Abstract
Saturated bicycles are becoming ever more important in the design and development of new pharmaceuticals. Here a new strategy for the synthesis of bicyclo[2.1.1]hexanes is described. These bicycles are significant because they have defined exit vectors, yet many substitution patterns are underexplored as building blocks. The process involves sensitization of a bicyclo[1.1.0]butane followed by cycloaddition with an alkene. The scope and mechanistic details of the method are discussed.
Graphical Abstract
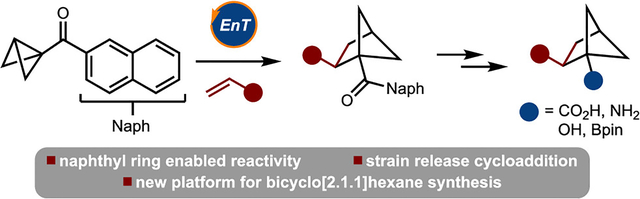
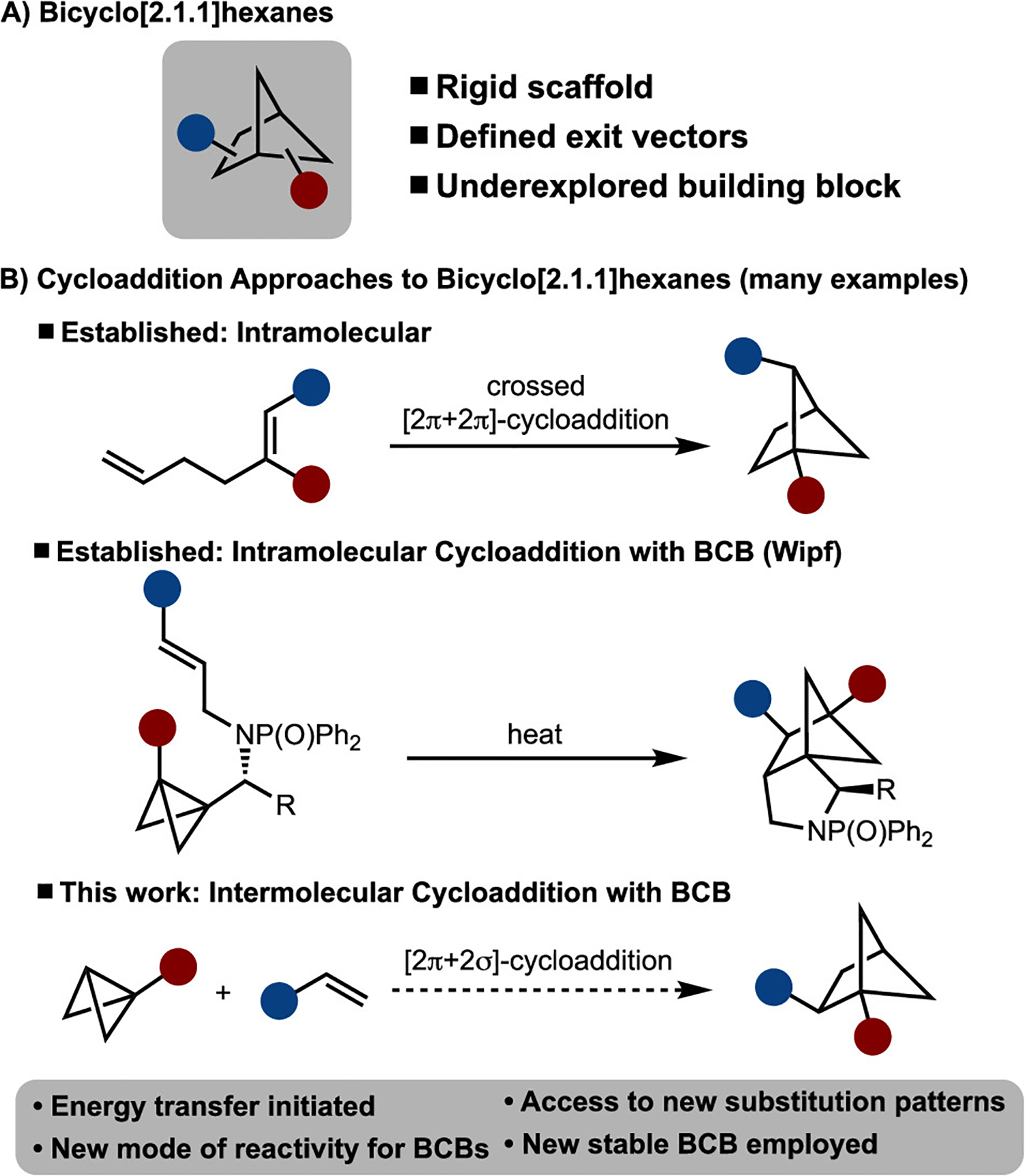
In recent years, a greater emphasis has been placed on the incorporation of saturated building blocks into chemical libraries for drug development.1 For example, bicyclo[1.1.1]pentane has garnered significant attention in the literature because it has attributes similar to those of a benzene ring.2 Many of the synthetic advances in this area are driven by the strain-release ring-opening reaction of [1.1.1]propellane. The bicyclo[2.1.1]hexane scaffold is significant because of its rigid nature and defined exit vectors, yet it has received significantly less attention (Scheme 1A).3
Scheme 1.
Cycloaddition Approaches toward Bicyclo[2.1.1]hexanes
The most common method for the synthesis of bicyclo[2.1.1]hexanes is via crossed [2 + 2] cycloaddition of 1,5-dienes.4 An alternative approach involves [2π + 2σ] cycloaddition of bicyclo[1.1.0]butanes (BCBs).5–7 Only three examples of the reaction of BCBs with alkenes are known.7,8 Among those, two are intermolecular and were reported in 1966 by Blanchard and 1986 by De Meijere.7a,b In each study, it was demonstrated in one example that bicyclo[2.1.1]hexanes could be generated by heating a mixture of either butadiene or an α-thioacrylonitrile. More recently, in 2006 Wipf reported a thermally induced intramolecular cycloaddition to arrive at complex tricyclic compounds (Scheme 1B).7c On the basis of these reports, it is clear that cycloadditions of BCBs to prepare bicyclo[2.1.1]hexanes are quite limited. Herein we provide a solution to this challenge by introducing a new mode of activation of bicyclo[1.1.1]butanes. The approach allows for intermolecular cycloaddition and thus access to a diverse range of bicyclo[2.1.1]hexanes with new substitution patterns.8
The reaction design is illustrated in Scheme 2A. We envisioned that the strained central C–C bond of BCB could be cleaved by single electron transfer (SET), thermolysis, direct excitation, or energy transfer to generate a diradical or radical anion intermediate. Capture of this intermediate with an alkene would lead to the formation of the desired product via stepwise cycloaddition. It should be noted that other strain-release methods to enable cycloaddition are known. The vast majority of known examples involve monocyclic systems (e.g., cyclopropane). For example, the chemistry of donor–acceptor cyclopropanes, which typically proceeds via two-electron pathways, is well-established.9 Reactions involving radical intermediates are also known. For example, single-electron reduction of cyclopropyl ketones followed by reaction with an alkene has been demonstrated in a variety of contexts.10 Finally, radical-initiated ring-opening reactions of substrates derived from cyclopropylamines,11 alcohols,12 and alkenes13 are known. Some of these methods have also been extended to cyclobutanes.14 In one very recent study, the Stephenson group utilized bicyclo[1.1.1]pentanes in a strain-release cycloaddition with an alkene.15
Scheme 2.
Initial Investigations
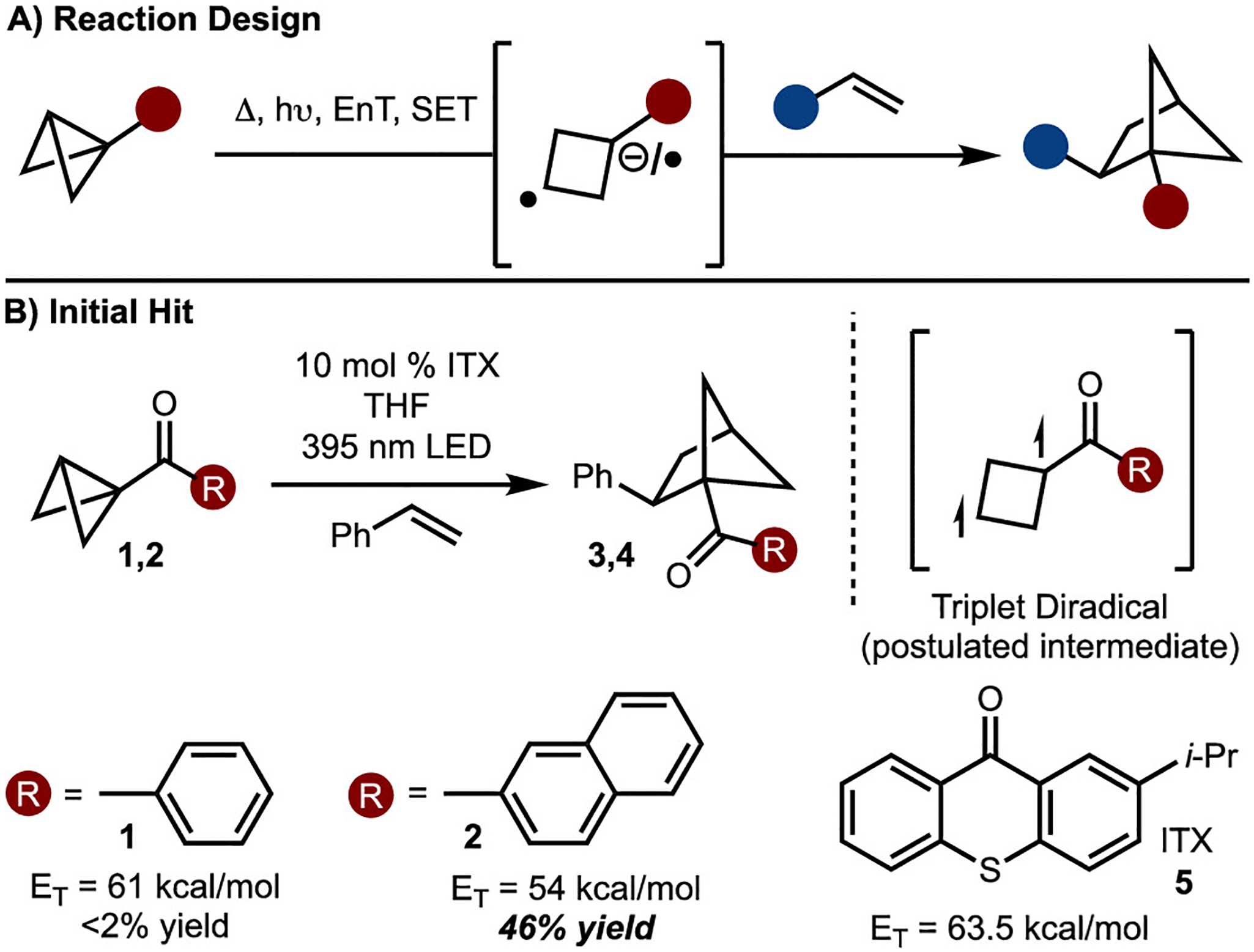
After considerable experimentation with thermally induced reactions as well as photoredox processes, no product from reaction between a BCB and an alkene was observed. In most cases, polymerization of the BCB was observed.16 At this stage, we decided to explore an alternative approach involving triplet energy transfer (Scheme 2B).17 In this scenario, the substituent bound to the BCB should be capable of being sensitized. We reasoned that an aryl ketone should undergo energy transfer with an appropriate sensitizer, as demonstrated previously.18 Initial studies with phenyl ketone 1 did not result in productive product formation (Scheme 2B). We reasoned that the similarity of the triplet energies of phenyl ketone 1 and styrene (ET = 61 kcal/mol) was likely the issue. Therefore, naphthyl ketone 2 was examined, as the calculated triplet energy (ET = 54 kcal/mol) indicated that this substitution should lead to more facile sensitization relative to styrene. An initial hit was observed when 2-isopropylthioxanthone (ITX, 5) was used, delivering the product in 46% yield.
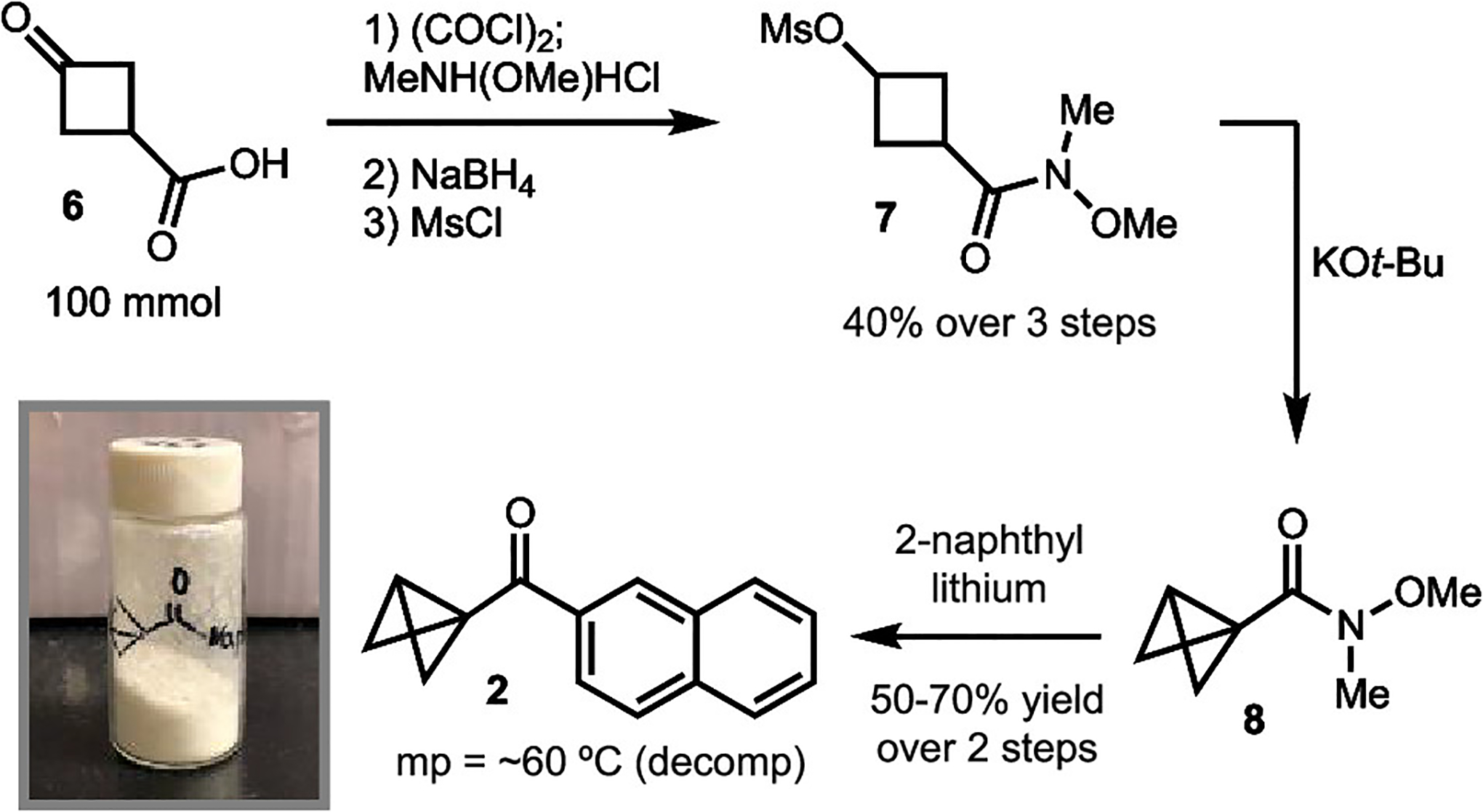
In addition to the promising results, we were also drawn to the straightforward synthesis and favorable properties of the naphthyl ketone-substituted bicyclo[1.1.0]butane 2 (Scheme 3). While known routes to BCBs typically rely on the use of t-BuLi,19 we explored a route that avoids use of this pyrophoric reagent. The synthesis commences with Weinreb amide formation followed by reduction and mesylation to generate 7.20 These first three steps were conducted without any precautions to exclude air or moisture. The transannular ring formation could be easily carried out by treatment with potassium tert-butoxide to provide Weinreb amide 8. Completion of the synthesis was accomplished by the addition of 2-naphthyllithium to 8. The final product is a solid (mp ≈ 60 °C (decomp); see the Supporting Information (SI) for DSC data) that is stable in air for <1 month at room temperature and indefinitely stable at −30 °C.
Scheme 3.
Synthesis of Bench-Stable Bicyclo[1.1.0]butane
With the identification of ITX as an effective sensitizer, optimization was conducted with this as a starting point. It was reasoned that the use of a thioxanthone-based sensitizer with a lower triplet energy might be beneficial to allow for more selective energy transfer to ketone 2 as opposed to styrene. It should be noted that with the use of ITX, extensive dimerization/polymerization of styrene was observed. Previous studies by Booker-Millburn showed that addition of electron-donating groups at the 2- and 2′-positions of thioxanthone lowers the triplet energy.21 Thus, we examined 2-OMeTX (9) and 2,2′-OMeTX (10) and found that the latter resulted in the formation of the product in 76% yield (Table 1, entries 1–3). For each sensitizer evaluated, a light source was used on the basis of prior efforts.22 While we proceeded with 10 to evaluate the substrate scope, further examination of Ir-based sensitizers22 (Table 1, entries 4–7) revealed that [Ir(ppy)2dtbbpy]PF6 (14) could also provide product 4, albeit in lower yield (Table 1, entry 7). While the absolute triplet energies of the catalysts evaluated in the Ir series and the TX series are not correlated, the efficiency of energy transfer is due to factors beyond simple comparison of triplet energies.23
Table 1.
Optimization of the Reaction Conditionsa
| |||||
|---|---|---|---|---|---|
| entry | sensitizer | ET (kcal/mol) | X | hυmax (LED) | yield (%) |
| 1 | 2-iPrTX (5) | 63.5 | 10 | 395 | 46 |
| 2 | 2-OMeTX (9) | 57.8 | 10 | 395 | 28 |
| 3 | 2,2′-OMeTX (10) | 55.2 | 10 | 450 | 76 |
| 4 | Ir[(dFCF3ppy)2dtbbpy]PF6 (11) | 60.1 | 1 | 450 | 58 |
| 5 | Ir[(dFppy)2dtbbpy]PF6 (12) | 57.1 | 1 | 450 | 60 |
| 6b | Ir[(Fppy)2dtbbpy]PF6 (13) | 53.3 | 1 | 450 | 18 |
| 7 | Ir[(ppy)2dtbbpy]PF6 (14) | 51.0 | 1 | 450 | 70 |
Reactions were run on a 0.1 mmol scale. Yields were determined by 1H NMR analysis of the unpurified reaction mixtures with CH2Br2 as an internal standard.
The limited solubility of the sensitizer resulted in a poor yield.
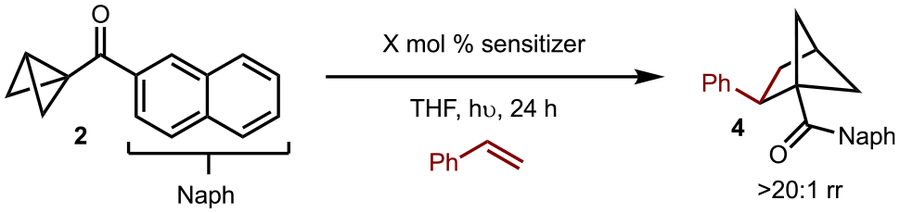
Under the optimized set of conditions, a variety of alkenes were examined with BCB 2 (Scheme 4). With simple vinyl arenes, substitution with electron-donating (products 16, 31) or electron-withdrawing substituents (products 17–19, 26, 30) were tolerated. In addition, sterically demanding groups did not greatly diminish the yield (products 22, 26). The reaction of heterocycles was met with mixed success (products 23–25, 27–29): while the desired product was observed in every case evaluated, the yields were generally lower. Overall, the yields tended to be better with pyridine-derived heterocycles (products 28 and 29). While reactions of 1,2-disubstituted alkenes also led to product formation (products 33–35), albeit in low to moderate yield, 1,1-disubstituted alkenes generally worked better (products 39–41). For reaction of cis- and trans-β-methylstyrene, stereoconvergence was observed (product 33). For substrates beyond vinyl arenes, success was found with a vinylBpin (product 38). Other terminal alkenes such as vinyl acetate (product 36) and vinylsilane (product 37) did lead to product formation, but in low yields. Electron-deficient alkenes and dienes do not allow for product formation (see the SI for details). In cases where low yields were observed, polymerization of the BCB was occurred.16 Finally, substitution on the BCB was also examined. 2-Methyl (product 43) and 2-phenyl (product 44)-derived BCBs were successfully synthesized, with more favorable results for the former. The lower yield for the 2-phenyl substrate may be due to competitive cleavage of the cyclobutane. In the case of 3-methyl (product 45) and 3-phenyl (product 46) derivatives, the yield was higher for the latter. It should be noted that the 3-substituted variants were prepared by a route analogous to that shown in Scheme 3,24 whereas 2-substituted analogues were synthesized by intramolecular cyclopropanation (see the SI for details).25
Scheme 4.
Substrate Scopea
aReactions were run on a 0.2 mmol scale. Yields of isolated and purified products that are averages of two separate experiments are shown.
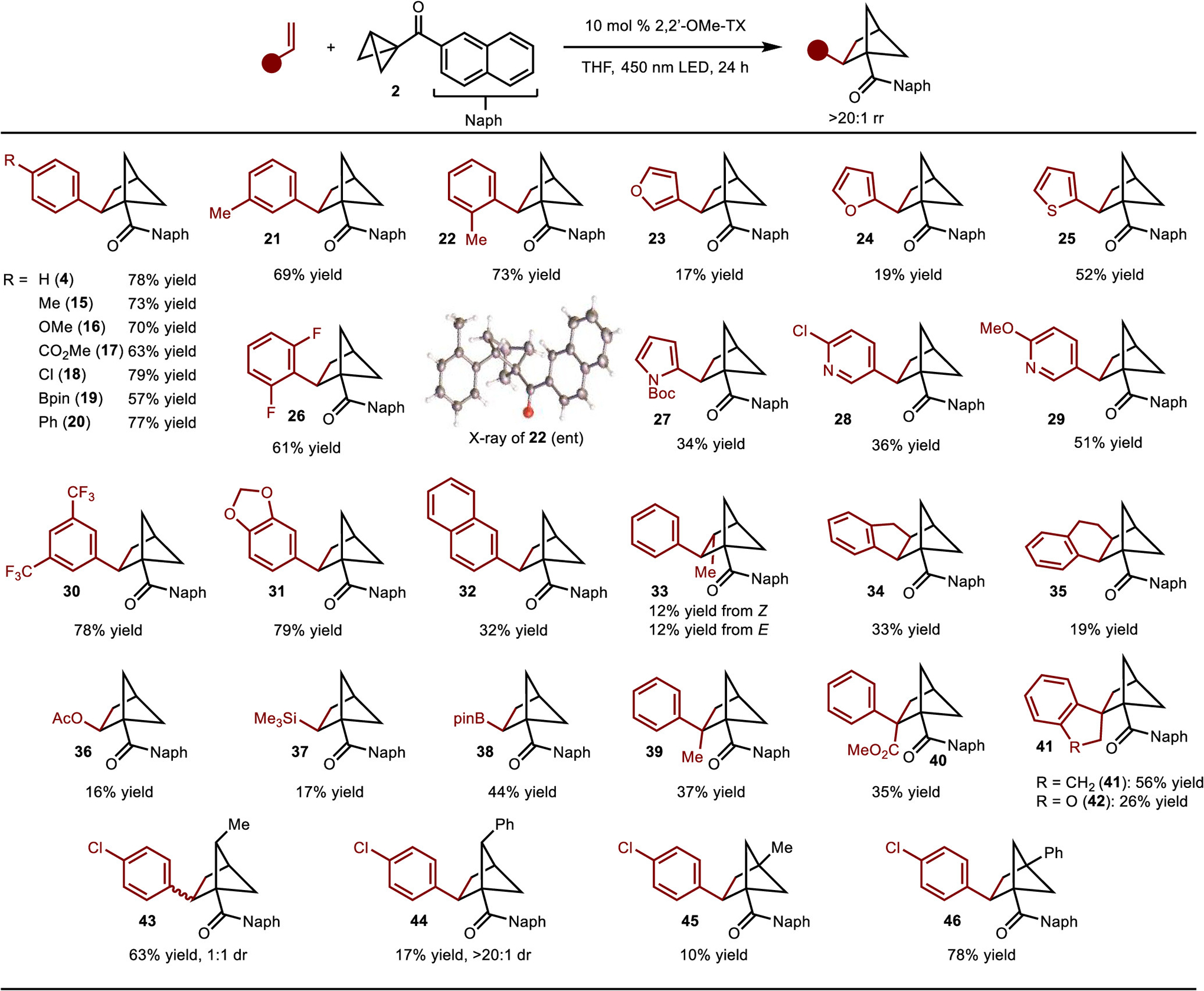
The products generated by this strategy can be further manipulated in various ways (Scheme 5). For example, the naphthyl group can be easily transformed to acid 47 by Baeyer–Villiger oxidation followed by hydrolysis. The acid can also be derivatized by a Curtius rearrangement to allow for synthesis of amine 48. In addition, the formation of redox-active ester 49 (NHPI = N-hydroxyphthalimide) followed by either decarboxylative Minisci reaction26 or borylation27 provides access to 50 or 51, respectively. Finally, oxidation of the Bpin group results in the formation of alcohol 52. Thus, the naphthyl ketone products can be easily elaborated to acid, amine, alcohol, and boronic ester functionality.
Scheme 5.
Further Transformationsa
aReaction conditions: (a) (i) DPPA, Et3N, PhMe, 110 °C, 2 h; (ii) 3 M HCl, AcOH, 0 °C to rt, 16 h. (b) NHPI, DCC, 10 mol % DMAP, CH2Cl2, rt, 12 h. (c) 4-Methylquinoline, TFA, 20 mol % NaI, 30 mol % PPh3, acetone, rt, 16 h, blue LED. (d) (Bcat)2, DMAc, rt, 16 h, blue LED, then pinacol, Et3N. (e) NaBO3·4H2O, THF/H2O, rt, 3 h.
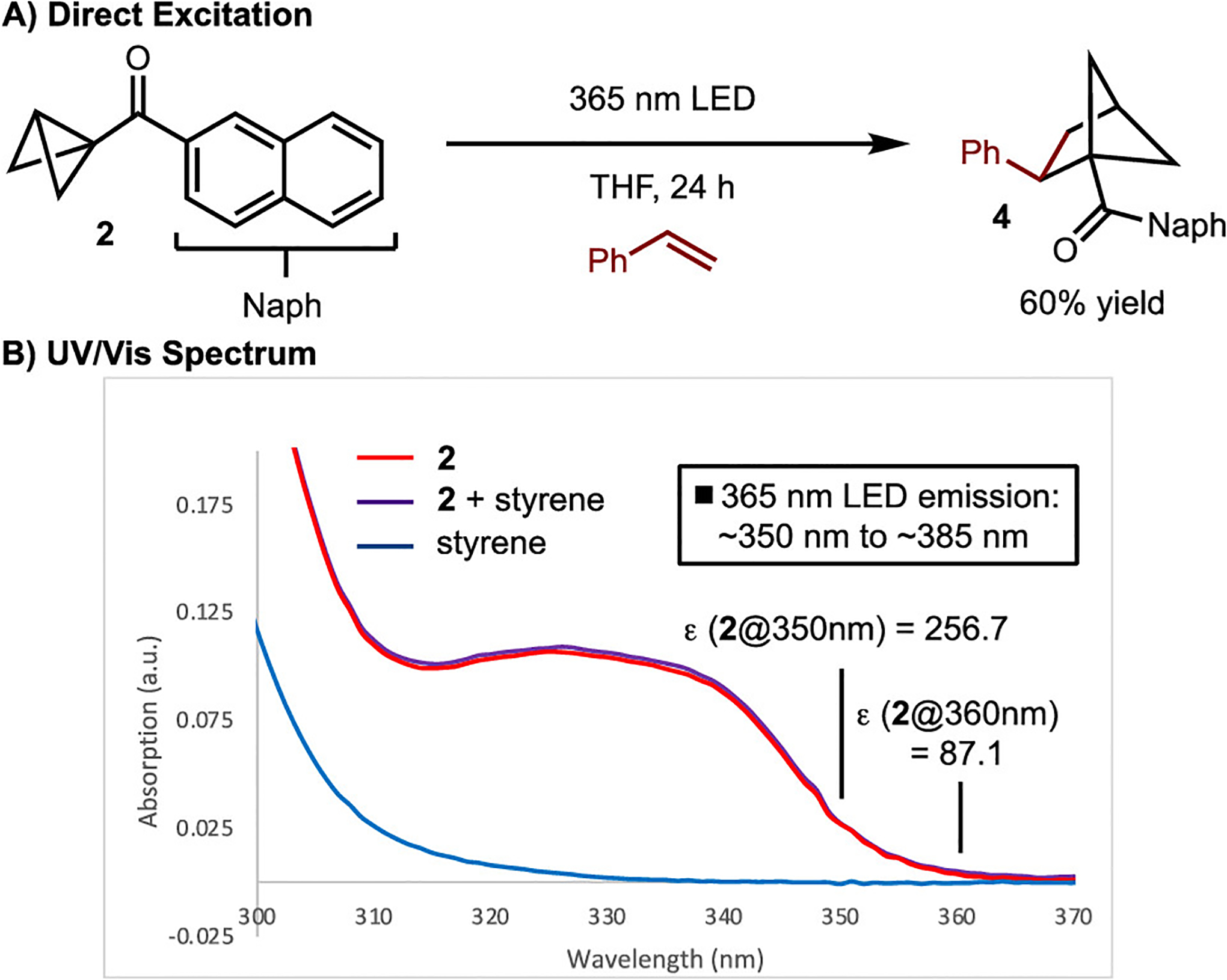
The mechanism of the reaction was also explored (Scheme 6). On the basis of a comparison of the triplet energies of 2,2′-OMe-TX (ET = 55 kcal/mol) and 2 (ET ≈ 54 kcal/mol) with the redox potentials ( vs Ered = −1.93 V, respectively), energy transfer seems more likely.28 Regardless, the most significant data suggesting that the reaction proceeds via excited-state intermediates are shown in Scheme 6. It was demonstrated that the reaction proceeds in 60% yield upon direct irradiation with 365 nm LEDs (emission spectral window of ~350 to ~385 nm) in the absence of the sensitizer.29 UV/vis measurements showed that the absorption spectrum of BCB 2 overlaps with the emission spectrum of the 365 nm LEDs (e.g., the molar absorptivity (ε) for 2 at 360 nm is 87.1 M−1·cm−1), whereas that of styrene does not (Scheme 6B). In addition, it was confirmed that an electron donor–acceptor complex between 2 and styrene does not occur, as evidenced by the lack of a change in the UV/vis spectrum (Scheme 6B).30
Scheme 6.
Mechanism Studies
On the basis on the studies outlined above, it is proposed that the reaction proceeds via energy transfer to generate the T1(π−π*) state of 2 (Scheme 7). This intermediate can also be accessed by direct excitation of 2 to the S1(n−π*) state followed by rapid intersystem crossing (ISC). Strain-release-induced bond cleavage then occurs to generate triplet diradical 53. Capture of the secondary radical with the alkene leads to the formation of 54. Finally, ISC and bond formation lead to the formation of the desired product.
Scheme 7.
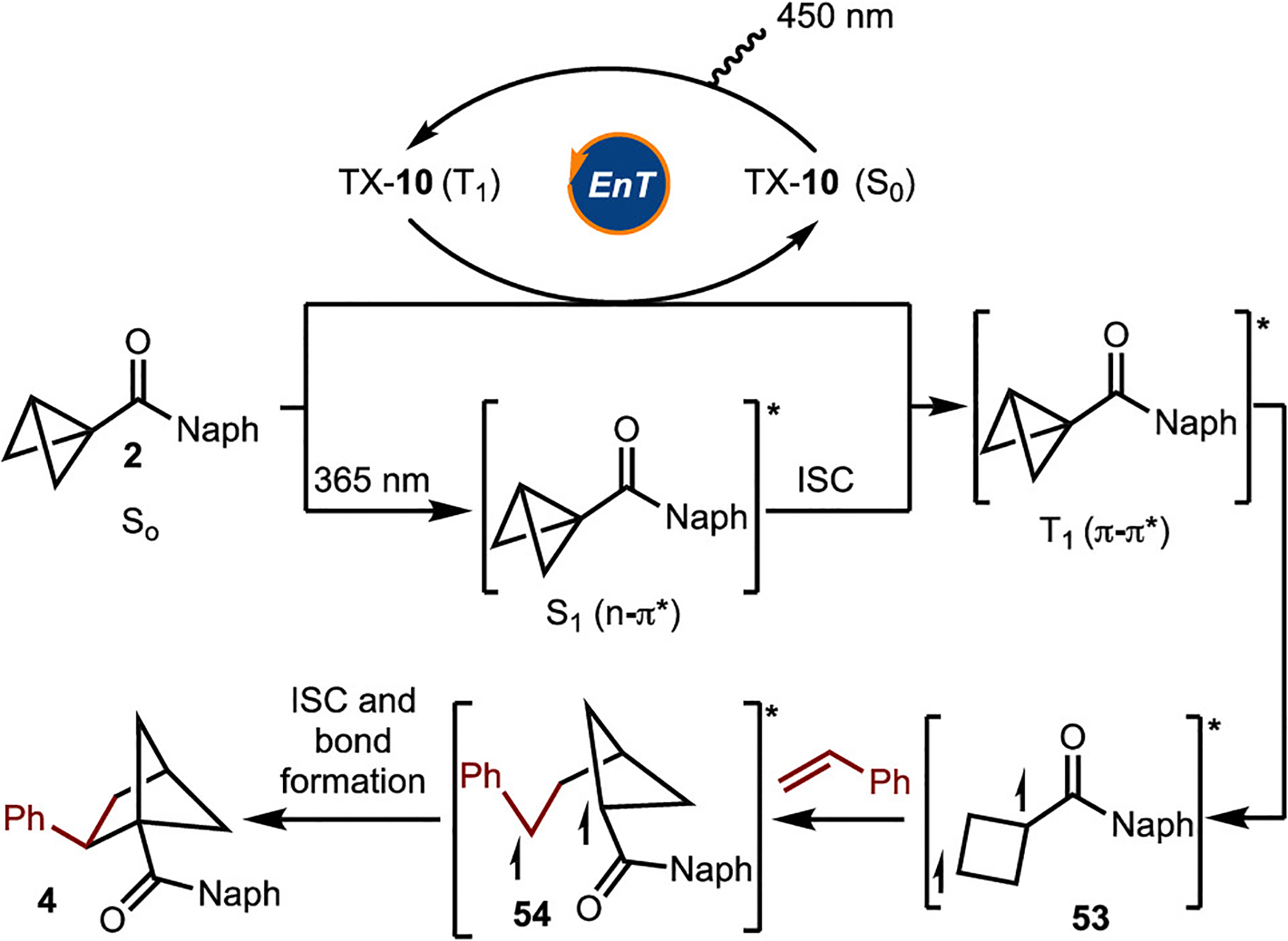
Proposed Mechanism
In conclusion, a new strategy for the synthesis of bicyclo[2.1.1]hexanes has been presented. The reaction operates by energy transfer, which is a new approach for strain-release-driven transformations. While this strategy has been demonstrated for the synthesis of bicyclo[2.1.1]hexanes, we expect that the principles delineated here will be more broadly applicable to access a diverse range of saturated bicyclic structures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Indiana University and the NIH (R35GM131755). This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF MRI Program (CHE-1726633 and CHE-1920026). L.H. received funding from the European Union’s Horizon 2020 Research and Innovation Programme Marie Sklodowska Curie Action ITN under Grant Agreement 859458. We thank Dr. Maren Pink and Dr. Veronica Carta of the IU Molecular Structure Center for acquisition of X-ray crystal structure data. Support for the acquisition of the Bruker Venture D8 diffractometer through the Major Scientific Research Equipment Fund from the President of Indiana University and the Office of the Vice President for Research is gratefully acknowledged. Calculations using the Big Red 3 supercomputer were supported in part by Lilly Endowment, Inc., through its support for the Indiana University Pervasive Technology Institute.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c02976.
Experimental procedures and analytical data for all new compounds (PDF)
Accession Codes
CCDC 2114082 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.2c02976
The authors declare no competing financial interest.
Contributor Information
Renyu Guo, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States.
Yu-Che Chang, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States.
Loic Herter, SpiroChem AG, 4058 Basel, Switzerland; Bio-Functional Chemistry (UMR 7199), LabEx Medalis, University of Strasbourg, 67400 Illkirch-Graffenstaden, France.
Christophe Salome, SpiroChem AG, 4058 Basel, Switzerland.
Sarah E. Braley, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States
Thomas C. Fessard, SpiroChem AG, 4058 Basel, Switzerland
M. Kevin Brown, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States.
REFERENCES
- (1).(a) Ritchie TJ; Macdonald SJF The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discovery Today 2009, 14, 1011–1020. [DOI] [PubMed] [Google Scholar]; (b) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (c) Lovering F Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar]; (d) Mykhailiuk PK Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem 2019, 17, 2839–2849. [DOI] [PubMed] [Google Scholar]; (e) Subbaiah MAM; Meanwell NA Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem 2021, 64, 14046–14128. [DOI] [PubMed] [Google Scholar]
- (2).(a) Delia EW; Lochert IJ Synthesis of Bridgehead-Substituted Bicyclo[1.1.1]pentanes. A Review. J. Org. Prep. Proced. Int 1996, 28, 411–441. [Google Scholar]; (b) Levin MD; Kaszynski P; Michl J Bicyclo[1.1.1]pentanes, [n]Staffanes, [1.1.1]Propellanes, and Tricyclo[2.1.0.02,5]pentanes. Chem. Rev 2000, 100, 169–234. [DOI] [PubMed] [Google Scholar]; (c) Dilmaç AM; Spuling E; de Meijere A; Bräse S Propellanes—From a Chemical Curiosity to “Explosive” Materials and Natural Products. Angew. Chem., Int. Ed 2017, 56, 5684–5718. [DOI] [PubMed] [Google Scholar]; (d) Kanazawa J; Uchiyama M Recent Advances in the Synthetic Chemistry of Bicyclo[1.1.1]pentane. Synlett 2019, 30, 1–11. [Google Scholar]; (e) Ma X; Pham LN Selected Topics in the Syntheses of Bicyclo[1.1.1]pentane (BCP) Analogues. Asian J. Org. Chem 2020, 9, 8–22. [Google Scholar]
- (3).(a) Denisenko A; Garbuz P; Shishkina SV; Voloshchuk NM; Mykhailiuk PK Saturated Bioisosteres of ortho-Substituted Benzenes. Angew. Chem., Int. Ed 2020, 59, 20515. [DOI] [PubMed] [Google Scholar]; (b) Yang Y; Tsien J; Hughes JME; Peters BK; Merchant RR; Qin T An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem 2021, 13, 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected recent examples, see:; (a) Saya JM; Vos K; Kleinnijenhuis RA; van Maarseveen JH; Ingemann S; Hiemstra H Total Synthesis of Aquatolide. Org. Lett 2015, 17, 3892–3894. [DOI] [PubMed] [Google Scholar]; (b) Kleinnijenhuis RA; Timmer BJJ; Lutteke G; Smits JMM; de Gelder R; van Maarseveen JH; Hiemstra H Formal Synthesis of Solanoeclepin A: Enantioselective Allene Diboration and Intramolecular [2 + 2] Photocycloaddition for the Construction of the Tricyclic Core. Chem. - Eur. J 2016, 22, 1266–1269. [DOI] [PubMed] [Google Scholar]; (c) Takao K.-i.; Kai H; Yamada A; Fukushima Y; Komatsu D; Ogura A; Yoshida K Total Syntheses of (+)-Aquatolide and Related Humulanolides. Angew. Chem., Int. Ed 2019, 58, 9851–9855. [DOI] [PubMed] [Google Scholar]
- (5).For reaction of BCBs with azo compounds, see:; (a) Amey RL; Smart BE Bicyclo[1.1.0]butanes. Reactions with cyclic azo compounds. J. Org. Chem 1981, 46, 4090–4092. [Google Scholar]; (b) Chang MH; Dougherty DA 2,3-Diazabicyclo[2.1.1]hex-2-ene. Synthesis and thermal decomposition. J. Org. Chem 1981, 46, 4092–4093. [Google Scholar]; (c) Schwartz BD; Smyth AP; Nashar PE; Gardiner MG; Malins LR Investigating Bicyclobutane–Triazolinedione Cycloadditions as a Tool for Peptide Modification. Org. Lett 2022, 24, 1268. [DOI] [PubMed] [Google Scholar]
- (6).For reactions of BCBs with imines, see:; Dhake K; Woelk KJ; Becica J; Un A; Jenny SE; Leitch DC Modular Synthesis of Azabicyclohexanes and Cyclobutenyl Amines. ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-p0fzf, (accessed 2022–02-01). [DOI] [PubMed] [Google Scholar]
- (7).(a) Cairncross A; Blanchard EP Bicyclo[1.1.0]butane Chemistry. II. Cycloaddition Reactions of 3-Methylbicyclo[1.1.0]-butanecarbonitriles. The Formation of Bicyclo[2.1.1]hexanes. J. Am. Chem. Soc 1966, 88, 496–504. [Google Scholar]; (b) De Meijere A; Wenck H; Seyed-Mahdavi F; Viehe HG; Gallez V; Erden I Cycloadditions of methylenecyclopropanes and strained bicyclo[n.1.0]alkanes to radicophilic olefins. Tetrahedron 1986, 42, 1291–1297. [Google Scholar]; (c) Wipf P; Walczak MAA Pericyclic Cascade Reactions of (Bicyclo[1.1.0]-butylmethyl)amines. Angew. Chem., Int. Ed 2006, 45, 4172–4175. [DOI] [PubMed] [Google Scholar]
- (8).After the submission of this Communication, two studies describing [2π + 2σ] cycloaddition of BCBs and alkenes were reported. See:; (a) Kleinmans R; Pinkert T; Dutta S; Paulisch TO; Keum H; Daniliuc CG; Glorius R Intermolecular [2π+2σ]-Photocycloaddition Enabled by Triplet Energy Transfer. Nature 2022, DOI: 10.1038/s41586-022-04636-x. [DOI] [PubMed] [Google Scholar]; (b) Agasti S; Beltran F; Pye E; Kaltsoyannis N; Crisenza G; Procter D A Catalytic Alkene Insertion Approach to Bicyclo[2.1.1]hexane Bioisosteres. ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-v93kv, (accessed 2022–03-23). [DOI] [PubMed] [Google Scholar]
- (9).For selected reviews, see:; (a) De Simone F; Waser J Cyclization and Cycloaddition Reactions of Cyclopropyl Carbonyls and Imine. Synthesis 2009, 2009, 3353–3374. [Google Scholar]; (b) Schneider TF; Kaschel J; Werz DB A New Golden Age for Donor–Acceptor Cyclopropanes. Angew. Chem., Int. Ed 2014, 53, 5504–5523. [DOI] [PubMed] [Google Scholar]; (c) Cavitt MA; Phun LH; France S Intramolecular donor–acceptor cyclopropane ring-opening cyclizations. Chem. Soc. Rev 2014, 43, 804–818. [DOI] [PubMed] [Google Scholar]; (d) Wang L; Tang Y Asymmetric Ring-Opening Reactions of Donor-Acceptor Cyclopropanes and Cyclobutanes. Isr. J. Chem 2016, 56, 463–475. [Google Scholar]
- (10).For selected recent studies, see:; (a) Amador AG; Sherbrook EM; Yoon TP Enantioselective Photocatalytic [3 + 2] Cycloadditions of Aryl Cyclopropyl Ketones. J. Am. Chem. Soc 2016, 138, 4722–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang X; Lin J; Shen T; Harms K; Marchini M; Ceroni P; Meggers E Asymmetric [3 + 2] Photocycloadditions of Cyclopropanes with Alkenes or Alkynes through Visible-Light Excitation of Catalyst-Bound Substrates. Angew. Chem., Int. Ed 2018, 57, 5454–5458. [DOI] [PubMed] [Google Scholar]; (c) Hao W; Harenberg JH; Wu X; MacMillan SN; Lin S Diastereo- and Enantioselective Formal [3 + 2] Cycloaddition of Cyclopropyl Ketones and Alkenes via Ti-Catalyzed Radical Redox Relay. J. Am. Chem. Soc 2018, 140, 3514–3517. [DOI] [PubMed] [Google Scholar]; (d) Schmalz V; Koert U Visible-Light-Induced Photoannulation of α-Naphthyl Cyclopropane Carboxylic Esters to Functionalized Dihydrophenalenes. Org. Lett 2022, 24, 152–157. [DOI] [PubMed] [Google Scholar]
- (11).For selected recent studies, see:; (a) Maity S; Zhu M; Shinabery RS; Zheng N Intermolecular [3 + 2] Cycloaddition of Cyclopropylamines with Olefins by Visible-Light Photocatalysis. Angew. Chem., Int. Ed 2012, 51, 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Staveness D; Sodano TM; Li K; Burnham EA; Jackson KD; Stephenson CRJ Providing a New Aniline Bioisostere through the Photochemical Production of 1-Amino-norbornanes. Chem 2019, 5, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Muriel B; Gagnebin A; Waser J Synthesis of Bicyclo[3.1.0]-hexanes by (3 + 2) Annulation of Cyclopropenes with Amino-cyclopropanes. Chem. Sci 2019, 10, 10716–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Staveness D; Collins JL; McAtee RC; Stephenson CRJ Exploiting Imine Photochemistry for Masked N-Centered Radical Reactivity. Angew. Chem., Int. Ed 2019, 58, 19000–19006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yin Y; Li Y; Goncalves TP; Zhan Q; Wang G; Zhao X; Qiao B; Huang K-W; Jiang Z All-Carbon Quaternary Stereocenters α to Azaarenes via Radical-Based Asymmetric Olefin Difunctionalization. J. Am. Chem. Soc 2020, 142, 19451–19456. [DOI] [PubMed] [Google Scholar]; (f) Uraguchi D; Kimura Y; Ueoka F; Ooi T Urea as a Redox Active Directing Group under Asymmetric Photocatalysis of Iridium-Chiral Borate Ion Pairs. J. Am. Chem. Soc 2020, 142, 19462–19467. [DOI] [PubMed] [Google Scholar]; (g) Sowden MJ; Collins JL; Staveness D; Stephenson C A One Pot Photochemical Method for the Generation of Functionalized Aminocyclopentanes. ChemRxiv 2020, DOI: 10.26434/chemrxiv.13079159.v1, (accessed 2022–02-01). [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) White DH; Noble A; Booker-Milburn KI; Aggarwal VK Diastereoselective Photoredox-Catalyzed [3 + 2] Cycloadditions of N-Sulfonyl Cyclopropylamines with Electron-Deficient Olefins. Org. Lett 2021, 23, 3038–3042. [DOI] [PubMed] [Google Scholar]
- (12).Woźniak Ł; Magagnano G; Melchiorre P Enantioselective Photochemical Organo-Cascade Catalysis. Angew. Chem., Int. Ed 2018, 57, 1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).For selected recent examples, see:; (a) Hashimoto T; Kawamata Y; Maruoka K An organic thiyl radical catalyst for enantioselective cyclization. Nat. Chem 2014, 6, 702–705. [DOI] [PubMed] [Google Scholar]; (b) Ryss JM; Turek AK; Miller SJ Disulfide-Bridged Peptides That Mediate Enantioselective Cycloadditions through Thiyl Radical Catalysis. Org. Lett 2018, 20, 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu M; Huang X-L; Sun S; Zheng C; You S-L Visible-Light-Induced Dearomatization of Indoles/Pyrroles with Vinylcyclopropanes: Expedient Synthesis of Structurally Diverse Polycyclic Indolines/Pyrrolines. J. Am. Chem. Soc 2021, 143, 13441–13449. [DOI] [PubMed] [Google Scholar]
- (14).(a) Wang J; Zheng N The Cleavage of a C-C Bond in Cyclobutylanilines by Visible-Light Photoredox Catalysis: Development of a [4 + 2] Annulation Method. Angew. Chem., Int. Ed 2015, 54, 11424–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hu A; Chen Y; Guo J-J; Yu N; An Q; Zuo Z Cerium-Catalyzed Formal Cycloaddition of Cycloalkanols with Alkenes through Dual Photoexcitation. J. Am. Chem. Soc 2018, 140, 13580–13585. [DOI] [PubMed] [Google Scholar]
- (15).Harmata AS; Spiller TE; Sowden MJ; Stephenson CRJ Photochemical Formal (4 + 2)-Cycloaddition of Imine-Substituted Bicyclo[1.1.1]pentanes and Alkenes. J. Am. Chem. Soc 2021, 143, 21223–21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For a review, see:; Hall HK Jr.; Padias AB Bicyclobutanes and Cyclobutenes: Unusual Carbocyclic Monomers. J. Polym. Sci., Part A: Polym. Chem 2003, 41, 625–635. [Google Scholar]
- (17).For selected reviews regarding energy transfer, see:; (a) Strieth-Kalthoff F; James MJ; Teders M; Pitzer L; Glorius F Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev 2018, 47, 7190–7202. [DOI] [PubMed] [Google Scholar]; (b) Zhou Q-Q; Zou Y-Q; Lu L-Q; Xiao W-J Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem., Int. Ed 2019, 58, 1586–1604. [DOI] [PubMed] [Google Scholar]; (c) Strieth-Kalthoff F; Glorius F Triplet Energy Transfer Photocatalysis: Unlocking the Next Level. Chem 2020, 6, 1888–1903. [Google Scholar]; (d) Großkopf J; Kratz T; Rigotti T; Bach T Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev 2022, 122, 1626–1653. [DOI] [PubMed] [Google Scholar]
- (18).James MJ; Schwarz JL; Strieth-Kalthoff F; Wibbeling B; Glorius F Dearomative Cascade Photocatalysis: Divergent Synthesis through Catalyst Selective Energy Transfer. J. Am. Chem. Soc 2018, 140, 8624–8628. [DOI] [PubMed] [Google Scholar]
- (19).(a) Walczak MAA; Krainz T; Wipf P Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res 2015, 48, 1149–1158. [DOI] [PubMed] [Google Scholar]; (b) Bennett SH; Fawcett A; Denton EH; Biberger T; Fasano V; Winter N; Aggarwal VK Difunctionalization of C–C σ-Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins. J. Am. Chem. Soc 2020, 142, 16766–16775. [DOI] [PubMed] [Google Scholar]; (c) Schwartz BD; Zhang MY; Attard RH; Gardiner MG; Malins LR Structurally Diverse Acyl Bicyclobutanes: Valuable Strained Electrophiles. Chem. - Eur. J 2020, 26, 2808–2812. [DOI] [PubMed] [Google Scholar]
- (20).For related strategies for the synthesis of BCBs, see:; (a) Wiberg KB; Ciula RP Ethyl Bicyclo[1.1.0]- butane-1-carboxylate. J. Am. Chem. Soc 1959, 81, 5261–5262. [Google Scholar]; (b) Hall HK; Blanchard EP; Cherkofsky SC; Sieja JB; Sheppard WA Synthesis and polymerization of 1-bicyclobutanecarbonitriles. J. Am. Chem. Soc 1971, 93, 110–120. [Google Scholar]; (c) Applequist DE; Renken TL; Wheeler JW Polar substituent effects in 1,3-disubstituted bicyclo[1.1.1]pentanes. J. Org. Chem 1982, 47, 4985–4995. [Google Scholar]; (d) Hoz S; Livneh M J. Am. Chem. Soc 1987, 109, 7483–7488. [Google Scholar]; (e) Measom ND; Down KD; Hirst DJ; Jamieson C; Manas ES; Patel VK; Somers DO Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS Med. Chem. Lett 2017, 8, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ociepa M; Wierzba AJ; Turkowska J; Gryko D Polarity-Reversal Strategy for the Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc 2020, 142, 5355–5361. [DOI] [PubMed] [Google Scholar]
- (21).Elliott LD; Kayal S; George MW; Booker-Milburn K Rational Design of Triplet Sensitizers for the Transfer of Excited State Photochemistry from UV to Visible. J. Am. Chem. Soc 2020, 142, 14947–14956. [DOI] [PubMed] [Google Scholar]
- (22).Daub ME; Jung H; Lee BJ; Won J; Baik M-H; Yoon TP Enantioselective [2 + 2] Cycloadditions of Cinnamate Esters: Generalizing Lewis Acid Catalysis of Triplet Energy Transfer. J. Am. Chem. Soc 2019, 141, 9543–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).For example, see:; Singh A; Fennell CJ; Weaver JD Photocatalyst Size Controls Electron and Energy Transfer: Selectable E/Z Isomer Synthesis via C–F Alkenylation. Chem. Sci 2016, 7, 6796–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ma X; Sloman DL; Han Y; Bennett DJ A Selective Synthesis of 2,2-Difluorobicyclo[1.1.1]pentane Analogues: “BCP-F2”. Org. Lett 2019, 21, 7199–7203. [DOI] [PubMed] [Google Scholar]
- (25).Panish R; Chintala SR; Boruta DT; Fang Y; Taylor MT; Fox JM Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc 2013, 135, 9283–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fu M-C; Shang R; Zhao B; Wang B; Fu Y Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 2019, 363, 1429–1434. [DOI] [PubMed] [Google Scholar]
- (27).Fawcett A; Pradeilles J; Wang Y; Mutsuga T; Myers EL; Aggarwal VK Photoinduced decarboxylative borylation of carboxylic acids. Science 2017, 357, 283–286. [DOI] [PubMed] [Google Scholar]
- (28).The success of Ir[(ppy)2dtbby]PF6 (ET = 51 kcal/mol) and the calculated triplet energy of 2 suggest endergonic triplet energy transfer, which has been described previously. See:; Strieth-Kalthoff F; Henkel C; Teders M; Kahnt A; Knolle W; Gómez-Suárez A; Dirian K; Alex W; Bergander K; Daniliuc CG; Abel B; Guldi DM; Glorius F Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach. Chem 2019, 5, 2183–2194. [Google Scholar]
- (29).Wagner PJ Photoinduced Ortho [2 + 2] Cycloaddition of Double Bonds to Triplet Benzenes. Acc. Chem. Res 2001, 34, 1–8. [DOI] [PubMed] [Google Scholar]
- (30).Crisenza GEM; Mazzarella D; Melchiorre P Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc 2020, 142, 5461–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


