Abstract
Solitary fibrous tumor (SFT) is a rare tumor of mesenchymal origin that most commonly involves the pleura but can be found anywhere in the body. SFT can range from indolent benign tumors to aggressive malignant tumors, and pre-operative diagnosis is based mainly on imaging. In this case report, we describe an extremely rare case of SFT of left maxilla on F-18 FDG PET-CT and Ga-68 DOTANOC PET-CT. Very few cases of PET-CT findings in SFT are reported in literature; and to our knowledge, none involving the maxilla. By highlighting the findings on F-18 FDG and GA-68 DOTANOC PET-CT, we aim to further add on to the role of both the tracers in the diagnosis and management of this tumor group.
Keywords: Solitary fibrous tumor, FDG PET-CT, DOTANOC PET-CT, Positron emission tomography
Introduction
Solitary fibrous tumor (SFT) is a rare mesenchymal neoplasm that makes up for less than 2% of all soft tissue tumors [1]. SFT most commonly affects adults and ranges from indolent tumors to more aggressive masses. Hemangiopericytoma is included as a phenotypic variant within the spectrum of solitary fibrous tumor. Histologically, SFT is reactive for CD34 and STAT6 (most sensitive and specific) and has a dilated “staghorn”-like vascular network [2–4]. It may be found anywhere in the body and is divided into two entities: pleural and extrapleural [5]. Pre-operative diagnosis is mainly done by imaging. There are very few descriptions of positron emission tomography-computed tomography (PET-CT) findings of SFT in literature, majority being pleural SFT (SFTP), limited to case reports and case series [6–9]. A case series described 5 patients with SFTP imaged with Ga-68 DOTATOC. All tumors showed uptake of G-68 DOTATOC, indicating somatostatin receptor (SSTR) expression by these tumors [6]. Herein, we describe the PET-CT findings of an extremely rare case of SFT of maxilla imaged with two PET tracers, F-18 fluorodeoxyglucose (FDG) and Ga-68 DOTANOC. To our knowledge, there is no description of PET-CT manifestations of maxillary SFT in literature or any report comparing the SFT uptake of these two PET tracers. We aim to describe the PET-CT findings of this tumor, highlighting the imaging results with both PET tracers; thereby, aiding in the diagnosis and management of the tumor group.
Case report
A 32-year-old female presented with complaints of left nasal obstruction, left-sided facial pain, left eye watering, and left-sided heaviness in the face. Magnetic resonance imaging (MRI) revealed a heterogeneous necrotic mass lesion measuring 5.3 cm × 6.6 cm × 5.4 cm in the left maxillary sinus. Excision biopsy yielded histopathological features of non-specific inflammation. The patient then underwent endoscopic and sub-labial excision of the mass. Post-operative histopathological examination (HPE) of the excised tumor tissue revealed features suggestive of solitary fibrous tumor. Immunohistochemistry (IHC) was positive for STAT6. Following surgery, the patient was asymptomatic for a period of 7 months, after which she became symptomatic again. Contrast-enhanced CT (CECT) revealed a large well-defined heterogeneously enhancing lesion measuring 5.6 cm × 5.2 cm × 4 cm in the left maxillary sinus, involving the left pterygopalatine fossa, left infratemporal fossa, and left nasal cavity with extensive bony erosions. HPE of biopsied tissue from left nasal cavity and maxillary sinus mass revealed features suggestive of osteogenic sarcoma. HPE review of the biopsy tissue block was suggestive of solitary fibrous tumor with areas of hyalinization (Fig. 1a). Tumor cells were immunopositive for STAT6 (nuclear) (Fig. 1b) while negative for CD34, SMA, and desmin. There were no areas of dedifferentiation. F-18 FDG PET-CT, done for restaging the tumor, revealed metabolically active heterogeneously enhancing mass involving the left masticator space and left infratemporal fossa with destruction of the left maxillary sinus (Fig. 2). The SUVmax of the tumor was 5.13. No other metabolically active lesions were seen in the body. On multi-disciplinary discussion, the tumor was found to be unresectable. Patient was started on Axitinib and Temozolamide 1 week after FDG PET-CT and showed clinical benefit with reduction in pain and swelling on follow up visit 1 week later. The patient was referred for a Ga-68 DOTANOC PET-CT, which was done after 2 weeks of F-18 FDG PET-CT. Ga-68 DOTANOC PET-CT revealed a tracer avid soft tissue density left sino-nasal mass occupying left maxillary sinus region and left infratemporal fossa with lytic destruction of left side of maxilla (Fig. 2). The SUVmax of tumor was 6.21. Tumor to background ratio (TBRmax) was calculated in both scans using the SUVmax of the tumor and the SUVmax of mediastinal blood pool as background. The TBRmax in F-18 FDG PET-CT was 2.44 while the TBRmax in Ga-68 DOTANOC PET-CT was 8.87.
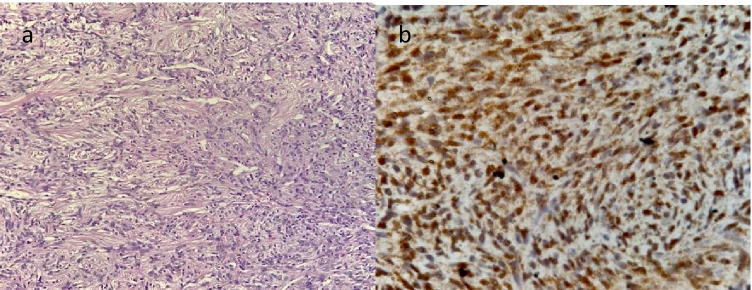
Fig. 1.
Histology section (H and E, 200 ×) of the tumor showing cells arranged in vague fascicles with traversing hemangiopericytoma-like thin-walled vascular channels. Tumor cells show mild to moderate atypia with finely dispersed chromatin and ropy collagen in background (a). Immunostain (400 ×) for STAT6 showing nuclear immunoreactivity in tumor cells (b)
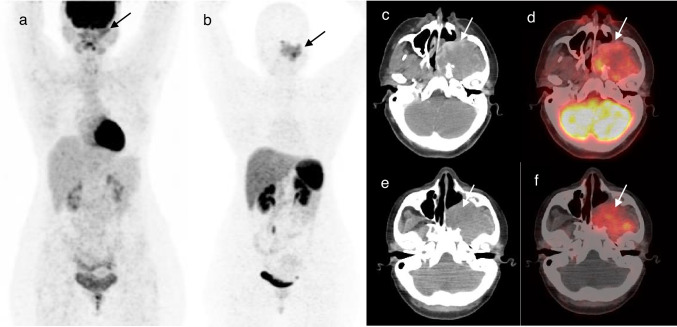
Fig. 2.
Maximum intensity projection (MIP) images showing metabolically active mass (arrow) in the left maxillary region on F-18 FDG PET-CT (a) which shows tracer avidity (arrow) on Ga-68 DOTANOC PET-CT (b). Axial CT (c) and axial fused PET-CT (d) images of F-18 FDG PET-CT showing a metabolically active heterogeneously enhancing mass (arrows) involving the left maxillary sinus and left infratemporal fossa. Axial CT (e) and axial fused PET-CT (f) images of Ga-68 DOTANOC PET-CT showing the same mass (arrows) with tracer avidity
Discussion
Solitary fibrous tumors most commonly involve the pleura while other less common sites include peritoneum, liver, spleen, extremities, and retroperitoneum. Very rarely, SFT originates in the intracranial or extracranial head and neck regions. The differential diagnosis on imaging depends on the location of the tumor and mainly includes meningioma, soft tissue sarcoma, and neurogenic tumor. The clinical presentation is related to the mass effect caused by this tumor; therefore, tumors involving head and neck tend to present earlier than those involving pleura and abdomen [4]. Head and neck SFT tend to have a good prognosis and are more likely to be benign than SFT in other sites.
Radiographs and CT alone generally yield non-specific findings for SFT. On MRI, low-signal-intensity foci on T1- and T2-weighted images, representing the collagen content, is a frequent and unifying feature of SFT. Being a highly vascular tumor, SFT is avidly enhancing on both CT and MRI. This combination gives a “chocolate chip cookie” appearance, which can help in pre-operative diagnosis. Whole body imaging with PET-CT can be performed to look for multiple SFT’s and to detect metastatic lesions in case of malignant SFT. Increased uptake of F-18 FDG, which corresponds to glucose metabolism, may indicate aggressive or malignant variety of SFT [10]. Complete resection is the treatment of choice for these tumors while chemotherapy can be given for unresectable tumors.
In this case report, F-18 FDG PET-CT showed good uptake in the primary tumor and no other lesions were detected (Fig. 2). Thus, F-18 FDG PET-CT helped in staging of this tumor. Considering the surgical unresectability of the tumor in this young female patient, the limited maximal potential reduction in tumor bulk offered by chemotherapeutic drugs in SFT [11] and SSTR expression shown by SFT on Ga-68 DOTATOC scans in literature [6], we wanted to explore additional therapeutic options such as peptide receptor radionuclide therapy (PRRT). Therefore, the patient was referred for a Ga-68 DOTANOC PET-CT, which showed SSTR expression in the tumor tissue resulting in increased tracer uptake (Fig. 2). Although Ga-68 DOTANOC PET-CT was done 2 weeks after F-18 FDG PET-CT and 1 week after starting Axitinib and Temozolamide, which could have affected the uptake on Ga-68 DOTANOC PET-CT, both SUVmax and TBRmax were higher in DOTANOC PET-CT compared to FDG PET-CT. These factors, coupled with lesser physiological uptake of G-68 DOTANOC in brain and nasopharyngeal region compared to F-18 FDG, helped in better tumor delineation with Ga-68 DOTANOC PET-CT. Since SFT’s can occur anywhere in the body, it could be hypothesized that Ga-68 DOTANOC may be a better PET tracer than F-18 FDG, especially when the tumor is arising from or near regions where F-18 FDG uptake is physiologically high. Similar to F-18 FDG, Ga-68 DOTANOC can also help in detecting other lesions and metastatic spread of SFT; however; the lesion detectability rate may be higher owing to the higher tumor to background ratio of SFT in Ga-68 DOTANOC PET-CT. It should be noted that the HPE revealed no areas of dedifferentiation in this case which could be the reason behind better DOTANOC uptake compared to FDG as metabolic activity is generally higher in more aggressive and undifferentiated tumors [10]. It is also important to note that SFT should be considered as a rare differential diagnosis while reporting Ga-68 DOTANOC PET-CT in patients with the relevant clinical and imaging features. Furthermore, SSTR expression of SFT warrants further studies on the role of radionuclide therapy for unresectable SFT as one of the management options.
Author Contribution
The study was designed by SAS. First draft of the manuscript was written by SP. Histopathology and immunohistochemistry images and their descriptions were provided by AB. The draft of the manuscript was reviewed by SAS and SR. All authors read and approved the final manuscript.
Data Availability
Contact the corresponding author for data requests.
Declarations
Competing Interests
Sneha Prakash, Shamim Ahmed Shamim, Sameer Rastogi, and Adarsh Barwad declare that they have no conflict of interest.
Ethics Approval
The study was approved by the institutional review board of All India Institute of Medical Sciences. Informed consent was obtained from the participant in the study. All procedures performed in the study were in accordance with the Helsinki declaration as revised in 2013 and its later amendments.
Consent to Participate
The patient has consented to the submission of the case report to the journal.
Consent for Publication
The patient has consented to the submission of the case report to the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sneha Prakash, Email: sneha.4teen@gmail.com.
Shamim Ahmed Shamim, Email: sashamim2002@gmail.com.
Sameer Rastogi, Email: samdoc_mamc@yahoo.com.
Adarsh Barwad, Email: adawad@gmail.com.
References
- 1.Davanzo B, Emerson RE, Lisy M, Koniaris LG, Kays JK. Solitary fibrous tumor. Transl Gastroenterol Hepatol. 2018;3:94. doi: 10.21037/tgh.2018.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chick JFB, Chauhan NR, Madan R. Solitary fibrous tumors of the thorax: nomenclature, epidemiology, radiologic and pathologic findings, differential diagnoses, and management. Am J Roentgenol. 2013;200:W238–W248. doi: 10.2214/AJR.11.8430. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 4.Tariq MU, Din NU, Abdul-Ghafar J, Park YK. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn Pathol. 2021;16:32. doi: 10.1186/s13000-021-01095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruzzone A, Varaldo M, Ferrarazzo C, Tunesi G, Mencoboni M. Solitary fibrous tumor. Rare Tumors. 2010;2:e64. doi: 10.4081/rt.2010.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lococo F, Rufini V, Filice A, Paci M, Rindi G. 68Ga-DOTATOC PET/CT in pleural solitary fibrous tumors. Clin Nucl Med. 2021;46:e336–e338. doi: 10.1097/RLU.0000000000003570. [DOI] [PubMed] [Google Scholar]
- 7.Shinya T, Masaoka Y, Sando M, Tanabe S, Okamoto S, Ihara H, et al. Imaging an intrapulmonary solitary fibrous tumor with CT and F-18 FDG PET/CT. Radiol Case Rep. 2019;14:755–758. doi: 10.1016/j.radcr.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R, Liu Q, Sui H, Zhang M, Zhu Z, Cui R. 68Ga-FAPI outperforms 18F-FDG PET/CT in identifying solitary fibrous tumor. Eur J Nucl Med Mol Imaging. 2021;48:2055–2056. doi: 10.1007/s00259-020-05181-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Zhu W, Zhou X, Yao H, Su J, Ouyang X, et al. 18F-FDG PET/CT imaging findings of multiple solitary fibrous tumor: a case report. Medicine (Baltimore) 2019;98:e16743. doi: 10.1097/MD.0000000000016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. Am J Roentgenol. 2011;196:487–495. doi: 10.2214/AJR.10.4948. [DOI] [PubMed] [Google Scholar]
- 11.de Bernardi A, Dufresne A, Mishellany F, Blay JY, Ray-Coquard I, Brahmi M. Novel therapeutic options for solitary fibrous tumor: antiangiogenic therapy and beyond. Cancers. 2022;14:1064. doi: 10.3390/cancers14041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Contact the corresponding author for data requests.