Abstract
Introduction
Little is known about the consequences of deranged chronic kidney disease–mineral and bone disorder (CKD-MBD) parameters on kidney allograft function in children. We examined a relationship between these parameters over time and allograft outcome.
Methods
This registry study from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) collected data at baseline, months 1, 3, 6, 9, and 12 after transplant; and every 6 months thereafter up to 5 years. Survival analysis for a composite end point of graft loss or estimated glomerular filtration rate (eGFR) ≤30 ml/min per 1.73 m2 or a ≥50% decline from eGFR at month 1 posttransplant was performed. Associations of parathyroid hormone (PTH), calcium, phosphate, and 25-hydroxyvitamin D (25(OH)D) with allograft outcome were investigated using conventional stratified Cox proportional hazards models and further verified with marginal structural models with time-varying covariates.
Results
We report on 1210 patients (61% boys) from 16 European countries. The composite end point was reached in 250 grafts (21%), of which 11 (4%) were allograft losses. In the conventional Cox proportional hazards models adjusted for potential confounders, only hyperparathyroidism (hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.82–4.74) and hyperphosphatemia (HR, 1.94; 95% CI, 1.28–2.92) were associated with the composite end point. Marginal structural models showed similar results for hyperparathyroidism (HR, 2.74; 95% CI, 1.71–4.38), whereas hyperphosphatemia was no longer significant (HR, 1.35; 95% CI, 0.87–2.09), suggesting that its association with graft dysfunction can be ascribed to a decline in eGFR.
Conclusion
Hyperparathyroidism is a potential independent risk factor for allograft dysfunction in children.
Keywords: allograft outcome, hyperparathyroidism, kidney transplantation, pediatric, structural marginal models
Graphical abstract
See Commentary on Page 8
The main factors contributing to CKD-MBD after kidney transplantation (KTx) are mineral metabolism disturbances before transplant, side effects of immunosuppressants, and graft dysfunction. Clinical and laboratory manifestations of CKD-MBD may be prevalent even in the presence of restored kidney function.1,2 In a large European cohort study of 1237 pediatric KTx recipients, hypocalcemia and hypophosphatemia were observed in 19% and 14% of patients, hypercalcemia and hyperphosphatemia in 11% each, and hyperparathyroidism in 41%.3 Both early hypocalcemia and hypercalcemia have been reported in KTx recipients, whereby pretransplant plasma PTH appears to be predictive of post-transplant calcium levels.4 Pretransplant, plasma PTH, fibroblast growth factor 23, and phosphate increase as kidney function declines. Post-transplant plasma fibroblast growth factor 23 and PTH may remain elevated and contribute to the development of early hypophosphatemia by stimulation of phosphaturia.1,5 Long-term mineral metabolism dysregulation is increasingly driven by gradual graft function decline, resulting in an increase in fibroblast growth factor 23, elevated PTH, hypocalcemia, and hyperphosphatemia.6
Our understanding of the clinical consequences of deranged CKD-MBD parameters in pediatric KTx recipients is mainly based on data from pretransplant CKD. Secondary hyperparathyroidism is associated with high bone turnover and mineralization defects, risk of fractures, ectopic calcifications, and vascular stiffness.7, 8, 9 Although hypocalcemia and hypophosphatemia can result in impaired bone mineralization in children with CKD, hypercalcemia and hyperphosphatemia may be associated with vascular calcifications.9,10 In turn, vascular calcifications in adult KTx recipients are associated with an increased risk of cardiovascular events.11 Indeed, a recent study in adult KTx recipients reported an association between hypercalcemia and mortality, independent of eGFR.12 Long-term data on the association between deranged CKD-MBD parameters and graft outcome in pediatric KTx recipients are scarce. It is difficult to show whether these associations are merely a consequence of declining kidney function, or whether they independently contribute to allograft dysfunction.
As per recent recommendations, the assessment of CKD-MBD should be based on serial measurements of plasma markers rather than single time point values.8,13 Therefore, the primary objective of our study was to analyze the relationship between time-varying PTH, calcium, phosphate, and 25(OH)D, and allograft outcome in a large cohort of pediatric KTx recipients. In addition, we aimed to estimate the effect of CKD-MBD disturbances on allograft dysfunction by adjusting for time-varying confounding due to eGFR.14 Furthermore, we aimed to analyze the natural evolution of CKD-MBD markers after KTx as well as the association between hyperparathyroidism and hypophosphatemia at month 1 posttransplant and long-term allograft outcome.
Methods
Patients and Follow-up
Pediatric KTx recipients younger than 19 years at time of first KTx who were registered in the CERTAIN Registry were considered for inclusion in this retrospective, multicenter, longitudinal cohort study. Patients with multiorgan transplants, and those who experienced graft loss or died within 4 weeks after transplant were excluded.
The CERTAIN Registry captures detailed clinical and laboratory longitudinal data and applies rigorous validity check procedures (http://www.certain-registry.eu, see Supplementary Material for details on the quality of data). Participation in the CERTAIN Registry is approved by the ethics committee in each center. Informed consent must be obtained from the parents or legal caregivers before patient inclusion, with assent from patients when appropriate for their age. For this study, data up to 5 years after transplant were analyzed. The time points of data collection and the corresponding time intervals were as follows: baseline (before transplant), at 1, 3, 6, 9, and 12 months after transplant, and every 6 months thereafter. All procedures and immunosuppressive regimens were applied as per local institutional protocols in each center. Delayed graft function was defined as acute kidney injury with the need for dialysis within the first 7 days after transplant.
The study was performed in accordance with the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The study was designed, analyzed, and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (https://www.strobe-statement.org).
Laboratory Measurements
Plasma calcium and phosphate levels were adjusted for age-specific reference values as per recent clinical practice recommendations8,13 and categorized as hypocalcemia or hypercalcemia and hypophosphatemia or hyperphosphatemia, respectively. Plasma PTH was determined by assays measuring either only the bioactive PTH form or the “intact” PTH, which includes other carboxyl-terminal forms.15 Therefore, the local PTH reference values were obtained from each participating center (see Supplementary Material). The input PTH levels were then converted accordingly and expressed as a ratio of the upper limit of normal (ULN). Hyperparathyroidism was defined as values above ULN in each center, corresponding to 65 ng/l,13 and 25(OH)D deficiency was defined as values below 30 ng/ml.8 eGFR was calculated using the revised 2009 bedside Schwartz formula.16
Statistical Analysis
For descriptive purposes, continuous variables with normal distribution were reported as mean ± SD, whereas median with first (quartile 1) and third (quartile 3) quartile was given for skewed variables. Categorical variables were reported as number and percentage. To explore the rank-based association between 2 skewed continuous measures, Spearman r was reported.
Because graft loss is a rare event in pediatric KTx recipients, the primary outcome measure we used was a death-censored composite end point named allograft dysfunction, which is defined as either graft loss, or eGFR ≤30 ml/min per 1.73 m2, or a ≥50% decline from baseline eGFR at month 1 after transplant. eGFR values were above 120 ml/min per 1.73 m2 at month 1 after transplant in 125 patients (10.3%) with a median age of 4.6 years and a median weight of 15.9 kg. These eGFR values were set to 120 ml/min per 1.73 m2. We performed a survival analysis, where the associations between calcium, phosphate, PTH, 25(OH)D, and time to composite end point were investigated. Because data were available in follow-up time intervals, the assumption was made that time-dependent covariates change at the beginning, whereas the event takes place at the end of an interval. In case of missing data for calcium, phosphate levels, and eGFR during follow-up, the last available observation was carried forward. First, unadjusted cumulative probabilities of allograft dysfunction for PTH, calcium, phosphate, and 25(OH)D as categorical variables (hyperparathyroidism or no hyperparathyroidism; hypocalcemia, normocalcemia or hypercalcemia; and hypophosphatemia, normophosphatemia, or hyperphosphatemia; hypovitaminosis D or no hypovitaminosis D, respectively) as time-varying covariates and as time-fixed covariates at month 1 after transplant for PTH only were visualized using the classical and extended Kaplan-Meier methods for time-fixed and time-varying factors, respectively. Next, unadjusted Cox regression analyses stratified by center were performed to assess the association of PTH, calcium, and phosphate values and time to composite end point. Finally, conventional center-stratified Cox proportional hazards models were fitted to estimate the independent association of time-varying hyperparathyroidism, hypocalcemia, and hyperphosphatemia with allograft dysfunction, after adjustments for potential confounders, including the time-varying covariates allograft rejection and systolic blood pressure Z scores. Before inclusion in the model, the proportional hazards assumption and linearity were assessed in each covariate. In case of nonlinearity, a smoothing spline using a pspline basis was fitted and stratified analyses were performed to account for nonproportional hazards. Stratified proportional hazard assumptions were subsequently checked by applying the statistical test per stratum.
The association of time-varying hyperparathyroidism, hypocalcemia, and hyperphosphatemia with allograft dysfunction was further verified by marginal structural models. eGFR varies over time and causes disturbances in PTH, calcium, and phosphate levels. Conversely, allograft function may be affected by derangements in CKD-MBD parameters. In marginal structural models, inverse probability weighting is applied to construct a pseudopopulation in which the exposure variable is not confounded by time-varying eGFR and thereby provides a more accurate estimation of the independent association between them.14 We considered eGFR as a time-varying confounder, whereas hyperparathyroidism, hypocalcemia, and hyperphosphatemia were time-varying exposures of interest. Time-varying acute rejection and systolic blood pressure Z scores were included as time-varying covariates in the marginal structural Cox proportional hazards model. The secondary analyses included associations between time-fixed PTH and hypophosphatemia at month 1 after transplant, and time to composite end point.
All statistical analyses were performed using R version 4.2.1 (Vienna, Austria) and R libraries survival (version 3.3-1)17, 39 and ipw (version 1.0-11) (http://www.jstatsoft.org/v43/i13/).39
Results
Cohort Characteristics and Incidence of Death, Graft Loss, and Allograft Dysfunction
Data on 1231 grafts in 1218 patients younger than 19 years were reported to the CERTAIN Registry by 40 centers in 16 countries (see Supplementary Material). During the first month after transplant, 3 children died and 5 experienced graft loss; they were therefore excluded from analyses. We included 1210 patients (61% boys), first graft available in the registry for each patient, who underwent KTx between September 1993 and February 2019. Patient characteristics are shown in Table 1.
Table 1.
Patient and transplant characteristics
| Baseline characteristics | Patient cohort (n = 1210) |
|---|---|
| Recipient age, yr | 9.7 (5.1) |
| Male sex, n (%) | 740 (61.2) |
| Weight, kg | 30.2 (16.9) |
| Weight Z score | −1.6 (1.6) |
| Height Z score | −2.0 (1.6) |
| BMI Z score | −0.4 (1.3) |
| Primary kidney disease, n (%) | |
| CAKUT | 54 (45.2) |
| Glomerulopathies | 251 (20.7) |
| Tubulo-interstitial nephritis and cystic kidney disease | 225 (18.6) |
| (atypical) HUS | 57 (4.7) |
| Other/unknown | 130 (10.8) |
| First KTx, n (%) | 1126 (93.1) |
| Second, n (%) | 80 (6.6) |
| Third, n (%) | 4 (0.3) |
| Donor source, n (%) | |
| Deceased | 853 (70.5) |
| Living-related | 357 (29.5) |
| Delayed graft function, n (%) | 92 (7.6) |
| Initial immunosuppressive therapy, n (%) | |
| Glucocorticoids | 1185 (97.9) |
| Calcineurin inhibitors | 1204 (99.5) |
| Tacrolimus | 759 (63.0) |
| Mycophenolate mofetil | 948 (78.4) |
BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; HUS, hemolytic uremic syndrome; KTx, kidney transplantation.
Data are presented as number (%) or mean (SD) for normally distributed variables or as median as first (quartile 1) and third (quartile 3) quartile for skewed variables.
Five (0.4%) primary KTx recipients died during the 5-year follow-up, of whom 3 children had reached the composite end point of the study before death. The causes of death included infection (n = 3), respiratory failure (n = 1), and hyperkalemia (n = 1). Graft loss during follow-up occurred in 11 patients (0.9%) who were all primary KTx recipients. Further 18 grafts, of which 5 were second transplants, were lost after the composite end point of the study had been reached. The causes of graft loss were as follows: allograft rejection (n = 11), recurrence of primary kidney disease (n = 8), loss of kidney perfusion because of vascular complications (n = 3), and unknown (n = 7). After 1, 2, 3, 4, and 5 years of follow-up, data were available for 1069 (88%), 819 (68%), 622 (51%), 418 (34%), and 302 (25%) of allografts, respectively. Until the composite end point, 400 patients (33%) experienced acute rejection. The total number of patients who reached the composite end point was 250 (20.7%) with the following distribution: graft losses (n = 11), eGFR < 30 ml/min per 1.73 m2 (n = 121), and ≥50% decline from baseline eGFR at month 1 after transplant (n = 118).
Evolution of PTH, Calcium, Phosphate, and 25(OH)D
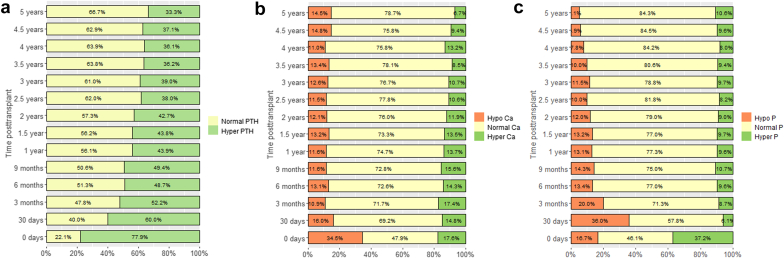
The evolution of plasma PTH, calcium, and phosphate over time is shown in Figure 1. Pretransplant PTH levels were available in 411 grafts (33%) with 320 patients (78%) having PTH levels higher than the ULN. The percentage of patients with PTH below and above ULN at different time points including baseline and 1 month is depicted in Figure 1a. The median pretransplant PTH was 156 ng/l, whereas median PTH at 1 month was 80 ng/l. In stable KTx recipients at ≥6 months after transplant with an eGFR ≥60, 45 to 59, and 30 to 44 ml/min per 1.73 m2, the respective median (interquartile range) PTH ratio of ULN were as follows: 0.85 (0.58–1.25), 0.95 (0.62–1.38), and 1.25 (0.8–1.87), respectively. These values correspond to PTH plasma concentrations of 55.2 (37.7–81.3), 61.8 (40.3–89.7), and 81.3 (52–121.6) ng/l, respectively. At months 1 and 3, and years 1, 3, and 5, posttransplant data on 25(OH)D concentrations were available in 139 (12%), 135 (12%), 328 (31%), 170 (27%), and 98 (33%) patients, respectively. At these time points, 91%, 74%, 66%, 49%, and 50% of patients, respectively, had hypovitaminosis D. At 1 and 5 years after transplant, there was an inverse correlation between 25(OH)D and PTH levels (Spearman r = −0.26, P < 0.001 and r = −0.21, P = 0.02, respectively).
Figure 1.
(a) Rate and number of patients with hyperparathyroidism, (b) hypocalcemia or hypercalcemia, and (c) hypophosphatemia or hyperphosphatemia during the follow-up of up to 5 years after transplant. Yellow indicates no hyperparathyroidism, normocalcemia, and normophosphatemia. Orange indicates hypocalcemia and hypophosphatemia. Green indicates hyperparathyroidism, hypercalcemia, and hyperphosphatemia.
Association Between PTH, Calcium, Phosphate, 25(OH)D, and Allograft Dysfunction
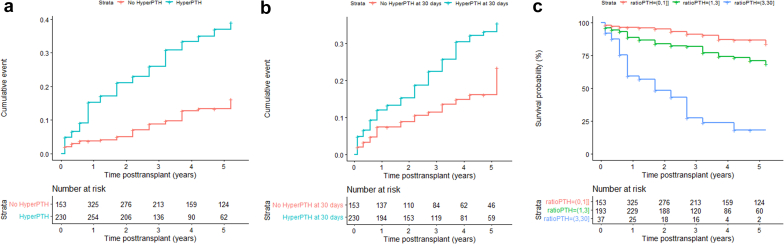
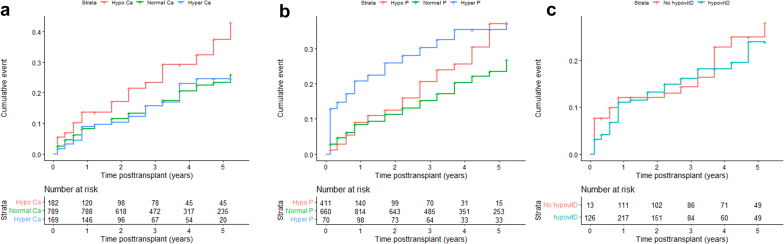
The unadjusted cumulative probabilities for allograft dysfunction with time-varying covariates hyperparathyroidism, hypocalcemia, and hyperphosphatemia were associated with time to composite end point (Figure 2a and Figure 3a and b). Hypercalcemia and hypophosphatemia were not significant and therefore not investigated further. There was no association between 25(OH)D and time to composite end point (Figure 3c). The significant difference in unadjusted cumulative probabilities for allograft dysfunction between patients with and without hyperparathyroidism at month 1 after transplant is shown in Figure 2b. The association between the degree of time-varying hyperparathyroidism and time to composite end point is shown in Figure 2c. There was a stepwise increase of cumulative events with higher PTH plasma values. Patients with both hypocalcemia and hyperphosphatemia had the highest risk of allograft dysfunction as shown in Figure 4.
Figure 2.
(a) Association between the cumulative incidence of time to composite end point and hyperparathyroidism versus no hyperparathyroidism (HR, 3.4; 95% CI, 2.1–5.2; P < 0.001), (b) the cumulative incidence of time to composite end point plotted for patients with and without hyperparathyroidism using single PTH values at 30 days after transplant (HR, 1.85; 95% CI, 1.13–3.08; P = 0.014), and (c) association between the degree of time-varying hyperparathyroidism and time to composite end point (HR, 2.64; 95% CI, 1.64–4.24; P < 0.001 for PTH ratio 1–3 times above ULN; HR, 13.73; 95% CI, 7.11–26.5; P < 0.001 for PTH ratio above 3 times ULN). Number at risk in time-varying analysis (a and c) corresponds to the number of patients with available PTH levels at a given time point. CI, confidence interval; HR, hazard ratio; PTH, parathyroid hormone; ULN, upper limit of normal.
Figure 3.
(a) Association between the cumulative incidence of event and hypocalcemia versus normocalcemia or hypercalcemia: hypocalcemia versus normocalcemia HR, 1.7; 95% CI, 1.2–2.3; P = 0.003; hypercalcemia-normocalcemia HR, 0.9; 95% CI, 0.6–1.4; P = 0.79; (b) hyperphosphatemia versus normophosphatemia or hypophosphatemia: hypophosphatemia-normophosphatemia HR, 1.0; 95% CI, 0.7–1.4; P = 0.97; hyperphosphatemia-normophosphatemia HR, 1.9; 95% CI, 1.3–2.8; P < 0.001; (c) hypovitaminosis D versus no hypovitaminosis D HR, 1.4; 95% CI, 0.6–3.2; P = 0.42. Number at risk corresponds to the number of patients with available Ca, P, and 25-hydroxyvitamin D levels at a given time point. Ca, calcium; D, vitamin D; P, phosphate.
Figure 4.
Combined hypocalcemia and hyperphosphatemia and the cumulative incidence of event: hypocalcemia HR, 1.6; 95% CI, 1.2–2.3; P = 0.004; hyperphosphatemia HR, 1.9; 95% CI, 1.3–2.8; P = 0.001; no significant hypocalcemia and hyperphosphatemia interaction (P = 0.49). Ca, calcium; P, phosphate.
There was no association between early posttransplant hypophosphatemia at month 1 and allograft dysfunction (P = 0.95) (Supplementary Figure S1). There was an inverse correlation between hypophosphatemia and PTH at months 1 and 3 after transplant (Spearman r = −0.19, P < 0.001 and r = −0.20, P < 0.001, respectively).
Because of 67% to 88% of missing values, the association between 25(OH)D concentrations and allograft outcome was not analyzed further. The respective number of composite end points, patients, and laboratory values available for the survival analysis were as follows: 104, 1002, and 4375 for hyperparathyroidism; 234, 1132, and 9582 for hypocalcemia; and 233, 1131, and 9583 for hyperphosphatemia. To verify the association of time-varying hyperparathyroidism, hypocalcemia, and hyperphosphatemia with allograft dysfunction in the presence of time-varying confounding eGFR, marginal structural models were applied and compared with conventional time-dependent Cox proportional hazards models. When adjusted for time-varying eGFR only in the marginal structural model, both analyses showed that hyperparathyroidism and hypocalcemia were associated with a higher risk of allograft dysfunction (Table 2). For hyperphosphatemia, there was an association with allograft dysfunction in the conventional Cox model (HR 1.99; 95% CI, 1.36–2.91; P < 0.001), but not in the marginal structural model (HR 1.32; 95% CI, 0.87–2.00; P = 0.193). The results of the marginal structural models and the conventional time-dependent Cox proportional hazards models after adjustment for potential confounders for graft dysfunction are displayed in Table 3. The association of hyperparathyroidism with allograft dysfunction was shown on both analyses, whereas the association with hypocalcemia was no longer significant or borderline significant. For hyperphosphatemia, the association with allograft dysfunction was shown only in the conventional Cox model (HR 1.94; 95% CI 1.28–2.92; P = 0.002) but not in the marginal structural model (HR 1.35; 95% CI 0.87–2.09; P = 0.177). The reciprocal relationship between eGFR and PTH is shown in Figure 5. The full structural marginal model for the assessment of the association of time-varying hyperparathyroidism with allograft dysfunction, adjusted for other risk factors for allograft dysfunction is given in Table 4.
Table 2.
Comparison between the conventional Cox proportional hazards models and the structural marginal models, the latter adjusted only for time-varying estimated glomerular filtration rate, for the assessment of the association of time-varying hyperparathyroidism, hypocalcemia, and hyperphosphatemia with allograft dysfunction
| Cox proportional hazards model (unadjusted) | HR | 95% CI | P value |
|---|---|---|---|
| Hyperparathyroidism | 3.13 | 1.99–4.92 | <0.001 |
| Hypocalcemia | 1.62 | 1.16–2.27 | 0.005 |
| Hyperphosphatemia | 1.99 | 1.36–2.91 | <0.001 |
| Marginal structural model (unadjusted) | |||
| Hyperparathyroidism | 2.79 | 1.80–4.34 | <0.001 |
| Hypocalcemia | 1.89 | 1.37–2.60 | <0.001 |
| Hyperphosphatemia | 1.32 | 0.87–2.00 | 0.193 |
CI, confidence interval; HR, hazard ratio.
Table 3.
Comparison between the conventional Cox proportional hazards models and the structural marginal models for the assessment of the association of time-varying hyperparathyroidism, hypocalcemia, and hyperphosphatemia with allograft dysfunction
| Cox proportional hazards model (adjusted) | HR | 95% CI | P value |
|---|---|---|---|
| Hyperparathyroidism | 2.94 | 1.82–4.74 | <0.001 |
| Hypocalcemia | 1.31 | 0.92–1.87 | 0.132 |
| Hyperphosphatemia | 1.94 | 1.28–2.92 | 0.002 |
| Marginal structural model (adjusted) | |||
| Hyperparathyroidism | 2.74 | 1.71–4.38 | <0.001 |
| Hypocalcemia | 1.47 | 1.04–2.09 | 0.030 |
| Hyperphosphatemia | 1.35 | 0.87–2.09 | 0.177 |
CI, confidence interval; HR, hazard ratio.
The models were adjusted for baseline and time-varying risk factors for allograft dysfunction (baseline confounders: primary kidney disease, sex, recipient age, donor source, year of transplantation, disease vintage, dialysis vintage, dialysis mode, graft sequence, cold ischemia time, body mass index Z score, and delayed graft function [stratified until and after 1 year posttransplant]); time-varying confounders: allograft rejection, spline for systolic blood pressure Z score.
Figure 5.
Relationship between eGFR, PTH, and other clinical and biochemical factors whereupon the structural marginal model was based. The red arrows represent the reciprocal relationship between eGFR and PTH. eGFR, estimated glomerular filtration rate; KTx, kidney transplantation; PTH, parathyroid hormone; X, not available for analysis.
Table 4.
Full structural marginal model for the assessment of the association of time-varying hyperparathyroidism with allograft dysfunction, adjusted for baseline and time-varying risk factors for allograft dysfunction
| Full marginal structural modela | HR | 95% CI | P value |
|---|---|---|---|
| Hyperparathyroidism (exposure) | 2.74a | 1.71–4.38a | <0.001a |
| Acute rejection | 6.53a | 3.86–11.06a | <0.001a |
| Era of KTx 2001–2010 | 1.23 | 0.23–6.75 | 0.80 |
| Era of KTx 2010–2019 | 0.47 | 0.08–2.71 | 0.39 |
| Gender | 0.92 | 0.58–1.45 | 0.71 |
| Age at KTx | 0.98 | 0.91–1.04 | 0.39 |
| Donor source (living) | 2.013 | 0.87–4.67 | 0.10 |
| Disease vintage | 1.01a | 1.00–1.01a | 0.03a |
| Dialysis vintage | 1.00 | 0.98–1.01 | 0.32 |
| Dialysis mode (peritoneal) | 1.97a | 1.04–3.72a | 0.04a |
| Dialysis mode (hemodialysis) | 1.13 | 0.54–2.38 | 0.75 |
| Transplant sequence > 1 | 1.50 | 0.58–3.87 | 0.40 |
| Kidney disease (glomerular) | 0.96 | 0.55–1.70 | 0.90 |
| Kidney disease (tubulointerstitial) | 0.93 | 0.55–1.56 | 0.78 |
| Kidney disease (atypical HUS) | 0.54 | 0.13–2.21 | 0.39 |
| Kidney disease (other/unknown) | 0.74 | 0.32–1.66 | 0.4621 |
| Cold ischemia time | 1.00 | 1.00–1.00 | 0.51 |
| Body mass index | 1.093 | 0.88–1.36 | 0.42 |
| Systolic blood pressure Z score (spline)a | NAa | NAa | 0.04a |
| Delayed graft function (first year after KTx) | 3.548a | 1.61–7.84a | 0.002a |
| Delayed graft function (beyond 1 yr from KTx) | 1.034 | 0.26–4.07 | 0.96 |
CI, confidence interval; HR, hazard ratio; HUS, hemolytic uremic syndrome; KTX, kidney transplantation; NA, not applicable.
Baseline confounders: primary kidney disease, sex, recipient age, donor source, year of transplantation, disease vintage, dialysis vintage, dialysis mode, graft sequence, cold ischemia time, body mass index Z score, and delayed graft function stratified until and after 1 year KTx; time-varying confounders: allograft rejection and spline for systolic blood pressure Z score.
Factors associated with allograft dysfunction.
Discussion
To our knowledge, this is the first study that identifies time-varying hyperparathyroidism as an independent risk factor for allograft dysfunction in a large cohort of pediatric KTx recipients, after adjustment for covariates known to affect graft survival such as allograft rejection or systolic blood pressure Z score. The distinctive feature of our analysis is the estimation of the relationship between CKD-MBD parameters and allograft dysfunction by using both the conventional Cox proportional hazards and the marginal structural models. In the latter, eGFR affecting both the outcome and time-varying exposure was considered as time-varying confounder. In case of a reciprocal relationship between time-varying exposure (e.g., hyperparathyroidism or hyperphosphatemia) and time-varying confounder (eGFR), a conventional Cox proportional hazards models may yield biased estimates of the exposure–outcome association.14,18,19 In this study, the marginal structural models showed similar results for hyperparathyroidism as the conventional Cox proportional hazards models, whereas hyperphosphatemia was no longer significant, suggesting that its association with graft dysfunction can be ascribed to a decline in eGFR.
The association between hyperparathyroidism, CKD progression, vascular events, and death has been reported in large observational studies of adult CKD patients before transplantation.20,21 Potential explanations for a causative role of PTH for kidney dysfunction include its promotion of osteoblastic differentiation into endothelial cells leading to vascular calcification22 and its role in the pathogenesis of interstitial calcification.23 For example, in a study on 213 adult kidney transplant recipients, 56 (26.3%) had interstitial calcification in 1 or more protocol biopsies.24 Calcification was not related to donor or recipient characteristics or posttransplant immunologic events, but patients with calcification had significantly higher serum PTH levels. In patients with calcification, high PTH levels correlated with an inferior outcome of graft function at year 1 posttransplant.24
Hyperparathyroidism can easily be detected by routine laboratory assessment and is a modifiable risk factor. The prevalence and clinical consequences of hyperparathyroidism may be distinct in pediatric versus adult KTx recipients given the increased calcium requirements during statural growth, lower pediatric target PTH levels in most European centers and the proportion of patients in whom these targets are attained. The Kidney Disease: Improving Global Outcomes guidelines recommend measurement of PTH in children with CKD stage 2 and above.13 Moreover, in our cohort, we observed higher posttransplant values of PTH than in pretransplant children with comparable eGFR.25 This taken together with our observation that the severity of hyperparathyroidism is related to allograft outcome indicates a need for a PTH surveillance even in patients with a good allograft function. This might require a change of current practice, as PTH was measured only in 33 of 40 centers contributing data to the CERTAIN Registry (see Supplementary Table S1). Factors associated with the persistence of hyperparathyroidism after pediatric KTx include older age at transplantation, longer dialysis vintage, deceased donor KTx, high body mass index, pretransplant hyperparathyroidism, and 25(OH)D deficiency or insufficiency.26, 27, 28 We report an association between time-fixed PTH levels at month 1 posttransplant and long-term allograft outcome. In this early postoperative period, PTH is still closely related to pretransplant values, suggesting that pretransplant PTH control might contribute to posttransplant kidney function. An association between pretransplant PTH and the risk of graft failure has previously been reported in adults.29
Hypovitaminosis D is widely prevalent in pediatric KTx recipients.1,30 In children with pretransplant CKD, the progressive loss of eGFR is inversely associated with 25(OH)D levels.31 Low levels of 25(OH)D have also been linked with an accelerated decline in eGFR in some studies of adult KTx recipients,32,33 but a recent randomized controlled trial showed that cholecalciferol supplementation does not affect eGFR change compared with placebo among incident KTx recipients.34 In our study, hypovitaminosis D was not associated with allograft outcome, suggesting that the effect of PTH may be independent of 25(OH)D levels. A previous study in pediatric kidney transplant recipients also did not observe an association of vitamin D levels with lower kidney function.35
Hypophosphatemia was highly prevalent in our cohort with the peak of 36% at month 1 posttransplant, but it was not related to graft outcome. In contrast, in adult KTx recipients the degree of hypophosphatemia was associated with a lower risk of death-censored graft failure and cardiovascular mortality.36 Interestingly, hypophosphatemia was more pronounced in the adult cohort with 47% of kidney transplant recipients displaying values lower than 0.5 mmol/l within the first 3 months after transplantation. This may reflect the tendency to low-threshold treatment of hypophosphatemia in pediatric patients.37
The strengths of our study include multicenter design, which allowed a robust statistical analysis in the largest cohort of pediatric KTx recipients to date with 5-year follow-up. Data from the academic CERTAIN Registry closely reflect European clinical practice. Our study also has several limitations. Because this is a retrospective registry analysis, we cannot state causalities but only associations. We cannot exclude residual confounding by factors not included in the multivariable analysis. Furthermore, we cannot exclude that a proportion of PTH measurements was performed on indication, such as poor graft function, rather than as routine follow-up. Finally, the available data on 25(OH)D levels were limited.
In conclusion, in this observational cohort study of pediatric KTx recipients, we identified hyperparathyroidism as factor of allograft dysfunction, whereas the association between hyperphosphatemia and allograft outcome was not confirmed in the structural marginal model. Our findings emphasize the need for prospective studies to analyze the potential causal relationship between hyperparathyroidism and allograft dysfunction, to elucidate mechanisms underlying this relationship and to define target PTH levels in pediatric KTx recipients. Interventions aimed at strict PTH control may contribute to preserving allograft function.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to thank all the following contributors to the Cooperative European Paediatric Renal Transplant Initiative Registry for providing patient data: M. Bald (Stuttgart), A. Bouts (Amsterdam), S. Caliskan (Istanbul), K. Heindl-Rusai (Vienna), C. Hempel (Leipzig), M. Kaabak (Moscow), S. Marks (London), H. Nalcacioglu (Samsun), A. Noyan (Adana), L. Peruzzi (Turin), S. Saygılı (Istanbul), K. Sauerstein (Erlangen), T. Seeman (Prague), G. Schalk (Bonn), R. Topaloglu (Hacettepe), S. Testa (Milan), O. Yavascan (Izmir), and F. Yalcinkaya (Ankara).
Funding Statement
This study was supported by a research grant from the European Society for Paediatric Nephrology to AP. The authors gratefully acknowledge the funding of the Cooperative European Paediatric Renal Transplant Initiative Registry by a grant from the Dietmar Hopp Stiftung, the European Society for Paediatric Nephrology, and the German Society for Paediatric Nephrology and by grants from the pharmaceutical companies Astellas and Novartis.
Footnotes
Description of the CERTAIN Registry: Completeness and Quality of Data.
Patient Inclusion per Country.
Figure S1. Time to composite end point in patients with hypophosphatemia at month 1 posttransplant.
Table S1. Center-specific reference values for parathyroid hormone
Appendix
List of Members of Working Groups “Transplantation” and “CKD-MBD” of the European Society for Paediatric Nephrology and the Cooperative European Paediatric Renal Transplant Initiative Research Network
Transplantation Working Group Member List: Gema Ariceta, Atif Awan, Sevcan Bakkaloğlu, Marjolein Bonthuis, Charlotte Bootsma Robroeks, Antonia Bouts, Martin Christian, Marlies Cornelissen, Ali Duzova, Nasrin Esfandiar, Luciana Ghio, Ryszard Grenda, Isabella Guzzo, Maria Herrero Goni, Julien Hogan, Nattaphorn Hongsawong, Nele Kanzelmeyer, Aysun Karabay Bayazit, Gülşah Kaya Aksoy, Noel Knops, Linda Koster Kamphuis, Daniella Levy Erez, Victor Lopez-Baez, Alvaro Madrid, Stephen Marks, Anette Melk, Luisa Murer, Lars Pape, Licia Peruzzi, Edita Petrosyan, Evgenia Preka, Nikoleta Printza, Andreea Liana Rachisan, Ann Raes, Mohan Shenoy, Oguz Soylemezoglu, Luca Dello Strologo, Ana Teixeira, Rezan Topaloglu, Markus Weitz, Jakub Zieg, Galia Zlatanova, Christian Patry, Jerome Harambat.
CKD Mineral and Bone Disorder (CKD-MBD) Working Group Member List: Ayşe Ağbaş, Varvara Askiti, Marina Avramescu, Justine Bacchetta, Sevcan Bakkaloglu, Marjolein Bontuis, Caroline Booth, Laurene Dehoux, Giacomo Dizazzo, Dorota Drozdz, Ismail Dursun, Michaela Gessner, Jaap Groothoff, Giuliana Guido, Isabella Guzzo, Aysun Karabay Bayazit, Guenter Klaus, Linda Koster-Kamphuis, Alexander Lalayiannis, Maren Leifheit-Nestler, Sinha Manish, Chiara Matteucci, Jun Oh, Ozan Ozkaya, Edita Petrosyan, Christine Pietrement, Agnieszka Prytula, George Reusz, Franz Schaefer, Claus Peter Schmitt, Anne Schön, Fatma Lale Sever, Stella Stabouli, Serra Sürmeli Döven, Camilla Tondel, Enrico Verrina, Enrico Vidal, Dean Wallace, Zainab Arslan.
Supplementary Material
Description of the CERTAIN Registry: Completeness and Quality of Data.
Patient Inclusion per Country.
Figure S1. Time to composite end point in patients with hypophosphatemia at month 1 posttransplant.
Table S1. Center-specific reference values for parathyroid hormone.
References
- 1.Haffner D., Leifheit-Nestler M. CKD-MBD post kidney transplantation. Pediatr Nephrol. 2021;36:41–50. doi: 10.1007/s00467-019-04421-5. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen H.S., Behets G., Bammens B., et al. Natural history of bone disease following kidney transplantation. J Am Soc Nephrol. 2022;33:638–652. doi: 10.1681/ASN.2021081081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonthuis M., Busutti M., van Stralen K.J., et al. Mineral metabolism in European children living with a renal transplant: a European Society for Paediatric Nephrology/European Renal Association-European Dialysis and Transplant Association Registry study. Clin J Am Soc Nephrol. 2015;10:767–775. doi: 10.2215/CJN.06200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evenepoel P., Van Den Bergh B., Naesens M., et al. Calcium metabolism in the early posttransplantation period. Clin J Am Soc Nephrol. 2009;4:665–672. doi: 10.2215/CJN.03920808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wesseling-Perry K., Pereira R.C., Tsai E., et al. FGF23 and mineral metabolism in the early post-renal transplantation period. Pediatr Nephrol. 2013;28:2207–2215. doi: 10.1007/s00467-013-2547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drueke T.B., Massy Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289–302. doi: 10.1016/j.kint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Denburg M.R., Kumar J., Jemielita T., et al. Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J Am Soc Nephrol. 2016;27:543–550. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakkaloglu S.A., Bacchetta J., Lalayiannis A.D., et al. Bone evaluation in paediatric chronic kidney disease: clinical practice points from the European Society for Paediatric Nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol Dial Transplant. 2021;36:413–425. doi: 10.1093/ndt/gfaa210. [DOI] [PubMed] [Google Scholar]
- 9.Shroff R., Long D.A., Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 10.Shroff R. Phosphate is a vascular toxin. Pediatr Nephrol. 2013;28:583–593. doi: 10.1007/s00467-012-2347-x. [DOI] [PubMed] [Google Scholar]
- 11.Podesta M.A., Cucchiari D., Ciceri P., et al. Cardiovascular calcifications in kidney transplant recipients. Nephrol Dial Transplant. 2021;37:2063–2071. doi: 10.1093/ndt/gfab053. [DOI] [PubMed] [Google Scholar]
- 12.van der Plas W.Y., Gomes Neto A.W., Berger S.P., et al. Association of time-updated plasma calcium and phosphate with graft and patient outcomes after kidney transplantation. Am J Transplant. 2020;21:2437–2447. doi: 10.1111/ajt.16457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketteler M., Block G.A., Evenepoel P., et al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92:26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Robins J.M., Hernan M.A., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Vieira J.G. PTH assays: understanding what we have and forecasting what we will have. J Osteoporos. 2012;2012:523246. doi: 10.1155/2012/523246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz G.J., Munoz A., Schneider M.F., et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therneau T.M., Grambsch P.M. 1st ed. Springer; New York: 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 18.Park S., Kang E., Park S., et al. Metabolic acidosis and long-term clinical outcomes in kidney transplant recipients. J Am Soc Nephrol. 2017;28:1886–1897. doi: 10.1681/ASN.2016070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E.D., Famure O., Li Y., Kim S.J. Uric acid and the risk of graft failure in kidney transplant recipients: a re-assessment. Am J Transplant. 2015;15:482–488. doi: 10.1111/ajt.13000. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y., Evans M., Soro M., et al. Secondary hyperparathyroidism and adverse health outcomes in adults with chronic kidney disease. Clin Kidney J. 2021;14:2213–2220. doi: 10.1093/ckj/sfab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozic M., Diaz-Tocados J.M., Bermudez-Lopez M., et al. Independent effects of secondary hyperparathyroidism and hyperphosphatemia on chronic kidney disease progression and cardiovascular events: an analysis from the NEFRONA cohort. Nephrol Dial Transplant. 2021;37:663–672. doi: 10.1093/ndt/gfab184. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Z.Y., Ye T., Ling Q.Y., et al. Parathyroid hormone promotes osteoblastic differentiation of endothelial cells via the extracellular signal-regulated protein kinase 1/2 and nuclear factor-kappaB signaling pathways. Exp Ther Med. 2018;15:1754–1760. doi: 10.3892/etm.2017.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berchtold L., Ponte B., Moll S., et al. Phosphocalcic markers and calcification propensity for assessment of interstitial fibrosis and vascular lesions in kidney allograft recipients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwinner W., Suppa S., Mengel M., et al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. 2005;5:1934–1941. doi: 10.1111/j.1600-6143.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 25.Portale A.A., Wolf M., Juppner H., et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9:344–353. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda-Abedini M., Portale A.A., Shah A., et al. Persistent secondary hyperparathyroidism after renal transplantation in children. Pediatr Nephrol. 2006;21:413–418. doi: 10.1007/s00467-005-2113-4. [DOI] [PubMed] [Google Scholar]
- 27.Guzzo I., Di Zazzo G., Laurenzi C., et al. Parathyroid hormone levels in long-term renal transplant children and adolescents. Pediatr Nephrol. 2011;26:2051–2057. doi: 10.1007/s00467-011-1896-8. [DOI] [PubMed] [Google Scholar]
- 28.Vanderstraeten K., De Pauw R., Knops N., et al. Body mass index is associated with hyperparathyroidism in pediatric kidney transplant recipients. Pediatr Nephrol. 2021;36:977–986. doi: 10.1007/s00467-020-04796-w. [DOI] [PubMed] [Google Scholar]
- 29.Roodnat J.I., van Gurp E.A., Mulder P.G., et al. High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation. 2006;82:362–367. doi: 10.1097/01.tp.0000228923.75739.88. [DOI] [PubMed] [Google Scholar]
- 30.Tuchman S., Kalkwarf H.J., Zemel B.S., et al. Vitamin D deficiency and parathyroid hormone levels following renal transplantation in children. Pediatr Nephrol. 2010;25:2509–2516. doi: 10.1007/s00467-010-1612-0. [DOI] [PubMed] [Google Scholar]
- 31.Shroff R., Aitkenhead H., Costa N., et al. Normal 25-hydroxyvitamin D Levels are associated with less proteinuria and attenuate renal failure progression in children with CKD. J Am Soc Nephrol. 2016;27:314–322. doi: 10.1681/ASN.2014090947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obi Y., Hamano T., Ichimaru N., et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:527–535. doi: 10.1210/jc.2013-2421. [DOI] [PubMed] [Google Scholar]
- 33.Keyzer C.A., Riphagen I.J., Joosten M.M., et al. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J Clin Endocrinol Metab. 2015;100:81–89. doi: 10.1210/jc.2014-3012. [DOI] [PubMed] [Google Scholar]
- 34.Doi Y., Tsujita M., Hamano T., et al. The effect of cholecalciferol supplementation on allograft function in incident kidney transplant recipients: a randomized controlled study. Am J Transplant. 2021;21:3043–3054. doi: 10.1111/ajt.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosca M., Lion-Lambert M., Bienaime F., et al. Association between 25(OH) vitamin D and graft survival in renal transplanted children. Pediatr Transplant. 2020;24 doi: 10.1111/petr.13809. [DOI] [PubMed] [Google Scholar]
- 36.van Londen M., Aarts B.M., Deetman P.E., et al. Post-transplant hypophosphatemia and the risk of death-censored graft failure and mortality after kidney transplantation. Clin J Am Soc Nephrol. 2017;12:1301–1310. doi: 10.2215/CJN.10270916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakhaee K. Post-renal transplantation hypophosphatemia. Pediatr Nephrol. 2010;25:213–220. doi: 10.1007/s00467-009-1252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Wal W.M., Geskus R.B. ipw: An R package for inverse probability weighting. J Stat Softw. 2011;43:1–23. [Google Scholar]
- 39.Therneau T. A Package for Survival Analysis in R_. R package version 3.3-1. 2022. https://CRAN.R-project.org/package=survival
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.