Abstract
Aim
To investigate the impact of postoperative infection (PI), surgical site infection, and remote infection (RI), on long‐term outcomes in patients with colorectal cancer (CRC).
Methods
The Japan Society for Surgical Infection conducted a multicenter retrospective cohort study involving 1817 curative stage I/II/III CRC patients from April 2013 to March 2015. Patients were divided into the No‐PI group and the PI group. We examined the association between PI and oncological outcomes for cancer‐specific survival (CSS) and overall survival (OS) using Cox proportional hazards models and propensity score matching.
Results
Two hundred and ninety‐nine patients (16.5%) had PIs. The 5‐year CSS and OS rates in the No‐PI and PI groups were 92.8% and 87.6%, and 87.4% and 83.8%, respectively. Both the Cox proportional hazards models and propensity score matching demonstrated a significantly worse prognosis in the PI group than that in the No‐PI group for CSS (hazard ratio: 1.60; 95% confidence interval: 1.10‐2.34; P = .015 and P = .031, respectively) but not for OS. RI and the PI severity were not associated with oncological outcomes. The presence of PI abolished the survival benefit of adjuvant chemotherapy.
Conclusions
These results suggest that PI after curative CRC surgery is associated with impaired oncological outcomes. This survival disadvantage of PI was primarily derived from surgical site infection, not RI, and PI induced lower efficacy of adjuvant chemotherapy. Strategies to prevent PI and implement appropriate postoperative treatment may improve the quality of care and oncological outcomes in patients undergoing curative CRC surgery.
Keywords: colorectal cancer, postoperative infection, remote infection, surgical site infection, survival
This multicenter retrospective cohort study suggested that postoperative infection (PI) after curative colorectal surgery (CRC) is associated with impaired oncological outcomes. Strategies to prevent PI and implement appropriate postoperative treatment may improve the quality of care and oncological outcomes in patients undergoing curative CRC surgery.
1. INTRODUCTON
Colorectal cancer (CRC) is one of the most common cancers worldwide. CRC killed approximately 880 000 people worldwide in 2018 and is the fourth leading cause of cancer death. 1 Despite recent advances in CRC treatments, particularly chemotherapies and molecular‐targeted agents, surgery is still a mainstay for localized CRC treatment. Despite recent improvements in minimally invasive procedures and perioperative management, surgical site infections (SSI) remain the most frequent surgical complications and could result in increased medical costs, prolonged hospital stay, and deteriorated patient quality of life. 2 , 3 Remote infection (RI) occurring at various distant sites after surgery is also a major concern, and RI frequently overlaps with SSI and is reported to be associated with longer hospital stays and increased medical costs. 4 The incidence of SSI in CRC surgery is gradually decreasing globally, including in Japan, owing to the increased use of laparoscopic surgery. 5 However, studies reporting the detailed epidemiology of RI are scarce; therefore, comprehensive consideration of SSI and RI, as postoperative infections (PI), is required to assess their influence in CRC surgical patients.
In addition to the mentioned disadvantages of PIs, previous studies have shown increased recurrence and worse survival in patients who developed PIs, especially for anastomotic leakage (AL), in CRC surgeries. 6 , 7 , 8 A recent meta‐analysis of 154 981 CRC surgical patients demonstrated that PIs had a significant negative impact on overall survival (OS) and cancer‐specific survival (CSS). 9 However, other studies demonstrated no oncological impact in patients who developed AL. 10 , 11 , 12 These heterogeneous results across studies might be affected by the study design (i.e., impossibility of randomized controlled trial), unvalidated definitions of PI, and perioperative management changes during the patient inclusion period.
In this study conducted by the Japan Society for Surgical Infection, the authors aimed to investigate the impact of PI after CRC surgery on survival using multicenter retrospective cohort data.
2. METHODS
2.1. Study design and setting
This retrospective cohort study investigating the association between PI and oncological survival in patients with gastrointestinal cancer was conducted by the Clinical Trial Committee of the Japan Society of Surgical Infection. The current a priori planned study was performed using CRC surgery cohorts at 16 centers, namely 13 university hospitals and three general hospitals. The protocol for this study was approved by the Ethics Committee of Nippon Medical School Tama Nagayama Hospital (Approval No. 694), and the study conforms to the provisions of the Declaration of Helsinki. The need to obtain written informed consent from the included patients was waived because of the retrospective nature of the study.
2.2. Eligibility
The inclusion criteria were as follows: (1) histologically proven pathological stage I/II/III CRC, and (2) open or laparoscopic curative primary tumor resection performed from April 1, 2013, to March 31, 2015. Patients with more than one active cancer, missing and insufficient survival data, and short follow‐up period of <6 months were excluded because these factors could interfere when investigating the direct association between PI and oncological survival. Data were collected for the following variables of interest: patient demographics, tumor characteristics, preoperative blood test results, surgical procedures, presence of a stoma, operation time, blood loss volume, and adjuvant chemotherapy.
2.3. Definitions of PI
PI was defined as SSI and PI occurring with 30 postoperative days. SSI comprised superficial SSI, deep SSI, and organ/space SSI, 13 and RI comprised respiratory tract infections, urinary tract infections, antibiotic‐associated diarrhea, catheter‐related bloodstream infections, drain infections, and bacteremia of unknown origin. 14 PI severity was categorized using the Clavien‐Dindo (CD) system. 15 In this study, we divided the patients into two groups for analysis: patients who developed PI (PI group) and those who did not (No‐PI group).
2.4. Oncological outcomes
Cancer‐specific survival (CSS) and overall survival (OS) were calculated from the date of surgery to the date of targeted cancer death and all‐cause death, respectively. Postoperative cancer surveillance and the use of adjuvant therapy were based on the Japanese guidelines for the treatment of colorectal cancer in place during the study period. 16
2.5. Statistics
Continuous variables are expressed as median with interquartile range. The two‐tailed Student's t test and Mann‐Whitney U test were used to compare continuous variables, and the χ2 test and Fisher's exact test were used to compare discrete variables. A P value of <.05 was considered statistically significant. Survival curves were created using Kaplan‐Meier estimates, and the curves were compared using the log‐rank test. Bonferroni's correction was applied to control for multiple comparisons. Univariate analysis, and multivariate analysis comprising variables with a P‐value of <.05 in the univariate analysis, and Cox proportional hazards models were used to examine the association between the selected variables and CSS or OS. Propensity score matching (PSM) was conducted to balance the covariates and reduce the selection bias between the PI and No‐PI groups. Possible variables were comprehensively selected for one‐to‐one PSM. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Tokyo, Japan) and R, version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patient characteristics
Data were collected for 1968 patients with CRC who underwent curative resection at the registered institutions. Among the 1968 patients, 151 patients were excluded in accordance with the strict data screening process shown in Figure S1. Ultimately, 1817 patients were included and divided into the No‐PI group (n = 1518; 83.5%) and PI group (n = 299; 16.5%). The patients' characteristics are shown in Table 1. Compared with the No‐PI group, the PI group had statistically significant differences in the percentages of men and patients with rectal cancer; higher American Society of Anesthesiologists scores; higher rates of emergency surgery, open surgery, and stoma creation; longer operation times; higher blood loss volumes; higher blood transfusion rates; and lower preoperative albumin concentrations.
TABLE 1.
Patients' backgrounds in No‐PI and PI groups
| Variables | No‐PI (n = 1518) | PI (n = 299) | P value |
|---|---|---|---|
| Age (years) a | 70 (62‐76) | 68.0 (60‐76) | .182 |
| Sex (male: female) | 823 (54.2): 695 (45.8) | 187 (62.5): 112 (37.5) | .009 |
| Body mass index (kg/m2) a | 22.5 (20.0‐24.8) | 22.3 (19.7‐24.6) | .320 |
| Location (colon: rectum) (%) | 1001 (65.9): 517 (34.1) | 145 (48.5): 154 (51.5) | <.001 |
| Smoking (yes: no) (%) | 405 (26.7)/1102 (72.6) | 85 (28.4)/213 (71.2) | .569 |
| ASA score (I, II/III, IV) (%) | 1356 (89.6)/158 (10.4) | 249 (83.3)/50 (16.7) | .003 |
| Emergency surgery (yes: no) (%) | 29 (1.9): 1488 (98.1) | 16 (5.4): 283 (94.6) | .002 |
| Surgical approach (open: laparoscopic) (%) | 275 (18.1): 1243 (81.9) | 94 (31.4): 205 (68.6) | <.001 |
| Stoma creation (yes: no) (%) | 219 (14.4): 1298 (85.6) | 99 (33.1): 200 (66.9) | <.001 |
| Operation time (min) a | 233 (184–296) | 273 (200–393) | <.001 |
| Blood loss (ml) a | 30 (5–103) | 90 (20–319) | <.001 |
| Blood transfusion (yes: no) (%) | 78 (5.1): 1440 (94.9) | 43 (14.4): 256 (85.6) | <.001 |
| Lymph node dissection (D1, 2/D3) b (%) | 435 (28.7)/1068 (70.4) | 83 (27.8)/215 (71.9) | .727 |
| Pathological stage (I/II/III) c (%) | 440 (29.0)/530 (34.9)/548 (36.1) | 74 (24.7)/101 (33.8)/124 (41.5) | .165 |
| Adjuvant chemotherapy (yes: no) (%) | 527 (34.7)/991 (65.3) | 103 (34.4)/196 (65.6) | .947 |
| Preoperative blood exam | |||
| Hemoglobin (g/dL) a | 12.7 (11.1‐14.0) | 12.7 (11.0‐14.2) | .993 |
| Albumin (g/dL) a | 4.1 (3.7‐4.4) | 4.0 (3.5‐4.3) | <.001 |
| CEA (ng/mL) a | 3.3 (2.1‐6.3) | 4.1 (2.2‐9.2) | .526 |
| CA19‐9 (U/mL) a | 9.9 (5.0‐20.1) | 11.0 (5.8‐22.2) | .262 |
Abbreviations: ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; PI, postoperative infections.
Median (interquartile range).
Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, 9th edition.
UICC Cancer Staging Manual for Colorectal Cancer, 8th edition.
3.2. Details of the PIs
The details of the PIs are shown in Table 2. SSI and RI accounted for 13.7% and 3.5% of the PIs, respectively, and 0.7% of the cases were overlapping cases. Among SSIs, organ/space SSI accounted for 6.9% of the cases, and the majority of these were cases with AL, which accounted for 5.7% of the cases. Superficial SSI accounting for 5.2% followed next. Among RIs, the most common was urinary tract infection accounting for 1.3% and pneumonia, catheter‐related bloodstream infection, and antibiotic‐associated diarrhea followed next (1.0%, 0.4%, and 0.4%, respectively).
TABLE 2.
Details of postoperative infections
| Types of complication | Incidence |
|---|---|
| Overall postoperative infection (PI) | 299 (16.5) |
| Surgical site infection (SSI) | 249 (13.7) |
| Remote infection (RI) | 63 (3.5) |
| SSI and RI (overlapped) | 13 (0.7) |
| Details of SSI | |
| Superficial SSI | 95 (5.2) |
| Deep SSI | 19 (1.0) |
| Organ/space SSI | 125 (6.9) |
| Anastomotic leakage | 104 (5.7) |
| Details of RI | |
| Pneumonia | 19 (1.0) |
| Urinary tract infection | 24 (1.3) |
| Catheter‐related bloodstream infection | 8 (0.4) |
| Antibiotic‐associated diarrhea | 8 (0.4) |
| Drain infection | 4 (0.2) |
| Bacteremia of unknown origin | 7 (0.4) |
| Severity of all complications a | |
| Clavien‐Dindo grading ≧II | 350 (19.3) |
| ≧III | 149 (8.2) |
Note: The values are presented as n (%).
Including both infectious and non‐infectious complications.
3.3. PI and survival
The median follow‐up periods in the No‐PI and PI groups were 62.7 (55.0‐76.5) months and 62.3 (48.2‐78.0) months, respectively. Kaplan‐Meier curves for CSS and OS stratified by with or without PI are shown in Figures 1A, S2A, respectively. The 5‐year CSS and OS rates in the No‐PI and PI groups were 92.8% and 87.6%, and 87.4% and 83.8%, respectively. The PI group had significantly worse survival compared with the No‐PI group for both CSS and OS (P < .001 for both). In the multivariate Cox proportional hazards models, laparoscopic surgery, advanced pathological stage, adjuvant chemotherapy, and PI were significantly associated with poor CSS (hazard ratios: 0.49, 5.24, 0.60, and 1.60; 95% confidence intervals: 0.33‐0.71, 3.61‐7.61, 0.41‐0.88, and 1.10‐2.34; P values: <.001, <.001, .010, and .015; respectively) after adjusting for each patient characteristic. Age, sex, laparoscopic surgery, blood loss volume, and pathological stage were significantly associated with poor OS (hazard ratios: 1.03, 0.59, 0.64, 1.00, and 1.85; 95% confidence intervals: 1.02‐1.04, 0.44‐0.78, 0.47‐0.87, 1.00‐1.00, and 1.51‐2.27; P values: <.001, <.001, .005, .032, and <.001; respectively) (Table 3).
FIGURE 1.
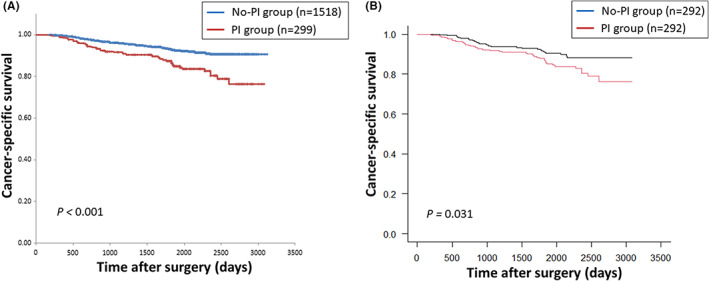
Kaplan‐Meier curves for cancer‐specific survival in all included patients (A) and propensity score matched patients (B) according to the presence or absence of postoperative infection
TABLE 3.
Univariate and multivariate Cox proportional hazard models in cancer‐specific survival and overall survival
| Cancer‐specific survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Variables | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value |
| Age | 1.010 | 0.996‐1.024 | .175 | 1.037 | 1.025‐1.050 | <.001 | 1.030 | 1.016‐1.045 | <.001 | |||
| Sex (female vs male) | 0.996 | 0.716‐1.386 | .982 | 0.634 | 0.485‐0.830 | <.001 | 0.585 | 0.437‐0.784 | <.001 | |||
| Body mass index (kg/m2) | 0.953 | 0.911‐0.997 | .035 | 0.997 | 0.952‐1.044 | .896 | 0.943 | 0.910‐0.976 | <.001 | 0.970 | 0.934‐1.010 | .135 |
| Location (rectum vs colon) | 1.365 | 0.996‐1.872 | .053 | 1.084 | 0.846‐1.389 | .524 | ||||||
| Smoking | 0.989 | 0.691‐1.415 | .950 | 1.291 | 0.995‐1.675 | .055 | ||||||
| Steroid use | 0.907 | 0.224‐3.660 | .891 | 1.337 | 0.551‐3.240 | .521 | ||||||
| Hemoglobin (g/dL) | 0.888 | 0.826‐0.955 | .001 | 0.964 | 0.879‐1.057 | .437 | 0.853 | 0.806‐0.901 | <.001 | 0.928 | 0.859‐1.002 | .057 |
| Albumin (g/dL) | 0.511 | 0.391‐0.666 | <.001 | 0.935 | 0.671‐1.302 | .689 | 0.433 | 0.355‐0.527 | <.001 | 0.759 | 0.572‐1.007 | .056 |
| CEA (ng/mL) | 1.001 | 1.000‐1.002 | .037 | 1.001 | 1.000‐1.003 | .135 | 1.001 | 1.000‐1.002 | .110 | |||
| CA19‐9 (U/mL) | 1.002 | 1.001‐1.003 | .003 | 1.001 | 1.000‐1.002 | .164 | 1.001 | 1.000‐1.002 | .013 | 1.001 | 1.000‐1.002 | .163 |
| Emergency surgery | 2.511 | 1.281‐4.923 | .007 | 1.476 | 0.598‐3.122 | .308 | 1.627 | 0.865‐3.061 | .131 | |||
| Stoma creation | 1.946 | 1.376‐2.752 | <.001 | 1.343 | 0.897‐2.012 | .152 | 1.580 | 1.194‐2.092 | .001 | 1.281 | 0.905‐1.814 | .163 |
| Laparoscopic surgery | 0.333 | 0.241‐0.459 | <.001 | 0.489 | 0.335‐0.713 | <.001 | 0.447 | 0.346‐0.578 | <.001 | 0.642 | 0.472‐0.872 | .005 |
| ASA score | 1.123 | 0.850‐1.496 | .404 | 1.526 | 1.229‐1.895 | <.001 | 1.226 | 0.947‐1.586 | .121 | |||
| Operation time (min) | 1.001 | 1.000‐1.002 | .080 | 1.000 | 0.999‐1.001 | .543 | ||||||
| Blood loss (ml) | 1.001 | 1.000‐1.001 | <.001 | 1.000 | 1.000‐1.001 | .109 | 1.001 | 1.000‐1.001 | <.001 | 1.000 | 1.000‐1.001 | .032 |
| Transfusion | 2.081 | 1.287‐3.365 | <.001 | 0.659 | 0.352‐1.232 | .191 | 2.140 | 1.484‐3.085 | <.001 | 0.713 | 0.439‐1.160 | .174 |
| Lymphnode dissection (D) a | 1.077 | 0.784‐1.480 | .647 | 0.886 | 0.705‐1.114 | .301 | ||||||
| Pathological stage | 4.228 | 3.115‐5.739 | <.001 | 5.242 | 3.611‐7.610 | <.001 | 1.762 | 1.493‐2.079 | <.001 | 1.851 | 1.509‐2.269 | <.001 |
| Adjuvant chemotherapy | 1.987 | 1.451‐2.722 | <.001 | 0.603 | 0.411‐0.885 | .010 | 0.910 | 0.766‐1.268 | .910 | |||
| Postoperative infection | 2.188 | 1.551‐3.087 | <.001 | 1.602 | 1.098‐2.337 | .015 | 1.621 | 1.219‐2.156 | <.001 | 1.268 | 0.916‐1.756 | .152 |
Abbreviations: ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; CI, confidence interval;
Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, 9th edition.
The propensity score model variables were age, sex, American Society of Anesthesiologists score, laparoscopic surgery, tumor location, and pathological stage. After PSM for the clinical and oncological factors, 292 balanced pairs were identified, and CSS and OS were analyzed. The PI group had significantly worse CSS compared with the No‐PI group (P = .031) (Figure 1B). In the OS analysis, the difference between the groups was not statistically significant (P = .303) (Figure S2B).
3.4. Type and severity of PI and survival
To investigate the survival impact by the type of PI, patients were divided into No‐PI (n = 1518), SSI only (n = 236), RI only (n = 50), and SSI plus RI (n = 13) groups. Kaplan‐Meier curves for CSS and OS stratified by the type of PI are shown in Figures 2, S3. The SSI only and SSI plus RI groups had significantly worse survival compared with that of the No‐PI group in both the CSS and OS analyses; RI had no survival impact in both analyses. To investigate the survival impact by the PI severity, patients were divided into a PI with CD grade ≥ III group (n = 68) and a PI with CD grade < III group (n = 145). Patients with non‐infectious complications were excluded to assess the effect of PI severity alone, in this analysis. The Kaplan‐Meier curves for both groups for CSS and OS almost overlapped, and there were no significant differences between the groups (Figure S4A,B; CSS and OS, respectively).
FIGURE 2.
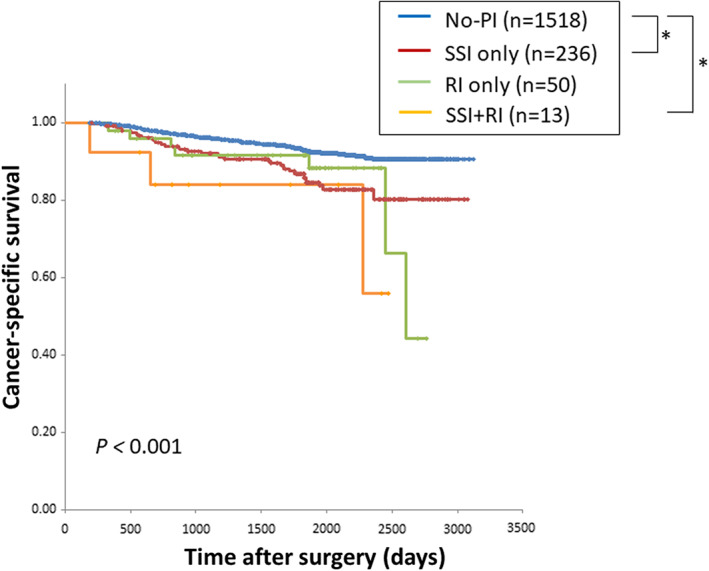
Kaplan‐Meier curve for cancer‐specific survival according to the type of postoperative infection. *P < .05
3.5. The effect of pathological stage on survival with or without PI
The Kaplan‐Meier curves for CSS and OS stratified by pathological stage are shown in Figures 3A‐C, S5A‐C, respectively. The frequencies of PI for each pathological stage were 14.4% for stage I, 19.1% for stage II, and 22.6% for stage III. PI had a significant oncological impact for stage II and III patients in the CSS analyses, and for stage III patients in the OS analyses; no differences were seen for stage I patients in both analyses.
FIGURE 3.
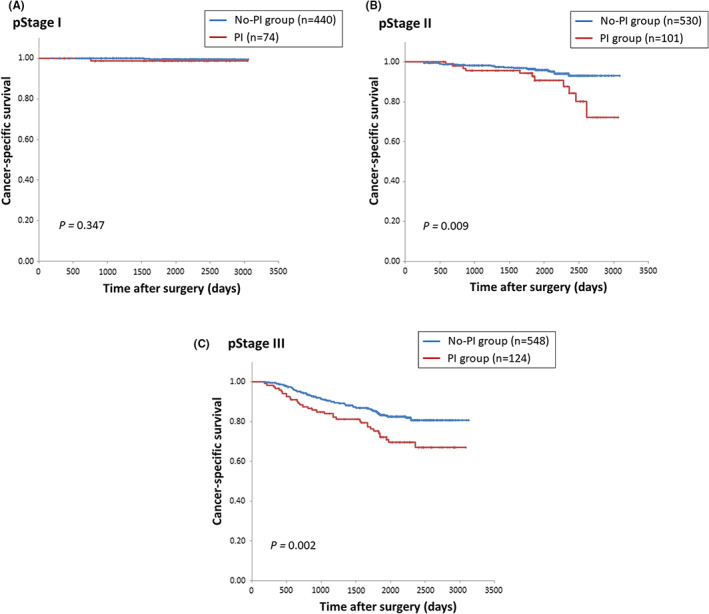
Kaplan‐Meier curves for cancer‐specific survival in patients with pStage I (A), pStage II (B), pStage III (C) according to the presence or absence of postoperative infection
3.6. Recurrence pattern according to PI
The total recurrence rate was significantly higher in the PI group than that in the No‐PI group (23.75% vs 15.48%, respectively; P = .001). Among the various recurrence sites, the PI group had a significantly higher locoregional recurrence rate than that in the No‐PI group (7.36% vs 4.35%, respectively; P = .038) (Table 4).
TABLE 4.
Details of recurrence pattern according to postoperative infection
| Recurrence site | No‐PI (n = 1518) | PI (n = 299) | P value |
|---|---|---|---|
| Total | 235 (15.48) | 71 (23.75) | .001 |
| Liver | 85 (5.60) | 20 (6.69) | .497 |
| Lung | 50 (3.29) | 13 (4.35) | .386 |
| Locoregional | 66 (4.35) | 22 (7.36) | .038 |
| Dissemination | 25 (1.65) | 9 (3.01) | .156 |
| Others | 6 (0.40) | 7 (2.34) | .002 |
| Interval to recurrence (days) a | 400 (235‐819) | 367 (185‐665) | .282 |
Note: The values are presented as n (%).
Median (interquartile range).
3.7. Influence of PI on the efficacy of adjuvant chemotherapy
To investigate the influence of PI on the efficacy of adjuvant chemotherapy, pathological stage III patients were divided into four groups according to PI and adjuvant chemotherapy. The Kaplan‐Meier curves for CSS and OS in the four groups are shown in Figures 4, S6, respectively. Although adjuvant chemotherapy significantly improved CSS and OS in patients without PI, the presence of PI abolished the survival benefit. Additionally, the presence of PI still had a negative survival impact, even if adjuvant chemotherapy was administered.
FIGURE 4.
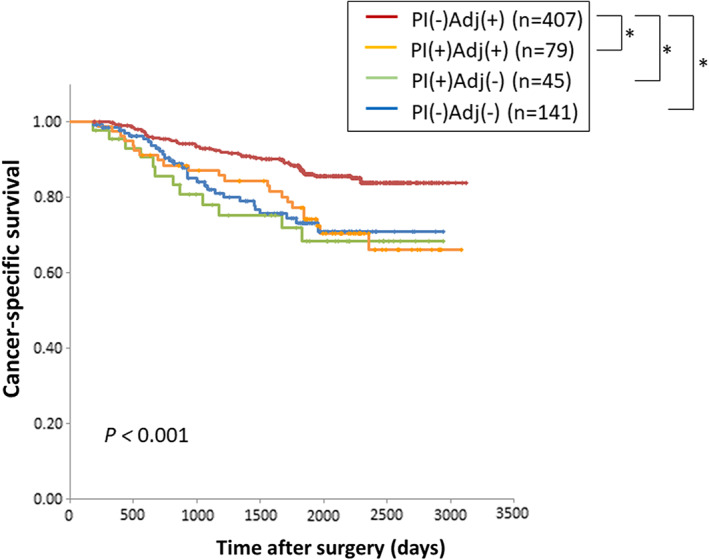
Kaplan‐Meier curves for cancer‐specific survival according to the presence or absence of postoperative infection and adjuvant chemotherapy. *P < .05
4. DISCUSSION
This was a multi‐institutional retrospective study comprising 1817 patients with stage I‐III CRC who underwent curative surgery. We demonstrated that patients with PI had a poor prognosis regarding CSS but not OS. This association was confirmed by multivariate Cox proportional hazard models and PSM. As additional findings, we showed that 1) RI had no negative oncological impact, 2) PI severity was not associated with oncological outcomes, 3) more advanced pathological stage had a greater negative oncological impact in the presence of PI, and 4) the presence of PI abolished the survival benefit of adjuvant chemotherapy.
Among the varied incidences of PI worldwide, the Japanese retrospective survey data in 2015 reported that the PI incidence was 13.7% in colorectal surgery, 14 which is consistent with the frequency of 16.5% in this study. SSIs are receiving greater attention. The negative impact of SSIs on short‐term outcomes, including longer hospital stays and increased medical costs, as well as the deleterious effects on oncological outcomes, especially regarding AL, are well‐reported in curative CRC surgery. 6 , 7 , 8 However, several studies showed heterogenous results. 10 , 11 , 12 A plausible reason for the discrepancies between study results is the long patient inclusion periods in previous studies, which affects the difference in treatment efficacy for metastasized patients because of the dramatic improvements in chemotherapeutic and molecular‐targeted agents over time. The current study included patients during only a 2‐year period, which might eliminate the confounding effect of medical advances over time.
In contrast to SSI, RI after gastrointestinal surgery has not attracted much research. The main cause of SSIs is intraoperative contamination with bacteria, including intestinal flora and resident skin flora. However, most RIs are caused by cross‐infection with bacterial contaminants in the hospital environment via the hands of medical staff. 17 Studies reporting the epidemiology and influence of RIs on oncological outcomes are scarce. Niitsuma et al 14 reported that the incidence of RI in Japanese digestive surgical patients was 3.7%, which is consistent with the result of 3.5% in our study of patients undergoing CRC surgery. These incidences were lower than those of reports in other countries, 18 , 19 which could be because of the specific medical systems in Japan in which surgeons manage all aspects of perioperative patient care, including PI prevention and treatment. Although the reported incidences of PI are lower in the Japanese cohort, similar disadvantages regarding the length of hospital stay and medical costs associated with RI and SSI make it reasonable to comprehensively analyze the oncological impact of PI.
In the comparison of the PI and No‐PI groups, the patients' backgrounds were potentially biased, as shown in Table 1. These background differences, such as sex, tumor location, and nutritional condition, could influence both PI occurrence and patient survival. Therefore, we performed both multivariate analyses and PSM to minimize potential bias and were able to conclude that PI had a negative oncological impact on CSS. However, the sub‐analysis demonstrated that RI had no impact on both CSS and OS. Wide variations in the RI incidence and the small sample size in the RI cohort (n = 63) may have affected the sub‐analysis, and a future larger‐scale study is warranted.
The potential mechanism underlying impaired survival by PI may involve the production and activation of proinflammatory cytokines and mediators both locally and systemically, which has been shown to promote micrometastasis. 20 Tsujimoto et al 21 recently explored the finding that PI induced higher circulating hepatocyte growth factor concentrations, which was followed by tumor progression and metastasis via the hepatocyte growth factor/c‐Met signaling pathway. Perego et al 22 reported that stress‐induced oxidized lipids can upregulate the fibroblast growth factor pathway in cancer cells, drive the reaction of dormant tumor cells, and promote the development of metastasis. As additional possible mechanisms underlying impaired survival by PI, we speculated on the following: 1) the association of PI with increased stage, 23 which was also shown in this study; 2) delayed or canceled adjuvant chemotherapy; 24 and 3) abdominal implantation of intraluminal cancer cells in the case of AL. 25
Although we defined PI as all postoperative infectious complications regardless of the type, whose influences to systemic inflammatory responses and tumor immunity may differ, the negative prognostic impact of the mildest PI, namely superficial SSI, is skeptical. To investigate it, we performed additional analyses. Patients with superficial SSI only (excluding overlapped patients with other types of PI) had no significant impact on CSS compared with the No‐PI group (P = .429) (Figure S7A). The PI (excluding superficial SSI) group a had significantly worse survival compared with the No‐PI (including superficial SSI) group for CSS (P < .001) (Figure S7B). The hazard ratio in the univariate CSS analysis was larger than the hazard ratio in the comparison between the PI and the No‐PI groups (2.414 and 2.188, respectively). Taken together, the negative prognostic impact of superficial SSI might be marginal, and PI excluding superficial SSI had an enhanced negative prognostic impact. Additionally, we compared CSS between patients with No‐PI and organ/space SSI only. The analysis demonstrated a statistically significant difference between the groups (P = .001) (Figure S7C). Although the difference of direct comparison between patients with superficial SSI only and organ/space SSI only was not statistically significant (P = .280), the negative prognostic impact on CCS might be at least equivalent or higher in organ/space SSI than that of superficial SSI.
Interestingly, our data demonstrated that the severity of PI did not affect the degree of negative oncological impact. Previous studies reported heterogenous results regarding this issue. Duraes et al 26 reported that postoperative complications with higher CD grades had a greater negative impact on OS and relapse‐free survival in CRC surgery. In contrast, Oh et al 27 demonstrated no significant difference in disease‐free survival between major (CD grade ≥ III) and minor postoperative complications (CD grade < III) in patients who underwent CRC surgery. A meta‐analysis by McSorley et al 28 involving 1879 patients who underwent primary CRC surgery also reported that complication severity had no significant impact on disease‐free survival. The underlying mechanism linking the severity of PI and oncological outcomes is not currently understood. The degree of exaggerated postoperative systemic inflammatory host responses after developing a PI, which could be a trigger for cancer progression followed by the development of recurrence, is not only dependent on the PI severity itself, but is also strongly dependent on the patient's anti‐tumor immunological potential. 29
Our results demonstrated that stoma creation was associated with PI occurrence and was identified as a prognostic factor for both CSS and OS in the univariate analyses, although these did not reach statistically significances in multivariate analyses. Stoma creation, which is a distinct procedure in CRC surgeries and is prone to be performed in high‐risk patients, could be a considerable confounding factor for oncological outcomes in CRC surgical patients. Therefore, future studies were warranted to clarify the negative oncological impact focusing on stoma creation. This study demonstrated that patients with PI had significantly higher rates of total and locoregional recurrence. Substantial evidence reported that intraluminal viable cancer cells shed from the bowel may attach to stapling devices, resulting in enhanced tumor dissemination in the event of AL or reoperation. 30 , 31 , 32 AL, which was the most frequent PI in this study, could induce extraluminal implantation and has the effect of upstaging the disease and increasing the locoregional recurrence rate.
Regarding adjuvant chemotherapy, the proportion of stage III patients who received adjuvant chemotherapy was significantly lower in the PI group than that in the No‐PI group (63.7% vs 74.3%, respectively; P = .020). Moreover, a significant delay in initiating adjuvant chemotherapy was observed in the PI group compared with the No‐PI group in stage III patients (median [interquartile range]) (53 (40‐77) days vs 36 (29‐46) days, respectively; P < .001). This delay and the tumor‐promoting inflammatory microenvironment induced by a PI could be the mechanism underlying the abolished survival benefit with adjuvant chemotherapy in patients with PI. 33 , 34 , 35 To improve oncological outcomes in patients with PI, earlier implementation of adjuvant chemotherapy as well as intensive regimens should be considered.
Several limitations in this study warrant mention. This was a retrospective cohort study; therefore, there might have been unmeasurable confounding factors that could have influenced the study results, even though we tried to minimize bias using multivariate analysis and PSM. The protocol of this study did not define and require the criteria of surveillance program in each institute. However, since the participating institutions in this study were leading institutions in the Japan Society of Surgical Infection, we believe that a certain level of quality of the surveillance was ensured. As another limitation, detailed information regarding the regimens and the dose intensity of adjuvant chemotherapy was not available. Finally, the current study did not evaluate biological mechanisms or how they adversely affect PI and oncological outcomes.
In conclusion, this study indicated that PI after CRC surgery was associated with impaired oncological outcomes, ever after adjusting for differences in the patients' backgrounds. This survival disadvantage of PI was primarily derived from SSI, not RI, and PI induced lower efficacy of adjuvant chemotherapy. However, the weak implementation of adjuvant chemotherapy, such as less intensive regimen and lower dose intensity than the standardized therapy, might be the plausible reason for the less oncological benefit of adjuvant chemotherapy in patients with PI. Based on our findings, strategies to prevent PI and implement appropriate postoperative treatment may improve the quality of care and oncological outcomes in patients undergoing curative CRC surgery.
DISCLOSURE
Author Contributions: Conception/design (Matsuda A, Maruyama H, Akagi S, Inoue T, Uemura K, Kobayashi M, Shiomi H, Watanabe M); data acquisition (all authors); data interpretation (all authors); critical revisions (all authors); final approval (all authors).
Conflict of Interest: Y. Kitagawa received designated donation from Chugai Pharmaceutical Co., Ltd, TAIHO Pharmaceutical Co., Ltd, ASAHI KASEI Pharma Corporation, Otsuka Pharmaceutical Factory Inc, ONO Pharmaceutical Co., Ltd, SHIONOGI & Co., Ltd, Nippon Covidien Inc, AstraZeneca K. K, Ethicon Inc, Bristol‐Myers Squibb K.K, Olympus Corporation, MSD K.K, Smith & Nephew K.K, KAKEN Pharmaceutical Co., Ltd, ASKA Pharmaceutical Co., Ltd,, Miyarisan Pharmaceutical Co., Ltd, Yakult Honsha Co., Ltd, TSUMURA & Co, DAINIPPON SUMITOMO Pharma Co., Ltd, EA Pharma Co., Ltd, Eisai Co., Ltd, MEDICON Inc, Kyowa Hakko Kirin Co., Ltd, Takeda Pharmaceutical Co., Ltd, and Toyama Chemical Co., Ltd, Asellas Pharma Inc, TEIJIN Pharma Limited, Nihon Pharmaceutical Co., Ltd. His institution has endowed chairs of Chugai Pharmaceutical Co., Ltd and TAIHO Pharmaceutical Co., Ltd. Y. Kitagawa is a chief editor of the Annals of Gastroenterological Surgery. He is not involved in the editorial of or decision to accept this article for publication.
Ethics Approval: The protocol for this study was approved by the Ethics Committee of Nippon Medical School Tama Nagayama Hospital (Approval No. 694).
Informed Consent: N/A (The need to obtain written informed consent from the included patients was waived because of the retrospective nature of the study).
Registry and the Registration No. of the study/Trial: N/A (The registration was not required because of the retrospective nature of the study).
Animal Studies: N/A.
Supporting information
Figure S1
Figure S2A
Figure S2B
Figure S3
Figure S4A
Figure S4B
Figure S5A
Figure S5B
Figure S5C
Figure S6
Figure S7A
Figure S7B
Figure S7C
ACKNOWLEDGEMENTS
The authors would like to extend our deep appreciation to Prof. Shinya Kusachi, immediate past president of the society, and Prof. Kazuo Tanemoto, director of the clinical trial committee of the society for their considerable cooperation. The authors also thank Jane Charbonneau, DVM, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Matsuda A, Maruyama H, Akagi S, Inoue T, Uemura K, Kobayashi M, et al. Do postoperative infectious complications really affect long‐term survival in colorectal cancer surgery? A multicenter retrospective cohort study. Ann Gastroenterol Surg. 2023;7:110–120. 10.1002/ags3.12615
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Anderson DJ, Kaye KS, Classen D, Arias KM, Podgorny K, Burstin H, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S51–61. [DOI] [PubMed] [Google Scholar]
- 3. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–97. [DOI] [PubMed] [Google Scholar]
- 4. Nishimuta H, Kusachi S, Watanabe M, Asai K, Kiribayashi T, Niitsuma T, et al. Impact of postoperative remote infection on length of stay and medical costs in hospitals in Japan. Surg Today. 2021;51(2):212–8. [DOI] [PubMed] [Google Scholar]
- 5. Ohge H, Mayumi T, Haji S, Kitagawa Y, Kobayashi M, Kobayashi M, et al. The Japan Society for Surgical Infection: guidelines for the prevention, detection, and management of gastroenterological surgical site infection, 2018. Surg Today. 2021;51(1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long‐term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497–505. [DOI] [PubMed] [Google Scholar]
- 7. Goto S, Hasegawa S, Hida K, Uozumi R, Kanemitsu Y, Watanabe T, et al. Multicenter analysis of impact of anastomotic leakage on long‐term oncologic outcomes after curative resection of colon cancer. Surgery. 2017;162(2):317–24. [DOI] [PubMed] [Google Scholar]
- 8. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta‐analysis. Ann Surg. 2011;253(5):890–9. [DOI] [PubMed] [Google Scholar]
- 9. Lawler J, Choynowski M, Bailey K, Bucholc M, Johnston A, Sugrue M. Meta‐analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open. 2020;4(5):737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espin E, Ciga MA, Pera M, Ortiz H, Spanish Rectal Cancer Project . Oncological outcome following anastomotic leak in rectal surgery. Br J Surg. 2015;102(4):416–22. [DOI] [PubMed] [Google Scholar]
- 11. Jang JH, Kim HC, Huh JW, Park YA, Cho YB, Yun SH, et al. Anastomotic leak does not impact oncologic outcomes after preoperative chemoradiotherapy and resection for rectal cancer. Ann Surg. 2019;269(4):678–85. [DOI] [PubMed] [Google Scholar]
- 12. Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256(6):1034–8. [DOI] [PubMed] [Google Scholar]
- 13. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132; quiz 3‐4. discussion 96. [PubMed] [Google Scholar]
- 14. Niitsuma T, Kusachi S, Takesue Y, Mikamo H, Asai K, Watanabe M. Current status of postoperative infections after digestive surgery in Japan: The Japan Postoperative Infectious Complications Survey in 2015. Ann Gastroenterol Surg. 2019;3(3):276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maruyama H, Kusachi S, Yoshida H, Makino H, Nishimuta H, Niitsuma T. Association of Respiratory Tract Infection after Gastroenterological Surgery with postoperative duration of hospitalization and medical expenses: subanalysis of data from a multicenter study. J Nippon Med Sch. 2020;87(5):252–9. [DOI] [PubMed] [Google Scholar]
- 18. Chenoweth CE, Gould CV, Saint S. Diagnosis, management, and prevention of catheter‐associated urinary tract infections. Infect Dis Clin North Am. 2014;28(1):105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yun JS, Na KJ, Song SY, Kim S, Jeong IS, Oh SG. Comparison of perioperative outcomes following hybrid minimally invasive versus open Ivor Lewis esophagectomy for esophageal cancer. J Thorac Dis. 2017;9(9):3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bohle B, Pera M, Pascual M, Alonso S, Mayol X, Salvado M, et al. Postoperative intra‐abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery. 2010;147(1):120–6. [DOI] [PubMed] [Google Scholar]
- 21. Tsujimoto H, Horiguchi H, Matsumoto Y, Takahata R, Shinomiya N, Yamori T, et al. A potential mechanism of tumor progression during systemic infections via the Hepatocyte Growth Factor (HGF)/c‐met signaling pathway. J Clin Med. 2020;9(7):2074. 10.3390/jcm9072074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, et al. Reactivation of dormant tumor cells by modified lipids derived from stress‐activated neutrophils. Sci Transl Med. 2020;12(572):eabb5817. 10.1126/scitranslmed.abb5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richards CH, Platt JJ, Anderson JH, McKee RF, Horgan PG, McMillan DC. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg. 2011;254(1):83–9. [DOI] [PubMed] [Google Scholar]
- 24. Krarup PM, Nordholm‐Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long‐term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259(5):930–8. [DOI] [PubMed] [Google Scholar]
- 25. Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra‐abdominal abscess on cancer‐related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum. 2009;52(3):380–6. [DOI] [PubMed] [Google Scholar]
- 26. Duraes LC, Stocchi L, Steele SR, Kalady MF, Church JM, Gorgun E, et al. The relationship between clavien‐dindo morbidity classification and oncologic outcomes after colorectal cancer resection. Ann Surg Oncol. 2018;25(1):188–96. [DOI] [PubMed] [Google Scholar]
- 27. Oh CK, Huh JW, Lee YJ, Choi MS, Pyo DH, Lee SC, et al. Long‐term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol. 2020;36(4):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McSorley ST, Horgan PG, McMillan DC. The impact of the type and severity of postoperative complications on long‐term outcomes following surgery for colorectal cancer: a systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2016;97:168–77. [DOI] [PubMed] [Google Scholar]
- 29. Roxburgh CS, Horgan PG, McMillan DC. The perioperative immune/inflammatory insult in cancer surgery: time for intervention? Onco Targets Ther. 2013;2(12):e27324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gertsch P, Baer HU, Kraft R, Maddern GJ, Altermatt HJ. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35(3):238–41. [DOI] [PubMed] [Google Scholar]
- 31. Jenner DC, de Boer WB, Clarke G, Levitt MD. Rectal washout eliminates exfoliated malignant cells. Dis Colon Rectum. 1998;41(11):1432–4. [DOI] [PubMed] [Google Scholar]
- 32. Matsuda A, Kishi T, Musso G, Matsutani T, Yokoi K, Wang P, et al. The effect of intraoperative rectal washout on local recurrence after rectal cancer surgery: a meta‐analysis. Ann Surg Oncol. 2013;20(3):856–63. [DOI] [PubMed] [Google Scholar]
- 33. Gao P, Huang XZ, Song YX, Sun JX, Chen XW, Sun Y, et al. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population‐based study. BMC Cancer. 2018;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda A, Yamada T, Matsumoto S, Sakurazawa N, Kawano Y, Shinozuka E, et al. Pretreatment neutrophil‐to‐lymphocyte ratio predicts survival after TAS‐102 treatment of patients with metastatic colorectal cancer. Anticancer Res. 2019;39(8):4343–50. [DOI] [PubMed] [Google Scholar]
- 35. Merkow RP, Bentrem DJ, Mulcahy MF, Chung JW, Abbott DE, Kmiecik TE, et al. Effect of postoperative complications on adjuvant chemotherapy use for stage III colon cancer. Ann Surg. 2013;258(6):847–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2A
Figure S2B
Figure S3
Figure S4A
Figure S4B
Figure S5A
Figure S5B
Figure S5C
Figure S6
Figure S7A
Figure S7B
Figure S7C
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


