Abstract
Introduction
Proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID) occasionally presents refractory nephrotic syndrome resulting in poor renal prognosis, but its etiology is not fully elucidated. Given that glomerular endothelial cell (GEC) stress or damage may lead to podocytopathy and subsequent proteinuria, as in thrombotic microangiopathy (TMA), diabetic kidney disease, and focal segmental glomerulosclerosis, we investigated the evidence of glomerular endothelial injury by evaluating the expression of plasmalemmal vesicle-associated protein-1 (PV-1), a component of caveolae in the cases of PGNMID.
Methods
We measured the immunofluorescent PV-1 intensities of 23 PGNMID cases and compared with those of primary membranoproliferative glomerulonephritis (MPGN) (n = 5) and IgA nephropathy (IgAN) (n = 54) cases. PV-1 localization was evaluated with Caveolin-1, and CD31 staining, and the ultrastructural analysis was performed using a low-vacuum scanning electron microscope (LVSEM). To check the association of podocyte injury, we also conducted 8-oxoguanine and Wilms tumor 1 (WT1) double stain. We then evaluated PV-1 expression in other glomerulitis and glomerulopathy such as lupus nephritis and minimal change disease.
Results
The intensity of glomerular PV-1 expression in PGNMID is significantly higher than that in the other glomerular diseases, although the intensity is not associated with clinical outcomes such as urinary protein levels or renal prognosis. Immunostaining and LVSEM analysis revealed that glomerular PV-1 expression is localized in GECs in PGNMID. 8-oxoguanine accumulation was detected in WT1-positive podocytes but not in PV-1–expressing GECs, suggesting GEC-derived podocyte injury in PGNMID.
Conclusion
PV-1 overexpression reflects glomerular endothelial injury, which could be associated with podocyte oxidative stress in PGNMID cases.
Keywords: caveolae, glomerular endothelium cells, low-vacuum scanning electron microscope (LVSEM), plasmalemmal vesicle-associated protein-1 (PV-1), proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID)
Graphical abstract
Since the establishment of the concept of proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID) by Nasr et al.,1 approximately 100 PGNMID cases have been reported in the literature.2, 3, 4 PGNMID is diagnosed based on immunofluorescence (IF) findings indicating monoclonal IgG deposits with IgG subclass and light-chain staining. In transmission electron microscope (TEM) analysis, PGNMID is distinguished on the basis of the pattern of the structure of deposits from other deposition diseases, such as light-chain and/or heavy-chain deposition diseases.2 Histologic patterns of glomerulonephritis in PGNMID were reported as membranoproliferative, membranous, mesangial proliferative, and endocapillary proliferative glomerulonephritis. Most patients with PGNMID manifest nephritic proteinuria, hematuria, and variable kidney dysfunction. Some case reports have suggested that an intensive immunosuppressive regimen, including rituximab, may be effective for PGNMID.5,6 However, certain cases exhibit nephrosis, which is resistant to immunosuppression, and rapidly or gradually progresses to end-stage kidney disease, including recurrent cases of renal allograft.7,8 Nevertheless, the pathologic and clinical features of PGNMID have not been completely elucidated.
GECs, which are covered by glycocalyx and fenestrated, play vital roles such as regulation of glomerular filtration, vascular tone, coagulation, inflammation, and homeostasis of glomerular functions.9 Injury and dysfunction of GECs have been reported in TMA,10 diabetic nephropathy,11 focal segmental glomerulosclerosis,12 crescentic glomerulonephritis,13 or other glomerulonephritis.9 Anti–vascular endothelial growth factor therapy (representatively, bevacizumab) has been proved to cause TMA and subsequent podocytopathy because podocytes depend on vascular endothelial growth factor from GECs to maintain cytoskeletal and Akt signaling pathways for survival.14,15 However, TMA-like endothelial injury is not common in PGNMID cases.
Previous work highlighted that caveolae and transendothelial channels could be involved in transcapillary exchanges between GECs and stomatal diaphragms at their orifices in fenestrated capillaries.16 PV-1 is known as a component of endothelial fenestra and stomatal diaphragms.17 In the human kidney, PV-1 expression is detected in the peritubular capillaries and vasa recta, but not in the glomerular artery.17,18 PV-1 expression is also generally negative in glomerulonephritis such as IgAN (Supplementary Figure S1A–D) and primary MPGN.19 A clinicopathological study reported that PV-1 is prominently expressed in the glomeruli in transplant glomerulopathy and correlated with the grade of proteinuria.20
We recently reported a case of a patient with de novo PGNMID that was subsequently complicated after recurrent (or de novo) IgAN in the renal allograft, who showed marked injuries in GEC in the TEM analysis as endothelial proliferation.21 In this case, the allograft biopsies revealed that PV-1 expression was negative in the glomerulus on postoperative day (POD) 1463 at the stage of development of IgAN (Supplementary Figure S1E–H), however, glomerular PV-1 expression started when PGNMID was complicated on POD 1549 (Supplementary Figure S1I–L). This case suggested that glomerular PV-1 expression is unique in PGNMID, which is not observed in IgAN, and MPGN as previously reported.19 In this study, we evaluated glomerular PV-1 expression among PGNMID cases and compared with other glomerulonephritis and glomerulopathy including IgAN and MPGN cases. We also show PV-1 localization in GECs by immunohistochemistry and LVSEM analysis. Finally, we propose that the overexpression of PV-1 in GECs could be associated with podocyte injury (or stress) such as 8-oxoguanine accumulation.
Methods
Patients
This study was approved by the ethics committee at Tokyo Women’s Medical University (No. 5415). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” Details regarding PGNMID and other GN cases as control were extracted from the database of 2208 native renal biopsies and 12,316 renal allograft biopsies, which were performed from 1995 to 2017 in Tokyo Women’s Medical University. All renal biopsies included in this study were reviewed independently by 3 pathologists (AS, KK, and JK).
Pathologic Diagnosis
All biopsy samples were examined using a standard light microscope, IF, and TEM. For light microscope examinations, all biopsies were routinely stained with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome, and periodic acid-methenamine silver. For IF examinations, 2-μm cryostat sections were dried and stained with fluorescein isothiocyanate–conjugated polyclonal antibodies against IgG, IgA, IgM, C3c, C1q, fibrinogen, C4d, κ light-chain, and λ light-chain (Dako Corp., Carpinteria, CA). IgG subclass measurements were performed using fluorescein isothiocyanate–conjugated monoclonal antibodies against IgG1, IgG2, IgG3, and IgG4 (The Binding Site Inc., San Diego, CA). TEM analysis was conducted for all PGNMID cases to identify the nonorganized electron-dense deposits in the glomerular regions. Renal allograft specimens were histologically diagnosed according to Banff classification 2017.22
Histologic Evaluations
Fluorescein isothiocyanate–conjugated monoclonal antibody against PV-1 and Alexa Fluor 568–conjugated polyclonal antibody against caveolin-1 using frozen sections were stained to evaluate the endothelial changes in the glomeruli (PV-1: Progen, Heidelberg, Germany; caveolin-1: Santa Cruz Biotechnology, Dallas, TX). Rabbit polyclonal antibody against PV-1; mouse monoclonal antibody against platelet endothelial cell adhesion molecule (CD31) were used for formalin-fixed paraffin-embedded sections to confirm the localization of PV-1 expression (PV-1: Novus Biologicals, Centennial, CO; CD31: DAKO, Agilent Technologies Inc., CA). Mouse monoclonal antibody against 8-hydroxy-deoxyguanosine (NIKKEN SEIL Co., Ltd., Shizuoka, Japan) and rabbit polyclonal WT1 antibody (Santa Cruz Biotechnology, Dallas, TX), biotinylated Maackia Amurensiis Lectin II (Vector Laboratories, Burlingame, CA) were used to evaluate podocyte injury. Goat antirabbit IgG (H+L), Alexa Fluor 488, goat antimouse IgG (H+L) Alexa 568, and Alexa Fluor 647 streptavidin were used as secondary reagents, respectively. IF images were captured with AX-80 (OLYMPUS, Tokyo, Japan) or BZ-X800 (KEYENCE, Osaka, Japan). For LVSEM (Hitachi, Tokyo, Japan) analysis, 5-μm formalin-fixed paraffin-embedded sections were stained with periodic acid-methenamine silver to evaluate the glomerular basement membrane as described previously.23,24 Gold (III) chloride solution was used after 3,3′-diaminobenzidine staining of 3-μm formalin-fixed paraffin-embedded sections (CD31 and PV-1 immunostaining) to enhance the 3,3′-diaminobenzidine–stained areas as white areas in LVSEM as described previously.25 PV-1 intensities were calculated by ImageJ software, according to the area of glomeruli without global sclerosis (Supplementary Figure S2). All statistical analyses were performed using the Prism software (version 9; GraphPad Software). Pathologic and clinical variables were compared using the Mann-Whitney test or Kruskal-Wallis test. Correlation between proteinuria and PV-1 intensity was evaluated with the Spearman rank correlation coefficient. All P values < 0.05 were considered statistically significant.
Results
Clinical Manifestations
The clinical manifestations of PGNMID cases are shown in Table 1, and those of IgAN and MPGN are shown in Supplementary Table S1. A total of 23 PGNMID cases (13 males, 10 females) were identified in the renal biopsy database during 1995 to 2017. Two cases were renal allografts. One case was diagnosed with PGNMID in both native kidney and renal allograft. The prevalence rate in native renal biopsies was approximately 1%. The median age of the patients was 47 years (range, 7–70 years). Parvovirus infection was confirmed in one case, and another case had a history of hepatitis B virus infection. Two cases exhibited a monoclonal spike in their serum and were diagnosed with monoclonal gammopathy of renal significance. The median serum creatinine level was 1.02 mg/dl (range, 0.42–2.03 mg/dl). The median proteinuria level was 1.88 g/gCr (range, negative to 12.6 g/gCr), and 11 cases had a nephrotic-range proteinuria. Urine occult blood was found in 20 cases. The median follow-up period was 3 years (range, 0.5–13 years). Steroids combined with other immunosuppressive treatments were administered to 11 patients, and 10 patients received oral or intravenous steroid treatment alone. Two cases (parvovirus associated case; an asymptomatic case found in protocol biopsy after renal transplantation) did not receive either steroid or immunosuppressive treatment. Regarding prognosis, 4 cases progressed to end-stage kidney disease, and the other 3 cases persisted with the nephrotic-range proteinuria. Two patients died because of infection during immunosuppressive treatment. Nine patients responded to the treatments; their urinary protein levels were reduced to half.
Table 1.
Clinical manifestations of PGNMID cases
| Characteristics | Value |
|---|---|
| Age, yr, median (range) | 47 (7–70) |
| Sex, male, n (%) | 13 (56.5) |
| U-pro, g/d or g/g.cr, median (range) | 1.88 (negative to 12.6) |
| sCr, mg/dl, median (range) | 1.02 (0.42–2.03) |
| U-OB, n (%) | 20 (87.0) |
| Renal allograft, n (%) | 3 (13.0) |
| Follow-up duration, yr, median (range) | 3 (0.5–13) |
| ESKD, n (%) | 4 (17.4) |
| Death, n (%) | 2 (8.7) |
| Medication | |
| Prednisone, n (%) | 21 (91.3) |
| Other immunosuppressive agents, n (%) | 11 (47.8) |
ESKD, end-stage kidney disease; PGNMID, proliferative glomerulonephritis with monoclonal IgG deposits; sCr, serum creatinine; U-OB, urine occult blood; U-Pro, urinary protein.
Renal Histopathology
The pathologic characteristics of PGNMID are presented in Table 2. The pattern of glomerulonephritis was as follows: MPGN (n = 18), membranous nephropathy (n = 3), endocapillary proliferative glomerulonephritis (n = 1), and minor glomerular abnormalities (n = 1). In 3 cases of renal allograft, the Banff score was all negative. In the IF analysis, all cases demonstrated light-chain isotype restriction, including 19 cases with exclusive positivity for κ chain and 4 cases with exclusive positivity for λ chain. Deposit patterns with clinical features are shown in Table 3. No case exhibited positivity for IgG4. C1q and C3 depositions were found in 16 and 21 cases, respectively. In the TEM analysis, all cases exhibited nonorganized electron-dense deposits in the glomerular compartment. The electron-dense deposits were located in the subepithelial (n = 10), intramembranous (n = 16), subendothelial (n = 19), and mesangial (n = 19) regions. Two cases demonstrated hump and nodules, respectively.
Table 2.
Pathologic characteristics of PGNMID cases
| Characteristics | Value |
|---|---|
| The pattern of glomerulonephritis, n | |
| Membranoproliferative glomerulonephritis | 18 |
| Membranous nephropathy | 3 |
| Endocapillary proliferative glomerulonephritis | 1 |
| Minor glomerular abnormalities | 1 |
| Monoclonality | |
| IgG1 kappa | 6 |
| IgG2 lambda | 1 |
| IgG3 kappa | 16 |
| IgG3 lambda | 3 |
| C1q deposition, n | 16 |
| C3 deposition, n | 21 |
| The location of EDD, n | |
| Subepithelial | 10 |
| Intramembranous | 16 |
| Subendothelial | 19 |
| Mesangial | 19 |
| Hump, n | 1 |
| Nodule, n | 1 |
C1q, complement 1q; C3, complement C; EDD, electron-dense deposit; PGNMID, proliferative glomerulonephritis with monoclonal IgG deposits.
Table 3.
Clinicopathological characteristics of PGNMID cases
| Monoclonality | No remission (%) | ESKD or death (%) | Transplanted kidney (%) |
|---|---|---|---|
| IgG1 κ | 8.7 | 0 | 4.3 |
| IgG2 λ | 0 | 4.3 | 0 |
| IgG3 κ | 8.7 | 17.4 | 4.3 |
| IgG3 λ | 0 | 8.7 | 4.3 |
ESKD, end-stage kidney disease; PGNMID, proliferative glomerulonephritis with monoclonal IgG deposits.
No remission is defined as proteinuria ≥ 3.5 g/d after treatment.
PV-1 Staining on the Glomerulus
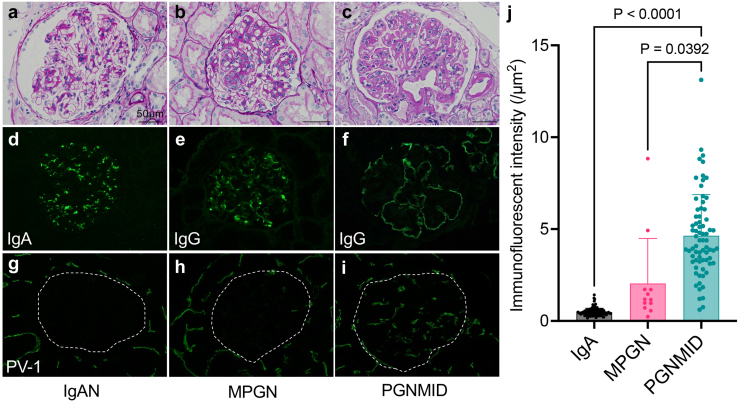
All evaluable glomeruli in IF images obtained from PGNMID, MPGN, and IgAN (representative images in Figure 1a-h) were collected and evaluated for PV-1 intensity. As a result, a total of 72 glomeruli from 30 biopsies of PGNMID, 12 glomeruli from 5 biopsies of primary MPGN, and 93 glomeruli from 54 biopsies of IgAN were evaluated (Figure 1j). The intensity of PV-1 in PGNMID is significantly higher than that in MPGN and IgAN (P < 0.05 and P < 0.001, respectively) (Figure 1j). However, no significant correlations were found between the PV-1 intensity and proteinuria or renal prognosis (Supplementary Figure S3).
Figure 1.
PV-1 expression in glomerular endothelial cells is significant in PGNMID cases. (a, d, g) PV-1 is negative in glomeruli of IgAN and (b, e, h) MPGN. (c, f, i) Alternatively, PV-1 is globally expressed in glomeruli of PGNMID. (j) The intensity of PV-1 in PGNMID is significantly higher than that in primary MPGN and IgA nephropathy (mean with SD, Kruskal-Wallis test). (a–c) Periodic acid-methenamine silver staining (black bars = 50 μm); (d) immunofluorescent staining for IgA, (e, f) IgG, and (g–i) PV-1. IgAN, IgA nephropathy; MPGN, membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits.
Overexpressed PV-1 Is Localized in GECs
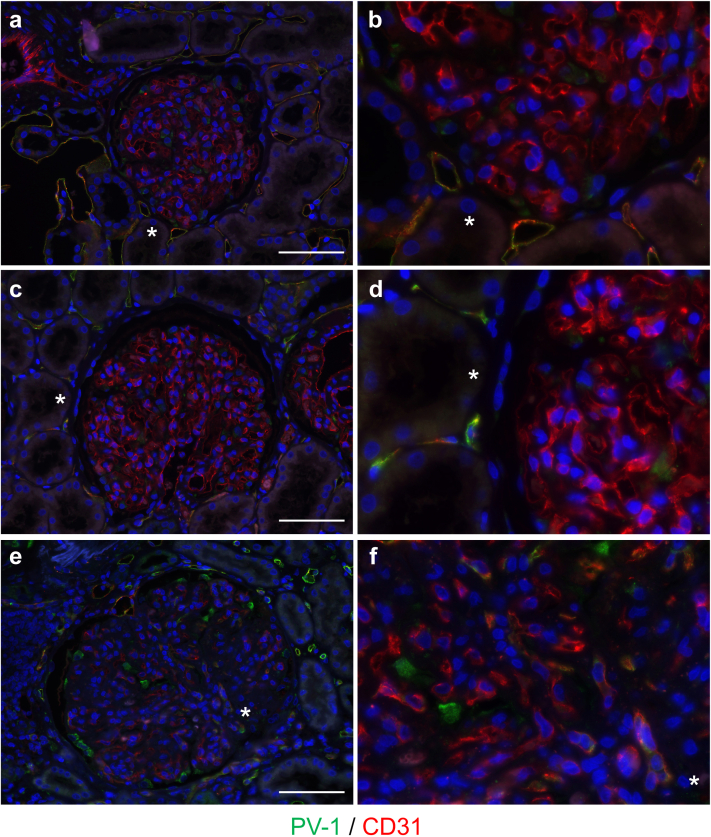
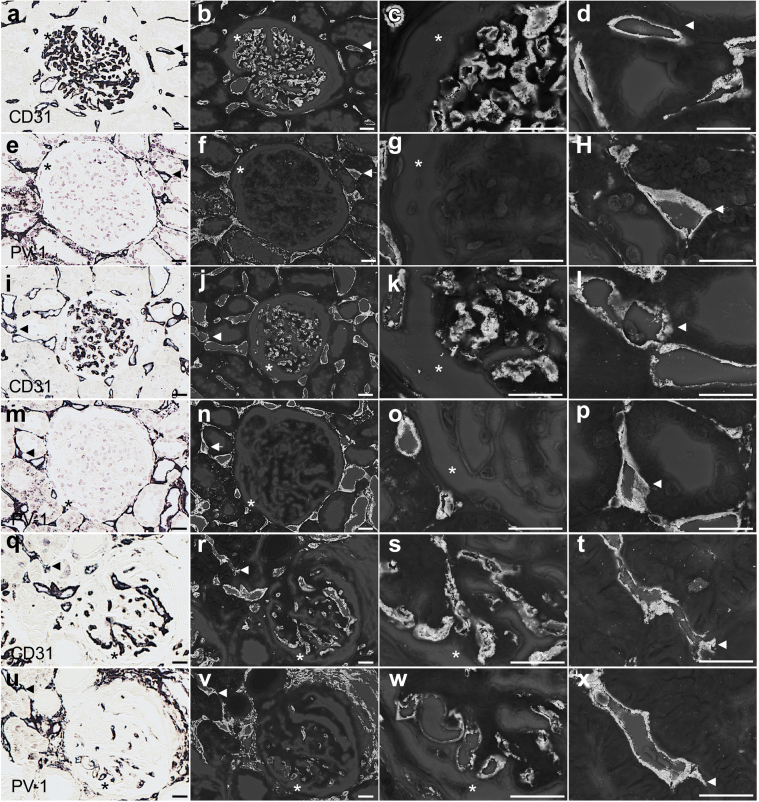
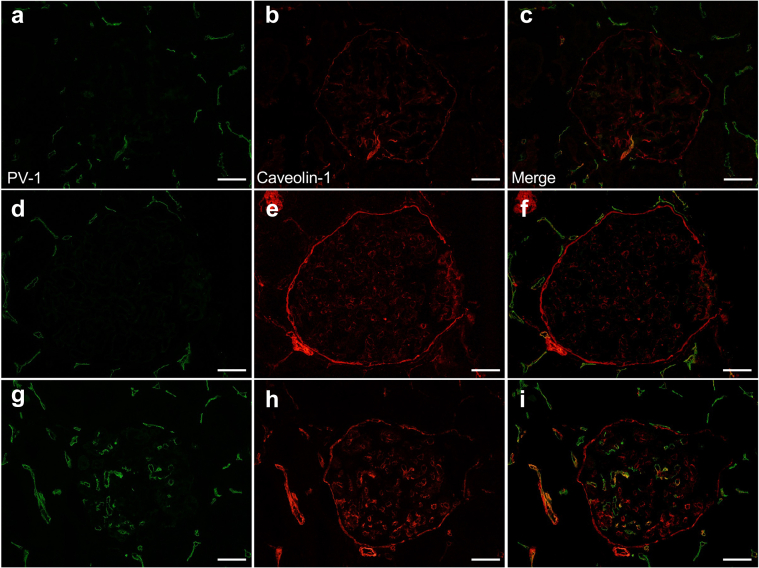
Although PV-1 and CD31 were merged in peritubular capillaries in all cases, glomerular PV-1 expression was not detected in IgAN and MPGN cases (Figure 2a and b and Figure 2c and d, respectively). PV-1 and CD31 were merged in the glomeruli only in PGNMID case (Figure 2e and f), suggesting that PV-1 expression localized in GECs. LVSEM analysis showed that the presence of enhanced white signals of PV-1 and CD31 in the surface of both peritubular capillaries and GECs (Figure 3). Negative control stain and basement membrane analyses are shown in Supplementary Figure S4. Moreover, the glomerular PV-1 staining pattern of PGNMID mostly merged with caveolin-1, whereas it rarely occurred in IgAN and MPGN (Figure 4).
Figure 2.
PV-1 expression is colocalized with CD31 in GEC of PGNMID. (a, b) PV-1 and CD31 colocalization was found in the peritubular capillaries, but not in the glomeruli in IgA nephropathy and (c, d) primary MPGN. (e, f) In PGNMID, PV-1 and CD31 were merged in glomeruli. 4',6-diamidino-2-phenylindole was used for nuclear counterstaining. White bars = 50 μm, and asterisk shows same lesions. GEC, glomerular endothelial cell; MPGN, membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits; PV-1, plasmalemmal vesicle-associated protein-1.
Figure 3.
LVSEM analysis of PV-1 and CD31 expression in PGNMID. (a–h) Representative case of IgA, (i–p) MPGN, and (q–x) PGNMID were examined by LVSEM. The lesions were enhanced by methenamine silver or gold (III) chloride combined with 3,3′-diaminobenzidine after immunohistochemistry of CD31 (a–d, IgAN; i–l, MPGN; q–t, PGNMID) and PV-1 (e–h, IgAN; m–p, MPGN; u–x, PGNMID). The panels display peritubular capillaries (arrowheads) and glomerular capillaries (asterisk). White bars = 20 μm. LVSEM, low-vacuum scanning electron microscope; MPGN, membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits; PV-1, plasmalemmal vesicle-associated protein-1.
Figure 4.
The glomerular PV-1 is colocalized with caveolin-1 in PGNMID. (a–c) PV-1 expression was localized only in peritubular capillaries, and caveolin-1 showed glomerular endothelium pattern in IgA nephropathy and (d–f) primary MPGN. (g–i) In PGNMID, PV-1 was globally positive in glomeruli and merged with caveolin-1. White bars = 20 μm. MPGN, membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits; PV-1, plasmalemmal vesicle-associated protein-1.
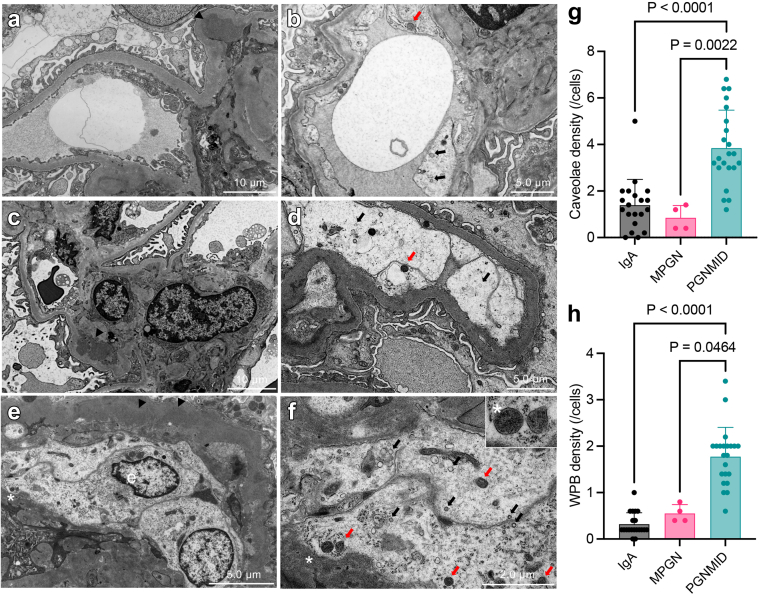
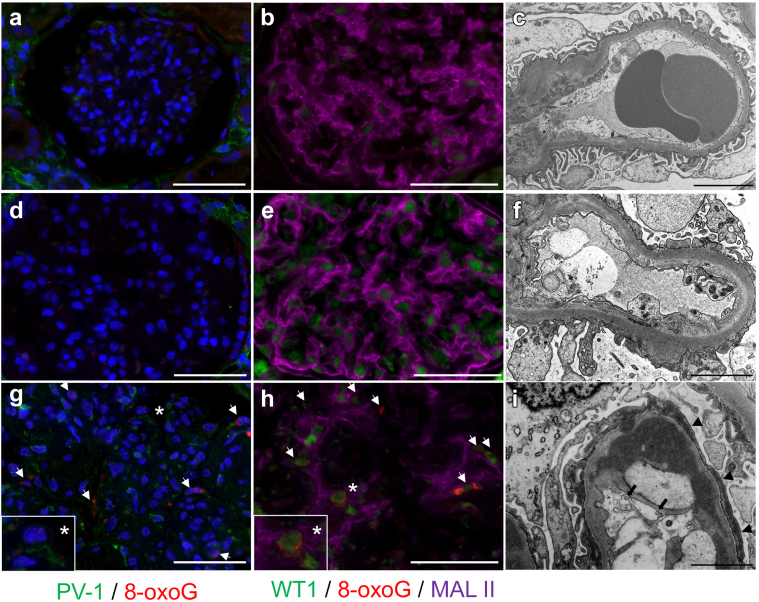
Correspondingly, the numbers of Weibel-Palade body (WPB) and caveolae were increased in GECs in PGNMID cases in TEM analysis (Figure 5a−f). In fact, the density of caveolae and WPB in PGNMID is significantly higher than in IgAN and MPGN (Figure 5g and h), but those are not directly associated with endothelial stress, such as accumulation of 8-oxoG (Figure 6g). Interestingly, accumulation of 8-oxoG was observed in WT1-positive (nucleus) area but not in Maackia Amurensiis Lectin II–positive (cytoplasm or membrane) area of podocyte (Figure 6g and h) with foot process effacement (Figure 6i), suggesting oxidative stress in podocytes of the PGNMID cases. Importantly, accumulation of 8-oxoG was not observed in IgAN and MPGN (Figure 6a–c and d–f).
Figure 5.
Caveolae and WPBs were increased in PGNMID. (a, b) Caveolae (black arrow) and (c, d) WPBs (red arrow) were found in a IgAN case and/or a primary MPGN case. (e, f) Those were increased within enlarged glomerular endothelium in PGNMID cases. WPBs contain microtubular structure inside a single membrane (asterisk indicates same lesion). Arrowheads indicate deposits. (e) Glomerular endothelial cell. Cellular density of (g) caveolae and (h) WPB was shown (mean with SD, Kruskal-Wallis test). MPGN, primary membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits; WPB, Weibel-Palade body.
Figure 6.
Accumulation of 8-oxoG is unique in glomeruli of PGNMID. There were no PV-1 expression and accumulation of 8-oxoG in glomeruli, and/or podocyte effacement in (a–c) IgA nephropathy and (d–f) primary MPGN. Diffuse accumulation of 8-oxoG was observed and merged with WT1, and Maackia Amurensiis Lectin II(MAL II)–positive podocytes were detected in PGNMID. (g, h) Asterisk shows same lesions. (i) Caveolin-like particles were observed in glomerular endothelium (black arrow), accompanied with podocyte effacement (arrowheads), which is compatible with PV-1 expression and 8-oxoG accumulation. White bars = 50 μm and asterisks show same lesions. PV-1, plasmalemmal vesicle-associated protein-1; WT-1, Wilms tumor 1.
Glomerular PV-1 Expression in Various Glomerular Disease
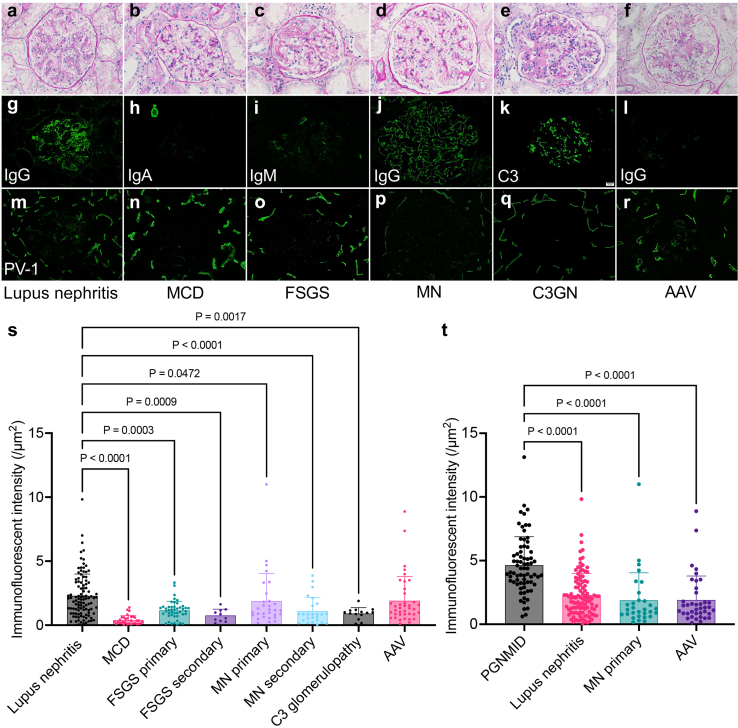
To test whether the PV-1 overexpression is unique in PGNMID, we next examined glomerular PV-1 intensities in various glomerular disease. All evaluable glomeruli were obtained in IF images from the patients of lupus nephritis (105 glomeruli from 51 cases), minimal change disease (32 glomeruli from 21 cases), primary and secondary focal segmental glomerulosclerosis (primary, 45 glomeruli from 21 cases; secondary, 10 glomeruli from 6 cases), primary and secondary membranous nephropathy (primary, 28 glomeruli from 15 cases; secondary, 25 glomeruli from 9 cases), C3 glomerulopathy (14 glomeruli from 6 cases), and antineutrophil cytoplasmic antibody–associated vasculitis (39 glomeruli from 16 cases) (Figure 7a–r). The clinical information of each disease is presented in Supplementary Tables S2 and S3. Among them, the intensity of PV-1 is significantly stronger in lupus nephritis and antibody-associated vasculitis than in other glomerular diseases (Figure 7s). We also added IgG monoclonal pattern analysis for PGNMID, International Society of Nephrology/Renal Pathology Society classification for lupus nephritis26 and IgG-positive or IgG-negative analysis for antibody-associated vasculitis, but there were no significant findings (Supplementary Figure S5). Finally, PGNMID showed significantly higher glomerular PV-1 intensity than the other top 3 glomerular diseases (Figure 7t). Therefore, we conclude that the glomerular PV-1 expression is unique in PGNMID among various types of glomerular diseases.
Figure 7.
Glomerular PV-1 expression in various glomerular disease. (a–r) Glomerular PV-1 expression is confirmed in lupus nephritis and antineutrophil cytoplasmic AAV among various glomerular disease. (s, t) The intensity of PV-1 is significantly stronger in lupus nephritis and AAV than in other glomerular diseases, but it is significantly higher in (t) PGNMID (intensity data were reposted from Figure 1j) (mean with SD, Kruskal-Wallis test). (a–f) Periodic acid-methenamine silver staining; (g–l) immunofluorescent staining; (m–r) PV-1. AAV, antibody-associated vasculitis; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin G deposits; PV-1, plasmalemmal vesicle-associated protein-1; SLE, systemic lupus erythematosus.
Discussion
Studies have reported that PGNMID occurs in 0.17% to 3.7% of native kidney biopsies.2,3,27 Although monoclonal IgG deposits are found in the glomeruli, serologic monoclonal IgG levels are detected in 20% to 30% cases.3,4 Histologically, most cases exhibit the MPGN and/or IgG3κ patterns.2,3 IgG3κ type of PGNMID was also reported to have a higher recurrence rate in renal allografts.4,28
In our study, the largest single-center case series, except that from Mayo Clinic,3,4 the most frequent deposit pattern was also IgG3κ, which exhibited significant complement component depositions (C3 and C1q) in the IF analysis and various subendothelial and mesangial EDDs in the TEM analysis (Table 2) with worse clinical outcomes than other deposit types (Table 3), as reported previously.2 These findings indicate that PGNMID exhibits common clinicopathological characteristics irrespective of race or region.
Next, we demonstrated the pathologic characteristic of PGNMID, especially, overexpression of PV-1 in GECs, which belongs to a type II transmembrane glycoprotein and is known as a component of caveolae.29,30 Caveolae show 50- to 100-nm flask-shaped structures and invaginations of the plasma membrane.17 Caveolin (homologous genes, CAV1, CAV2, and CAV3) contains cytoplasmic N-terminus, transmembrane domain, and cytoplasmic C-terminus and maintain the structure of caveolae.31 CAV1 expression was confirmed in the glomerular endothelium, mesangial cells, podocytes, and Bowman’s parietal epithelial cells in the glomeruli.32, 33, 34, 35 Previous research demonstrated that the intensity of caveolin-1 expression in the glomeruli is associated with urinary albumin excretion in human glomerular disease.36 Caveolae present in endothelial cells are considered to play a vital role in the profibrotic signal transduction and the pathogenesis of albuminuria by promoting endothelial cell albumin uptake.37,38 Phosphorylation of CAV1 (Y14) is an important regulator of these functions.39 Several studies have reported that inhibition of caveolin-1 Y14 phosphorylation ameliorates proteinuria and kidney dysfunction in an animal model of diabetic kidney disease.40,41
In contrast, PV-1 is an essential molecule that forms both stomatal and fenestrated diaphragms17 and plays a vital role in the formation of endothelial fenestrations.42 PV-1 expression and diaphragmed fenestrate were detected in embryotic rat glomeruli, whereas PV-1 expression was not found in adult glomeruli.43 Moreover, in human transplant glomerulopathy, PV-1 overexpression and increases in the number of caveolae in the glomeruli were found to be associated with proteinuria, probably because of the loss of fenestrations,20 which is also found in patients with diabetic nephropathy with albuminuria.44 These findings suggest that PV-1 expression serves as a possible marker of glomerular capillary remodeling.
In PGNMID, we confirmed the colocalization of PV-1 and caveolin-1 in GECs (Figure 4) for the first time, and increased WPB in the luminal surface of GECs (Figure 5), as previously reported.45 WPB contains the von Willebrand factor that forms an adhesive extracellular matrix and allows in situ platelet aggregation and microangiopathy.46 WPB is also associated with vascular endothelial inflammation47 because it secretes interleukin-8, eotaxin-3, endothelin-1, and angiopoietin-2.48 Exocytosis of WPB represents a distinct response of endothelial cells to stressors, and local release of its contents causes escalation of endothelial injury.49 In this study, we found glomerular PV-1 overexpression in lupus nephritis, antibody-associated vasculitis, and PGNMID (Figure 7), suggesting that PV-1 expression serves as a possible marker of glomerular capillary injury (or remodeling).
Moreover, we confirmed that glomerular PV-1 overexpression in PGNMID is related to not only the endothelial cell damage but also podocyte injury via 8-oxoG accumulation, which is an indicator of oxidative stress. In addition, 8-oxoG accumulation could be induced via endothelin-1 signaling pathway and could be associated with subsequent progression of chronic kidney disease as in focal segmental glomerulosclerosis12; however, PV-1 expression is not significant either in primary or secondary focal segmental glomerulosclerosis in our study (Figure 7). Importantly, 8-oxoG accumulation was observed in GECs not in podocytes in the previous study,12 whereas we found that 8-oxoG accumulation was localized in podocytes that is accompanied with PV-1–expressing GECs (Figure 6), suggesting that PV-1 overexpression is a different entity from endothelial endothelin receptor A expression in GECs.12 Another study revealed that endothelial endothelin receptor A expression in GECs could be induced by endothelin-1 from podocytes in focal segmental glomerulosclerosis model mice, suggesting “podocyte derived crosstalk with GECs,”50 which is compatible with the other clinical study.12 We speculated that PV-1 expression accompanied with caveolae and WPB increasing in GECs could cause podocyte injury, suggesting “GEC-derived crosstalk with podocyte.” Nonetheless, TMA lesion usually lacks IgG deposition and is clearly distinguished from PGNMID in the diagnosis criteria,51 although a monoclonal gammopathy of renal significance case, complicated with TMA and PGNMID, was recently reported.52 Nevertheless, monoclonal gammopathy was present in 13.7% of patients with TMA and in 21% of patients older than 50 years.53 It might be related to the monoclonal Ig acting as an autoantibody against a complement regulatory protein, which could trigger the development of another forms of TMA.54 Although standard therapeutic regimens for PGNMID have not been established, there are reports on the effectiveness of the clone-directed approach from the viewpoint of its relationship with monoclonal gammopathy of renal significance.5,55,56 Moreover, in the renal allograft, some reports have indicated that treatment of early PGNMID recurrence with rituximab was effective, resulting in reasonable, long-term graft survival.27 Importantly, there seem to be benign subgroups of PGNMID, which would not require an exclusive immunosuppressor.2 A recent case report also suggested that protection of GECs, using renin angiotensin system blockades, is one of the therapeutic candidates, especially in the cases without M-spike or identified clone.57 Combining previous reports and ours, in PGNMID, an endothelial stress response different from TMA was elicited, leading to podocyte damage through the deposition of “M protein” in the glomerulus, which activates the complement causing an inflammatory response.
We need to acknowledge some limitations in this study. First, we could not find the correlation between the intensity of glomerular PV-1 expression and clinical manifestation, although a previous report showed that glomerular PV-1 expression is related to proteinuria in transplant glomerulopathy.20 It is probably because PGNMID develops rapidly and there is a wide variation in biopsy timing, whereas transplant glomerulopathy develops in gradual stages, and episodic biopsies for renal allograft are generally acceptable. Second, some cases (n = 4) were not examined for paraprotein using immunofixation, immune electrophoresis, or kappa/lambda ratio measurement, although paraprotein was not detected by immune electrophoresis in other cases. We also did not perform more sensitive evaluation techniques, such as serum immunoblotting and molecular studies of bone marrow, to detect subtle plasma cell proliferation, as reported in a case of IgA-proliferative glomerulonephritis with monoclonal immunoglobulin deposits.58 Because many of our cases were originally diagnosed with MPGN or MN and re-diagnosed with PGNMID on the basis of IgG subclasses and light-chain staining in this study, it was difficult to normalize or categorize the clinical situation, especially, the immunosuppressor choice between patients with PGNMID and other patients with GN. In the future study, evaluating the treatment regimen with a detailed analysis of the hematological and histologic findings, such as glomerular PV-1 expression, would be worthy.
In conclusion, we discovered that PV-1 overexpression in GECs might be a useful histologic marker for glomerular endothelial injury in PGNMID cases. Further investigation is required to assess whether these endothelial alterations are associated with clinical prognosis and treatment.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was approved and registered by the ethics committee at Tokyo Women’s Medical University (No. 5415). The authors thank Shigeru Horita, Hideki Nakayama and Mayuko Ohno, Division of Pathology, Kidney Center, Tokyo Women's Medical University, Tokyo, Japan, for their technical support. This work was supported by a JSPS KAKENHI Grant Number JP 19K17730 (to KK), 20J12294 (to AS), JST START University Promotion Type, Grant Number JPMJST2052 (to KK), and LVSEM device support from the LVSEM Study Group of Renal Biopsy and Hitachi High-Technologies Corporation in Japan Grant Number 007 (to KK). The authors would like to thank Enago (www.enago.jp) for the English language review.
Author Contributions
KK conceptualized the study. AS and KK contributed to methodology, formal analysis, and original draft writing. AS, KK, and JK contributed to investigation. KK, MH, HI, KT, YN, and KN contributed to resources. Review and editing were done by AS, KK, YI, ST, JK, and KH. Supervision was performed YN and KN. KK, YN, and KN were responsible for project administration. KK and KN were responsible for funding acquisition.
Footnotes
Figure S1. PGNMID is characterized by glomerular overexpression of PV-1.
Figure S2. Measurements of glomerular PV-1 intensity.
Figure S3. The correlations between the PV-1 intensity and proteinuria or renal prognosis in PGNMID.
Figure S4. Low-vacuum scanning electron microscopy analysis.
Figure S5. Analysis for the PV-1 intensity and immunoglobin deposition.
Table S1. IgA nephropathy and MPGN characteristics.
Table S2. Lupus nephritis, MCD, and FSGS characteristics.
Table S3. MN, C3 glomerulopathy, and AAV characteristics.
Contributor Information
Anri Sawada, Email: anri-sawada@nms.ac.jp.
Kunio Kawanishi, Email: kukawanishi@md.tsukuba.ac.jp.
Supplementary Material
Figure S1. PGNMID is characterized by glomerular overexpression of PV-1.
Figure S2. Measurements of glomerular PV-1 intensity.
Figure S3. The correlations between the PV-1 intensity and proteinuria or renal prognosis in PGNMID.
Figure S4. Low-vacuum scanning electron microscopy analysis.
Figure S5. Analysis for the PV-1 intensity and immunoglobin deposition.
Table S1. IgA nephropathy and MPGN characteristics.
Table S2. Lupus nephritis, MCD, and FSGS characteristics.
Table S3. MN, C3 glomerulopathy, and AAV characteristics.
References
- 1.Nasr S.H., Markowitz G.S., Stokes M.B., et al. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Nasr S.H., Satoskar A., Markowitz G.S., et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhutani G., Nasr S.H., Said S.M., et al. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc. 2015;90:587–596. doi: 10.1016/j.mayocp.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Said S.M., Cosio F.G., Valeri A.M., et al. Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits is associated with high rate of early recurrence in the allograft. Kidney Int. 2018;94:159–169. doi: 10.1016/j.kint.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Gumber R., Cohen J.B., Palmer M.B., et al. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int. 2018;94:199–205. doi: 10.1016/j.kint.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Von Visger J., Cassol C., Nori U., et al. Complete biopsy-proven resolution of deposits in recurrent proliferative glomerulonephritis with monoclonal IgG deposits (PGNMIGD) following rituximab treatment in renal allograft. BMC Nephrol. 2019;20:53. doi: 10.1186/s12882-019-1239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawanishi K., Honda K., Horita S., et al. Recurrent proliferative glomerulonephritis with monoclonal immunoglobulin G deposits leads to rapid graft loss after kidney transplantation: a case report. CEN Case Rep. 2014;3:139–144. doi: 10.1007/s13730-013-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing G., Gillespie R., Bedri B., et al. Proliferative glomerulonephritis with monoclonal IgG deposits in children and young adults. Pediatr Nephrol. 2018;33:1531–1538. doi: 10.1007/s00467-018-3949-8. [DOI] [PubMed] [Google Scholar]
- 9.Jourde-Chiche N., Fakhouri F., Dou L., et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15:87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y.F., Tan Y., Yu X.J., et al. Podocyte involvement in renal thrombotic microangiopathy: a clinicopathological study. Am J Nephrol. 2020;51:752–760. doi: 10.1159/000510141. [DOI] [PubMed] [Google Scholar]
- 11.Fu J., Lee K., Chuang P.Y., et al. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Ren Physiol. 2015;308:F287–F297. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Lest N.A., Bakker A.E., Dijkstra K.L., et al. Endothelial endothelin receptor a expression is associated with podocyte injury and oxidative stress in patients with focal segmental glomerulosclerosis. Kidney Int Rep. 2021;6:1939–1948. doi: 10.1016/j.ekir.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M., Kallenberg C.G. New advances in the pathogenesis of ANCA-associated vasculitides. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S108–S114. doi: 10.1038/nrrheum.2010.158. [DOI] [PubMed] [Google Scholar]
- 14.Hanna R.M., Tran N.T., Patel S.S., et al. Thrombotic microangiopathy and acute kidney injury induced after intravitreal injection of vascular endothelial growth factor inhibitors VEGF blockade-related TMA after intravitreal use. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.579603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ollero M., Sahali D. Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol Dial Transplant. 2015;30:1449–1455. doi: 10.1093/ndt/gfu368. [DOI] [PubMed] [Google Scholar]
- 16.Parton R.G., del Pozo M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 17.Stan R.V. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Niemela H., Elima K., Henttinen T., Irjala H., Salmi M., Jalkanen S. Molecular identification of PAL-E, a widely used endothelial-cell marker. Blood. 2005;106:3405–3409. doi: 10.1182/blood-2005-01-0254. [DOI] [PubMed] [Google Scholar]
- 19.Nishi Y., Namikoshi T., Sasaki T., et al. Histopathological manifestations of membranoproliferative glomerulonephritis and glomerular expression of plasmalemmal vesicle-associated protein-1 in a patient with polycythemia vera. Clin Nephrol. 2010;74:393–398. [PubMed] [Google Scholar]
- 20.Yamamoto I., Horita S., Takahashi T., et al. Glomerular expression of plasmalemmal vesicle-associated protein-1 in patients with transplant glomerulopathy. Am J Transplant. 2007;7:1954–1960. doi: 10.1111/j.1600-6143.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 21.Sawada A., Kawanishi K., Horita S., et al. Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits complicated by immunoglobulin A nephropathy in the renal allograft. Nephrology (Carlton) 2016;21(Suppl 1):48–52. doi: 10.1111/nep.12775. [DOI] [PubMed] [Google Scholar]
- 22.Haas M., Loupy A., Lefaucheur C., et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaga S., Kato M., Hirashima S., et al. Rapid three-dimensional analysis of renal biopsy sections by low vacuum scanning electron microscopy. Arch Histol Cytol. 2010;73:113–125. doi: 10.1679/aohc.73.113. [DOI] [PubMed] [Google Scholar]
- 24.Okada S., Inaga S., Kawaba Y., et al. A novel approach to the histological diagnosis of pediatric nephrotic syndrome by low vacuum scanning electron microscopy. Biomed Res. 2014;35:227–236. doi: 10.2220/biomedres.35.227. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki H., Itoh T., Takaku Y., et al. The NanoSuit method: a novel histological approach for examining paraffin sections in a nondestructive manner by correlative light and electron microscopy. Lab Investig. 2020;100:161–173. doi: 10.1038/s41374-019-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajema I.M., Wilhelmus S., Alpers C.E., et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Gowda K.K., Nada R., Ramachandran R., et al. Proliferative glomerulonephritis with monoclonal immunoglobulin deposition disease: the utility of routine staining with immunoglobulin light chains. Indian J Nephrol. 2015;25:344–348. doi: 10.4103/0971-4065.151354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxeda A., Said S.M., Nasr S.H., et al. Recurrent proliferative glomerulonephritis with monoclonal immunoglobulin deposits in kidney allografts treated with anti-CD20 antibodies. Transplantation. 2019;103:1477–1485. doi: 10.1097/TP.0000000000002577. [DOI] [PubMed] [Google Scholar]
- 29.Rothberg K.G., Heuser J.E., Donzell W.C., et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 30.Keuschnigg J., Tvorogov D., Elima K., et al. PV-1 is recognized by the PAL-E antibody and forms complexes with NRP-1. Blood. 2012;120:232–235. doi: 10.1182/blood-2012-01-406876. [DOI] [PubMed] [Google Scholar]
- 31.Schlegel A., Lisanti M.P., A molecular dissection of caveolin-1 membrane attachment and oligomerization Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605–21617. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 32.Ostalska-Nowicka D., Nowicki M., Zachwieja J., et al. The significance of caveolin-1 expression in parietal epithelial cells of Bowman’s capsule. Histopathology. 2007;51:611–621. doi: 10.1111/j.1365-2559.2007.02844.x. [DOI] [PubMed] [Google Scholar]
- 33.Sorensson J., Fierlbeck W., Heider T., et al. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–2647. doi: 10.1097/01.asn.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- 34.Fujita Y., Maruyama S., Kogo H., et al. Caveolin-1 in mesangial cells suppresses MAP kinase activation and cell proliferation induced by bFGF and PDGF. Kidney Int. 2004;66:1794–1804. doi: 10.1111/j.1523-1755.2004.00954.x. [DOI] [PubMed] [Google Scholar]
- 35.Tamai O., Oka N., Kikuchi T., et al. Caveolae in mesangial cells and caveolin expression in mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:471–480. doi: 10.1046/j.1523-1755.2001.059002471.x. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama T., Tsuruta Y., Shimizu A., et al. The significance of caveolae in the glomeruli in glomerular disease. J Clin Pathol. 2011;64:504–509. doi: 10.1136/jcp.2010.087023. [DOI] [PubMed] [Google Scholar]
- 37.Van Krieken R., Krepinsky J.C. Caveolin-1 in the pathogenesis of diabetic nephropathy: potential therapeutic target? Curr Diab Rep. 2017;17:19. doi: 10.1007/s11892-017-0844-9. [DOI] [PubMed] [Google Scholar]
- 38.Moriyama T., Takei T., Itabashi M., et al. Caveolae may enable albumin to enter human renal glomerular endothelial cells. J Cell Biochem. 2015;116:1060–1069. doi: 10.1002/jcb.25061. [DOI] [PubMed] [Google Scholar]
- 39.Lee H., Volonte D., Galbiati F., et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 40.Wu D., Yang X., Zheng T., et al. A novel mechanism of action for salidroside to alleviate diabetic albuminuria: effects on albumin transcytosis across glomerular endothelial cells. Am J Physiol Endocrinol Metab. 2016;310:E225–E237. doi: 10.1152/ajpendo.00391.2015. [DOI] [PubMed] [Google Scholar]
- 41.Sun L.N., Chen Z.X., Liu X.C., et al. Curcumin ameliorates epithelial-to-mesenchymal transition of podocytes in vivo and in vitro via regulating caveolin-1. Biomed Pharmacother. 2014;68:1079–1088. doi: 10.1016/j.biopha.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Satchell S.C., Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Ren Physiol. 2009;296:F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichimura K., Stan R.V., Kurihara H., Sakai T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol. 2008;19:1463–1471. doi: 10.1681/ASN.2007101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toyoda M., Najafian B., Kim Y., et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 45.Green D.F., Hwang K.H., Ryan U.S., Bourgoignie J.J. Culture of endothelial cells from baboon and human glomeruli. Kidney Int. 1992;41:1506–1516. doi: 10.1038/ki.1992.220. [DOI] [PubMed] [Google Scholar]
- 46.Hannah M.J., Williams R., Kaur J., et al. Biogenesis of Weibel-Palade bodies. Semin Cell Dev Biol. 2002;13:313–324. doi: 10.1016/s1084-9521(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 47.Rondaij M.G., Bierings R., Kragt A., et al. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 48.Frank P.G., Woodman S.E., Park D.S., Lisanti M.P. Caveolin, caveolae, and endothelial cell function Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda K., Vasko R., Hayek P., et al. Functional consequences of inhibiting exocytosis of Weibel-Palade bodies in acute renal ischemia. Am J Physiol Ren Physiol. 2012;302:F713–F721. doi: 10.1152/ajprenal.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daehn I., Casalena G., Zhang Taoran, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung N., Bridoux F., Batuman V., et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De La Flor J.C., Alonso M., Sandoval E., Marschall A., Rodeles M. Monoclonal gammopathy of renal significance with deposits of peculiar morphology and injuries of secondary thrombotic microangiopathy: a case report and review of the literature. Case Rep Nephrol. 2020;2020 doi: 10.1155/2020/6679857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravindran A., Go R.S., Fervenza F.C., Sethi S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017;91:691–698. doi: 10.1016/j.kint.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 54.Sethi S., Rajkumar S.V., D’Agati V.D. The complexity and heterogeneity of monoclonal immunoglobulin–associated renal diseases. J Am Soc Nephrol. 2018;29:1810–1823. doi: 10.1681/ASN.2017121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J., Wang W., Xu F., et al. Clinicopathological analysis of proliferative glomerulonephritis with monoclonal IgG deposits in 5 renal allografts. BMC Nephrol. 2018;19:173. doi: 10.1186/s12882-018-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kousios A., Duncan N., Tam F.W.K., et al. Proliferative glomerulonephritis with monoclonal Ig deposits (PGNMID): diagnostic and treatment challenges for the nephrologist. Kidney Int. 2019;95:467–468. doi: 10.1016/j.kint.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Rosenstock J.L., Vynnyk M., DeVita M.V., D’Agati V.D. Two cases of proliferative glomerulonephritis with monoclonal IgG deposits treated with renin angiotensin inhibition alone with long-term follow-up Kidney Int Rep. 2021;2021:2218–2222. doi: 10.1016/j.ekir.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignon M., Cohen C., Faguer S., et al. The clinicopathologic characteristics of kidney diseases related to monotypic IgA deposits. Kidney Int. 2017;91:720–728. doi: 10.1016/j.kint.2016.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.