Abstract
Clonal Borrelia burgdorferi N40 (cN40) passaged 75 times in vitro (N40-75) infects mice but does not cause disease. N40-75 passaged 45 times further in vitro (N40-120) was no longer infectious and lacked genes encoded on linear plasmids 38 and 28-1, among other differences. These data suggest that B. burgdorferi cN40, N40-75, and N40-120 have distinct phenotypes that can be used to dissect the genetic elements responsible for pathogenicity and infectivity.
Lyme borreliosis, which is caused by Borrelia burgdorferi, can result in infection and disease involving the skin, joints, and heart, both in humans and in experimental models (2, 3, 18, 27). Comparisons of B. burgdorferi isolates cultured in vitro have helped to identify B. burgdorferi genes that may be associated with infectivity. Noninfectious B. burgdorferi isolates have been shown to lack, or have reduced expression of or mutations in, ospB, ospD, ospC, or vls, among other genes, and the loss of several plasmids, including linear plasmid (lp) 25, lp28.4, and circular plasmid 9 (5, 16, 19, 20, 24, 25, 28, 31–34). A direct cause and effect relationship has not yet been established for any single gene or plasmid: for example, some noninfectious B. burgdorferi B31 clones express ospD and other infectious clones do not (15, 19–21). Recent studies have also suggested an important role for lp28-1 in B. burgdorferi infectivity (14, 22). The genetic factors that contribute to B. burgdorferi infectivity and pathogenicity (i.e., the ability to cause arthritis and carditis) are likely to be both multifactorial and distinct.
Several B. burgdorferi isolates that were initially nonclonal have been shown to lose infectivity fairly quickly upon in vitro passage (19, 20, 25). In contrast, a clonal isolate of B. burgdorferi N40 (cN40) that is highly pathogenic in C3H/HeN (C3H) mice, causing severe arthritis and carditis, retains infectivity after multiple passages (1). However, cN40 passaged 75 times in vitro (N40-75) and individual N40-75 clones were infectious but nonpathogenic in mice, thereby enabling us to begin to dissociate these two processes (1). N40-75 also remained nonpathogenic when passaged one time through immunocompetent mice (data not shown), but the stability of this phenotype in vivo must be explored further, because N40-75 can cause disease in severe combined immunodeficient (SCID) mice (1). The inability of N40-75 to cause arthritis and carditis in immunocompetent mice has been associated with the lack of expression of several genes that are preferentially expressed in vivo rather than the loss of specific genetic material in DNA analyses or of antigens in protein profiles or immunoblots (1). We have now passaged N40-75 further to develop specific derivatives of the spirochete that are noninfectious in mice and that can be used to understand the differences that contribute to both the infectivity and pathogenicity of B. burgdorferi.
N40-75 passaged in vitro retained infectivity in C3H mice until passage 120 (N40-120). Spirochetes were passaged by cultivation in Barbour-Stoenner-Kelly II medium as described previously (1). Every five passages, the spirochetes were tested for their ability to infect mice; representative passaged isolates (i.e., N40-90, N40-105, and N40-120) are shown in Table 1. When B. burgdorferi was noted to lose infectivity (N40-120), the previous five individual passaged isolates were then also tested for infectivity, such as N40-119 (Table 1). Mice were intradermally inoculated with 104 N40-120 spirochetes in the dorsal midthorax, and 2 weeks later, skin, bladder, heart, and joints were examined for B. burgdorferi by using PCR, culture, and histopathology (Table 1) (1). Infection or disease was not evident in mice challenged with N40-120. Five individual N40-120 clones were also noninfectious in mice (data not shown). N40-120 remained noninfectious even when 107 organisms were injected into C3H mice or when spirochetes were administered to immunodeficient C3H-scid mice.
TABLE 1.
Noninfectious derivatives of B. burgdorferi N40
| B. burgdorferi derivative (dose)a | Mouse strain | Incidence of infectiond | Incidence of diseasee |
|---|---|---|---|
| cN40b (104) | C3H | 5/5 | 5/5 |
| N40-75c (104) | C3H | 5/5 | 0/5 |
| N40-90c (104) | C3H | 4/5 | 0/5 |
| N40-105c (104) | C3H | 5/5 | 0/5 |
| N40-119c (104) | C3H | 4/5 | 0/5 |
| N40-120c (104) | C3H | 0/5 | 0/5 |
| C3H-scid | 0/5 | 0/5 | |
| N40-120c (107) | C3H | 0/5 | 0/5 |
C3H mice were inoculated with either 104 or 107 organisms in the midline of the back.
cN40 is the initial clonal isolate of B. burgdorferi N40.
N40-75 is the described infectious but nonpathogenic cN40 derivative (1). N40-90, N40-105, N40-119, and N40-120 have been passaged 90, 105, 119, and 120 times, respectively.
Infection was evaluated by PCR and/or culture at 2 weeks following inoculation; data are number infected/number of mice exposed.
Joints and hearts were histopathologically examined for inflammation; data are number of mice with evidence of disease/number of mice exposed.
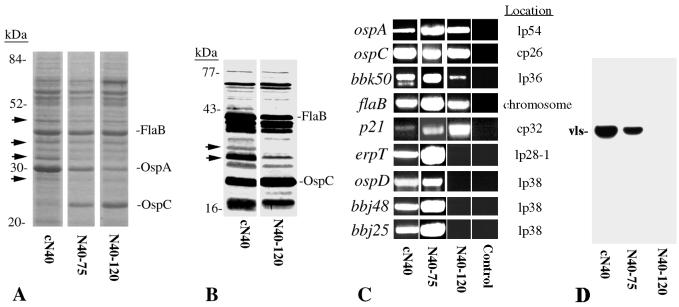
To identify the factors that correlate with infectivity of N40-120, protein profiles of cN40 and N40-120 were compared (Fig. 1A). Previous studies have demonstrated that consistent differences between cN40 and N40-75 were not apparent from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or immunoblotting, in part because subclones of N40-75 had variable patterns of protein expression (1). Several protein bands were somewhat less intense in N40-120 than in cN40 on Coomassie blue-stained SDS–12% PAGE gels (Fig. 1A). In addition, cN40 had several bands between 26 to 35 kDa that were not as evident in N40-120 upon immunoblotting using sera from B. burgdorferi cN40-infected mice (Fig. 1B). These studies suggested that specific genetic elements might have been lost in N40-120.
FIG. 1.
Characterization of noninfectious B. burgdorferi N40-120. (A) Coomassie blue-stained SDS-PAGE gel of cN40, N40-75, and N40-120. (B) Immunoblot of cN40 and N40-120 probed with sera from B. burgdorferi cN40-infected mice (needle inoculation with 104 spirochetes). Some potential differences are shown with arrows. (C) PCR analysis of DNA from cN40, N40-75, and N40-120. PCR was performed with primers specific for ospA, ospC, bbk50, flaB, p21, erpT, ospD, bbj48, and bbj25. Amplified DNA was visualized by ethidium bromide incorporation. Reactions in which water served as the template were used as a control (Control). (D) Southern blot of cN40, N40-75, and N40-120 probed with horseradish peroxidase-labeled vls DNA fragment.
PCR of selected B. burgdorferi genes demonstrated the loss of some genes that are either antigenic or have been associated with infectivity. PCR was performed for 30 cycles with primers specific for each gene, as described previously (1). Several genes, including ospA (which is down-regulated during tick engorgement [6, 8]), ospC (which is up-regulated during tick feeding [26]), bbk50 (which is preferentially expressed in vivo [11]), flaB (which is involved in skeletal structure and motility [17]), and p21 (a member of the ospE-related protein [erp] gene family [7]), were present in cN40, N40-75, and N40-120 (Fig. 1C). The amplified p21 PCR product was lower in intensity in cN40 than in N40-120 but was always detectable in repeated assays. The ospD gene, which is on lp38 and encodes a 28-kDa protein, was not present in N40-120 (Fig. 1C). bbj25 and bbj48 (13), which are two additional genes on lp38, were also not evident in N40-120, suggesting that this plasmid had been lost in N40-120 (Fig. 1C). Previous studies have shown that infectivity does not correlate strictly with the presence of ospD (15, 19–21). We therefore assessed whether erpT, a gene that is preferentially expressed in deep tissues and is present on the vls-containing plasmid lp28-1, was present in N40-120 (10, 12, 13). erpT DNA was not detected in N40-120 (Fig. 1C). The vls locus has been strongly correlated with infectivity (33, 34), and Southern blotting revealed that N40-120 lacked the vls gene locus, which is consistent with the loss of lp28-1 (Fig. 1D). PCR and Southern blotting demonstrated that N40-119 contained genes located on lp28-1 and lp38 (results not shown), indicating that these two plasmids were lost during the subsequent passage. These data provide initial insight into some of the genetic differences between cN40 and N40-120, but they are not meant to be comprehensive.
We have demonstrated that passaged isolates of B. burgdorferi N40—cN40, N40-75, and N40-120—can be distinguished with respect to infectivity and pathogenicity. The number of passages required to generate N40 spirochetes with alterations in infectivity suggests that previously infectious organisms can be converted into noninfectious spirochetes by the loss of genetic material but that conversion does not occur equally during all passages. These B. burgdorferi N40 descendants may provide new opportunities for targeted genetic manipulation of the spirochete that can then be studied in the murine model. Initial studies have demonstrated that cN40 and infectious clones derived from cN40 can be transformed by electroporation, although the frequency of transformation was approximately 100-fold lower than what was observed with a noninfectious clone of B. burgdorferi B31 (P. Rosa, unpublished results). Transformation of B. burgdorferi has previously only been demonstrated in noninfectious B. burgdorferi B31 (4, 9, 23, 29). Recently, Stewart and his colleagues succeeded in transforming infectious spirochetes with a shuttle vector capable of autonomous replication in B. burgdorferi (30). These developments should facilitate efforts to define the contributions of specific B. burgdorferi genes to infection and disease.
The factors contributing to the ability of B. burgdorferi to infect the mammalian host and to cause specific disease manifestations, such as arthritis and carditis, are likely to be multifactorial. Both the diversity of the host response to the spirochete and the virulence of the incoming B. burgdorferi isolate may influence the outcome of exposure to this pathogen. B. burgdorferi cN40, N40-75, and N40-120 have well-defined phenotypes in the experimental murine model of Lyme disease. These organisms should prove useful for distinguishing the spirochete genotypes that are important for B. burgdorferi persistence throughout its life cycle and for the ability of this organism to cause disease that affects selected organ systems.
Acknowledgments
Grants from the National Institutes of Health, Arthritis Foundation, and American Heart Association and a gift from SmithKline Beecham Biologicals supported this work. E. Fikrig is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
REFERENCES
- 1.Anguita J, Samanta S, Revilla B, Suk K, Das S, Barthold S W, Fikrig E. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect Immun. 2000;68:1222–1230. doi: 10.1128/iai.68.3.1222-1230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi: dissemination and evolution of disease following intradermal inoculation of mice. Am J Pathol. 1991;163:263–273. [PMC free article] [PubMed] [Google Scholar]
- 4.Bono J L, Elias A F, Kupko J J, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll J A, Gherardini F C. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect Immun. 1996;64:392–398. doi: 10.1128/iai.64.2.392-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Barthold S W, Giles S S, Montgomery R R, Telford S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias A F, Bono J L, Carroll J A, Stewart P, Tilly K, Rosa P. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J Bacteriol. 2000;182:2909–2918. doi: 10.1128/jb.182.10.2909-2918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Hodzic E, Barthold S W. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect Immun. 2000;68:4169–4173. doi: 10.1128/iai.68.7.4169-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 12.Fikrig E, Chen M, Barthold S W, Anguita J, Feng W, Telford S R, Flavell R A. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol Microbiol. 1999;31:281–290. doi: 10.1046/j.1365-2958.1999.01171.x. [DOI] [PubMed] [Google Scholar]
- 13.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 14.Labandeira-Rey M, Skare J T. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuzawa T, Kurita T, Kawabata H, Yanagihara Y. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol Lett. 1994;123:319–324. doi: 10.1111/j.1574-6968.1994.tb07242.x. [DOI] [PubMed] [Google Scholar]
- 17.Motaleb M A, Corum L, Bono J L, Elias A F, Rosa P, Samuels D S, Charon N W. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci USA. 2000;97:10899–10904. doi: 10.1073/pnas.200221797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadelman R B, Wormser G P. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 19.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris S J, Howell J K, Garza S A, Ferdows M S, Barbour A G. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun. 1995;63:2206–2212. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Probert W S, Lefebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB or OspC but not OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purser J E, Norris S J. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa P, Samuels D S, Hogan D, Stevenson B, Casjens S, Tilly K. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J Bacteriol. 1996;178:5946–5953. doi: 10.1128/jb.178.20.5946-5953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadziene A, Barbour A G, Rosa P A, Thomas D D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiler K P, Weis J J. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 28.Simpson W J, Garon C F, Schwan T G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson B, Bono J L, Elias A, Tilly K, Rosa P. Transformation of the Lyme disease spirochete Borrelia burgdorferi with heterologous DNA. J Bacteriol. 1998;180:4850–4855. doi: 10.1128/jb.180.18.4850-4855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart P, Thalken R, Bono J, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–722. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Goldberg M S, Popova T G, Schoeler G B, Wikel S K, Hagman K E, Norgard M V. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J R, Norris S J. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]