Abstract
Introduction
Sotrovimab, a recombinant human monoclonal antibody (mAb) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had US Food and Drug Administration Emergency Use Authorization for the treatment of high-risk outpatients with mild-to-moderate coronavirus disease 2019 (COVID-19) from 26 May 2021 to 5 April 2022. Real-world clinical effectiveness of sotrovimab in reducing the risk of 30-day all-cause hospitalization and/or mortality was evaluated for the period when the prevalence of circulating SARS-CoV-2 variants changed between Delta and Omicron in the USA.
Methods
A retrospective analysis was conducted of de-identified patients diagnosed with COVID-19 between 1 September 2021 to 30 April 2022 in the FAIR Health National Private Insurance Claims database. Patients meeting high-risk criteria were divided into two cohorts: sotrovimab and not treated with a mAb (“no mAb”). All-cause hospitalizations and facility-reported mortality ≤ 30 days of diagnosis (“30-day hospitalization or mortality”) were identified. Multivariable and propensity score-matched Poisson and logistic regressions were conducted to estimate the adjusted relative risk (RR) and odds of 30-day hospitalization or mortality in each cohort.
Results
Compared with the no mAb cohort (n = 1,514,868), the sotrovimab cohort (n = 15,633) was older and had a higher proportion of patients with high-risk conditions. In the no mAb cohort, 84,307 (5.57%) patients were hospitalized and 8167 (0.54%) deaths were identified, while in the sotrovimab cohort, 418 (2.67%) patients were hospitalized and 13 (0.08%) deaths were identified. After adjusting for potential confounders, the sotrovimab cohort had a 55% lower risk of 30-day hospitalization or mortality (RR 0.45, 95% CI 0.41–0.49) and an 85% lower risk of 30-day mortality (RR 0.15, 95% CI 0.08–0.29). Monthly, from September 2021 to April 2022, the RR reduction for 30-day hospitalization or mortality in the sotrovimab cohort was maintained, ranging from 46% to 71% compared with the no mAb cohort; the RR estimate in April 2022 was uncertain, with wide confidence intervals due to the small sample size.
Conclusion
Sotrovimab was associated with reduced risk of 30-day all-cause hospitalization and mortality versus no mAb treatment. Clinical effectiveness persisted during Delta and early Omicron variant waves and among all high-risk subgroups assessed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00755-0.
Keywords: COVID-19, Effectiveness, Monoclonal antibody, SARS-CoV-2, Sotrovimab, Real-world
Key Summary Points
| Why carry out this study? |
| It remains uncertain how in vitro antibody neutralization activity translates to clinical effectiveness for sotrovimab, a dual-action monoclonal antibody against SARS-CoV-2, for high-risk outpatients with mild-to-moderate COVID-19. |
| We conducted a retrospective observational cohort study of patients diagnosed with COVID-19 between 1 September 2021 and 30 April 2022 to evaluate the real-world clinical effectiveness of sotrovimab when the prevalence of circulating SARS-CoV-2 variants changed between Delta and Omicron in the USA. |
| What was learned from the study? |
| Compared with no mAb treatment, sotrovimab was associated with reduced risk of 30-day hospitalization and/or facility-reported mortality among high-risk COVID-19 patients. |
| Sotrovimab demonstrated real-world clinical effectiveness beyond clinical trial data, including benefits among populations not previously studied in clinical trials such as immunocompromised, those with ≥ 1 documented COVID-19 vaccine, and pregnant women. |
Introduction
As of 22 September 2022, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 610 million confirmed cases of coronavirus disease 2019 (COVID-19) globally [1]. Although most cases of COVID-19 are mild to moderate, approximately 5–10% of cases are severe [2]. An estimated 18.2 million excess deaths worldwide were attributed to COVID-19 in 2020 and 2021 [3].
On 26 May 2021, sotrovimab, an anti-SARS-CoV-2 recombinant human monoclonal antibody (mAb) received US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) to treat mild-to-moderate COVID-19 in SARS-CoV-2-positive adults and children (≥ 12 years, weighing ≥ 40 kg) at high risk for severe COVID-19 [4]. This EUA was issued based on interim results from the phase 3 randomized controlled clinical trial COMET-ICE, which showed that, compared with placebo, sotrovimab was associated with 85% [97.24% confidence interval (CI) 44–96%] relative risk (RR) reduction of all-cause hospitalization (lasting > 24 h) or death due to any cause within 29 days of treatment among high-risk outpatients [4, 5]. In the final analysis of the primary endpoint, 29-day hospitalization or mortality events occurred in 1% (6/528) of the sotrovimab cohort and 6% (30/529) of the placebo cohort, RR 0.21 (95% CI 0.09–0.50), a 79% RR reduction [5].
Sotrovimab development leading up to the EUA occurred prior to the SARS-CoV-2 Delta variant wave, when the population was mostly unvaccinated. Since then, new SARS-CoV-2 variants of concern (VOC) have emerged and shifts in sotrovimab’s in vitro neutralization activity relative to wild-type strain have been observed [6]. The FDA deauthorized sotrovimab for COVID-19 on a rolling basis across states with > 50% Omicron BA.2 prevalence and, on 5 April 2022, deauthorized it across the country [7].
Uncertainty remains regarding how in vitro antibody neutralization activity translates to clinical effectiveness, especially for dual-action antibodies such as sotrovimab, which have potent effector function, including antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). We used a large, nationally representative insurance claims database to retrospectively analyze patients diagnosed with COVID-19 at high risk of disease progression. Our objectives were to examine patient characteristics and real-world effectiveness of sotrovimab on the risk of 30-day hospitalization and/or mortality in all treated patients and high-risk subgroups during the SARS-CoV-2 Delta and early Omicron variant waves in the USA.
Methods
Study Design and Data Source
We used the FAIR Health National Private Insurance Claims (FH NPIC) database, which includes medical and dental claims submitted by over 70 private insurers across 50 US states, Puerto Rico, and the US Virgin Islands. FAIR Health researchers were responsible for operationalizing all analyses upon study initiation in February 2022, pursuant to a data license and use agreement. Study sponsors did not have access to the database or to any patient-level data, and were provided with monthly reports of deidentified, aggregated cohort-level data in summary tables. This research did not require institutional review board (IRB) or ethics review, as analyses with these data do not meet the definition of “research involving human subjects” as defined within 45 CFR 46.102(f). This study followed the STROBE reporting guidelines.
Study Population
The analysis included aggregated claims records for 1,530,501 deidentified patients with a diagnosis of COVID-19 (International Classification of Diseases, 10th Revision [ICD-10]: U07.1) recorded from 1 September 2021 to 30 April 2022. The database is not linked to electronic health records; therefore, diagnoses, disease severity, and COVID-19-related hospitalizations or deaths could not be confirmed by laboratory test results or medical records.
Patients at high risk of disease progression were identified via ICD-10-CM (Clinical Modification) diagnosis codes aligned to sotrovimab’s EUA in the 24 months leading up to their first COVID-19 diagnosis date. Prespecified high-risk conditions included: age (≥ 65 years), body mass index (BMI) ≥ 25 kg/m2, pregnancy, chronic kidney disease (CKD; any stage), type 1 or 2 diabetes, immunocompromising conditions [human immunodeficiency virus (HIV), autoimmune disease, Hodgkin’s lymphoma, Non-Hodgkin’s lymphoma, leukemia, solid cancer], immunosuppressive therapy [systemic corticosteroids and noncorticosteroid immunosuppressants billed using Healthcare Common Procedure Coding System (HCPCS) codes], chronic obstructive pulmonary disease (COPD), asthma, chronic lung disease, sickle cell disease, congenital heart disease, acquired heart disease, cardiovascular disease, hypertension, and neurodevelopmental disorders. Additionally, patients were considered high risk if prespecified claims for a medical device (or components) outside of a nonacute care facility setting were identified, including noninvasive ventilation, oxygen therapy, renal replacement therapy, total parenteral nutrition, tracheostomy/other endotracheal airway, ventilation, ventricular assist, gastro- or jejunostomy, or Mitrofanoff catheter. We excluded patient records that were missing age, gender (patient reported), geographic data, or those with a claim for tixagevimab and cilgavimab pre-exposure prophylaxis (Supplementary Table 1).
The diagnosis month was the month and year of the first COVID-19 diagnosis recorded in a patient’s claims record between 1 September 2021 and 30 April 2022. As the study was conducted using a healthcare claims database, variant sequencing data for patients were unavailable. Therefore, the study was conducted over a period of time (September 2021–April 2022) for which the predominant variants in the USA changed between Delta, Omicron BA.1, and BA.2. The diagnosis month category served as a proxy for the predominant circulating variant(s) or time period when a circulating variant became predominant in the USA per the Centers for Disease Control and Prevention (CDC) COVID data tracker, Nowcast [8]. The predominant circulating SARS-CoV-2 variant (> 99% prevalence) was Delta from 1 September to 30 November 2021. Omicron BA.1 (and sublineages) became the predominant variant between 1 December 2021 and 28 February 2022, and Omicron BA.2 (and sublineages) between 1 March and 30 April 2022.
Based on the state in which the COVID-19 diagnosis was recorded, patients were assigned to a US Department of Health and Human Services designated region used by CDC COVID data tracker, Nowcast, for variant monitoring and reporting.
The FH NPIC database included only COVID-19 vaccinations with claims submitted to a contributing insurer (“documented COVID-19 vaccine”). Those without a documented COVID-19 vaccine were of uncertain vaccination status as they could be unvaccinated or vaccinated without a submitted claim to a contributing insurer. The FH NPIC database captures limited data for oral drugs sold in the retail pharmacy setting or billed using National Drug Codes (NDC). Although antiviral therapy was present in the data, the variable was omitted from the analysis because of insufficient sample size for analysis (≤ seven patients were identified in each cohort with a claim for an antiviral therapy after the COVID-19 diagnosis date).
Exposure and Outcome Variables
We used claimed HCPCS codes (Supplementary Table 1) for drug or infusion outpatient administration ≤ 7 days of recorded diagnosis to divide high-risk patients into two cohorts: one treated with sotrovimab and one not treated with a mAb authorized for early treatment of COVID-19.
Assessed outcomes were all-cause hospitalization within 30 days of claimed COVID-19 diagnosis, 30-day facility-reported all-cause mortality (“mortality”), and the composite outcome of 30-day all-cause hospitalization or mortality. All-cause hospitalizations were identified in claims by bill types with beginning digits 11*, 12*, and 18*, corresponding to hospital facility inpatient care. Intensive care unit (ICU)-related hospitalizations were identified by revenue codes 0200, 0202, 0203, 0206, 0208, and 0209. Deaths were identified from claims records based on discharge status reported by health care facilities for which a billable medical service was provided. Information regarding cause of death was not available.
Statistical Analyses
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina). Given this study was a retrospective, real-world analysis of all COVID-19 patients who met the study eligibility criteria in the FH NPIC database, a sample size calculation was not warranted. Baseline patient characteristics were described for high-risk COVID-19 patients by treatment cohort. Chi-square tests for categorical variables and t-tests for continuous variables were performed to compare statistical differences by treatment cohort. P-values were not adjusted for multiplicity. All statistical comparisons were two-sided, with significance assumed at α ≤ 0.05.
Multivariate Poisson and logistic regression analyses were conducted to assess the impact of sotrovimab versus no mAb on 30-day hospitalization and/or mortality, adjusting for potential confounders. Confounders were selected from demographic and clinical characteristics, based on a priori evidence that these factors are associated with receipt of treatment or risk of hospitalization and/or death [9]. Covariates in the Poisson and logistic regression models were the same and included diagnosis month category, region, gender, age, rurality (urban/rural based on geozip where COVID-19 diagnosis was recorded), obesity (BMI ≥ 30 kg/m2), pregnancy, CKD, diabetes, immunocompromising conditions (includes immunosuppressive therapy), lung disease (includes COPD, asthma, chronic lung disease), cardiovascular disease (includes acquired heart disease, congenital heart disease, cardiovascular disease, hypertension), medical device, and documented COVID-19 vaccine. Adjusted RR and 95% confidence intervals (CIs) were derived from the Poisson regression model with robust error variances and presented as primary results in this manuscript. Adjusted odds ratios (OR) and 95% CIs were estimated from the logistic regression model and are presented in the Supplementary Tables. For analyses stratified by diagnosis month, high-risk conditions, and documented COVID-19 vaccine, the multivariable Poisson and logistic regression models were run separately for each subgroup.
As a sensitivity analysis for the comparison between the sotrovimab and no mAb cohorts, we conducted propensity score (PS)-matched Poisson and logistic regression analyses to estimate the RR and OR, respectively, of 30-day hospitalization or mortality among PS-matched cohorts. The PS matching balanced the study cohorts to reduce potential bias associated with treatment selection. Multinomial logistic regression was used to calculate the propensity score—the conditional probability that each patient would be assigned to a specific treatment group given that patient’s pretreatment variables. The treatment cohorts were matched on diagnosis month, region, gender, age, rurality, obesity, pregnancy, CKD, diabetes, immunocompromising conditions (including immunosuppressive therapy), lung disease (including asthma, COPD, chronic lung disease), cardiovascular disease (including heart disease and hypertension), and medical device. Greedy nearest neighbor matching with caliper of 0.2 and matching ratio of 1:4 (one patient in the sotrovimab cohort was matched to four patients in the no-mAb cohort) without replacement was performed. The PS match was determined successful if all the covariates included in the PS model had a standardized mean difference (SMD) of ≤ 0.10 between the sotrovimab and no mAb cohorts.
Results
Cohort Characteristics
Among 1,530,501 high-risk patients diagnosed with COVID-19 during the study period, 15,633 had a claim for sotrovimab and 1,514,868 did not have any claims for mAbs (Table 1). The sotrovimab cohort was older than the no mAb cohort (20.44% versus 12.84% aged ≥ 65 years; P < 0.001). The majority of patients were diagnosed with COVID-19 between 1 December 2021 and 28 February 2022, when Omicron BA.1 became the predominant circulating variant [8]. Compared with the no mAb cohort, the sotrovimab cohort had a higher proportion of patients across most high-risk conditions. Notably, 41.74% of the sotrovimab cohort had a diagnosis of an immunocompromising condition and/or immunosuppressive therapy compared with 25.02% of the no mAb cohort.
Table 1.
Demographic and clinical characteristics by treatment cohort
| Cohort characteristics | High risk Sotrovimab N = 15,633 |
High risk No mAba, N = 1,514,868 |
P-value |
|---|---|---|---|
| Diagnosis month category,b no. (%) | |||
| 1 September 2021–30 November 2021 | 2143 (13.71) | 511,292 (33.75) | < 0.001 |
| 1 December 2021–28 February 2022 | 12,376 (79.17) | 820,817 (54.18) | |
| 1 March 2022–30 April 2022 | 1114 (7.13) | 182,759 (12.06) | |
| Regionc, no. (%) | |||
| 1 and 2 | 5450 (34.86) | 516,964 (34.13) | < 0.001 |
| 3 and 4 | 3336 (21.34) | 324,250 (21.40) | |
| 5 and 7 | 3277 (20.96) | 205,595 (13.57) | |
| 6 and 8 | 2055 (13.15) | 271,037 (17.89) | |
| 9 and 10 | 1506 (9.63) | 197,022 (13.01) | |
| Rurality, no. (%) | |||
| Rural | 2847 (18.21) | 208,637 (13.77) | < 0.001 |
| Urban | 12,786 (81.79) | 1,306,231 (86.23) | |
| Gender, no. (%) | |||
| Female | 9188 (58.77) | 855,221 (56.46) | < 0.001 |
| Male | 6445 (41.23) | 659,647 (43.54) | |
| Age, years, no. (%) | |||
| 0–17 | 87 (0.56) | 117,021 (7.72) | < 0.001 |
| 18–34 | 1909 (12.21) | 279,322 (18.44) | |
| 35–49 | 3815 (24.40) | 411,711 (27.18) | |
| 50–64 | 6627 (42.39) | 512,330 (33.82) | |
| 65–74 | 2348 (15.02) | 131,456 (8.68) | |
| 75 + | 847 (5.42) | 63,028 (4.16) | |
| Mean (SD) | 52.98 (14.53) | 45.90 (18.05) | < 0.001 |
| Median (IQR) | 55.00 (20) | 48.00 (25) | < 0.001 |
| Documented COVID-19 vaccine, no. (%) | |||
| Yes | 3177 (20.32) | 229,770 (15.17) | < 0.001 |
| No/unknown | 12,456 (79.68) | 1,285,098 (84.83) | |
| High-risk conditions (EUA) | |||
| Obesity (BMI ≥ 30 kg/m2) | 4335 (27.73) | 379,463 (25.05) | < 0.001 |
| Pregnant | 1203 (7.70) | 75,133 (4.96) | < 0.001 |
| CKD | 1571 (10.05) | 68,168 (4.50) | < 0.001 |
| Diabetes | 4081 (26.11) | 268,798 (17.74) | < 0.001 |
| Immunocompromising conditions/immunosuppressive therapy | 6525 (41.74) | 379,002 (25.02) | < 0.001 |
| COPD | 1105 (7.07) | 63,497 (4.19) | < 0.001 |
| Asthma | 709 (4.54) | 45,930 (3.03) | < 0.001 |
| Chronic lung disease | 2509 (16.05) | 193,149 (12.75) | < 0.001 |
| Sickle cell disease | 22 (0.14) | 3727 (0.25) | 0.007 |
| Congenital heart disease | 265 (1.70) | 23,741 (1.57) | 0.223 |
| Acquired heart disease | 5135 (32.85) | 323,485 (21.35) | < 0.001 |
| Cardiovascular disease | 4118 (26.34) | 227,764 (15.04) | < 0.001 |
| Hypertension | 8319 (53.21) | 627,283 (41.41) | < 0.001 |
| Neurodevelopmental disorder | 970 (6.20) | 188,172 (12.42) | < 0.001 |
| Medical device | 1462 (9.35) | 110,341 (7.28) | < 0.001 |
SD standard deviation, IQR interquartile range, EUA Emergency Use Authorization, BMI body mass index, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease
aIncludes 131 patients identified based on history of drug-induced anaphylaxis, which is not an EUA high-risk condition
bDiagnosis month category reflects the time period for when a circulating variant was or became predominant
cRegion: region 1 (CT, ME, MA, NH, RI, VT), region 2 (NJ, NY), region 3 (DE, DC, MD, PA, VA, WV), region 4 (AL, FL, GA, KY, MS, NC, SC, TN), region 5 (IL, IN, MI, MN, OH, WI), region 6 (AR, LA, NM, OK, TX), region 7 (IA, KS, MO, NE), region 8 (CO, MT, ND, SD, UT, WY), region 9 (AZ, CA, HI, NV), and region 10 (AK, ID, OR, WA)
30-Day All-Cause Hospitalization and Facility-Reported Mortality
In the sotrovimab cohort, 418 (2.67%) patients were hospitalized ≤ 30 days of diagnosis (Table 2), including 65 (15.55%) patients admitted to an intensive care unit (ICU). In the no mAb cohort, 84,307 (5.57%) patients were hospitalized ≤ 30 days of diagnosis, including 24,489 (29.05%) patients with an ICU admission. There were 13 (0.08%) deaths identified ≤ 30 days of diagnosis in the sotrovimab cohort and 8167 (0.54%) in the no mAb cohort.
Table 2.
Adjusted and PS-matched risk of 30-day all-cause hospitalization or facility-reported mortality
| Outcome | High risk Sotrovimab, no. (%), N = 15,633 |
High risk No mAb, no. (%) N = 1,514,868 |
RRa,b (95% CI) | PS matched RRa,c (95% CI) | P-valued |
|---|---|---|---|---|---|
| 30-day hospitalization | 418 (2.67) | 84,307 (5.57) | 0.45 (0.41, 0.49) | 0.39 (0.36, 0.43) | < 0.001 |
| 30-day mortality | 13 (0.08) | 8167 (0.54) | 0.15 (0.08, 0.29) | 0.12 (0.06, 0.24) | < 0.001 |
| 30-day hospitalization or mortality | 419 (2.68) | 84,720 (5.59) | 0.45 (0.41, 0.49) | 0.39 (0.35, 0.43) | < 0.001 |
PS propensity score, mAb monoclonal antibody, RR relative risk, CI confidence interval
aReference group = high-risk no mAb
bAdjusted for diagnosis month category, age, gender, region, rurality, high-risk conditions, and documented COVID-19 vaccine
c15,633 sotrovimab patients were matched with 62,532 no mAb patients on diagnosis month, age, gender, region, rurality, and selected high-risk conditions
dP-value applies to RR and PS-matched RR
Relative Risk of 30-Day All-Cause Hospitalization or Facility-Reported Mortality
After adjusting for all other covariates in the Poisson regression model, sotrovimab was associated with a 55% lower risk of 30-day hospitalization (RR 0.45, 95% CI 0.41–0.49), 85% lower risk of 30-day mortality (RR 0.15, 95% CI 0.08–0.29), and 55% lower risk of 30-day hospitalization or mortality (RR 0.45, 95% CI 0.41–0.49) compared with no mAb (Table 2). Older age, male gender, or having a specific high-risk condition (versus not having a specific high-risk condition) increased the odds of 30-day hospitalization or mortality (Supplementary Table 2).
We PS-matched 15,633 patients treated with sotrovimab to 62,532 patients not treated with a mAb (1:4 ratio) and eliminated statistically significant differences between cohorts (Supplementary Table 3); covariates included in the PS model had a SMD of ≤ 0.10 between the sotrovimab and no mAb cohorts. In the PS-matched analysis, the sotrovimab cohort had 61% lower risk (RR 0.39, 95% CI 0.36–0.43) of 30-day hospitalization or mortality and an 88% lower risk (RR 0.12, 95% CI 0.06–0.24) of 30-day mortality compared with the no mAb cohort (Table 2). Logistic regression analysis of 30-day hospitalization and/or mortality data are summarized in Supplementary Table 4.
Relative Risk of 30-Day All-Cause Hospitalization or Facility-Reported Mortality by Diagnosis Month
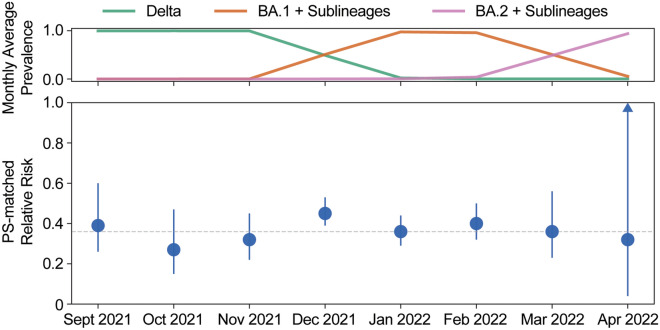
In the no mAb cohort, rates of 30-day hospitalization or mortality were lower from December 2021 to April 2022, when Omicron sublineages became the predominant circulating variants, than from September to November 2021, when Delta was the predominant variant (Table 3; Fig. 1). The sotrovimab cohort had statistically significant lower risks of 30-day hospitalization or mortality from September 2021 to March 2022. The estimated RR for sotrovimab was similar across the Delta and early Omicron waves (Fig. 1). Logistic regression results for 30-day hospitalization or mortality, by month of diagnosis, are summarized in Supplementary Table 5.
Table 3.
Adjusted and PS-matched RR of 30-day all-cause hospitalization or facility-reported mortality by diagnosis month
| Diagnosis month | High-risk sotrovimab, no. (%)a | High-risk no mAb, no. (%)a | RRb,c (95% CI) | PS-matched RRb,d (95% CI) | P-valuee |
|---|---|---|---|---|---|
| September 2021 | 460 (4.78) | 307,096 (9.53) | 0.48 (0.32, 0.72) | 0.39 (0.26, 0.60) | < 0.001 |
| October 2021 | 451 (2.66) | 84,367 (8.62) | 0.29 (0.17, 0.51) | 0.27 (0.15, 0.47) | < 0.001 |
| November 2021 | 1232 (2.76) | 119,829 (7.81) | 0.33 (0.23, 0.45) | 0.32 (0.22, 0.45) | < 0.001 |
| December 2021 | 5188 (3.14) | 455,706 (3.90) | 0.49 (0.43, 0.57) | 0.45 (0.39, 0.53) | < 0.001 |
| January 2022 | 4859 (1.85) | 261,765 (3.37) | 0.36 (0.30, 0.44) | 0.36 (0.29, 0.44) | < 0.001 |
| February 2022 | 2329 (3.26) | 103,346 (6.90) | 0.43 (0.34, 0.54) | 0.40 (0.32, 0.50) | < 0.001 |
| March 2022 | 1046 (2.01) | 65,521 (4.37) | 0.41 (0.27, 0.62) | 0.36 (0.23, 0.56) | < 0.001 |
| April 2022 | 68 (1.47) | 117,238 (1.90) | 0.54 (0.08, 3.54) | 0.32 (0.04, 2.38) | 0.519 |
PS propensity score, RR relative risk, mAb monoclonal antibody, CI confidence interval
aThe reported % = number hospitalized or died in diagnosis month in treatment cohort/number in diagnosis month in treatment cohort
bReference group = high-risk no mAb
cAdjusted for diagnosis month category, age, gender, region, rurality, high-risk conditions, and documented COVID-19 vaccine
d15,633 sotrovimab patients were matched with 62,532 no mAb patients on diagnosis month, age, gender, region, rurality, and selected high-risk conditions
eP-value applies to RR and PS-matched RR
Fig. 1.
Propensity score-matched RR of 30-day all-cause hospitalization or facility-reported mortality and relative COVID-19 variant prevalence over time. Monthly US average prevalences of Delta (green), BA.1 + sublineages (orange), and BA.2 + sublineages (pink) are depicted in the upper panel based on data from the Global Initiative on Sharing All Influenza Data [20] and are not necessarily representative of the study population. The lower panel shows the PS-matched RR by month for the sotrovimab cohort relative to the no mAb cohort. The 95% CI for the PS-matched RR for April 2022 is 0.04–2.38. PS propensity score, RR relative risk, mAb monoclonal antibody, CI confidence interval
Relative Risk of 30-Day All-Cause Hospitalization or Facility-Reported Mortality by High-Risk Condition
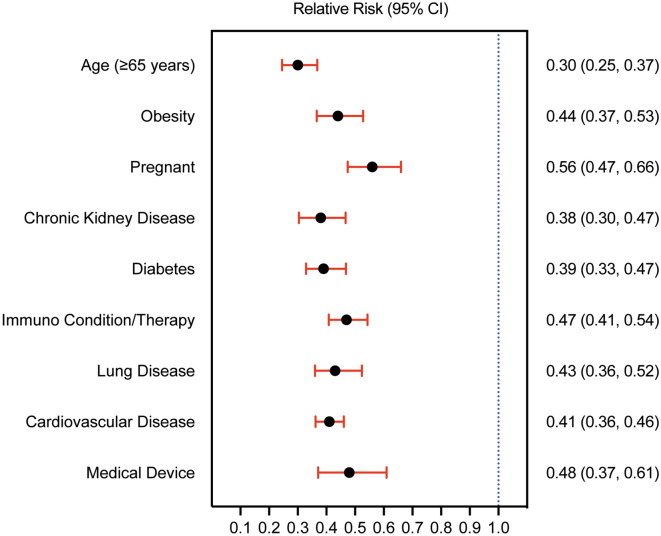
The sotrovimab cohort showed statistically significant RR reductions of 30-day hospitalization or mortality compared with the no mAb cohort in a multivariate regression model stratified by high-risk condition (Fig. 2), ranging from 44% among pregnant women to 70% among patients aged ≥ 65 years. The subgroup of patients with an immunocompromised condition and/or immunosuppressive therapy had a 53% lower risk (RR 0.47, 95% CI 0.41–0.54) of 30-day hospitalization or mortality.
Fig. 2.
RR of 30-day all-cause hospitalization or facility-reported mortality by high-risk condition. Adjusted RR for patients with certain high-risk conditions in the sotrovimab cohort versus the no mAb cohort; P < 0.001 for all high-risk conditions. RR relative risk, mAb monoclonal antibody
Impact of Documented COVID-19 Vaccine
Among patients with ≥ one documented COVID-19 vaccine, hospitalizations ≤ 30 days of diagnosis were seen in 59 of 3177 patients (1.86%) in the sotrovimab cohort and 7030 of 229,770 patients (3.06%) in the no mAb cohort. In this population with a documented vaccine, sotrovimab compared with no mAb was associated with a 57% lower risk (RR 0.43, 95% CI 0.34–0.56) of 30-day hospitalization or mortality.
Discussion
In this real-world analysis of high-risk patients with reported COVID-19 during the Delta and early Omicron waves, sotrovimab was associated with a 55% lower risk of 30-day hospitalization or mortality and an 85% lower risk of 30-day mortality compared with no mAb. In the PS-matched analysis, the sotrovimab cohort showed a 61% lower risk of 30-day all-cause hospitalization or mortality and an 88% lower risk of mortality compared with the no mAb cohort, similar to the COMET-ICE trial results (RR 0.21, 95% CI 0.09–0.50) [10]. During the September 2021–March 2022 study period, the sotrovimab cohort maintained a RR reduction in 30-day hospitalization or mortality compared with the no mAb cohort. Sotrovimab effectiveness in April 2022 was uncertain due to the small sample size (sotrovimab had been deauthorized across the country on 5 April 2022), together with mixed variant prevalence and lack of specific sequencing data.
It is well documented that immunocompromised/immunosuppressed populations are at higher risk of severe disease due to challenges in mounting robust antibody responses to the COVID-19 vaccine [11, 12]. In our study, despite the higher prevalence of immunocompromising conditions and/or immunosuppressive therapy in the sotrovimab cohort compared with the no mAb cohort (42% versus 25%, respectively), sotrovimab was associated with a statistically significant 53% RR reduction of 30-day hospitalization or mortality in immunocompromised/immunosuppressed patients. Furthermore, sotrovimab was associated with lower risk of 30-day hospitalization or mortality across all high-risk conditions examined.
Approximately 20% of patients in the sotrovimab cohort and 15% of patients in the no mAb cohort had ≥ one claim for a COVID-19 vaccine. It cannot be determined whether these patients were partially or fully vaccinated. The vaccination status of those without a documented COVID-19 vaccine is uncertain and likely reflective of incomplete billing by vaccine providers due to US Government procurement and distribution. Sotrovimab was associated with a 57% lower risk of 30-day hospitalization or mortality compared with no mAb treatment among patients with ≥ one documented COVID-19 vaccine. This estimate is consistent with the overall treated population estimate and suggests that sotrovimab might provide treatment benefits in the presence of COVID-19 vaccine antibodies.
Aggarwal and colleagues used a similar approach to the current study to assess sotrovimab effectiveness among high-risk outpatients diagnosed with COVID-19 in Colorado state, when Delta was the predominant circulating variant. Similar to our findings, sotrovimab was associated with 63% (OR 0.37, 95% CI 0.19–0.66) lower odds of 28-day all-cause hospitalization and 89% (OR 0.11, 95% CI 0.0–0.79) lower odds of 28-day all-cause mortality [13]. A subsequent study during the Omicron BA.1 wave found nonsignificant lower odds of 28-day all-cause hospitalization or mortality [14]. This may be due to smaller sample size and lower power to detect differences between treatment groups.
In a real-world study conducted in England among high-risk nonhospitalized patients, treatment with sotrovimab was associated with 46% lower risk [hazard ratio (HR) 0.54, 95% CI 0.33–0.88] of 28-day hospitalization or death during the Omicron BA.1 period (16 December 2021–10 February 2022) and 56% lower risk (HR 0.44, 95% CI 0.27–0.71) during the Omicron BA.2 period (16 February 2022–1 May 2022) compared with treatment with molnupiravir [15].
The clinical effectiveness of sotrovimab 500 mg intravenously (IV) for Omicron BA.1 and BA.2 was also evaluated in a multicenter, prospective observational study of high-risk COVID-19 patients with laboratory-confirmed variant in France. After 28 days post sotrovimab treatment, similar rates of hospitalization occurred between the BA.1 cohort [2.4% (3/125), 95% CI 1–7%] and the BA.2 cohort [2.4% (1/42), 95% CI 0–13%] [16].
A small study in Qatar conducted during the reported Omicron BA.2 wave found nonsignificant higher odds (OR 2.67, 95% CI 0.60–11.91) of progression to severe, critical, or fatal COVID-19 in sotrovimab-treated patients compared with those untreated [17]. However, patients were excluded from the control group if they showed signs or symptoms of severe COVID-19 within 7 days of diagnosis. This study design likely biased the sotrovimab-treated group to be sicker and at higher risk of disease progression compared with the control group, which may explain the observed results.
During 2021 and the first half of 2022, the COVID-19 pandemic continued with SARS-CoV-2 Delta and Omicron VOC with no clear end in sight [18]. The rapid rate of SARS-CoV-2 mutations makes it challenging to assess the effectiveness of interventions against emerging VOC, especially when confounded by population immunity from vaccination or natural infection, which may lower the mAb titers needed for neutralization in humans. Recent evidence suggests that in vitro neutralization activity may only be a partial determinant of sotrovimab efficacy, and Fc-mediated effector functions, such as ADCC and ADCP, may contribute additional antiviral effects against SARS-CoV-2 Omicron variants based on in vivo studies in mice [19]. Effector function may explain the notable lack of correlation between in vitro neutralization and in vivo activity in animal studies and is a possible contribution to clinical effectiveness [19]. As such, real-world evidence obtained from usual clinical practice has become increasingly important to assess treatment effectiveness outside of preclinical studies or clinical trials.
Strengths and Limitations
The FH NPIC claims database is a useful resource to examine therapeutic strategies in actual clinical practice settings. To our knowledge, with over 1.5 million patients, our study is one of the largest to assess sotrovimab effectiveness among high-risk COVID-19 patients with geographic representation across the USA. However, the primary purpose of insurance claims data is for billing and reimbursement as opposed to research. Therefore, the use of claims data limits the ability to verify the diagnostic conditions reported, disease severity, or treatment pathways. The data are also limited in capturing vaccinations (unbilled service due to US Government procurement and distribution) and oral therapies dispensed through retail pharmacy or billed using National Drug Codes (e.g., oral noncorticosteroids). We assume more complete capture of sotrovimab treatment in the database because it was a billable healthcare service. Further, the dataset did not include populations covered by public programs (e.g., Medicare, Medicaid) or individuals without health insurance. However, given that the population identified in this study were patients at high risk of severe disease who were more likely to utilize health care services, we expect the findings to be generalizable to other high-risk subpopulations. Facility-reported mortality likely underestimated deaths, as deaths were only identifiable from the discharge status reported by health care facilities for which a billable medical service was provided. We cannot exclude the possibility of residual confounding due to the absence of race/ethnicity and variant sequencing data in the dataset.
Given the absence of variant sequencing data, we used the month of COVID-19 diagnosis as a proxy for the likelihood of a given case of COVID-19 being attributable to the Delta or Omicron BA.1 or BA.2 variants. Circulating variants may influence clinicians’ decisions to treat and/or the specific regimen to administer, and this proxy variable may not adequately assess variant exposure for all patients in our study. We also adopted several sensitivity approaches to account for the effect of changing COVID-19 variants on treatment effectiveness, including propensity score matching as well as stratified Poisson and logistic regression analyses.
Patients in the no mAb cohort appeared to be eligible for sotrovimab per EUA criteria but were untreated for unknown reasons. Applying the adjusted RR reductions in hospitalizations and mortality with sotrovimab to the no mAb cohort results in an estimated 46,000 (43,000–50,000) hospitalizations and 7000 (5800–7500) deaths that might have been avoided in this study population. Reducing the disparity of nonclinical factors in the receipt of effective COVID-19 therapies will help improve outcomes among high-risk patients. Further research utilizing sequencing data is warranted to confirm our study findings.
Conclusions
In this large US real-world study of high-risk patients with COVID-19 during the Delta and early Omicron waves, sotrovimab was associated with reduced risk of 30-day all-cause hospitalization or facility-reported mortality compared with no mAb treatment. The protective effect also persisted among all high-risk subgroups assessed. Interventions with demonstrated real-world clinical effectiveness against emerging variants of concern should be accessible to patients at high risk of severe COVID-19 disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Importantly, we thank the anonymous patients whose data enabled this study.
Funding
This study and journal rapid service publication fees were funded by Vir Biotechnology, Inc. and GSK. The funder/sponsor had a role in the design and conduct of the study, interpretation of the data, and preparation and review of the manuscript. The authors made the decision to submit the manuscript for publication.
Medical writing/Editorial assistance
We thank Ali Russo, Alexander Mizenko, and Randi Scott from FAIR Health for conducting the data extraction, data analyses, and statistical analyses for this study. We also thank Wenjie Wang, Hong Wang, Rajendra Joshi, and Yimeng Lu for their assistance with the visual presentation of the data. We thank Tamara Palagashvili and Don Hoang for publication support. Editorial assistance was provided by Lumanity Scientific Inc. and was funded by Vir Biotechnology.
Prior presentation
Some of the data reported in this manuscript were presented at the European Respiratory Society International Congress 2022 (oral presentation; September 6, 2022; Barcelona, Spain) and at IDWeek 2022 (poster presentation; October 21, 2022; Washington, DC, USA). A preprint version of this manuscript was previously posted to medRxiv.
Author contributions
All authors had full access to the aggregated data from the study and take responsibility for the integrity of the findings and interpretation of the data analyses. Research for this study was based upon the data compiled and maintained by FAIR Health, Inc. The authors are solely responsible for the research and conclusions reflected in this article. FAIR Health is not responsible for any of the conclusions or opinions expressed herein. Concept and design: Mindy M. Cheng, Carolina Reyes, Sacha Satram, Helen Birch, Daniel C. Gibbons, Myriam Drysdale and Christopher F. Bell. Acquisition, analysis, or interpretation of data: Mindy M. Cheng, Carolina Reyes, Sacha Satram, Helen Birch, Daniel C. Gibbons, Myriam Drysdale, Christopher F. Bell, Anvar Suyundikov, Xiao Ding, M. Cyrus Maher, Wendy Yeh, Amalio Telenti and Lawrence Corey. Drafting of the manuscript: Mindy M. Cheng, Carolina Reyes and Sacha Satram. Critical revisions of the manuscript for important intellectual content: Mindy M. Cheng, Carolina Reyes, Sacha Satram, Helen Birch, Daniel C. Gibbons, Myriam Drysdale, Christopher F. Bell, Anvar Suyundikov, Xiao Ding, M. Cyrus Maher, Wendy Yeh, Amalio Telenti and Lawrence Corey. Statistical analysis: FAIR Health conducted the statistical analyses. Mindy M. Cheng, Carolina Reyes, Sacha Satram, Helen Birch, Daniel C. Gibbons, Myriam Drysdale, Christopher F. Bell, Anvar Suyundikov, Xiao Ding, M. Cyrus Maher, Wendy Yeh, Amalio Telenti and Lawrence Corey are responsible for the interpretation of the analyses. Administrative, technical, or material support: Mindy M. Cheng, Carolina Reyes, Sacha Satram, Helen Birch, Daniel C. Gibbons, Myriam Drysdale, Christopher F. Bell, Anvar Suyundikov, Xiao Ding, M. Cyrus Maher, Wendy Yeh, Amalio Telenti and Lawrence Corey.
Disclosures
Mindy M. Cheng, Carolina Reyes, Sacha Satram, Xiao Ding, M. Cyrus Maher, Wendy Yeh and Amalio Telenti are full-time employees and shareholders of Vir Biotechnology. Helen Birch, Daniel C. Gibbons, Myriam Drysdale and Christopher F. Bell are full-time employees and shareholders of GSK. Lawrence Corey has no conflicts of interest to disclose.
Compliance with ethics guidelines
The authors conducted a retrospective cohort study (secondary research) using de-identified and aggregated data licensed from a third party, FAIR Health, in compliance with 45 CFR 164.514(a)-(c) and the Health Insurance Portability and Accountability Act. Patient level identifiers were removed and were coded in such a way that the data could not be linked back to subjects from whom they were originally collected prior to the authors gaining access to it. This research, which used the de-identified licensed data described above, does not require IRB or ethics review, as analyses with these data do not meet the definition of 'research involving human subjects' as defined within 45 CFR 46.102(f) which stipulates human subjects as living individuals about whom an investigator obtains identifiable private information for research purposes.
Data availability
The datasets generated and/or analyzed during this study were licensed from FAIR Health, Inc. and are not publicly available.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 22 Sep 2022.
- 2.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21(2):167–179. doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19 related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. News Release. FDA; May, 26, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19. Accessed 20 May 2022.
- 5.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 6.Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2021 doi: 10.1101/2021.03.09.434607. [DOI] [Google Scholar]
- 7.Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Sotrovimab. 2022. https://www.fda.gov/media/149534/download. Accessed 13 May 2022.
- 8.Centers for Disease Control and Prevention. COVID Data Tracker, Nowcast. https://covid.cdc.gov/covid-data-tracker/#circulatingVariants. Accessed 15 Mar 2022.
- 9.Centers for Disease Control and Prevention. Assessing Risk Factors for Severe COVID-19 Illness. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/assessing-risk-factors.html. Accessed 15 Feb 2022.
- 10.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10(3):e326–e328. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal NR, Beaty LE, Bennett TD, et al. Real world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022 doi: 10.1093/infdis/jiac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Ginde AA. Change in effectiveness of sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients during the BA.1 and BA.1.1-predominant phase. Int J Infect Dis. 2022 doi: 10.1016/j.ijid.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: an observational cohort study using the OpenSAFELY platform. BMJ. 2022;379:e071932. doi: 10.1136/bmj-2022-071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Blondel G, Marcelin AG, Soulie C, et al. Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2. J Infect. 2022;85(4):e104–e108. doi: 10.1016/j.jinf.2022.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaqout A, Almaslamani MA, Chemaitelly H, et al. Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar. Int J Infect Dis. 2022;124:96–103. doi: 10.1101/2022.04.21.22274060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telenti A, Hodcroft EB, Robertson DL. The evolution and biology of SARS-CoV-2 variants. Cold Spring Harb Perspect Med. 2022 doi: 10.1101/cshperspect.a041390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Case JB, Mackin S, Errico JM, et al. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat Commun. 2022;13(1):3824. doi: 10.1038/s41467-022-31615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during this study were licensed from FAIR Health, Inc. and are not publicly available.