Abstract
Background & Aims
Efruxifermin has shown clinical efficacy in patients with non-alcoholic steatohepatitis (NASH) and F1–F3 fibrosis. The primary objective of the BALANCED Cohort C was to assess the safety and tolerability of efruxifermin in patients with compensated NASH cirrhosis.
Methods
Patients with NASH and stage 4 fibrosis (n = 30) were randomized 2:1 to receive efruxifermin 50 mg (n = 20) or placebo (n = 10) once-weekly for 16 weeks. The primary endpoint was safety and tolerability of efruxifermin. Secondary and exploratory endpoints included evaluation of non-invasive markers of liver injury and fibrosis, glucose and lipid metabolism, and changes in histology in a subset of patients who consented to end-of-study liver biopsy.
Results
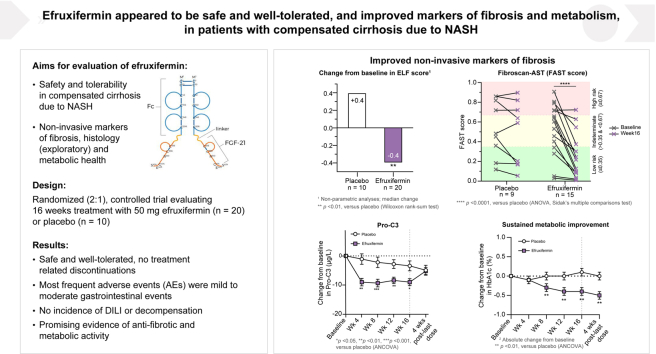
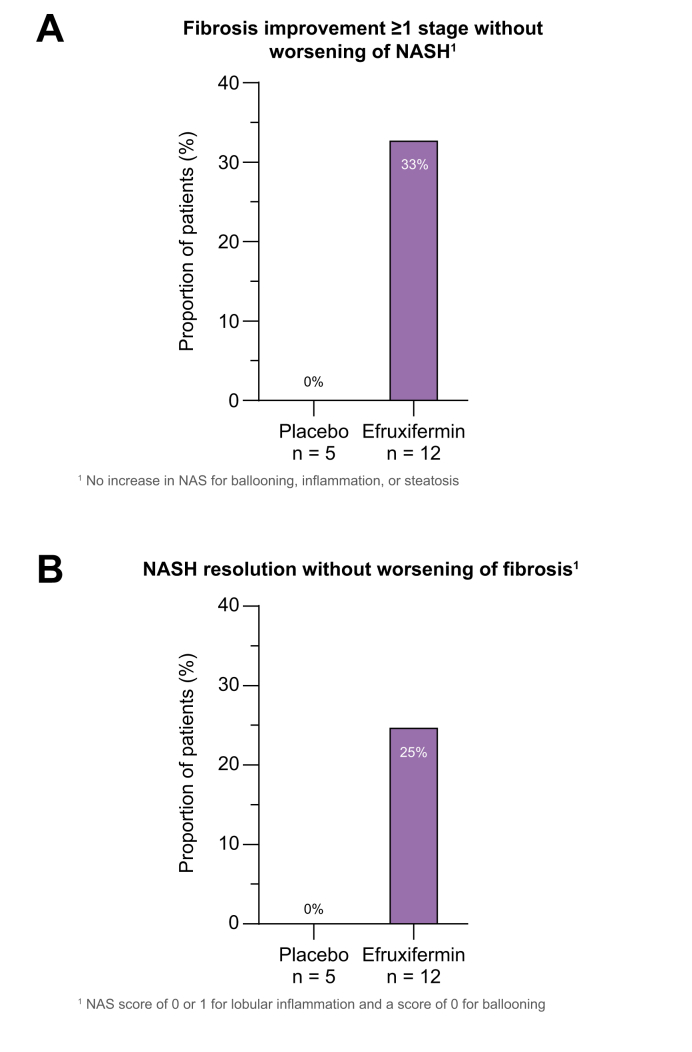
Efruxifermin was safe and well-tolerated; most adverse events (AEs) were grade 1 (n = 7, 23.3%) or grade 2 (n = 19, 63.3%). The most frequent AEs were gastrointestinal, including transient, mild to moderate diarrhea, and/or nausea. Significant improvements were noted in key markers of liver injury (alanine aminotransferase) and glucose and lipid metabolism. Sixteen-week treatment with efruxifermin was associated with significant reductions in non-invasive markers of fibrosis including Pro-C3 (least squares mean change from baseline [LSMCFB] −9 μg/L efruxifermin vs. −3.4 μg/L placebo; p = 0.0130) and ELF score (−0.4 efruxifermin vs. +0.4 placebo; p = 0.0036), with a trend towards reduced liver stiffness (LSMCFB −5.7 kPa efruxifermin vs. −1.1 kPa placebo; n.s.). Of 12 efruxifermin-treated patients with liver biopsy after 16 weeks, 4 (33%) achieved fibrosis improvement of at least one stage without worsening of NASH, while an additional 3 (25%) achieved resolution of NASH, compared to 0 of 5 placebo-treated patients.
Conclusions
Efruxifermin appeared safe and well-tolerated with encouraging improvements in markers of liver injury, fibrosis, and glucose and lipid metabolism following 16 weeks of treatment, warranting confirmation in larger and longer term studies.
Lay summary
Cirrhosis resulting from non-alcoholic steatohepatitis (NASH), the progressive form of non-alcoholic fatty liver disease, represents a major unmet medical need. Currently there are no approved drugs for the treatment of NASH. This proof-of-concept randomized, double-blind clinical trial demonstrated the potential therapeutic benefit of efruxifermin treatment compared to placebo in patients with cirrhosis due to NASH.
Clinical Trial Number
Keywords: FGF21, non-alcoholic steatohepatitis/NASH, cirrhosis, clinical trial, liver disease, efruxifermin, nonalcoholic fatty liver disease/NAFLD, histopathology
Abbreviations: ADA(s), anti-drug antibody(ies); AE, adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANCOVA, analysis of covariance; AST, aspartate aminotransferase; CFB, change from baseline; C–P, Child-Pugh; CTX-1, C-terminal telopeptide of type 1 collagen; DXA, dual-energy X-ray absorptiometry; ELF, enhanced liver fibrosis; FGF21, fibroblast growth factor-21; FGFR, fibroblast growth factor receptor; GGT, gamma-glutamyltransferase; HbA1c, hemoglobin A1c; HDL-C, HDL-cholesterol; HPA, hypothalamic-pituitary-adrenal; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; INR, international normalized ratio; IRT, interactive response technology; LDL-C, LDL-cholesterol; LS, least squares; MELD, model for end-stage liver disease; NAb, neutralizing antibody; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; Non-HDL-C, non-HDL-cholesterol; PAI-1, plasminogen activator inhibitor-1; P1NP, procollagen type-I N-terminal propeptide; P3NP, procollagen type III N-terminal propeptide; Pro-C3, N-terminal type III collagen propeptide; TEAE, treatment-emergent adverse event; TIMP-1, tissue inhibitor of metalloproteinase-1; ULN, upper limit of normal
Graphical abstract
Highlights
-
•
Efruxifermin appeared safe and well-tolerated in patients with NASH and compensated cirrhosis.
-
•
Improvements were noted in serum markers of liver injury and fibrosis – consistent with histological trends in efruxifermin-treated patients.
-
•
Improvements in lipoprotein profile, insulin sensitivity, and glycemic control indicate added beneficial effects of efruxifermin.
-
•
These data support the conduct of larger, adequately powered studies to test the findings from this hypothesis-generating study.
Introduction
Non-alcoholic steatohepatitis (NASH), the most severe and progressive form of non-alcoholic fatty liver disease (NAFLD), is characterized by abnormal accumulation of fat in the liver (steatosis), hepatocellular ballooning, and inflammation.[1], [2], [3] The global prevalence of NAFLD is increasing with recent estimates ranging from 25% to 30% worldwide, with 3% to 5% progressing to NASH with advanced fibrosis or cirrhosis.[4], [5], [6]
Fibrosis stage is the strongest predictor of adverse clinical outcomes in NASH.[7], [8], [9], [10], [11] In a post hoc analysis of phase III interventional studies, an improvement of ≥1 stage from F4 to ≤F3 was associated with a reduction in incidence of liver-related outcomes from 7.2% to 1.1%, corresponding to a greater than 80% reduction in liver-related complications.12
In the most advanced stages, NASH may present as cryptogenic cirrhosis or burned-out NASH,13 which is associated with higher probability of liver failure, hepatocellular carcinoma14 and/or major adverse cardiac events.7 NASH is among the leading causes of liver transplantation,15 a particularly acute need for patients with cirrhosis,9 who have an estimated transplant-free survival rate of approximately 60% over 5 years.8
With no approved drugs for treating NASH there is a clear unmet medical need to slow, halt, or reverse progression of disease, particularly for patients with cirrhosis.16 Optimal therapy would improve liver health (steatosis, inflammation, and fibrosis) while restoring whole-body metabolic health by reducing prevalent comorbidities of dyslipidemia, impaired glycemic control, obesity, and hyperuricemia. To date, potential drug candidates have tended to target either the underlying metabolic dysfunction of the liver, or the downstream consequences of hepatocyte stress and injury, inflammation, and fibrosis.17
Fibroblast growth factor 21 (FGF21) analogues have emerged as a promising therapeutic target possessing many of the characteristics of an ideal NASH therapy. FGF21 not only addresses the underlying disease driver (i.e., excessive accumulation of fat in the liver), but also the downstream sequelae of inflammation and fibrosis.[18], [19], [20], [21], [22], [23], [24], [25] FGF21 appears to act directly and indirectly to reduce pro-fibrogenic signaling.26 Acting indirectly, FGF21 reduces liver fat and lipotoxicity while simultaneously protecting against cell stress and activation of apoptosis, in turn suppressing activation of Kupffer cells and hepatic stellate cells.20,21,24,27 Acting directly, FGF21 appears to inhibit activation of hepatic stellate cells into collagen-secreting myofibroblasts.26 An increasing number of FGF21 analogues are under clinical evaluation with differences evident in their therapeutic profiles. Variation in the extent of stimulation of FGF21’s cognate receptors (FGFR1c, FGFR2c, FGFR3c) in the two tissues contributing most to the accumulation of fat in the liver, i.e. adipose tissue (via FGFR1c) or liver (via FGFR2c/3c), may at least partly explain these differences.26 For example, induction of maximal adiponectin response downstream of FGFR1c signaling in adipose tissue appears to be weaker for the PEGylated FGF21 analogue pegbelfermin,28 than reported for other FGF21 analogues at comparable or lower moles of FGF21.29 Such differences may in part be attributable to lower exposure of PEGylated analogues in peripheral adipose tissue than the liver, or the differences in half-life of pegbelfermin30 and Fc-fusion FGF21 proteins.29
Efruxifermin is a long-acting FGF21 analogue comprised of a modified sequence of native human FGF21 fused to the Fc region of human IgG1. The mutations in the FGF21 moiety specific to efruxifermin increase half-life in humans to more than three days, and enhance binding affinity to the obligate co-receptor, beta-klotho.29,31 Efruxifermin has balanced in vitro potency across FGF21’s receptors, FGFR1c, FGFR2c, and FGFR3c31 and high systemic exposure in vivo,29 enabling it to act on adipose tissue and the liver, the major sources of liver fat. It is inactive at FGFR4, thereby avoiding an undesirable increase in LDL-cholesterol (LDL-C) levels. In a phase IIa study of patients with F1–F3 NASH, efruxifermin significantly reduced liver fat and markers of liver injury, decreased fibrosis, improved glucose and lipid metabolism, and reduced hyperuricemia, with a trend to weight loss.32 Notable among these improvements was a 2-stage reversal of fibrosis in 11 of 22 (50%) patients with F2 or F3 NASH after only 16 weeks of treatment.32
This study reports results from Cohort C, an expansion cohort of the phase IIa study, in patients with compensated cirrhosis (F4) due to NASH. The primary objective was to assess safety and tolerability of efruxifermin in these patients, who are at the highest risk of progressing to hepatic decompensation and liver failure, and therefore have the greatest unmet medical need. Secondary objectives were to establish proof-of-concept for efficacy of efruxifermin in patients with compensated cirrhosis by evaluating its effects on liver stiffness and biomarkers of liver fibrosis. Exploratory objectives included evaluation of effects on markers of liver injury, function, and histology, as well as whole-body metabolism.
Patients and methods
Study design and participants
Cohort C was a randomized, double-blind, placebo-controlled phase IIa expansion cohort of the BALANCED study (NCT03976401) that evaluated safety and tolerability of efruxifermin in patients with NASH and compensated cirrhosis (F4 fibrosis). This study was conducted at 18 gastroenterology or hepatology clinics in the USA.
Eligible patients were 18 to 80 years of age with biopsy-confirmed compensated cirrhosis due to NASH, as documented by a local pathologist. Participants were required to have a FibroScan measurement >13.1 kPa and enhanced liver fibrosis (ELF) score >9.8, and no evidence of worsening levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) between screening and randomization. Patients must have had a glomerular filtration rate ≥60 ml/min; hemoglobin A1c (HbA1c) ≤9.5%; hemoglobin ≥11 g/dl; international normalized ratio (INR) ≤1.3; direct bilirubin ≤0.3 mg/dl; total bilirubin ≤1.3 × upper limit of normal (ULN); creatine kinase <3 × ULN; platelet count ≥125,000/μl; serum triglyceride level ≤500 mg/dl; ALT and AST <5 × ULN; alkaline phosphatase (ALP) <2 × ULN; and albumin ≥3.5 g/dl. Patients with decompensated liver disease, liver transplantation, hepatocellular carcinoma or other chronic liver diseases including viral hepatitis were excluded. Patients were allowed to continue with their anti-diabetic medications including glucagon like peptide-1 receptor agonists or sodium-glucose transport protein 2 inhibitors, provided they were on a stable dose for at least 6 months prior to screening. All participants provided written informed consent before enrollment. All inclusion and exclusion criteria are provided in the supplementary information (Table S1).
Eligible participants were randomly assigned in a 1:2 ratio to placebo or efruxifermin 50 mg treatment groups, for a total study period of 26 weeks: 6 weeks for screening, 16 weeks on-treatment, 4 weeks of safety follow-up (Fig. S1). A qualified staff member administered the study drug (1.0 ml s.c. injection) into the abdomen of each participant at weekly intervals. The 50 mg dose of efruxifermin was chosen based on results from the BALANCED main study,32 as optimally balancing efficacy with safety and tolerability.
Interactive response technology (IRT) was used for centralized randomization and treatment assignment. Study site personnel obtained the treatment assignment from the IRT.
Study drug (efruxifermin or placebo) was prepared by an independent, unblinded, qualified healthcare professional (such as a pharmacist not associated with the study). Efruxifermin and placebo were identical in appearance and administered in a blinded fashion to the participants. All investigators, staff, sponsor, and patients were blinded to treatment assignment.
Study assessments
Safety and tolerability
Safety and tolerability of efruxifermin were assessed through the reporting of adverse events (AEs), treatment-emergent adverse events (TEAEs), clinical laboratory tests, electrocardiograms, vital sign assessments, body weight, anti-drug antibodies (ADA) including neutralizing activity (NAb), and concomitant medication usage. Markers of liver function (albumin, bilirubin, model for end-stage liver disease [MELD] score, Child-Pugh score [C–P]); hemostasis (INR, fibrinogen, platelets, and plasminogen activator inhibitor-1 [PAI-1]), a marker of systemic inflammation (high-sensitivity C-reactive protein [hs-CRP]), biomarkers of bone turnover (osteocalcin, procollagen type-I N-terminal propeptide [P1NP], C-terminal telopeptide of type 1 collagen [CTX-1], bone specific alkaline phosphatase, parathyroid hormone, and vitamin D), and a marker of hypothalamus-pituitary-adrenal (HPA) axis activation (salivary cortisol) were obtained at baseline and every 4 weeks throughout the study. Dual-energy X-ray absorptiometry (DXA) scan measurements, including bone mineral density of the lumbar spine, femoral neck, total hip, and percent body fat, were performed at baseline and week 16. The safety follow-up visit was performed at week 20, 4 weeks after the last dose of study drug.
Clinical laboratory tests were performed by Medpace Reference Laboratories (Cincinnati, Ohio). ADA and NAb assays were performed by Precision for Medicine Bioanalytical Laboratory (Redwood City, California). Immunogenicity data analysis and reporting were conducted by B2S Life Sciences (Franklin, Indiana).
Non-invasive markers of fibrosis and liver injury
Efficacy was evaluated as change from baseline to week 16 in non-invasive markers of fibrosis, including liver stiffness by transient elastography (FibroScan) and serum markers N-terminal type III collagen propeptide (Pro-C3), ELF score and its components (hyaluronic acid; procollagen type III N-terminal propeptide [P3NP]; and tissue inhibitor of metalloproteinase-1 [TIMP-1]). ALT, AST, bilirubin, gamma-glutamyltransferase (GGT), ALP, and urate were measured every 4 weeks as biomarkers of liver injury.
In response to requests from participants and investigators, the protocol was amended to assess the impact of treatment on NASH histopathology by liver biopsy obtained on a voluntary basis at week 16. Baseline and end-of treatment biopsies were read by a single pathologist (central reader, Pacific Rim Pathology, San Diego, California). For all biopsies, the reader was blinded to treatment, sequence, and participant. Imaging aspects of the study were conducted by Perspectum Diagnostics (Oxford, United Kingdom).
Markers of metabolism
Markers of lipid metabolism (triglycerides, LDL-C, HDL-cholesterol [HDL-C], non-HDL-cholesterol [non-HDL-C]), insulin sensitivity (C-peptide, insulin, adiponectin), glycemic control (HbA1c), and body weight were measured at baseline and at weeks 4, 8 12, 16, and 20.
Outcome measures
The primary endpoint was safety and tolerability of efruxifermin in patients with compensated cirrhosis due to NASH. Secondary endpoints included change from baseline to week 16 in liver stiffness and non-invasive biomarkers of fibrosis: ELF score and serum levels of Pro-C3. Exploratory endpoints included changes in liver histopathology, as well as in markers of liver injury and of glucose and lipid metabolism. A full list of exploratory endpoints is provided in the supplementary information (Table S2).
Statistical analyses
Since the primary endpoint was safety and tolerability, sample size was determined based on clinical considerations. Cross-treatment comparison of secondary and exploratory endpoints was not powered to detect pre-specified differences. Statistical analyses were conducted by Medpace (Cincinnati, Ohio).
Demographic and baseline characteristics were summarized with descriptive statistics. For the primary endpoint, safety and tolerability of efruxifermin, the safety set included all individuals who received at least one dose of study drug. All data collected during treatment through 30 days after the last dose of study drug were included in the safety analyses.
For the secondary endpoint of liver stiffness, analysis of covariance (ANCOVA) was used to determine the absolute and percent change from baseline to week 16, with treatment group as a factor and baseline liver stiffness as a covariate using the full analysis set. Normality of the residuals was tested by the Shapiro-Wilk test.
For the secondary endpoints of non-invasive biomarkers of fibrosis (ELF test score and Pro-C3) and exploratory endpoints including markers of liver injury (AST, ALT, GGT, ALP), liver function (albumin, bilirubin, MELD score, C–P score), hemostasis (platelet count, INR,), HPA (salivary cortisol) and body weight, an ANCOVA model with treatment group as a factor and baseline value as a covariate was used. Exploratory biomarkers of lipid metabolism, insulin sensitivity (C-peptide, insulin, adiponectin), glycemic control (HbA1c), and DXA scan measurements were also analyzed as absolute and percent change from baseline by treatment using an ANCOVA model with treatment group as a factor and baseline value as a covariate. Analysis of urate, fibrinogen, PAI-1 and hs-CRP was performed using mixed-model repeated measures with absolute change from baseline as the dependent variable and treatment group and visit as factors and baseline value as a covariate, as well as interaction of treatment group by visit. For all of the above, least squares (LS) mean, 95% CIs, and p values for change from baseline as well as difference from placebo were calculated. The Shapiro-Wilk normality test was performed for residuals from the model; if the p value was <0.01, non-parametric analyses were conducted. Non-parametric analyses are presented for the ELF score and for its individual components (P1NP, hyaluronic acid, TIMP-1), where p values are from the Wilcoxon rank-sum test.
The liver biopsy analysis set included all patients with week 16 liver biopsies whose stage-4 fibrosis at baseline was confirmed by the central reader. Data were analyzed according to the proportion of individuals at week 16 whose fibrosis regressed by ≥1 stage; fibrosis regressed by ≥1 stage with no worsening in non-alcoholic fatty liver disease activity score (NAS); NAS improved by ≥2 points; NAS improved by ≥1-point in any of steatosis, lobular inflammation, or hepatocellular ballooning; or NASH resolved (i.e., 0- or 1-point inflammation, 0-point ballooning).
Study oversight
The study protocol and all amendments were approved by an institutional review board and independent ethics committee for each site in compliance with the ethical principles of the Declaration of Helsinki, and consistent with the International Conference on Harmonization of Good Clinical Practice and applicable regulatory guidelines. The study was designed and conducted according to the Sponsor’s (Akero Therapeutics, Inc) protocol. An independent data monitoring committee reviewed progress and provided oversight. Further information regarding the methods is available in the CONSORT statement.
Results
Patient disposition
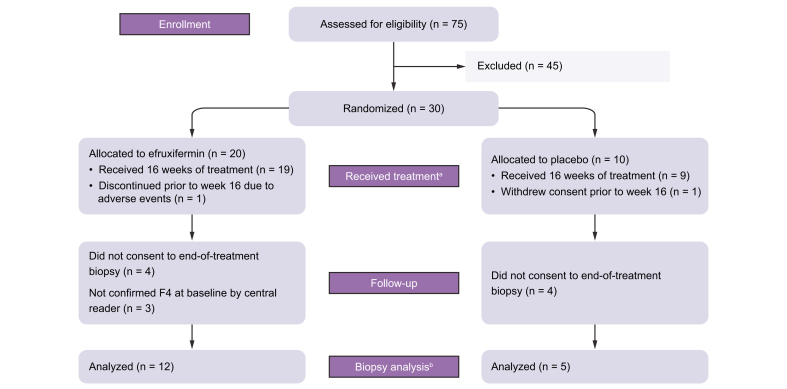
Between 4 June 2020 and 23 September 2020, 75 patients were screened at 18 sites in the USA. A total of 30 patients with compensated cirrhosis due to NASH were enrolled and randomized to receive either efruxifermin (n = 20) or placebo (n = 10). Follow-up continued until 10 February 2021. All patients started the study, with two patients discontinuing prior to week 16: one placebo patient withdrew consent, and one efruxifermin patient withdrew due to adverse events. The remaining patients (19 efruxifermin and 9 placebo) completed 16 weeks of treatment. Eight patients (4 in each group) did not consent to optional end-of-treatment biopsy. Three efruxifermin patients assessed originally by a local pathologist as F4 were not confirmed as F4 by the central reader, thus were not included in the biopsy analysis set. Of these three patients, two were considered difficult to stage by the central pathologist and one patient was identified as a borderline F3. The remaining 17 patients with end-of-treatment biopsies were confirmed as F4 patients by the central reader and comprised the biopsy analysis set: 12 efruxifermin-treated and 5 in the placebo group (Fig. 1). Non-invasive markers of fibrosis were evaluated in all individuals (full analysis set) and in the liver biopsy analysis set.
Fig. 1.
CONSORT clinical study flow diagram.
aComprises the full analysis set and bcomprises the biopsy analysis set.
Baseline demographics and disease characteristics
The demographics and baseline characteristics of participants in this study are representative of patients with compensated cirrhosis due to NASH and were generally balanced across treatment groups with a few exceptions (Table 1), potentially due to the small sample size. The mean age was 61 and 57 years in efruxifermin and placebo groups, respectively, while baseline body weight was higher in the placebo group. The efruxifermin-treated group was predominantly female (n = 16; 80%), while the placebo group was mostly male (n = 7; 70%). Pathophysiological characteristics reflect the advanced NASH stage of F4 patients, as indicated by mean AST and ALT values in the low thirties, ELF scores ranging between 9.7 and 10.4, and relatively high values for Pro-C3 (22.6 to 25.6 μg/L) and liver stiffness (22.1 to 25.8 kPa).
Table 1.
Baseline demographics and disease characteristics.
| Parameter | Placebo (n = 10) | Efruxifermin (n = 20) |
|---|---|---|
| Mean age, years (SD) | 57.1 (14.4) | 61.1 (10.0) |
| Sex, n (%) | ||
| Male | 7 (70.0) | 4 (20.0) |
| Female | 3 (30.0) | 16 (80.0) |
| Race or ethnicity, n (%) | ||
| White | 10 (100.0) | 18 (90.0) |
| Black or African American | 0 | 1 (5.0) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (5.0) |
| Hispanic or Latino | 5 (50.0) | 8 (40.0) |
| Metabolic risk factors and parameters, mean (SD) | ||
| Body weight, kg | 119.1 (30.5) | 97.9 (19.8) |
| Body mass index, kg/m2 | 39.1 (8.2) | 36.0 (5.6) |
| Type 2 diabetes, n (%) | 5 (50.0) | 10 (50.0) |
| Markers of liver health, mean (SD) | ||
| Alanine aminotransferase, U/L | 32.7 (20.0) | 31.7 (16.8) |
| Aspartate aminotransferase, U/L | 28.9 (21.1) | 31.4 (13.7) |
| Gamma-glutamyltransferase, U/L | 46.7 (17.7) | 75.8 (39.2) |
| Alkaline phosphatase, U/L | 67.2 (19.0) | 77.9 (24.6) |
| Urate, mg/dl | 5.9 (1.4) | 6.0 (1.2) |
| High-sensitivity C-reactive protein, mg/L | 4.9 (5.8) | 6.7 (8.4) |
| Liver steatosis, CAP score, dB/m | 336.9 (48.5) | 299.2 (59.3) |
| Markers of fibrosis, mean (SD) | ||
| Pro-C3, μg/L | 22.6 (11.8) | 25.6 (27.5) |
| ELF score | 9.7 (0.8) | 10.4 (1.2) |
| Liver stiffness, kPA | 25.8 (13.2) | 22.1 (10.8) |
| Markers of liver function, mean (SD) | ||
| Bilirubin, mg/dl | 0.8 (0.2) | 0.7 (0.3) |
| Albumin, g/dl | 4.3 (0.2) | 4.2 (0.3) |
| Platelets, 10ˆ9/L | 166.8 (29.5) | 191.7 (34.5) |
| Fibrinogen, mg/dl | 407.0 (93.7) | 452.2 (64.6) |
| International normalized ratio | 1.1 (0.1) | 1.1 (0.1) |
| Plasminogen activator inhibitor-1, IU/ml | 14.0 (11.8) | 14.5 (10.6) |
| Model for end-stage liver disease score | 6.9 (0.9) | 7.4 (1.0) |
| Child-Pugh score | 5.0 (0.0) | 5.1 (0.2) |
| NAFLD activity score, mean (SD) | 3.3 (2.1) | 4.1 (1.7) |
| Markers of lipid metabolism, mean (SD) | ||
| Total cholesterol, mg/dl | 157.1 (42.9) | 167.4 (40.4) |
| Triglycerides, mg/dl | 121.7 (59.6) | 134.6 (62.8) |
| LDL-C, mg/dl | 89.5 (34.7) | 90.1 (34.4) |
| HDL-C, mg/dl | 43.3 (12.4) | 50.4 (13.8) |
| Non-HDL-C, mg/dl | 113.8 (43.3) | 117.1 (41.2) |
| Apolipoprotein B, mg/dl | 82.8 (26.1) | 82.6 (26.5) |
| Apolipoprotein C-III, mg/dl | 6.3 (3.3) | 7.8 (4.0) |
| Lipoprotein-a (nmol/L) | 48.7 (71.9) | 61.9 (66.7) |
| Beta -hydroxybutyrate (mmol/L) | 0.1 (0.1) | 0.1 (0.1) |
| Bile acids (μmol/L) | 10.3 (6.7) | 9.5 (8.8) |
| Markers of glycemic control, mean (SD) | ||
| Fasting serum glucose, mg/dl | 122.9 (26.4) | 107.3 (16.4) |
| HbA1c, % | 6.6 (1.4) | 6.1 (1.0) |
| Patients with type 2 diabetes | 7.3 (1.6), n = 5 | 6.5 (1.1), n = 10 |
| Patients without type 2 diabetes | 5.8 (0.7), n = 4 | 5.7 (0.5), n = 9 |
| C-peptide, μg/L | 5.7 (2.1) | 5.1 (1.0) |
| Insulin, mIU/L | 38.7 (21.9) | 29.4 (9.3) |
| HOMA-IR | 12.5 (8.1) | 8.4 (3.7) |
| Adiponectin, mg/L | 4.8 (2.5) | 5.8 (2.9) |
| Concomitant medications of interest, n (%) | ||
| Statins | 3 (30.0) | 6 (30.0) |
| GLP-1 receptor agonists | 3 (30.0) | 3 (15.0) |
CAP, controlled attenuation parameter; HOMA-IR, homeostatic model assessment of insulin resistance.
Mean triglyceride levels are also lower than earlier stages of NASH, likely due to lower capacity for secretion of VLDL resulting from fewer healthy hepatocytes in patients with cirrhosis. Half of the patients had type 2 diabetes, with mean baseline HbA1c values of 6.5–7.3%, compared to 5.7% in patients without diabetes.
In the full analysis set, based on levels of fibrosis biomarkers Pro-C3 and ELF, the efruxifermin-treated group appears to be slightly more advanced compared to the placebo group. However, in the liver biopsy analysis set (n = 17), baseline levels of these fibrosis biomarkers were comparable across both treatment groups as shown in Table S3. The small number of patients in each group likely contributed to the apparent variability across some of the baseline characteristics.
Safety and tolerability
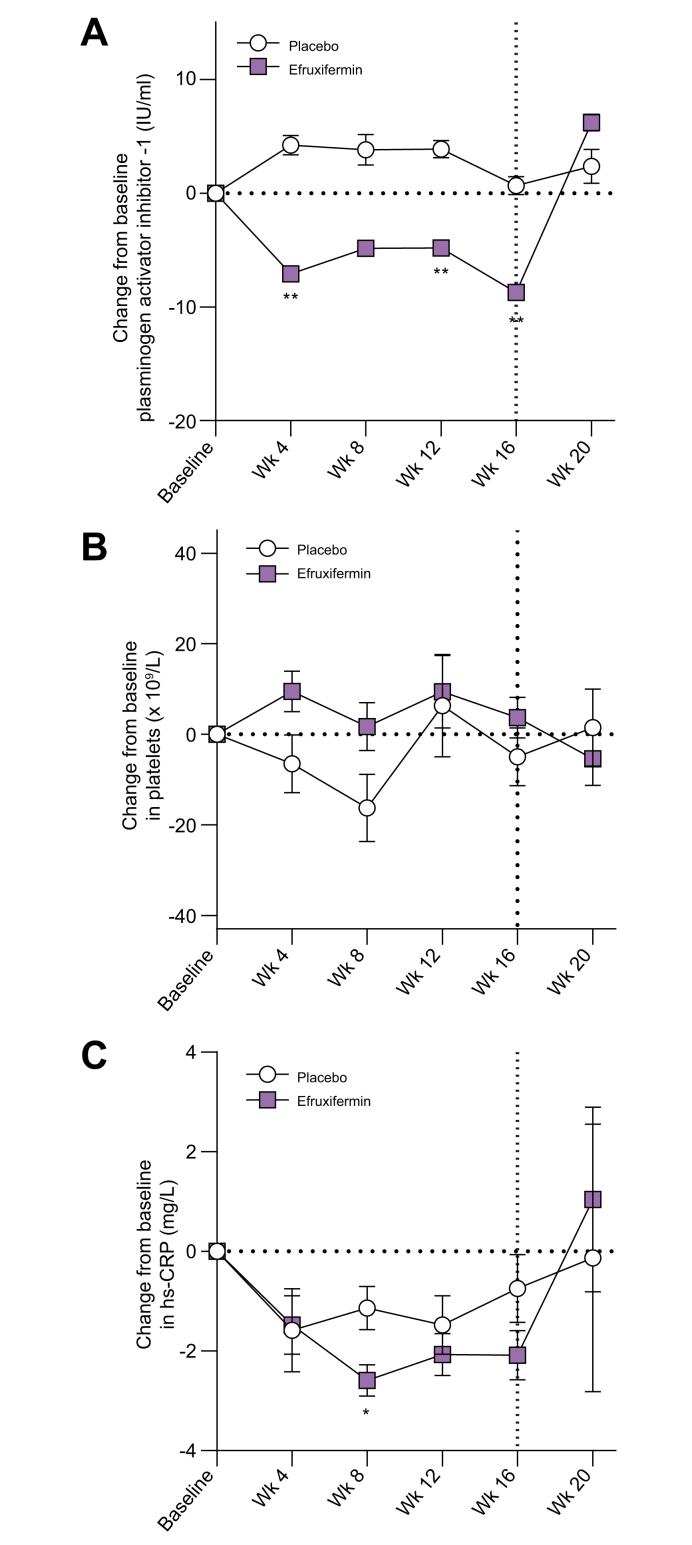
The primary objective was to determine safety and tolerability of efruxifermin in patients with compensated cirrhosis due to NASH. Overall, efruxifermin appeared to be well-tolerated, with a similar safety profile to that observed in patients with fibrosis stage 1-3.32 No new population-specific safety concerns were noted. Twenty-seven (90%) participants reported one TEAE: 19 (95.0%) in the efruxifermin group and 8 (80.0%) in the placebo group. The majority of TEAEs were grade 1 (n = 7, 23.3%) or grade 2 (n = 19, 63.3%). Table 2 lists all TEAEs reported in ≥15% of patients in either treatment group. Compared to placebo, more patients in the efruxifermin group experienced TEAEs of special interest, which included diarrhea, injection site reactions (including bruising, erythema, pruritus, and rash), and decreased blood glucose. The most frequent AEs were gastrointestinal, predominantly mild or moderate, occurring primarily during the first 4 weeks of treatment, generally transient and resolving without treatment. Injection site reactions were infrequent, transient, and of mild severity (all grade 1). There were no serious AEs in the efruxifermin group. One placebo-treated patient (3.3%) experienced a grade 4 TEAE of pulmonary embolism, considered unrelated to study treatment or procedures. One patient treated with efruxifermin discontinued at week 5 due to adverse events, experiencing abdominal distension (grade 1), constipation (grade 1), diarrhea (grade 1) and pruritus (grade 1). There were no deaths and no cases of hepatic decompensation or drug-induced liver injury. Most hemostasis parameters either remained stable (INR) or trended to improve (platelets, PAI-1) (Table S5, Fig. 2). There were no increases in MELD or C–P score, and markers of liver function (e.g., total/direct bilirubin, albumin) were unchanged. A marker of systemic inflammation, hs-CRP, was significantly reduced from baseline at week 8 and trended lower after 12 and 16 weeks of efruxifermin treatment (Fig. 2).
Table 2.
Safety and tolerability.
| Safety overview | Placebo (n = 10) | Efruxifermin (n = 20) |
|---|---|---|
| Study discontinuations | 1a | 1b |
| Deaths | 0 | 0 |
| Any treatment-emergent adverse events, n (%) | 8 (80) | 19 (95) |
| Treatment-emergent adverse events, n (%) | ||
| Life-threatening | 1 (10) | 0 |
| Severe | 0 | 0 |
| Moderate | 5 (50) | 14 (70) |
| Mild | 2 (20) | 5 (25) |
| Study procedure related | 1 (10) | 7 (35) |
| Treatment-emergent events leading to discontinuation, n (%) | 0 | 1 (5) |
| Drug-related treatment-emergent adverse events, n (%) | 3 (30) | 13 (65) |
| Serious adverse events | 1c | 0 |
| Treatment-emergent events occurring in ≥15% of patients in any group, n (%) | ||
| Gastrointestinal disorders | 6 (60) | 14 (70) |
| Diarrhea | 1 (10) | 10 (50) |
| Nausea | 2 (10) | 9 (45) |
| Vomiting | 0 | 4 (20) |
| Abdominal pain | 2 (20) | 3 (15) |
| Constipation | 0 | 4 (20) |
| Gastroesophageal reflux disease | 2 (20) | 0 |
| Nervous system disorders | 4 (40) | 8 (40) |
| Headache | 1 (10) | 4 (20) |
| General disorders and administration site conditions | 1 (10) | 10 (50) |
| Injection site reaction | 0 | 6 (30) |
| Injection site bruising | 1 (10) | 4 (20) |
| Injection site erythema | 0 | 5 (25) |
| Infections and infestations | 2 (20) | 7 (35) |
| Sinusitis | 0 | 4 (20) |
| Skin and subcutaneous tissue disorders | 0 | 9 (45) |
| Pruritus | 0 | 3 (15) |
Withdrawal of consent.
Abdominal distention, constipation, diarrhea, pruritus.
Pulmonary embolism.
Fig. 2.
Time courses for absolute change from baseline in levels of plasminogen activator inhibitor-1, platelets, and high-sensitivity C-reactive protein.
Significance is indicated by asterisks: ∗p <0.05, ∗∗p <0.01 vs. placebo (MMRM). The vertical line at week 16 indicates the end of the treatment period, with safety follow-up continuing to week 20.
Vital signs including heart rate and blood pressure were unchanged. Bone mineral density was unchanged over 16 weeks of treatment. Consistent with this, levels of CTX-1 (a bone resorption marker) were unchanged relative to baseline (p = 0.2445) and to placebo (p = 0.7114). However, levels of P1NP (a biomarker of type-I collagen synthesis in EFX-treated group any tissue or organ in the body) were approximately 20% lower (p = 0.0211 vs. placebo). Levels of salivary cortisol (a marker of HPA axis activation) were unchanged throughout the treatment period.
Of the patients who received efruxifermin, 11 of 20 (55%) tested positive for treatment-emergent ADAs by week 20. The observed titers were low (1:26.01 or lower) and were first detected at week 8. Sensitivity of the ADA assay, at 5.574 ng/ml, is >15-fold more sensitive than the FDA-recommended 100 ng/ml.33 No patients developed NAbs to efruxifermin. One ADA-positive patient had high triglyceride levels which could have interfered with detection of NAbs. One efruxifermin-treated patient (8.3% of ADA-positive patients) had antibodies which cross-reacted with endogenous FGF21. Utilizing individual patient-level elevation of serum adiponectin or reduction in serum triglycerides as pharmacodynamic markers, there was no discernible attenuation of response to efruxifermin associated with development of anti-efruxifermin antibodies.
Non-invasive markers of fibrosis
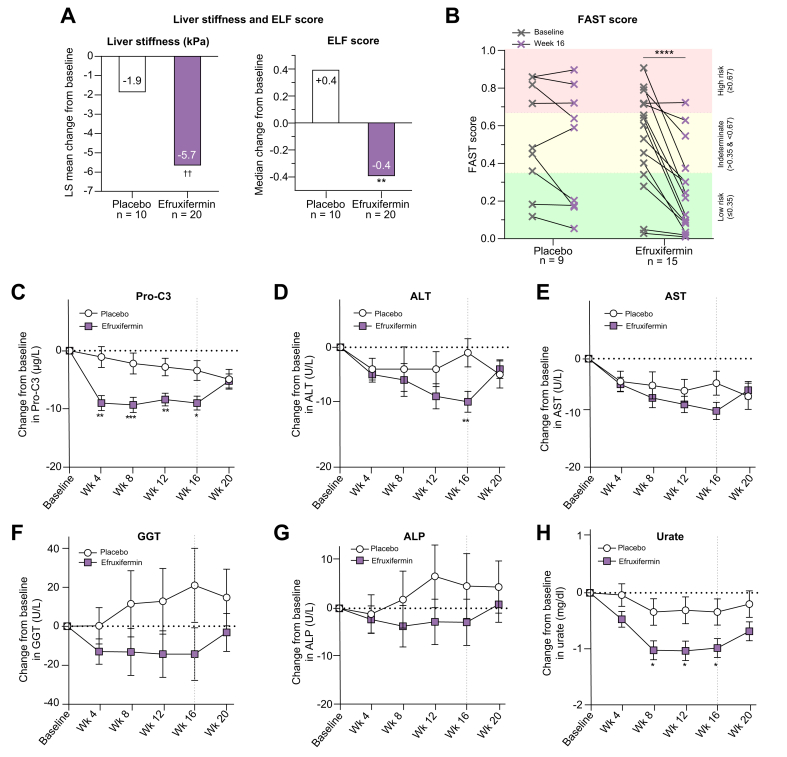
Liver stiffness was significantly decreased over 16 weeks of efruxifermin treatment (LS mean absolute change from baseline [CFB] −5.7 kPa, p = 0.0036 vs. baseline [LS mean relative CFB −24.4%, p = 0.0007 vs. baseline]) compared to a smaller decrease for placebo (LS mean absolute CFB −1.9 kPa, p = 0.4519 vs. baseline; LS mean relative CFB of −7.6% [p = 0.3970 vs. baseline]) (Fig. 3A). The reduction in liver stiffness in efruxifermin-treated patients over 16 weeks was not statistically significantly different from placebo (p = 0.1326), and was comparable in patients with or without type 2 diabetes.
Fig. 3.
Absolute change from baseline to week 16 for liver stiffness and ELF score, pre- and post-treatment FAST score, time course for Pro-C3 and liver enzymes, and absolute change in urate from baseline.
Values are shown as LS mean CFB values ± SEs, with p values from an ANCOVA model for FibroScan liver stiffness, Pro-C3, ALT, AST, GGT, ALP; Wilcoxon rank-sum test for median change in ELF score; and an MMRM for urate. p values for FAST score are from repeated-measures two-way ANOVA followed by Sidak’s multiple comparisons test. Refer to statistical analysis in methods for details. For ALT, AST, GGT, and ALP, the last observation carried forward method was used for patients with missing values. For urate, only individuals from the full analysis set with non-missing baseline values and the specified visit are included. Significance is indicated by asterisks: ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001 vs. placebo; ††p <0.01 vs. baseline. The vertical line at 16 weeks indicates end-of-treatment period. ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANCOVA, analysis of covariance; AST, aspartate aminotransferase; CFB, change from baseline; ELF, enhanced liver fibrosis; FAST, FibroScan-AST; GGT, gamma-glutamyltransferase; LS, least-square; MMRM, mixed-model repeated measures; Pro-C3, N-terminal type III collagen propeptide.
Serum markers of fibrosis and fibrogenesis, ELF score and Pro-C3, improved significantly following 16 weeks of treatment with efruxifermin. ELF score decreased by 0.4 (median absolute CFB, p = 0.0036 vs. placebo) over 16 weeks of treatment with efruxifermin, while placebo was associated with a median absolute CFB of +0.4 (Fig. 3A). Components of the ELF score (Table 3) also appeared to improve, with significant reductions in P3NP in the treated group (−3.2 μg/L; p = 0.0019 vs. placebo) compared to placebo (+1.8 μg/L). Significant reductions from baseline were also noted in hyaluronic acid (CFB −19.4 μg/L in the efruxifermin-treated group compared to +16.4 μg/L in the placebo group; p = 0.0139) and in TIMP-1 (CFB -35.7 μg/L in the treated group compared to +17.6 μg/L in placebo; p = 0.0415).
Table 3.
Change from baseline to week 16 in non-invasive markers of fibrosis, ELF score and its components, and markers of liver injury.
| Placebo (n = 10) |
Efruxifermin (n = 20) |
p vs. placebo | |||
|---|---|---|---|---|---|
| Value | p vs. baseline | Value | p vs. baseline | ||
| Liver stiffness (kPa) | |||||
| Baseline mean (SD) | 25.8 (13.2) | 22.6 (10.9) | |||
| LS mean absolute CFB (95% CI)1 | −1.9 (−7.0, 3.2) | 0.4519 | −5.7 (−9.4, −2.0) | 0.0036 | 0.2226 |
| LS mean percent CFB (95% CI)1 | −7.6 (−25.6, 10.5) | 0.3970 | -24.4 (−37.5, −11.4) | 0.0007 | 0.1326 |
| Pro-C3 (μg/L) | |||||
| Baseline mean (SD) | 22.6 (11.8) | 28.9 (30.8) | |||
| LS mean absolute CFB (95% CI)1 | −3.4 (−6.9, 0.2) | 0.0620 | −9.0 (−11.5, −6.5) | <0.0001 | 0.0130 |
| LS mean percent CFB (95% CI)1 | 3.9 (−17.7, 25.4) | 0.7168 | −16.2 (−31.4, −1.0) | 0.0379 | 0.1309 |
| ELF score | |||||
| Baseline, median | 9.5 | 10.4 | |||
| Median absolute CFB (IQR)2 | 0.4 (0.0, 0.6) | NC | −0.4 (−0.9, −0.0) | NC | |
| Median difference vs. placebo2 | −0.8 (−1.3, −0.4) | 0.0036 | |||
| Hyaluronic acid, μg/L | |||||
| Baseline, median | 63.8 | 116.9 | |||
| Median absolute CFB (IQR)2 | 16.4 (1.8, 69.9) | NC | −19.4 (−58.3, 6.4) | NC | |
| Median difference vs. placebo2 | −59.3 (−86.6, −11.1) | 0.0139 | |||
| P3NP, μg/L | |||||
| Baseline, median | 8.2 | 11.6 | |||
| Median absolute CFB (IQR)2 | 1.8 (0.3, 3.4) | NC | −3.2 (−5.7, −1.2) | NC | |
| Median difference vs. placebo2 | −5.9 (−9.0, −2.7) | 0.0019 | |||
| TIMP-1, μg/L | |||||
| Baseline, median | 230.2 | 279.5 | |||
| Median absolute CFB (IQR)2 | 17.6 (−14.3, 43.0) | NC | −35.7 (−74.1, 3.6) | NC | |
| Median difference vs. placebo2 | −52.3 (−112.9, −6.9) | 0.0415 | |||
| ALT, U/L | |||||
| Baseline mean (SD) | 32.7 (20.0) | 31.8 (17.2) | |||
| LS mean absolute CFB (95% CI)1 | −1.3 (−6.7, 4.1) | 0.6289 | −10.3 (−14.3, −6.4) | <0.0001 | 0.0098 |
| LS mean percent CFB (95% CI)1 | 3.0 (−14.5, 20.4) | 0.7301 | −22.1 (−34.8, −9.5) | 0.0014 | 0.0244 |
| AST, U/L | |||||
| Baseline mean (SD) | 28.9 (21.1) | 31.6 (14.0) | |||
| LS mean absolute CFB (95% CI)1 | −4.5 (−9.0, 0.1) | 0.0566 | −9.6 (−12.9, −6.2) | <.0001 | 0.0752 |
| LS mean percent CFB (95% CI)1 | −5.5 (−17.9, 6.8) | 0.3654 | −25.8 (−34.8, −16.9) | <.0001 | 0.0112 |
| GGT, U/L | |||||
| Baseline mean (SD) | 46.7 (17.7) | 76.2 (40.2) | |||
| LS mean absolute CFB (95% CI)1 | 21.3 (−18.4, 61.0) | 0.2808 | −14.5 (−42.6, 13.6) | 0.2996 | 0.1569 |
| LS mean percent CFB (95% CI)1 | 42.2 (−16.1, 100.5) | 0.1487 | −23.0 (−64.2, 18.3) | 0.2624 | 0.0817 |
| ALP, U/L | |||||
| Baseline mean (SD) | 67.2 (19.0) | 77.9 (24.6) | |||
| LS mean absolute CFB (95% CI)1 | 4.6 (−9.2, 18.4) | 0.4988 | −2.9 (−12.9, 7.0) | 0.5468 | 0.3748 |
| LS mean percent CFB (95% CI)1 | 6.5 (−10.6, 23.7) | 0.4412 | −2.4 (−14.7, 10.0) | 0.6956 | 0.3999 |
| Urate, mg/dl | |||||
| Baseline mean (SD) | 5.87 (1.41) | 6.04 (1.22) | |||
| LS mean absolute CFB (95% CI)3 | −0.34 (−0.82, 0.14) | 0.1526 | -0.98 (−1.32, −0.63) | <.0001 | 0.0357 |
| LS mean percent CFB (95% CI)3 | −4.56 (−11.95, 2.83) | 0.2164 | −16.19 (−21.52, −10.86) | <.0001 | 0.0143 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CFB, change from baseline; ELF, enhanced liver fibrosis; GGT, gamma-glutamyltransferase; NC, not calculated; LS, least squares; Pro-C3, N-terminal type III collagen propeptide; P3NP, procollagen type III N-terminal propeptide; TIMP-1, tissue inhibitor of metalloproteinase-1.
p values from ANCOVA.
p values from Wilcoxon rank-sum test.
p values from mixed-effects model of repeated measure.
Sixteen weeks of treatment with efruxifermin significantly reduced the FibroScan-AST or FAST score, a composite biomarker for non-invasively identifying patients at risk of progressive NASH, combining liver stiffness measurement, controlled attenuation parameter (CAP), and AST (Fig. 3B).34
Pro-C3 was also significantly decreased over 16 weeks (LS mean absolute CFB −9.0 μg/L, p = 0.0130 vs. placebo [LS mean relative CFB -16.2%]) compared to a smaller decrease on placebo (LS mean absolute CFB −3.4 μg/L [LS mean relative CFB +3.9%]) (Fig. 3C, Table 3). Although Pro-C3 declined over time in the placebo group, potentially as a result of implementation of lifestyle intervention and regular clinical evaluation per standard of care, the magnitude of reduction was significantly less than in efruxifermin-treated patients.
Non-invasive markers of fibrosis were also evaluated in the liver biopsy analysis set comprising those individuals with available end-of-treatment biopsies and confirmed cirrhosis at baseline; the results were comparable to those observed in the full analysis set (Table S4).
Exploratory endpoints
Markers of liver injury
ALT levels were significantly decreased over 16 weeks of efruxifermin treatment (LS mean absolute CFB −10.3 U/L [LS mean relative CFB −22.1%]) by comparison with placebo (LS mean absolute CFB −1.3 U/L [LS mean relative CFB +3.0%], p = 0.0098, efruxifermin vs. placebo) (Fig. 3D, Table 3). Four weeks after the last dose, ALT had returned to near-baseline levels. AST showed a similar numerical trend to ALT for LS mean absolute CFB (SE) in treated patients of −9.6 (1.6) U/L compared to an LS mean absolute CFB of −4.5 (2.2) U/L for placebo (p = 0.0752, efruxifermin vs. placebo). The relative reduction in AST at week 16 (LS mean CFB of −25.8%) for the treated group was significantly (p = 0.0112) greater than −5.5% for placebo (Fig. 3E, Table 3). Of the other markers of liver injury, GGT (Fig. 3F, Table 3) and ALP (Fig. 3G, Table 3) showed numerical but not statistically significant reductions in treated patients as early as week 8 that appeared to be sustained through week 16, compared to increases in the placebo group. For GGT, the LS mean (SE) absolute CFB was -14.5 (13.7) U/L for treated patients, compared to +21.3 (19.3) U/L for placebo (p = 0.1569, efruxifermin vs. placebo). For ALP, the LS mean (SE) absolute CFB to week 16 was -2.9 (4.8) U/L for treated patients, compared to +4.6 (6.7) for placebo (p = 0.3748, efruxifermin vs. placebo). Levels of urate, another marker of hepatocyte stress, decreased significantly more in treated patients (LS mean absolute CFB of −0.98 mg/dl; p = 0.0357 vs. placebo [relative CFB -16.19%]; p = 0.0143 vs. placebo), compared to an LS mean absolute CFB of -0.34 mg/dl (relative CFB −4.56%) for the placebo group (Fig. 3H, Table 3).
Histology of liver biopsies
The apparent reduction in non-invasive markers of fibrosis with efruxifermin treatment was associated with evidence of fibrosis regression in this small cohort of patients with paired biopsies. Four (33%) of 12 efruxifermin-treated patients with confirmed cirrhosis at baseline and available end-of-treatment biopsies achieved fibrosis improvement of ≥1 stage without worsening of NASH. By comparison, in the placebo group, none of the 5 patients with available end-of-treatment biopsies achieved this threshold (Fig. 4A). Three (25%) efruxifermin-treated patients achieved NASH resolution (0 to 1-point inflammation, 0-point ballooning) compared to none in the placebo group (Fig. 4B). Patients whose fibrosis improved without NAS worsening were distinct from those who had NASH resolution. Seven of 12 patients (58%) in the efruxifermin group at week 16 had a ≥2-point improvement in NAS compared with 1 of 5 patients (20%) in the placebo group. Overall, 9/12 efruxifermin patients (75%) with confirmed cirrhosis at baseline and end-of-treatment biopsies experienced either fibrosis improvement without worsening of NASH, NASH resolution, or improvement in NAS by ≥2-points, compared to 1 of 5 (20%) placebo patients.
Fig. 4.
Proportion of patients with NASH cirrhosis achieving an improvement in fibrosis stage or NASH resolution after 16 weeks.
The study was not powered to assess statistical significance of histological endpoints. NAS, non-alcoholic fatty liver disease activity score; NASH, non-alcoholic steatohepatitis.
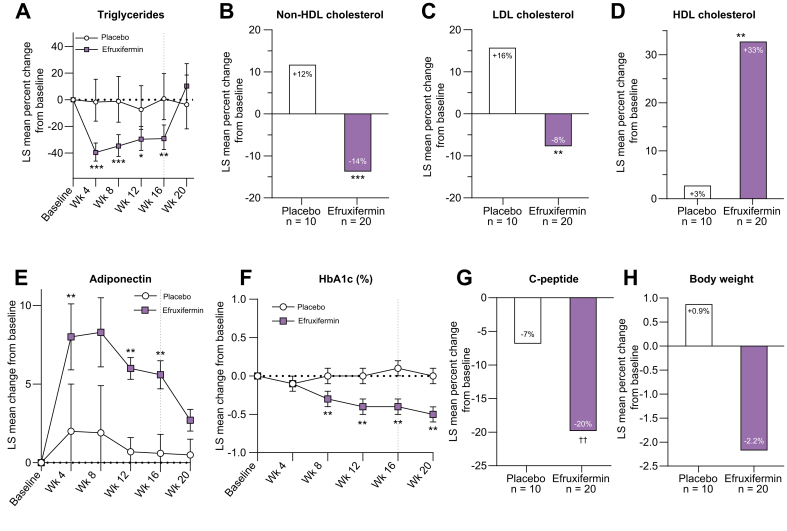
Markers of lipid and glucose metabolism
Markers of lipid (Fig. 5A-D) and glucose metabolism (Fig. 5E-G) are consistent with efruxifermin restoring a healthier whole-body metabolic phenotype. Lipoprotein profiles were significantly improved after 16 weeks treatment, with reductions of 29% for triglycerides (p <0.0001 vs. baseline; p = 0.0020 vs. placebo), 14% for non-HDL-C (p = 0.0004 vs. baseline; p = 0.0002 vs. placebo), and 8% for LDL-C (p =0.0961 vs. baseline; p = 0.0056 vs. placebo), compared to placebo patients, who experienced increases of 0.8% for triglycerides (p = 0.9289 vs. baseline), 11.8% for non-HDL-C (p = 0.0211 vs. baseline), and 16% for LDL-C (p = 0.0198 vs. baseline). Consistent with restoration of a healthier lipoprotein profile, HDL-C increased by 33% (p <0.0001 vs. baseline; p = 0.0062 vs. placebo) in efruxifermin-treated patients compared to 3% for placebo (p = 0.7581 vs. baseline). Among other evaluated markers of lipid metabolism, efruxifermin treatment appeared to significantly (p = 0.0004 vs. placebo) reduce apolipoprotein B levels (LS mean CFB: -11.1%) compared to an increase of 7.1% for placebo, but apolipoprotein C-III was unchanged (LS mean CFB +15% for the treated group compared to +20% for placebo; p = 0.7220), and lipoprotein-a levels appeared to increase significantly (LS mean CFB +32.7% for treated group compared to +8.4% for placebo, p = 0.0203). Efruxifermin treatment was associated with a numerically higher serum concentration of beta-hydroxybutyrate (LS mean CFB +68.4% for the treated group compared to +37.8% for placebo, p = 0.4448). Levels of bile acids in the treated group did not appear to be different from placebo (LS mean CFB +39% for the treated group compared to +54% for placebo, p = 0.7083).
Fig. 5.
Mean change (%) from baseline to week 16 of markers of lipid metabolism, glycemic control, and body weight.
Relative (A, B, C, D, G, H) or absolute (E, F) change from baseline. ∗∗∗p <0.0001, ∗∗p <0.01, ∗p <0.05 vs. placebo (ANCOVA); ††p <0.01 vs. baseline (ANCOVA). ANCOVA, analysis of covariance; HbA1c, hemoglobin A1c.
After 16 weeks of treatment, efruxifermin elicited statistically significant and potentially clinically meaningful improvements in multiple serum markers of glucose metabolism. Mean HbA1c declined by 0.4% (absolute CFB, p = 0.0011 vs. placebo) across all treated patients, compared with an increase of 0.1% for placebo. For the subset of patients with type 2 diabetes, all of whom remained on their existing anti-diabetic medications, the absolute change from baseline in HbA1c was -0.5% (p = 0.0013 vs. baseline; p = 0.0579 vs. placebo) compared to a 0.1% decrease for the corresponding subset of placebo patients. In patients without type 2 diabetes, efruxifermin was associated with a decrease of 0.3% (p = 0.0019 vs. baseline; p = 0.0023 vs. placebo) compared to an increase of 0.2% for placebo. Efruxifermin appeared to improve glucose control by enhancing peripheral insulin sensitivity, as indicated by a 20% LS mean relative reduction in C-peptide (p = 0.0073 vs. baseline) compared to a decrease of 7% for placebo (p = 0.2817 vs. placebo). Reflecting the enhanced insulin sensitivity associated with efruxifermin treatment, adiponectin increased by 95.2% (p <0.0001 vs. baseline) compared to an increase of 4.1% for placebo (p <0.0001 vs. placebo).
As shown in Fig. 5A,E,F, changes in metabolic parameters (triglycerides, adiponectin, and HbA1c) occurred early and were sustained throughout 16 weeks of treatment.
In contrast to the insulin-sensitizing peroxisome proliferator activating receptor gamma class of oral anti-diabetic medications, efruxifermin treatment was associated with a trend toward reduction in body weight (Fig. 5H) of −2.2 kg (p = 0.0814 vs. baseline; p = 0.1325 vs. placebo) or a relative loss of 2.2% (p = 0.0628 vs. baseline; p = 0.1490 vs. placebo), as opposed to a trend toward body weight gain observed in the placebo group of 1.2 kg (absolute CFB, p = 0.5004 vs. baseline) or 0.9% (relative CFB, p = 0.6124 vs. baseline).
Significant improvement was also noted in liver steatosis assessed by CAP (FibroScan) with an absolute change from baseline (LS mean) of −37.6 dB/m, p = 0.0027 vs. placebo (relative CFB: −10.5%; p = 0.0037 vs. placebo) in the efruxifermin-treated group compared to an increase of 12.1 dB/m (relative CFB: 4.6%) for the placebo group.
Discussion
Up to 20% of patients with NASH develop cirrhosis (F4) within 10 years.35 Patients with NASH cirrhosis have a poor prognosis, carrying a high risk of liver decompensation and progression to end-stage liver disease, including ascites, variceal hemorrhage, hepatic encephalopathy, liver failure, and liver cancer,35 as well as liver-related and all-cause mortality.9,17 In a recent post hoc analysis of two interventional studies, reversal of cirrhosis in patients with NASH, i.e. 1-stage improvement of fibrosis, was associated with a greater than 80% reduction in relative risk of liver-related clinical events.12 Such a reduction in risk represents significant potential medical benefit for patients who may otherwise progress to decompensation and end-stage liver disease. Treatment of this population should aim to prevent hepatic decompensation and liver-related and all-cause mortality, reverse cirrhosis, and ultimately restore liver function. To date, experimental medicines with diverse modes of action have failed to meaningfully improve fibrosis in patients with compensated cirrhosis due to NASH, despite showing promising results in less advanced NASH in prior clinical studies (simtuzumab,35 selonsertib,36 cilofexor,37 firsocostat,37 semaglutide [NCT03987451], pegbelfermin [Abdelmalek, 2021]).
While patients with compensated cirrhosis due to NASH suffer from more advanced disease, efruxifermin appeared to demonstrate a tolerability and safety profile similar to that reported for patients with less advanced disease (F1–F3 NASH) in the BALANCED main study.32 Although the trial’s duration was not long enough to evaluate clinical outcomes, there were no decompensation events reported for patients with cirrhosis treated with efruxifermin. Circulating biomarkers of liver function (albumin, bilirubin, MELD, C–P scores) appeared unchanged, while most markers of hemostasis were either preserved (platelets, clotting time) or showed a trend towards improvement (PAI-1).
Although this study was not designed to evaluate histologic improvements, an exploratory analysis demonstrated a numeric difference between treated patients compared to placebo following 16 weeks of treatment. Four of 12 patients (33%) treated with 50 mg efruxifermin who opted to receive an end-of-study biopsy showed an improvement in fibrosis stage without worsening of NASH, and an additional 25% showed NASH resolution. Consistent with the apparent improvement in histopathology, liver stiffness was reduced by 26%, a magnitude previously associated with a 1-stage improvement in fibrosis in F3–F4 patients.36,38 The association between liver stiffness or other non-invasive tests and resolution of features of NASH histopathology remains to be validated.
Along with regression of fibrosis in this small patient cohort, reductions were observed in markers of liver injury and inflammation (ALT, urate, hs-CRP) as well as liver-specific biomarkers of fibrogenesis and fibrosis (Pro-C3, ELF). While decreases over time were also noted in the placebo group for Pro-C3, it is not unusual to see clinical improvement in individuals receiving placebo, possibly due to them adopting a healthier lifestyle when receiving regular clinical evaluation (Hawthorne effect). However, the magnitude of changes in Pro-C3 in efruxifermin-treated patients were significantly larger than for placebo. Another biomarker of fibrogenesis in both soft tissues and bone, P1NP, which is elevated in patients suffering from cirrhosis,39 was also significantly reduced. As with patients with F1–F3 NASH, the decline in serum markers of fibrogenesis or fibrosis was rapid, reaching a nadir after as few as 4-8 weeks treatment.32
In F1–F3 patients, the reduction in serum markers of fibrogenesis and fibrosis preceded the maximal decrease in liver fat,32 suggesting part of the reduction may be ascribable to a direct antifibrotic action of efruxifermin, as opposed to indirect effects mediated by improvements in liver metabolic health. There is preclinical evidence of direct inhibition of fibrogenesis by FGF21 in vitro26,40,41 and in vivo,42 independent of metabolic improvement. Direct antifibrotic activity is also consistent with the high proportion (50%) of patients with F2/F3 NASH achieving 2-stage regression of fibrosis,32 as well as reversal of cirrhosis in 33% of patients with cirrhotic NASH after only 16 weeks efruxifermin treatment, despite high baseline levels of collagen present in cirrhosis.43
The magnitude of improvement in markers of liver injury (ALT, AST, ALP, GGT, urate) associated with efruxifermin treatment was somewhat smaller for Cohort C than for the BALANCED main study.32 This may be ascribed to a declining population of hepatocytes as fibrosis progresses to cirrhosis,14 consistent with the lower baseline levels of these markers in Cohort C compared to the main study. Nevertheless, the overall pattern of improvement in liver histopathology, as well as whole-body and liver metabolic health, is consistent with that observed in patients with F1–F3 NASH.32
Improved lipid and glucose metabolism were also observed in an earlier study in patients with type 2 diabetes treated with efruxifermin for 4 weeks.29 The consistency of responses across three distinct patient populations highlights the reproducible effects of efruxifermin in addressing components of metabolic syndrome. In patients with or without type 2 diabetes, efruxifermin treatment has consistently improved markers of glucose metabolism and insulin sensitivity while restoring a healthy lipoprotein profile without increasing body weight. Should these broad improvements be confirmed with longer term treatment in a larger patient population, efruxifermin could be of considerable therapeutic utility since type 2 diabetes is frequently inadequately controlled and highly prevalent among patients with NASH, increasing from 30-40% in F1/F2[44], [45], [46] to 70-90% in F3/4 NASH.47
The time courses for changes in serum markers of metabolism and liver health during 16 weeks of treatment with efruxifermin appeared to reach a maximum after 4-8 weeks, which was maintained through 16 weeks in both F1-332 and F4 patients with NASH; this durability will be further assessed in longer-duration, ongoing clinical trials. This pattern contrasts with the waning magnitude of responses for adiponectin and triglyceride during treatment for 24-to-48 weeks with the FGF21 analogue pegbelfermin (Sanyal 2021). A potential explanation could be insufficient agonism by pegbelfermin of the FGF21 receptors (FGFR1c/2c/3c) throughout the dosing interval. Biologically active (i.e., intact C-terminal) pegbelfermin has a reported half-life of approximately one day which appears unlikely to sustain high levels of receptor agonism with once-weekly dosing.30 In contrast, efruxifermin has a 3–4-day half-life and 2–3.5-day Tmax.29 Maintaining high levels of agonism of FGF21’s receptors between doses of FGF21 analogues appears necessary to maximize the full range of metabolic improvements. Dosing either efruxifermin or pegozafermin (BIO89-100), which have similar half-lives, once-every-two-weeks was associated with diminished metabolic responses relative to once-weekly dosing.29,48
Based on preclinical reports of effects of FGF21 on bone health, the impact of efruxifermin on biomarkers of bone metabolism was evaluated. No changes were observed in CTX-1, a widely reported biomarker of type-I collagen degradation linked to bone resorption. However, decreases were observed in P1NP, a biomarker of type-I collagen synthesis linked to bone formation. While P1NP and CTX-1 have been proposed to be standard markers for evaluating bone health,39,49,50 type-I collagen is a ubiquitously expressed protein not specific to bone. Active fibrogenesis in soft tissues such as the liver, lungs, or heart contributes significantly to circulating levels of P1NP and C-terminal type-1 collagen extension peptide.39 In this study, baseline PINP levels were similar in F4 (56.00 ug/L), and F1-3 BALANCED32 (55.14 ug/L) patients. Given the reduction of liver fibrosis by efruxifermin, as indicated by histology and serum-based markers of soft tissue fibrosis, i.e., ELF score and Pro-C3, decreases in P1NP may be attributable to reduced synthesis of fibrils (type-I and -III collagen) and extracellular matrix (type-I and -IV collagen) in the liver.39,51 The reduction of P1NP levels associated with efruxifermin may therefore not reflect altered bone turnover, especially in the context of no change in CTX-1.
Limitations to this study include relatively short (16-week) duration of treatment and a small sample size. The observations of acceptable tolerability and safety and encouraging albeit preliminary signs of efficacy will require confirmation in larger and longer-duration studies of efruxifermin. While liver biopsies were obtained from 12 out of 20 efruxifermin- and 5 out of 10 placebo-treated patients, (i.e., all of those who consented to biopsy), the small sample size for evaluating histology precluded statistical analysis. Despite the relatively short treatment period, the pattern and extent of improvement in histopathology was consistent with that seen with F1–F3 patients treated over the same duration of treatment. Moreover, the pattern of improvements in markers of liver health, of liver fibrosis, and of whole-body metabolism was also consistent with that observed in F1–3 patients with NASH.
The breadth of desirable effects in patients with cirrhosis, if confirmed in larger and longer term studies, potentially sets efruxifermin apart from other treatments under development for NASH. Efruxifermin was generally safe and well-tolerated. Improvements in non-invasive markers of liver injury and fibrosis appeared consistent with a trend towards inhibition of fibrogenesis and reversal of cirrhosis, as indicated by a greater number of patients whose fibrosis improved by ≥1-stage on end-of-treatment biopsy. The observed results are encouraging, and further evaluation of efruxifermin as a treatment for compensated cirrhosis due to NASH, in sufficiently powered, longer-duration studies, is warranted.
Financial support
This study was funded by Akero Therapeutics.
Authors’ contributions
The protocol was designed by KY, TR, BdT, EF, AC, HC, SH. Data were acquired by SH, PJR, BF, GF, RP, CB.The data were analyzed by KY, TR, BdT, EF, AC, SH, EJT, RS, HC. A statement indicating that at least one author had access to all of the data and can vouch for the integrity of the data analyses [KY].
Data availability statement
The datasets generated and/or analyzed in this study are considered commercially sensitive and, therefore, are not publicly available. Requests for data supporting findings in the manuscript should be made to the corresponding author and will be reviewed individually. Data might be shared in the form of aggregate data summaries and via a data transfer agreement. Individual participant-level raw data containing confidential or identifiable patient information are subject to patient privacy and cannot be shared.
Conflict of interest
Stephen A. Harrison holds a leadership or fiduciary role at, advises, consults for, receives grants or contracts from Northsea Therapeutics. He advises, consults for, receives grants or contracts, support for attending meetings and/or travel from Madrigal Pharmaceuticals, Inc. He advises, consults for, and receives grants or contracts from Akero Therapeutics, Inc. He advises, consults for, and receives grants or contracts from Axcella Health, Inc., Cymabay Therapeutics, Inc., Galectin Therapeutics, Inc., Hepion Pharmaceuticals, Inc., Hightide Therapeutics, Inc., Intercept Pharmaceuticals, Inc., Metacrine Inc., NGM Biopharmaceuticals Inc., Genfit Corp, Novo Nordisk, Poxel, Sagimet Biosciences. He advises and receives grants or contracts from Gilead Sciences, Inc., Galmed Research & Dev. LTD., and Novartis Pharmaceuticals Corp. He consults for and receives grants or contracts from Viking Therapeutics, Inc. and Enyo Pharma S.A. He advises and consults for Altimmune, Echosens North America Inc. Foresite Labs, LLC, HistoIndex PTE LTD, Medpace Inc., Prometic, Pharma SMT LTD, Ridgeline and Sonic Incytes Medical Corp, Terns Inc. He advises for 89bio, Arrowhead, Chronwell, CiVi, Indalo, PathAI, and Theratechnologies. He consults for AgomAB, Alentis Therapeutics AG, Alimentiv, Inc, Boston Pharmaceuticals, B Riley FBR Inc. BVF Partners LP, Cohbar, Inc. Canfite, Corcept Therapeutics, Inc, Fibronostics, Fortress Biotech, Inc GNS, Inipharm, Ionis, Kowa Research Institute, Microba, Nutrasource, Perspectum Diagnostics, and Piper Sandler. He receives grants or contracts from Cirius Therapeutics, Inc., and CiVi Biopharma Inc. He holds stock or stock options at Akero Therapeutics, Inc., Chronwell Inc., Cirius Therapeutics, Inc, Galectin Therapeutics, Inc., Genfit Corp, Hepion Pharmaceuticals Inc., HistoIndex PTE LTD, Metacrine Inc., NGM Biopharmaceuticals., Northsea Therapeutics B.V, and Sonic Incytes Medical Corp. Peter J. Ruane: none. Bradley Freilich: none. Guy Neff has received payment or honoraria from Intercept Pharmaceuticals. Rashmee Patil has received grants to institution from 89 Bio, AltImmune, Boehringer Ingelheim, Bristol Myers Squibb, Corcept Therapeutics, Fibronostics, Galectin Therapeutics, Genentech, Gilead, Helio Health, Hepagene, Madrigal Pharmaceuticals, NGMBio, NorthSea Therapeutics, Poxel, Sagimet Biosciences, and Viking Therapeutics. He consults for Intercept Pharmaceuticals. Cynthia Behling has received payment via Pacific Rim Pathology for liver biopsy scoring. She has received grants as a co-investigator under N100 K U01 DK61734. She has received honoraria for lectures from Pfizer and Alimentev. She is a co-chair on NASH CRN Pathology Committee. Chen Hu: none. Reshma Shringarpure, Brittany de Temple, Erica Fong, Erik J. Tillman, Timothy Rolph, Andrew Cheng, and Kitty Yale are employees of Akero Therapeutics and own stock and/or stock options of Akero Therapeutics.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The initial draft of the manuscript was prepared by Andrea R. Gwosdow, Ph.D. of Gwosdow Associates Science Consultants, LLC on behalf of Akero Therapeutics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100563.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y., Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:15539–15548. doi: 10.3748/wjg.v20.i42.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Murag S., Ahmed A., Kim D. Recent epidemiology of nonalcoholic fatty liver disease. Gut Liver. 2021;15:206–216. doi: 10.5009/gnl20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y., et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 6.Le M.H., Yeo Y.H., Li X., Li J., Zou B., Wu Y., et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.12.002. S1542-3565(21)01280-01285, [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M., Hagström H., Nasr P., Fredrikson M., Stål P., Kechagias S., et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z., et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review ad meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyal A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R.S., Taylor R.J., Bayliss S., Hagström H., Nasr P., Schattenberg J.M., et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal A.J., Anstee Q.M., Trauner M., Lawitz E.J., Abdelmalek M.F., Ding D., et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2022;75:1235–1246. doi: 10.1002/hep.32204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Poorten D., Samer C.F., Ramezani-Moghadam M., Coulter S., Kacevs ka M., Schrijnders D., et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57:2180–2188. doi: 10.1002/hep.26072. [DOI] [PubMed] [Google Scholar]
- 14.Heyens L.J.M., Busschots D., Koek G.H., Robaeys G., Francque S. Liver fibrosis in non-alcoholic fatty liver disease: from biopsy to non-invasive biomarkers in diagnosis and treatment. Front Med. 2021;8:615978. doi: 10.3389/fmed.2021.615978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noureddin M., Vipani A., Bresse C., Todo T., Kim I.K., Alkhouri N., et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA Guidance . 2019. Nonalcoholic steatohepatitis with compensated cirrhosis: developing drugs for treatment guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nonalcoholic-steatohepatitis-compensated-cirrhosis-developing-drugs-treatment-guidance-industry [Google Scholar]
- 17.Albhaisi S., Sanyal A. Recent advances in understanding and managing non-alcoholic fatty liver disease. F1000 Res. 2018;7:720. doi: 10.12688/f1000research.14421.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z., Tian H., Lam K.S.L., Lin S., Hoo R.C.L., Konishi M., et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Camporez J.P.G., Jornayvaz F.R., Petersen M.C., Pesta D., Guigni B.A., Serr J., et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–3109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu J., Pinkstaff J., Li Z., Skidmore L., Li N., Myler H., et al. FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes. 2012;61:505–512. doi: 10.2337/db11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G., et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 23.Kim A.M., Somayaji V.R., Dong J.Q., Rolph T.P., Weng Y., Chabot J.R., et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab. 2017;19:1762–1772. doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- 24.Wu G., Li H., Fang Q., Zhang J., Zhang M., Zhang L., et al. Complementary role of fibroblast growth factor 21 and cytokeratin 18 in monitoring the different stages of nonalcoholic fatty liver disease. Sci Rep. 2017;7:5095. doi: 10.1038/s41598-017-05257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23:427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Tillman E.J., Rolph T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol. 2020;11:601290. doi: 10.3389/fendo.2020.601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher F.M., Chui P.C., Nasser I.A., Popov Y., Cunniff J.C., Lundasen T., et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073–1083. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charles E.D., Neuschwander-Tetri B.A., Pablo Frias J., Kundu S., Luo Y., Tirucherai G.S., et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and Type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring) 2019;27:41–49. doi: 10.1002/oby.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman A., Abuqayyas L., Denney W.S., Tillman E.J., Rolph T. AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep Med. 2020;1:100057. doi: 10.1016/j.xcrm.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirucherai G.S., Mora J., Revankar R., Charles E.D. Pharmacokinetics and safety of pegbelfermin (BMS-986036) administered in the abdomen and upper arm to normal, overweight, and obese healthy participants. J Hepatol. 2019;70:E798. [Google Scholar]
- 31.Stanislaus S., Hecht R., Yie J., Hager T., Hall M., Spahr C., et al. A novel Fc-FGF21 with improved resistance to proteolysis, increased affinity toward β-Klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology. 2017;158:1314–1327. doi: 10.1210/en.2016-1917. [DOI] [PubMed] [Google Scholar]
- 32.Harrison S.A., Ruane P.J., Freilich B.L., Neff G., Patil R., Behling C.A., et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2a trial. Nat Med. 2021;27:1262–1271. doi: 10.1038/s41591-021-01425-3. [DOI] [PubMed] [Google Scholar]
- 33.FDA Guidance . 2019. Immunogenicity testing of therapeutic protein products-developing and validating assays for anti-drug antibody detection.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-testing-therapeutic-protein-products-developing-and-validating-assays-anti-drug [Google Scholar]
- 34.Newsome P.N., Sasso M., Deeks J.J., Paredes A., Boursier J., Chan W.-K., et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison S.A., Abdelmalek M.F., Caldwell C., Shiffman M.L., Diehl A.M., Ghalib R., et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Harrison S.A., Wong V.W.-S., Okanoue T., Bzowej N., Vuppalanchi R., Younes Z., et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Dufour J.-F., Caussy C., Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877–1884. doi: 10.1136/gutjnl-2019-319104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anstee Q.M., Lawitz E.J., Alkhouri N., Wong V.W.-S., Romero-Gomez M., Okanoue T., et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70:1521–1530. doi: 10.1002/hep.30842. [DOI] [PubMed] [Google Scholar]
- 39.Szulc P. Bone turnover: biology and assessment tools. Best Prac Res Clin Endocrinol Metabol. 2018;32:725–738. doi: 10.1016/j.beem.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Xu P., Zhang Y., Liu Y., Yuan Q., Song L., Liu M., et al. Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways. Toxicol Appl Pharmacol. 2016;290:43–53. doi: 10.1016/j.taap.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Opoku Y.K., Liu Z., Afrifa J., Kumi A.K., Liu H., Ghartey-Kwansah G., et al. Fibroblast Growth Factor-21 ameliorates hepatic encephalopathy by activating the STAT3-SOCS3 pathway to inhibit activated hepatic stellate cells. EXCLI J. 2020;19:567–581. doi: 10.17179/excli2020-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S., Yu D., Wang M., Huang T., Wu H., Zhang Y., et al. FGF21 attenuates pulmonary fibrogenesis through ameliorating oxidative stress in vivo and in vitro. BioMed Pharmacother. 2018;103:1516–1525. doi: 10.1016/j.biopha.2018.03.100. [DOI] [PubMed] [Google Scholar]
- 43.Masugi Y., Abe T., Tsujikawa H., Effendi K., Hashiguchi A., Abe M., et al. Quantitative assessment of liver fibrosis reveals a nonlinear association with fibrosis stage in nonalcoholic fatty liver disease. Hepatol Comm. 2018;2:58–68. doi: 10.1002/hep4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison S.A., Neff G., Guy C.D., Bashir M.R., Paredes A.H., Frias J.P., et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–231. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Francque S.M., Bedossa P., Ratziu V., Anstee Q.M., Bugianesi E., Sanyal A.J., et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 46.Harrison S.A., Gawrieh S., Roberts K., Lisanti C.J., Schwope R.B., Cebe K.M., et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75:284–291. doi: 10.1016/j.jhep.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 47.Abdelmalek M.F., Charles E.D., Sanyal A.J., Harrison S.A., Neuschwander-Tetri B.A., Goodman Z., et al. The FALCON program: two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp Clin Trials. 2021;104:106335. doi: 10.1016/j.cct.2021.106335. [DOI] [PubMed] [Google Scholar]
- 48.Frias J.P., Lawitz E.J., Ortiz-LaSanta G., Franey B., Morrow L., Chen C.Y., et al. BIO89-100 demonstrated robust reductions in liver fat and liver fat volume (LFV) by MRI-PDFF, favorable tolerability and potential for weekly (QW) or every 2 weeks (Q2W) dosing in a phase 1b/2a placebo-controlled, double-blind, multiple ascending dose study in NASH. J Endocr Soc. 2021;5(Suppl 1):A5–A6. [Google Scholar]
- 49.Chavassieux P., Portero-Muzy N., Roux J.P., Garnero P., Chapurlat R. Are biochemical markers of bone turnover representative of bone histomorphometry in 370 postmenopausal women? J Clin Endocrinol Metab. 2015;100:4662–4668. doi: 10.1210/jc.2015-2957. [DOI] [PubMed] [Google Scholar]
- 50.Bhattoa H.P., Cavalier E., Eastell R., Heijboer A.C., Jørgensen N.R., Makris K., et al. Analytical considerations and plans to standardize or harmonize assays for the reference bone turnover markers PINP and β-CTX in blood. Clin Chim Acta. 2021;515:16–20. doi: 10.1016/j.cca.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veidal S.S., Vassiliadis E., Bay-Jensen A.C., Tougas G., Vainer B., Karsdal M.A. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrogen Tissue Repair. 2010;3:5. doi: 10.1186/1755-1536-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed in this study are considered commercially sensitive and, therefore, are not publicly available. Requests for data supporting findings in the manuscript should be made to the corresponding author and will be reviewed individually. Data might be shared in the form of aggregate data summaries and via a data transfer agreement. Individual participant-level raw data containing confidential or identifiable patient information are subject to patient privacy and cannot be shared.