Abstract
Objectives
To review the drug-drug interactions between tacrolimus and lopinavir/ritonavir in 23 patients who received solid organ transplant during the first wave of COVID-19 and to determine the efficacy as well as safety of prednisone monotherapy.
Methods
Observational study performed between March and June 2020 in solid organ transplant recipients admitted with an established diagnosis of SARS-CoV-2 infection who received lopinavir/ritonavir (≥2 doses). Once lopinavir/ritonavir therapy was initiated, calcineurin inhibitor treatment was temporarily switched to prednisone monotherapy (15–20 mg/d) to avoid drug-drug interactions and toxicity. After lopinavir/ritonavir treatment completion, immunosuppressive treatment was restarted with reduced doses of prednisone-tacrolimus (target minimum blood concentration –C0- approximately 5 ng/mL). Patients were observed for 3 months to confirm the absence of rejection.
Results
The median time from discontinuation of tacrolimus to initiation of lopinavir/ritonavir was 14 hours (interquartile range [IQR], 12–15) and from discontinuation of lopinavir/ritonavir to resumption of tacrolimus 58 hours (IQR, 47–81). The duration of lopinavir/ritonavir treatment was 7 days (IQR, 5–7). Nine of the 21 (42.8%) patients on tacrolimus treatment had C0 above the cutoff point after lopinavir/ritonavir initiation, despite having been substituted with prednisone before lopinavir/ritonavir initiation. Three patients had very high concentrations (>40 ng/mL) and developed toxicity. No episodes of acute rejection were diagnosed.
Discussion
We did not observe toxicity in patients for whom tacrolimus was discontinued 24 hours before starting lopinavir/ritonavir and reintroduced at half dose 48 to 72 hours after lopinavir/ritonavir discontinuation. Prednisone monotherapy during lopinavir/ritonavir therapy was safe with no episodes of acute rejection. Experience with lopinavir/ritonavir may be applicable to the use of nirmatrelvir/ritonavir, but larger multicentre studies are needed to confirm these findings.
Keywords: Clinical research/practice, Drug interaction, Immunosuppressant, Lopinavir/ritonavir, SARS-CoV-2/COVID-19, Solid organ transplantation
Introduction
Nirmatrelvir/ritonavir is the first approved oral SARS-CoV-2 protease inhibitor [1]. It is indicated for the treatment of COVID-19 in adults who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19. Drug-drug interactions (DDIs) are a major constraint because of the potent inhibition of CYP3A4 and, to a lesser extent, of CYP2D6, by ritonavir. Ritonavir also inhibits P-glycoprotein. Studies show lopinavir/ritonavir, used during the first wave of COVID-19 pandemic, to be ineffective [2]. Nevertheless, experience in its management in vulnerable groups, e.g. patients who received solid organ transplant (SOT) may be useful for managing nirmatrelvir/ritonavir and other ritonavir-boosted therapies today.
The calcineurin inhibitor (CNI), tacrolimus, is the cornerstone of current immunosuppressive treatment in SOT recipients, sometimes administered in combination with mycophenolic acid or an inhibitors of the mammalian target of rapamycin (mTORi). Mycophenolic acid (MPA) is metabolized mainly by glucuronyl transferase. Although ritonavir is an inducer of this enzyme, in short-term use it has no inducing effect; therefore, the risk of interaction with MPA is low. In contrast, CNI and mTORi are major substrates of CYP3A4 and P-glycoprotein, and ritonavir may increase their systemic concentrations. Toxicity has been described when associating lopinavir/ritonavir to everolimus [3] and tacrolimus [4] in clinical practice. To reduce immunosuppression and avoid DDI in SOT recipients, in March 2020, the usual immunosuppressive treatment was replaced with prednisone monotherapy [5] during lopinavir/ritonavir treatment.
This study reviewed DDIs between tacrolimus and lopinavir/ritonavir in SOT recipients treated with lopinavir/ritonavir during the first COVID-19 wave and determined the efficacy and safety of prednisone monotherapy.
Methods
Design
We conducted a retrospective review of all SOT recipients admitted with a confirmed diagnosis of COVID-19 and having received lopinavir/ritonavir (≥2 doses) between March and June 2020 at the Hospital Clinic Barcelona, a tertiary-care teaching hospital in Barcelona, Spain. Patients were followed up to 3 months after resuming their usual immunosuppression treatment to rule out transplant-organ rejection. The study was approved by the Drug Research Ethics Committee (CEIm) Hospital Clinic Barcelona (HCB/2021/0630).
Immunosuppressive management
According to centre policy, mycophenolate and mTORi were initially withdrawn in all admitted SOT recipients with COVID-19. Furthermore, patients also had CNI temporarily discontinued before lopinavir/ritonavir therapy initiation. Interaction management was performed on a case-by-case basis according to our centre's experience with SOT in HIV. Maintenance immunosuppression was prednisone monotherapy (15–20 mg/day) [5]. Once lopinavir/ritonavir treatment was completed and after the acute phase of infection, immunosuppressive treatment was restarted according to the clinical situation, renal function, and residual tacrolimus levels. Prednisone was reduced whereas tacrolimus progressively increased (minimum blood concentration –C0- around 5ng/mL). Ritonavir causes irreversible inhibition of CYP3A4 (i.e. mechanism-based inhibition) which takes several days to resolve upon discontinuation of ritonavir [6]. Hence, tacrolimus should not be reinitiated immediately after the last dose of a SARS-CoV-2 ritonavir-based regimen.
Variables
The variables collected are depicted in Table S1. In patients with elevated tacrolimus concentrations, risk factors were assessed. We considered a tacrolimus C0 > 12 ng/mL (4 ng/mL in association with everolimus) outside the therapeutic range [7]. Blood levels of tacrolimus in admitted patients were closely monitored every 24 to 72 hours.
Results
Twenty-three patients who received SOT were admitted for COVID-19 during the period: 18 kidneys (86.9%), 2 hearts (8.7%), 1 liver (4.3%), and 2 combined: kidney-pancreas and kidney-heart. Table S1 shows the baseline characteristics and immunosuppressive treatment: tacrolimus (20 cases), everolimus (5 cases), and sirolimus (3 cases). Median age was 55.0 years (interquartile range [IQR], 46.0–65.5); 17 men (73.9%). Median time from transplant to COVID-19 was 4.4 years (IQR, 2.1–6.2). None of the patients diagnosed with COVID-19 had been recently transplanted (<3 months), except for one patient who presented with nosocomial COVID-19 during the postoperative period of a heart transplant.
Median time from tacrolimus suspension to lopinavir/ritonavir initiation was 14 hours (IQR, 12–15), and from lopinavir/ritonavir discontinuation to tacrolimus reinitiation 58 hours (IQR, 47–81). Lopinavir treatment duration was 7 days (IQR, 5–7). The percentages of the usual daily tacrolimus dose used to restart therapy and ongoing after 1 week were 45% (IQR, 25%–69%) and 60% (IQR, 37%–96%). No correlation was observed between patients' renal or hepatic function and drug elimination.
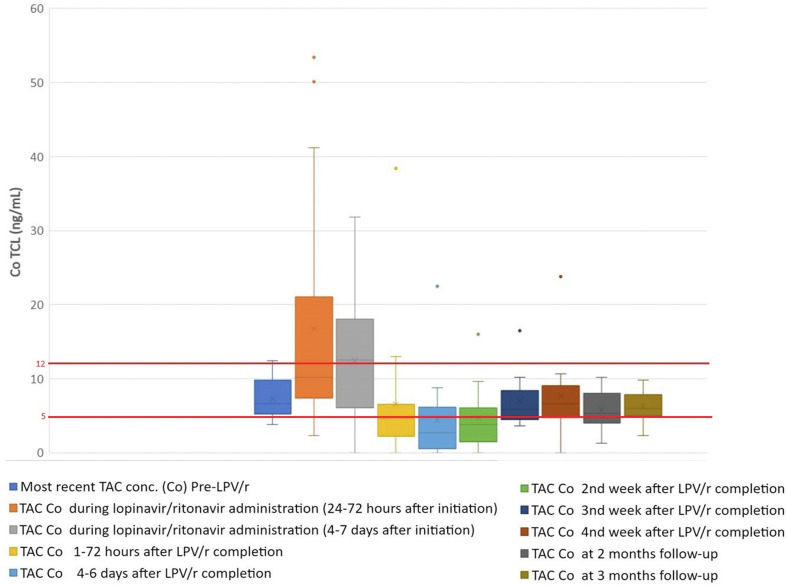
Fig. 1 depicts median blood tacrolimus concentrations over time. Nine (42.8%) of 21 patients had C0 above the limit after starting lopinavir/ritonavir, despite it being replaced with prednisone before lopinavir/ritonavir initiation. Three patients presented very high concentrations: 41.2 ng/mL, 50.1 ng/mL, and 53.4 ng/mL and developed toxicity possibly related to tacrolimus: tremors, metabolic acidosis, hypocalcaemia, and acute renal failure. One of them mistakenly received 2 lopinavir/ritonavir doses 3 hours apart, approximately 11 hours after tacrolimus. Although lopinavir/ritonavir was withdrawn, tacrolimus reached 53.44 ng/mL. Of note, 14 and 18 hours elapsed between the last dose of tacrolimus and the lopinavir/ritonavir initiation in the remaining 2 patients. None of those with tacrolimus C0 above the cutoff received a blood transfusion, had bleeding or vomiting, or had significant elevations of liver enzymes. No supratherapeutic concentrations were observed in 3 patients initiating lopinavir/ritonavir 24 hours or more after discontinuation of tacrolimus. No elevated everolimus or sirolimus concentrations were observed.
Fig. 1.
Box plot chart of tacrolimus (tacrolimus) trough (Co) blood concentrations. The first box represents the most recent tacrolimus C0 before receipt of lopinavir/ritonavir. All patients received lopinavir/ritonavir for a median of 7 days (interquartile range [IQR], 5–7). The second and third plots represent tacrolimus C0 during lopinavir/ritonavir administration (24–72 hours and 4–7 days after starting Lopinavir/ritonavir). The 4th to the 10th plots represent tacrolimus C0 at different times after Lopinavir/ritonavir completion and tacrolimus restart (1–72 hours, 4–6 days, 2 weeks, 3 weeks, 4 weeks, and 2 months and 3 months of follow-up). The boxes represent median and IQR and the end of the lines the values Q1-1.5 × IQR and Q3 + 1.5 × IQR. The dots are outliers. The red horizontal lines represent the minimum or maximum target values of tacrolimus blood concentrations.
Once immunosuppression resumed, 20 restarted tacrolimus and 2 were switched from sirolimus to tacrolimus. One heart-transplant patient presented supratherapeutic concentrations (tacrolimus, 22.5 ng/mL) after restarting tacrolimus (70 hours after lopinavir/ritonavir therapy completion), switching to cyclosporine, mycophenolate mofetil, and prednisone before transfer to another centre. Interestingly, we observed no other elevated blood tacrolimus concentrations.
No SOT rejection was observed during the 3-month follow-up. Four patients died during admission: two from COVID-19 pneumonia and two from other complications. None of the deaths were related to DDI.
Discussion
Using ritonavir-boosted therapies in SOT is a challenge. Despite extreme care in managing CNI under our protocol, we observed supratherapeutic tacrolimus concentrations with related toxicity. Bartiromo M et al. [8] and Prikis M et al. [9] also reported renal toxicity after DDI between tacrolimus and nirmatrelvir/ritonavir.
The interval between the last dose of immunosuppressant and initiation of lopinavir/ritonavir may be a key factor. The terminal elimination half-life of tacrolimus ranges between 4 and 41 hours [7]. Therefore, if lopinavir/ritonavir is added soon after tacrolimus discontinuation, concentrations may still be detectable when lopinavir/ritonavir is added. We observed supratherapeutic concentrations in 9 patients (in 3: >40 ng/mL), but not in the 3 initiating lopinavir/ritonavir ≥24 hours after tacrolimus discontinuation. Optimally, tacrolimus concentrations should be detectable but low enough so that the addition of lopinavir/ritonavir does not lead to supratherapeutic concentrations.
After completing lopinavir/ritonavir, we observed no toxicity upon resuming tacrolimus. Wang et al. [10] also described no toxicity in 4 patients who received SOT and recommended reintroducing tacrolimus in partial or full doses 2 to 4 days after completing nirmatrelvir/ritonavir. However, Salerno et al. [4] observed supratherapeutic tacrolimus concentrations in 4 of 21 patients with SOT, when reintroducing tacrolimus at 82% of usual dose at a median of 8 days from starting nirmatrelvir/ritonavir (i.e. 48–96 hours after nirmatrelvir/ritonavir discontinuation). Of note, tacrolimus C0 measured after ending nirmatrelvir/ritonavir was normal. Similarly, we restarted tacrolimus a median of 58 hours (IQR, 47–81) after lopinavir/ritonavir withdrawal, but, unlike them, we used just 45% (IQR, 25–71) of the usual tacrolimus dose and observed no toxicity. Therefore, it appears that the percentage of the administered dose may be relevant when reintroducing tacrolimus shortly after ritonavir discontinuation. We observed no toxicity with everolimus or sirolimus. However, fewer patients received them.
This study has some limitations because of the exceptional pandemic situation. The area under the curve was not determined. In addition, the CYP3A4 and CYP3A5 genetic polymorphisms, relevant for tacrolimus, were not genotyped. In addition, some patients took the last dose of tacrolimus at home; therefore, the times elapsed before lopinavir/ritonavir are estimates based on usual dosing. However, these results correspond to real clinical practice.
Some authors prefer to switch immunosuppressive agents to corticosteroids in SOT with SARS-CoV-2 infection to minimize immunosuppression and reduce adverse events [11,12]. In our experience, using prednisone monotherapy while patients were on lopinavir/ritonavir was safe and successful. Based on this, if ritonavir-boosted therapy, e.g. nirmatrelvir/ritonavir, is clinically indicated, tacrolimus should be discontinued without delay and preferably ≥24 hours before starting nirmatrelvir/ritonavir, thus avoiding toxicity. Conservatively, we suggest that tacrolimus be restarted 48 to 72 hours after ritonavir discontinuation at 50% of the basal dose. In the case of very high tacrolimus C0, it may take longer (up to 6–7 days after nirmatrelvir/ritonavir withdrawal) to reach normal concentrations [8], and clinical decisions should be based on therapeutic drug monitoring.
In conclusion, in our case series, we did not observe toxicity in any of the patients for whom tacrolimus was discontinued 24 hours before starting lopinavir/ritonavir and reintroduced at half dose 48 to 72 hours after discontinuation. Nevertheless, close monitoring of tacrolimus plasma levels is advised after the last dose of ritonavir to guide the reinitiation of tacrolimus. Prednisone monotherapy during lopinavir/ritonavir therapy was safe with no episodes of acute rejection. Experience with lopinavir/ritonavir may be applicable to the use of nirmatrelvir/ritonavir.
Author contributions
M.B.,.J.M.M., and M.T. contributed equally as senior authors.
Transparency declaration
J.M.M. received a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–23 and has received consulting honoraria and/or research grants from Angelini, Contrafect, Genentech, Gilead Sciences, Janssen, Lysovant, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work.. M.T. has received honoraria from Janssen, Gilead Sciences, MSD, ViiV Healthcare, and Shionogi. All other authors declare that they have no conflicts of interest.
Data availability statement
The data that support the findings of this study are available on request from mtuset@clinic.cat.
Editor: K. Andre
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.01.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/nejmoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P.W., Mafham M., Bell J.L., Linsell L., Staplin N., Emberson J., et al. RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/s0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meziyerh S., Zwart T.C., van Etten R.W., Janson J.A., van Gelder T., Alwayn I.P.J., et al. Severe COVID-19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transplant. 2020;20:1896–1901. doi: 10.1111/ajt.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salerno D.M., Jennings D.L., Lange N.W., Kovac D.B., Shertel T., Chen J.K., et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22:2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montagud-Marrahi E., Cofan F., Torregrosa J.V., Cucchiari D., Ventura-Aguiar P., Revuelta I., et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single center cohort of kidney recipients. Am J Transplant. 2020;20:2958–2959. doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stader F., Khoo S., Stoeckle M., Back D., Hirsch H.H., Battegay M., et al. Stopping lopinavir/ritonavir in COVID-19 patients: duration of the drug interacting effect. J Antimicrob Chemother. 2020;75:3084–3086. doi: 10.1093/jac/dkaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet M., van Gelder T., Åsberg A., Haufroid V., Hesselink D.A., Langman L., et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307. doi: 10.1097/ftd.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 8.Bartiromo M., Borchi B., Botta A., Bagalà A., Lugli G., Tilli M., et al. Threatening drug-drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID-19) Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prikis M., Cameron A. Paxlovid (Nirmatelvir/Ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Transplant Proc. 2022;54:1557–1560. doi: 10.1016/j.transproceed.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang A.X., Koff A., Hao D., Tuznik N.M., Huang Y. Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: early experience in four SARS-CoV-2 infected kidney transplant recipients. Am J Transplant. 2022;22:2117–2119. doi: 10.1111/ajt.16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A., et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875. doi: 10.1111/ajt.15874. –8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forns X., Navasa M. Liver transplant immunosuppression during the Covid-19 pandemic. Gastroenterol Hepatol. 2020;43:457–463. doi: 10.1016/j.gastrohep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from mtuset@clinic.cat.