distance; 2) quality of life as assessed by the Minnesota Living with Heart Failure Questionnaire score; 3) LV systolic function as assessed by 2-dimensional LV ejection fraction; 4) LV filling pressure (E/e′); 5) LV GLS, 6) LV end-diastolic volume; and 7) LV end-systolic volume. No on-trial, between-group differences were detected for these exploratory endpoints (Supplemental Table 1).
Discussion
This trial evaluated 30 participants with clinically stable, American College of Cardiology/American Heart Association stage C HFrEF. The 20 participants randomized to NR received high-dose NR (1,000 mg twice daily) for the final 9 weeks of the 12-week trial. This NR dose equals the largest employed in any NR study to date, and this is the first report of a randomized trial of NR in HF. The trial met its primary endpoint of safety, as there were no significant differences in on-trial rates of AEs, serious AEs, or treatment-emergent laboratory abnormalities. In addition, NR treatment approximately doubled whole blood NAD+ levels in these clinically stable HF patients, a finding similar to those of our previously reported, nonrandomized open-label studies in both 8 healthy volunteers12 and 4 patients with end-stage HF.20 Moreover, correlations were observed for increases in on-trial NAD+ levels with improved PBMC maximal mitochondrial respiration and decreased PBMC expression of proinflammatory markers, suggesting potential beneficial effects of raising NAD+ levels on both mitochondrial function and inflammatory activation in patients with HFrEF. Unsurprisingly, owing to its small size, the trial did not find any significant differences between groups in exploratory, surrogate clinical endpoints.
A feature of failing myocardium is a relative decrease in both NAD+ levels and the ratio of NAD+ to NADH.5, 6, 7 NAD+ is a co-substrate for multiple enzymes, including sirtuin deacetylases. It also plays critical roles in redox balance, cell death, inflammation, and posttranslational protein modifications important to energy transduction and cellular metabolic processes.8 Recent studies have found that administration of NAD+,22 or of the NAD+ precursors nicotinamide mononucleotide6,23 or NR,7 slowed disease development in murine models of HF with HFrEF, reviewed in Zhou and Tian,8 O’Brien and Tian,9 and Tian et al.10 However, determining the safety and tolerability of NR in patients with HF is a crucial translational step9 that has not been studied previously in a randomized, placebo-controlled trial.
In addition, elevations in circulating cytokine levels are known to correlate with both HF severity24,25 and mortality.26,27 Early clinical studies targeting TNF-α in HF were negative.28,29 A more recent report has suggested that high-dose, though not intermediate-dose, treatment with the IL-1β antagonist canakinumab may decrease risk for HF hospitalization in patients with prior myocardial infarction and elevated C-reactive protein.30 The divergent results of trials targeting individual cytokines, each of which might contribute to HF progression raises the possibility that targeting inflammatory activation at a more proximal step, such as the NLRP3 inflammasome,19 may represent a more effective therapeutic strategy. The findings of the present trial and those of a previous nonrandomized study20 also raise the intriguing possibility that boosting NAD+ with NR may protect PBMC mitochondria and reduce mitochondrial reactive oxygen species–induced activation of the NLRP3 inflammasome, thereby inhibiting downstream expression of multiple individual cytokines.20,31
NR previously has been reported to improve mitochondrial respiration and decrease NLRP3 inflammasome activation in healthy individuals.19 In a recent, open-label, nonrandomized study in 4 stage D HFrEF patients, short-term (5-9 days) NR treatment increased whole blood NAD+ levels, improved PBMC mitochondrial respiration, and decreased PBMC cytokine expression.20 The present trial extends those preliminary findings in HF patients in several important respects: 1) the present trial is randomized and placebo controlled; 2) participants had less severe HF and were treated for a longer period; 3) it confirms the substantial, interindividual variability in whole blood NAD+ level change in response to NR12; and 4) correlation of the magnitude of NAD+ whole blood level increase with the extent of improvement in mitochondrial respiration and decreases in PBMC inflammatory gene expression suggest that differences in NR bioavailability or metabolism may have substantial effects on therapeutic response.
Beyond data on safety and tolerability, this trial provides important information for the design of future clinical studies. First, the results of this trial and a prior trial12 indicate substantial interindividual variability in clearance rate coupled with a relatively short blood half-life for NR, as evidenced by the lack of definable changes in trough NR levels during dose titration in the NR group. Thus, monitoring of blood NR, which also is challenging to measure, may not be helpful in assessing response to NR treatment. Second, trough NAD+ levels showed a distinct, dose-related elevation during titration. Steady-state NR levels were achieved by week 4 (while on 1,000 mg twice daily for a full week) and did not further increase at 4 hours following the regular morning dose, suggesting that a plateau in blood NAD+ can be achieved fairly rapidly, within a week or less.12 Moreover, blood NAD+ levels remain stable throughout the daily dosing cycle, which offers great convenience in blood sampling for monitoring of peripheral NAD+ status. This also implies that blood NAD+ (in particular, morning trough levels) may serve as an easily accessible, peripheral biological index or marker for gauging an individual’s response to therapy with the current NR preparation. Thus, this and a prior study12 suggest that whole blood NAD+ levels might be a useful screen for NR “responders.”
While we currently do not have sufficient data to confirm the optimal oral dose of NR, several lines of evidence suggest that the dose employed in this trial obtains the maximal increase in blood NAD+ that can be achieved with NR as it is currently formulated: 1) the present study (Figures 3B and 3D) and prior, nonrandomized studies in healthy volunteers12 and stage D HF patients20 demonstrated an increase in whole blood NAD+ levels; 2) pharmacokinetic data obtained while on the maximal NR dose of 1,000 mg twice daily in this and a prior study12 found no further increase in NAD+ levels following a morning dose of 1,000 mg, as would be expected if the dose was submaximal; and 3) parenthetically, we note that the dose employed in this and both of our prior studies12,20 is twice that used in many human studies of NR.11,13,17 We suspect that the heterogeneity in response likely is due to interindividual heterogeneity in absorption or metabolism of this highly hydrophilic molecule.
Unresolved issues and trial limitations
First, it is not known whether the observed effects of orally administered NR on raising blood NAD+ levels and improving PBMC mitochondrial function also are seen in human myocardium. This question is being addressed in an ongoing, mechanistic clinical trial of NR pretreatment in patients scheduled for LV assist device implantation (NCT04528004). A corollary question also being addressed in that ongoing trial is whether blood NAD+ levels can be used as a surrogate for mitochondrial and inflammatory effects at the myocardial level.
Second, in order to fully realize the potential benefit of NAD+ precursor treatment in HF, the pharmacokinetic mechanisms responsible for the wide, interindividual variation in blood NAD+ elevation in response to NR administration must be better understood and overcome. Individual variations in NR bioavailability, pharmacokinetics, and metabolism in human subjects are poorly understood. Unfortunately, we were unable to accurately analyze NR metabolites. We did attempt to identify the mass-ion peaks of Me2PY, NAAD, NMN, and NADH in blood extracts but lacked appropriate calibration standards for Me2PY and NAAD to allow their quantification, while NMN and NADH peaks consistently fell below the detection limit of our current liquid chromatography tandem mass spectrometry method. In addition, it is possible that diseases or other environmental factors, such as gut microbiota, may play important roles in determining individual responsiveness to NAD+ precursor administration.
Third, whether raising NAD+ levels may ultimately lead to improvement in clinical outcomes will need to be addressed in larger clinical trials that are adequately powered to assess NAD+ precursor effects on surrogate cardiovascular outcomes and, ultimately, on clinical HF events and mortality.
Finally, by virtue of the trial’s small sample size, it was underpowered for the exploratory endpoints. For example, using the reported standard deviation for changes in LV ejection fraction as measured by 2-dimensional echocardiography in a study of 50 patients with LV dysfunction due to previous MI,32 a power calculation performed prior to trial initiation indicated that a trial with 80% power to detect a between-group LV ejection fraction difference of 3% would require a total sample size of 172 participants. In addition, the very large, interindividual variability in NAD+ response to NR therapy makes it even more challenging to detect differences between NR and placebo groups. Therefore, the trial did not allow us to draw positive or negative conclusions about the effect of NR on the surrogate HF outcomes assessed.
Conclusions
This trial met its primary goal by finding that, in patients with stage C HFrEF, high-dose NR is well tolerated and has a favorable safety profile, thereby meeting an early HF translational milestone for this novel potential therapy.9,10 Importantly, this randomized, placebo-controlled trial of HF patients demonstrates that NR approximately doubles whole blood NAD+ levels, and that relative increases in blood NAD+ levels correlate with both increased PBMC mitochondrial respiratory function and decreased inflammatory gene (NLRP3) expression. Therefore, larger studies of patients with HFrEF that are sufficiently powered to assess the effect of NR on clinically relevant, surrogate endpoints as well as inflammation are warranted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Chronic HFrEF is associated with significant morbidity and mortality and is characterized by a reduction in myocardial NAD+ levels. This trial demonstrates that the NAD+ precursor NR is safe and well tolerated and raises blood NAD+ levels in this vulnerable population.
TRANSLATIONAL OUTLOOK: A feature of failing myocardium is a relative decrease in NAD+ levels and in the ratio of NAD+ to NADH. Boosting cellular NAD+ with NR and other NAD+ precursors has been shown to favorably affect cardiac function in murine cardiomyopathy models. The findings of this randomized trial and a previous nonrandomized trial suggest that boosting NAD with NR may improve mitochondrial respiratory function and reduce activation of the NLRP3 inflammasome, thereby inhibiting downstream expression of multiple individual cytokines. Future studies to assess the effect of NR on clinically relevant, surrogate endpoints and inflammation are warranted.
Funding Support and Author Disclosures
This trial was funded by National Heart, Lung, and Blood Institute grants HL126209 (to Drs Tian and O’Brien), HL126209-01S1 (to Drs O’Brien and Tian), and HL144937 (to Drs O’Brien and Tian) and T32 Fellowship HL007828 (to Dr Wang); American Heart Association Postdoctoral Fellowship Awards 15POST22560002 (to Dr Airhart) and 18POST34030098 (to Dr Wang); and a grant from the John L. Locke, Jr. Foundation (to Dr Wang). The University of Washington Institute for Translational Health Sciences is funded by UL1 TR002319 from the National Institutes of Health National Center for Advancing Translational Sciences. Nicotinamide riboside and matching placebo were supplied by ChromaDex, Inc. The company played no role in trial funding, design, conduct, data analysis or interpretation, nor in manuscript drafting or revision. Dr Tian is a member of the Scientific Advisory Board of Cytokinetics, USA. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table and figures, please see the online version of this paper.
Contributor Information
Rong Tian, Email: rongtian@uw.edu.
Kevin D. O’Brien, Email: cardiac@uw.edu.
Appendix
References
- 1.Fiuzat M., Ezekowitz J., Alemayehu W., et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT Trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2020;5:757–764. doi: 10.1001/jamacardio.2020.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaduganathan M., Fonarow G.C., Greene S.J., et al. Contemporary treatment patterns and clinical outcomes of comorbid diabetes mellitus and HFrEF: the CHAMP-HF registry. J Am Coll Cardiol HF. 2020;8:469–480. doi: 10.1016/j.jchf.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Virani S.S., Alonso A., Benjamin E.J., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Tian R., Colucci W.S., Arany Z., et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure-a report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation. 2019;140:1205–1216. doi: 10.1161/CIRCULATIONAHA.119.040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamanlidis G., Lee C.F., Garcia-Menendez L., et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.F., Chavez J.D., Garcia-Menendez L., et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diguet N., Trammell S.A.J., Tannous C., et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137:2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien K.D., Tian R. Boosting mitochondrial metabolism with dietary supplements in heart failure. Nat Rev Cardiol. 2021;18:685–686. doi: 10.1038/s41569-021-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian R., Colucci W.S., Arany Z., et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure—a report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation. 2019;140:1205–1216. doi: 10.1161/CIRCULATIONAHA.119.040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trammell S.A., Schmidt M.S., Weidemann B.J., et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7 doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Airhart S.E., Shireman L.M., Risler L.J., et al. An open-label, nonrandomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martens C.R., Denman B.A., Mazzo M.R., et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dollerup O.L., Christensen B., Svart M., et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108:343–353. doi: 10.1093/ajcn/nqy132. [DOI] [PubMed] [Google Scholar]
- 15.Dollerup O.L., Trammell S.A.J., Hartmann B., et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab. 2019;104:5703–5714. doi: 10.1210/jc.2019-01081. [DOI] [PubMed] [Google Scholar]
- 16.Dolopikou C.F., Kourtzidis I.A., Margaritelis N.V., et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur J Nutr. 2020;59:505–515. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 17.Elhassan Y.S., Kluckova K., Fletcher R.S., et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28:1717–1728. doi: 10.1016/j.celrep.2019.07.043. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simic P., Vela Parada X.F., Parikh S.M., Dellinger R., Guarente L.P., Rhee E.P. Nicotinamide riboside with pterostilbene (NRPT) increases NAD(+) in patients with acute kidney injury (AKI): a randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol. 2020;21:342. doi: 10.1186/s12882-020-02006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traba J., Kwarteng-Siaw M., Okoli T.C., et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125:4592–4600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B., Wang D.D., Qiu Y., et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020;130:6054–6063. doi: 10.1172/JCI138538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Pillai V.B., Sundaresan N.R., Kim G., et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin A.S., Abraham D.M., Hershberger K.A., et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich's ataxia cardiomyopathy model. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torre-Amione G., Kapadia S., Benedict C., Oral H., Young J.B., Mann D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 25.Seta Y., Shan K., Bozkurt B., Oral H., Mann D.L. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 26.Mann D.L. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 27.Deswal A., Petersen N.J., Feldman A.M., Young J.B., White B.G., Mann D.L. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 28.Chung E.S., Packer M., Lo K.H., Fasanmade A.A., Willerson J.T. Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 29.Mann D.L., McMurray J.J., Packer M., et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 30.Everett B.M., Cornel J.H., Lainscak M., et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.F., Tian R. Mitochondrion as a target for heart failure therapy- role of protein lysine acetylation. Circ J. 2015;79:1863–1870. doi: 10.1253/circj.CJ-15-0742. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins C., Bricknell K., Chan J., Hanekom L., Marwick T.H. Comparison of 2- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol. 2007;99:300–306. doi: 10.1016/j.amjcard.2006.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.