Abstract
Objective
To test the hypothesis that the Monoclonal Antibody Screening Score performs consistently better in identifying the need for monoclonal antibody infusion throughout each “wave” of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant predominance during the coronavirus disease 2019 (COVID-19) pandemic and that the infusion of contemporary monoclonal antibody treatments is associated with a lower risk of hospitalization.
Patients and Methods
In this retrospective cohort study, we evaluated the efficacy of monoclonal antibody treatment compared with that of no monoclonal antibody treatment in symptomatic adults who tested positive for SARS-CoV-2 regardless of their risk factors for disease progression or vaccination status during different periods of SARS-CoV-2 variant predominance. The primary outcome was hospitalization within 28 days after COVID-19 diagnosis. The study was conducted on patients with a diagnosis of COVID-19 from November 19, 2020, through May 12, 2022.
Results
Of the included 118,936 eligible patients, hospitalization within 28 days of COVID-19 diagnosis occurred in 2.52% (456/18,090) of patients who received monoclonal antibody treatment and 6.98% (7,037/100,846) of patients who did not. Treatment with monoclonal antibody therapies was associated with a lower risk of hospitalization when using stratified data analytics, propensity scoring, and regression and machine learning models with and without adjustments for putative confounding variables, such as advanced age and coexisting medical conditions (eg, relative risk, 0.15; 95% CI, 0.14-0.17).
Conclusion
Among patients with mild to moderate COVID-19, including those who have been vaccinated, monoclonal antibody treatment was associated with a lower risk of hospital admission during each wave of the COVID-19 pandemic.
Abbreviations and Acronyms: CMH, Cochran-Mantel-Haenszel; COVID-19, coronavirus disease 2019; EHR, electronic health record; GBM, gradient boosting machine; MASS, Monoclonal Antibody Screening Score; RR, relative risk; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Most therapies for coronavirus disease 2019 (COVID-19) have targeted disease progression or death in hospitalized patients. However, the US Food and Drug Administration issued emergency use authorization for several monoclonal antibody treatments for outpatient use after data reported decreases in incidences of disease progression and hospitalization associated with neutralizing antispike monoclonal antibody treatment.1, 2, 3, 4, 5, 6, 7 Monoclonal antibody treatments have evolved throughout the COVID-19 pandemic because of concerns related to evolutions of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, including monoclonal antibody–resistant SARS-CoV-2 variants,8,9 and greater virulence and transmissibility in emerging SARS-CoV-2 variants.10, 11, 12
Initially, the COVID-19 pandemic was associated with a heterogeneous array of SARS-CoV-2 wild-type genotypes, which were supplanted by the Alpha (B.1.1.7) and Beta (B.1.351) variants in early 2021, the Delta (B.1.617.2) variant in the middle months of 2021, and, subsequently, the Omicron variant and subvariants (BA.1, BA.2, BA.2.12.1, BA.4, BA.5), which became the predominant SARS-CoV-2 lineage in late 2021.13,14 There were demographic and clinical differences in patients who tested positive for COVID-19 during different periods (“waves”) of variant predominance. Patients infected during the Delta variant wave were more likely to be younger and have fewer comorbidities; however, these patients had higher odds of both developing severe COVID-19 and mortality compared with those who were infected before the Delta variant wave.10 However, patients infected during the Omicron variant wave were younger with lower hospitalization rates, had reduced length of hospitalization, and had an increased breakthrough infection rate after COVID-19 vaccination.13 The genetic variants of the SARS-CoV-2 virus developed sequence variations in the spike protein that allowed the virus to escape neutralization by monoclonal antibody treatment. This monoclonal antibody escape led to diminished responsiveness of the viral variants to monoclonal antibody treatment and subsequent changes to indication for monoclonal antibody treatment over time, including US Food and Drug Administration authorization for some monoclonal antibody treatments to be restricted or withdrawn.15,16 The Monoclonal Antibody Screening Score (MASS) was used to identify patients deemed eligible for monoclonal antibody treatment.7,17, 18, 19, 20 In this retrospective cohort study, we tested the hypothesis that infusion of contemporary monoclonal antibody treatments would be associated with a lower risk of hospitalization throughout each “wave” of SARS-CoV-2 variant predominance during the COVID-19 pandemic. To address this hypothesis, we evaluated the incidence of hospitalization among outpatient adults with COVID-19 who received monoclonal antibody treatment in a real-world clinical setting.
Patients and Methods
Design and Oversight
We conducted a retrospective cohort study including data from all Mayo Clinic sites in the United States, representing 4 states—Arizona, Florida, Minnesota, and Wisconsin. The Mayo Clinic Institutional Review Board determined that this study met the criteria for exemption. Informed consent was waived. Only Mayo Clinic patients with research authorization were included. The following data elements were obtained from Mayo Clinic electronic health records (EHRs): age, sex, race and ethnic groups, comorbidities, COVID-19 vaccination, anti–COVID-19 therapies, and history of hospitalization. Vaccination records are routinely updated through state and federal reporting mechanisms.
Patients
Eligible patients were those aged 18 years or older with a confirmed diagnosis of COVID-19 with a positive nasopharyngeal polymerase chain reaction or antigen test result for SARS-CoV-2 from November 19, 2020, through May 12, 2022 (Figure 1A). Patients were classified into one of 3 waves on the basis of their test date: pre-Delta predominant, Delta predominant, and Omicron predominant. Patients who tested positive for COVID-19 between these waves were classified as washout periods 1 and 2. Although Omicron subvariants persist, a cutoff date for the Omicron wave was selected to examine similar blocks of time for monoclonal antibodies to be in use. The exclusion criteria included previous COVID-19–related hospitalization, receipt of other COVID-19 outpatient therapies (including remdesivir, convalescent plasma, and oral antivirals), and receipt of monoclonal antibody treatments, such as pre-exposure prophylaxis before COVID-19 diagnosis. These patients were excluded to create a comparison between monoclonal antibody therapy and no treatment. Pregnant patients and those who had received a COVID-19 live attenuated vaccine before or during follow-up were eligible. Patients who met the inclusion criteria were categorized into 2 cohorts on the basis of monoclonal antibody treatment: infused with monoclonal antibody before hospitalization (infused cohort) and either not infused or infused with monoclonal antibody after hospital admission (not-infused cohort). This classification of cohorts was used for all analyses, including tables and figures.
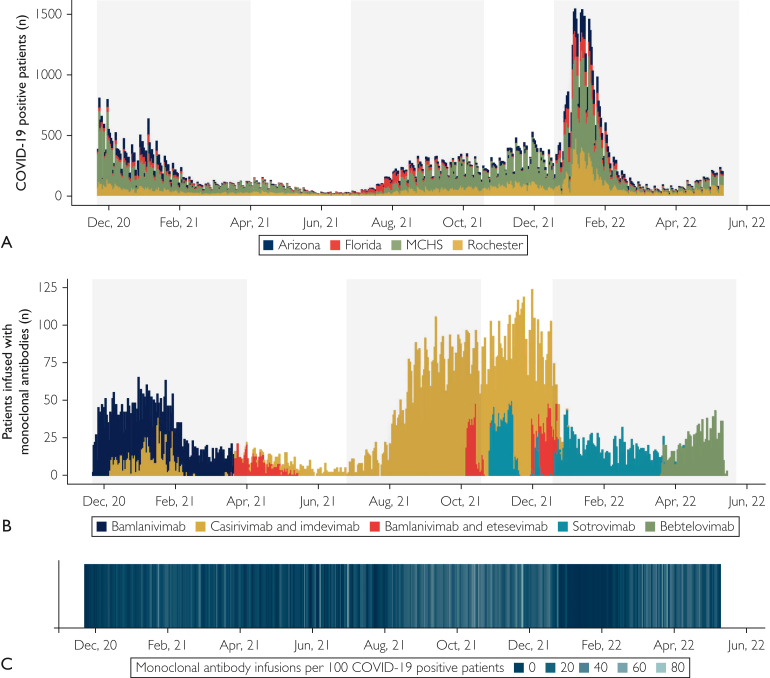
Figure 1.
Study population and temporal patterns of coronavirus disease 2019 (COVID-19) positive test results and monoclonal antibody infusions. (A) Patients testing positive for COVID-19 between November 19, 2020, and June 2, 2022 and meeting inclusion criteria across the 3 main Mayo Clinic campuses and the Mayo Clinic Health System (MCHS). The height of each bar represents the number of positive test results on each day stratified by hospital: Arizona (blue), Florida (red), MCHS (green), and Rochester (gold). (B) Patients received monoclonal antibody treatment on the basis of emergency use authorizations that were in effect at the time and available supply. Each bar represents the number of treated patients stratified by monoclonal antibody therapy type: bamlanivimab (blue), bamlanivimab and etesevimab (red), bebtelovimab (green), casirivimab and imdevimab (gold) and sotrovimab (teal). (C) The number of monoclonal antibody infusions per 100 COVID-19 positive test results shows that the rate of infusion was highest from mid-August through December 2021. Lighter shades of blue represent periods of higher rates of monoclonal antibody treatment.
Intervention
In November 2020, Mayo Clinic established a Monoclonal Antibody Treatment program to facilitate the administration of monoclonal antibody treatment and developed MASS to identify patients who were at an elevated risk of hospitalization and who would benefit the most from monoclonal antibody treatment.19 Originally MASS was intended to risk-stratify patients to guide the allocation of scarce monoclonal antibody treatments; however, because the availability of monoclonal antibody treatments improved, MASS was subsequently used as a screening tool to identify patients who were eligible for monoclonal antibody treatment.7,18 A numeric value is provided by MASS to stratify patient risk on the basis of risk factors for progressing from mild or moderate COVID-19 to severe disease; the scoring methodology is defined in Supplemental Table 1 (available online at http://www.mcpiqojournal.org).7,17, 18, 19, 20 The MASS scale ranges from 0 to 19, with higher scores indicating a greater number of risk factors for progressing to severe COVID-19 and higher prioritization for monoclonal antibody treatment.
Antispike monoclonal antibodies were distributed to infusion facilities on behalf of the US government. The specific monoclonal antibody administered to any eligible patient was based solely on the product available at the infusion facility during the date of treatment (types and dates are shown in Figure 1B, and infusions per 100 COVID-19 patients are shown in Figure 1C).
Primary Outcome
The primary outcome was hospitalization among patients with COVID-19 within 28 days after their positive test result. This was assessed as the cumulative incidence of hospital admission in the cohort infused with monoclonal antibody compared with the cohort that did not receive monoclonal antibody infusion. The decision to hospitalize patients was at the discretion of local providers.
Statistical Analyses
As patients were not randomly assigned to treatment and controls in this retrospective study, the analysis plan aimed to limit confounding effects through multiple complementary statistical approaches, including stratification analyses, regression techniques, and machine learning approaches. Although each method was distinct in approach, each attempted to account for potential confounding and risk modification with the use of different statistical techniques. Owing to the large sample size, we used absolute standardized differences to compare baseline characteristics and comorbidities in the 2 treatment cohorts, with a cutoff of 10% to indicate large effect sizes.21
The primary analysis focused on estimating the association of monoclonal antibody infusions with the risk of hospital admission. To start this analysis, crude cumulative incidence rates for hospital admission were compared across MASS values using chi-square tests for independence. To control for differing baseline characteristics in the 2 treatment cohorts, propensity scores (PS) were generated for every patient on the basis of propensity to be infused with monoclonal antibodies using a gradient-boosted logistic regression model matched on hospital admission risk factors and baseline characteristics (age, race and ethnic group, COVID-19 vaccination status, wave of SARS-CoV-2 variant predominance, hospital location, body mass index [calculated as the weight in kilograms divided by the height in meters squared] classification, and recent or coexisting medical conditions). For a stratified analysis, patient cohorts were categorized on the basis of propensity score quintile and MASS into 5 subgroups. These subgroups were then used in a Cochran-Mantel-Haenszel (CMH) analysis and an analysis using both unconditional and conditional logistic regression models on the matched data. Three additional CMH analyses were also performed grouped by MASS and key demographic characteristics, including race, age, and sex.
For a model-based analysis of the risk of hospital admission, a gradient boosting machine (GBM) was constructed to predict admission within 28 days after testing positive for COVID-19. A GBM model was constructed by performing a Cartesian grid search across a series of hyperparameters (interaction depth, number of trees, learning rate, and column and row sampling rates). Each model was assessed using 5-fold cross-validation, and the final model was chosen to optimize the area under the curve. Key covariates, identified through GBM-based variable importance, were then included in a series of relative risk (RR) regression models with varying levels of adjustments and weighting factors. Additionally, a weighted model using the propensity scores described earlier in the article was evaluated. Finally, a regression model was constructed using the propensity scores as the distance metric in a 1:1 nearest neighbor matching algorithm without replacement.
The GBM model was further accessed for interpretability by generating SHapley Additive exPlanation, partial dependence, and variable importance plots. To identify patient profiles or characteristics that would most likely benefit from monoclonal antibody treatments, the finalized GBM model was used to predict the probability of hospital admission in 2 scenarios, including both infusion with monoclonal antibodies and no infusion of monoclonal antibodies. These probabilities were then subtracted to generate a predicted change in the probability of hospital admission when infused and grouped into categories of least to most likely to benefit from monoclonal antibody treatments. Lastly, the inverse of the difference in probabilities was calculated to capture the number of patients needed to treat to prevent 1 admission.22 This metric was then modeled using a classification and regression tree to identify primary covariates and splits that contribute to the number needed to treat.
Data were used as reported in the EHR and an EHR-based registry built specifically for tracking COVID-19–related outcomes and complications. No imputation program was applied to the data. Analyses were performed using the R software (R Core Team).23 Descriptive statistics are presented as frequencies and percentages. Interpretation of findings was based on the 95% CIs for the estimated measures of association. Point estimates were calculated for the crude cumulative incidence of COVID-19 hospitalization with Wilson CIs for binomial proportions. The widths of the CIs were not adjusted for multiplicity; hence, the intervals should not be used to infer definitive treatment effects. The reported P values are 2-sided.
Results
Patients
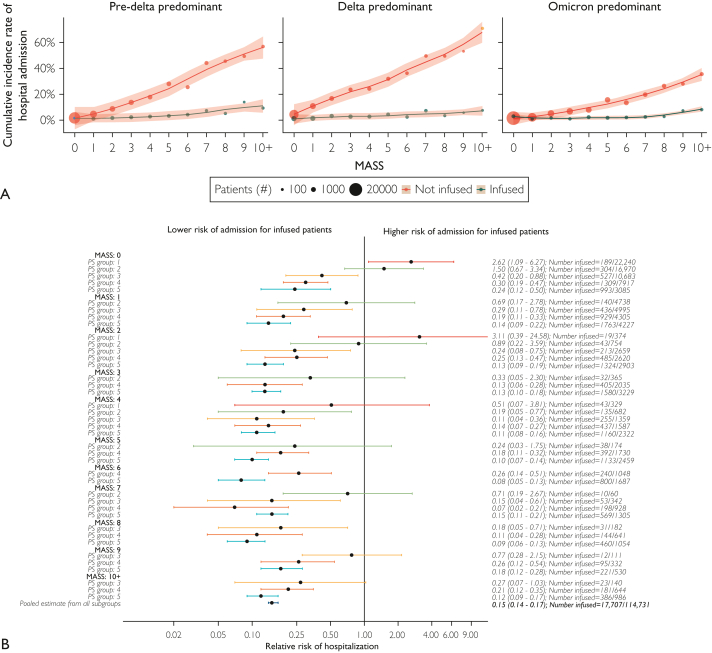
Of the 118,936 eligible patients who tested positive for COVID-19 during the study period (Figure 1A), 18,090 (15.2%) received monoclonal antibody treatments (Figure 1B). The number of monoclonal antibody infusions per 100 patients with COVID-19 is depicted in Figure 1C. Key patient characteristics stratified by monoclonal antibody treatment status are presented in Table 1. Patients who received monoclonal antibody treatment were generally older, more likely to be White and not Hispanic or Latino, had higher MASS, were less likely to have received COVID-19 vaccination, and had more coexisting medical conditions (standardized difference, >10%) compared with patients who did not receive monoclonal antibody treatment. There was a greater rate of monoclonal antibody treatments during the Delta predominant wave than during the other waves (pre-Delta predominant wave, 12.9%; Delta predominant wave, 28.4%; Omicron predominant wave, 6.3%; P<.001). Cumulative incidence rates for hospital admission across MASS values stratified by wave of SARS-CoV-2 variant predominance and monoclonal antibody infusion status are shown in Figure 2A and Supplemental Table 2 (available online at http://www.mcpiqojournal.org).
Table 1.
Characteristics of Patients with Coronavirus Disease 2019 According to Monoclonal Antibody Infusion Statusa
| Variable | Not Infused (N=100,846) | Infused (N=18,090) | All Patients (N=118,936) | Standardized Differencef |
|---|---|---|---|---|
| Sex | 3.6% | |||
| Female | 52,891 (52.5%) | 9813 (54.3%) | 62,704 (52.7%) | |
| Male | 47,937 (47.5%) | 8275 (45.7%) | 56,212 (47.3%) | |
| Age category at COVID-19 test | 66.2% | |||
| 18-39 y | 44,770 (44.4%) | 3495 (19.3%) | 48,265 (40.6%) | |
| 40-59 y | 33,948 (33.7%) | 5943 (32.9%) | 39,891 (33.5%) | |
| 60-69 y | 12,250 (12.1%) | 4268 (23.6%) | 16,518 (13.9%) | |
| 70-79 y | 6492 (6.4%) | 3095 (17.1%) | 9587 (8.1%) | |
| ≥80 y | 3386 (3.4%) | 1289 (7.1%) | 4675 (3.9%) | |
| Race or ethnic groupb | 23.1% | |||
| White | 88,010 (87.3%) | 16,991 (93.9%) | 105,001 (88.3%) | |
| Black or African American | 3988 (4.0%) | 388 (2.1%) | 4376 (3.7%) | |
| Other or unknown race | 8848 (8.8%) | 711 (3.9%) | 9559 (8.0%) | |
| Hispanic or Latino | 6982 (7.2%) | 755 (4.2%) | 7737 (6.7%) | |
| Not Hispanic or Latino | 90,147 (92.8%) | 17,053 (95.8%) | 107,200 (93.3%) | |
| BMI classification | 38.4% | |||
| Underweight or normal weight | 23,852 (27.4%) | 2586 (14.8%) | 26,438 (25.3%) | |
| Overweight | 26,318 (30.3%) | 4851 (27.8%) | 31,169 (29.8%) | |
| Class 1 obesity | 19,453 (22.4%) | 4362 (25.0%) | 23,815 (22.8%) | |
| Class 2 obesity | 9631 (11.1%) | 2925 (16.7%) | 12,556 (12.0%) | |
| Class 3 obesity | 7694 (8.8%) | 2752 (15.7%) | 10,446 (10.0%) | |
| Hospital location according to statec | 11.3% | |||
| Arizona | 11,966 (11.9%) | 2223 (12.3%) | 14,189 (11.9%) | |
| Florida | 10,530 (10.4%) | 1385 (7.7%) | 11,915 (10.0%) | |
| Minnesota | 52,544 (52.1%) | 9273 (51.3%) | 61,817 (52.0%) | |
| Wisconsin | 25,806 (25.6%) | 5209 (28.8%) | 31,015 (26.1%) | |
| Wave | 76.6% | |||
| Pre-Delta predominant | 26,565 (26.3%) | 3933 (21.7%) | 30,498 (25.6%) | |
| Washout period 1 | 3637 (3.6%) | 524 (2.9%) | 4161 (3.5%) | |
| Delta predominant | 14,240 (14.1%) | 5651 (31.2%) | 19,891 (16.7%) | |
| Washout period 2 | 12,073 (12.0%) | 4992 (27.6%) | 17,065 (14.3%) | |
| Omicron predominant | 44,331 (44.0%) | 2990 (16.5%) | 47,321 (39.8%) | |
| MASS category | 90.7% | |||
| 0 | 57,573 (57.1%) | 3322 (18.4%) | 60,895 (51.2%) | |
| 1-3 | 28,378 (28.1%) | 7522 (41.6%) | 35,900 (30.2%) | |
| 4-6 | 10,009 (9.9%) | 4861 (26.9%) | 14,870 (12.5%) | |
| 7-9 | 3706 (3.7%) | 1795 (9.9%) | 5501 (4.6%) | |
| 10+ | 1180 (1.2%) | 590 (3.3%) | 1770 (1.5%) | |
| Vaccination statusd | 20.3% | |||
| Unvaccinated | 56,235 (55.8%) | 8274 (45.7%) | 64,509 (54.2%) | |
| Partially vaccinated | 3279 (3.3%) | 816 (4.5%) | 4095 (3.4%) | |
| Fully vaccinated | 41,332 (41.0%) | 9000 (49.8%) | 50,332 (42.3%) | |
| Monoclonal antibody type | ||||
| Bamlanivimab | 0 | 2990 (16.5%) | 2990 (16.5%) | |
| Bamlanivimab and etesevimab | 0 | 1432 (7.9%) | 1432 (7.9%) | |
| Bebtelovimab | 0 | 1122 (6.2%) | 1122 (6.2%) | |
| Casirivimab and imdevimab | 0 | 10,022 (55.4%) | 10,022 (55.4%) | |
| Sotrovimab | 0 | 2524 (14.0%) | 2524 (14.0%) | |
| Comorbiditiese | ||||
| Myocardial infarction | 3178 (3.2%) | 1290 (7.1%) | 4468 (3.8%) | 18.1% |
| Congestive heart failure | 4800 (4.8%) | 1952 (10.8%) | 6752 (5.7%) | 22.7% |
| Peripheral vascular disease | 8085 (8.0%) | 3445 (19.0%) | 11,530 (9.7%) | 32.7% |
| Cerebrovascular disease | 5349 (5.3%) | 2061 (11.4%) | 7410 (6.2%) | 22.1% |
| Dementia | 945 (0.9%) | 194 (1.1%) | 1139 (1.0%) | 1.4% |
| Long-term pulmonary disease | 22,631 (22.4%) | 6604 (36.5%) | 29,235 (24.6%) | 31.2% |
| Connective tissue disease rheumatic disease | 2702 (2.7%) | 1337 (7.4%) | 4039 (3.4%) | 21.7% |
| Peptic ulcer disease | 2270 (2.3%) | 858 (4.7%) | 3128 (2.6%) | 13.6% |
| Mild liver disease | 7139 (7.1%) | 2634 (14.6%) | 9773 (8.2%) | 24.3% |
| Paraplegia and hemiplegia | 1050 (1.0%) | 299 (1.7%) | 1349 (1.1%) | 5.3% |
| Renal disease | 6195 (6.1%) | 2587 (14.3%) | 8782 (7.4%) | 27.2% |
| Cancer | 6481 (6.4%) | 2484 (13.7%) | 8965 (7.5%) | 24.4% |
| Moderate or severe liver disease | 667 (0.7%) | 216 (1.2%) | 883 (0.7%) | 5.6% |
| Metastatic carcinoma | 1885 (1.9%) | 741 (4.1%) | 2626 (2.2%) | 13.1% |
| Diabetes | 9391 (9.3%) | 4078 (22.5%) | 13,469 (11.3%) | 36.8% |
BMI, body mass index; COVID-19, coronavirus disease 2019; MASS, Monoclonal Antibody Screening Score. Data are represented as counts and column percentages. Missing data were present for sex (N=20), ethnicity (N=3999) and body mass index classification (N =14,512).
In the “other or unknown race” category, 540 patients were American Indian/Alaskan Native, 3208 patients were Asian, 213 patients were Native Hawaiian/Pacific Islander, 2850 patients were reported as ”other,” and data on 2748 patients were missing.
Minnesota includes Rochester campus of Mayo Clinic and Mayo Clinic Health System in Southeast/Southwest Minnesota. Wisconsin includes Mayo Clinic Health System in Northwest/Southwest Wisconsin.
Patients are fully vaccinated if their coronavirus disease 2019 positive test was 14 or more days after their first shot of a viral vector vaccine or their second messenger RNA dose. Patients who had received at least 1 shot but were not considered fully vaccinated were categorized as partially vaccinated.
Comorbidities were defined by standard International Classification of Diseases (ICD) 9/10 code sets. All diagnoses on or before the date of the coronavirus disease 2019 test were included.
Standardized difference = difference in proportions divided by SE; imbalance is defined as an absolute value greater than 0.1 and are shown in bold.
Figure 2.
Stratified analysis of cumulative incidence and relative risk of 28-day hospital admission. (A) Crude cumulative incidence of hospital admission 28 days after coronavirus disease 2019 (COVID-19)-positive test result by wave and infusion status. Monoclonal Antibody Screening Score (MASS) values were winsorized at 10 owing to sparse cell counts. Lower overall rates of admission were observed during the Omicron predominant wave than during the other variant waves; however, rates remained lower in patients treated with monoclonal antibody therapy. The size of each dot represents the number of patients within a given wave, MASS, and infusion group. Color captures whether a patient was treated with monoclonal antibody therapy (green) or not (red). (B) A forest plot of the Cochran-Mantel-Haenszel analysis of risk for hospital admission by MASS and propensity score (PS) quintile. Each row in the figure represents a mutually exclusive combination of MASS and propensity score group. Estimates are the relative risk of 28-day hospital admission. Results show lower risk of hospital admission for those patients who were infused across nearly all MASS and propensity score combinations. Patients in group 1 were younger, had fewer baseline comorbidities, and were tested in the pre-delta predominant or omicron-predominant waves (Supplemental Table 3). Color represents the propensity score quintile: 1 (red), 2 (green), 3 (gold), 4 (orange), and 5 (teal). Dots represent point estimates, and bars represent 95% CIs. On the right, relative risk and CIs are displayed along with the number of treated patients out of the total subgroup size.
Unadjusted Analyses
Hospital admission within 28 days after COVID-19 diagnosis occurred in 6.30% of all patients (7493 of 118,936 patients; 95% CI, 6.16-6.44). This primary outcome event occurred in 2.52% (456 of 18,090 patients; 95% CI, 2.30-2.76) of the patients in the cohort who received monoclonal antibody treatment and 6.98% (7037 of 100,846 patients; 95% CI, 6.82-7.14) of the patients in the cohort who did not receive monoclonal antibody treatment. Patients in the cohort treated with monoclonal antibodies had a lower RR of hospital admission within 28 days after COVID-19 diagnosis than patients in the cohort not treated with monoclonal antibodies (RR, 0.36; 95% CI, 0.33-0.40).
Stratified Analyses
The findings of lower RR of hospital admission among patients who received monoclonal antibody therapy were further supported by a stratified-data analytic approach that provided direct analytical control for the key variables associated with increased risk of hospital admission (Figure 2B). The CMH pooled RR of hospital admission within 28 days of COVID-19 diagnosis among patients who received monoclonal antibody therapy compared with that in the cohort not infused with monoclonal antibody was 0.15 (95% CI, 0.14-0.17). Although the rate of hospital admission was low in this group, monoclonal antibody treatment may have been associated with an increased risk of hospital admission among patients with very low MASSs (ie, MASS = 0) and low propensity to be given monoclonal antibodies (PS group 1; Figure 2B). Supplemental Table 3 (available online at http://www.mcpiqojournal.org) shows patient characteristics stratified by propensity score quintile. Cochran-Mantel-Haenszel analyses stratified by race, age, and sex reported similar trends over values of MASS.
Machine Learning
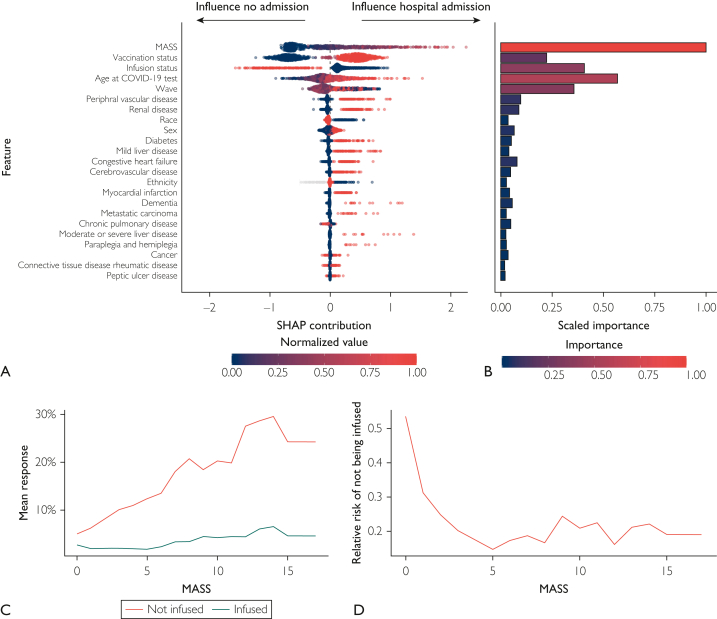
The GBM analysis of hospital admissions reported that higher MASS values were associated with a higher risk of hospital admission, and monoclonal antibody infusions were associated with a lower probability of admission (Figure 3A). In rank order, MASS, COVID-19 vaccination status, and monoclonal antibody infusion status were the 3 most influential variables in predicting hospital admission according to Shapley Additive Explanations contribution and the first, fifth, and third most important, respectively, according to variable importance (Figure 3A and B). Additionally, age and wave of SARS-CoV-2 variant predominance were important variables. Using estimates of partial dependence, the mean predicted response for hospital admission was lower for patients who received monoclonal antibody treatment across all MASS values and each wave (Figure 3C and D). Among patients who received monoclonal antibody treatment, the risk of hospital admission was similar across each wave. Among patients who did not receive monoclonal antibody treatment, the risk of hospital admission was lowest in the Omicron predominant wave and highest in the Delta predominate wave. Thus, the RR of hospital admission based on infusion status varied by wave, particularly in patients with low MASS values.
Figure 3.
Shapley Additive Explanation (SHAP) plot, variable importance plot, partial dependence plots, and relative risk of admission based on a gradient boosting machine model. (A) SHAP contribution is displayed in overall rank order, with the variables most influential in predicting 28-day hospital admission at the top of the plot. Each dot represents a single patient, with large SHAP values indicating influence toward hospital admission (positive values) or no hospital admission (negative values). Large absolute SHAP values indicate that a patient’s overall prediction was highly influenced by that variable’s value. Color represents the normalized values for each variable, with high values in red and low values in dark blue. (B) A variable importance plot ordered on the basis of SHAP feature importance. Bar width and color specify scaled importance (ie, selection order in the boosted trees and relative changes in split purity in the tree). (C) In the top row, partial dependence plots show the relationship between Monoclonal Antibody Screening Score (MASS), monoclonal antibody treatment, and 28-day hospital admission stratified by wave after accounting for all other variables in the model. The mean predicted probability of hospital admission was lower across all MASS values and waves for patients treated with monoclonal antibody therapy (blue line) compared with those who were not (red line). (D) The relative risk of hospital admission in patients who did not receive monoclonal antibody therapy derived from the partial dependence estimates. COVID-19, coronavirus disease 2019.
Regression Models
Model-based estimates adjusting for patient demographic characteristics (age, sex, and race) and clinical characteristics (MASS, COVID-19 vaccination status, and wave of SARS-CoV-2 variant predominance) reported the association between greater risk of hospital admission and a greater MASS value (RR, 1.32; 95% CI, 1.31-1.33 per unit increase in MASS; Table 2, base model). These regression models also reported a lower risk of hospital admission among patients who received monoclonal antibody treatment (RR, 0.20; 95% CI, 0.18-0.22). The adjusted models (as defined in Table 2) generally reported similar associations—a lower RR of hospital admission among patients with lower MASS values and patients who received monoclonal antibody treatment. The relationships between hospital admission and both MASS and monoclonal antibody treatment were further supported by conditional logistic regressions stratified on the 1:1 nearest neighbor matched pairs (MASS, RR, 1.24; 95% CI, 1.23-1.25; monoclonal antibody treatment status, RR, 0.14; 95% CI, 0.13-0.16).
Table 2.
Models of the Association of Neutralizing Antispike Monoclonal Antibody Treatment With Coronavirus Disease 2019 Hospitalizationa
| Model | No. of Patients | Estimated Relative Risk (95% CI) |
|---|---|---|
| Base model | 118,936 | |
| MASS | 1.32 (1.31-1.33) | |
| Infusion status (infused) | 0.20 (0.18-0.22) | |
| Model 2 | 97,692 | |
| MASS | 1.22 (1.21-1.22) | |
| Infusion status (infused) | 0.18 (0.16-0.20) | |
| Vaccination status (partially vaccinated) | 0.79 (0.71-0.89) | |
| Vaccination status (fully vaccinated) | 0.31 (0.29-0.33) | |
| Wave (Delta predominant) | 2.48 (2.34-2.63) | |
| Wave (Omicron predominant) | 1.21 (1.14-1.29) | |
| Age at COVID-19 test | 1.04 (1.03-1.04) | |
| Sex (male) | 1.18 (1.13-1.24) | |
| Race (non-White or unknown) | 1.42 (1.32-1.53) | |
| Model 3 | 85,646 | |
| MASS | 1.13 (1.12-1.14) | |
| Infusion status (infused) | 0.18 (0.16-0.20) | |
| Vaccination status (partially vaccinated) | 0.78 (0.70-0.87) | |
| Vaccination status (fully vaccinated) | 0.31 (0.29-0.32) | |
| Wave (Delta predominant) | 2.45 (2.31-2.60) | |
| Wave (Omicron predominant) | 1.18 (1.11-1.26) | |
| Age at COVID-19 test | 1.03 (1.03-1.03) | |
| Sex (male) | 1.23 (1.17-1.29) | |
| Race (non-White or unknown) | 1.53 (1.43-1.65) | |
| BMI classification (overweight) | 0.90 (0.85-0.96) | |
| BMI classification (class 1 obesity) | 0.93 (0.87-0.99) | |
| BMI classification (class 2 obesity) | 0.99 (0.91-1.07) | |
| BMI classification (class 3 obesity) | 1.23 (1.13-1.34) | |
| Peripheral vascular disease | 1.29 (1.21-1.37) | |
| Renal disease | 1.24 (1.16-1.32) | |
| Mild liver disease | 1.21 (1.13-1.28) | |
| Diabetes | 1.15 (1.08-1.22) | |
| Congestive heart failure | 1.18 (1.11-1.26) | |
| Cerebrovascular disease | 1.10 (1.03-1.17) | |
| Dementia | 1.42 (1.30-1.56) | |
| Model 4b | 118,936 | |
| MASS | 1.25 (1.24-1.25) | |
| Infusion status (infused) | 0.15 (0.13-0.16) | |
| Model 5c | 36,180 | |
| MASS | 1.24 (1.23-1.25) | |
| Infusion status (infused) | 0.14 (0.13-0.16) |
BMI, body mass index; COVID-19, coronavirus disease 2019; MASS, Monoclonal Antibody Screening Score. Relative risk regression models were constructed using a generalized linear model framework. A log link and the robust variance estimator (Poisson distribution) were included to correct for the misspecified variance structure. Models were only adjusted for the covariates listed. Models 4 and 5 also used the results of propensity matching.
Model 4 was weighted on the basis of propensity scores calculated using a gradient boosting machine. Matching was on the basis of age at coronavirus disease 2019 test, sex, race, wave, hospital, body mass index classification, myocardial infarction, congestive heart failure, peripheral vascular disease, long-term pulmonary disease, connective tissue disease rheumatic disease, mild liver disease, diabetes, renal disease, cancer, vaccination status, and distance from hospital.
In Model 5, the cohort was identified by using the propensity scores from the gradient-boosted model as the distance metric in a 1:1 nearest neighbor matching algorithm with no replacement.
Number Needed to Treat
The results of the classification and regression tree model reported that the primary nodes influencing the number needed to treat were MASS, COVID-19 vaccination status, wave of SARS-CoV-2 variant predominance, and age. Among patients with a MASS greater than 1, treatment of 34.1 patients with monoclonal antibodies would prevent 1 hospitalization (Supplemental Figure, available online at http://www.mcpiqojournal.org). Patient characteristics stratified by the number needed to treat are shown in Supplemental Table 4 (available online at http://www.mcpiqojournal.org).
Discussion
In this retrospective analysis performed on a geographically diverse set of patients with COVID-19, neutralizing antispike monoclonal antibody treatment was associated with a lower risk of hospital admission. This principal finding (association between monoclonal antibody treatment and lower risk of hospital admission) was consistent across a range of modeling approaches and methods to adjust for confounding variables, and the results of our analyses are consistent with those of prior studies on the efficacy of monoclonal antibody treatments in reducing hospital admission in mild to moderate COVID-19 cases.1, 2, 3, 4, 5, 6, 7 Available data provide support for the observation that monoclonal antibody treatment leads to a reduced risk of COVID-19 disease progress when administered early and in high doses in both outpatients and seronegative inpatients.
Although monoclonal antibody treatments are widely available in high-income countries, there is concern about the emergence of antibody resistance because monoclonal antibody treatments have limited targeted effect that may not keep up with contemporary, locally circulating SARS-CoV-2 variants.8, 9, 10,15,24 In this context, both monoclonal antibody treatments and predominant SARS-CoV-2 variants have evolved throughout the COVID-19 pandemic.10, 11, 12, 13, 14 Our findings demonstrate that the risk of hospitalization remained low because monoclonal antibody treatments varied on the basis of the emergence of resistant variants. Hence, because systems have been developed to qualify monoclonal antibody treatments as new SARS-CoV-2 variants evolve, monoclonal antibody treatments have thus far been an effective therapeutic option for patients with mild to moderate COVID-19.
Collectively, our results confirm that despite the emergence of new variants, the increasing prevalence of the vaccinated population, and the SARS-CoV-2 variants’ responsiveness toward monoclonal antibodies, the clinical performance of MASS as a prioritization tool has been maintained throughout the entire timeline. Accordingly, we encourage its continued use as a prognostic tool to identify patients who would most benefit from monoclonal antibody treatment, leading to a continued predicted reduction in hospital admissions.
Several limitations should be noted. First, this was a retrospective cohort study and not a randomized clinical trial; as such, these data should not be misconstrued as definitive evidence of effectiveness for monoclonal antibody treatment. As such, during this time period, multiple monoclonal antibody treatments were available to target variants and subvariants, and the frequency of vaccinations and hospitalizations changed over time. Similarly, these analyses were exploratory by nature, and the findings should be interpreted as such. Second, the program was not large enough for definitive subgroup analyses according to coexisting medical conditions. Third, treatments and outcomes recorded outside of Mayo Clinic are generally not captured; as such, we conducted a sensitivity analysis of only paneled patients to assess the potential bias of patients likely to seek care elsewhere (Supplemental Table 5, available online at http://www.mcpiqojournal.org). Finally, sequencing analyses were not performed, and available data did not allow for associations at the variant or subvariant level.
Conclusion
In summary, our analyses found that antispike monoclonal antibody treatment was associated with lower hospitalization rates among outpatients with COVID-19. The relationship between monoclonal antibody treatment and reduced risk of hospitalization was supported by several separate analyses and after multiple complementary means for adjusting for confounding. These real-world clinical data support observations from controlled clinical trials that monoclonal antibody treatments are associated with lower rates of hospitalization if administered early in the COVID-19 disease course. Monoclonal antibody treatment remains an important treatment tool to reduce the risk of hospitalization for patients with COVID-19 even as monoclonal antibody–resistant SARS-CoV-2 variants emerge.
Potential Competing Interests
Dr O’Horo received grants from nference, Inc, and the MITRE Corporation and consulting fees from Bates College, all of which was outside the present work. Dr Razonable received grants from Roche, Regeneron, and Gilead; received honoraria from Integritas and Medscape; is on the Data Safety Monitoring Board or Advisory Board for Novartis; and has a leadership role in the American Society of Transplantation.
Acknowledgments
Mr Johnson and Dr Kunze had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Mr. Johnson and Dr. Kunze contributed equally as first authors to the work of the study and manuscript. Additionally, Drs Razonable, Carter, Sanghavi, and Speicher contributed equally as senior authors to the work of the study and manuscript.
Footnotes
Grant Support: This work was supported in part by National Center for Advancing Translational Sciences (grant UL1TR002377); National Heart, Lung, and Blood Institute (grant 1F32HL154320 to J.W.S.); and Mayo Clinic Research Funds on Monoclonal Antibodies (R.R.R.).
Data Sharing: Study data cannot be shared publicly because of institutional review board restrictions. Limited and deidentified data sets will be deposited into a research data repository and may be shared with investigators under controlled access procedures if approved by the Mayo Clinic Institutional Review Board. A scientific committee will review requests for the conduct of protocols approved or determined to be exempt by an Institutional Review Board. Requestors may be required to sign a data use agreement. Data sharing must be compliant with all applicable Mayo Clinic policies. Interested parties may contact Dr Leigh Speicher at speicher.leigh@mayo.edu.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 3.Dougan M., Nirula A., Azizad M., et al. Bamlanivimab plus Etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385(15):1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesh R., Philpot L.M., Bierle D.M., et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis. 2021;224(8):1278–1286. doi: 10.1093/infdis/jiab377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jary A., Marot S., Faycal A., et al. Spike gene evolution and immune escape mutations in patients with mild or moderate forms of COVID-19 and treated with monoclonal antibodies therapies. Viruses. 2022;14(2):226. doi: 10.3390/v14020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pommeret F., Colomba J., Bigenwald C., et al. Bamlanivimab + etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann Oncol. 2021;32(11):1445–1447. doi: 10.1016/j.annonc.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisman D.N., Tuite A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ. 2021;193(42):E1619–E1625. doi: 10.1503/cmaj.211248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong S.W.X., Chiew C.J., Ang L.W., et al. Clinical and virological features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2022;75(1):e1128–e1136. doi: 10.1093/cid/ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh A., McMenamin J., Taylor B., Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen P.A., Olsen R.J., Long S.W., et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemieux J.E., Siddle K.J., Shaw B.M., et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science. 2021;371(6529) doi: 10.1126/science.abe3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S., Chandele A., Sharma A. Current status of therapeutic monoclonal antibodies against SARS-CoV-2. PLoS Pathog. 2021;17(9) doi: 10.1371/journal.ppat.1009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashita E., Kinoshita N., Yamayoshi S., et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med. 2022;386(10):995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierle D.M., Ganesh R., Tulledge-Scheitel S., et al. Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities. J Infect Dis. 2022;225(4):598–602. doi: 10.1093/infdis/jiab570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bierle D.M., Ganesh R., Wilker C.G., et al. Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19. J Prim Care Community Health. 2021;12(Jan-Dec) doi: 10.1177/21501327211019282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razonable R.R., Aloia N.C.E., Anderson R.J., et al. A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate Coronavirus Disease-19: the Mayo Clinic model. Mayo Clin Proc. 2021;96(5):1250–1261. doi: 10.1016/j.mayocp.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razonable R.R., Ganesh R., Bierle D.M. Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate COVID-19. Mayo Clin Proc. 2022;97(1):26–30. doi: 10.1016/j.mayocp.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas J., Sharp C. Nomogram for number needed to treat will be of limited use. BMJ. 1996;312(7040):1229. doi: 10.1136/bmj.312.7040.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 24.Kunze K.L., Johnson P.W., van Helmond N., et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun. 2021;12(1):4864. doi: 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.