Abstract
A previous study has shown that YopB of Yersinia spp. is essential for translocation of Yop effectors across the eucaryotic plasma membrane (M.-P. Sory and G. R. Cornelis, Mol. Microbiol. 14:583–594, 1994). However, this role was recently challenged (V. T. Lee and O. Schneewind, Mol. Microbiol. 31:1619–1629, 1999). Using protease protection and digitonin extraction, we reconfirm that YopB of Yersinia enterocolitica is essential for the translocation of YopE into HeLa cell monolayers.
Human pathogenic yersiniae cause disease by utilizing adherence to, type III-machinery-mediated secretion in, and translocation of numerous antihost Yop effector proteins into target cells. A subset of genes located on a common virulence plasmid of Yersinia spp. encode a type III delivery apparatus that is essential for the translocation of these effectors across the eucaryotic plasma membrane (5). Specifically, YopB, YopD, and LcrV are essential for this process (1, 6, 8, 9, 14, 16). In contrast, however, a recent publication by Lee and Schneewind (12) suggests that YopB is dispensable for the translocation of Yop effectors, such as YopE, into the eucaryotic cell.
To reconcile this obvious discrepancy, in this study we have used the Yersinia enterocolitica strains W22703 (wild type) and MC4 (yopB1; insertion of a stop codon after amino acid 8), kindly provided by O. Schneewind, to show that YopB is essential for Yop effector translocation. In particular, the pool of YopE extracted with digitonin from HeLa cells infected with a yopB mutant was degraded if cultures were treated with proteinase K (PK) prior to extraction. However, a protease-resistant YopE fraction was recovered from cell monolayers infected with wild-type bacteria. Therefore, YopB is required to target YopE into the eucaryotic cytoplasm by pathogenic Yersinia.
Experiments were performed as follows. Bacterial cultures were prepared prior to infection by inoculating Y. enterocolitica overnight cultures grown in Luria broth into 2 ml of modified Eagle medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (hi-FCS). All samples were incubated at 26°C for 30 min and then at 37°C for 60 min. HeLa cell monolayers were grown to 80% confluence in 10-cm-diameter tissue culture dishes with MEM plus 10% hi-FCS plus ampicillin. Before infection (30 min), monolayers were washed twice with phosphate-buffered saline (PBS) and overlaid with 2 ml of MEM plus 10% hi-FCS plus 1.0 μg of cytochalasin D per ml (to block uptake of the yopB mutant by HeLa cells [7, 10, 15]). Monolayers were separately infected with either the Y. enterocolitica wild-type strain or a yopB mutant at a multiplicity of infection of 10.
Following incubation at 37°C for 3 h, the HeLa cells were washed twice with PBS to remove the cell culture medium and nonadherent bacteria. To some of the monolayers, 500 μl of PK (500 μg/ml in PBS) was added. The PK solution was removed after 30 s to leave only a thin film of liquid on the cells. After 20 min at room temperature, 500 μl of freshly prepared phenylmethylsulfonyl fluoride (4 mM in PBS) was added to all monolayers to block protease activity. HeLa cells were lysed by addition of 400 μl of digitonin (1% in PBS) or 400 μl of sodium dodecyl sulfate (SDS) (1% in PBS), cells were collected into Eppendorf tubes, and the incubation was continued at room temperature for 20 min, with occasional vortexing. Cell debris and attached bacteria were removed from the lysate by centrifugation for 10 min at 4°C. The resulting supernatant (∼1.1 ml) was collected and mixed with an equal volume of 2× SDS sample buffer. Aliquots corresponding to approximately 6 × 104 infected HeLa cells were analysed by SDS-polyacrylamide gel electrophoresis and Western blotting using antiserum raised against YopE or its intrabacterial chaperone SycE.
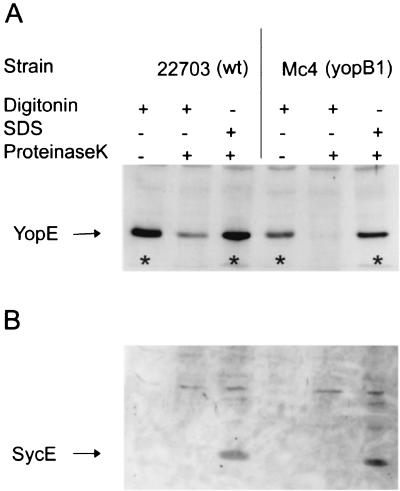
In the absence of PK treatment, infection of HeLa cells with either the wild-type strain or the yopB1 mutant of Y. enterocolitica resulted in digitonin extracts containing large amounts of YopE (Fig. 1A). Moreover, if the infected cells were first treated with PK and then lysed with SDS, large amounts of YopE were recovered regardless of the strain (Fig. 1A). These results are in agreement with the results presented by Lee and Schneewind (12) (Fig. 1A). In contrast, if the infected monolayers were treated with PK prior to digitonin lysis, a reduction in the level of YopE was found after infection with the wild type. Thus, this fraction of YopE was protected from PK activity presumably due to its cytosolic location within HeLa cells. However, after PK treatment of HeLa cells infected with the yopB1 mutant, no YopE could be recovered (Fig. 1A). In this case, YopE was likely of extracellular origin and therefore proteolytically sensitive. All lysates were, in addition, analyzed for the presence of intrabacterial SycE (17). Significantly, this chaperone was detected only after extraction with SDS (Fig. 1B). These results suggest that the YopE detected after protease treatment followed by SDS lysis of a yopB1 mutant originates from an intrabacterial pool and not from the HeLa cell cytosol.
FIG. 1.
YopE extracted with digitonin from HeLa cell monolayers infected with Yersinia defective in YopB is degraded in a protease protection assay. HeLa cell monolayers, infected for 3 h with wild-type Y. enterocolitica (wt) or a yopB1 mutant were either treated (+) or not treated (−) with PK. Phenylmethylsulfonyl fluoride was added to all cultures to block protease activity. Infected cultures were then extracted with either digitonin or SDS. Resulting lysates were centrifuged, and soluble proteins were separated on an SDS–12% polyacrylamide gel and subjected to Western blot analysis with antibodies against YopE (A) or with antibodies against SycE (B). ∗, results correspond to data in reference 12.
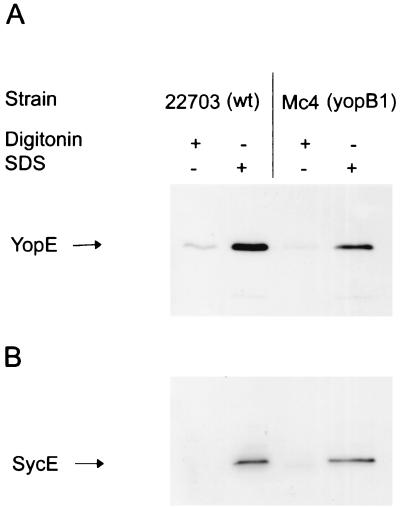
To determine the fate of the intrabacterial YopE pool in vitro upon digitonin or SDS treatment, we incubated bacteria, grown without HeLa cells, with the detergents. The wild-type and the yopB1 mutant strains were incubated with MEM plus 10% hi-FCS and cytochalasin D in tissue culture dishes in the absence of HeLa cells. After 4 h at 37°C, the bacteria were pelleted and resuspended in 500 μl of PBS, to which 500 μl of either 1% digitonin or 1% SDS was added. Digitonin-prepared soluble lysates, recovered from both the wild-type and yopB1 bacteria, contained only trace amounts of YopE. In contrast, when the bacteria were extracted with SDS, YopE was abundant in lysates from both the wild type and the yopB mutant (Fig. 2A). This result was not surprising since SDS is routinely used to lyse gram-negative bacteria (11). Total bacterial lysis by SDS was confirmed, since SycE was also detected in the SDS lysates (Fig. 2B). The amounts of YopE or SycE found in bacteria lysed with 1% SDS for 20 min were equal to amounts detected in bacteria boiled in SDS sample buffer (data not shown). This indicates that 1% SDS was enough to completely solubilize the bacteria. Importantly, digitonin treatment did not release any soluble SycE (Fig. 2B). Taken together, these results show that the yopB1 mutant still secretes YopE during a HeLa cell infection but, importantly, that it is unable to translocate YopE. Thus, it can be concluded that YopB is an essential component of the Yop translocation machinery. In conclusion, protease protection in combination with SDS lysis (as performed in studies reported in references 2 to 4 and 11 to 13) is a technique unsuitable for determination of the subcellular localization of translocated proteins, since this detergent leads to bacterial lysis. In addition, proteins recovered from digitonin extracts cannot automatically be considered to be derived from the eucaryotic cytosol, since they can originate equally well from the bacterial cell surface and the space between the bacterium and the eucaryotic cell. Our results show that experiments using digitonin extraction without prior protease treatment to remove protein, secreted but not translocated, are inconclusive and may be misleading. Therefore, no reliable conclusions can be made about type III-machinery-mediated protein translocation based on experiments using digitonin only (see references 2 to 4 and 11 to 13).
FIG. 2.
SDS extraction of bacteria solubilizes both YopE and the intrabacterial chaperone SycE. Wild-type Y. enterocolitica or a yopB1 mutant was grown in tissue culture dishes without HeLa cells for 4 h. Collected bacteria were centrifuged and resuspended in 500 μl of PBS containing either 500 μl of digitonin (1%) or SDS (1%). Bacteria were incubated at room temperature for 20 min. Cleared lysates were fractionated by SDS-polyacrylamide gel electrophoresis and subjected to Western blot analysis with antibodies against YopE (A) or SycE (B).
Acknowledgments
We acknowledge M. Frances for critically reading the manuscript.
This work was supported by grants from the Swedish Foundation for Strategic Research and from the Swedish Medical Research Council.
REFERENCES
- 1.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5–1.8 macrophages by YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 2.Cambronne E, Cheng L W, Schneewind O. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone dependent mechanism. Mol Microbiol. 2000;37:263–273. doi: 10.1046/j.1365-2958.2000.01974.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L W, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L W, Schneewind O. Yersinia enterocolitica type III secretion. On the role of SycE in targeting YopE into HeLa cells. J Biol Chem. 1999;274:22102–22108. doi: 10.1074/jbc.274.31.22102. [DOI] [PubMed] [Google Scholar]
- 5.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields K A, Straley S C. LcrV of Yersinia pestis enters infected eucaryotic cells by a virulence plasmid-independent mechanism. Infect Immun. 1999;67:4801–4813. doi: 10.1128/iai.67.9.4801-4813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay B B, Falkow S. Comparision of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 8.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effectors across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 9.Holmström A, Olsson J, Cherepanov P, Maier E, Nordfelth R, Pettersson J, Benz R, Wolf-Watz H, Forsberg Å. LcrV is a channel size determining component of the Yop effector translocation of Yersinia. Mol Microbiol. 2001;39:620–632. doi: 10.1046/j.1365-2958.2001.02259.x. [DOI] [PubMed] [Google Scholar]
- 10.Kihlström E, Nilsson L. Endocytosis of Salmonella typhimurium 395MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand Sect B. 1977;85:322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee V T, Anderson M, Schneewind O. Targeting of yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eucaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. . (Erratum, 34:196, 1999.) [DOI] [PubMed] [Google Scholar]
- 13.Lee V T, Tam C, Schneewind O. Yersinia enterocolitica type III secretion. LcrV, a substrate for type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 14.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosqvist R, Håkansson S, Forsberg Å, Wolf-Watz H. Functional conservation of the secretion machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 17.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]