Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target inhibitory receptors expressed by immune cells and tumor cells. These targets include CTLA-4, PD-1, and PD-L1. As a result of their mechanism of action, these medications can lead to immune-related adverse events involving different organs. Kidney involvement is less common and typically presents as acute tubulointerstitial nephritis.1 This report describes a case linking ICI therapy to a novel form of renal tubular acidosis (RTA).
Case Presentation
A 46-year-old woman with metastatic lung cancer and squamous cell cancer of the tonsil was initially treated with 6 cycles of carboplatin-paclitaxel-pembrolizumab over 4 months, followed by maintenance pembrolizumab. Three months after starting pembrolizumab maintenance therapy, she developed a normal-gap metabolic acidosis that gradually worsened. Her total serum CO2 decreased to 15 mmol/l; hypokalemia developed simultaneously with a serum potassium of 3.2 meq/l. Renal function was preserved with a serum creatinine of 0.6 mg/dl. No diarrhea was noted. Urinary studies showed a urine anion gap of +27 meq/l, a urine osmole gap of 83 mOsmol/kg H2O, and a urine pH of 6.0. Direct urine ammonia measurements, although preferred to urine anion gap and urine osmole gap to assess ammonia excretion,S1 were not clinically available locally at the time of this study. Urinary citrate in a 24-hour urine collection was undetectable.
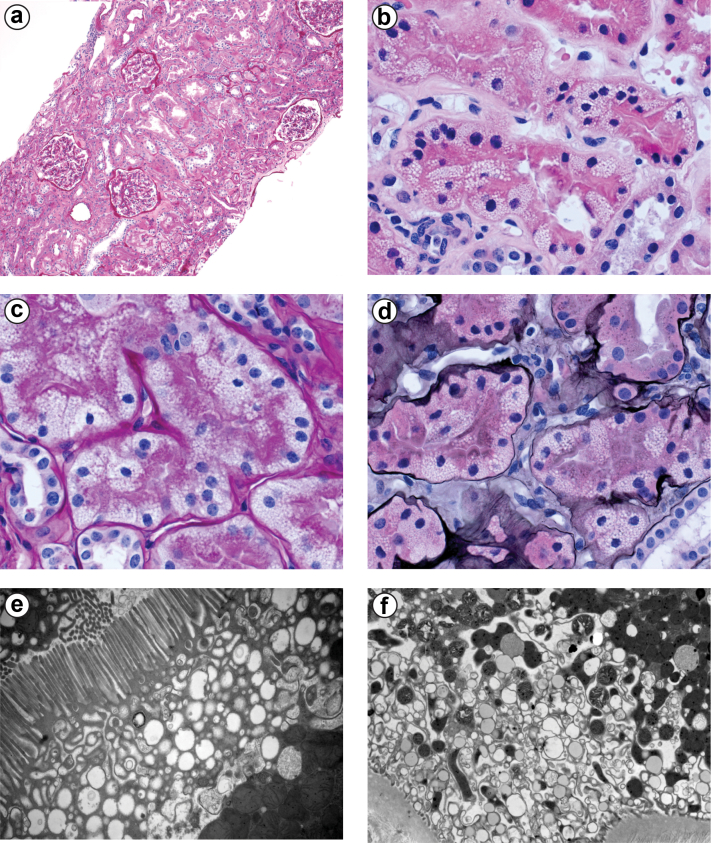
A diagnosis of RTA secondary to pembrolizumab was made. Additional treatment with ICI was held. A kidney biopsy, performed 2 months after the last dose of ICI therapy and while RTA was still present, showed no evidence of tubulitis, interstitial nephritis, or interstitial inflammation (Figure 1a). However, significant vacuolization of the proximal tubule was evident by light microscopy and by ultrastructural analysis (Figure 1b–f). Glucocorticoid therapy was not used because of the absence of tubulitis or interstitial nephritis. Her RTA was treated with oral potassium citrate, 45 meq 3 times a day (total, 135 meq/d), correcting the acidosis and hypokalemia. Urine pH remained 6.0 during treatment with potassium citrate. Over a period of 3 months following the kidney biopsy, the RTA spontaneously resolved, and potassium citrate was discontinued gradually. Total serum CO2 remained at approximately 25 mmol/l with a serum potassium of 4.3 meq/l 4 months after withdrawal of the alkali supplement and again at 1 year of follow-up. The patient was not rechallenged with ICI therapy because of good tumor response without evidence of progression of metastatic disease.
Figure 1.
Renal tissue morphology. Panel a shows a low-power micrograph of the renal cortex using a periodic acid-Schiff stain. Glomeruli are histologically normal, and there is no tubule necrosis or interstitial inflammation. Original magnification ×100. Panel b shows hematoxylin and eosin–stained section and demonstrates focal proximal tubule vacuolization. Original magnification ×400. Panel c shows PAS-stained tissue and also demonstrates proximal tubule cytoplasmic vacuolization. Original magnification ×400. Panel d shows Jones Silver–stained tissue with dramatic proximal tubule cytoplasmic vacuolization (original magnification ×630). Panel e shows a transmission electron micrograph of the apical region of the proximal tubule. Substantial subapical cytoplasmic vacuolization is present (original magnification ×15,000). Panel f shows transmission electron micrograph of the basal region of the proximal tubule. Diffuse basal vacuolization is present (original magnification ×15,000).
Investigative Results
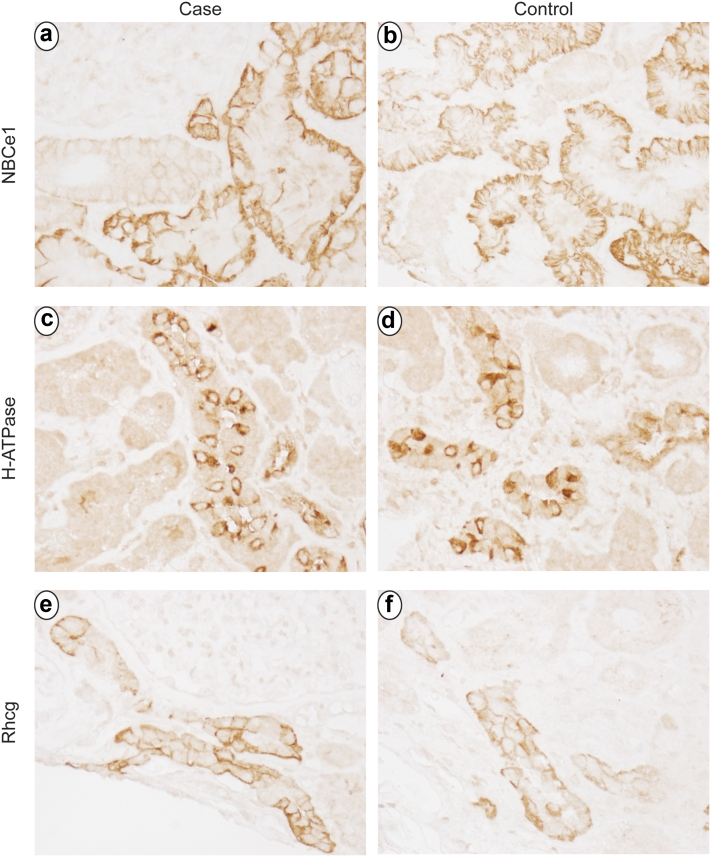
To investigate the mechanism of the RTA, we evaluated expression of critical acid-base transporters, electrogenic sodium bicarbonate cotransporter 1, H⁺-ATPase, and Rhesus C glycoprotein. We used tissue from the renal biopsy that was not needed for routine clinical evaluation, and as control tissue, we used tissue from an allograft biopsy of a patient of similar age who received no pathologic diagnosis. We used standard immunohistochemistry techniques described previously.S2 Immunohistochemistry for sodium bicarbonate cotransporter 1, the proximal tubule’s primary basolateral bicarbonate transporter, whose expression is necessary for normal ammonia metabolism,2,S3,S4 showed normal proximal tubule expression not evidently different from control tissue (Figure 2a and b). H⁺-ATPase, critical for collecting duct H⁺ secretion, exhibited normal expression and localization in collecting duct type A intercalated cells that did not differ detectably from control tissue (Figure 2c and d). Rhesus C glycoprotein is an ammonia transporter critical for collecting duct ammonia secretion3 and showed normal apical and basolateral expression and intensity not detectably different from control tissue (Figure 2e and f).
Figure 2.
Immunohistochemistry examination for critical acid-base transporters. Panels a and b show expression of the electrogenic sodium bicarbonate cotransporter, isoform 1. Panel a shows intact basolateral sodium bicarbonate cotransporter, isoform 1 immunolabel in proximal tubule segments in this patient. Panel b shows sodium bicarbonate cotransporter, isoform 1 immunolabel expression in control tissue. Sodium bicarbonate cotransporter, isoform 1 immunolabel does not differ detectably between case and control tissues. Panels c and d show immunohistochemistry for H⁺-ATPase in the case and control tissues, respectively. In both, intact H⁺-ATPase expression with apical polarization in type A intercalated cells is not detectably different between case and control tissues. Panels e and f show Rhesus C glycoprotein immunolabel expression in the case and control tissue. Panels e and f show Rhesus C glycoprotein immunolabel expression in the case and control tissues, respectively. Apical and basolateral Rhesus C glycoprotein immunolabel in intercalated cells is present in both and does not differ detectably between case and control tissues.
Discussion
ICI immune-modulatory therapy is being increasingly used as first-line therapy for many forms of cancers. With more widespread use, more adverse events are being recognized. We describe a case of RTA associated with ICI use. Following ICI therapy, this patient developed hypokalemic, nonanion gap metabolic acidosis associated with an intact ability to acidify the urine but without evidence of increased urinary ammonia excretion. Thus, a diagnosis of RTA was made. The hypokalemic metabolic acidosis resolved spontaneously over several months following ICI withdrawal without treatment with steroids. Investigation of the cause of RTA showed evidence of impaired ammonia excretion despite intact urinary acidification, undetectable urinary citrate excretion, and no evidence of loss of sodium bicarbonate cotransporter 1, H⁺-ATPase, or Rhesus C glycoprotein expression.
This case represents a form of RTA that may not be easily classifiable by current criteria. Given the persistent urine acidification during alkali therapy, proximal (type II) RTA cannot explain the observed findings. Furthermore, the undetectable urinary citrate indicates that proximal tubule citrate reabsorption is intact, which contrasts with “classic proximal RTA” where urinary citrate excretion is not suppressed.4 Hyperkalemic (type IV) RTA presents with an intact ability to acidify the urine but requires the concomitant presence of hyperkalemia, which directly causes metabolic acidosis.5 Thus, this patient does not have hyperkalemic (type IV) RTA. Distal (type I) RTA is associated with an impaired ability to reabsorb filtered bicarbonate completely, leading to a relatively alkaline urine pH.6 The patient’s urine pH was not less than 6.0 during the metabolic acidosis, which may give an impression of submaximal distal acidification (i.e., distal RTA). However, several features lead us to suggest that this patient did not have a classic form of distal RTA. Considering that the patient was hypokalemic, which normally causes urine alkalinization, a urine pH of 6.0 actually indicates enhanced urine acidification. Second, the patient’s urine pH did not increase when the acidosis was corrected, indicating an intact ability to reabsorb filtered bicarbonate even when filtered levels were normal. Finally, all genetic forms of distal RTA involving deletion of AE1, H⁺-ATPase, or carbonic anhydrase isoform II7 are associated with impaired ability to reabsorb filtered bicarbonate leading to substantially alkaline urine, which this patient did not have. Indeed, if the term distal RTA is used to imply a distal, that is, collecting duct, defect in net acid excretion, urine pH may not be ideal as a diagnostic criterion. Ammonia excretion is the primary component of net acid excretion, and the deletion of proteins necessary for collecting duct ammonia secretion leads to metabolic acidosis with an excessively acidic urine pH.8,9,S5–S8 Thus, we suggest that this patient has a novel form of RTA that we term type V RTA, which is characterized by nonanion gap metabolic acidosis associated with evidence of impaired urinary ammonia excretion, intact urine acidification, absence of hyperkalemia, and intact ability to decrease urinary citrate excretion.
The mechanism of this patient’s RTA is likely to be a primary defect in proximal tubule ammonia generation. Ammonia excretion in this patient appears to be decreased, as suggested by the urine anion gap and osmolar gap findings. Unfortunately, direct measurements of urine ammonia excretion were not available. The proximal tubule apical and basal vacuolization is consistent with abnormal proximal tubule function, which may have altered ammoniagenesis. Defective proximal tubule ammoniagenesis is known to cause a failure of the normal ammonia excretion response to metabolic acidosis despite an intact ability to acidify the urine.2,S3 In addition, it blunts the renal ability to conserve potassium,S4 which could have contributed to hypokalemia present in this case. Impaired collecting duct ammonia secretion is unlikely because H-ATPase and Rhesus C glycoprotein expression was intact.
Several previous studies have reported the association of RTA with ICI use.S9–S13 The key features of these cases are summarized in Table 1. Three of the 7 cases reported urine pH of 6.0, consistent with the findings in this study. In the other 4 cases, urine pH was 6.3 (1 case), 6.5 (2 cases), and 6.7 (1 case). Thus, intact urine acidification is frequently observed in patients with ICI-associated RTA. This case further shows that RTA can occur without acute kidney injury or interstitial inflammation. Therefore, the presence of what we term type V RTA may be a common finding in ICI-associated RTA and may be independent of associated acute kidney injury.
Table 1.
Summary of previous reports of metabolic acidosis with ICI therapy
| Case # [Ref] | Cancer | Drug | Target | Urine pH | Serum creatinine (mg/dl) | Peak creatinine (mg/dl) | Urine anion gap | AIN on kidney biopsy |
|---|---|---|---|---|---|---|---|---|
| 1S11 | Lung | Pembrolizumab | PD-1 | 6.5 | 1.5 | 1.5 | 10 | Yes |
| 2 S11 | Melanoma | Nivolumab | PD-1 | 6.3 | 1.5 | 4.9 | 48 | Yes |
| 3S11 | Renal Cell | Nivolumab | PD-1 | 6.7 | 2.1 | 2.6 | NA | NA |
| 4S10 | Lung | Nivolumab followed by pembrolizumab | PD-1 | 6.5 | 0.55 | 1.67 | 36 | NA |
| 5S10 | Melanoma | Pembrolizumab | PD-1 | 6.0 | 1.4 | 2.0 | 49 | Yes |
| 6S13 | Lung | Pembrolizumab | PD-1 | 6.0 | 0.8 | 0.8 | 36 | Yes |
| 7S12 | Squamous cell carcinoma of the skull | Cemiplimab | PD-1 | 6.0 | 1.3 | 2.8 | 39 | Yes |
| Case | Lung | Pembrolizumab | PD-1 | 6.0 | 0.6 | 0.6 | 26 | No |
AIN, acute interstitial nephritis; ICI, immune checkpoint inhibitor; NA, not available; PD-1, programmed death-1.
In this case, as in all previously reported cases of ICI-associated RTA, anti–PD-1 immunotherapy was used (Table 1). Notably, the target ligand for PD-1, PD-L1, is expressed in the proximal tubule in the kidney.S14 We suggest that anti-PD1 immunotherapy can, in selected cases, cause proximal tubule vacuolization and impaired ammoniagenesis, leading to a novel form of RTA, for which we propose the terminology type V RTA.
Conclusion
In conclusion, ICI anti-PD1 therapy appears to have led to a novel form of RTA that is characterized by impaired urinary ammonia excretion, hypokalemia, intact ability to acidify the urine, and evidence of intact proximal tubule citrate transport (Table 2).
Table 2.
Teaching points
| Teaching points |
|---|
| ICI-RTA is a unique immune-related adverse event. |
| ICI-RTA is not easily classifiable with our current criteria for RTA. |
| The novel form of RTA, which we term type V RTA, is characterized by impaired urinary ammonia excretion, hypokalemia, intact ability to acidify the urine, and evidence of intact proximal tubule citrate transport. |
| The presence of this unique RTA may be a common finding in ICI-associated RTA and may be independent of associated AKI. |
AKI, acute kidney injury; ICI, immune checkpoint inhibitor; RTA, renal tubular acidosis.
Disclosures
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in this report.
Acknowledgments
Funding
This work was supported by funds from the National Institutes of Health (R01-DK107798 to IDW).
Footnotes
Supplementary References.
Supplementary Material
Supplementary References.
References
- 1.Perazella M.A., Shirali A.C. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020;97:62–74. doi: 10.1016/j.kint.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.W., Osis G., Harris A.N., et al. NBCe1-A regulates proximal tubule ammonia metabolism under basal conditions and in response to metabolic acidosis. J Am Soc Nephrol. 2018;29:1182–1197. doi: 10.1681/ASN.2017080935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner I.D., Verlander J.W. Ammonia transporters and their role in acid-base balance. Physiol Rev. 2017;97:465–494. doi: 10.1152/physrev.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemann J., Jr., Adams N.D., Wilz D.R., Brenes L.G. Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int. 2000;58:1267–1277. doi: 10.1046/j.1523-1755.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 5.Harris A.N., Grimm P.R., Lee H.W., et al. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol. 2018;29:1411–1425. doi: 10.1681/ASN.2017111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unwin R.J., Shirley D.G., Capasso G. Urinary acidification and distal renal tubular acidosis. J Nephrol. 2002;15(suppl 5):S142–S150. [PubMed] [Google Scholar]
- 7.Batlle D., Haque S.K. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27:3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 8.Biver S., Belge H., Bourgeois S., et al. A role for rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature. 2008;456:339–343. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- 9.Lee H.W., Verlander J.W., Bishop J.M., et al. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol. 2009;296:F1364–F1375. doi: 10.1152/ajprenal.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.