Abstract
As life expectancy increases, the older population continues to grow rapidly, resulting in increased requirement for surgery for older patients with gastrointestinal cancer. Older individuals represent a heterogeneous group in terms of physiological reserves, co‐morbidity, cognitive impairment, and disability. Owing to the lack of treatment guidelines for vulnerable patients with gastrointestinal cancer, these patients are more likely to be at risk of undertreatment or overtreatment. Hence, the identification of frail patients with gastrointestinal cancer would improve cancer treatment outcomes. Although there is no standardized geriatric assessment tool, a growing body of research has shown associations of frailty with adverse postoperative outcomes and poor prognosis after resection of gastrointestinal tract and hepatobiliary‐pancreatic cancers. Emerging evidence suggests that prehabilitation, which includes exercise and nutritional support, can improve preoperative functional capacity, postoperative recovery, and surgical outcomes, particularly in frail patients with gastrointestinal cancer. We reviewed major geriatric assessment tools for identification of frail patients and summarized clinical studies on frailty and surgical outcomes, as well as prehabilitation or rehabilitation in gastrointestinal tract and hepatobiliary‐pancreatic cancers. The integration of preoperative geriatric assessment and prehabilitation of frail patients in clinical practice may improve surgical outcomes. In addition, improving preoperative vulnerability and preventing functional decline after surgery is important in providing favorable long‐term survival in patients with gastrointestinal cancer. Further clinical trials are needed to examine the effects of minimally invasive surgery, and chemotherapy in frail patients with gastrointestinal cancer.
Keywords: disability, elderly, impairment, oncology, sarcopenia
Accumulating evidence demonstrates that the integration of preoperative geriatric assessment and prehabilitation for frail patients into clinical practice may improve perioperative and long‐term outcomes in patients with gastrointestinal cancer. We proposed an example for the integration of older patients‐specific care into treatment strategies for gastrointestinal cancer.
1. INTRODUCTION
Gastrointestinal carcinomas are a leading cause of death worldwide. 1 As life expectancy increases, the number of older individuals with gastrointestinal cancer has increased in Japan and worldwide. 2 , 3 , 4 Older individuals are a heterogeneous group, in terms of physiologic reserves, co‐morbidity, cognitive impairment, and disability. 5 Because older patients with gastrointestinal cancer are underrepresented in clinical cancer trials, those patients are more likely to be at risk for undertreatment or overtreatment. 6 , 7 , 8
Frailty is a syndrome characterized by reduced physiological reserve from stressors due to age‐related disability. 9 , 10 Poor treatment tolerance, adverse postoperative outcomes, and poor prognosis have been associated with frail patients with cancer. 4 , 11 , 12 Hence, the identification of frail patients with cancer would improve cancer treatment outcomes through patient selection and optimization prior to surgery. Geriatric assessment (GA) is an assessment that includes functional capacity, mobility, cognition, emotional status, nutritional status, comorbidities, polypharmacy, and social support. 13 , 14 According to this assessment, frailty can be improved by perioperative rehabilitation. Prehabilitation is a preoperative multidisciplinary intervention to prevent or minimize functional decline after surgery and improve postoperative outcomes. 15
Herein, we reviewed major GA tools to identify frail patients, and summarized clinical studies on frailty and surgical outcomes as well as prehabilitation or rehabilitation in gastrointestinal tract and hepatobiliary‐pancreatic (HBP) cancers.
2. GA TOOLS FOR PATIENTS WITH GASTROINTESTINAL CANCERS
Table 1 shows the diagnostic properties of eight GA tools that have been reported in studies on gastrointestinal tract and HBP cancers. The Geriatric‐8 (G8) comprises seven items, as well as an indication of age, as follows: nutritional status, weight loss, body mass index, motor skills, psychological status, number of medications, and self‐perception of health. 16 , 17 Age was considered in three categories (<80, 80‐85, and >85 years). Overall, the G8 score ranges from 0 (heavily impaired) to 17 (not at all impaired). The diagnostic accuracy of the G8 has been investigated in three studies. 18 , 19 , 20 The G8 score had moderate‐to‐high sensitivity (77%‐97%), low‐to‐moderate specificity (44%‐64%), and moderately high accuracy for frailty (AUC, 0.71‐0.80).
TABLE 1.
Accuracy of geriatric assessment tools in patients with cancer
| Assessment tool | Evaluation items | Author (year) | Sensitivity % | Specificity % | Area under curve |
|---|---|---|---|---|---|
| Geriatric‐8 | (1) age, (2) food intake, (3) weight loss, (4) body mass index, (5) motor skills, (6) psychological status, (7) number of medications, (8) self‐perception of health | Kenig et al (2014) | 97% | 44% | 0.71 |
| Soubeyran et al (2014) | 77% | 64% | 0.80 | ||
| Russo et al (2018) | 89% | 49.5% | 0.79 | ||
| Abbreviated Comprehensive Geriatric Assessment | (items 1‐4) geriatric depression scale, (5) bathing, (6) transfer, (7) continence, (8) shopping, (9) preparing own meals, (10) doing housework, (11) doing own laundry, (12) attention and calculation, (13) reading, (14) writing, (15) copying | Kenig et al (2014) | 84% | 86% | 0.85 |
| Fried Frailty Criteria | (1) weight loss, (2) self‐reported exhaustion, (3) grip strength, (4) walking speed, (5) physical activity | Kenig et al (2014) | 52% | 92% | 0.72 |
| Groningen Frailty Indicator | (1) shopping, (2) walk outside house, (3) getting (un)dressed, (4) visiting restroom, (5) impaired vision, (6) impaired hearing, (7) weight loss, (8) co‐morbidity, (9) cognition, (items 10‐14) depression scale, (15) physical fitness | Kenig et al (2014) | 64% | 86% | 0.74 |
| Clinical Frailty Scale | (1) very fit, (2) well, (3) managing well, (4) vulnerable, (5) mildly frail, (6) moderately frail, (7) severely frail | Kenig et al (2014) | 54% | 100% | 0.77 |
| Balducci Frailty Criteria | (1) age, (2) ADL, (3) co‐morbidity, (4) geriatric syndromes: delirium, dementia, depression, osteoporosis, incontinence, falls, neglect and abuse, and failure to thrive | Kenig et al (2014) | 84% | 50% | 0.67 |
| Kihon Checklist | Instrumental (3 questions) and social (4 questions) activities of daily living, physical functions (5 questions), nutritional status (2 questions), oral function (3 questions), cognitive function (3 questions), and depressive mood (5 questions) | Satake et al (2016) | 90% | 81% | 0.89 |
| Usual gait speed | <1 m/s | Pamoukdjian et al (2017) | 79% | 65% | 0.82 |
The diagnostic accuracy of the following tools was investigated in the study by Kenig et al 18 : abbreviated Comprehensive Geriatric Assessment (CGA), the Fried Frailty Criteria, the Groningen Frailty Indicator, Balducci Frailty Criteria, and the Clinical Frailty Scale (Table 1). The abbreviated CGA is composed of items from the Geriatric Depression Scale, Mini‐Mental State Examination, activities of daily living (ADL), and the independent activities of daily living (IADL). 21 The abbreviated CGA had high sensitivity (84%), specificity (86%), and accuracy for frailty (AUC, 0.85).
The Fried Frailty Criteria defined frailty as a clinical syndrome in which three or more of the following criteria were present: unintentional weight loss, self‐reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity. 22 The Fried Frailty Criteria had low sensitivity (52%), high specificity (92%), and moderate accuracy for frailty (AUC, 0.72). The Groningen Frailty Indicator consists of 15 questionnaire screenings for self‐reported limitations. 23 The Groningen Frailty Indicator had moderate sensitivity (64%), high specificity (86%), and moderate‐to‐high accuracy for frailty (AUC, 0.74).
The Balducci Frailty Criteria defined frailty based on fulfilling any of the following criteria from the components of CGA: dependence in one or more ADL, three or more comorbidities, and/or one or more geriatric syndromes. 24 The Balducci Frailty Criteria had high sensitivity (84%), low specificity (50%), and moderate accuracy for frailty (AUC, 0.67). The Clinical Frailty Scale (CFS) is a semiquantitative tool that provides a global score ranging from 1 (very fit) to 9 (terminally ill) to reflect the following domains: disability for basic and instrumental activities of daily living, mobility, activity, energy, and disease‐related symptoms. 25 The CFS had low sensitivity (54%), high specificity (100%), and moderate‐to‐high accuracy for frailty (AUC, 0.77). Usual gait speed had moderate sensitivity (79%), moderate specificity (81%), and moderate‐to‐high accuracy for frailty (AUC, 0.82) in a study by Pamoukdjian et al. 26
The Kihon checklist (KCL) can identify individuals at an increased risk of requiring care or support. It consists of 25 questions regarding ADLs, physical strength, nutrition, cognition, and mood. 27 Satake et al 28 found that the KCL had high sensitivity (90%), high specificity (81%), and high accuracy for frailty (AUC, 0.89).
A variety of GA tools have been used due to the lack of an exact definition. According to studies evaluating the diagnostic accuracy of the GA tools, the abbreviated CGA and the KCL may be useful in assessing frailty. However, those studies were mostly retrospective. Hence, geriatric‐specific data should be collected routinely in future clinical trials for gastrointestinal cancer to establish a standardized assessment of frailty.
3. FRAILTY AND OUTCOMES FOLLOWING SURGERY FOR GASTROINTESTINAL TRACT CANCERS
Table 2 shows the association of frailty with outcomes following surgery for gastrointestinal tract cancer. In upper gastrointestinal tract cancers, one study by Tanaka et al 29 examined CFS retrospectively in 96 patients aged over 65 years who underwent laparoscopic gastrectomy for gastric cancer. The authors found that scores of ≥5 on the CFS were associated with worse overall survival (multivariable hazard ratio [HR]: 3.43, 95% confidence interval [CI]: 1.43‐8.22, P = 0.006) and cancer‐specific survival (multivariable HR: 4.00, 95%CI: 1.20‐13.3, P = 0.024). However, the CFS scores were not significantly associated with the length of postoperative hospital stay or the incidence of overall complications (≥ Clavien‐Dindo [CD] grade II) (multivariable odds ratio [OR]: 2.72, 95%CI: 0.81‐9.20, P = 0.11).
TABLE 2.
Major studies on geriatric assessment and perioperative outcomes in gastrointestinal tract cancers
| Author (year) | Study cohort | Geriatric assessment tool/cut‐off value (% of frail patients) | Postoperative complications | Postoperative mortality | Length of hospital stay | Patient survival |
|---|---|---|---|---|---|---|
| Gastric cancer | ||||||
| Tanaka et al (2019) |
|
|
NS | NS | NS |
↓ OS: HR 3.4 (95%CI, 1.4‐8.2) CSS: HR 4.0 (95%CI, 1.2‐13.3) |
| Colorectal cancer | ||||||
| Tamura et al (2021) |
|
|
↑ (CD ≥I, 28% vs 15%, P = 0.001) |
– | – | – |
| Bessems et al (2021) |
|
|
↑ (CD ≥I, 62% vs 28%, P < 0.001) |
NS |
↑ (9 days vs 8 days, P = 0.009) |
– |
| Mima et al (2020) |
|
|
NS | NS | – |
↓ OS: HR 2.0 (95%CI, 1.4‐3.0) RFS: HR 1.7 (95%CI, 1.3‐2.3) |
| Okabe et al (2019) |
|
|
↑ (CD III/IV, 23% vs 8%, P = 0.001) |
NS |
↑ (13 days vs 10 days, P < 0.001) |
– |
| Fagard et al (2017) |
|
|
↑ (CD ≥II, 48% vs 31%, P = 0.020) |
– | – | – |
| Reisinger et al (2015) |
|
|
↑ (Sepsis, 15% vs 4.4%, P = 0.03) |
NS | – | – |
| Ommundsen et al (2014) |
|
|
– | – | – |
↓ OS: HR 3.6 (95%CI, 2.3‐5.5) |
| Tan et al (2012) |
|
|
↑ (CD ≥II, 48% vs 18%, P = 0.006) |
– | – | – |
| Kristjansson et al (2010) |
|
|
↑ (CD ≥II, 62% vs 33%, P = 0.002) |
– | – | – |
Note: ↑, higher incidence of postoperative complications or mortality, and longer length of hospital stay in frail patients; ↓, shorter overall, cancer‐specific, or recurrence‐free survival in frail patients; −, not examined.
Abbreviations: CD, Clavien‐Dindo grade; CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; NS, not significant (P > 0.05); OS, overall survival; RFS, recurrence‐free survival.
An increasing number of studies have investigated the associations of geriatric assessment tools, including the Kihon Checklist, G8, gait speed test, CFS, Balducci Frailty Criteria, Groningen Frailty Indicator, Fried Frailty Criteria, and abbreviated CGA, with postoperative outcomes after resection of colorectal cancer. Tamura and colleagues examined frailty with the use of the Kihon checklist in 500 patients over 65 years who underwent elective colorectal cancer resection. 30 They found that a score of ≥8 on the Kihon checklist was a significant predictor of postoperative overall complications (≥ CD grade I) with an OR of 1.88 (95% CI: 1.66‐3.04) in the multivariate analysis.
Two studies examined the associations between CFS score and postoperative outcomes after resection of colorectal cancer. In the study by Okabe, scores of ≥4 on CFS were significantly associated with longer length of postoperative hospital stay (13 days vs 10 days, P < 0.001) and higher incidence of severe complications (≥ CD grade III or IV, 23% vs 8%, P = 0.001). 31 Mima et al 12 examined the CFS in 729 colorectal cancer patients undergoing curative resection. The authors found that scores of ≥4 on the CFS were independently associated with shorter OS (multivariable HR: 2.04, 95% CI: 1.40‐2.99, P < 0.001) and recurrence‐free survival (multivariable HR: 1.70, 95%CI: 1.25‐2.31, P < 0.001) in multivariable analyses adjusting for potential confounders, including age and disease stage. However, the CFS scores were not significantly associated with the incidence of severe complications (CD ≥ grade III, P = 0.67). In a retrospective study by Fagard and colleagues, a G8 score of ≤14 was associated with a higher incidence of postoperative complications (≥ CD grade II). 32 Bessems et al 33 screened frailty combining G8 and 4‐m gait speed test (4MGST) in 149 patients over 70 years who underwent elective colorectal cancer resection. They found that G8 ≤14 and/or 4MGST <1 m/s were associated with a higher incidence of postoperative overall complications (≥ CD grade I, 62% vs 28%, P < 0.001) and longer length of hospital stay (9 days vs 8 days, P = 0.009).
Ommundsen et al 34 examined the Balducci Frailty Criteria in 178 patients with colorectal cancer over 70 years of age who underwent elective colorectal cancer resection. The authors found that frailty based on the Balducci Frailty Criteria was independently associated with shorter OS (multivariable HR: 3.6, 95% CI: 2.3‐5.5) in multivariable analyses adjusting for disease stage. Tan et al 35 examined the ability of the Fried criteria for frailty to predict postoperative complications after resection of colorectal cancer. They found frailty to be an independent predictor of postoperative complications (CD ≥ grade II). The OR of these complications was 4.08 (95% CI: 1.43‐11.64). In a retrospective study by Kristjansson et al, 36 frailty based on the abbreviated CGA was an independent predictor of postoperative complications (≥ CD grade II, multivariable OR: 3.13, 95% CI: 1.65‐5.92). Reisinger et al 37 examined associations of the Groningen Frailty Indicator, which screened physical, cognitive, social, and emotional status, with postoperative complications after resection of colorectal cancer. The Groningen Frailty Indicator score of ≥5 was associated with a higher incidence of postoperative sepsis (univariable OR: 3.96, 95%CI: 1.14‐13.83, P = 0.03). The Groningen Frailty Indicator score ≥5 was not significantly associated with the incidence of postoperative mortality (P = 0.72) or anastomotic leakage (P = 0.62).
4. FRAILTY AND OUTCOMES FOLLOWING SURGERY FOR HBP CANCERS
Table 3 shows the association between frailty and outcomes following surgery for HBP cancers. Rostoft et al 38 also reviewed the associations between preoperative frailty, geriatric assessment, and surgical outcomes, especially in HBP cancers. Studies from Japan have investigated the associations of geriatric assessment tools, including the Kihon Checklist, G8, and CFS, with postoperative outcomes after resection of hepatocellular carcinoma (HCC). Two studies examined the Kihon Checklist in relation to postoperative outcomes after HCC resection. Tanaka et al 39 screened preoperative frailty using the Kihon Checklist in 217 patients aged ≥65 years who underwent HCC resection. They found that the Kihon Checklist score of ≥8 was associated with a higher incidence of bile leakage (CD ≥ grade III, 11% vs 3.2%, P = 0.021), delirium (CD ≥ grade I, 13% vs 1.9%, P = 0.003), and 90‐day mortality (4.8% vs 0%, P = 0.024) in univariable analyses. They also demonstrated that the Kihon Checklist score of ≥8 was an independent predictor of postoperative age‐related events, including major respiratory complications, major cardiac events, delirium requiring medication, transfer to rehabilitation facility, and dependency, with an OR of 5.16 (95% CI: 2.30‐11.56) in the multivariate analysis. 39 In the study by Ishihara, which included 295 patients with HCC over 65 years of age, the Kihon Checklist score was independently associated with a higher incidence of postoperative delirium (per increase of one point, multivariable OR: 1.14, 95%CI: 1.03‐1.26, P = 0.010) in the multivariable analyses. 40 They also demonstrated that the optimal cutoff total KCL score for predicting postoperative delirium was 6 points with the use of receiver operating characteristic curves (area under the ROC curves, 0.74). 40 Kaibori et al 41 screened preoperative frailty using the G8 in 71 patients aged >70 years who underwent HCC resection. They found that a G8 score of <14 was independently associated with a higher incidence of postoperative complications (CD ≥ grade II, multivariable OR: 24.36, 95%CI: 1.66‐157.08, P = 0.020). In another study by Kaibori et al 42 that included 100 patients with HCC over 70 years, patients were screened and reassessed for frailty at 1, 3, and 6 months postoperatively with the use of G8. The authors found that the reduction in the G8 at 6 months was associated with shorter OS (univariable HR: 8.09, 95% CI: 4.03‐16.27, P < 0.001; multivariable HR: 12.5, 95% CI: 4.54‐33.3, P < 0.001) and RFS (univariable HR: 5.35, 95%CI: 3.18‐9.01, P < 0.001; multivariable HR: 6.25, 95%CI: 2.94‐12.25, P < 0.001) in univariable and multivariable analyses. In one study by Yamada et al 43 that included 92 patients with HCC over 75 years of age, preoperative frailty was screened using the CFS. They found that CFS score ≥4 was independently associated with shorter CSS (multivariable HR: 7.85, 95% CI: 1.57‐38.1, P = 0.01) in multivariable analyses. CFS score ≥4 appeared to be associated with a longer length of postoperative hospital stay (20 days vs 15 days, P = 0.08) and a higher incidence of severe complications (≥ CD grade III, 24% vs 8.5%, P = 0.06); however, the differences were not statistically significant.
TABLE 3.
Major studies on geriatric assessment and perioperative outcomes in hepatobiliary‐pancreatic cancers
| Author (year) | Study cohort | Geriatric assessment tool/cut‐off value (% of frail patients) | Postoperative complications | Postoperative mortality | Length of hospital stay | Patient survival |
|---|---|---|---|---|---|---|
| HCC | ||||||
| Ishihara et al (2021) |
|
|
↑ (Postoperative delirium, 15% vs 1.8%, P < 0.001) |
– | – | – |
| Kaibori et al (2021) |
|
|
NS | – | – |
↓ OS: HR 8.1 (95%CI, 4.0‐16.3) RFS: HR 5.4 (95%CI, 3.2‐9.0) |
| Yamada et al (2021) |
|
|
NS | NS | NS |
↓ CSS: HR 7.9 (95%CI, 1.6‐38.1) |
| Tanaka et al (2018) |
|
|
↑ (Bile leakage, 11% vs 3.2%, P = 0.021; delirium, 13% vs 1.9%, P = 0.003) |
↑ (90‐day mortality, 4.8% vs 0%, P = 0.024) |
‐ | ‐ |
| Kaibori et al (2016) |
|
|
↑ (CD ≥II, 44% vs 3%, P < 0.001) |
NS |
↑ (Postoperative hospital stay ≥13 days, 67% vs 38%, P = 0.014) |
‐ |
| Pancreatic cancer | ||||||
| Mima et al (2021) |
|
|
NS | NS | – |
↓ OS: HR 2.3 (95%CI, 1.1‐4.4) CSS: HR 2.5 (95%CI, 1.1‐5.3) |
| Ngo‐Huang et al (2019) |
|
|
– | – | – |
↓ (P = 0.038) |
| Mogal et al (2017) |
|
|
↑ (CD III/IV, 41% vs 29%, P < 0.001) |
↑ (6.3% vs 2.7%, P < 0.001) |
‐ | ‐ |
| Augustin et al (2016) |
|
|
↑ (CD IV, PD: 37% vs 7.1%, P < 0.001; DP, 28% vs 3.4%, P < 0.001) |
↑ (PD, 22% vs 1.6%, P < 0.001; DP, 11% vs 0.6%, P < 0.001) |
↑ (PD, 20 days vs 13 days, P < 0.001; DP, 17 days vs 8 days, P < 0.001) |
‐ |
| Dale et al (2014) |
|
|
↑ (CD ≥III, OR, 4.1, P = 0.01) |
– |
↑ (P = 0.02) |
‐ |
Note: ↑, higher incidence of postoperative complications or mortality, and longer length of hospital stay in frail patients; ↓, shorter overall, cancer‐specific, or recurrence‐free survival in frail patients; −, not examined.
Abbreviations: CD, Clavien‐Dindo grade; CI, confidence interval; CSS, cancer‐specific survival; DP, distal pancreatectomy; HCC, hepatocellular carcinoma; HR, hazard ratio; NS, not significant (P > 0.05); OS, overall survival; PD, pancreaticoduodenectomy.
In pancreatic resection, frailty according to GA tools, including the CFS, Fried Frailty Criteria, and modified frailty index (mFI), has been associated with worse postoperative outcomes. Mima et al 44 examined CFS in 142 patients with pancreatic cancer who underwent curative resection. The authors found that CFS score ≥5 was independently associated with shorter OS (multivariable HR: 2.25, 95% CI: 1.05‐4.43, P = 0.038) and CSS (multivariable HR: 2.49, 95%CI: 1.05‐5.34, P = 0.039) in multivariable analyses adjusted for age, disease stage, and other potential confounders. However, CFS scores were not significantly associated with the incidence of severe complications (CD ≥ grade III, P = 0.67) or postoperative mortality. The authors also found that CFS score ≥5 was significantly associated with the absence of adjuvant chemotherapy (P < 0.001).
Two studies examined the Fried Frailty Criteria in relation to outcomes after palliative chemotherapy or resection for pancreatic cancer. In a study by Ngo‐Huang, frailty based on the Fried Frailty Criteria was associated with shorter OS in patients with pancreatic cancer who underwent palliative chemotherapy or resection for pancreatic cancer in the univariable analysis (univariable HR: 2.50, 95%CI: 1.57‐3.98, P < 0.001). 45 However, they did not examine the associations between the Fried Frailty Criteria and adverse events during palliative chemotherapy or postoperative complications. Dale et al 46 screened preoperative frailty using the Fried Frailty Criteria in 76 patients aged >18 years who underwent PD for pancreatic tumors. They found that self‐reported exhaustion based on the Fried Frailty Criteria was an independent predictor of severe postoperative complications (CD ≥ grade III, multivariable OR: 4.06, P = 0.01) and longer length of hospital stay (P = 0.02) after adjusting for potential confounders, including age, BMI, comorbidities, and ASA score.
The mFI has been validated in a population of patients with vascular diseases. 47 Augustin et al 48 examined the mFI in relation to postoperative outcomes by utilizing the National Surgical Quality Improvement Program (NSQIP) database that included more than 13 020 patients who underwent pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) for pancreatic cancer. The authors found that scores of ≥5 on the mFI were associated with higher incidence of postoperative complications (CD grade IV, PD: 37% vs 7.1%, P < 0.001; DP: 28% vs 3.4%, P < 0.001) and 30‐day mortality (PD: 22.2% vs 1.6%, P < 0.001; DP: 11.1% vs 0.6%, P < 0.001), and longer length of hospital stay (PD: 19.7 days vs 12.5 days, P < 0.001; DP: 17.4 days vs 8.2 days, P < 0.001), compared to mFI of 0. In multivariable analyses adjusting for age, BMI, serum albumin levels, and type of pancreatic resection, scores of ≥5 on the mFI were independently associated with a higher incidence of postoperative complications (CD grade IV, multivariable OR: 6.17, 95%CI: 3.34‐11.40, P < 0.001) and 30‐day mortality (multivariable OR: 10.9, 95%CI: 4.92‐24.01, P < 0.001), compared to an mFI of 0. These findings have been validated in another study by Mogal et al 49 that included 9986 patients with pancreatic cancer who underwent PD in the NSQIP database. Higher scores on the mFI were independently associated with a higher incidence of postoperative complications (CD grade III or IV, multivariable OR: 1.54, 95%CI: 1.29‐1.85) and postoperative mortality (multivariable OR: 1.54, 95%CI: 1.05‐2.25).
Studies have demonstrated that preoperative frailty is associated with higher incidence of postoperative complications and mortality, and worse prognosis in patients with gastrointestinal tract and HBP cancers. These findings suggest that integration of the GA assessment into clinical practice may improve risk assessment in patients with gastrointestinal cancer, although geriatric‐specific data should be collected routinely in future clinical trials to establish a standardized assessment of frailty and treatment strategies for frail patients with gastrointestinal cancer.
5. EFFECTS OF PRE‐ OR REHABILITATION ON PERIOPERATIVE OUTCOMES IN PATIENTS UNDERGOING RESECTION OF GASTROINTESTINAL CANCERS
Table 4 shows findings from randomized clinical trials on prehabilitation and rehabilitation in gastrointestinal tract and HBP cancers. Two randomized controlled trials (RCTs) of patients undergoing resection for gastrointestinal cancers, including colorectal cancer, demonstrated that prehabilitation can reduce the incidence of postoperative complications, compared to usual care. In the RCT by Barberan‐Garcia and colleagues that included 125 patients over 70 years and/or the American Society of Anesthesiologists (ASA)‐physical status (PS) classification score III or IV who underwent major abdominal surgery, including 96 undergoing oncologic surgery, preoperative 60‐minute supervised training sessions (1‐3 sessions per week; mean duration of 6 weeks) and home‐based training enhanced aerobic capacity, and reduced the incidence of postoperative overall complications, compared to usual care (31% vs 62%, P = 0.001). 50 In the RCT by Berkel and colleagues that included 57 patients with colorectal cancer or premalignant lesions over 60 years who had a metabolic equivalent of task score ≤7 on the veteran‐specific activity questionnaire, 3 weeks of exercise prehabilitation, consisting of 60 minutes of supervised exercise, improved the incidence of postoperative overall complications (43% vs 72%, P = 0.024), compared to usual care. 51 No differences in length of hospital stay (8.4 days vs 9.1 days, P = 0.14) and hospital readmission rates (14% vs 17%, P > 0.99) were found between the two groups. Two RCTs by Gillis et al and Carli et al evaluated prehabilitation versus postoperative rehabilitation; both interventions included exercise, nutrition, and psychologic interventions in patients who underwent resection for colorectal cancer. 52 , 53 However, no differences were observed in postoperative overall complication rates, length of hospital stay, and hospital readmission rates between the prehabilitation and rehabilitation groups in the two RCTs. There are very few previous prehabilitation studies on upper gastrointestinal tract cancers. In colorectal cancer, prehabilitation for 3‐4 weeks prior to surgery can improve functional capacity and survival, and reduce the incidence of overall complication. 51 , 52 , 54 Delaying elective colorectal cancer surgery for more than 4 weeks has been associated with increased mortality. 55 These findings suggest that prehabilitation for 3‐4 weeks prior to surgery or rehabilitation may improve clinical outcomes in colorectal cancer, although future studies are needed to establish optimal protocols and durations of prehabilitation or rehabilitation.
TABLE 4.
Randomized clinical trials on prehabilitation and rehabilitation in gastrointestinal tract and hepatobiliary‐pancreatic cancers
| Author (year) | Participants | Intervention | Main findings |
|---|---|---|---|
| Gastrointestinal tract cancers | |||
| Berkel et al (2022) |
|
|
|
| Carli et al (2020) |
|
|
|
| Barberan‐Garcia et al (2018) |
|
|
|
| Gillis et al (2014) |
|
|
|
| HBP cancers | |||
| Nakajima et al (2019) |
|
|
|
| Ausania et al (2019) |
|
|
|
| Dunne et al (2016) |
|
|
|
| Kaibori et al (2013) |
|
|
|
Abbreviations: CD, Clavien‐Dindo grade; HBP, hepatobiliary‐pancreatic; HCC, hepatocellular carcinoma; ICU, intensive care unit; QOL, quality of life.
In HBP cancers, studies have investigated the effects of exercise prehabilitation or multimodal prehabilitation, including exercise and nutrition, on functional capacity, surgical outcomes, and quality of life (QOL) after surgery. Two studies have examined the effects of exercise prehabilitation in patients who underwent hepatectomy. In a prospective study by Kaibori et al 56 that included 51 patients with HCC, 4 weeks of preoperative exercise and 24 weeks of postoperative exercise, in addition to nutrition therapy, improved postoperative oxygen uptake at anaerobic threshold at 6 months (% of baseline, 115% vs 102%, P = 0.038), compared to nutrition therapy alone; however, no differences in postoperative overall complication rates and length of hospital stay between the two groups were observed. In the RCT that included 38 patients with colorectal liver metastasis, Dunne et al 57 demonstrated that 4 weeks of exercise prehabilitation, comprising 30 minutes of supervised exercise, improved preoperative oxygen uptake at anaerobic threshold (P = 0.023) and preoperative QOL (P = 0.028), compared to usual care.
Nakajima et al 58 investigated the effect of exercise and nutritional therapy, consisting of 60 minutes of home‐based training (at least three sessions per week) and leucine‐rich essential amino acid supplement within 30 minutes after the start and end of exercise therapy, on postoperative outcomes of patients undergoing invasive HBP surgery, including major hepatectomy with at least 3 Couinaud segments, PD, or hepato‐pancreatoduodenectomy. The authors found that exercise and nutritional therapy were associated with lower incidence of postoperative bile leakage (11% vs 25%, P = 0.020) and shorter length of postoperative hospital stay (23 days vs 30 days, P = 0.045), compared to usual care.
In an RCT that included 40 patients with pancreatic or peripancreatic malignancies who underwent PD, Ausania et al 59 demonstrated that 60 minutes of in‐hospital supervised exercise and home‐based training, and nutritional intervention, consisting of liquid oral nutrition supplements, vitamin supplements, and pancreatic enzyme replacement therapy, reduced the incidence of delayed gastric emptying, compared to usual care (5.6% vs 41%, P = 0.010). However, no differences in postoperative overall complication rates (33% vs 55%, P = 0.18), postoperative pancreatic fistula (11% vs 27%, P = 0.20), and length of hospital stay (11 days vs 13 days, P = 0.45) were found between the two groups.
The impact of prehabilitation or rehabilitation in frail patients with HBP cancers remains uncertain. Two studies suggest that prehabilitation for 4 weeks prior to surgery may improve preoperative oxygen uptake at anaerobic threshold and preoperative QOL in patients with HCC or colorectal liver metastasis. 57 , 60 Prehabilitation for at least 4 weeks prior to surgery might be necessary to improve functional capacity. Neoadjuvant and adjuvant chemotherapy have been shown to improve prognosis in pancreatic cancer. It would be reasonable to assess frailty and perform prehabilitation and rehabilitation during neoadjuvant and adjuvant chemotherapy in frail patients with pancreatic cancer, although future studies are needed.
Three RCTs in hepatocellular carcinoma, colorectal liver metastasis, or pancreatic tumors have evaluated the effects of prehabilitation for older patients without assessing frailty. In those trials, no differences were found between prehabilitation and usual care groups in the incidence of overall complication. In contrast, two RCTs in colorectal cancer have evaluated the effects of prehabilitation especially in high‐risk or vulnerable patients, and demonstrated that prehabilitation significantly reduced the incidence of overall postoperative complications, compared to usual care. These findings suggest that prehabilitation may improve preoperative functional capacity and postoperative outcomes, especially in frail patients with gastrointestinal cancer, although future clinical trials are needed to establish optimal protocols and durations of prehabilitation or rehabilitation for frail patients with gastrointestinal cancer.
6. MINIMALLY INVASIVE SURGERY FOR FRAIL PATIENTS WITH GASTROINTESTINAL CANCERS
Minimally invasive surgery, including laparoscopic or robotic surgery, has been shown to improve postoperative outcomes, compared to open surgery, in terms of less postoperative pain, early postoperative recovery, lower complications, and mortality rates in resections of gastrointestinal tract 61 , 62 , 63 , 64 and HBP cancers. 65 , 66 , 67 , 68 However, the impact of minimally invasive surgery in frail patients remains uncertain.
In a prospective, observational, multicenter study that included 2968 patients who underwent resection for colorectal cancer by open or laparoscopic approach, Santacruz et al 69 found that laparoscopic surgery was associated with a lower incidence of postoperative overall complications (28% vs 37%, P < 0.001) and shorter length of postoperative hospital stay (7 days vs 10 days, P < 0.001), compared to open surgery, in high‐risk patients with an ASA‐PS score of III or IV. Mosquera et al examined associations of surgical approach with postoperative outcomes according to the mFI, utilizing the NSQIP database, which included over 94 811 patients who had undergone resection for colorectal cancer. The authors found that in frail patients with mFI ≥3, the laparoscopic approach was associated with a lower incidence of postoperative complications (35% vs 51%, P < 0.001) and 30‐day mortality (5.1% vs 11%, P < 0.001) compared to open surgery. These findings have been validated in another study by Kothari et al 70 that included 117 064 patients in the NSQIP database. The authors found that in frail patients with mFI ≥4, the laparoscopic approach was associated with a lower incidence of postoperative complications (36% vs 59%, P < 0.05) and 30‐day mortality (5.4% vs 20.3%, P < 0.05), compared to open surgery. Lo et al 71 examined associations of surgical approach with postoperative complications according to the mFI, utilizing the NSQIP database, which included 81 803 patients who had undergone open, laparoscopic, or robotic resection for colorectal cancer. The authors found that in frail patients with an mFI of 3 or 4, the robotic approach was associated with a higher incidence of postoperative major complications, compared to open or laparoscopic surgery (OR: 3.15, 95%CI: 1.34‐7.45, P = 0.009).
Minimally invasive distal pancreatectomy (MIDP), including laparoscopic or robotic approach, has been shown to improve postoperative outcomes, in terms of less postoperative pain, early postoperative recovery, lower complications, or mortality rates, compared to open distal pancreatectomy (ODP) for treatment of pancreatic body‐tail neoplasms. 72 , 73 Konstantinidis et al 74 examined associations of surgical approach with postoperative outcomes according to the mFI utilizing the NSQIP database, which included 1038 patients who had undergone distal pancreatectomy. The authors found that in frail patients with mFI >0, the laparoscopic or robotic approach was associated with a lower incidence of postoperative CD grade IV complications (2.4% vs 8.3% vs 12%, P = 0.007) and mortality (0% vs 2% vs 5.8%, P = 0.009), compared to open surgery or converted‐to‐open surgery.
Studies have demonstrated that laparoscopic surgery is associated with better surgical outcomes in frail patients with colorectal and distal pancreatic cancers, compared to open surgery. These findings suggest that laparoscopic surgery in combination with prehabilitation or rehabilitation may improve clinical outcomes in those patients. Further studies are needed to investigate the safety and effectiveness of minimally invasive surgery, including robotic approach, in high‐risk or frail patients with upper gastrointestinal tract and hepatobiliary cancers.
7. CHEMOTHERAPY IN FRAIL PATIENTS WITH GASTROINTESTINAL CANCER
Table 5 shows the association between frailty and chemotherapy outcomes in patients with gastrointestinal cancer. Huang et al 75 screened pretreatment frailty in 87 patients with esophageal cancer who received neoadjuvant radiotherapy and concurrent chemotherapy with weekly administration of carboplatin and paclitaxel for 5 weeks. They found that pretreatment frailty was associated with a higher incidence of at least one severe hematological adverse event (63.4% vs 19.6%, P < 0.001) and poor prognosis (HR: 2.12, 95%CI: 1.01‐4.42, P = 0.046) during concurrent chemoradiotherapy, when compared to fit patients. In a study by Jespersen 76 that included 170 patients with metastatic gastrointestinal cancers (colorectal cancer, esophagus‐gastric cancer, biliary and pancreatic cancer, gastrointestinal stromal tumor) over 70 years of age, pretreatment frailty was associated with a higher incidence of functional decline (OR: 3.5, 95%CI: 1.0‐11.6, P = 0.04), rapid progressive disease (OR: 3.5, 95%CI: 1.5‐8.4, P = 0.005), and shorter OS (HR: 1.7, 95%CI: 1.2‐2.4, P = 0.01) during palliative chemotherapy. Mima et al examined CFS in 196 patients with high‐risk stage II or stage III colorectal cancer who underwent curative resection and adjuvant chemotherapy. They found that during oxaliplatin‐based adjuvant chemotherapy, frail patients were more likely to experience severe adverse events, compared to non‐frail patients (43% vs 9.4%, P = 0.036). 77
TABLE 5.
Major studies on geriatric assessment and chemotherapy outcomes in patients with gastrointestinal cancers
| Author (year) | Study cohort | % of frail patients | Adverse events during chemotherapy | Patient survival according to frailty |
|---|---|---|---|---|
| Huang et al (2021) |
|
n = 41, 47% |
↑ (Severe hematological adverse event, 63% vs 20%, P < 0.001; emergent room visiting, P = 0.009) |
↓ OS: HR 2.1 (95%CI, 1.0‐4.4) |
| Jespersen et al (2021) |
|
n = 49, 29% |
↑ [Functional decline, OR: 3.5 (95%CI, 1.0‐11.6, P = 0.04)] |
↓ OS: HR 1.7 (95%CI, 1.2‐2.4) |
| Mima et al (2021) |
|
n = 36, 18% |
↑ (Severe adverse events during oxaliplatin‐based chemotherapy, 43% vs 9.4%, P = 0.036) |
‐ |
| Rittberg et al (2021) |
|
n = 14, 16% | NS | NS |
| Ngo‐Huang et al (2019) |
|
n = 29, 31% | NS |
↓ (P = 0.003) |
Note: ↑, higher incidence of adverse events during chemotherapy in frail patients; ↓, shorter overall survival in frail patients; −, not examined.
Abbreviations: CI, confidence interval; HR, hazard ratio; NS, not significant (P > 0.05); OS, overall survival.
Rittberg et al examined the mFI in relation to outcomes of palliative chemotherapy in 87 patients with pancreatic cancer over 65 years of age. The authors found that frailty according to the mFI was not associated with the incidence of adverse events (P > 0.16) or OS (P = 0.60) during chemotherapy. 78 In a study by Ngo‐Huang, frailty based on the Fried Frailty Criteria was associated with shorter OS in patients with pancreatic cancer who underwent palliative chemotherapy (P = 0.003), although they did not examine the associations between the Fried Frailty Criteria and adverse events during palliative chemotherapy. 45
There are few previous studies on geriatric assessment and outcomes of chemotherapy in patients with gastrointestinal cancers. Future clinical trials are needed to establish optimal chemotherapy regimens for frail patients with gastrointestinal cancer.
8. FUTURE CHALLENGES AND CONCLUSIONS
In this review, we summarized the major GA tools and clinical studies on associations between frailty and perioperative outcomes, and the effects of prehabilitation or rehabilitation on these outcomes in patients with gastrointestinal cancers. A variety of frail measures have been used due to the lack of an exact definition. An increasing number of studies have investigated the association between preoperative frailty and postoperative outcomes after resection of gastrointestinal tract and HBP cancers, especially in older patients. Although these studies were mostly retrospective, and the few prospective studies conducted were small, the results consistently suggest that preoperative frailty is a negative predictor of postoperative outcomes, such as postoperative complications, postoperative mortality, readmission, reoperation, and length of hospital stay.
Evidence on the effects of prehabilitation or rehabilitation on postoperative outcomes in patients with gastrointestinal cancer is still limited. Only two RCTs demonstrated better effects of preoperative exercise on the incidence of postoperative complications, especially in frail patients. Hence, standardized methods must be developed to identify patients who may benefit from prehabilitation and/or rehabilitation. Further clinical trials are required to establish a standardized assessment of frailty in patients with gastrointestinal cancer. In addition, future research should focus on exploring the benefits of combining these GA tools.
Utilizing the NSQIP database, minimally invasive surgery for frail patients with gastrointestinal cancer has been associated with a lower incidence of postoperative complications than open surgery. Neoadjuvant and adjuvant chemotherapy have been shown to improve prognosis in gastrointestinal cancer. 79 , 80 , 81 , 82 , 83 Mima et al reported that frail patients with colorectal and pancreatic cancers are less likely to receive adjuvant chemotherapy, which leads to poor prognosis. 12 , 44 , 77 , 84 Improvement of perioperative frailty would be key in providing favorable long‐term outcomes in patients with gastrointestinal cancers. Further clinical trials are needed to examine the effects of minimally invasive surgery and neoadjuvant and adjuvant chemotherapy in patients with frailty. We proposed an example for the integration of older‐patient‐specific care into treatment strategies for gastrointestinal cancer, as outlined in Figure 1.
FIGURE 1.
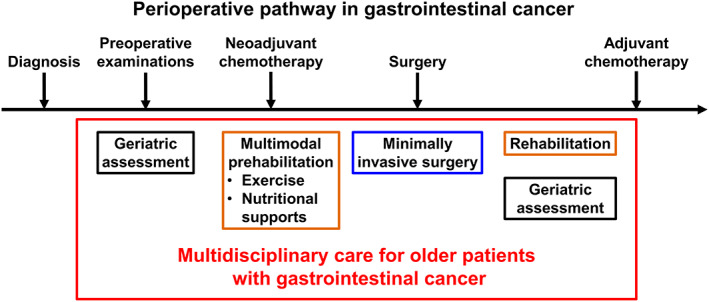
Perioperative pathway in gastrointestinal cancer
In conclusion, accumulating evidence demonstrates that the integration of preoperative geriatric assessment and prehabilitation for frail patients into clinical practice may improve perioperative and long‐term outcomes in patients with gastrointestinal cancer. The increasing numbers of older or frail patients with gastrointestinal cancers require multidisciplinary care; hence, there is an urgent need for future research to establish treatment strategies for these vulnerable patients.
DISCLOSURE
Funding: This study was funded by JSPS KAKENHI (Grant number 20 K17658), The Medical Research Encouragement Prize of The Japan Medical Association, The SGH Foundation, and The Shinnihon Foundation of Advanced Medical Treatment Research.
Conflict of Interest: Prof. Hideo Baba is a current editorial board member of the Annals of Gastroenterological Surgery. The authors declare no other conflicts of interest.
Author Contribution: conception and design: Kosuke Mima; provision of study materials or patients: all authors; collection and assembly of data: Kosuke Mima; data analysis and interpretation: Kosuke Mima; manuscript writing: Kosuke Mima; final approval of manuscript: all authors.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Mima K, Nakagawa S, Miyata T, Yamashita Y‐i, Baba H. Frailty and surgical outcomes in gastrointestinal cancer: Integration of geriatric assessment and prehabilitation into surgical practice for vulnerable patients. Ann Gastroenterol Surg. 2023;7:27–41. 10.1002/ags3.12601
REFERENCES
- 1. Cortes J, Perez‐Garcia JM, Llombart‐Cussac A, et al. Enhancing global access to cancer medicines. CA Cancer J Clin. 2020;70:105–24. [DOI] [PubMed] [Google Scholar]
- 2. Marubashi S, Takahashi A, Kakeji Y, Hasegawa H, Ueno H, Eguchi S, et al. Surgical outcomes in gastroenterological surgery in Japan: report of the National Clinical Database 2011‐2019. Ann Gastroenterol Surg. 2021;5:639–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 4. Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–77. [DOI] [PubMed] [Google Scholar]
- 5. Sedrak MS, Gilmore NJ, Carroll JE, Muss HB, Cohen HJ, Dale W. Measuring biologic resilience in older cancer survivors. J Clin Oncol. 2021;39:2079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DuMontier C, Loh KP, Bain PA, Silliman RA, Hshieh T, Abel GA, et al. Defining Undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol. 2020;38:2558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DuMontier C, Loh KP, Soto‐Perez‐de‐Celis E, Dale W. Decision making in older adults with cancer. J Clin Oncol. 2021;39:2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter B, Law J, Hewitt J, Parmar KL, Boyle JM, Casey P, et al. Association between preadmission frailty and care level at discharge in older adults undergoing emergency laparotomy. Br J Surg. 2020;107:218–26. [DOI] [PubMed] [Google Scholar]
- 11. Takeda T, Sasaki T, Suzumori C, Mie T, Furukawa T, Yamada Y, et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol. 2021;26:1293–303. [DOI] [PubMed] [Google Scholar]
- 12. Mima K, Miyanari N, Morito A, Yumoto S, Matsumoto T, Kosumi K, et al. Frailty is an independent risk factor for recurrence and mortality following curative resection of stage I‐III colorectal cancer. Ann Gastroenterol Surg. 2020;4:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia MV, Agar MR, Soo WK, To T, Phillips JL. Screening tools for identifying older adults with cancer who may benefit from a geriatric assessment: a systematic review. JAMA Oncol. 2021;7:616–27. [DOI] [PubMed] [Google Scholar]
- 14. Rostoft S, O'Donovan A, Soubeyran P, Alibhai SMH, Hamaker ME. Geriatric assessment and management in cancer. J Clin Oncol. 2021;39:2058–67. [DOI] [PubMed] [Google Scholar]
- 15. Carli F, Baldini G. From preoperative assessment to preoperative optimization of frail older patiens. Eur J Surg Oncol. 2021;47:519–23. [DOI] [PubMed] [Google Scholar]
- 16. Bellera CA, Rainfray M, Mathoulin‐Pelissier S, et al. Screening older cancer patients: first evaluation of the G‐8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. [DOI] [PubMed] [Google Scholar]
- 17. van Walree IC, Scheepers E, van Huis‐Tanja L, Emmelot‐Vonk MH, Bellera C, Soubeyran P, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10:847–58. [DOI] [PubMed] [Google Scholar]
- 18. Kenig J, Zychiewicz B, Olszewska U, Richter P. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J Geriatr Oncol. 2015;6:52–9. [DOI] [PubMed] [Google Scholar]
- 19. Soubeyran P, Bellera C, Goyard J, Heitz D, Curé H, Rousselot H, et al. Screening for vulnerability in older cancer patients: the ONCODAGE prospective multicenter cohort study. PLoS One. 2014;9:e115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russo C, Giannotti C, Signori A, Cea M, Murialdo R, Ballestrero A, et al. Predictive values of two frailty screening tools in older patients with solid cancer: a comparison of SAOP2 and G8. Oncotarget. 2018;9:35056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol. 2005;54:129–36. [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 23. Bielderman A, van der Schans CP, van Lieshout MR, et al. Multidimensional structure of the Groningen frailty indicator in community‐dwelling older people. BMC Geriatr. 2013;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–37. [DOI] [PubMed] [Google Scholar]
- 25. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pamoukdjian F, Canoui‐Poitrine F, Longelin‐Lombard C, Aparicio T, Ganne N, Wind P, et al. Diagnostic performance of gait speed, G8 and G8 modified indices to screen for vulnerability in older cancer patients: the prospective PF‐EC cohort study. Oncotarget. 2017;8:50393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arai H, Satake S. English translation of the Kihon checklist. Geriatr Gerontol Int. 2015;15:518–9. [DOI] [PubMed] [Google Scholar]
- 28. Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, et al. Validity of the Kihon checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16:709–15. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka T, Suda K, Inaba K, Umeki Y, Gotoh A, Ishida Y, et al. Impact of frailty on postoperative outcomes for laparoscopic gastrectomy in patients older than 80 years. Ann Surg Oncol. 2019;26:4016–26. [DOI] [PubMed] [Google Scholar]
- 30. Tamura K, Matsuda K, Fujita Y, Iwahashi M, Mori K, Yamade N, et al. Optimal assessment of frailty predicts postoperative complications in older patients with colorectal cancer surgery. World J Surg. 2021;45:1202–9. [DOI] [PubMed] [Google Scholar]
- 31. Okabe H, Ohsaki T, Ogawa K, Ozaki N, Hayashi H, Akahoshi S, et al. Frailty predicts severe postoperative complications after elective colorectal surgery. Am J Surg. 2019;217:677–81. [DOI] [PubMed] [Google Scholar]
- 32. Fagard K, Casaer J, Wolthuis A, Flamaing J, Milisen K, Lobelle JP, et al. Value of geriatric screening and assessment in predicting postoperative complications in patients older than 70 years undergoing surgery for colorectal cancer. J Geriatr Oncol. 2017;8:320–7. [DOI] [PubMed] [Google Scholar]
- 33. Bessems SAM, Konsten JLM, Vogelaar JFJ, Csepán‐Magyar R, Maas HAAM, van de Wouw YAJ, et al. Frailty screening by Geriatric‐8 and 4‐meter gait speed test is feasible and predicts postoperative complications in elderly colorectal cancer patients. J Geriatr Oncol. 2021;12:592–8. [DOI] [PubMed] [Google Scholar]
- 34. Ommundsen N, Wyller TB, Nesbakken A, Jordhøy MS, Bakka A, Skovlund E, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–43. [DOI] [PubMed] [Google Scholar]
- 36. Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–17. [DOI] [PubMed] [Google Scholar]
- 37. Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–52. [DOI] [PubMed] [Google Scholar]
- 38. Rostoft S, van Leeuwen B. Frailty assessment tools and geriatric assessment in older patients with hepatobiliary and pancreatic malignancies. Eur J Surg Oncol. 2021;47:514–8. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, Hirokawa F, et al. Preoperative assessment of frailty predicts age‐related events after hepatic resection: a prospective multicenter study. J Hepatobiliary Pancreat Sci. 2018;25:377–87. [DOI] [PubMed] [Google Scholar]
- 40. Ishihara A, Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, et al. Preoperative risk assessment for delirium after hepatic resection in the elderly: a prospective multicenter study. J Gastrointest Surg. 2021;25:134–44. [DOI] [PubMed] [Google Scholar]
- 41. Kaibori M, Ishizaki M, Matsui K, Iida H, Inoue K, Nagashima F, et al. Geriatric assessment as a predictor of postoperative complications in elderly patients with hepatocellular carcinoma. Langenbecks Arch Surg. 2016;401:205–14. [DOI] [PubMed] [Google Scholar]
- 42. Kaibori M, Matsushima H, Ishizaki M, Kosaka H, Matsui K, Ogawa A, et al. Perioperative geriatric assessment as a predictor of long‐term hepatectomy outcomes in elderly patients with hepatocellular carcinoma. Cancers (Basel). 2021;13:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamada S, Shimada M, Morine Y, Imura S, Ikemoto T, Arakawa Y, et al. Significance of frailty in prognosis after hepatectomy for elderly patients with hepatocellular carcinoma. Ann Surg Oncol. 2021;28:439–46. [DOI] [PubMed] [Google Scholar]
- 44. Mima K, Hayashi H, Nakagawa S, Matsumoto T, Kinoshita S, Matsumura K, et al. Frailty is associated with poor prognosis after resection for pancreatic cancer. Int J Clin Oncol. 2021;26:1938–46. [DOI] [PubMed] [Google Scholar]
- 45. Ngo‐Huang A, Holmes HM, des Bordes JKA, Parker NH, Fogelman D, Petzel MQB, et al. Association between frailty syndrome and survival in patients with pancreatic adenocarcinoma. Cancer Med. 2019;8:2867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dale W, Hemmerich J, Kamm A, Posner MC, Matthews JB, Rothman R, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg. 2014;259:960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ehlert BA, Najafian A, Orion KC, Malas MB, Black JH 3rd, Abularrage CJ. Validation of a modified frailty index to predict mortality in vascular surgery patients. J Vasc Surg. 2016;63:1595–1601 e2. [DOI] [PubMed] [Google Scholar]
- 48. Augustin T, Burstein MD, Schneider EB, Morris‐Stiff G, Wey J, Chalikonda S, et al. Frailty predicts risk of life‐threatening complications and mortality after pancreatic resections. Surgery. 2016;160:987–96. [DOI] [PubMed] [Google Scholar]
- 49. Mogal H, Vermilion SA, Dodson R, Hsu FC, Howerton R, Shen P, et al. Modified frailty index predicts morbidity and mortality after Pancreaticoduodenectomy. Ann Surg Oncol. 2017;24:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barberan‐Garcia A, Ubre M, Roca J, et al. Personalised Prehabilitation in high‐risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267:50–6. [DOI] [PubMed] [Google Scholar]
- 51. Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM, et al. Effects of community‐based exercise Prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275:e299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–47. [DOI] [PubMed] [Google Scholar]
- 53. Carli F, Bousquet‐Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal Prehabilitation vs postoperative rehabilitation on 30‐day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trepanier M, Minnella EM, Paradis T, et al. Improved disease‐free survival after Prehabilitation for colorectal cancer surgery. Ann Surg. 2019;270:493–501. [DOI] [PubMed] [Google Scholar]
- 55. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaibori M, Ishizaki M, Matsui K, Nakatake R, Yoshiuchi S, Kimura Y, et al. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013;206:202–9. [DOI] [PubMed] [Google Scholar]
- 57. Dunne DF, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–12. [DOI] [PubMed] [Google Scholar]
- 58. Nakajima H, Yokoyama Y, Inoue T, Nagaya M, Mizuno Y, Kadono I, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing Hepato‐Pancreato‐biliary surgeries for malignancy. Ann Surg Oncol. 2019;26:264–72. [DOI] [PubMed] [Google Scholar]
- 59. Ausania F, Senra P, Melendez R, Caballeiro R, Ouvina R, Casal‐Nunez E. Prehabilitation in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Rev Esp Enferm Dig. 2019;111:603–8. [DOI] [PubMed] [Google Scholar]
- 60. Kaibori M, Ishizaki M, Matsui K, Nakatake R, Sakaguchi T, Habu D, et al. Assessment of preoperative exercise capacity in hepatocellular carcinoma patients with chronic liver injury undergoing hepatectomy. BMC Gastroenterol. 2013;13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gottlieb‐Vedi E, Kauppila JH, Malietzis G, Nilsson M, Markar SR, Lagergren J. Long‐term survival in esophageal cancer after minimally invasive compared to open Esophagectomy: a systematic review and meta‐analysis. Ann Surg. 2019;270:1005–17. [DOI] [PubMed] [Google Scholar]
- 62. Muller‐Stich BP, Probst P, Nienhuser H, et al. Meta‐analysis of randomized controlled trials and individual patient data comparing minimally invasive with open oesophagectomy for cancer. Br J Surg. 2021;108:1026–33. [DOI] [PubMed] [Google Scholar]
- 63. Cuk P, Kjaer MD, Mogensen CB, Nielsen MF, Pedersen AK, Ellebaek MB. Short‐term outcomes in robot‐assisted compared to laparoscopic colon cancer resections: a systematic review and meta‐analysis. Surg Endosc. 2022;36:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kikuchi K, Suda K, Shibasaki S, Tanaka T, Uyama I. Challenges in improving the minimal invasiveness of the surgical treatment for gastric cancer using robotic technology. Ann Gastroenterol Surg. 2021;5:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ciria R, Berardi G, Alconchel F, et al. The impact of robotics in liver surgery: a worldwide systematic review and short‐term outcomes meta‐analysis on 2,728 cases. J Hepatobiliary Pancreat Sci. 2022;29:181–97. [DOI] [PubMed] [Google Scholar]
- 66. Korrel M, Vissers FL, van Hilst J, de Rooij T, Dijkgraaf MG, Festen S, et al. Minimally invasive versus open distal pancreatectomy: an individual patient data meta‐analysis of two randomized controlled trials. HPB (Oxford). 2021;23:323–30. [DOI] [PubMed] [Google Scholar]
- 67. Nakata K, Nakamura M. The current status and future directions of robotic pancreatectomy. Ann Gastroenterol Surg. 2021;5:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ishihara A, Tanaka S, Shinkawa H, Yoshida H, Takemura S, Amano R, et al. Superiority of laparoscopic liver resection to open liver resection in obese individuals with hepatocellular carcinoma: a retrospective study. Ann Gastroenterol Surg. 2022;6:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cerdan Santacruz C, Frasson M, Flor‐Lorente B, et al. Laparoscopy may decrease morbidity and length of stay after elective colon cancer resection, especially in frail patients: results from an observational real‐life study. Surg Endosc. 2017;31:5032–42. [DOI] [PubMed] [Google Scholar]
- 70. Kothari P, Congiusta DV, Merchant AM. Laparoscopic versus open colectomy: the impact of frailty on outcomes. Updates Surg. 2019;71:89–96. [DOI] [PubMed] [Google Scholar]
- 71. Lo BD, Leeds IL, Sundel MH, Gearhart S, Nisly GRC, Safar B, et al. Frailer patients undergoing robotic colectomies for colon cancer experience increased complication rates compared with open or laparoscopic approaches. Dis Colon Rectum. 2020;63:588–97. [DOI] [PubMed] [Google Scholar]
- 72. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient‐blinded randomized controlled trial. Ann Surg. 2019;269:2–9. [DOI] [PubMed] [Google Scholar]
- 73. Klompmaker S, de Rooij T, Koerkamp BG, Shankar AH, Siebert U, Besselink MG, et al. International validation of reduced major morbidity after minimally invasive distal pancreatectomy compared with open pancreatectomy. Ann Surg. 2021;274:e966–73. [DOI] [PubMed] [Google Scholar]
- 74. Konstantinidis IT, Lewis A, Lee B, Warner SG, Woo Y, Singh G, et al. Minimally invasive distal pancreatectomy: greatest benefit for the frail. Surg Endosc. 2017;31:5234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang YH, Hung YS, Lai CC, et al. Impact of frailty on treatment outcome in patients with locally advanced esophageal cancer undergoing concurrent Chemoradiotherapy. Anticancer Res. 2021;41:5213–22. [DOI] [PubMed] [Google Scholar]
- 76. Jespersen E, Winther SB, Minet LR, Moller S, Pfeiffer P. Frailty screening for predicting rapid functional decline, rapid progressive disease, and shorter overall survival in older patients with gastrointestinal cancer receiving palliative chemotherapy ‐ a prospective, clinical study. J Geriatr Oncol. 2021;12:578–84. [DOI] [PubMed] [Google Scholar]
- 77. Mima K, Miyanari N, Kosumi K, Tajiri T, Kanemitsu K, Takematsu T, et al. The efficacy of adjuvant chemotherapy for resected high‐risk stage II and stage III colorectal cancer in frail patients. Int J Clin Oncol. 2021;26:903–12. [DOI] [PubMed] [Google Scholar]
- 78. Rittberg R, Zhang H, Lambert P, Kudlovich R, Kim CA, Dawe DE. Utility of the modified frailty index in predicting toxicity and cancer outcomes for older adults with advanced pancreatic cancer receiving first‐line palliative chemotherapy. J Geriatr Oncol. 2021;12:112–7. [DOI] [PubMed] [Google Scholar]
- 79. Mayanagi S, Irino T, Kawakubo H, Kitagawa Y. Neoadjuvant treatment strategy for locally advanced thoracic esophageal cancer. Ann Gastroenterol Surg. 2019;3:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ishido K, Hakamada K, Kimura N, Miura T, Wakiya T. Essential updates 2018/2019: current topics in the surgical treatment of pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2021;5:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ali F, Keshinro A, Weiser MR. Advances in the treatment of locally advanced rectal cancer. Ann Gastroenterol Surg. 2021;5:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Irino T, Matsuda S, Wada N, Kawakubo H, Kitagawa Y. Essential updates 2019/2020: perioperative and surgical management of gastric cancer. Ann Gastroenterol Surg. 2021;5:162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oki E, Ando K, Taniguchi H, Yoshino T, Mori M. Sustainable clinical development of adjuvant chemotherapy for colon cancer. Ann Gastroenterol Surg. 2022;6:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mima K, Kosumi K, Miyanari N, Tajiri T, Kanemitsu K, Takematsu T, et al. Impairment of activities of daily living is an independent risk factor for recurrence and mortality following curative resection of stage I‐III colorectal cancer. J Gastrointest Surg. 2021;25:2628–36. [DOI] [PubMed] [Google Scholar]


