Abstract
The Pseudomonas aeruginosa major constitutive outer membrane porin protein OprF, which has previously been shown to be a protective antigen, was targeted as a DNA vaccine candidate. The oprF gene was cloned into plasmid vector pVR1020, and the plasmid vaccines were delivered to mice by biolistic (gene gun) intradermal inoculation. Antibody titers in antisera from immunized mice were determined by enzyme-linked immunosorbent assay, and the elicited antibodies were shown to be specifically reactive to OprF by immunoblotting. The immunoglobulin G (IgG) immune response was predominantly of the IgG1 isotype. Sera from DNA vaccine-immunized mice had significantly greater opsonic activity in opsonophagocytic assays than did sera from control mice. Following the initial immunization and two consecutive boosts, each at 2-week intervals, protection was demonstrated in a mouse model of chronic pulmonary infection by P. aeruginosa. Eight days postchallenge, both lungs were removed and examined. A significant reduction in the presence of severe macroscopic lesions, as well as in the number of bacteria present in the lungs, was seen. Based on these findings, genetic immunization with oprF has potential for development as a vaccine to protect humans against infection by P. aeruginosa.
Pseudomonas aeruginosa, an opportunistic pathogen, is a leading cause of life-threatening infections in immunocompromised individuals. It is an etiological agent of bacteremia, urinary tract infections, and pneumonia in such patients. The mortality from bacteremia and pneumonia caused by P. aeruginosa infections can exceed 50%. Each year, over two million patients develop hospital-associated infections, and an estimated 88,000 patients die as a result. A report on nosocomial infection surveillance places P. aeruginosa among the three most frequently reported nosocomial pathogens (26). P. aeruginosa is also the cause of chronic, serious pulmonary infection in cystic fibrosis patients. Recent reports list P. aeruginosa among the most serious antibiotic-resistant bacteria and one for which effective vaccines are needed (11, 42).
The observation that genetic immunization, more commonly referred to as DNA vaccination, is able to elicit an immune response has fostered a new generation in vaccine development (37, 49, 50). Production of an effective immune response against selected target antigens has been successfully demonstrated using recombinant retroviral vectors, encapsulation of DNA in liposomes, DNA-coated gold particles introduced by particle bombardment (29, 54), and pure plasmid DNA (naked DNA) injected into muscle tissue in mice (12, 49). DNA immunization has been used to elicit protective antibody and cell-mediated immune responses in a wide variety of preclinical animal models for viral and bacterial diseases (14, 15). The antigen is produced in vivo by the host and is appropriately presented on major histocompatibility complex I or II molecules (2, 6, 8). DNA vaccination represents a novel way to induce a specific immune response in a host organism. DNA vaccines compared to earlier generations of vaccines have many advantages, such as ease of construction, low cost of mass production, high-temperature stability, and ability to induce many different long-lasting immune responses, including cytotoxic T cells as well as recognition by B cells to induce antibody production.
Vaccination with outer membrane protein antigens has been shown to be efficacious against P. aeruginosa infection in a number of studies using killed whole cells (9), purified outer membrane preparations (32, 33), isolated outer membrane proteins (18, 20, 39, 53), protein fusions (38), or synthetic peptides representing protective epitopes (22, 23). The P. aeruginosa major constitutive porin protein, OprF, which has previously been shown to be antigenic (3, 20, 25) and has high homology among Pseudomonas strains (18, 34, 40), was chosen as a vaccine target. This protein has been shown to provide protection in a mouse model of systemic infection (20), a mouse burn infection model (39), and rodent models of acute (28) and chronic lung infection (18, 47). Based on these previous findings, we designed and tested the efficacy of a DNA vaccine based upon outer membrane protein F for immunoprotection against P. aeruginosa.
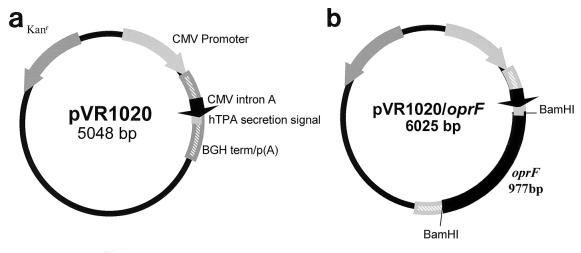
The eukaryotic expression plasmid pVR1020 (Vical, Inc., San Diego, Calif.) was used in this study (Fig. 1). This plasmid contains an immediate-early cytomegalovirus promoter to ensure efficient expression in a eukaryotic host as well as the human tissue plasminogen activator secretion signal to facilitate secretion of the target antigen (OprF) from the eukaryotic cell (31, 45). The 977-bp oprF gene was cloned from P. aeruginosa PAO1 genomic DNA using a Taq polymerase core kit (Qiagen, Inc., Santa Clarita, Calif.) with primers engineered with BamHI- and BglII-cut sites at the 5′ and 3′ ends, respectively. This PCR product and pVR1020 were digested with BamHI and BglII. The plasmid was subsequently dephosphorylated with calf intestinal alkaline phosphatase, and the resulting products were ligated with T4 DNA ligase (Life Technologies, Gaithersburg, Md.). The resulting plasmid, pVR1020/oprF, was transformed into Escherichia coli DH5α, purified by anion-exchange chromatography using Qiagen-tip 2500, and resuspended in cell culture-grade phosphate-buffered saline (PBS) (Life Technologies) to a final concentration of 1 mg/ml.
FIG. 1.
Construction of plasmids for DNA immunization. (a) Plasmid pVR1020, a eukaryotic expression vector, was used as the negative control for immunization. Kanr, kanamycin resistance gene; CMV promoter, cytomegalovirus immediate-early promoter; CMV intron A, intron A of the CMV immediate-early promoter; hTPA, human tissue plasminogen activator secretion signal; BGH term/p(A), bovine growth hormone terminator and polyadenylation sequence. (b) oprF was cloned into pVR1020 using the BamHI restriction endonuclease site, resulting in the plasmid pVR1020/oprF, which was used as the DNA vaccine for immunization.
Initial experiments were performed to compare the effectiveness of the pVR1020/oprF vaccine administered either by gene gun or by intramuscular (i.m.) inoculation. Inoculation by gene gun yielded results superior to i.m. inoculation in that i.m. inoculation elicited reactive antibodies at a lower rate and to a lower final titer, with the elicited antibodies being less opsonic and nonprotective than gene gun-elicited antibodies. Thus, our preliminary results agreed with previous reports (4, 5, 17, 55) that gene gun inoculation is superior to i.m. inoculation. We therefore adopted gene gun inoculation as the route of immunization for our standard procedure.
Mice (5-week-old, female, specific-pathogen-free ICR mice) were obtained from Harlan Sprague-Dawley, Indianapolis, Ind. All mice were housed in the Animal Resources Facility at Louisiana State University Health Sciences Center-Shreveport and handled according to American Association for Laboratory Animal Sciences guidelines. At 14-day intervals, groups of 30 mice were inoculated a total of three times in the abdomen via biolistic particle injection (Helios Gene Gun kit; Bio-Rad, Richmond, Calif.) on days 0, 14, and 28 with 2 μg of either pVR1020 (control) or pVR1020/oprF, which was used to coat 1-μm-diameter gold beads. Serum samples were taken from nonimmunized mice on day zero and from each immunized group on days 14, 28, and 42.
To obtain sera for use in the opsonophagocytic assays, mice were immunized with protein F purified from the PAO1 strain as previously described (20). Each mouse was immunized with four doses of 10 μg of protein F given i.m. in alternate hips at 2-week intervals, with aluminum hydroxide used as adjuvant for the first and second doses and no adjuvant included for the last two doses. Two weeks after the last immunization, the mice were bled and the serum samples obtained were pooled for use in the assay.
To demonstrate that pVR1020/oprF was expressed in eukaryotic cells, human melanoma UM449 cells were transiently transfected using Lipofectin (Life Technologies) (36). Briefly, cells were transfected in duplicate with 2 μg of either pVR1020/oprF or pVR1020. Following 48 h of incubation, the culture medium was removed from the UM449 cell layers and the supernatant fraction resulting from centrifugation (10,000 × g for 10 min at 4°C) of that culture medium was used for immunoblot analysis to detect in situ synthesis of OprF from pVR1020/oprF. The cell layer was washed once in PBS. The cells were resuspended in PBS and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoreis sample buffer prior to use in immunoblot analysis. The immunoblot protocol has been described previously (24).
All bacterial strains used in this study were grown at 30°C with shaking in BBL nutrient broth (Becton-Dickinson Microbiological Systems, Cockeysville, Md.) or on nutrient agar (Difco Laboratories, Detroit, Mich.) plates. The following strains of P. aeruginosa were used: Fisher-Devlin (FD) immunotype 1 (ATCC 27584; Difco 0–6), FD immunotype 2 (ATCC 27313; Difco 0–11), FD immunotype 3 (ATCC 27314; Difco 0–2), FD immunotype 4 (ATCC 27315; Difco 0–1), FD immunotype 5 (ATCC 27585; Difco 0–10), FD immunotype 6 (ATCC 27579; Difco 0–7 and 0–8), FD immunotype 7 (PAO1; Difco 0–5), and KG1077, which is a protein F-deficient mutant of PAO1.
Polyclonal antiserum specificity in immunized mice was subjected to immunoblot analysis against P. aeruginosa PAO1 cell lysates using antisera diluted 1:1,000 as previously described (13). Nitrocellulose membranes were blocked in 2% (wt/vol) nonfat milk in 0.1% Tween 20–Tris-buffered saline and probed with polyclonal mouse sera from immunized mice. The membrane was washed three times in 1% Tween 20–Tris-buffered saline, and rabbit anti-mouse immunoglobulin (Ig) conjugated to horseradish peroxidase (Amersham Life Science, Arlington Heights, Ill.) was added as a secondary antibody. Reactive polypeptides were detected using a chemiluminescent substrate (National Diagnostics, Inc., Atlanta, Ga.) and visualized on film.
Sera recovered from DNA vaccine-immunized and control mice were analyzed by enzyme-linked immunosorbent assay (ELISA) for reactivity against bacterial cells, OprF peptides 9 (14 mer) and 10 (14 mer) (27), and purified protein F according to published procedures (27, 39). The wells of microtiter plates (polyvinyl chloride or Immulon 1 [Dynatech, Chantilly, Va.]) were coated with either bacterial whole cells of KG1077 or each FD immunotype strain, 2.5 μg of synthetic peptide 9 (14 mer), 2.5 μg of synthetic peptide 10 (14 mer), or 10 μg of protein F. Dilutions of sera pooled from similarly immunized mice were incubated with the prepared microtiter plate at 37°C for 1 h. Bound IgG was detected by alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology Assoc. Inc., Birmingham, Ala.) using p-nitrophenylphosphate (Sigma-Aldrich, St. Louis, Mo.) as the substrate. Endpoint titers for P. aeruginosa whole cell-, peptide-, or protein F-specific antibodies were expressed as the inverse of the dilution that gave a mean value for the optical density at 405 nm (OD405) greater than the OD405 of a 1:50 dilution of normal mouse serum obtained from unimmunized mice. All sera were analyzed in at least two separate assays.
Antibody isotypes in antisera from immunized mice were analyzed by ELISA as previously described (52) as modified by Abdillahi and Poolman (1). Briefly, Immulon 3 plates (Dynatech) were coated with 100 μl of whole P. aeruginosa PAO1 cells suspended in PBS at an OD620 of 0.09. Plates were then placed at 37°C overnight to allow the buffer to evaporate. Serum samples were serially diluted in PBS containing 0.025% Tween 20 and added to the plate; anti-mouse IgG1 or anti-mouse IgG2a conjugated to alkaline phosphatase was used as the secondary antibody (Zymed Laboratories, Inc., San Francisco, Calif.). The presence of bound antibody was detected using p-nitrophenylphosphate (Sigma) as the substrate, with absorbance read at 410 nm on a Bio-Rad model 550 plate reader. Antibody quantitation was determined by ELISA analysis using a standard curve with purified IgG2a and IgG1 antibody reagents (Zymed Laboratories, Inc.).
Sera from DNA vaccine-immunized mice taken 14 days after the last immunization and from control mice were tested for the ability to mediate the opsonophagocytic uptake of P. aeruginosa by human polymorphonuclear leukocytes (PMNs) as described previously (19). All sera were heated at 56°C for 30 min to inactivate complement. Cultures of FD immunotype 2 and 4 strains were diluted to a cell density of 108 cells/ml for use in the assay. The reaction mixtures included 50 μl of bacterial culture plus 50 μl of serum. These mixtures were incubated for 30 min at 37°C with shaking. Then, 100 μl of human venous blood was added to each mixture, and this final combination was incubated for 30 min at 37°C with shaking. Blood smears were prepared from each of the reaction mixtures and stained with Giemsa stain. For each assay three slides per reaction mixture were examined microscopically, and the number of bacterial cells contained within the first 75 isolated, intact PMNs encountered (25 PMNs per slide) was determined for each reaction mixture. Each assay was performed in triplicate and the results were combined to yield a total of 225 PMNs counted for each reaction mixture. The number (mean ± standard deviation) of bacterial cells per PMN was calculated. Statistical analyses of the differences in the opsonophagocytic uptake of bacteria by PMNs were performed by comparing the means by the unpaired two-tailed Student t test using a Pro-Stat program.
The immunoprotective potential of the DNA vaccine was tested in a mouse model of chronic pulmonary infection (7, 18, 47, 48). Two weeks after the final immunization, the mice were challenged with agar beads containing the P. aeruginosa FD immunotype 4 strain. The mice were first anesthetized with an intraperitoneal injection of sodium pentobarbital and then inoculated via a tracheal incision with 50 μl of an agar bead slurry encasing approximately 7 × 102 CFU of P. aeruginosa. A beaded-tip 22-gauge needle was gently guided to favor inoculation of the left lung. The incision was closed with sterile wound clips. Eight days after the challenge, protection afforded to the immunized mice by the DNA vaccine was assessed by two methods. First, the lungs were examined macroscopically for the presence of lesions (7, 18, 47) according to the following scoring scheme: 0, absense of any macroscopic lesion; 1+, presence of one or two small lesions not exceeding 1 mm in diameter; 2+, presence of three or more small lesions not exceeding 1 mm in diameter; 3+, presence of a medium lesion 2 to 5 mm in diameter; and 4+, presence of a large lesion exceeding 5 mm in diameter. Scoring of the pulmonary lesions was done by an investigator with more than 15 years of experience in macroscopic lung lesion scoring. Second, bacterial quantitation of the number of P. aeruginosa cells present in the lungs was performed as described previously (7, 18). Briefly, both lungs were aseptically removed and homogenized in a 1:10 (wt/vol) dilution of sterile saline with a tissue grinder. Dilutions of these lung tissues were plated onto nutrient agar for quantitative determination of the numbers of P. aeruginosa cells present. The presence of fewer than 5 × 103 CFU was selected as a significant cutoff value because previously it has been shown to represent a >95% (or 2-log) reduction from the mean number of bacteria found in the lungs of challenged control mice 8 days after challenge (47). Duplicate experiments were performed and the results were compiled for analysis. Statistical analyses of the differences in the number of severe lung lesions and in bacterial quantitation between mice immunized with pVR1020 or pVR1020/oprF were performed with the IBM EpiStat Basic Statistics program. P values were calculated by the Fisher exact test, and values of ≤0.05 were considered to be significant.
Production of OprF by eukaryotic cells.
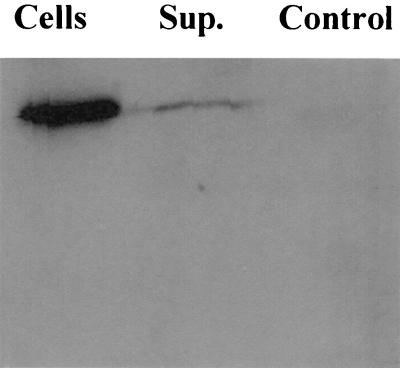
To verify expression of the target gene and synthesis of the target protein in mammalian cells, immunoblot analysis of transfected cells was performed. The adherent human melanoma cell line UM449 was transiently transfected with plasmid VR1020/oprF or pVR1020 (negative control). Following a 48-h incubation, culture supernatants and cell lysates were examined by immunoblot analysis with antisera to OprF to detect the presence of OprF. As illustrated in Fig. 2, upon transfection with pVR1020/oprF, the protein was found not only in the UM449 cells but also in the supernatant fraction, which was expected since the pVR1020 plasmid is designed to include an N-terminal secretion signal sequence to facilitate translocation of OprF to the endoplasmic reticulum and eventual release from the cell.
FIG. 2.
Immunoblot of eukaryotic cells with polyclonal anti-OprF mouse sera. Lanes (labeled as follows): Cells, lysed UM449 cells that had been transfected with pVR1020/oprF, incubated for 48 h, washed in PBS, and then resuspended in PBS for lysis; Sup., the initial 48-hour culture supernatant from UM449 cells transfected with pVR1020/oprF; and Control, lysed UM449 cells transfected with pVR1020. Note that OprF was seen predominantly in the pelleted cell fraction but was also found in the supernatant.
Characteristics of elicited antibodies.
Sera from pVR1020/oprF-immunized mice were evaluated for the ability to react specifically with protein F from P. aeruginosa strain PAO1. Whereas sera from vector-immunized mice did not react, sera from DNA-vaccinated animals reacted with protein F, F′, and protein H (Fig. 3). Cross-reaction with protein H has been shown previously with sera from mice immunized with protein F (25, 39). Antibody responses induced by gene gun inoculation were predominantly of the IgG1 isotype, indicating a Th2 (humoral) type of immune response as the calculated IgG1:IgG2a ratio was 10.33 (93 ng of IgG1/ml of serum versus 9 ng of IgG2a/ml of serum). This is in agreement with the findings of previous reports (4, 13, 35) that gene gun inoculation with various antigens results in a predominant IgG1 response.
FIG. 3.
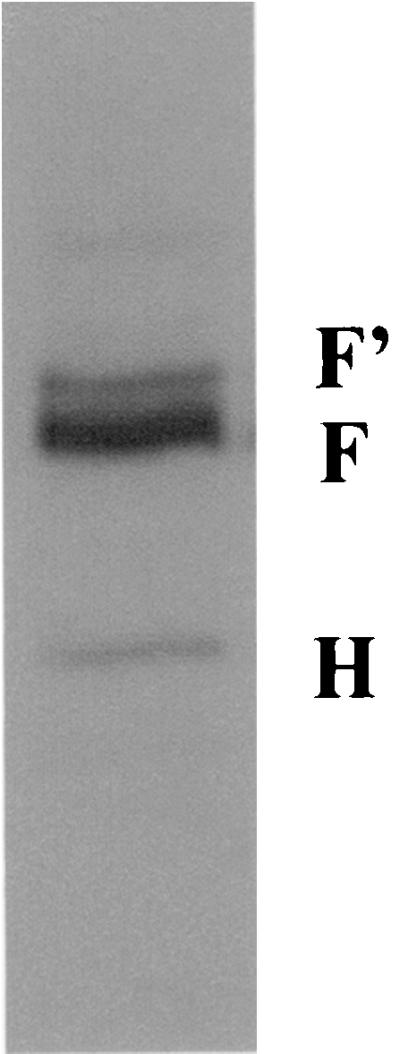
Immunoblot of proteins extracted from whole cells of the PAO1 strain of P. aeruginosa with polyclonal anti-OprF sera obtained from DNA-immunized (pVR1020/oprF) mice. Note that the antisera reacted at bands corresponding to F (OprF), F′ (denatured OprF), and H (OprH).
The sera from the vector control-immunized mice had no reactivity by ELISA with any of the whole cells of the various strains of P. aeruginosa, with peptides 9 and 10, or with purified protein F. The sera from mice biolistically immunized with the pVR1020/oprF vaccine reacted strongly by ELISA with whole cells of each of the seven FD immunotype strains, with a titer of IgG antibodies against each in the range of 12,800 to 25,600. The sera did not react significantly (titer of 50) with the protein F-deficient KG1077 strain or with protein F peptide 9 or 10 (titer of 100). The sera did react well against purified intact protein F, with titers in the range of 51,200 to 102,400. Thus, biolistic inoculation of mice with the pVR1020/oprF vaccine did elicit a strong IgG antibody response to purified protein F and whole cells of heterologous immunotypes of P. aeruginosa.
Sera from vector control- and pVR1020/oprF-immunized mice were tested for opsonic activity in an opsonophagocytic assay. P. aeruginosa cells were mixed with undiluted heat-inactivated sera obtained from the control- and pVR1020/oprF- immunized mice and incubated with human whole blood. The sera recovered from mice immunized with the pVR1020/oprF vaccine mediated opsonophagocytic uptake of P. aeruginosa by PMNs significantly greater than that following exposure to the vector-immunized mouse sera. As shown for the FD 4 strain (Table 1), the sera from pVR1020/oprF-immunized mice had an opsonic ratio of 1.34 compared to that of the sera from vector control-immunized mice. This ratio compares favorably with the opsonic ratio of 1.56 obtained for sera from mice immunized with purified protein F versus normal mouse sera (P value for the mean for sera from pVR1020/oprF-immunized mice compared to the mean for sera from protein F-immunized mice is 0.084; i.e., there is no statistically significant difference between these two values). Similar results were obtained for FD 2 cells, which yielded an opsonic ratio of 1.37 for sera from pVR1020/oprF-immunized mice compared to sera from vector control-immunized mice (data not shown).
TABLE 1.
Quantitation of phagocytic uptake by PMNs of P. aeruginosa FD 4 cells exposed to antisera from immunized mice
| Test serum | No. of bacteria/PMN (mean ± SD) | Opsonic ratioa (P) |
|---|---|---|
| Vector control | 9.48 ± 7.46 | |
| DNA-protein F | 12.69 ± 9.31 | 1.34 (<0.00001) |
| NMSb | 9.21 ± 8.32 | |
| Protein F | 14.41 ± 11.56 | 1.56 (<0.00001) |
Opsonic ratio is the mean number of bacteria associated with test sera per PMN divided by the mean number of bacteria associated with the appropriate control sera per PMN. P values were determined by unpaired two-tailed Student t test.
NMS, pooled normal mouse sera collected from nonimmunized, specific-pathogen (P. aeruginosa)-free mice.
Protection in the murine chronic pulmonary infection model.
The immunoprotective potential of the pVR1020/oprF vaccine was tested in a mouse model of chronic pulmonary infection. Eight days after challenge with approximately 7 × 102 agar-encased P. aeruginosa FD 4 cells delivered via intratracheal inoculation into the left lung of each mouse, the lungs were removed and examined for evidence of vaccine protection by two means. First, the presence of severe lung lesions, i.e. lesions scored as ≥2+, was determined (Table 2). Second, the lungs were assayed to quantitate the number of P. aeruginosa cells present in the lungs (Table 3). The pVR1020/oprF-immunized mice had significantly fewer severe lung lesions (43.2%) than did the vector control-immunized mice (76.2%). A similar reduction in the number of lungs containing >5 × 103 CFU of P. aeruginosa was seen (40.9 and 69% for pVR1020/oprF-immunized mice and vector control-immunized mice, respectively). The number of lungs from which P. aeruginosa had been completely cleared, as indicated by no growth upon quantitation, also was significantly higher in the pVR1020/oprF-immunized mice than in the vector control-immunized mice. Thus, the pVR1020/oprF vaccine afforded significant protection to the immunized mice against subsequent pulmonary challenge with P. aeruginosa.
TABLE 2.
Scoring of macroscopic lung lesions in immunized mice following challenge with FD 4 P. aeruginosa in a chronic pulmonary infection model
| Immunization group | No. of mice scored asa:
|
No. of mice with severe (≥2+) lung lesions/total no. of mice (%) | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | ||
| Vector control | 9 | 1 | 6 | 12 | 14 | 32/42 (76.2) |
| DNA-protein F | 18 | 7 | 5 | 9 | 5 | 19/44 (43.2) |
Lesions were scored as follows: 0, absence of any macroscopic lesion; 1+, presence of one or two small lesions not exceeding 1 mm in diameter; 2+, presence of three or more small lesions not exceeding 1 mm in diameter; 3+, presence of a medium lesion 2 to 5 mm in diameter; 4+, presence of a large lesion exceeding 5 mm in diameter. The P value was 0.002 as determined by Fisher's exact test.
TABLE 3.
Quantitation of P. aeruginosa present in the lungs of immunized mice following challenge with FD 4 P. aeruginosa in a chronic pulmonary infection model
| Immunization group | No. of mice with lungs yielding no growth/total no. of mice (%)a | No. of mice with lungs yielding >5 × 103 CFU/total no. of mice (%)b |
|---|---|---|
| Vector control | 12/42 (28.6) | 29/42 (69.0) |
| DNA-protein F | 22/44 (50.0) | 18/44 (40.9) |
P value was 0.035 as determined by Fisher's exact test.
The P value was 0.008 as determined by Fisher's exact test.
Potential of an oprF DNA vaccine against P. aeruginosa infections in humans.
Herein, we have reported the successful construction of a DNA vaccine using the oprF gene of P. aeruginosa (Fig. 1). The efficacy of this vaccine in eliciting anti-OprF (Fig. 3), whole cell-reactive, opsonic (Table 1) IgG antibodies of the IgG1 isotype and in affording protection (Tables 2 and 3) in a mouse model of chronic pulmonary infection was demonstrated. The DNA vaccine produced results comparable to previously published results for protection obtained in rodent models of chronic pulmonary infection following immunization with purified protein F from P. aeruginosa (16, 18, 21, 47).
Protection in animal models provides only a suggestion of the potential effectiveness of that vaccine in humans. In the case of a pVR1020/oprF vaccine specifically, additional vaccine components, such as P. aeruginosa PcrV (43), outer membrane protein I (30, 53), or elastase epitopes (46), all of which have previously been shown to be antigenic, might be combined with oprF to augment the final vaccine's effectiveness. Moreover, the effectiveness of the final vaccine in humans may depend on determining the optimal vaccination protocol. This may require using a different DNA vector delivery system or using a combination of delivery systems, such as an attenuated Salmonella strain (10) or chimeric virus to maximize the immunogenic response in humans. Alternatively, a combination of initial immunization with a DNA oprF vaccine followed by booster immunization(s) with a protein F or protein F epitope peptide vaccine might be required for optimal effectiveness of the vaccine in humans (41, 44). Another consideration is that treatment of Pseudomonas infections in humans will no doubt involve other simultaneous therapeutic modalities, such as antibiotics and pulmonary or respiration therapy, and these additional treatments will influence the outcome of human infections in vaccinated individuals. The results presented in this report using a DNA oprF vaccine for protection against P. aeruginosa in a mouse model of chronic pulmonary infection indicates that a DNA vaccine for protection of humans against P. aeruginosa infection warrants continued development.
Acknowledgments
This research was funded in part by grant RO1-AI44424 from the National Institutes of Health to J.S. and H.E.G. and by a grant from the Shriners' Hospitals for Children (Cincinnati, Ohio) to D.R.G. and N.R.B.
REFERENCES
- 1.Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. [PubMed] [Google Scholar]
- 2.Allsopp C E, Plebanski M, Gilbert S, Sinden R E, Harris S, Frankel G, Dougan G, Hioe C, Nixon D, Paoletti E, Layton G, Hill A V. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur J Immunol. 1996;26:1951–1959. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- 3.Battershill J L, Speert D P, Hancock R E W. Use of monoclonal antibodies to protein F of Pseudomonas aeruginosa as opsonins for phagocytosis by macrophages. Infect Immun. 1987;55:2531–2533. doi: 10.1128/iai.55.10.2531-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belperron A A, Feltquate D, Fox B A, Horii T, Bzik D J. Immune responses induced by gene gun or intramuscular injection of DNA vaccines that express immunogenic regions of the serine repeat antigen from Plasmodium falciparum. Infect Immun. 1999;67:5163–5169. doi: 10.1128/iai.67.10.5163-5169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett A M, Phillpotts R J, Perkins S D, Jacobs S C, Williamson E D. Gene gun mediated vaccination is superior to manual delivery for immunisation with DNA vaccines expressing protective antigens from Yersinia pestis or Venezuelan equine encephalitis virus. Vaccine. 1999;18:588–596. doi: 10.1016/s0264-410x(99)00317-5. [DOI] [PubMed] [Google Scholar]
- 6.Bohm W, Mertens T, Schirmbeck R, Reimann J. Routes of plasmid DNA vaccination that prime murine humoral and cellular immune responses. Vaccine. 1998;16:949–954. doi: 10.1016/s0264-410x(97)00302-2. [DOI] [PubMed] [Google Scholar]
- 7.Brennan F R, Jones T D, Gilleland L B, Bellaby T, Xu F, North P C, Thompson A, Staczek J, Lin T, Johnson J E, Hamilton W D O, Gilleland H E., Jr Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology. 1999;145:211–220. doi: 10.1099/13500872-145-1-211. [DOI] [PubMed] [Google Scholar]
- 8.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 9.Cripps A W, Dunkley M L, Clancy R L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun. 1994;62:1427–1436. doi: 10.1128/iai.62.4.1427-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darji A, Guzman C A, Gerstel B, Wachholz P, Timmis K N, Wehland J, Chakraborty T, Weiss S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 11.Davies J. Bacteria on the rampage. Nature. 1996;383:219–220. doi: 10.1038/383219a0. [DOI] [PubMed] [Google Scholar]
- 12.Davis H L, Michel M L, Mancini M, Schleef M, Whalen R G. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994;12:1503–1509. doi: 10.1016/0264-410x(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 13.Denis-K S, Mize, Price B M, Baker N R, Galloway D R. Analysis of immunization with DNA encoding Pseudomonas aeruginosa exotoxin A. FEMS Immunol Med Microbiol. 2000;27:147–154. doi: 10.1111/j.1574-695X.2000.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 15.Ertl H C, Xiang Z Q. Genetic immunization. Viral Immunol. 1996;9:1–9. doi: 10.1089/vim.1996.9.1. [DOI] [PubMed] [Google Scholar]
- 16.Fox C W, Campbell G D, Jr, Anderson M M, Zavecz J H, Gilleland L B, Gilleland H E., Jr Preservation of pulmonary function by an outer membrane protein F vaccine: a study in rats with chronic pulmonary infection caused by Pseudomonas aeruginosa. Chest. 1994;105:1545–1550. doi: 10.1378/chest.105.5.1545. [DOI] [PubMed] [Google Scholar]
- 17.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilleland H E, Jr, Gilleland L B, Matthews-Greer J M. Outer membrane protein F preparation of Pseudomonas aeruginosa as a vaccine against chronic pulmonary infection with heterologous immunotype strains in a rat model. Infect Immun. 1988;56:1017–1022. doi: 10.1128/iai.56.5.1017-1022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilleland H E, Jr, Gilleland L B, Hughes E E, Matthews-Greer J M. Recombinant outer membrane protein F of Pseudomonas aeruginosa elicits antibodies that mediate opsonophagocytic killing, but not complement-mediated bacteriolysis, of various strains of P. aeruginosa. Curr Microbiol. 1992;24:1–7. [Google Scholar]
- 20.Gilleland H E, Jr, Parker M G, Matthews-Greer J M, Berg R D. Use of purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984;44:49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilleland H E, Gilleland L B, Fowler M R. Vacine efficiencies of elastase, exotoxin A, and outer membrane protein F in preventing chronic pulmonary infection by Pseudomonas aeruginosa in a rat model. J Med Microbiol. 1993;38:79–86. doi: 10.1099/00222615-38-2-79. [DOI] [PubMed] [Google Scholar]
- 22.Gilleland H E, Jr, Gilleland L B, Staczek J, Harty RN, Garcia-Sastre A, Palase P, Brennan F R, Hamilton W D O, Bendahmane M, Beachy R N. Chimeric animal and plant viruses expressing epitopes of outer membrane protein F as a combined vaccine against Pseudomonas aeruginosa lung infection. FEMS Immunol Med Microbiol. 2000;27:291–297. doi: 10.1111/j.1574-695X.2000.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilleland L B, Gilleland H E., Jr Synthetic peptides representing two protective, linear B-cell epitopes of outer membrane protein F of Pseudomonas aeruginosa elicit whole-cell-reactive antibodies that are functionally pseudomonad specific. Infect Immun. 1995;63:2347–2351. doi: 10.1128/iai.63.6.2347-2351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 471–510. [Google Scholar]
- 25.Hedstrom R C, Pavlovskis O R, Galloway D R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984;43:49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horan T C, White J W, Jarvis W R, Emori G, Culver D H, Munn V P, Thornsberry C, Olson D R, Hughes J M. Nosocomial infection surveillance. Morb Mortal Wkly Rep. 1986;35:17–29. [PubMed] [Google Scholar]
- 27.Hughes E E, Gilleland L B, Gilleland H E., Jr Synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa that elicit antibodies reactive with whole cells of heterologous immunotype strains of P. aeruginosa. Infect Immun. 1992;60:3497–3503. doi: 10.1128/iai.60.9.3497-3503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes E E, Gilleland H E., Jr Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against P. aeruginosa in a murine acute pneumonia model. Vaccine. 1995;13:1750–1753. doi: 10.1016/0264-410x(95)00166-x. [DOI] [PubMed] [Google Scholar]
- 29.Johnston S A, Tang D C. Gene gun transfection of animal cells and genetic immunization. Methods Cell Biol. 1994;43:353–365. doi: 10.1016/s0091-679x(08)60612-3. [DOI] [PubMed] [Google Scholar]
- 30.Knapp B, Huntz E, Lenz U, Hungerer K-D, Gabelsberger J, Domdey H, Mansouri E, Li Y, von Specht B-U. A recombinant hybrid outer membrane protein for vaccination against Pseudomonas aeruginosa. Vaccine. 1999;17:1663–1666. doi: 10.1016/s0264-410x(98)00420-4. [DOI] [PubMed] [Google Scholar]
- 31.Le T P, Coonan K M, Hedstrom R C, Charoenvit Y, Sedegah M, Epstein J E, Kumar S, Wang R, Doolan D L, Maguire J D, Parker S E, Hobart P, Norman J, Hoffman S L. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–1901. doi: 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee N, Ahn B, Jung S B, Kim Y G, Kim H, Park W J. Conformation-dependent antibody response to Pseudomonas aeruginosa outer membrane proteins induced by immunization in humans. FEMS Immunol Med Microbiol. 2000;27:79–85. doi: 10.1111/j.1574-695X.2000.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee N, Jung S B, Ahn B Y, Kim Y H, Kim J, Kim D, Kim I, Yoon S M, Nam S W, Kim H, Park W J. Immunization of burn-patients with a Pseudomonas aeruginosa outer membrane protein vaccine elicits antibodies with protective efficacy. Vaccine. 2000;18:1952–1961. doi: 10.1016/s0264-410x(99)00479-x. [DOI] [PubMed] [Google Scholar]
- 34.Lee N-G, Ahn B Y, Jung S B, Lee Y, Kim Y G, Kim H-S, Park W J. Human anti-Pseudomonas aeruginosa outer membrane proteins IgG cross-protective against infection with heterologous immunotype strains of P. aeruginosa. FEMS Immunol Med Microbiol. 1999;25:339–347. doi: 10.1111/j.1574-695X.1999.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 35.Leitner W W, Seguin M C, Ballou W R, Seitz J P, Schultz A M, Sheehy M J, Lyon J A. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159:6112–6119. [PubMed] [Google Scholar]
- 36.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 37.Manickan E, Karem K L, Rouse B T. DNA vaccines—a modern gimmick or a boon to vaccinology? Crit Rev Immunol. 1997;17:139–154. doi: 10.1615/critrevimmunol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri E, Gabelsberger J, Knapp B, Hundt E, Lenz U, Hungerer K-D, Gilleland H E, Jr, Staczek J, Domdey H, von Specht B-U. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect Immun. 1999;67:1461–1470. doi: 10.1128/iai.67.3.1461-1470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews-Greer J M, Gilleland H E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987;155:1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- 40.Mutharia L M, Nicas T I, Hancock R E W. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982;146:770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- 41.Okuda K, Xin K O, Tsuji T, Bukawa H, Tanaka S, Koff W C, Tani K, Honma K, Kawamoto S, Hamajima K, Fukushima J. DNA vaccination followed by macromolecular multicomponent peptide vaccination against HIV-1 induces strong antigen-specific immunity. Vaccine. 1997;15:1049–1056. doi: 10.1016/s0264-410x(97)00045-5. [DOI] [PubMed] [Google Scholar]
- 42.Robinson H L. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–787. doi: 10.1016/s0264-410x(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 43.Sawa T, Yahr T L, Ohara M, Kurahashi K, Gropper M A, Wiener-Kronish J P, Frank D W. Active and passive immunization with Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 44.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smooker P M, Steeper K R, Drew D R, Strugnell R A, Spithill T W. Humoral responses in mice following vaccination with DNA encoding glutathione S-transferase of Fasciola hepatica: effects of mode of vaccination and the cellular compartment of antigen expression. Parasite Immunol. 1999;21:357–364. doi: 10.1046/j.1365-3024.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- 46.Sokol P A, Kooi C, Hodges R S, Cachia P, Woods D E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J Infect Dis. 2000;181:1682–1692. doi: 10.1086/315470. [DOI] [PubMed] [Google Scholar]
- 47.Staczek J, Gilleland H E, Jr, Gilleland L B, Harty R N, Garcia-Sastre A, Engelhardt O G, Palese P. A chimeric influenza virus expressing an epitope of outer membrane protein F of Pseudomonas aeruginosa affords protection against challenge with P. aeruginosa in a murine model of chronic pulmonary infection. Infect Immun. 1998;66:3990–3994. doi: 10.1128/iai.66.8.3990-3994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starke J R, Edwards M S, Langston C, Baker C J. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22:698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Tang D C, De Vit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 50.Taubes G. Salvation in a snippet of DNA? Science. 1997;278:1711–1714. doi: 10.1126/science.278.5344.1711. [DOI] [PubMed] [Google Scholar]
- 51.Tuteja R. DNA vaccines: a ray of hope. Crit Rev Biochem Mol Biol. 1999;34:1–24. doi: 10.1080/10409239991209165. [DOI] [PubMed] [Google Scholar]
- 52.Voller A, Bidwell D E, Bartlett A. The enzyme linked immunosorbent assay. Alexandria, Va: Dynatech Laboratories, Inc.; 1979. [Google Scholar]
- 53.von Specht B-U, Christian L H, Blum B, Schmitt A, Hungerer K-D, Domdey H. Safety and immunogenicity of a Pseudomonas aeruginosa outer membrane protein I vaccine in human volunteers. Vaccine. 1996;14:1111–1117. doi: 10.1016/0264-410x(96)00054-0. [DOI] [PubMed] [Google Scholar]
- 54.Waine G J, McManus D P. Nucleic acids: vaccines of the future. Parasitol Today. 1993;11:113–116. doi: 10.1016/0169-4758(95)80172-3. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine. 2000;18:1725–1729. doi: 10.1016/s0264-410x(99)00432-6. [DOI] [PubMed] [Google Scholar]