Abstract
Capparis zeylanica L. is a climbing shrub distributed in Indian subcontinent and Mediterranean region. Almost all parts of the plant are used in folk medicine and traditional practices to treat several human ailments. The present study was aimed to investigate the role of C. zeylanica L. root extract in preventing cancerous cells growth and proliferation, as well as promoting apoptosis and cell cycle arrest in MDA-MB-231 and MCF-7 breast cancer cells. Methanolic extract of C. zeylanica L. (MECz) was prepared and characterized by LC–ESI–MS/MS analysis. In vitro cytotoxicity and anti-proliferative activity of MECz was evaluated by MTT assay, while cell viability, apoptosis and cell cycle progression by Muse Cell analyzer. Furthermore, the mRNA and protein expressions of EMT markers were assessed using qRT-PCR and western blotting techniques, respectively. The MECz was found to be rich in phenolic compounds including chlorogenic acid, 6-gingerol, and certain triterpenes like ursolic acid etc. The apparent anti-metastasis activity of MECz was evident from IC50 value of 19.12 and 24.22 μg/mL, respectively, on MDA-MB-231 and MCF-7 cells in MTT assay. An absolute decrease in cell viability (78.1–53.4% and 89.9–49.0%), augmented apoptosis (90.98–48.25% and 88.25–47.70%) and S phase, G2/M phase cell cycle arrest was found by MECz treatment on MDA-MB-231 and MCF-7 cells. The gene expression studies revealed that MECz could significantly (p < 0.001) regulate the expression of EMT markers such as snail, slug, zeb-1, twist-1, fibronectin, vimentin and E-cadherin at molecular level. These findings demonstrate that C. zeylanica L. root extract inhibits breast cancer cells growth and proliferation through regulating the expression of key EMT marker genes and proteins. Thus, MECz may be suggested as a potential anti-metastasis agent in the treatment of breast cancer.
Keywords: Apoptosis, Breast cancer, Capparis zeylanica, Cell cycle, Cytotoxicity, EMT markers
Introduction
Cancer is the second most prominent cause of death worldwide in people with age of 70 or less and about 30% of cancer deaths are owing to breast cancer (Siegel et al. 2018). The high incidence of cancer is majorly owing to steady rise in life expectancy, increasing urbanization; changes in environmental conditions, food habits and lifestyle (Jemal et al. 2011). According to GLOBOCAN 2020, an estimated 19.3 million people worldwide were diagnosed with cancer in 2020, with 10 million deaths from cancer (Sung et al. 2021). It is predicted that, by 2040, the global prevalence of cancer will be doubled from its current prevalence (29–37%), with low- and middle-income countries (LMICs) account for two-thirds of the prevalence (Shah et al. 2019). Breast cancer is the most widespread cancer among women and in 2022, approximately 2.9 million female breast cancer cases were diagnosed worldwide, accounting for nearly one-fourth of all cancer cases in women. Australia, New Zealand, United Kingdom, Sweden, Finland, Denmark, Belgium, Netherlands, France, Italy, and Northern America have the highest rates of breast cancer. The most rapid increases are occurring in transitional countries in South America, Africa, and Asia (Bray et al. 2004). While, actual pathogenesis of cancer still needs to be elucidated.
Epithelial–mesenchymal transition (EMT) is a biological phenomenon that happens when epithelial cells acquire mesenchymal properties and is linked to numerous aspects of cancer malignancy, including metastasis, tumor invasion, and therapeutic resistance (Yang et al. 2020). Certain transcription factors, such as members of the Snail, Twist, Zeb, and Slug families, are reported to suppress the expression of epithelial phenotypic genes like E-cadherin, claudins, and occludin and induce the expression of mesenchymal genes like fibronectin, vimentin, and N-cadherin, resulting in the EMT phenomenon. To alter cell behavior, EMT is mediated by a set of signaling pathways that in turn influence downstream and up effectors. Understanding the interplay of cell signaling pathways to regulate the repression of E-cadherin by Snail, Slug, Zeb-1, Twist-1, etc., in the process of EMT and metastasis could help in novel and effective drug development (Gonzalez and Medici 2014; Maurya et al. 2021). Therefore, novel, target-specific, safe and cost-effective therapeutic approaches as well as drug candidates need to be developed to treat cancer.
Since ancient times natural products, medicinal plants and traditional medicine have played pivotal role in the treatment of several human health disorders. According to WHO report, about 80% of the world’s population use mostly plant/herbal drugs to deal with different health matters (Locatelli et al. 2017; Zhang and Ma 2018; Yao et al. 2022). For instance, in industrialized nation like Germany, 90% of the population depend on natural and traditional remedies to contain health matters (Calapai 2008).
Capparis genus belongs to Capparidaceae family and its plants have been widely used in traditional and folk medicine in several Asian and Mediterranean countries from historical times (Tlili et al. 2009). The link between capers (Capparis species) and human beings’ dates back to the Stone Age. Caper has been used for fragrance and flavoring purpose from ancient times in food preparation. From 1970s, the capers are cultivated in some European and Mediterranean countries such as Italy, Spain, Turkey, Tunisia and Iran (Infantino et al. 2007).
Various plant species of Capparis are found useful to cure fevers, cough, inflammation, cholera, asthma etc. (Chopra et al. 1986; Kirtikar and Basu 1987). People living in arid and semi-arid locations use caper species-berry pickle from ancient times as a best source of nutrients to maintain good health (Soetan et al. 2010). Caper fruit pickle is reported to be useful in treating Cirrhosis (Angulo 2002), type 2 diabetes (Matthaus and Ozcan 2005), and many other health issues, and it has traditionally been used as an anti-hyperglycemic, anti-hyperlipidemic food by Iranian diabetic patients (Matthaus and Ozcan 2005; Huseini et al. 2013).
Capparis zeylanica L., commonly known as Indian caper, is a climbing shrub belongs to the family Capparidaceae, distributed in the major regions of India, China, Nepal, Sri Lanka, Malaysia, Bangladesh, Philippines and some regions of Pakistan (Nilavukkarasi et al. 2020a, b). C. zeylanica has been used as a “Rasayana” drug in Ayurvedic systems of medicine. In fact, the seeds, fruits, bark, roots and leaves of C. zeylanica are used in folk medicine and traditional practices to cure immune disorders and other human ailments (Perry 1980; Ghule et al. 2006). For instance, in Philippines, the bark of C. zeylanica is used in the treatment of cholera and has stomachic properties (Perry 1980). In Northern India, the leaves are widely used as counter-irritant, febrifuge and as a cataplasm in swellings, boils and piles (Chopra et al. 1986). The leaf extract of C. zeylanica exhibits analgesic and antipyretic effects (Ghule et al. 2007). Its leaves and roots are used to treat inflammation and helminthic parasites (Assam et al. 2010; Arulmozhi et al. 2019). The entire C. zeylanica plant extracts are applied to treat paralysis and rheumatism (Kirtikar and Basu 1987). The leaf methanolic extract of C. zeylanica was reported to possess memory enhancement effect (Ghule et al. 2006). However, the anti-cancer effects of C. zeylanica are still not investigated.
Therefore, the present study was conducted to investigate the anti-cancer activity of C. zeylanica root extract through in vitro cell culture model by measuring cell viability, apoptosis, cell cycle arrest and expression of key mRNA and protein markers involved in EMT using MDA-MB-231 and MCF-7 breast cancer cells.
Materials and methods
Chemicals and materials
Dulbecco's modified Eagle’s medium (DMEM), Fetal Bovine Serum (FBS), Insulin, Streptomycin and Penicillin were purchased from Gibco (St W Ste, US). Propidium iodide, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethyl benzothiazoline-6-sulphonic acid) diammonium salt (ABTS), DMSO, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), p-nitro phenyl butyrate (p-NPB), Nonidet P-40, isopropyl alcohol, ellagic acid, gallic acid and quercetin and ethidium bromide were procured from Sigma-Aldrich, Bangalore, India. Enzyme-Linked Immunosorbent Assay (ELISA) kits (Texas, US) and other chemicals used were of analytical grade and purchased from local manufacturers in India.
Plant material collection and phytochemical analysis
Capparis zeylanica roots were collected from Seshachalam hill ranges spread in Chittoor and Kadapa districts of Andhra Pradesh, India. The plant was authenticated by a taxonomist from the Department of Botany, at Sri Venkateswara University in Tirupati, the specimen was deposited in the departmental herbarium and the voucher number is 1654. C. zeylanica roots were shade dried, ground fine powder, and soaked in (50 g/250 mL) ethyl acetate, acetone, methanol and aqueous solvent system with fitful shaking. The solvent extracts were collected and concentrated under reduced pressure in a rotary evaporator (Heidolph, Germany) to obtain respective semisolid extracts namely EAECz, AcECz, MECz and AqECz (15%, w/w), which were stored at − 20 °C until further analysis. Initially, C. zeylanica’s phytochemical analysis was performed using standard methods and procedures (Williams and Grayer 2004). These phytochemical constituents such as anthraquinones, alkaloids, carbohydrates, polyphenols, flavonoids, saponins, steroids, tannins, triterpenoids etc., were quantitatively analyzed by LC–ESI–MS/MS as described below. Further, from all these extracts, total phenolic contents were determined by measuring absorbance at 470 nm (Ainsworth and Gillespie 2007), and expressed as mg of gallic acid equivalents (GAE). The total flavonoid contents were estimated using aluminum chloride method (Chang et al. 2002) by taking absorbance at 520 nm and expressed as mg of quercetin equivalent (QE). The total steroidal saponins present in solvent extracts were quantified by measuring absorbance at 570 nm (Baccou et al. 1977).
LC–ESI–MS/MS analysis of MECz
Liquid Chromatography–Electron Spray Ionization-MS/MS (LC–ESI–MS/MS) analysis was performed on a 6520 Accurate Q-TOF (Agilent Santa Clara, CA) HPLC coupled with mass spectrometer equipped with a UV–Vis detector to identify the phytochemicals present in the methanol extract of C. zeylanica root. Zorbax SB C18 column was used at the following conditions: (A) 0.1% v/v formic acid (B) acetonitrile + 0.1% formic acid; gradient (in solvent B): (i) 20% from 0 to 20 min, (ii) 95% from 20 to 27 min and (iii) 35% at 27–30 min which was the total run time; 0.2 mL/min flow rate; 3 μL injection volume when ESI parameters included both negative and positive ion modes. A mass range of 100–1200 m/z, a spray voltage of 4 kV, a gas temperature of 325°C, a gas flow of 10 mL/min, a nebulizer pressure of 40 psi were used, and the mass analysis was conducted using an Agilent Technologies Mass-Hunter software 2.5.
Assessment of anti-oxidant activity
DPPH radical scavenging assay
The DPPH radical scavenging potency of MECz was determined using the 2,2-diphenyl-2-picryl-hydrazyl assay according to the method (Cano-Lamadrid et al. 2018). Briefly, 4 mg of DPPH was dissolved in 100 mL of methanol and this solution was used as a stock solution. The working standard was prepared using a diluted stock solution with methanol. Then, 4 mL of the DPPH standard solution was mixed with different concentrations of MECz dissolved in methanol (5–25 μg/mL); then, a 100 μL of methanol was added to samples before incubated in the dark at room temperature for 20 min. The absorbance was measured at 517 nm. The absorbance for parallel control (without MECz) and standard ascorbic acid were also measured in the similar manner. DPPH radical scavenging activity was calculated using the following equation:
where Acontrol absorbance of the control; Atest absorbance of the MECz.
The radical scavenging activity results were expressed as the half maximal inhibitory concentration (IC50) compared to standard. All measurements were done in triplicate and the mean and SD values were calculated.
ABTS radical scavenging assay
The 2,2-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging assay was performed according to the method described (Re et al. 1999) to measure the anti-oxidant activity of the MECz. The 50 μL of the different concentrations of MECz dissolved in methanol (5–25 μg/mL) was made up to 400 μL using ABTS solution, incubated at room temperature for 30 min and the absorbance was measured at 734 nm. All measurements were done in triplicate and mean and SD values were calculated. The anti-oxidant ability of extract was calculated using the following equation and the results were expressed as the half of maximal inhibitory concentration (IC50) with standard compared:
where Acontrol absorbance of the control; Atest absorbance of the MECz.
Superoxide radical scavenging assay
The free radical scavenging activity of MECz was determined using the method (Sharma and Singh 2012). In brief, 3 mL of 100 mM sodium phosphate buffer (pH 7.4) containing 1 mL of 150 μM nitro blue tetrazolium (NBT) solution, 468 μM NADH solution, and different concentrations of the MECz dissolved in methanol (5–25 μg/mL) were taken in test tube. The total reaction mixture was incubated at 25°C for 5 min. The gradual reduction of nitro blue tetrazolium (NBT) was determined with a spectrophotometer at 560 nm. A parallel control (without MECz) and standard ascorbic acid was used as a positive control. The superoxide radical scavenging activity was calculated as follows:
where Acontrol absorbance of the control; Atest absorbance of the MECz.
In vitro cell culture experiments
Breast cancer cell lines, namely MDA-MB-231 and MCF-7 were obtained from National Centre for Cell Science, Pune, India. They were maintained in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% Fetal Bovine Serum (FBS) and 10 μg/mL streptomycin and penicillin, incubated at 37°C in an incubator with 5% CO2.
MTT assay for cytotoxicity
The cytotoxic effect of MECz was determined by 3-[4, 5-dimethylthiazole-2-yl] 2,5-di-phenyl-tetrazoliumbromide (MTT) assay. Briefly, 100 μL of MDA-MB-231 and MCF-7 cells were aliquoted, placed into two separate 96-well plate at a density of 1.5 × 104 cells/well and left for 24 h, to attain 70–80% of cell confluency. Then, the cells were treated with various concentrations of MECz (5, 10, 15, 20, and 25 μg/mL) and incubated for 24 h. After the treatment, 0.8 mg/mL of MTT solution was added to each well of the cells and after 4-h incubation, the supernatant was removed, then 100 μL of DMSO was added into each well to dissolve the formazan crystals, and the plates were incubated on a shaker and the absorption was read at 570 nm using a microplate reader (BioRad). All experiments were performed in triplicate. The relative cytotoxicity was compared using untreated control cells as the baseline (Alessandria et al. 2014).
Cell viability assay
Cell viability assay was performed using Muse Cell Analyzer (Luminex, USA). The MDA-MB-231 and MCF-7 cells were cultured in separate 6-well plates at a density of 1.5 × 104 cells/well and incubated for 24 h at 37°C in CO2 incubator to reach 70–80% of cell confluency. After treatment with different doses of MECz (5, 10, 15, 20, and 25 μg/mL) for 24 h, the cells were collected and centrifuged at 3000 rpm for 4 min, followed by washing the pellet with TE buffer. Then, the cell viability was measured by following instructions of commercially available kit using Muse cell analyzer.
Measurement of cell apoptosis
The MDA-MB-231 and MCF-7 cells were seeded in separate 6-well plates at a density of 1.5 × 104 cells/well with 70–80% of cell confluency and incubated for 24 h. Then, the cells were treated with various concentrations of MECz (5, 10, 15, 20, and 25 μg/mL) and incubated for 24 h. Afterwards, the collected cells were washed by TE buffer followed by adding 5 μL of annexin-V reagent and propidium iodide (PI) into the suspension, and incubated for 30 min in the dark. Quantification of apoptosis was reported as a percentage of fluorescence according to the criteria of the kit, using cell analyzer (Jin et al. 2012).
Cell cycle analysis
The MDA-MB-231 and MCF-7 cells were cultured in separate 6-well plates at a density of 1.5 × 104 cells/well and incubated for 24 h in a CO2 incubator to achieve 70–80% of cell confluency. The cells were washed thrice with Phosphate Buffer Saline and then cells were incubated for 24 h at 37°C in an incubator after treated with the different doses of MECz, then, cells were harvested using trypsin–EDTA. This was followed by cell fixation in 30 mL of 70% ethanol overnight at 4°C, followed by centrifuged at 1500 rpm for 5 min. The pellet was resuspended in 200 μL of buffer saline containing 0.1% Triton X-100 and RNase A at 100 mg/mL final concentration and incubated for 2 h at 37°C. Then, cells were centrifuged at 1500 rpm at 4°C for 5 min, the pellet was stained with propidium iodide at 30 μg/mL concentration and incubated at room temperature in the dark for 30 min. Cell cycle analysis was carried out using a muse cell analyzer (Jin et al. 2012).
Expression of EMT marker genes by qRT-PCR analysis
The mRNA expression of EMT marker genes of untreated (control) and 25 μg/mL of MECz-treated samples were estimated by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA was isolated from MDA-MB-231 and MCF-7 cells using tri-reagent (Sigma-Aldrich, USA) according to manufacturer’s protocol and reverse-transcribed to obtain cDNA using DNA synthesis kit (Applied Biosystems, Foster City, USA). About 20 ng of cDNA was used for RT-PCR (BioRad CFX96, Real-Time PCR) using SYBR green master mix and the conditions were kept as follows: 95°C for 30 s, 95°C for 5 s (denaturation), 60°C for 30 s (annealing) followed by 40 cycles. Data were analyzed using ΔΔCt and 2n − ΔΔCt methods and values were expressed in terms of relative fold change (RFC). PCR reactions were run in triplicate for each sample using the following specific primers. The PCR primers for
Snail is:
5'-AAGATGCACATCCGAAGCCA-3'(sense), 5'-CAAAAACCCACGCAGACAGG-3'(antisense).
Slug is:
5'-CTTCCTGGTCAAGAAGCA-3'(sense), 5'-GGGAAATAATCACTGTATGTGTG-3'(antisense).
Zeb1 is:
5'-GCACAACCAAGTGCAGAAGA-3'(sense), 5'-GCCTGGTTCAGGAGAAGATG-3'(antisense).
Twist1 is:
5'-GGCTCAGCTACGCCTTCTC-3'(sense), 5'-CTAGTGGGACGCGGACAT-3'(antisense).
Fibronectin is:
5'-ATGATGAGGTGCACGTGTGT-3'(sense), 5'-CTCTGAATCCTGGCATTGGT-3'(antisense).
Vimentin is:
5'-AGATGGCCCTTGACATTGAG-3'(sense), 5'-TGGAAGAGGCAGAGAAATCC-3'(antisense).
E-Cadherin is:
5'-TGCTCTTGCTGTTTCTTCGG-3′(sense), 5'-TGCCCCATTCGTTCAAGTAG-3′(antisense).
GAPDH is:
5'-ACCACAGTCCATGCCATCAC-3'(sense), 5'-TCCACCACCCTGTTGCTGTA-3'(antisense) and transcription levels of every gene were normalized to the level of GAPDH gene.
Western blot analysis
The untreated (control) and 25 μg/mL of MECz-treated MDA-MB-231 and MCF-7 breast cancer cells were washed with PBS (1×) and homogenized in RIPA lysis buffer [0.1% SDS, 0.25% sodium deoxycholate, 150 mM NaCl, 1% Triton X-100, 50 mM Tris–Cl (pH 7.4), 1% Nonidet P-40] containing protease inhibitors (PI) (RIPA + PI: 1 mL + 40 μL), centrifuged at 14,000 rpm/10 min/4°C, the supernatant was collected. The concentration of proteins was measured using the Bradford method. Proteins (40 μg) were resolved on 10% SDS-PAGE gels and transferred onto PVDF membrane (Billerica, USA). The membrane was blocked with 5% (w/v) skimmed milk for 1 h on shaking rocker followed by overnight incubation at 4 °C with specific primary antibodies against anti-snail, anti-slug, anti-zeb1, anti-twist1, anti-fibronectin, anti-vimentin, anti-E-cadherin (1:1000 dilution) and anti-GADPH (Abclonal technology, United States). The membrane was then incubated with HRP-conjugated secondary antibody for 1 h at room temperature, followed by washing with TBST (1 ×), the membrane was treated with chemiluminescent ECL detection reagent (BioRad) and bands were visualized by Chemi Scope western blot imaging system.
Statistical analysis
All the experiments were carried out in triplicates. Data were loaded to Microsoft Excel, Prism 8.0 and one-way analysis of variance (ANOVA) was used to analyze the data. All the data were presented as mean ± SD; the significant differences between samples were determined by Student’s t test. The experimental data were considered significant at p < 0.05.
Results
Phytochemical analysis and quantification
Phytochemical examination of ethyl acetate and methanolic extracts revealed the presence of phenols, alkaloids, glycosides, steroids, and saponins, but, tannins and phytosterols were present only in ethyl acetate extract. The aqueous extract contains alkaloids, flavonoids, phenols, and saponins. The acetone extract contained no other phytochemicals other than glycosides, phenols, flavonoids, and steroids. Table 1 shows the results of phytochemical analysis, when Table 2 reports the quantification results of total phenolics, flavonoids and steroidal saponins present in different solvent extracts. When compared to other solvent extracts of C. zeylanica, the methanolic extract has the highest total phenolics content (142.4 ± 4.20 mg GAE/g), highest total flavonoids content (72.18 ± 2.32 mg QE/g) and highest total steroidal saponin contents (16.72 ± 1.05 mg SAE/g).
Table 1.
Phytochemical analysis of solvent extracts of Capparis zeylanica L. roots
| Phytochemicals | Aqueous extract | Ethyl acetate extract | Methanol extract | Acetone extract |
|---|---|---|---|---|
| Phenols | + | + + | + + | + |
| Alkaloids | + | + | + | − |
| Terpenoids | − | − | + + | − |
| Flavonoids | + | − | + + | + |
| Tannins | − | + | − | − |
| Saponins | + | − | − | − |
| Glycosides | − | + | + | + |
| Steroids | − | + | + + | + |
| Proteins | − | − | − | − |
+ indicates presence, − indicates absence
Table 2.
Quantification of total phenol content (TPC), total flavonoid content (TFC), and total steroidal saponin content (TSSC) in C. zeylanica root extracts
| Extracts | TPC (μg/mg GAE/g) | TFC (μg/mg QE/g) | TSSC (μg/mg SAE/g) |
|---|---|---|---|
| EAECz | 122.24 ± 3.20 | 18.35 ± 0.82 | 4.20 ± 0.12 |
| AECz | 46.13 ± 2.33 | 61.17 ± 1.47 | 12.17 ± 0.65 |
| MECz | 142.4 ± 4.20 | 72.18 ± 2.32 | 16.72 ± 1.05 |
| AqECz | 93.15 ± 2.80 | 40.73 ± 1.13 | 7.15 ± 0.34 |
EAECz ethyl acetate extract of Capparis zeylanica; AECz acetone extract of Capparis zeylanica; MECz methanol extract of Capparis zeylanica; AqECz aqueous extract of Capparis zeylanica
LC–ESI–MS/MS analysis of MECz
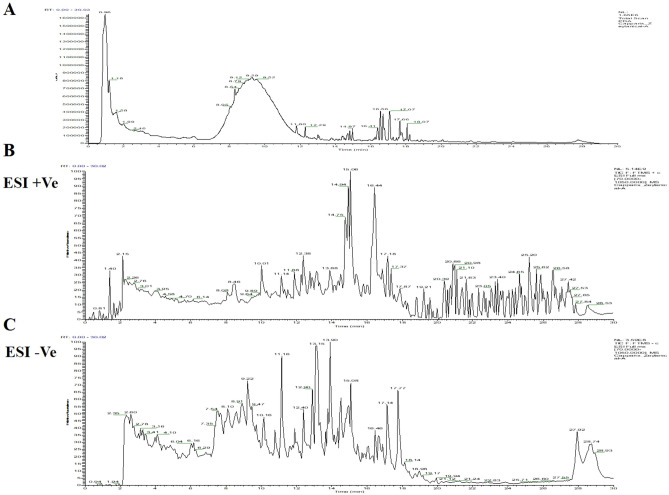
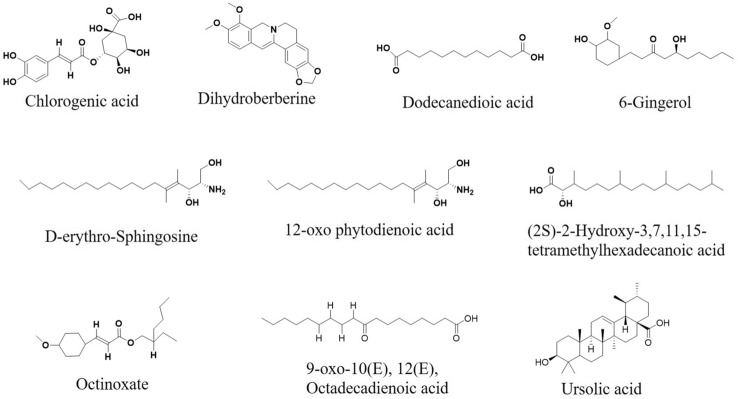
In the present study, 10 major compounds were detected in MECz by LC–ESI–MS/MS analysis, based on their masses, molecular formula and retention time (Fig. 1). They are Chlorogenic acid, Dihydroberberine, Dodecanedioic acid, 6-Gingerol, d-erythro-Sphingosine, 12-oxo-phytodienoic acid, (2S)-2-hydroxy-3,7,11,15-tetramethylhexadecanoic acid, Octinoxate, 9-oxo-10(E), 12(E), Octadecadienoic acid and Ursolic acid as shown in Fig. 2. Table 3 represents the identified list of the 10 compounds, based on their retention time, molecular formula and mass.
Fig. 1.
LC–ESI–MS/MS spectrum of MECz. A Spectral data of ESI + mode and ESI − mode. B Spectral data of ES + mode. C Spectral data of ES − mode
Fig. 2.
Phytochemicals structures of methanol extract of Capparis zeylanica
Table 3.
List of compounds present in MECz with their retention times, molecular formula and molecular weights
| RT (min) | Name of the compounds in methanol extract | Molecular formula | Mass |
|---|---|---|---|
| 2.15 | Chlorogenic acid | C16 H18 O9 | 354.09 |
| 9.11 | Dihydroberberine | C20 H19 N O4 | 337.13 |
| 11.16 | Dodecanedioic acid | C12 H22 O4 | 230.15 |
| 12.38 | 6-Gingerol | C13 H22 N6 O2 | 294.18 |
| 12.90 | D-erythro-Sphingosine | C18 H37 N O2 | 299.28 |
| 13.15 | 12-oxo phytodienoic acid | C18 H28 O3 | 274.19 |
| 13.90 | (2S)-2-Hydroxy-3,7,11,15-tetramethylhexadecanoic acid | C20 H40 O3 | 328.29 |
| 14.94 | Octinoxate | C18 H26 O3 | 290.18 |
| 15.06 | 9-oxo-10(E), 12(E), Octadecadienoic acid | C18H30O3 | 294.21 |
| 16.44 | Ursolic acid | C30H48 O | 456.36 |
Antioxidant activity
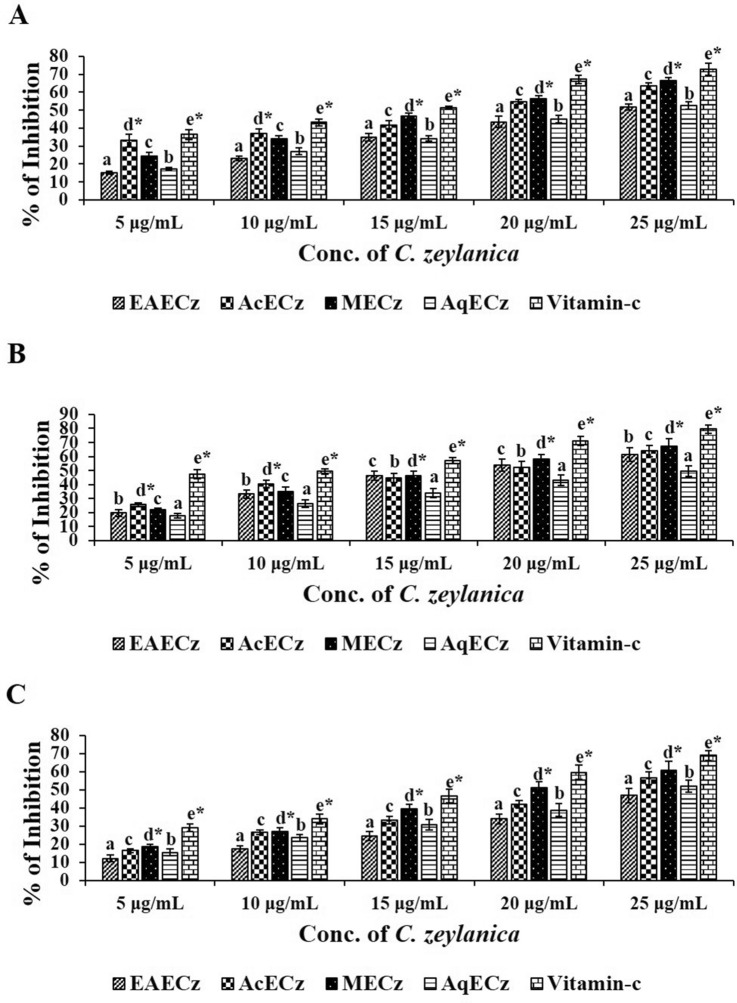
The DPPH, superoxide (O2−), and ABTS radical scavenging assays were performed to determine the anti-oxidant activity of C. zeylanica root extracts. The results of DPPH showed that, among all the extracts, MECz at 25 μg/mL had the strongest free radical scavenging activity (66.36 ± 1.9) followed by acetone and aqueous extracts. The IC50 value of MECz was 16.06 μg/mL which is nearer to ascorbic acid (14.61 μg/mL) (Fig. 3A). On the same lines, MECz showed the strongest ability to eliminate O2− radicals with a value of 67.28 ± 4.1 followed by acetone and aqueous extracts (Fig. 3B). The IC50 value of MECz for superoxide scavenging activity was 13.49 μg/mL (data not shown). Furthermore, as shown in Fig. 3C, ABTS radical scavenging activity of MECz had the highest (60.98 ± 5.0) when the IC50 value is 19.48 μg/mL (data not shown).
Fig. 3.
Anti-oxidant activities of MECz. A DPPH radical scavenging activity. B Superoxide radical scavenging activity. C ABTS radical scavenging activity. Data are represented as mean ± SD of triplicates
MTT assay for cytotoxicity
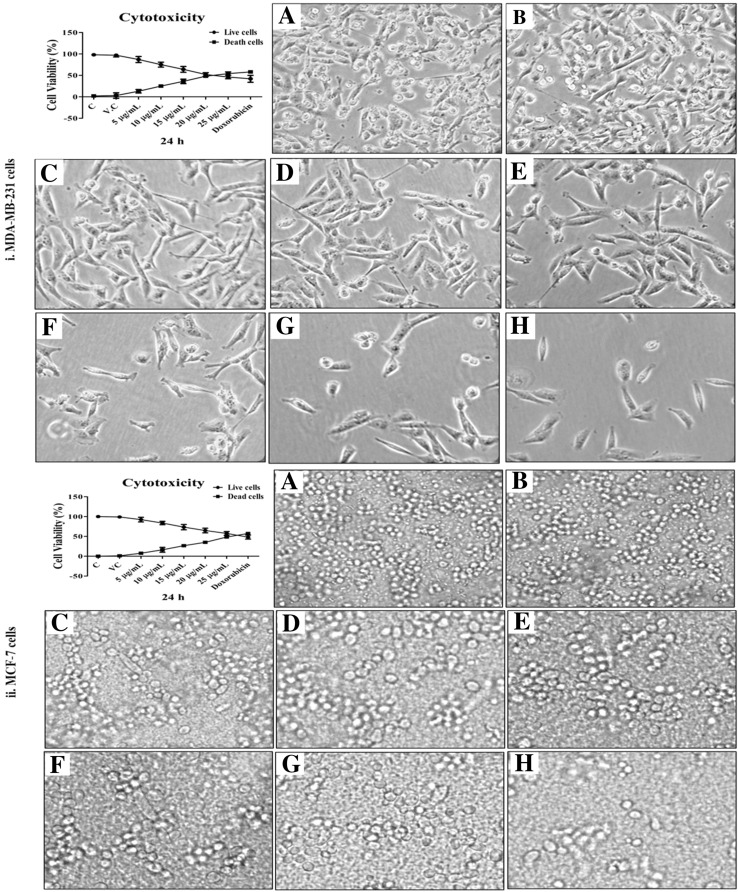
The MTT assay was conducted to assess the IC50 value of methanolic extract of C. zeylanica (MECz) root against MDA-MB-231 and MCF-7 breast cancer cell lines at different concentrations (5, 10, 15, 20, and 25 μg/mL) for 24-h treatment. MECz showed considerable and dose-dependent cytotoxicity on MDA-MB-231 and MCF-7 cells, with IC50 values of 19.12 μg/mL, and 24.22 μg/mL, respectively (Fig. 4). Doxorubicin was used as the standard drug for comparison.
Fig. 4.
Cytotoxicity by MTT assay: MECz significantly showed toxic effects on breast cancer cells and their microscopic evaluation. (i) MDA-MB-231 cells structure. (ii) MCF-7 cells structure. A Control (untreated cells), B Vehicle control (DMSO-treated cells), C–H Cancer cells treated with 5–25 μg/mL of MECz and 0.5 μM of Doxorubicin. Data are represented as mean ± SD of triplicates
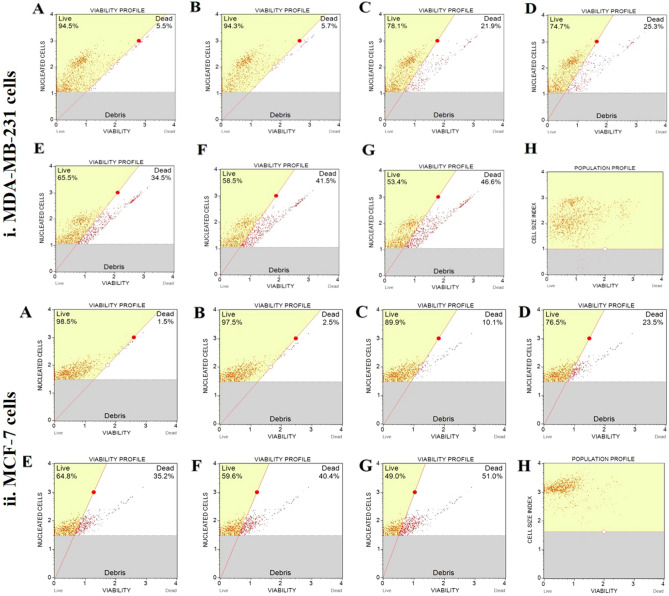
Effect of MECz on cell viability
The MDA-MB-231 and MCF-7 breast cancer cells were cultured in 12-well plates and treated with MECz (5, 10, 15, 20, 25 μg/mL) to determine its effect on cell viability. The positive control was Doxorubicin (0.5 μM), a well-known cancer inhibitor. The viability of the cells was tested using a muse cell analyzer, and the results are represented as a percentage of control and vehicle controls (cells exposed to DMSO). The cell viability of both types of tumor cells was significantly inhibited in MECz-treated cells in a dose-dependent manner when compared to untreated control cells.
The MDA-MB-231 cell viability profile contents decreased by about 53.4–78.1% in 5–25 μg/mL treated MECz compared to control (94.5%) cells (Table 4), whereas the dead cell profile contents increased by around 21.9–46.6% in 5–25 μg/mL treated MECz compared to control (5.5%) cells (Fig. 5(i)A–H) (Table 4). Similarly, the MCF-7 cell viability profile contents decreased by 49.0–89.9% in 5–25 μg/mL treated MECz compared to control (98.5%) cells, while the dead cell profile contents increased by 10.1–51.0% in 5–25 μg/mL of MECz-treated cells compared to control (1.5%) cells (Fig. 5(ii)A–H). When compared to standard Doxorubicin, MDA-MB-231 and MCF-7 cells have shown 51.5% and 47.5% cell viability, respectively.
Table 4.
Effect of MECz on percentage of cell viability, apoptosis and cell cycle arrest in MDA-MB-231 and MCF-7 breast cancer cells
| MECz treatment (µg/mL) | Cell viability (% of live cells) | Apoptosis (% of dead cells) | Cell cycle (S phase) | Cell cycle (G2/M phase) | ||||
|---|---|---|---|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 | |
| Control | 94.5 | 98.5 | 0.43 | 0.35 | 18.8 | 17.6 | 9.8 | 9.7 |
| DMSO | 94.3 | 97.5 | 0.79 | 0.20 | 17.6 | 16.5 | 9.5 | 9.4 |
| MECz-5 µg/mL | 78.1* | 89.9* | 2.05* | 0.85 | 18.3 | 17.5 | 11.2 | 11.5* |
| MECz-10 µg/mL | 74.7* | 76.5* | 7.15* | 9.10* | 18.0 | 17.1 | 13.9* | 14.2* |
| MECz-15 µg/mL | 65.5* | 64.8* | 13.75** | 18.80** | 17.7 | 16.5 | 23.1* | 23.3* |
| MECz-20 µg/mL | 58.5** | 59.6** | 21.75** | 28.75** | 15.7* | 16.3* | 28.3** | 28.1** |
| MECz-25 µg/mL | 53.4*** | 49.0*** | 37.96*** | 48.10*** | 12.9** | 13.5** | 32.1*** | 32.3*** |
| Doxorubicin | 51.5*** | 47.5*** | 38.56*** | 49.25*** | 12.6** | 13.2** | 33.4*** | 33.5*** |
Data are represented as mean ± SD of triplicates, treated groups were compared with control using one-way ANOVA test, the values of DMSO (non-significant) and the values of treated groups are significant at *p < 0.05, **p < 0.01 and ***p < 0.001, respectively
Control untreated cells; DMSO vehicle control; MECz methanol extract of Capparis zeylanica; Doxorubicin standard drug
Fig. 5.
Cell Viability: MECz significantly reduced viability of the breast cancer cells. (i) MDA-MB-231cells structure. (ii) MCF-7 cells structure. A Control (untreated cells), B Vehicle control (DMSO-treated cells), C–H Cancer cells treated with 5–25 μg/mL of MECz and 0.5 μM of Doxorubicin. Cell debris were represented as gaiting
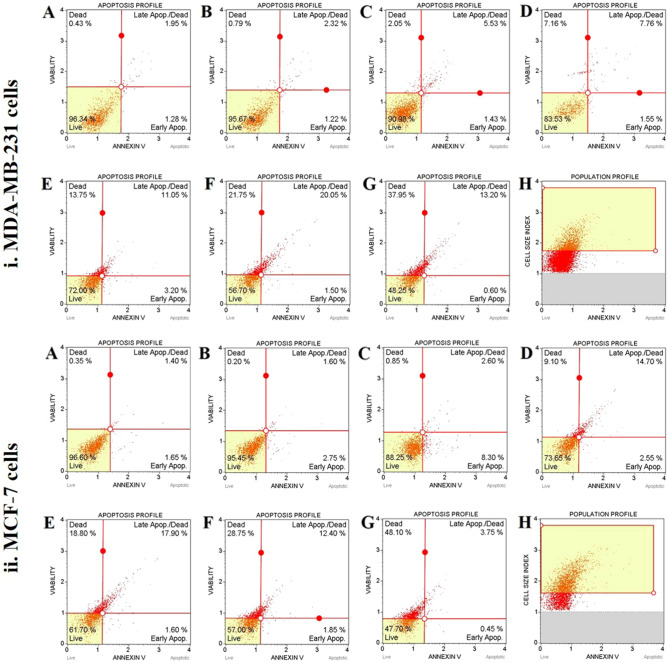
Effect of MECz on cell apoptosis
The Muse cell analyzer was used to measure the apoptosis profile and cell death using annexin-V. Representative trials at 5, 10, 15, 20, and 25 μg/mL of MECz for 24 h in the control and DMSO-treated vehicle control (A and B) and experimental (C, D, E, F, and G) groups (Fig. 6). The kit can distinguish its union with annexin-V and PI in the following measurements: live cells, lower left (annexin-V/PI = −/−), necrotic and late apoptotic cells, upper right (annexin-V/PI = + / +), early apoptotic cells, lower right (annexin-V/PI = +/−), and cell necrosis (cell death independent of apoptosis), upper left (annexin-V/PI = −/ +).There were significant changes (p < 0.05) in live, early, late apoptosis, and necrosis with 5, 10, 15, 20, and 25 μg/mL of MECz-treated cells that were subjected to 24-h treatment in the annexin-V experiment. Apoptosis was detected as 96.34 and 95.67% in control and DMSO-treated cells, respectively, according to the apoptotic profile. The cellular life of MECz at 5–25 μg/mL was reduced by 48.25–90.98%. With a dose of 5–25 μg/mL, late apoptosis and cell necrosis increased from 5.53% to 13.20% (late apoptosis) and 2.05% to 37.95% (necrosis) in MDA-MB-231 breast cancer cells. In Doxorubicin-treated cells, cell necrosis increased by 38.56% as compared to control cells (Fig. 6(i) A–H) (Table 4).
Fig. 6.
Cell apoptosis: MECz significantly induced breast cancer cell apoptosis. (i) MDA-MB-231 cells. (ii) MCF-7 cells. A Control, B Vehicle control, C–H Cancer cells treated for 24 h with 5–25 μg/mL of MECz and 0.5 μM of Doxorubicin. Cells were stained by Annexin-V and PI to detected apoptosis
There were significant differences in live cells of the experimental group of MCF-7 cells in terms of measuring apoptosis with annexin-V. It was found that the experimental group (5, 10, 15, 20, and 25 μg/mL of MECz) had fewer live cells than the control group. The apoptotic profile of control and DMSO-treated cells was 96.60% and 95.45%, respectively. The live cells were reduced from 47.70% to 88.25% when treated with 5–25 μg/mL of MECz. With a concentration of 5–25 μg/mL of MECz, late apoptosis and cell necrosis increased from 2.60% to 17.90% (late apoptosis) and 0.85% to 48.10% (necrosis) in MCF-7 breast cancer cells (Fig. 6(ii) A–H) (Table 4).
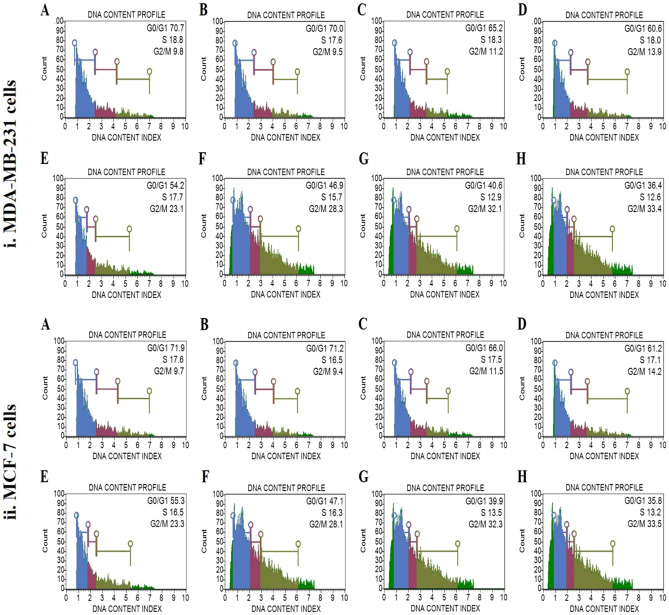
Effect of MECz on cell cycle arrest
The cell cycle of MDA-MB-231 and MCF-7 cells was examined using a muse cell analyzer. The MDA-MB-231 cellular DNA profile contents were decreased by about 36.4–65.2% in G0/G1 phase, 12.6–18.3% in S phase in 5–25 μg/mL treated MECz as compared to control cells, and increased by approximately 11.2–33.4% in G2/M phase in 5–25 μg/mL treated MECz as compared to control cells (Fig. 7(i) A–H). The DNA profile contents of MCF-7 cellular DNA were decreased by around 35.8–66.0% in G0/G1 phase, 13.2–17.5% in S phase in 5–25 μg/mL treated MECz as compared to control cells, while increased by around 11.5–33.5% in G2/M phase in 5–25 μg/mL treated MECz as compared to control cells (Fig. 7(ii) A–H) (Table 4).
Fig. 7.
Cell cycle: MECz significantly induced cell cycle arrest in S phase and G2/M phase in breast cancer cells. (i) MDA-MB-231 cells. (ii) MCF-7 cells. A Control (untreated), B Vehicle control (DMSO-treated), C–H Cancer cells treated for 24 h with 5–25 μg/mL of MECz and 0.5 μM of Doxorubicin. Cells were stained by PI, and then detected for cell cycle arrest. Cell debris were represented as gaiting
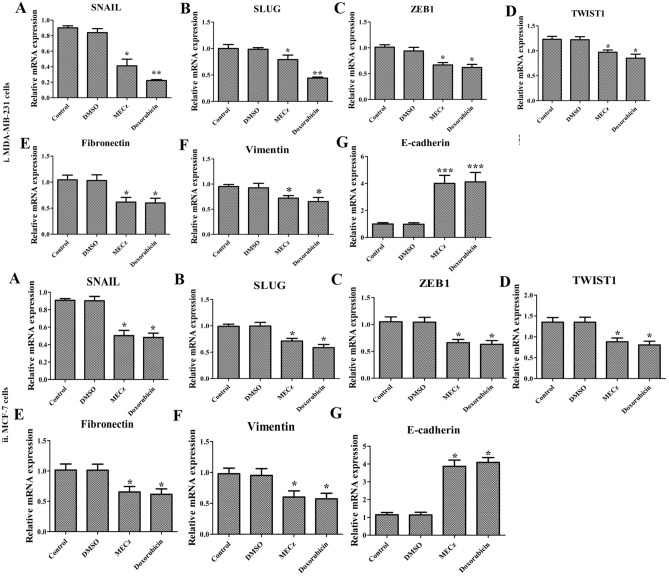
Effect of MECz on mRNA expression
The untreated MDA-MB-231 and MCF-7 breast cancer cells have shown relatively higher mRNA expression levels of EMT markers (Snail, Slug, Zeb-1, Twist-1, fibronectin, and vimentin) compared to adherent cells, which could repress E-cadherin levels. However, treatment with MECz (25 μg/mL) and Doxorubicin (0.5 μM of standard drug), decreased the growth of MDA-MB-231 and MCF-7 cells by down-regulating the mRNA levels of Snail, Slug, Zeb-1, Twist-1, fibronectin, and vimentin, which in turn up-regulated E-cadherin expression levels. Figure 8(i) and (ii)A–H shows relative mRNA expression of the mentioned EMT makers in MDA-MB-231 and MCF-7 breast cancer cells, indicating that, MECz suppressed the growth of cancer cells through regulation of the EMT process.
Fig. 8.
Quantitative RT-PCR analysis: mRNA expression profile of EMT marker genes at transcriptional level in control (untreated), DMSO (vehicle control), 25 μg/mL of MECz and 0.5 μM of Doxorubicin-treated breast cancer cells. (i) MDA-MB-231 cells. (ii) MCF-7 cells. A Snail, B Slug, C Zeb-1, D Twist-1, E Fibronectin, F Vimentin, and G E-cadherin. Data are represented as mean ± SD of triplicates, groups were compared using one-way ANOVA test and the values are significant at *p < 0.05, **p < 0.01 and ***p < 0.001, respectively
Effect of MECz on protein expression
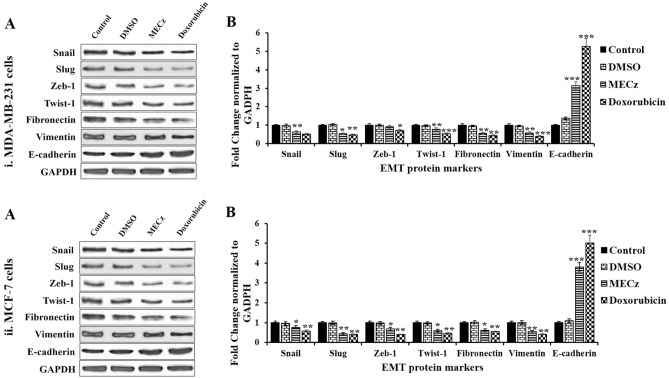
To assess the effect of MECz on the protein expression levels of EMT markers such as E-cadherin, and mesenchymal markers like Snail, Slug, Zeb-1, Twist-1, fibronectin and vimentin western blotting analysis was done using four groups such as control, DMSO (vehicle control), MECz (25 µg/mL) and Doxorubicin (0.5 µM of standard drug). After 24-h treatment, the Snail, Slug, Zeb-1, Twist-1, fibronectin, and vimentin levels were down-regulated in MDA-MB-231 and MCF-7 breast cancer cells, however, E-cadherin expression was up-regulated lines (Fig. 9(i) and (ii)A–B). The findings indicate that MECz may suppress the characteristics of epithelial–mesenchymal transition in breast cancer cells.
Fig. 9.
Western blotting analysis: protein expression profile of EMT marker at translational level in control (untreated), DMSO (vehicle control), 25 μg/mL of MECz and 0.5 μM of Doxorubicin-treated breast cancer cells. (i) MDA-MB-231 cells. (ii) MCF-7 cells. A Blotting images of EMT markers. B The protein expression levels of Snail, Slug, Zeb-1, Twist-1, Fibronectin, Vimentin and E-cadherin were normalized with a housekeeping gene GAPDH level. Data are represented as mean ± SD of triplicates, groups were compared using one-way ANOVA test and the values are significant at *p < 0.05, **p < 0.01 and ***p < 0.001, respectively
Discussion
The Capparis species have been traditionally used in the treatment of various ailments such as small pox, cholera, sores, neuralgia, pneumonia and also as antidote to snake bite (Ghule et al. 2007; Arulmozhi et al. 2018). Capparis zeylanica is commonly known as Indian caper shrub, with a number of divided thorny, sub-scandent climbing branches, used as an ingredient in various Ayurvedic preparations and folk medicine (Nilavukkarasi et al. 2020a, b). However, the anti-cancer activity of Capparis zeylanica, specifically on breast cancer remains unclear. Therefore, in the present study, the anti-cancer activity of methanol extract of C. zeylanica root (MECz) and its mechanism of action was studied on two breast cancer cell lines (MDA-MB-231 and MCF-7).
Oxidative stress is a potential pathological inducer of various human diseases including cancer. High doses of reactive oxygen species (ROS) disturb membrane dynamics and leads to depolarization of mitochondrial membranes and disturbs energy homeostasis. A variety of plant compounds have been characterized and reported for their anti-oxidant and other therapeutic efficacies against an array of diseases (Nahar et al. 2013). Capparis species have been earlier reported for their beneficial effects against oxidative stress, inflammation, microbial infections, and were reported to inhibit early stages of cancer development. The phenolic, alkaloid, terpenoid, saponins and other phytochemicals present in the Capparis plants could be responsible for the anti-oxidant, cytotoxic, immunomodulatory and other therapeutic effects (Rathee et al. 2012). In the current study, the phytochemical analysis of root extract of Capparis zeylanica (MECz) by LC–ESI–MS/MS revealed the presence of more than ten major biologically important compounds such as chlorogenic acid, dihydroberberine, dodecanedioic acid, 6-gingerol, d-erythro-sphingosine, 12-oxo-phytodienoic acid, (2S)-2-hydroxy-3,7,11,15-tetramethylhexadecanoic acid, octinoxate, 9-oxo-10(E), 12(E), octadecadienoic acid and ursolic acid having significant free radicals scavenging activity (Figs. 2, 3 and Table 3).
The results of anti-oxidant assays showed that Capparis zeylanica (MECz) exhibited potent and dose-dependent free radical scavenging activity (Fig. 3) that might augment its anti-cancer efficacy. Our results are supported by previous studies (Yu et al. 2017), who reported that, C. spinosa has high anti-oxidant, free radical elimination ability and promotes cellular apoptosis by regulating selective proteins like Bcl-2 and Bax expression which might be due to the presence of quaternary ammonium hydroxide. The leaf extract of C. brevispina showed high anti-oxidant property with three methods (DPPH, ABTS and phosphomolybdenum) and anti-cancer activity as well (Subramanian and Prasanna 2020).
Earlier research studies on C. zeylanica reported many phytochemicals with pharmaceutical value in leaf extract. For instance, phytol and its isomer is used for synthetic forms of vitamin E and vitamin K (SuffoKamela et al. 2016), colchicine is used for the treatment of gout (Thomopoulou et al. 2015), tocopherol is used as an anti-oxidant of unsaturated lipids (Rodriguez et al. 2021) and (3b)-stigmast-5-en-3-ol is used for the treatment of diabetes (Arulmozhi et al. 2018).
The major phytoconstituents mentioned above such as chlorogenic acid, 6-gingerol, ursolic acid, etc., identified in C. zeylanica and isolated from other plants have shown their versatile therapeutic activities. For example, the poly-phenolic nature of chlorogenic acid obtained from coffee and coffee beans activates endogenous anti-oxidant systems to defence and scavenge against free radicals. It also possesses multiple pharmacological properties like nephroprotective, anti-inflammatory, anti-diabetic, hepatoprotective, anti-hypertensive, neuroprotective, anti-obesity, cardioprotective, anti-angiogenic and to treat breast cancer as well (Onur and Arzu 2021; Nwafor et al. 2022). The phenolic nature of 6-gingerol has wide range of biological properties including anti-oxidant and anti-cancer (Hamza et al. 2021). The anti-oxidant property of ursolic acid, a natural pentacyclic triterpene compound present in a variety of traditional medicinal plants and most fruits and vegetables, exhibits a wide range of therapeutic potential, including anti-cancer activity (Li et al. 2022). Therefore, the strong anti-oxidant and free radical scavenging activity reported in this study for Capparis zeylanica (MECz) may be largely due to the presence of the above mentioned phytochemicals including chlorogenic acid,6-gingerol, and ursolic acid (Figs. 2, 3 and Table 3).
The anti-tumor activity of Capparis species have been reported using different experimental models. For instance, C. spinosa decreases in vitro cell viability, induces apoptosis, and exhibits anti-proliferative activity, which may be due to quercetin present in it (Yu et al. 2017; Moghadamnia et al. 2019). The isolated stachydrine from the Capparis decidua leaf extract has been reported to have potential cytotoxic effect on prostate cancer cells (Rathee et al. 2012). Capparis species, which contain numerous volatile and non-volatile compounds, play an important role in colon cancer prevention through G2/M phase cell cycle arrest, apoptosis stimulation, and suppression of the NF-kB activation pathway (Kulisic-Bilusic et al. 2012). In the present study, MECz showed potent anti-cancer activity on two breast cancer cell lines as demonstrated from cell viability, cell migration, and cell proliferation assays. Our results on cytotoxicity, cell cycle progression and apoptotic profiles showed that MECz at 25 μg/mL concentration significantly inhibited the growth and proliferation of MDA-MB-231 and MCF-7 breast cancer cells (Figs. 4, 5, 6, 7). However, MCF-7 cells were found to be more sensitive to MECz than MDA-MB-231 (Table 4). Apoptosis is a key process in degradation of disease and cancer cells. Therefore, drugs that activate proapoptotic markers play a key role in cancer drug development. Thus, C. zeylanica may be useful in inducing cancer cell death and preventing its progression.
The process of epithelial-to-mesenchymal transition (EMT) is a crucial step towards cancer progression through down-regulation of E-cadherin expression in different types of cancers including breast cancer, which is associated with invasion, metastasis, and therapy resistance (Smith et al. 2013; Zheng et al. 2014). The EMT process is controlled by up-regulation of zinc finger protein transcriptional factors and basic helix–loop–helix factors such as snail, slug, zeb-1, twist, fibronectin and vimentin. These transcription factors potentially bind to the E-box consensus sequence of promoter region of epithelial gene E-cadherin, leading to transcriptional repression of E-cadherin, that means loss of E-cadherin expression, which in turn leads to decreased cell adhesion to the neighboring cells (Zheng et al. 2014; Sinha et al. 2019; Strouhalova et al. 2020). In the present study, MECz at a dose of 25 μg/mL significantly down-regulated the mRNA and protein expression levels of snail, slug, zeb-1, twist, fibronectin and vimentin, but it up-regulated the expression of E-cadherin in MDA-MB-231 and MCF-7 breast cancer cells (Figs. 8, 9); thus, MECz efficiently suppressed the growth and proliferation of breast cancer cells through regulation of epithelial-to-mesenchymal transition (EMT) markers.
Conclusions
Based on the findings, MECz was found to be rich in phenolic compounds including chlorogenic acid, 6-gingerol, and certain triterpenes like ursolic acid, etc., and exhibited potent free radical scavenging and anti-oxidant activity. In vitro studies on both MDA-MB-231 and MCF-7 breast cancer cells demonstrated thats MECz potentially inhibited cell viability, induced apoptosis and caused cell cycle arrest through regulating the expression of key epithelial–mesenchymal transition markers such as snail, slug, zeb-1, twist-1, fibronectin, vimentin, and E-cadherin. Thus, the methanolic root extract of Capparis zeylanica (MECz) may be suggested beneficial in the treatment of breast cancer. Further research is being conducted to identify the specific phytochemical(s) of C. zeylanica and signaling mechanisms that can prevent breast cancer.
Acknowledgements
The author VM is highly debited to the Indian Council of Medical Research (ICMR), New Delhi, India for providing financial support in the form of Research Associate fellowship (45/09/2020/TRM/BMS).
Abbreviations
- C. zeylanica
Capparis zeylanica
- MECz
Methanolic extract of Capparis zeylanica
- EAECz
Ethyl acetate extract of Capparis zeylanica
- AcECz
Acetone extract of Capparis zeylanica
- AqECz
Aqueous extract of Capparis zeylanica
- EMT
Epithelial-to-mesenchymal transition
Author contributions
MV and MB conceived and developed the idea. MV and RSK have done extraction of MECz. MV and RSK conducted in vitro experiments (cell culture studies). MV, RSK and MB collected the data, performed statistical analysis, interpreted the results and wrote the manuscript. MdSI and MB were done final corrections and inputs.
Funding
Publication of this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The analyzed and/or used datasets presented herein are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All the authors hereby give their consent for publication of the revised manuscript.
References
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Alessandria L, Schiliro T, Degan R, Traversi D, Gilli G. Cytotoxic response in human lung epithelial cells and ion characteristics of urban-air particles from Torino, a northern Italian city. Environ Sci Pollut Res Int. 2014;21:5554–5564. doi: 10.1007/s11356-013-2468-1. [DOI] [PubMed] [Google Scholar]
- Angulo P. Non-alcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Arulmozhi P, Vijayakumar S, Kumar T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microb Pathog. 2018;123:219–226. doi: 10.1016/j.micpath.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Arulmozhi P, Vijayakumara S, Praseethab PK, Jayanthic S. Extraction methods and computational approaches for evaluation of antimicrobial compounds from Capparis zeylanica L. Anal Biochem. 2019;572:33–44. doi: 10.1016/j.ab.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Assam AJ, Dzoyem JP, Pieme CA, Penlap VB. In-vitro antibacterial activity and acute toxicity studies of aqueous-methanol extract of Sidarhombifolia Linn. (Malvaceae) BMC Complement Altern Med. 2010;10:40. doi: 10.1186/1472-6882-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst. 1977;102:458–465. doi: 10.1039/an9770200458. [DOI] [PubMed] [Google Scholar]
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G. European legislation on herbal medicines: a look into the future. Drug Saf. 2008;31:428–431. doi: 10.2165/00002018-200831050-00009. [DOI] [PubMed] [Google Scholar]
- Cano-Lamadrid M, Lech K, Calin-Sanchez A, Rosas-Burgos EC, Figiel A, Wojdyło A, Wasilewska M, Carbonell-Barrachina AA. Quality of pomegranate pomace as affected by drying method. J Food Sci Technol. 2018;55:1074–1082. doi: 10.1007/s13197-017-3022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen WM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colourimetric methods. J Food Drug Anal. 2002;10:178–182. doi: 10.38212/2224-6614.2748. [DOI] [Google Scholar]
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants (including the supplement), New Delhi. Council Sci Ind Res. 1986;1:56–92. [Google Scholar]
- Ghule BV, Murugananthan G, Nakhat PD, Yeole PG. Immunostimulant effects of Capparis zeylanica Linn. leaves. J Ethnopharmacol. 2006;108:311–315. doi: 10.1016/j.jep.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Ghule BV, Murugananthan G, Yeole PG. Analgesic and antipyretic effects of Capparis zeylanica leaves. Fitoterapia. 2007;78:365–369. doi: 10.1016/j.fitote.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial–mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza AA, Heeba GH, Hamza S, Abdalla A, Amin A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/inflammation pathway. Biomed Pharmacother. 2021;134:111102. doi: 10.1016/j.biopha.2020.111102. [DOI] [PubMed] [Google Scholar]
- Huseini HF, Hasani-Rnjbar S, Nayebi N, Heshmat R, Sigaroodi FK, Ahvazi M. Capparis spinosa L. (Caper) fruit extract in treatment of type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2013;21:447–452. doi: 10.1016/j.ctim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Infantino A, Tomassoli L, Peri E, Colazza S. Viruses, fungi and insect pests affecting caper. Eur J Plant Sci Biotechnol. 2007;1:170–179. [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jin HR, Jin SZ, Cai-Li XFD, Wu X, Nan JX, Lee JJ, Jin X. Cryptopleurine targets NF-κB pathway, leading to inhibition of gene products associated with cell survival, proliferation, invasion, and angiogenesis. PLoS One. 2012;7:e40355. doi: 10.1371/journal.pone.0040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun, India: International Book Distributors; 1987. pp. 195–197. [Google Scholar]
- Kulisic-Bilusic T, Schmoller I, Schnäbele K, Siracusa L, Ruberto G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.) Food Chem. 2012;132:261–267. doi: 10.1016/j.foodchem.2011.10.074. [DOI] [PubMed] [Google Scholar]
- Li H, Yu Y, Liu Y, Luo Z, Law B, Zheng Y, Huang X, Li W. Ursolic acid enhances the antitumor effects of sorafenib associated with Mcl-1-related apoptosis and SLC7A11-dependent ferroptosis in human cancer. Pharmacol Res. 2022;182:106306. doi: 10.1016/j.phrs.2022.106306. [DOI] [PubMed] [Google Scholar]
- Locatelli M, Zengin G, Uysal A, Carradori S, De Luca E, Bellagamba G, Aktumsek A, Lazarova I. Multicomponent pattern and biological activities of seven Asphodeline taxa: potential sources of natural-functional ingredients for bioactive formulations. J Enzyme Inhib Med Chem. 2017;32:60–67. doi: 10.1080/14756366.2016.1235041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaus B, Ozcan M. Glucosinolates and fatty acid, sterol, and tocopherol composition of seed oils from Capparis spinosa Var. spinosa and Capparis ovate Desf. Var. canescens (Coss) Heywood. J Agric Food Chem. 2005;53:7136–7141. doi: 10.1021/jf051019u. [DOI] [PubMed] [Google Scholar]
- Maurya N, Goel A, Singhai A. Gargcatenin/EMT markers as strong predictors of short survival in subsets of urothelial carcinoma of bladder patients. Adv Cancer Biol Metastasis. 2021;2:100006. doi: 10.1016/j.adcanc.2021.100006. [DOI] [Google Scholar]
- Moghadamnia Y, Mousavi Kani SN, Ghasemi-Kasman M, KazemiKani MT, Kazemi S. The Anti-cancer Effects of Capparis spinosa hydroalcoholic extract. Avicenna J Med Biotechnol. 2019;11:43–47. [PMC free article] [PubMed] [Google Scholar]
- Nahar T, Uddin B, Hossain S, Sikder AM, Ahmed S. Aloe vera gel protects liver from oxidative stress-induced damage in experimental rat model. J Complement Integr Med. 2013;10:1–7. doi: 10.1515/jcim-2012-0020. [DOI] [PubMed] [Google Scholar]
- Nilavukkarasi M, Vijayakumar S, Prathip KS. Biological synthesis and characterization ofsilver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti-proliferation efficiency. Mater Sci Energy Technol. 2020;3:371–376. doi: 10.1016/j.mset.2020.02.008. [DOI] [Google Scholar]
- Nilavukkarasi M, Vijayakumar S, Prathip KS. Capparis zeylanica mediated bio-synthesized ZnO nanoparticles as antimicrobial, photocatalytic and anti-cancer applications. Mater Sci Energy Technol. 2020;3:335–343. doi: 10.1016/j.mset.2019.12.004. [DOI] [Google Scholar]
- Nwafor EO, Lu P, Zhang Y, Liu R, Peng H, Xing B, Liu Y, Li Z, Zhang K, Zhang Y, Liu Z. Chlorogenic acid: potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl Oncol. 2022;15:101294. doi: 10.1016/j.tranon.2021.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onur B, Arzu A. Polyphenol chlorogenic acid, antioxidant profile, and breast cancer, Cancer. 2. Cambridge: Academic Press; 2021. pp. 311–321. [Google Scholar]
- Perry LM. Medicinal plants of East and Southeast Asia. Cambridge: MIT Press; 1980. pp. 68–69. [Google Scholar]
- Rathee P, Rathee D, Rathee D, Rathee S. In vitro anti-cancer activity of stachydrine isolated from Capparis decidua on prostate cancer cell lines. Nat Prod Res. 2012;26:1737–1740. doi: 10.1080/14786419.2011.608673. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Squeo G, Estivi L, Quezada Berru S, Buleje D, Caponio F, Brandolini A, Hidalgo A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sachainchi (Plukenetiavolubilis) oil during French fries deep-frying. Food Chem. 2021;340:127942. doi: 10.1016/j.foodchem.2020.127942. [DOI] [PubMed] [Google Scholar]
- Shah SC, Kayamba V, Peek RM, Jr-Heimburger D. Cancer control in low- and middle-income countries: is it time to consider screening? J Glob Oncol. 2019;5:1–8. doi: 10.1200/JGO.18.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Singh AP. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud. 2012;5:112–118. doi: 10.1016/j.jams.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Sinha N, Meher BR, Naik PP. p73 induction by Abrus agglutinin facilitates Snail ubiquitination to inhibit epithelial to mesenchymal transition in oral cancer. Phytomedicine. 2019;55:179–190. doi: 10.1016/j.phymed.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetan K, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants—a review. Afr J Food Sci. 2010;4:200–222. doi: 10.5897/AJFS.9000287. [DOI] [Google Scholar]
- Strouhalova K, Prechova M, Gandalovicova A, Brabek J, Gregor M, Rosel D. Vimentin intermediate filaments as potential target for cancer treatment. Cancers. 2020;12:184. doi: 10.3390/cancers12010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SK, Prasanna R. Antioxidant and cytotoxic activities of Indian caper (Capparis brevispina DC (Capparaceae)) leaf extracts. Eur J Integr Med. 2020;3:101038. doi: 10.1016/j.eujim.2019.101038. [DOI] [Google Scholar]
- SuffoKamela AL, Mouokeu RS, Ashish R, MaffoTazoho G, Glory Moh L, PamoTedonkeng E, Kuiate JR. Influence of processing methods on proximate composition and dieting of two Amaranthus species from West Cameroon. Int J Food Sci. 2016;13:1–8. doi: 10.1155/2016/6707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Thomopoulou P, Sachs J, Teusch N, Mariappan A, Gopalakrishnan J, Schmalz HG. New colchicine-derived triazoles and their influence on cytotoxicity and microtubule morphology. ACS Med Chem Lett. 2015;7:188–191. doi: 10.1021/acsmedchemlett.5b00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlili N, Nasri N, Saadaoui E, Khaldi A, Triki S. Carotenoid and tocopherol composition of leaves, buds, and flowers of Capparis spinosa grown wild in Tunisia. J Agric Food Chem. 2009;57:5381–5385. doi: 10.1021/jf900457p. [DOI] [PubMed] [Google Scholar]
- Williams CA, Grayer RJ. Anthocyanins and other flavonoids. Nat Prod Rep. 2004;21:539–573. doi: 10.1039/b311404j. [DOI] [PubMed] [Google Scholar]
- Yang AP, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang R, Kalcheim C, Kalluri R. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Qiu HM, Cheong KL, Zhong S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int J Biol Macromol. 2022;221:472–485. doi: 10.1016/j.ijbiomac.2022.09.055. [DOI] [PubMed] [Google Scholar]
- Yu L, Yang J, Wang X, Jiang B, Sun Y, Ji Y. Antioxidant and antitumor activities of Capparis spinosa L. and the related mechanisms. Oncol Rep. 2017;37(1):357–367. doi: 10.3892/or.2016.5249. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma ZF. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients. 2018;10:116. doi: 10.3390/nu10020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Shen M, Zha YL, Li W, Wei Y, Blanco MA, Ren G, Zhou T, Storz P, Wang HY, Kang Y. PKD1 phosphorylation-dependent degradation of SNAIL by SCF-FBXO11 regulates epithelial-mesenchymal transition and metastasis. Cancer Cell. 2014;26:358–373. doi: 10.1016/j.ccr.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed and/or used datasets presented herein are available from the corresponding author upon reasonable request.