Abstract
This narrative review summarizes the main findings of observational studies (case-control and cohort) as well as systematic reviews and meta-analyses on the role of nutrients and dietary patterns on pancreatic cancer (PC) risk and elucidates possible mechanisms for the association between nutrients or specific food components and the risk of PC. A literature search of MEDLINE (PubMed), Google Scholar, ScienceDirect, and Scopus was performed. An extensive search of related articles published in the English language from 1985 to 2022 was carried out. Our search included macro- and micronutrient intake as well as dietary patterns associated with PC. In conclusion, the consumption of a diet high in nutrients such as sugar, fats, and red and processed meats can increase the risk of PC. Conversely, a high dietary intake of fresh fruit and vegetables and their associated nutrients like fiber, antioxidants, and polyphenols may prevent PC. Dietary patterns loaded with red and processed meats were also linked to an increased risk of PC, whereas dietary patterns rich in plant-based foods like vegetables, fruits, whole grains, and legumes were associated with a reduced risk of PC. Dietary fiber, fat-soluble vitamins, water-soluble vitamins, and minerals might also play a protective role against PC.
Keywords: pancreatic cancer, macronutrients, micronutrients, dietary pattern
Introduction
Pancreatic cancer (PC) is one of the most rapidly fatal malignancies. Despite being the world’s 12th most common type of cancer, with 495,773 new cases in 2020,1 it is the seventh leading cause of cancer-related deaths worldwide, accounting for 4.7% of cancer deaths in 2020.1 PC is difficult to diagnose early because there are few signs and symptoms before it spreads beyond the pancreas.2 Other than that, the symptoms of PC are similar to those of many other illnesses, making diagnosis difficult, and more than half of the PC patients are diagnosed at a metastatic stage.3
Pancreatic cancer is multifactorial and has various risk factors, including gender, age, fat mass, overweight and obesity, smoking, heavy alcohol consumption, a medical history of diabetes and chronic pancreatitis, and genetic predisposition.4–6 Diet is a highly correlated risk factor that could be modified to reduce the risk of PC. Several epidemiological studies have examined the associations between individual foods or nutrients, for instance, red and processed meats,7,8 vegetables and fruits,9,10 foods rich in fat, sugar, and starch,6,11,12 vitamins and minerals,13–15 and fiber16,17 and the risk of PC, but results are still inconclusive.
A multiethnic cohort of smokers in the United States (US) showed an inverse association between a dietary pattern high in quercetin, kaempferol, and myricetin and the risk of PC.18 However, Arem et al found no significant association between flavonoid intake and PC risk.19 In the Lowa Women’s Health Study, it included 256 postmenopausal PC women, and the results revealed no significant association between the intake of nutrients and food groups or dietary patterns and PC, and this study did not support the association of fruits, vegetables, or red meat with PC.20
Recent studies present substantial controversies, thus, the current review has been directed to discuss the association between nutrient intake and dietary patterns with PC, as well as possible mechanisms for the association between diet and PC risk.
Methods
We performed a literature search of MEDLINE (PubMed), ScienceDirect, Google Scholar, and Scopus. Our search included macro- and micronutrients intake, as well as different dietary patterns that are associated with the risk of PC. Terms used in the search strategy included the exposures— dietary carbohydrates, carbohydrates-rich diet, sugar and sugar-sweetened soft drink, dietary fibers, high fiber diet, protein-rich diet, red meat and processed meat-rich dietary pattern, high fat diet, water-soluble vitamins, fat-soluble vitamins, major minerals, trace minerals, Mediterranean diet, plant-based diets, vegetable-rich diet, fruit-rich diet—and the risk of PC. Given that the present article included studies from different designs including cross-sectional, case-control, and cohorts studies, as well as systematic reviews and meta-analyses. This article is not a systematic review, and some studies might not have been identified. However, we did an extensive search for related articles published in the English language from 1985 to 2022. All authors conducted the literature search independently. Table 1 shows the summary of the search strategy.
Table 1.
The Search Strategy Summary
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | February–April, 2022 |
| Databases and other sources searched | MEDLINE (PubMed), ScienceDirect, Google Scholar, and Scopus |
|
Search terms used (including MeSH and free text search terms and filters) Note: please use an independent supplement table to present the detailed search strategy of one database as an example |
Dietary carbohydrates, carbohydrates rich diet, sugar and sugar-sweetened soft drink, dietary fibers, high fiber diet, protein-rich diet, red meat and processed meat-rich dietary pattern, high fat diet, water-soluble vitamins, fat-soluble vitamins, major minerals, trace minerals, Mediterranean diet, plant-based diets, vegetable-rich diet, fruit-rich diet—and the risk of PC. |
| Timeframe | 1985 to 2022 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) |
|
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | All of the authors conducted the literature search independently |
| Any additional considerations, if applicable | – |
Nutrients and PC
Dietary Carbohydrates and PC
The association between high carbohydrate intake and the risk of developing PC was not supported by some studies that were addressing the association between dietary carbohydrates, glycemic load (GL), glycemic index (GI) and the risk of PC.21,22 In addition, two prospective studies revealed that there was no association between GL, GI, total carbohydrates, total sugar, sucrose, or fructose,23,24 nor for high sugar foods and PC risk.25,26 In contrast, Meinhold et al27 reported that higher risk for PC was associated with higher percentiles of glycemic load (HR = 1.45, 95%CI: 1.05, 2.00), available carbohydrate (HR =1.47, 95% CI: 1.05, 2.06), and sucrose (HR = 1.37, 95% CI: 0.99, 1.89) intake. This was in line with a prospective study conducted by Michaud et al28 indicated that a greater risk of PC was linked to higher dietary GL, GI, and fructose intake in women with a body mass index (BMI) ≥ 25. Moreover, a positive association between sugars and increased risk of PC was reported in a study conducted by Genkinger et al29 and Mueller et al.30 Likewise, Nöthlings et al concluded from their multiethnic cohort study that high intakes of fructose and sucrose might also play a role in the etiology of PC.31
Some studies revealed no association between dietary carbohydrates, glycemic load (GL), glycemic index (GI) and the risk of PC; however, most of the studies emphasized that higher dietary GL plays a significant role as a risk factor for PC.
Dietary Fibers and PC
According to Bidoli et al,32 fiber plays a protective role against the risk of PC. Total fiber intake was found to be inversely related to the risk of PC. In the same context, Zhang et al33 and La Vecchia et al34 suggested that whole-grain consumption could be considered a protective factor against PC. Moreover, a recent systematic review conducted by Nucci et al 2021 revealed that dietary fiber intake is associated with a reduced risk of PC.16 Besides that, an epidemiological study indicated that the risk of developing PC is inversely associated with the intake of fruit and vegetables.35 Many factors could explain why certain types of fiber are protective against PC risk. This could be related to fiber’s beneficial effects on insulin metabolism via hormonal pathways linked to insulin-like growth factors, which have been linked to cancer promotion.36,37 Moreover, the beneficial effects of fiber include its role in reducing insulin resistance and improving glucose tolerance38 and this is attributed to decreasing food glycemic index (GI),39,40 improving subjects’ glucose homeostasis,39 and altering gut microbiota.41 Dietary fiber through its physicochemical properties plays a crucial role in delaying gastric emptying time and slowing down glucose absorption.36 However, Stolzenberg-Solomon et al 200242 found in their cohort study that there was no significant relationship between total intake of soluble or insoluble fiber and PC.
It could be concluded from the studies about dietary fibers and PC, that fibers from different food sources are considered a protective factor in reducing the risk of PC.
Dietary Proteins and PC
The association between protein intake (animal or plant-based protein) and the risk of PC is critical. In a large prospective cohort conducted by Ghorbani et al, results showed no clear or consistent association between the risk of PC and the intake of dietary protein.43 An increased risk of PC was reported by a case-control study to be associated with higher consumption of lamb, veal, and game.44 Larsson and Wolk45 found that every 50 g of processed meat contributes to a 19% increase in the risk of PC. The findings regarding poultry consumption are highly controversial. Rohrmann et al 2013,46 indicated a positive association with PC. However, a negative association has also been revealed by a different prospective study.47 In a meta-analysis conducted by Qin et al,48 no inverse association was detected between the consumption of fish or long-chain polyunsaturated fatty acids (LC-PUFA) and the risk of PC. They also revealed that only non-fried fish intake was inversely associated with the risk of PC. A recent systematic review and meta-analysis suggested that eating high amounts of may increase the risk of PC, whereas eating high amounts of fish is unlikely to increase the risk of PC.49 Moreover, previous research addressed the relationship between grain consumption as a source of dietary protein and the risk of PC and showed positive associations between the intake of refined grains and white bread and the risk of PC.50–52 whereas nut consumption was reported to be inversely associated with the risk of PC in a different study conducted by Bao et al.53
According to all studies mentioned about the association between dietary proteins and PC, it could be concluded that proteins from refined grains, processed meat, and non-fried fish are considered risk factors for PC. Also, contradicted results regarding poultry and the risk of PC. Finally, nuts may have a protective role from PC.
Dietary Fats and PC
It has been indicated that the long-term release of cholecystokinin (CCK) upon the presence of lipids in the duodenum, might lead to a high risk of PC.54 Some studies reported that the incidence of PC is high in countries that consume high-fat diets.55–57 Meanwhile, the incidence of PC is lower in countries that consume diets low in fat.58 Several cohort studies have indicated a positive association between the risk of PC and total fat consumption,59 saturated fatty acids (SFAs),42 and monounsaturated fatty acids (MUFAs).60 Nkondjock et al61 reported that substituting polyunsaturated fatty acids (PUFAs) with SFAs or MUFAs might reduce the risk of PC, independent of the total energy intake. Another recent cohort study revealed that unsaturated fatty acids including PUFAS, and especially MUFAS, had a protective effect against PC.62
Concerning omega-3 and omega-6 PUFAs, opposite associations were shown with the risk of PC in which the risk decreased with the omega-3 fatty acids (FAs)63,64 and increased with omega-6 FAs.65 Intake of other types of fat, such as cholesterol, was significantly associated with the risk of PC.17,66 In a meta-analysis by Chen et al,66 a dose-response relationship was reported with an 8% increase in the risk of PC with every 100 mg/ day intake of cholesterol. Another meta-analysis by Wang et al17 found ethnic variability in the association between dietary cholesterol and the risk of PC, in which dietary cholesterol may be associated with the risk of PC in worldwide populations, but not in Europeans. Several mechanisms may explain the possible role of cholesterol in the development of PC. This includes the cellular inflammation that could result from alterations in lipid and apolipoprotein levels67 and the high levels of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are related to the low levels of high-density lipoprotein cholesterol (HDL-C), high levels of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC).68
A conclusion from the studies between dietary fats and PC indicated that the high risk of PC is highly attributed to a high-fat diet. Moreover, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and dietary cholesterol are highly considered risk factors for PC. In contrast, polyunsaturated fatty acids (PUFAs) play an excellent role as protective factors for PC.
Water-Soluble Vitamins and PC
In a study conducted by Bravi et al in 2011, there was a clear protective role for thiamin, riboflavin, folate, and vitamin C.13 Multiple logistic regression models were developed to compare the highest and lowest quintile of intake, and results revealed a strong association with an Odd Ratio of ((OR) = 0.73, 0.73, 0.56, and 0.44) for thiamin, riboflavin, folate and vitamin C, respectively. The vitamin C results were in line with a case-control study conducted by Ji et al 199569 which revealed the same association. The protective role of vitamin C against PC is explained through its role in reactive oxygen species,70 autophagy71 and depletion of intracellular adenosine triphosphate.72 Moreover, a prospective nested case-control study indicated an inverse relationship between circulating levels of vitamin B12 and B6 and the risk of PC.73 In addition, a recent meta-analysis conducted by Wei1 and Mao74 examined the association between the risk of PC and the intake of vitamin B6 and vitamin B12. They reported a 9% decrease in the risk of PC for every 10 nmol/L increments in blood pyridoxal 5′-phosphate (PLP) levels, but there was no significant association between the risk of PC and vitamin B12 intake.74 On the other side, Gong et al 200975 suggested that a higher intake of vitamin B12 is associated with an increased risk of PC. Vitamin B6 plays a protective role in the development of PC.76 This is because it is a cofactor in DNA synthesis, and is involved in the methylation pathway of one-carbon metabolism.76 Low intake of vitamin B6 reduces the production of methylene- THF (methyl donor) which could lead to hypomethylation and promote oncogenesis.77
Generally concluded for the studies regarding the risk of PC and WSV, it is noted that vitamins such as B1, B2, B6, B12, folate, and vitamin C have a distinguished protective role from PC.
Fat-Soluble Vitamins and PC
Obvious conflict in the studies investigating the association between PC and fat-soluble vitamins has been documented.13,73,74 Bravi et al13 conducted an analysis comparing the highest to the lowest quintile of ß –carotene intake and the results showed no significant inverse relationship between the risk of PC and the intake of ß –carotene. On the other hand, a case-control study found an inverse relationship between ß-carotene and the risk of PC.78 Meanwhile, another study found no association.79 The most common mechanisms underlying the protective role of vitamin A in PC include retinoic acid receptor modulation,80 interaction with protein kinase,81 inhibition of cellular adhesion,82 and downregulation of IL-6.83 Two prospective cohort studies were conducted to investigate the association between vitamin D intake and PC risk.84,85 They found that a lower risk of PC was associated with a higher intake of vitamin D (≥ 600 IU per day). In contrast, a case-control study revealed that a higher intake of vitamin D (≥ 450 IU/day was associated with an increased risk of PC in men.86 However, a meta-analysis reported that dietary vitamin D is not associated with the risk of PC.87 Five common underlying mechanisms reveal the anti-pancreatic cancer effect of vitamin D. These mechanisms include vitamin D receptor agonist,88 AMP-activated protein kinase (AMPK)-dependent mechanisms,89 inhibition of phosphatidylinositol 3-kinases (PI3K)/protein kinase B (AKT) pathway,90 regulation of the cell cycle,91 and decreased cell migration and invasion.92 The protective role of vitamin E was clear in the study conducted by Bravi et al 2011.13 They demonstrated that after comparing the highest to the lowest quintile of intake, for vitamin E (OR= 0.60, 95% confidence interval (CI): 0.36–0.98). Another case control by Ji et al69 revealed the same associations. Mechanisms behind this association include the protection of vitamin E against PC through its role in the inhibition of nuclear factor-kB (NF-kB) activity,93 Ras-Raf-MEK-ERK pathway,94 regulation of the cell cycle,95 and induction of apoptosis.96 Previous studies indicated that vitamin K might act as a potential antitumor agent. From a metabolic point of view, vitamin K has a protective role from PC through its ability to suppress cancer growth and induce apoptosis and differentiation in PC cells.97,98
The studies mentioned regarding FSV and PC in general give important indicators that vitamin A, vitamin D, vitamin E, and vitamin K are having magical mechanisms behind their role as protective factors from PC.
Major Minerals and PC
A recent case-control study conducted by Fan et al 202199 indicated that the intake of calcium and phosphorus was significantly lower among cases compared to controls. Moreover, they found that there were no significant associations between the total intake of calcium and phosphorus with the risk of PC.99 In another case-control study, they reported that odds ratios of PC risk increased with higher calcium intake among men but not in women.86 In a prospective study conducted by Kesavan et al 2010,100 a 33% reduction in the risk of PC was associated with magnesium supplements in the study group. The protective role of both sodium and potassium against the risk of PC was emphasized in a study conducted by Bravi et al 2011.13 They found that comparing the highest to the lowest quintile, the OR significantly reduced (OR=0.57, 95% CI: 0.35–0.92) for potassium intake, while the increase in the OR was insignificant for sodium intake.
Upon the results of studies between major minerals and PC, it is concluded that calcium, phosphorus, magnesium, sodium, and potassium are highly considered protective factors against PC. An exception was found regarding calcium because gender may play a major role in the risk of PC with higher intakes of calcium among males.
Trace Minerals and PC
An exploratory analysis in the US discovered no association between iron intake and the risk of PC.56 This finding was also reported by the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort on studying the association between total iron and heme-iron and the risk of PC.101 The meta-analysis conducted by Li and Gai102 indicated the protective role of zinc against the risk of PC. In contrast, Bravi et al 201113 reported zinc intake is not associated with the risk of PC. Most of the studies confirm the significant role of chromium in enhancing insulin action and improving glucose tolerance and such roles require a healthy pancreas.103 Another study indicated the antioxidant capabilities of chromium in reducing oxidative stress.104 Epidemiological studies found a relationship between thyroid dysfunction and pancreas pathology and that thyroiditis could increase the risk of PC.105 Therefore, adequate iodine intake is associated with normal thyroid hormone levels and normal pancreatic function which may reduce the risk of PC.106 Several studies indicated that a high intake of selenium was associated with a reduced risk of PC.107–109 Finally, some trace minerals such as lead, cadmium and arsenic were significantly associated with increased risks of PC in the highest quartile.107 Table 2 summarizes the findings of these studies.
Table 2.
Summarization of the Studies on Trace Minerals and PC
| Trace Minerals | Findings |
|---|---|
| Iron | In an exploratory analysis in the US, it was found that there is no association between iron intake and the risk of PC.56 This was also reported by the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort on studying the association between total iron and heme-iron and the risk of PC.101 |
| Zinc | Li and Gai97 conducted a meta-analysis that included seven studies to investigate the association between zinc intake and the risk of PC. Their results indicated the protective role of zinc against the risk of PC. In addition, they found that the risk of PC significantly decreased in the group with the highest zinc intake as the PC risk for the highest versus the lowest categories of zinc intake was (RR= 0.798, 95% CI: 0.621–0.984). |
| Chromium | Most of the studies confirm the significant role of chromium in enhancing insulin action and improving glucose tolerance and such roles require a healthy pancreas.98 Another study indicated the antioxidant capabilities of chromium in reducing oxidative stress.99 |
| Iodine | Epidemiological studies found a relationship between thyroid dysfunction and pancreas pathology and that thyroiditis could increase the risk of PC.100 Therefore, adequate iodine intake is associated with normal thyroid hormone levels and normal pancreatic function which may reduce the risk of PC.101 |
| Selenium | Several studies indicated that a high intake of selenium was associated with a reduced risk of PC.102–104 |
| Lead, cadmium and arsenic | Lead, cadmium and arsenic were significantly associated with increased risks of PC in the highest quartile.102 |
| Nickel | Nickel was inversely associated with the risk of PC with (OR = 0.27, 95% CI: 0.12 to 0.59).102 |
The data collected regarding the associations between trace minerals and the risk of PC concluded that most of these minerals play a protective role while minors are considered as risk factors and others have no association. Zinc, chromium, iodine, selenium, and nickel represent the protective minerals while lead, cadmium and arsenic represent the risk factor minerals. No association was indicated between total iron and heme-iron and the risk of PC.
Dietary Patterns and PC
Mediterranean Dietary Pattern and PC
Mediterranean diet (MD), which is characterized mainly by high consumption of plant-based foods, has been linked to a decreased risk of various types of cancer. However, the evidence regarding its relationship with PC remains inconclusive. An Italian case-control study investigated the association between the MD and the risk of PC,110 and reported a decrease in PC risk by 15% per one-unit increase in adherence to the MD. Moreover, they found consistency in this association across sub-group analysis of age, BMI, smoking status, and alcohol consumption and it was stronger among non-diabetic participants compared to diabetics (OR= 0.84 and OR=0.99 respectively, P for interaction=0.01). The National Institutes of Health-AARP Diet and Health Study evaluated this association prospectively in diabetes-free individuals and included cases of PC.111 Adherence to the MD on of the components of the healthy lifestyle score which also considered other factors such as tobacco use, alcohol drinking, BMI, and physical activity. According to this study, those who scored the highest on the lifestyle score had a significantly lower risk of PC compared to those who scored lowest (Relative risk =0.42, 95% CI: 0.26–0.66).111 A plausible mechanism that can explain the role of MD as a cancer-preventive diet is based on the suppression of inflammation and carcinogenic pathways. This has been attributed to the powerful effect of nutritional components of the MD diet, which include healthy fats (omega 6 and omega 3), fibers, antioxidants, fruits, vegetables, legumes, and olive oil.112
However, this is inconsistent and limited among available studies. In a large European prospective cohort study,113 adherence to a non-alcohol-defined Mediterranean diet score (arMED) was not associated with PC risk, nor was there evidence of a significant association between the arMED score and risk of PC in stratified analyses by diabetes, smoking status, BMI, or European region. Moreover, A meta-analysis was conducted to investigate the association between adherence to the Mediterranean diet and the overall risk of cancer. The study results showed that colorectal cancer risk could decrease by 14% and prostate cancer risk could be reduced by 4%. However, the decrease in risk for breast, gastric, or PC was not significant.114 This was in line with a pooled analysis of two Dutch cohorts, which found no significant association between adherence to the Mediterranean diet and the risk of PC.115
Dietary Pattern Rich in Vegetables and Fruits and the Risk of PC
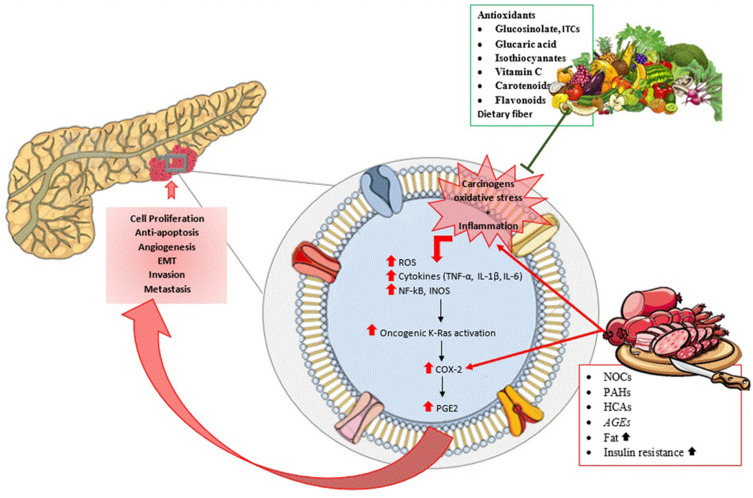
The majority of PC risk factors are thought to cause oxidative stress, activate inflammation, and, eventually result in high molecular alterations, which could lead to the prognosis of PC116,117 (Figure 1). Fruits and vegetables, which are high in antioxidants and anti-inflammatory nutrients, could result in a decrease in oxidative stress and inflammation.118
Figure 1.
Carcinogens, oxidative stress and inflammation can trigger inflammatory pathways that promote cell proliferation, anti-apoptosis, angiogenesis, epithelial-to-mesenchymal transition (EMT), invasion and metastasis. The presence of antioxidant nutrients (carotenoids and vitamin C), dietary fiber, and other phytochemicals (eg, flavonoids) provide a potential explanation for the protective effect of fruits and vegetables on PC by preventing the carcinogens, oxidative stress, and inflammation which initiate cancer pathogenesis. Cruciferous vegetables specifically have other potent antioxidants such as glucosinolate, indole-3-carbinol (ITCs), glucaric acid, and isothiocyanates. On the other hand, mechanisms that link red or processed meat to pancreatic carcinogenesis, include by-products of cooking methods such as polycyclic aromatic hydrocarbons (PAHs), heterocyclic amines (HCAs), N-nitroso compounds (NOCs), advanced glycation end-products (AGEs), increased fat intake, and insulin resistance.
A case-control study found that the majority of the nutrients in fruits and vegetables, both dietary and in supplements, are associated with a lower risk of PC.119 In this study, cases were more likely than controls to be older, males, non-smokers, and with a history of pancreatitis or diabetes. In other words, when compared to their controls, the cases had high-risk factors that have been consistently associated with PC risk, yet the consumption of fruits and vegetables was effective.119 The mechanisms of fruits and vegetables’ cancer-preventive effects could be due to various factors. The presence of antioxidant nutrients (eg, carotenoids and vitamin C), dietary fiber, and other phytochemicals (eg, flavonoids) provide a potential explanation for this protective effect.120 Vitamin C reduces free radicals and reactive oxygen molecules, protecting against oxidative damage while inhibiting carcinogen formation and shielding DNA from mutagenic attack.12 Also, flavones have been shown to inhibit the cell process linked to carcinogenesis.12 In a prospective Japanese study,9 they studied the relationship between fruit and vegetable consumption and PC and found that total fruit intake was negatively associated with PC risk and positively associated with total vegetable intake in every smoker. However, vegetable consumption may be associated with an increased risk due to the impact of smoking status on vegetable consumption.9
A hospital-based, case-control study included 183 PC patients and 732 to study the association between cruciferous vegetables (ie, broccoli, cauliflower, cabbage, and Brussels sprouts) and PC,121 and used a self-administered questionnaire to collect data. They found that usual intakes of raw cruciferous vegetables were negatively related to PC. where the highest level of raw cruciferous vegetable intake was associated with a 40% lower risk of PC in the study participants, and a 50–59% lower risk in groups that included non-modifiable risk factors, such as previous smokers, overweight participants, and males.121 This study supports the powerful anti-carcinogenic effect of cruciferous vegetables acting against PC.
Patients with pancreatic adenocarcinoma had significantly elevated levels of total unconjugated bile acids.122 One suggested plausible mechanism is related to the release of bile acid, in conjunction with a long common channel of the biliary and pancreatic ducts, which could result in bile acid reflux into the pancreatic duct and to the epithelial, or acinar, cells that give rise to pancreatic adenocarcinoma.123 Moreover, bile acids increase the expression of cyclooxygenase-2 (COX-2) in PC cells.124 COX-2, an enzyme that catalyzes prostaglandin synthesis, is known to be overexpressed in pancreatic adenocarcinoma.124 Cruciferous vegetables have properties that may help the bile production process. In an in-vitro study, cruciferous vegetables, indole-3-carbinol (ITC) and glucaric acid have bile acid binding properties and are powerful antioxidants and inducers of certain enzymes that act as detoxifying agents of harmful metabolites and bile acids, consequently inhibiting mechanisms of bile acid that potentially could result in PC.121 These vegetables also contain phytochemicals which help prevent PC. Glucosinolate is a dietary ITC precursor that has been shown to preserve chemically induced tumors by regulating epigenetic mechanisms.121 ITC and some isothiocyanates, compounds found in cruciferous vegetables, such as sulforaphane, benzyl isothiocyanate, and phenethyl isothiocyanate, have been shown in animal studies to have a suppressive effect on cancer cells of the pancreas.125
Despite that many case-control studies have found an inverse association between the consumption of fruits and vegetables and the risk of PC, A meta-analysis of prospective studies found that consuming large amounts of fruits and vegetables citrus fruit or cruciferous vegetables was not associated with a lower risk of PC.10 It was also stated that, while increased consumption of fruits is associated with a lower PC risk in men but not in women, the studies included are limited.10 Furthermore, dose-response analyses revealed no significant dose-response relationships between an increase in fruit and vegetable consumption of 100 g/d and PC risk. Moreover, the results of subgroup and subtype analyses were consistent with the results of the original analyses.10 Concluding that prospective study analyses revealed no evidence of the relationship between fruit and vegetable consumption and the risk of PC.
Overall, the association between the consumption of fruits and vegetables and the risk of PC is inconclusive. A better understanding and further examination of this association are required.
Dietary Patterns Rich in Red and Processed Meat and the Risk of PC
Based on a review of mechanistic and human evidence for colorectal cancer, the International Agency of Research on Cancer (IARC) has classified red and processed meats as probable and definite carcinogens, respectively.126 Yet, the association between red and processed meat and PC is very limited and inconclusive. According to a systematic review and meta-analysis, there was an association between the consumption of red and processed meat and the risk of PC in case-control studies but not in cohort studies.8 More recently, Petrick et al 20207 found a positive association between red and unprocessed red meat and the risk of PC in African American women with a mean age of 50 years, but not among women of a younger age. Consumption of red and processed meat, on the other hand, appeared to increase the risk of PC in men but not in women in cohort studies. In another meta-analysis conducted on studies investigating the association between red and processed meat intake and the risk of PC,45 it was found that consumption of processed meat has a statistically positive association with the risk of PC. A 50 g increase in processed meat consumption per day was linked to a 19% increased risk of PC. While consumption of red meat was only positively associated with PC in men, that was explained by suggesting a threshold effect in red meat consumption which could be detected only at higher amounts usually achieved by men than in women on average. However, in a longitudinal study done on African American women, higher intake of red meat compared to lower intake was associated with a 66% increase in the risk of PC in 50 years old women and older, while no significant association was found with the consumption of processed meat. In women aged 50 and older, intake of saturated fat was associated with an increase in the risk of PC, but the findings were not statistically significant.7
Several plausible mechanisms have been suggested to link red or processed meat to increased carcinogenesis in the pancreas, which includes the consumption of by-products of cooking such as polycyclic aromatic hydrocarbons, heterocyclic amines, N-nitroso compounds, advanced glycation end-products,127,128 and increased insulin resistance.129 Several studies aimed at investigating mutagens formed in meats as a result of grilling, barbecuing, or high-temperature cooking that increases the mutagenic activity in the body and leads to an increased risk of PC.45,130 In experimental models, N-nitroso compounds are known to be potent carcinogens,131 and tobacco smoking is a well-known risk factor for PC.132 Other than that, exposure to N-nitroso compounds could be through food consumption. N-nitrosamines form specifically in meat that has been preserved with nitrates, such as cured, smoked, and pickled meat, or meat that has been dried at high temperatures, which adds to the endogenously formed N-nitroso compounds that form in the stomach by the ingestion of nitrate and amides found in meat.133 Through the bloodstream, both consumed and endogenously formed N-nitroso compounds reach the pancreas and act as potent carcinogens there.7 Moreover, saturated fat and red and processed meat are linked to increased insulin resistance, which can lead to type 2 diabetes. Pre-existing type 2 diabetes is linked to an increased risk of PC.129
Dietary Patterns Rich in Sugar and Sugar-Sweetened Soft Drink Consumption and Risk of PC
Diabetes mellitus patients are at a higher risk of PC.134 Researchers found a dose-response relationship between PC risk and fasting glucose levels even in populations with a normal range of blood glucose,135 supporting a causal relationship between the two variables. Another study found an association between post-load serum glucose concentration and the risk of PC. Thereby, supporting the association of the role of impaired glucose intolerance, insulin resistance, and hyperinsulinemia in PC.136,137 Furthermore, a high glycemic load diet has been linked to an increased risk of diabetes and PC.136
Sugar-sweetened soft drinks are high in fructose corn syrup, which quickly increases serum glucose levels and consequently increase the risk of diabetes upon frequent consumption. Soft drinks deliver the greatest amounts of added sugar in the diet in America, adding to the high glycemic index content of the diet and driving the development of obesity and diabetes.138 Increased consumption of sugar-sweetened beverages was linked to an increase in weight gain and risk of type 2 diabetes in previous research of Nurses’ Health Study (NHS) participants, regardless of the risk factors.138 Because of their readily absorbable carbohydrates, sugar-sweetened soft drinks have been linked to an increased risk of type 2 diabetes. Sweetened soft drinks may contribute to the overall diet’s high glycemic load, which is a risk factor for PC.136 Furthermore, cola soft drinks have caramel coloring, which is high in advanced glycation end products, which may worsen insulin resistance and inflammation.138
On the other hand, a systematic review and meta-analysis conducted on dietary fructose, carbohydrate, glycemic indices, and risk of PC found that high glycemic index, glycemic load, total carbohydrates, or sucrose diets are not associated with the risk of PC. However, they reported an association between the consumption of fructose and an increased risk of PC.22 The exact mechanism underlying the association between fructose consumption and PC is unknown, but fructose metabolism differs from that of other carbohydrates such as glucose. Fructose contributes more to nucleic acid synthesis than glucose through the pentose phosphate pathway, which is catalyzed by transketolase.139 The synthesis of nucleic acids and nucleotides is required for the proliferation of tissues, particularly cancer cells. Transketolase-like protein 1 suppression reduces cancer cell proliferation, whereas transketolase activation promotes tumor growth.139 Furthermore, animal studies have shown that chronic fructose feeding causes insulin resistance and obesity.140 A positive relationship was found between fructose consumption and type 2 diabetes and obesity, establishing risk factors for PC.141 However, this association remains inconsistent and requires further investigation.
Conclusion
In summary, this narrative review showed inconsistent findings for associations between nutrient intake and dietary patterns and PC risk. The high intake of sugar, fructose, and fat may increase the risk of PC, whereas the consumption of dietary fiber, fat-soluble vitamins, water-soluble vitamins, and minerals might play a protective role against PC. Dietary patterns characterized by high consumption of red and processed meats, sugar, and sugar-sweetened soft drinks were associated with an elevated risk of PC, and dietary patterns rich in plant-based foods, vegetables, fruits, whole grains, legumes, and white meat and associated antioxidants and polyphenols reduced the risk of PC. Further studies are warranted to assess the potential anticarcinogenic role of specific foods against PC because the diet could have an impact on the development of PC. More studies are needed to verify and clarify the underlying reasons for the gender disparities in the association between nutrient intake and dietary patterns with PC risk.
Funding Statement
Open access funding provided by Qatar National Library for this review.
Ethics Policies
This research did not request ethical approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing or conflicts of interest for this work.
References
- 1.Global cancer observatory; 2020. Available from: https://gco.iarc.fr/. Accessed February 10, 2022.
- 2.Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24(19):2047. doi: 10.3748/wjg.v24.i19.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Khalaf N, El-Serag HB, Abrams HR, et al. Burden of pancreatic cancer: from epidemiology to practice. Clin Gastroenterol Hepatol. 2021;19(5):876–884. doi: 10.1016/j.cgh.2020.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63. doi: 10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J, Guinter MA, Merchant AT, et al. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev. 2017;75(11):883–908. doi: 10.1093/nutrit/nux038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrick JL, Castro-Webb N, Gerlovin H, et al. A prospective analysis of intake of red and processed meat in relation to pancreatic cancer among African American women. Cancer Epidemiol Prev Biomarkers. 2020;29(9):1775–1783. doi: 10.1158/1055-9965.EPI-20-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z, Yin Z, Pu Z, et al. Association between consumption of red and processed meat and pancreatic cancer risk: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(4):486–93. e10. doi: 10.1016/j.cgh.2016.09.143 [DOI] [PubMed] [Google Scholar]
- 9.Yamagiwa Y, Sawada N, Shimazu T, et al. Fruit and vegetable intake and pancreatic cancer risk in a population-based cohort study in Japan. Int J Cancer. 2019;144(8):1858–1866. doi: 10.1002/ijc.31894 [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Yu P, Feng X, et al. No associations between fruit and vegetable consumption and pancreatic cancer risk: a meta-analysis of prospective studies. Oncotarget. 2018;9(63):32250. doi: 10.18632/oncotarget.23128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alizadeh S, Shab-Bidar S, Mohtavinejad N, et al. A posteriori dietary patterns and risk of pancreatic and renal cancers. Nutr Food Sci. 2017;47:839–868. doi: 10.1108/NFS-03-2017-0053 [DOI] [Google Scholar]
- 12.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi: 10.1093/jn/nxz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravi F, Polesel J, Bosetti C, et al. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case–control study. Ann Oncol. 2011;22(1):202–206. doi: 10.1093/annonc/mdq302 [DOI] [PubMed] [Google Scholar]
- 14.Han X, Li J, Brasky TM, et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) study. Cancer. 2013;119(7):1314–1320. doi: 10.1002/cncr.27936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wang X, Sun X, et al. Vitamin intake and pancreatic cancer risk reduction: a meta-analysis of observational studies. Medicine. 2018;97(13):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nucci D, Santangelo OE, Provenzano S, et al. Dietary fiber intake and risk of pancreatic cancer: systematic review and meta-analysis of observational studies. Int J Environ Res Public Health. 2021;18(21):11556. doi: 10.3390/ijerph182111556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wang W-J, Zhai L, et al. Association of cholesterol with risk of pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21(12):3711. doi: 10.3748/wjg.v21.i12.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nöthlings U, Murphy SP, Wilkens LR, et al. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am J Clin Nutr. 2008;88(6):1653–1662. doi: 10.3945/ajcn.2008.26398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arem H, Bobe G, Sampson J, et al. Flavonoid intake and risk of pancreatic cancer in the National Institutes of Health-AARP diet and health study cohort. Br J Cancer. 2013;108(5):1168–1172. doi: 10.1038/bjc.2012.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue-Choi M, Flood A, Robien K, et al. Nutrients, food groups, dietary patterns and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol Prev Biomarkers. 2011;20:711–714. doi: 10.1158/1055-9965.EPI-11-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao L, Flood A, Subar AF, et al. Glycemic index, carbohydrates, glycemic load, and the risk of pancreatic cancer in a prospective cohort study. Cancer Epidemiol Prev Biomarkers. 2009;18(4):1144–1151. doi: 10.1158/1055-9965.EPI-08-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aune D, Chan D, Vieira A, et al. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23(10):2536–2546. doi: 10.1093/annonc/mds076 [DOI] [PubMed] [Google Scholar]
- 23.Johnson KJ, Anderson KE, Harnack L, et al. No association between dietary glycemic index or load and pancreatic cancer incidence in postmenopausal women. Cancer Epidemiol Prev Biomarkers. 2005;14(6):1574–1575. doi: 10.1158/1055-9965.EPI-05-0138 [DOI] [PubMed] [Google Scholar]
- 24.Silvera SA, Rohan TE, Jain M, et al. Glycemic index, glycemic load, and pancreatic cancer risk (Canada). Cancer Causes Control. 2005;16(4):431–436. doi: 10.1007/s10552-004-5028-7 [DOI] [PubMed] [Google Scholar]
- 25.Tseng T-S, Lin H-Y, Griffiths L, et al. Sugar intake from sugar-sweetened beverage among cancer and non-cancer individuals: the NHANES study. Transl Cancer Res. 2016;5(5):1019–1028. doi: 10.21037/tcr.2016.09.42 [DOI] [Google Scholar]
- 26.Wang F, Herrington M, Larsson J, et al. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2(1):1–5. doi: 10.1186/1476-4598-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinhold CL, Dodd KW, Jiao L, et al. Available carbohydrates, glycemic load, and pancreatic cancer: is there a link? Am J Epidemiol. 2010;171(11):1174–1182. doi: 10.1093/aje/kwq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaud DS, Liu S, Giovannucci E, et al. Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst. 2002;94(17):1293–1300. doi: 10.1093/jnci/94.17.1293 [DOI] [PubMed] [Google Scholar]
- 29.Genkinger JM, Li R, Spiegelman D, et al. Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer Epidemiol Prev Biomarkers. 2012;21(2):305–318. doi: 10.1158/1055-9965.EPI-11-0945-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller NT, Odegaard A, Anderson K, et al. Soft drink and juice consumption and risk of pancreatic cancer: the Singapore Chinese Health Study. Cancer Epidemiol Prev Biomarkers. 2010;19(2):447–455. doi: 10.1158/1055-9965.EPI-09-0862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nöthlings U, Murphy SP, Wilkens LR, et al. Dietary glycemic load, added sugars, and carbohydrates as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2007;86(5):1495–1501. doi: 10.1093/ajcn/86.5.1495 [DOI] [PubMed] [Google Scholar]
- 32.Bidoli E, Pelucchi C, Zucchetto A, et al. Fiber intake and pancreatic cancer risk: a case–control study. Ann Oncol. 2012;23(1):264–268. doi: 10.1093/annonc/mdr060 [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Dhakal IB, Gross MD, et al. Physical activity, diet, and pancreatic cancer: a population-based, case-control study in Minnesota. Nutr Cancer. 2009;61(4):457–465. doi: 10.1080/01635580902718941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Vecchia C, Chatenoud L, Negri E, et al. Whole grain cereals and cancer in Italy. Proc Nutr Soc. 2003;62(1):45–49. doi: 10.1079/PNS2002235 [DOI] [PubMed] [Google Scholar]
- 35.Chan JM, Wang F, Holly EA. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol Prev Biomarkers. 2005;14(9):2093–2097. doi: 10.1158/1055-9965.EPI-05-0226 [DOI] [PubMed] [Google Scholar]
- 36.Hallfrisch J, Behall KM. Mechanisms of the effects of grains on insulin and glucose responses. J Am Coll Nutr. 2000;19(sup3):320S–25S. doi: 10.1080/07315724.2000.10718967 [DOI] [PubMed] [Google Scholar]
- 37.Jensen MK, Koh-Banerjee P, Franz M, et al. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. Am J Clin Nutr. 2006;83(2):275–283. doi: 10.1093/ajcn/83.2.275 [DOI] [PubMed] [Google Scholar]
- 38.Garcia AL, Otto B, Reich SC, et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur J Clin Nutr. 2007;61(3):334–341. doi: 10.1038/sj.ejcn.1602525 [DOI] [PubMed] [Google Scholar]
- 39.Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19(2):71–84. doi: 10.1016/j.jnutbio.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 40.Delzenne NM, Cani PD. A place for dietary fibre in the management of the metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2005;8(6):636–640. doi: 10.1097/01.mco.0000171124.06408.71 [DOI] [PubMed] [Google Scholar]
- 41.Benítez-Páez A, Pulgar EMG, Kjølbæk L, et al. Impact of Dietary Fiber and Fat on Gut Microbiota Re-Modeling and Metabolic Health. Elsevier; 2016. [Google Scholar]
- 42.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155(9):783–792. doi: 10.1093/aje/155.9.783 [DOI] [PubMed] [Google Scholar]
- 43.Ghorbani Z, Pourshams A, Fazeltabar MA, et al. Major dietary protein sources in relation to pancreatic cancer: a large prospective study. 2016. [PubMed]
- 44.Ghadirian P, Nkondjock A. Consumption of food groups and the risk of pancreatic cancer: a case–control study. J Gastrointest Cancer. 2010;41(2):121–129. doi: 10.1007/s12029-009-9127-2 [DOI] [PubMed] [Google Scholar]
- 45.Larsson S, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer. 2012;106(3):603–607. doi: 10.1038/bjc.2011.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohrmann S, Linseisen J, Nöthlings U, et al. Meat and fish consumption and risk of pancreatic cancer: results from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2013;132(3):617–624. doi: 10.1002/ijc.27637 [DOI] [PubMed] [Google Scholar]
- 47.Larsson SC, Håkanson N, Permert J, et al. Meat, fish, poultry and egg consumption in relation to risk of pancreatic cancer: a prospective study. Int J Cancer. 2006;118(11):2866–2870. doi: 10.1002/ijc.21732 [DOI] [PubMed] [Google Scholar]
- 48.Qin B, Xun P, He K. Fish or long-chain (n-3) PUFA intake is not associated with pancreatic cancer risk in a meta-analysis and systematic review. J Nutr. 2012;142(6):1067–1073. doi: 10.3945/jn.111.156711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y, Ma Y, Yu M, et al. Poultry and fish intake and pancreatic cancer risk: a systematic review and meta-analysis. Nutr Cancer. 2020;2020:1–13. [DOI] [PubMed] [Google Scholar]
- 50.Gold EB, Gordis L, Diener MD, et al. Diet and other risk factors for cancer of the pancreas. Cancer. 1985;55(2):460–467. doi: [DOI] [PubMed] [Google Scholar]
- 51.Olsen GW, Mandel JS, Gibson RW, et al. A case-control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health. 1989;79(8):1016–1019. doi: 10.2105/AJPH.79.8.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raymond L, Infante F, Tuyns A, et al. Diet and cancer of the pancreas. Gastroenterol Clin Biol. 1987;11(6–7):488–492. [PubMed] [Google Scholar]
- 53.Bao Y, Hu F, Giovannucci E, et al. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. 2013;109(11):2911–2916. doi: 10.1038/bjc.2013.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Appel M, Meijers M, Garderen-Hoetmer V, et al. Role of cholecystokinin in dietary fat-promoted azaserine-induced pancreatic carcinogenesis in rats. Br J Cancer. 1992;66(1):46–50. doi: 10.1038/bjc.1992.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghadirian P, Lynch H, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27(2):87–93. doi: 10.1016/S0361-090X(03)00002-3 [DOI] [PubMed] [Google Scholar]
- 56.Heinen MM, Verhage BA, Goldbohm RA, et al. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer. 2009;125(5):1118–1126. doi: 10.1002/ijc.24387 [DOI] [PubMed] [Google Scholar]
- 57.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20(2):197–209. doi: 10.1016/j.bpg.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Jiao L, Chen L, White DL, et al. Low-fat dietary pattern and pancreatic cancer risk in the women’s health initiative dietary modification randomized controlled trial. J Natl Cancer Institute. 2018;110(1):49–56. doi: 10.1093/jnci/djx117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nöthlings U, Wilkens LR, Murphy SP, et al. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst. 2005;97(19):1458–1465. doi: 10.1093/jnci/dji292 [DOI] [PubMed] [Google Scholar]
- 60.Thiébaut AC, Jiao L, Silverman DT, et al. Dietary fatty acids and pancreatic cancer in the NIH-AARP diet and health study. J Natl Cancer Institute. 2009;101(14):1001–1011. doi: 10.1093/jnci/djp168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nkondjock A, Krewski D, Johnson K, et al. Specific fatty acid intake and the risk of pancreatic cancer in Canada. Br J Cancer. 2005;92(5):971–977. doi: 10.1038/sj.bjc.6602380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghamarzad Shishavan N, Masoudi S, Mohamadkhani A, et al. Dietary intake of fatty acids and risk of pancreatic cancer: golestan cohort study. Nutr J. 2021;20(1):1–14. doi: 10.1186/s12937-021-00723-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Ma DW. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014;6(11):5184–5223. doi: 10.3390/nu6115184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strouch MJ, Ding Y, Salabat MR, et al. A high omega-3 fatty acid diet mitigates murine pancreatic precancer development. J Surg Res. 2011;165(1):75–81. doi: 10.1016/j.jss.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, Qian SY. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed J. 2014;37(3):112. doi: 10.4103/2319-4170.131378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Qin S, Wang M, et al. Association between cholesterol intake and pancreatic cancer risk: evidence from a meta-analysis. Sci Rep. 2015;5(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferretti G, Bacchetti T, Nègre-Salvayre A, et al. Structural modifications of HDL and functional consequences. Atherosclerosis. 2006;184(1):1–7. doi: 10.1016/j.atherosclerosis.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 68.Haddy N, Sass C, Droesch S, et al. IL-6, TNF-α and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170(2):277–283. doi: 10.1016/S0021-9150(03)00287-9 [DOI] [PubMed] [Google Scholar]
- 69.Ji B-T, Chow W-H, Gridley G, et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiol Prev Biomarkers. 1995;4(8):885–893. [PubMed] [Google Scholar]
- 70.Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci. 2005;102(38):13604–13609. doi: 10.1073/pnas.0506390102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du J, Martin SM, Levine M, et al. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16(2):509–520. doi: 10.1158/1078-0432.CCR-09-1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci. 2007;104(21):8749–8754. doi: 10.1073/pnas.0702854104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schernhammer E, Wolpin B, Rifai N, et al. Plasma folate, vitamin B6, vitamin B12, and homocysteine and pancreatic cancer risk in four large cohorts. Cancer Res. 2007;67(11):5553–5560. doi: 10.1158/0008-5472.CAN-06-4463 [DOI] [PubMed] [Google Scholar]
- 74.Wei D-H, Mao -Q-Q. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: a meta-analysis. Nutr J. 2020;19(1):1–12. doi: 10.1186/s12937-020-00628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case–control study. Cancer Causes Control. 2009;20(8):1317–1325. doi: 10.1007/s10552-009-9352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Institute. 2017;109(3):djw230. doi: 10.1093/jnci/djw230 [DOI] [PubMed] [Google Scholar]
- 77.Timp W, Bravo HC, McDonald OG, et al. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. Genome Med. 2014;6(8):1–11. doi: 10.1186/s13073-014-0061-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olsen GW, Mandel JS, Gibson RW, et al. Nutrients and pancreatic cancer: a population-based case-control study. Cancer Causes Control. 1991;2(5):291–297. doi: 10.1007/BF00051668 [DOI] [PubMed] [Google Scholar]
- 79.Lin Y, Tamakoshi A, Hayakawa T, et al. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J Gastroenterol. 2005;40(3):297–301. doi: 10.1007/s00535-004-1537-0 [DOI] [PubMed] [Google Scholar]
- 80.Pili R, Salumbides B, Zhao M, et al. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer. 2012;106(1):77–84. doi: 10.1038/bjc.2011.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosewicz S, Brembeck F, Kaiser A, et al. Differential growth regulation by all-trans retinoic acid is determined by protein kinase C alpha in human pancreatic carcinoma cells. Endocrinology. 1996;137(8):3340–3347. doi: 10.1210/endo.137.8.8754760 [DOI] [PubMed] [Google Scholar]
- 82.Rosewicz S, Wollbergs K, Von Lampe B, et al. Retinoids inhibit adhesion to laminin in human pancreatic carcinoma cells via the alpha 6 beta 1-integrin receptor. Gastroenterology. 1997;112(2):532–542. doi: 10.1053/gast.1997.v112.pm9024307 [DOI] [PubMed] [Google Scholar]
- 83.Guan J, Zhang H, Wen Z, et al. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014;345(1):132–139. doi: 10.1016/j.canlet.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 84.Bao Y, Ng K, Wolpin B, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer. 2010;102(9):1422–1427. doi: 10.1038/sj.bjc.6605658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skinner HG, Michaud DS, Giovannucci E, et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Prev Biomarkers. 2006;15(9):1688–1695. doi: 10.1158/1055-9965.EPI-06-0206 [DOI] [PubMed] [Google Scholar]
- 86.Zablotska LB, Gong Z, Wang F, et al. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case–control study in the San Francisco Bay area. Cancer Causes Control. 2011;22(1):91–100. doi: 10.1007/s10552-010-9678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu S-L, Zhao Y-P, Dai M-H, et al. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J. 2013;126(17):3356–3359. [PubMed] [Google Scholar]
- 88.Pike J, Meyer MB, Bishop KA. Regulation of target gene expression by the vitamin D receptor-an update on mechanisms. Rev Endocr Metab Disord. 2012;13(1):45–55. doi: 10.1007/s11154-011-9198-9 [DOI] [PubMed] [Google Scholar]
- 89.Persons KS, Eddy VJ, Chadid S, et al. Anti-growth effect of 1, 25-dihydroxyvitamin D3-3-bromoacetate alone or in combination with 5-amino-imidazole-4-carboxamide-1-β-4-ribofuranoside in pancreatic cancer cells. Anticancer Res. 2010;30(6):1875–1880. [PubMed] [Google Scholar]
- 90.Robey RB, Hay N. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. In: Seminars in Cancer Biology. Elsevier; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiang K-C, Chen TC. Vitamin D for the prevention and treatment of pancreatic cancer. World J Gastroenterol. 2009;15(27):3349. doi: 10.3748/wjg.15.3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiang K-C, Yeh C-N, Hsu J-T, et al. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial–mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014;354(2):235–244. doi: 10.1016/j.canlet.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 93.Husain K, Francois RA, Yamauchi T, et al. Vitamin E δ-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-κB activation in pancreatic cancer. Mol Cancer Ther. 2011;10(12):2363–2372. doi: 10.1158/1535-7163.MCT-11-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shin-Kang S, Ramsauer VP, Lightner J, et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51(6):1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 95.Hodul PJ, Dong Y, Husain K, et al. Vitamin E δ-tocotrienol induces p27Kip1-dependent cell-cycle arrest in pancreatic cancer cells via an E2F-1-dependent mechanism. PLoS One. 2013;8(2):e52526. doi: 10.1371/journal.pone.0052526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greco E, Basso D, Fadi E, et al. Analogs of vitamin E epitomized by α-tocopheryl succinate for pancreatic cancer treatment: in vitro results induce caution for in vivo applications. Pancreas. 2010;39(5):662–668. doi: 10.1097/MPA.0b013e3181c8b48c [DOI] [PubMed] [Google Scholar]
- 97.Shibayama-Imazu T, Sakairi S, Watanabe A, et al. Vitamin K2 selectively induced apoptosis in ovarian TYK-nu and pancreatic MIA PaCa-2 cells out of eight solid tumor cell lines through a mechanism different from geranylgeraniol. J Cancer Res Clin Oncol. 2003;129(1):1–11. doi: 10.1007/s00432-002-0393-7 [DOI] [PubMed] [Google Scholar]
- 98.Wei G, Wang M, Carr BI. Sorafenib combined vitamin K induces apoptosis in human pancreatic cancer cell lines through RAF/MEK/ERK and c‐Jun NH2‐terminal kinase pathways. J Cell Physiol. 2010;224(1):112–119. doi: 10.1002/jcp.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan H, Yu Y, Nan H, et al. Associations between intake of calcium, magnesium and phosphorus and risk of pancreatic cancer: a population-based, case–control study in Minnesota. Br J Nutr. 2021;126(10):1549–1557. doi: 10.1017/S0007114521000283 [DOI] [PubMed] [Google Scholar]
- 100.Kesavan Y, Giovannucci E, Fuchs CS, et al. A prospective study of magnesium and iron intake and pancreatic cancer in men. Am J Epidemiol. 2010;171(2):233–241. doi: 10.1093/aje/kwp373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molina‐Montes E, Wark PA, Sánchez MJ, et al. Dietary intake of iron, heme‐iron and magnesium and pancreatic cancer risk in the European prospective investigation into cancer and nutrition cohort. Int J Cancer. 2012;131(7):E1134–E47. doi: 10.1002/ijc.27547 [DOI] [PubMed] [Google Scholar]
- 102.Li L, Gai X. The association between dietary zinc intake and risk of pancreatic cancer: a meta-analysis. Biosci Rep. 2017;37(3):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alkhatib AJ. Chromium in controlling diabetes and metabolic aspects. Adv Obes Weight Manag Control. 2021;11(3):86–88. doi: 10.15406/aowmc.2021.11.00340 [DOI] [Google Scholar]
- 104.Kim J, Chung K, Johnson BJ. Chromium acetate stimulates adipogenesis through regulation of gene expression and phosphorylation of adenosine monophosphate-activated protein kinase in bovine intramuscular or subcutaneous adipocytes. Asian Austral J Anim Sci. 2020;33(4):651. doi: 10.5713/ajas.19.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen C, Xie Z, Shen Y, et al. The roles of thyroid and thyroid hormone in pancreas: physiology and pathology. Int J Endocrinol. 2018;2018:1–14. doi: 10.1155/2018/2861034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thornburg KL, Chattergoon NN. Thyroid hormone and pancreas development: diabetes culprit or innocent bystander? J Physiol. 2017;595(11):3261. doi: 10.1113/JP274090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amaral AF, Porta M, Silverman DT, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61(11):1583–1588. doi: 10.1136/gutjnl-2011-301086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banim PJ, Luben R, McTaggart A, et al. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013;62(10):1489–1496. doi: 10.1136/gutjnl-2011-301908 [DOI] [PubMed] [Google Scholar]
- 109.Burney P, Comstock G, Morris J. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49(5):895–900. doi: 10.1093/ajcn/49.5.895 [DOI] [PubMed] [Google Scholar]
- 110.Bosetti C, Turati F, Dal Pont A, et al. The role of Mediterranean diet on the risk of pancreatic cancer. Br J Cancer. 2013;109(5):1360–1366. doi: 10.1038/bjc.2013.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiao L, Mitrou PN, Reedy J, et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med. 2009;169(8):764–770. doi: 10.1001/archinternmed.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giacosa A, Barale R, Bavaresco L, et al. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev. 2013;22(1):90–95. doi: 10.1097/CEJ.0b013e328354d2d7 [DOI] [PubMed] [Google Scholar]
- 113.Molina-Montes E, Sanchez M-J, Buckland G, et al. Mediterranean diet and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Br J Cancer. 2017;116(6):811–820. doi: 10.1038/bjc.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta‐analysis of observational studies. Int J Cancer. 2014;135(8):1884–1897. doi: 10.1002/ijc.28824 [DOI] [PubMed] [Google Scholar]
- 115.Schulpen M, Peeters PH, Brandt PA, van den Brandt PA. Mediterranean diet adherence and risk of pancreatic cancer: a pooled analysis of two Dutch cohorts. Int J Cancer. 2019;144(7):1550–1560. doi: 10.1002/ijc.31872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Polvani S, Tarocchi M, Tempesti S, et al. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J Gastroenterol. 2016;22(8):2441. doi: 10.3748/wjg.v22.i8.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Gioia F, Tzortzakis N, Rouphael Y, et al. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part II: leafy, fruit, and other vegetables. Antioxidants. 2020;9(2):97. doi: 10.3390/antiox9020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer. 2013;44(2):152–161. doi: 10.1007/s12029-012-9441-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakai M, Kitagawa Y, Saeki H, et al. Fruit and vegetable consumption and risk of esophageal cancer in the Asian region: a systematic review and meta-analysis. Esophagus. 2022;19(1):27–38. doi: 10.1007/s10388-021-00882-6 [DOI] [PubMed] [Google Scholar]
- 121.Morrison ME, Hobika EG, Joseph JM, et al. Cruciferous vegetable consumption and pancreatic cancer: a case-control study. Cancer Epidemiol. 2021;72:101924. doi: 10.1016/j.canep.2021.101924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rees DO, Crick PJ, Jenkins GJ, et al. Comparison of the composition of bile acids in bile of patients with adenocarcinoma of the pancreas and benign disease. J Steroid Biochem Mol Biol. 2017;174:290–295. doi: 10.1016/j.jsbmb.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng H-Y, Chen Y-C. Role of bile acids in carcinogenesis of pancreatic cancer: an old topic with new perspective. World J Gastroenterol. 2016;22(33):7463. doi: 10.3748/wjg.v22.i33.7463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong Z, Huang W, Wang B, et al. Interplay between cyclooxygenase‑2 and microRNAs in cancer. Mol Med Rep. 2021;23(5):1–10. doi: 10.3892/mmr.2020.11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casari I, Falasca M. Diet and pancreatic cancer prevention. Cancers. 2015;7(4):2309–2317. doi: 10.3390/cancers7040892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1 [DOI] [PubMed] [Google Scholar]
- 127.Ruan Y, Poirier AE, Hebert LA, et al. Estimates of the current and future burden of cancer attributable to red and processed meat consumption in Canada. Prev Med. 2019;122:31–39. doi: 10.1016/j.ypmed.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 128.Chiang VS-C, Quek S-Y. The relationship of red meat with cancer: effects of thermal processing and related physiological mechanisms. Crit Rev Food Sci Nutr. 2017;57(6):1153–1173. doi: 10.1080/10408398.2014.967833 [DOI] [PubMed] [Google Scholar]
- 129.Kim Y, Keogh J, Clifton P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism. 2015;64(7):768–779. doi: 10.1016/j.metabol.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 130.Anderson KE, Mongin SJ, Sinha R, et al. Pancreatic cancer risk: associations with meat‐derived carcinogen intake in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Mol Carcinog. 2012;51(1):128–137. doi: 10.1002/mc.20794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eichholzer M, Gutzwiller F. Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutr Rev. 1998;56(4):95–105. doi: 10.1111/j.1753-4887.1998.tb01721.x [DOI] [PubMed] [Google Scholar]
- 132.Korc M, Jeon CY, Edderkaoui M, et al. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2017;31(5):529–536. doi: 10.1016/j.bpg.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oostindjer M, Alexander J, Amdam GV, et al. The role of red and processed meat in colorectal cancer development: a perspective. Meat Sci. 2014;97(4):583–596. doi: 10.1016/j.meatsci.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 134.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47(13):1928–1937. doi: 10.1016/j.ejca.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 135.Koo D-H, Han K-D, Park C-Y. The incremental risk of pancreatic cancer according to fasting glucose levels: nationwide population-based cohort study. J Clin Endocrinol Metab. 2019;104(10):4594–4599. doi: 10.1210/jc.2019-00033 [DOI] [PubMed] [Google Scholar]
- 136.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51(1):64–74. doi: 10.1002/mc.20771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schernhammer ES, Hu FB, Giovannucci E, et al. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Prev Biomarkers. 2005;14(9):2098–2105. doi: 10.1158/1055-9965.EPI-05-0059 [DOI] [PubMed] [Google Scholar]
- 138.Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep. 2012;12(2):195–203. doi: 10.1007/s11892-012-0259-6 [DOI] [PubMed] [Google Scholar]
- 139.Liu H, Huang D, McArthur DL, et al. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010;70(15):6368–6376. doi: 10.1158/0008-5472.CAN-09-4615 [DOI] [PubMed] [Google Scholar]
- 140.Dekker MJ, Su Q, Baker C, et al. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metabol. 2010;299(5):E685–E94. doi: 10.1152/ajpendo.00283.2010 [DOI] [PubMed] [Google Scholar]
- 141.Kolderup A, Svihus B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J Nutr Metab. 2015;2015:1–12. doi: 10.1155/2015/823081 [DOI] [PMC free article] [PubMed] [Google Scholar]