Abstract
Introduction
Both coronary flow reserve (CFR) and chronic kidney disease (CKD) are known to be associated with adverse cardiac events. However, it is unclear how these prognostic factors are interrelated. This study evaluated the association between intracoronary physiologic indexes and CKD and their prognostic implications.
Methods
A total of 351 patients without left ventricular systolic dysfunction (ejection fraction ≥ 40%) and not on dialysis whose revascularization was deferred based on fractional flow reserve (FFR) > 0.80 were analyzed. Depressed CFR was defined as CFR ≤ 2.0. The primary outcome was a composite of cardiac death or hospitalization for heart failure at 3 years.
Results
Patients with CKD showed lower CFR than the non-CKD population (3.28 ± 1.77 vs. 2.60 ± 1.09, P < 0.001), mainly driven by increased resting coronary flow. There was no significant difference in hyperemic coronary flow, FFR, and index of microvascular resistance between the 2 groups. CFR was significantly associated with estimated glomerular filtration rate (eGFR) (P = 0.045), and the proportion of depressed CFR was significantly increased with higher CKD stages (P = 0.011). The risk of cardiac death or hospitalization for heart failure was the lowest in the non-CKD and preserved CFR group (11.9%) and the highest in the CKD and depressed CFR group (60.0%, overall log rank P < 0.001). Both CKD (adjusted hazard ratio [HRadj] 2.614, 95% confidence interval [CI] 1.505–4.539, P < 0.001) and depressed CFR (HRadj 3.237, 95% CI 2.015–5.199, P < 0.001) were independently associated with the risk of the primary outcome.
Conclusion
There was a significant association between severity of CKD and CFR. Both CKD and depressed CFR showed independent association with higher risk of cardiac death or hospitalization for heart failure.
Keywords: chronic kidney disease, coronary flow reserve, coronary microvascular dysfunction, index of microcirculatory resistance, prognosis
Graphical abstract
See Commentary on Page 10
CKD is a traditional comorbidity with cardiovascular diseases, which is the leading cause of early morbidity and mortality.1 Even after successful percutaneous coronary intervention, CKD is known to be associated with adverse cardiovascular outcome including nonfatal myocardial infarction, restenosis, or stent thrombosis.2, 3, 4 Furthermore, patients with CKD are prone to develop sudden death, fatal arrhythmia, diastolic dysfunction, and heart failure, even without significant epicardial coronary stenosis.4, 5, 6
Preclinical and postmortem studies have continuously reported the increased risk of sudden death or heart failure without significant epicardial stenosis in patients with CKD, and rarefaction of the myocardial microvasculature and/or microvascular dysfunction has been proposed as a pathophysiologic mechanism for nonatherosclerotic manifestation of CKD.7, 8, 9 In this regard, there have been few studies investigating the association between CKD and the presence of coronary microcirculatory dysfunction (CMD) by noninvasively measured CFR using positron emission tomography, magnetic resonance imaging, or Doppler echocardiography.6,10, 11, 12, 13 In these studies, noninvasively measured CFR was significantly associated with increased risk of cardiovascular mortality or adverse cardiac events.6,10,13
Presence of CMD can be also evaluated by invasive physiologic assessment using both CFR and index of microcirculatory resistance (IMR), which allows more accurate and comprehensive evaluation of entire coronary circulation.14,15 Nevertheless, limited data exist regarding the association between CKD and coronary microcirculatory function by invasive physiologic assessment. Accordingly, this study sought to evaluate the following: (i) the association between invasive physiologic indexes and CKD, and (ii) the differential prognostic implications by CKD and CMD dysfunction defined by invasive physiologic indexes.
Methods
Study Design and Population
Study population was derived from the DIAST-CMD (Prognostic Impact of Cardiac Diastolic Function and Coronary Microvascular Function, NCT05058833) registry, which prospectively enrolled patients who underwent clinically indicated invasive coronary angiography and comprehensive physiologic assessments, including FFR, CFR, and IMR measurements for at least 1 vessel. Patients with hemodynamic instability, severe left ventricular (LV) dysfunction, a culprit vessel of acute coronary syndrome, or severe valvular stenosis or regurgitation were excluded. All patients underwent echocardiography before or after coronary angiography. Among the registered population, patients with end-stage renal disease requiring dialysis, unavailable echocardiography data, LV ejection fraction < 40%, or functionally significant epicardial coronary stenosis (FFR ≤ 0.80) were excluded from this analysis, leaving 351 patients as the final sample size (Supplementary Figure S1). The study protocol was approved by the Institutional Review Board of Samsung Medical Center and conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent before registry enrollment.
Coronary Angiography and Intracoronary Physiologic Measurements
Coronary angiography was performed using standard techniques. All angiograms were analyzed at a core laboratory (Samsung Medical Center) in a blinded fashion using validated software (Centricity CA, GE, Waukesha, WI). Atherosclerotic burden in the epicardial coronary arteries was assessed by the SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score.
All coronary physiologic measurements were performed according to standard protocol by using a pressure-temperature sensor-tipped guide wire (PressureWire, Abbot Vascular, St. Paul, MN), after diagnostic angiography. Intracoronary nitrates (100 or 200 μg) were administered before each measurement. The guide wire was positioned at the distal segment of a target vessel. To derive resting mean transit time (Tmn), a thermodilution curve was obtained by using 3 injections of 4 ml room-temperature saline. Hyperemia was induced by intravenous infusion of adenosine (140 μg/kg/min) or intracoronary bolus injection of nicorandil (2 mg). Hyperemic proximal aortic pressure (Pa), distal arterial pressure (Pd), and hyperemic Tmn were measured during sustained hyperemia. After completion of measurements, the guide wire was pulled back to the guide catheter to check for the presence of a pressure drift. It was recommended to re-equalize and repeat measurements if the pressure drift was larger than > 0.03 of an FFR unit. CFR was calculated as resting Tmn/hyperemic Tmn. FFR was calculated as the lowest average of 3 consecutive beats during stable hyperemia. IMR was calculated by Pd × Tmn during hyperemia and expressed as unit (U). All coronary physiologic data were collected and validated at a core laboratory (Samsung Medical Center) in a blinded fashion.
Definitions and Clinical Outcomes
Based on the Kidney Disease: Improving Global Outcomes criteria,16 CKD was defined as having an eGFR of <60 ml/min per 1.73 m2 or the presence of albuminuria for longer than 3 months. Albuminuria was defined as the urine albumin-to-creatinine ratio (ACR) greater than 30 mg/g. Stages of CKD were defined as follows: stage 1 (eGFR ≥ 90 ml/min per 1.73 m2 and ACR ≥ 30 mg/g), stage 2 (eGFR 60–89 ml/min per 1.73 m2 and ACR ≥ 30 mg/g), stage 3 (eGFR 30–59 ml/min per 1.73 m2 regardless of ACR), stage 4 (eGFR 15–29 ml/min per 1.73 m2 regardless of ACR), and stage 5 (eGFR < 15 ml/min per 1.73 m2 regardless of ACR). Depressed CFR was defined as CFR ≤ 2.0 and elevated IMR was defined IMR ≥ 25 U, as previously described.17
Baseline characteristics, including demographics and cardiovascular risk factors, were recorded at the time of the index procedure. Follow-up was performed during outpatient visits or by telephone contact at 1, 6, 12, 24, and 36 months. For patients who were lost to follow-up, mortality data with cause of death were confirmed by National Death Records. The primary clinical outcome was a composite of cardiac death or hospitalization for heart failure at 3 years. All deaths were considered cardiovascular unless a definitive noncardiovascular cause was identified. Hospitalization for heart failure was defined as first admission due to heart failure. Hospitalization for heart failure should include all of the following criteria: (i) hospitalization with primary diagnosis of heart failure, (ii) hospitalization duration of at least 12 hours, (iii) new or worsening symptoms of heart failure, (iv) objective evidence of new or worsening heart failure on physical examination or laboratory findings, and (v) initiation or intensification of heart failure treatment.
Statistical Analysis
Data were analyzed on a per-patient basis. All discrete or categorical variables were presented as numbers and relative frequencies (percentages) and compared using the χ2 test or Fisher's exact test. Continuous variables were analyzed using the unpaired t tests or Mann-Whitney rank-sum test and presented as means and standard deviations according to their distributions, which were checked by the Kolmogorov-Smirnov test and visual inspection of Q-Q plots. Correlation coefficients were calculated to assess relationship between eGFR and physiologic indexes (Pearson or Spearman according to the normality). Multivariable linear regression analyses adjusted for confounding factors (age, diabetes mellitus, body mass index) were conducted to test the association between eGFR and invasive physiologic indexes. The proportion of patients with depressed CFR or elevated IMR across the stages of CKD were compared using Cochran-Armitage trend test. The cumulative incidence of clinical events was evaluated by Kaplan-Meier estimate and compared using a log-rank test. In comparisons of clinical outcomes according to the presence of CKD or depressed CFR, multivariable Cox proportional hazard regression was used to calculate the HRadj and 95% CI. The assumption of proportionality was assessed by the Schoenfeld residuals. Multivariable Cox regression analysis was fitted to evaluate the independent predictors associated with primary clinical outcome. The multivariable model was constructed using all variables that could be clinically relevant and were significant on univariable analysis with P < 0.05. Candidate variables are listed in Supplementary Table S1. The final model was selected using backward elimination based on the Akaike information criterion.
All probability values were 2-sided, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics of Patients
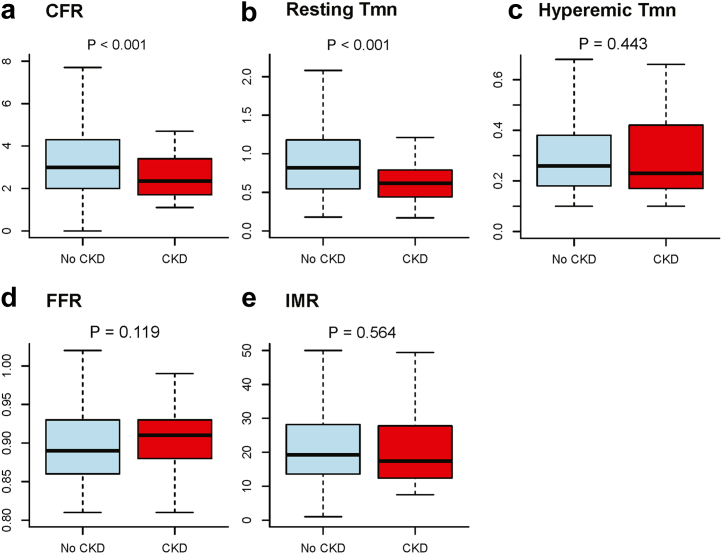
Overall, the mean age was 59.8 ± 13.7 years and 29.3% were female (Supplementary Table S2). CKD was present in 42 patients (12.0%). Patients with CKD showed higher prevalence of hypertension and diabetes mellitus (Table 1). There were significant differences in echocardiographic findings related to diastolic dysfunction, including left atrial volume index, E/e’, peak tricuspid regurgitation velocity, and right ventricular systolic pressure. The incidence of left atrial enlargement and left ventricular hypertrophy were significantly higher in the patients with CKD compared with those without CKD. Despite differences in angiographic disease severity, FFR was not different between the 2 groups. Patients with CKD had significantly lower CFR values (3.28 ± 1.77 vs. 2.60 ± 1.09, P < 0.001) originating from significantly higher resting coronary flow velocity, represented by lower resting Tmn (Figure 1). However, there was no significant difference in IMR according to the presence of CKD.
Table 1.
Baseline characteristics according to the presence of CKD
| Variables | No CKD (n = 309) | CKD (n = 42) | Standardized mean difference | P value |
|---|---|---|---|---|
| Age, yr | 59.7 ± 14.0 | 60.5 ± 11.1 | 0.064 | 0.671 |
| Female | 96 (31.1%) | 7 (16.7%) | 0.343 | 0.054 |
| Body mass index, kg/m2 | 23.6 ± 3.7 | 22.5 ± 3.6 | 0.294 | 0.076 |
| Hypertension | 176 (57.0%) | 33 (78.6%) | 0.475 | 0.007 |
| Diabetes mellitus | 136 (44.0%) | 27 (64.3%) | 0.416 | 0.013 |
| Chronic kidney disease | 0 (0%) | 42 (100%) | NA | <0.001 |
| Stage 1 | 0 (0%) | 7 (16.7%) | ||
| Stage 2 | 0 (0%) | 15 (35.7%) | ||
| Stage 3 | 0 (0%) | 16 (38.1%) | ||
| Stage 4–5 | 0 (0%) | 4 (9.5%) | ||
| Hyperlipidemia | 169 (54.7%) | 28 (66.7%) | 0.247 | 0.142 |
| Current smoking | 141 (45.6%) | 24 (57.1%) | 0.232 | 0.161 |
| Family history of cardiovascular disease | 76 (24.6%) | 13 (31.0%) | 0.142 | 0.374 |
| Laboratory findings | ||||
| High sensitivity CRP, mg/dl | 0.5 ± 1.6 | 0.5 ± 0.9 | 0.021 | 0.876 |
| Serum creatinine, mg/dl | 0.9 ± 0.3 | 1.4 ± 0.9 | 0.860 | <0.001 |
| Estimated GFR, ml/min per 1.73 m2 | 87.6 ± 24.5 | 62.2 ± 25.4 | 1.018 | <0.001 |
| NT-proBNP, pg/ml | 428.5 (94.0, 2,511.0) | 2,954.0 (1,942.0, 6,590.0) | 0.646 | <0.001 |
| Echocardiographic findings | ||||
| Ejection fraction, % | 62.1 ± 8.0 | 60.4 ± 7.6 | 0.231 | 0.158 |
| LVEDD, mm | 47.8 ± 5.3 | 47.0 ± 4.3 | 0.152 | 0.318 |
| LVESD, mm | 29.3 ± 4.9 | 29.2 ± 3.2 | 0.022 | 0.874 |
| Septal wall thickness, mm | 9.6 (8.5, 10.8) | 10.4 (9.2, 11.6) | 0.297 | 0.017 |
| Posterior wall thickness, mm | 9.2 (8.1, 10.2) | 10.1 (9.6, 11.0) | 0.540 | <0.001 |
| LA volume index, ml/m2 | 39.1 (30.1, 50.6) | 56.0 (43.5, 66.1) | 0.683 | <0.001 |
| Left atrial enlargementa | 199 (67.0%) | 39 (95.1%) | 0.769 | <0.001 |
| LVMI, g/m2 | 110.2 ± 35.4 | 114.0 ± 36.2 | 0.105 | 0.531 |
| Relative wall thickness | 0.40 ± 0.10 | 0.45 ± 0.10 | 0.488 | 0.005 |
| Left ventricular hypertrophyb | 55 (17.9%) | 14 (33.3%) | 0.359 | 0.032 |
| E velocity, cm/s | 71.2 ± 21.9 | 81.8 ± 24.7 | 0.457 | 0.023 |
| A velocity, cm/s | 62.8 ± 27.3 | 47.0 ± 30.1 | 0.550 | 0.012 |
| e’ velocity, cm/s | 6.4 ± 2.4 | 6.4 ± 2.5 | 0.003 | 0.985 |
| E/e’ | 10.9 (8.7, 13.9) | 13.3 (9.8, 16.1) | 0.288 | 0.018 |
| Peak TR velocity, m/s | 2.4 ± 0.4 | 2.5 ± 0.4 | 0.407 | 0.025 |
| RV systolic pressure, mm Hg | 28.6 ± 8.2 | 33.3 ± 9.4 | 0.528 | 0.007 |
| Coronary angiographic parameters | ||||
| Angiographic disease extent | 0.756 | 0.002 | ||
| Insignificant stenosis | 140 (45.3%) | 33 (78.6%) | ||
| 1-vessel disease | 62 (20.1%) | 2 (4.8%) | ||
| 2-vessel disease | 65 (21.0%) | 4 (9.5%) | ||
| 3-vessel disease | 40 (12.9%) | 3 (7.1%) | ||
| Reference vessel diameter, mm | 3.0 ± 0.6 | 2.9 ± 0.5 | 0.135 | 0.462 |
| Diameter stenosis, % | 38.9 ± 21.8 | 23.9 ± 20.1 | 0.713 | <0.001 |
| Lesion length, mm | 13.7 ± 10.1 | 10.5 ± 7.3 | 0.369 | 0.060 |
| SYNTAX score | 5.9 ± 7.2 | 2.4 ± 5.1 | 0.559 | <0.001 |
| Coronary physiologic parameters | ||||
| Interrogated vessels | 0.451 | 0.073 | ||
| Left anterior descending artery | 226 (73.1%) | 37 (88.1%) | ||
| Left circumflex artery | 41 (13.3%) | 1 (2.4%) | ||
| Right coronary artery | 42 (13.6%) | 4 (9.5%) | ||
| Resting Pd/Pa | 0.92 ± 0.50 | 0.95 ± 0.03 | 0.116 | 0.440 |
| FFR | 0.89 ± 0.05 | 0.90 ± 0.05 | 0.250 | 0.119 |
| Resting mean transit time, s | 0.92 ± 0.50 | 0.69 ± 0.33 | 0.556 | <0.001 |
| Hyperemic mean transit time, s | 0.33 ± 0.24 | 0.30 ± 0.21 | 0.121 | 0.443 |
| CFR | 3.28 ± 1.77 | 2.60 ± 1.09 | 0.465 | <0.001 |
| IMR, unit | 23.52 ± 15.54 | 22.22 ± 13.37 | 0.090 | 0.564 |
CFR, coronary flow reserve; CKD, chronic kidney disease; CRP, C-reactive protein; FFR, fractional flow reserve; GFR, glomerular filtration rate; IMR, index of microcirculatory resistance; LA, left atrium; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVMI, left ventricular mass index; NA, not applicable; NT-proBNP, N-terminal pro-B-type natriuretic peptide; Pa, aortic pressure; Pd, distal pressure; RV, right ventricle; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; TR, tricuspid regurgitation.
Data are presented as mean ± standard deviation and median with interquartile range or n (%).
Defined as LA volume index of >34 ml/m2.
Defined as LVMI ≥ 115/95 g/m2 (male/female) and relative wall thickness of >0.42.
Figure 1.
Comparison of physiologic indexes according to the presence of CKD. Box and whiskers plots representing physiologic indexes were compared according to the presence of CKD. Bold horizontal lines indicate the mean values, boxes extend from the 25th to 75th percentiles; and whiskers present standard deviations. CFR, coronary flow reserve; CKD, chronic kidney disease; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; Tmn, mean transit time.
The baseline patient characteristics according to CFR using a cutoff value of 2.0 is shown in Table 2. In the study population, 98 patients (27.9%) showed depressed CFR. Similar findings of echocardiographic parameters for diastolic dysfunction were observed in patients with depressed CFR. Angiographic disease severity was not different between the 2 groups; however, the depressed CFR group had lower resting Pd/Pa and higher value of IMR than the preserved CFR group.
Table 2.
Baseline characteristics according to CFR
| Variables | CFR > 2.0 (n = 253) | CFR ≤ 2.0 (n = 98) | Standardized mean difference | P value |
|---|---|---|---|---|
| Age, yr | 59.1 ± 13.1 | 61.7 ± 15.1 | 0.189 | 0.126 |
| Female | 67 (26.5%) | 36 (36.7%) | 0.222 | 0.058 |
| Body mass index, kg/m2 | 23.6 ± 3.7 | 23.0 ± 3.7 | 0.162 | 0.175 |
| Hypertension | 149 (58.9%) | 60 (61.2%) | 0.048 | 0.690 |
| Diabetes mellitus | 123 (48.6%) | 40 (40.8%) | 0.157 | 0.189 |
| Chronic kidney disease | 27 (10.7%) | 15 (15.3%) | 0.138 | 0.230 |
| Stage 1 | 6 (2.4%) | 1 (1.0%) | ||
| Stage 2 | 12 (4.7%) | 3 (3.1%) | ||
| Stage 3 | 8 (3.2%) | 8 (8.2%) | ||
| Stage 4–5 | 1 (0.4%) | 3 (3.1%) | ||
| Hyperlipidemia | 152 (60.1%) | 45 (45.9%) | 0.287 | 0.016 |
| Current smoking | 127 (50.2%) | 38 (38.8%) | 0.231 | 0.054 |
| Family history of cardiovascular disease | 72 (28.5%) | 17 (17.3%) | 0.267 | 0.032 |
| Laboratory findings | ||||
| High sensitivity CRP, mg/dl | 0.9 ± 7.1 | 0.5 ± 1.1 | 0.011 | 0.330 |
| Serum creatinine, mg/dl | 0.9 ± 0.4 | 1.0 ± 0.6 | 0.253 | 0.054 |
| Estimated GFR, ml/min per 1.73 m2 | 86.9 ± 24.4 | 78.7 ± 28.8 | 0.307 | 0.014 |
| NT-proBNP, pg/ml | 335.0 (85.8, 2578.0) | 2,119.0 (411.2, 4699.0) | 0.254 | <0.001 |
| Echocardiographic findings | ||||
| Ejection fraction, % | 62.1 ± 7.5 | 61.4 ± 9.0 | 0.084 | 0.499 |
| LVEDD, mm | 48.0 ± 5.0 | 46.8 ± 5.6 | 0.222 | 0.071 |
| LVESD, mm | 29.4 ± 4.6 | 29.0 ± 5.0 | 0.064 | 0.597 |
| Septal wall thickness, mm | 9.5 (8.5, 10.4) | 10.1 (8.8, 12.7) | 0.514 | <0.001 |
| Posterior wall thickness, mm | 9.1 (8.1, 10.1) | 10.1 (8.5, 11.7) | 0.517 | <0.001 |
| LA volume index, ml/m2 | 39.2 (30.1, 52.9) | 43.5 (35.2, 56.6) | 0.242 | 0.025 |
| Left atrial enlargementa | 164 (66.9%) | 74 (79.6%) | 0.288 | 0.032 |
| LVMI, g/m2 | 105.9 ± 30.7 | 123.1 ± 43.3 | 0.458 | <0.001 |
| Relative wall thickness | 0.39 ± 0.08 | 0.46 ± 0.13 | 0.552 | <0.001 |
| Left ventricular hypertrophyb | 28 (11.1%) | 41 (42.3%) | 0.753 | 0.032 |
| E velocity, cm/s | 70.1 ± 19.9 | 78.4 ± 27.5 | 0.347 | 0.014 |
| A velocity, cm/s | 60.3 ± 25.8 | 64.2 ± 33.7 | 0.130 | 0.367 |
| E’ velocity, cm/s | 6.8 ± 2.3 | 5.5 ± 2.3 | 0.575 | <0.001 |
| E/e’ | 10.4 (8.4, 13.0) | 14.2 (9.9, 20.0) | 0.736 | <0.001 |
| Peak TR velocity, m/s | 2.3 ± 0.3 | 2.5 ± 0.5 | 0.497 | <0.001 |
| RV systolic pressure, mm Hg | 27.5 ± 6.1 | 33.6 ± 11.6 | 0.650 | <0.001 |
| Coronary angiographic parameters | ||||
| Angiographic disease extent | 0.240 | 0.250 | ||
| Insignificant stenosis | 122 (48.2%) | 51 (52.0%) | ||
| 1-vessel disease | 46 (18.2%) | 18 (18.4%) | ||
| 2-vessel disease | 53 (20.9%) | 16 (16.3%) | ||
| 3-vessel disease | 32 (12.6%) | 11 (11.2%) | ||
| Reference vessel diameter, mm | 3.0 ± 0.6 | 2.9 ± 0.5 | 0.286 | 0.033 |
| Diameter stenosis, % | 36.6 ± 22.0 | 39.0 ± 22.6 | 0.107 | 0.473 |
| Lesion length, mm | 12.9 ± 9.8 | 14.4 ± 9.6 | 0.158 | 0.377 |
| SYNTAX score | 5.6 ± 7.2 | 5.2 ± 6.8 | 0.052 | 0.658 |
| Coronary physiologic parameters | ||||
| Interrogated vessels | 0.287 | 0.077 | ||
| Left anterior descending artery | 182 (71.9%) | 81 (82.7%) | ||
| Left circumflex artery | 32 (12.6%) | 10 (10.2%) | ||
| Right coronary artery | 39 (15.4%) | 7 (7.1%) | ||
| Resting Pd/Pa | 0.96 ± 0.04 | 0.94 ± 0.04 | 0.594 | <0.001 |
| FFR | 0.90 ± 0.05 | 0.89 ± 0.05 | 0.078 | 0.512 |
| Resting mean transit time, s | 0.99 ± 0.49 | 0.65 ± 0.39 | 0.749 | <0.001 |
| Hyperemic mean transit time, s | 0.28 ± 0.17 | 0.47 ± 0.31 | 0.762 | <0.001 |
| CFR | 3.89 ± 1.53 | 1.43 ± 0.40 | 2.200 | <0.001 |
| IMR, unit | 20.32 ± 12.04 | 31.22 ± 19.48 | 0.673 | <0.001 |
CFR, coronary flow reserve; CKD, chronic kidney disease; CRP, C-reactive protein; FFR, fractional flow reserve; GFR, glomerular filtration rate; IMR, index of microcirculatory resistance; LA, left atrium; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVMI, left ventricular mass index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; Pa, aortic pressure; Pd, distal pressure; RV, right ventricle; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; TR, tricuspid regurgitation.
Data are presented as mean ± SD and median with interquartile range or n (%).
Defined as LA volume index of >34 ml/m2.
Defined as LVMI ≥ 115/95 g/m2 (male/female) and relative wall thickness of >0.42.
Association between Severity of CKD and Intracoronary Physiologic Indexes
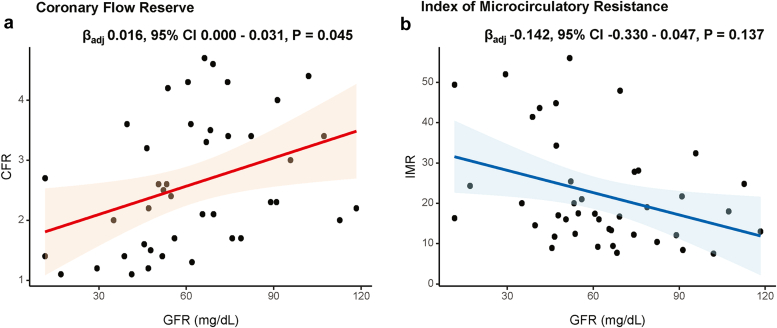
CFR showed significant association with eGFR in multivariable linear regression model (adjusted β = 0.016, P = 0.045). Conversely, IMR showed no significant association with eGFR (adjusted β = −0.142, P = 0.137) (Figure 2, Supplementary Table S3).
Figure 2.
Relationship between estimated GFR and physiologic indexes in patients with CKD. Linear regression analyses between the GFR and (a) CFR or (b) IMR are shown. CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; GFR, glomerular filtration rate; IMR, index of microcirculatory resistance.
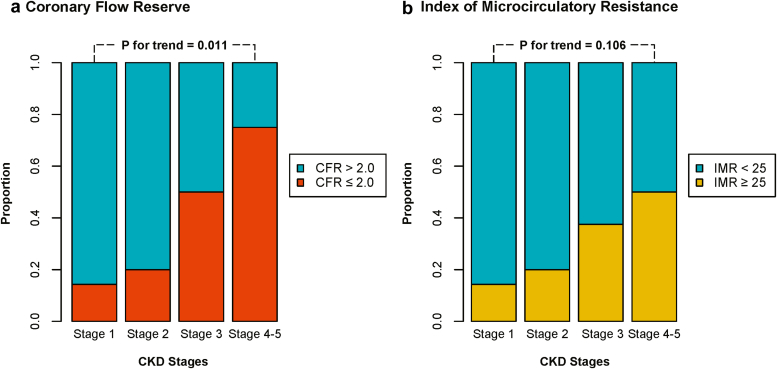
The proportion of patients with depressed CFR or elevated IMR according to CKD stages is shown in Figure 3. Patients with higher stages of CKD showed a higher proportion of depressed CFR (P for trend = 0.011). Conversely, proportion of patients with elevated IMR was not significantly increased with higher CKD stages (P for trend = 0.106).
Figure 3.
Proportion of abnormal microcirculatory function according to CKD stages. Proportions of (a) depressed CFR (≤2.0) and (b) increased IMR (≥25 unit) are shown according to the CKD stage. CFR, coronary flow reserve; CKD, chronic kidney disease; IMR, index of microcirculatory resistance.
Clinical Outcomes and Prognostic Implications According to CKD and CFR
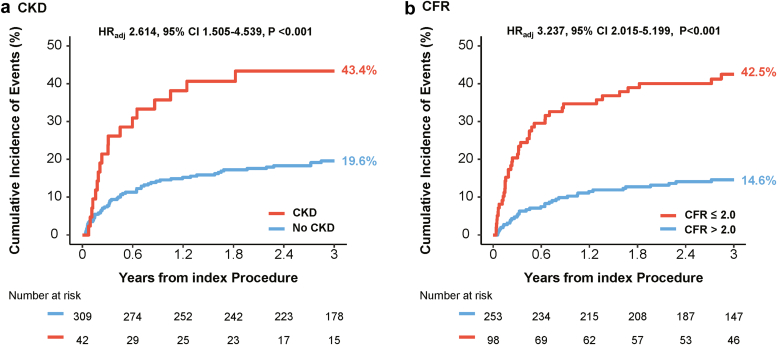
During median follow-up of 3.6 years (Q1–Q3: 2.5–4.6 years), patients with CKD showed higher risk of cardiac death or hospitalization for heart failure than those without CKD (43.4% vs. 19.6%, HRadj 2.614, 95% CI 1.505–4.539, P < 0.001). Patients with depressed CFR also had a significantly higher risk of cardiac death or hospitalization for heart failure than those with preserved CFR (42.5% vs. 14.6%, HRadj 3.237, 95% CI 2.015–5.199, P < 0.001) (Figure 4).
Figure 4.
Comparison of cardiac death or hospitalization for heart failure according to the presence of CKD and CFR. Comparison of cumulative incidence and Kaplan-Meier curves of primary outcome, a composite of cardiac death or hospitalization for heart failure, are presented according to the (a) presence of CKD and (b) depressed CFR (≤2.0). CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
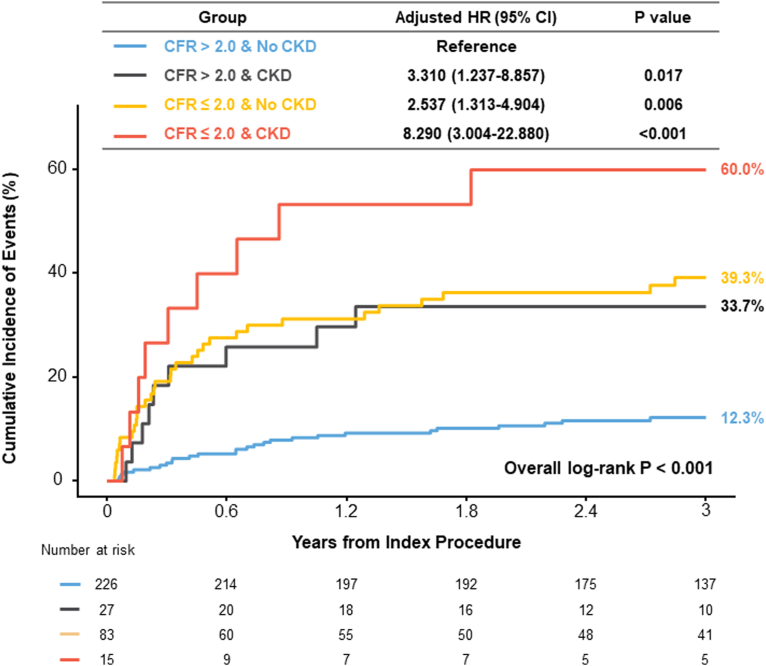
When patients were stratified by the presence of CKD and depressed CFR, patients without CKD and preserved CFR showed the lowest risk of cardiac death or hospitalization for heart failure. Conversely, patients with both CKD and depressed CFR showed the highest risk of cardiac death or hospitalization for heart failure than the reference group (HRadj 8.290, 95% CI 3.004–22.880, P < 0.001) (Table 3 and Figure 5). Similarly, patients with either CKD or depressed CFR showed significantly higher risk of cardiac death or hospitalization for heart failure than the reference group. In a multivariable model, both CKD (HRadj 2.614, 95% CI 1.505–4.539, P < 0.001) and depressed CFR (HRadj 3.237, 95% CI 2.015–5.199, P < 0.001) showed independent prognostic impact for the risk of cardiac death or hospitalization for heart failure (Table 4).
Table 3.
Comparison of clinical outcomes among 4 groups classified by CKD and CFR
| Clinical outcomes | CFR > 2.0 |
CFR ≤ 2.0 |
Overall P value | ||
|---|---|---|---|---|---|
| No CKD (n = 226) | CKD (n = 27) | No CKD (n = 83) | CKD (n = 15) | ||
| Cardiac death or hospitalization of heart failure | 12.3% (27) | 33.7% (9) | 39.3% (32) | 60.0% (9) | <0.001 |
| Cardiac death | 2.7% (6) | 8.1% (2) | 17.4% (13) | 22.0% (3) | <0.001 |
| Hospitalization of heart failure | 10.1% (22) | 33.7% (9) | 34.0% (27) | 41.3% (6) | <0.001 |
CFR, coronary flow reserve; CKD, chronic kidney disease.
Data are expressed as the cumulative incidence of clinical outcomes and the number of events. Cumulative incidence of clinical outcomes represents Kaplan-Meier estimates during 3 years. Overall P values were used for log-rank test in the survival analysis, comparing the 4 groups classified according to CFR ≤ 2.0 and the presence of CKD.
Figure 5.
Comparison of cardiac death or hospitalization for heart failure among 4 groups classified by CKD and CFR. Comparison of cumulative incidence and Kaplan-Meier curves of primary outcome, a composite of cardiac death or hospitalization for heart failure, are presented among the 4 groups classified by the presence of CKD and depressed CFR (≤2.0). Multivariable model included the variables of age, sex, hypertension, diabetes, dyslipidemia, smoking status, family history, ejection fraction, LA volume index, left ventricular mass index, and E/e’. CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
Table 4.
Independent predictors for cardiac death or hospitalization for heart failure
| Variable | Multivariable analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
| CFR ≥ 2.0 | 3.237 (2.015–5.199) | <0.001 |
| Chronic kidney disease | 2.614 (1.505–4.539) | <0.001 |
| Age | 0.980 (0.960–0.990) | 0.005 |
| Female | 1.400 (0.855–2.293) | 0.181 |
| Diabetes mellitus | 1.294 (0.808–2.071) | 0.283 |
| IMR ≤ 25 | 1.511 (0.933–2.446) | 0.093 |
CFR, coronary flow reserve; CI, confidence interval; HR, hazard ratio; IMR, index of microcirculatory resistance.
Discussion
In this study, we evaluated the association between CKD and CFR, and their prognostic implications in patients without significant LV systolic dysfunction and epicardial coronary stenosis. The main findings were as follows. First, patients with CKD had lower CFR than those without CKD, mainly due to increased resting flow, and not due to increased microcirculatory resistance. Second, severity of CKD was significantly correlated with CFR but not with IMR. Third, the presence of CKD or depressed CFR were each associated with the increased risk of cardiac death or hospitalization for heart failure. Fourth, both CKD and depressed CFR showed independent prognostic impact on the risk of cardiac death or hospitalization for heart failure.
Physiologic Mechanism of Depressed CFR in Patients with CKD
Limited studies have investigated the association between CFR and CKD using noninvasive imaging tests.6,10, 11, 12 In these studies, patients with CKD showed consistently lower CFR than patients with preserved renal function,6,10, 11, 12 and depressed CFR was significantly associated with the increased risk of mortality, even in early stages of CKD.6,10 Nevertheless, it should be noted that those results were derived from noninvasive imaging studies, which could not fully differentiate whether the cause of depressed CFR was flow-limiting epicardial stenosis or microcirculatory dysfunction. In this study, we evaluated the association of CKD with invasive physiologic indexes. Unlike the aforementioned studies, patients with functionally significant epicardial coronary stenosis (FFR ≤ 0.80), which is one of the major causes of depressed CFR, could be excluded. There was significant association between CFR and eGFR, and patients with higher severity of CKD showed a higher proportion of depressed CFR. These results support the view that microcirculatory dysfunction was a major cause of depressed CFR in patients with CKD and that microcirculatory dysfunction deteriorated according to the severity of CKD.
Moreover, because CFR can be affected by either increased resting coronary flow or reduced hyperemic coronary flow,18 the underlying mechanism of depressed CFR in patients with CKD could be different. Previous studies have presented heterogeneous results regarding the pattern of resting and hyperemic coronary flow as an underlying mechanism of depressed CFR.11, 12, 13 Bajaj et al.13 demonstrated that patients with CKD stage 3 or higher had significantly lower hyperemic myocardial blood flow (MBF) than patients with preserved eGFR (≥60 ml/min per 1.73 m2), which resulted in lower CFR values. Conversely, Koivuviita et al.11 reported that 31 individuals without cardiovascular disease who underwent [15O]H2O positron emission tomography imaging showed a trend of decreased CFR according to the stage of CKD, mainly driven by significantly increased resting MBF, and not by decrease of hyperemic MBF. Similarly, Charytan et al.12 analyzed 435 patients with CKD who underwent 13N-ammonia positron emission tomography and presented that those patients showed depressed CFR even in early stages of CKD, mainly driven by significantly higher resting MBF without difference in hyperemic MBF, compared with the preserved renal function group.
Clinical Relevance of CFR and CKD in Patients without Significant LV Systolic Dysfunction or Coronary Artery Disease
Although this study evaluated all stages of CKD, most patients with CKD had lower CKD severity based on mean eGFR of 62.2 ± 25.4 ml/min per 1.73 m2. Depressed CFR was mainly caused by increased resting coronary flow, and there were no significant trends of reduced hyperemic coronary flow and increased microcirculatory resistance, represented by IMR. Considering that the IMR value can be significantly increased when structural deformation has occurred in the microvasculature such as extensive myocardial infarction, infiltrative heart disease, or acute cellular rejection after heart transplantation,19, 20, 21 exclusion of end-stage renal disease requiring dialysis in this study might explain the preserved hyperemic coronary flow and IMR values in CKD population.
Microcirculatory dysfunction in patients with CKD even before significant structural deformation in microvasculature and subsequent LV systolic dysfunction might be explained by the pathophysiological background of CKD. Previous studies reported the presence of microvascular rarefaction distal to the level of smaller arterioles in patients with CKD and development of endothelial dysfunction with diminished myocardial perfusion.22,23 Experimental studies consistently demonstrated that myocardial capillary supply is reduced in uremic animal models.7,24 Neither treatment with erythropoietin nor in combination with antihypertensive therapy could ameliorate microvascular rarefaction in the uremic animal model, and these were indirectly indicating that the development of microcirculatory dysfunction among patients with CKD is an independent phenomenon, regardless of anemia and hypertension.25 Charytan et al.26 also showed similar results in an autopsy study, indicating that microvascular rarefaction and myocardial fibrosis were demonstrated in patients with CKD. Furthermore, higher incidence of LV hypertrophy and LV diastolic dysfunction in the current CKD population should be considered as another mechanism of microcirculatory dysfunction. These underlying pathophysiologic mechanisms are known to induce compensatory vasodilation of arterioles, increased resting coronary flow, and depressed coronary circulatory reserve, indicating disturbed autoregulatory mechanism in patients with CKD.23
Clinical Implications
It has been well known that cardiovascular disease, including ischemic heart disease and heart failure, is the major cause of death among patients with CKD.4 When we assessed 3-year rate of cardiovascular events, the patients with CKD showed significantly increased risk of cardiac death or hospitalization than those without CKD. Similarly, depressed CFR was also significantly associated with higher risk of cardiac death or hospitalization for heart failure. Notably, both CKD and depressed CFR were revealed to be independent predictors of the risk of cardiac death or hospitalization for heart failure, even after adjustment of variables of age, sex, diabetes mellitus and IMR.
These findings suggest that patients with CKD without LV dysfunction and epicardial coronary stenosis are at higher risk of future cardiovascular events and that the assessment of microvascular dysfunction can provide additive information on the prognostication of patients with CKD. Nevertheless, clinicians have focused on how to manage the traditional risk factors such as hypertension, diabetes, or dyslipidemia for patients with CKD to reduce the progression of cardiovascular disease.4 Our data suggest that microcirculatory dysfunction in patients with CKD can be an additional target for improving survival. Although the management of microcirculatory dysfunction has been an unmet need in contemporary practice, 2 ongoing trials, Women’s IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR: NCT 03417388) and International Coronary Microvascular Angina Trial (iCorMicA: NCT04674449), will evaluate the effective pharmacotherapy for microcirculatory dysfunction.
Study Limitations
There are several limitations in this study. First, the observational nature of study design has inherent limitations of residual confounding. Nevertheless, our results were derived from unrestricted prospective registry, which might increase the generalizability. Second, the data showing relationship between intracoronary physiologic indexes and CKD were cross-sectional; therefore, we could not assess the temporal changes in microcirculatory dysfunction in patients with CKD. Third, the exact etiology of CKD was not evaluated in the current registry, which could affect the clinical outcomes. However, we conducted rigorous adjustment for clinically relevant confounders, which related to the patients’ prognosis. Fourth, patients requiring dialysis were excluded from this study, which might have mitigated the association between advanced CKD and microcirculatory dysfunction. In addition, the relatively small sample size of advanced CKD in this study might mitigate the association between microcirculatory dysfunction and advanced CKD. Further study is needed to confirm the current results.
Conclusions
CFR was lower in patients with CKD due to increased resting coronary flow. Severity of CKD was inversely associated with CFR but not associated with IMR. Both CKD and depressed CFR showed independent association with higher risk of cardiac death or hospitalization for heart failure. Evaluation of microcirculatory dysfunction by CFR may improve the risk stratification in patients with CKD without LV systolic dysfunction and epicardial coronary stenosis. Further study is warranted to clarify the current results, given the relatively small sample size of advanced CKD.
Disclosure
SHL received a research grant from Abbott Vascular. JML received a research grant from Abbott Vascular, Boston Scientific, and Philips Volcano. J-YH received a research grant from Abbott Vascular, Boston Scientific, Biotronik, and Philips Volcano. All the other authors declared no competing interests.
Footnotes
Figure S1. Study flow.
Tables S1. Candidate variables for multivariable analyses.
Table S2. Baseline characteristics according to the presence of chronic kidney disease and CFR.
Table S3. Multivariable linear regression model of predictors of CFR or IMR.
STROBE Statement.
Supplementary Material
Figure S1. Study flow
Tables S1. Candidate variables for multivariable analyses
Table S2. Baseline characteristics according to the presence of chronic kidney disease and CFR
Table S3. Multivariable linear regression model of predictors of CFR or IMR
STROBE Statement.
References
- 1.Cai Q., Mukku V.K., Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331–339. doi: 10.2174/1573403x10666140214122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J.M., Kang J., Lee E., et al. Chronic kidney disease in the second-generation drug-eluting stent era: pooled analysis of the Korean multicenter drug-eluting stent registry. JACC Cardiovasc Interv. 2016;9:2097–2109. doi: 10.1016/j.jcin.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 3.Kim J., Lee J.M., Choi K.H., et al. Differential clinical outcomes between angiographic complete versus incomplete coronary revascularization, according to the presence of chronic kidney disease in the drug-eluting stent era. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jankowski J., Floege J., Fliser D., et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiuchi M.G., Mion D., Jr. Chronic kidney disease and risk factors responsible for sudden cardiac death: a whiff of hope? Kidney Res Clin Pract. 2016;35:3–9. doi: 10.1016/j.krcp.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charytan D.M., Skali H., Shah N.R., et al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 2018;93:501–509. doi: 10.1016/j.kint.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann K., Wiest G., Zimmer G., et al. Reduced capillary density in the myocardium of uremic rats--a stereological study. Kidney Int. 1992;42:1079–1085. doi: 10.1038/ki.1992.390. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi J., Porst M., Cordasic N., et al. Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int. 2006;69:2013–2021. doi: 10.1038/sj.ki.5000448. [DOI] [PubMed] [Google Scholar]
- 9.Amann K., Breitbach M., Ritz E., Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–1022. doi: 10.1681/ASN.V961018. [DOI] [PubMed] [Google Scholar]
- 10.Murthy V.L., Naya M., Foster C.R., et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5:1025–1034. doi: 10.1016/j.jcmg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koivuviita N., Tertti R., Jarvisalo M., et al. Increased basal myocardial perfusion in patients with chronic kidney disease without symptomatic coronary artery disease. Nephrol Dial Transplant. 2009;24:2773–2779. doi: 10.1093/ndt/gfp175. [DOI] [PubMed] [Google Scholar]
- 12.Charytan D.M., Shelbert H.R., Di Carli M.F. Coronary microvascular function in early chronic kidney disease. Circ Cardiovasc Imaging. 2010;3:663–671. doi: 10.1161/CIRCIMAGING.110.957761. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj N.S., Singh A., Zhou W., et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation. 2020;141:21–33. doi: 10.1161/CIRCULATIONAHA.119.043916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI Expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on coronary pathophysiology & microcirculation endorsed by Coronary Vasomotor Disorders International Study group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 16.Stevens P.E., Levin A., Kidney Disease Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.K., Lim H.S., Fearon W.F., et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Hoef T.P., Siebes M., Spaan J.A., Piek J.J. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36:3312–3319a. doi: 10.1093/eurheartj/ehv235. [DOI] [PubMed] [Google Scholar]
- 19.Fearon W.F., Low A.F., Yong A.S., et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.M., Choi K.H., Choi J.O., et al. Coronary microcirculatory dysfunction and acute cellular rejection after heart transplantation. Circulation. 2021;144:1459–1472. doi: 10.1161/CIRCULATIONAHA.121.056158. [DOI] [PubMed] [Google Scholar]
- 21.Choi K.H., Lee J.M., Kim S.R., et al. Prognostic value of the index of microcirculatory resistance over serum biomarkers in cardiac amyloidosis. J Am Coll Cardiol. 2020;75:560–561. doi: 10.1016/j.jacc.2019.11.045. [DOI] [PubMed] [Google Scholar]
- 22.Querfeld U., Mak R.H., Pries A.R. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond) 2020;134:1333–1356. doi: 10.1042/CS20200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul S., Jayaweera A.R. Myocardial capillaries and coronary flow reserve. J Am Coll Cardiol. 2008;52:1399–1401. doi: 10.1016/j.jacc.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Tornig J., Amann K., Ritz E., et al. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: the effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol. 1996;7:667–675. doi: 10.1681/ASN.V75667. [DOI] [PubMed] [Google Scholar]
- 25.Amann K., Odoni G., Benz K., et al. Sympathetic blockade prevents the decrease in cardiac VEGF expression and capillary supply in experimental renal failure. Am J Physiol Ren Physiol. 2011;300:F105–F112. doi: 10.1152/ajprenal.00363.2010. [DOI] [PubMed] [Google Scholar]
- 26.Charytan D.M., Padera R., Helfand A.M., et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol. 2014;176:99–109. doi: 10.1016/j.ijcard.2014.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.